Commentary: Clinical characteristics of male prolactinoma patients mainly presenting with severe obesity and the metabolic response to dopamine agonist therapy
- 1Department of Endocrinology, Key Laboratory of Endocrinology of National Health Commission, Peking Union Medical College Hospital, Peking Union Medical College, Chinese Academy of Medical Sciences, Beijing, China
- 2Shanxi Yuncheng Central Hospital, Yuncheng, China
Objective: To summarize the clinical characteristics of 4 male prolactinoma patients with severe obesity.
Methods: The clinical data of all the patients were retrospectively analyzed.
Results: All the patients visited our hospital for severe obesity at the age of 16-30 years old with their body mass index (BMI) of 37.9-55.9 kg/m2. All the patients were obese since childhood, even at birth. Hyperprolactinemia (72.3-273.0 ng/ml) was found during the etiological screening of obesity and MRI revealed pituitary adenomas. Additionally, all of them had multiple obesity related complications, such as hyperinsulinemia and dyslipidemia. Treatment of dopamine agonists (DAs) effectively normalized their prolactin level and the pituitary MRI reexamination after 6 months of DAs treatment showed the shrinkage of the pituitary adenomas in 3 patients. Their weight also decreased in different degrees (2.70~19.03% lower than the baseline) with improved metabolic profiles.
Conclusion: Serum prolactin level should be screened in obese patients, especially those with severe obesity.
Introduction
Obesity is an overwhelming prevalent chronic metabolic disease caused by a variety of factors. Previous studies have demonstrated that hyperprolactinemia, especially prolactinoma, can lead to obesity (1). Meanwhile, obesity has also been proved to be related to dysfunction of dopaminergic pathways (2). Here we summarized the clinical characteristics of 4 male prolactinoma patients, mainly presenting with severe obesity, which might provide us with further insights into the relationship between obesity and hyperprolactinemia.
Methods
Clinical data from 4 male prolactinoma patients with early-onset severe obesity in the endocrinology department of a tertiary medical center Peking Union Medical College Hospital of China from September 2016 to August 2022 were retrospectively analyzed.
Results
Baseline clinical characteristics
As shown in Table 1, all of the 4 patients (Case 1 to 4) visited our hospital due to severe obesity. Their age at the first visit was 16-30 years old, and their body mass index (BMI) was 37.9-55.9 kg/m2. All the patients had been obese since childhood, and the birth weight of 3 patients was over 4 kilograms. Hyperprolactinemia (72.3-273.0 ng/ml) was found during the aetiological screening of obesity. Further inquiry revealed that they denied any medical history of antipsychotics and gastric motility drugs. All of them denied delayed puberty. They had no complaints of headache or vision impairment. 3 patients reported hypolibido and erectile dysfunction. Physical examination showed that all of them had gynaecomastia without galactorrhea and their testes were normal in size. The evaluation of other anterior pituitary function showed that they all had hypogonadotropic hypogonadism, and 2 of them had decreased insulin like growth factor 1 (IGF1). None of the patients had secondary hypothyroidism or adrenal insufficiency. MRI revealed pituitary adenomas (the maximum diameter ranged from 9 to 17 mm). Therefore, prolactinoma was suspected. The levels of PTH, serum calcium, glucagon and gastrin were measured, which were all in normal range excluding the clinical diagnosis of multiple endocrine neoplasia type 1. No genetic screenings of AIP or MEN1 mutations were performed.
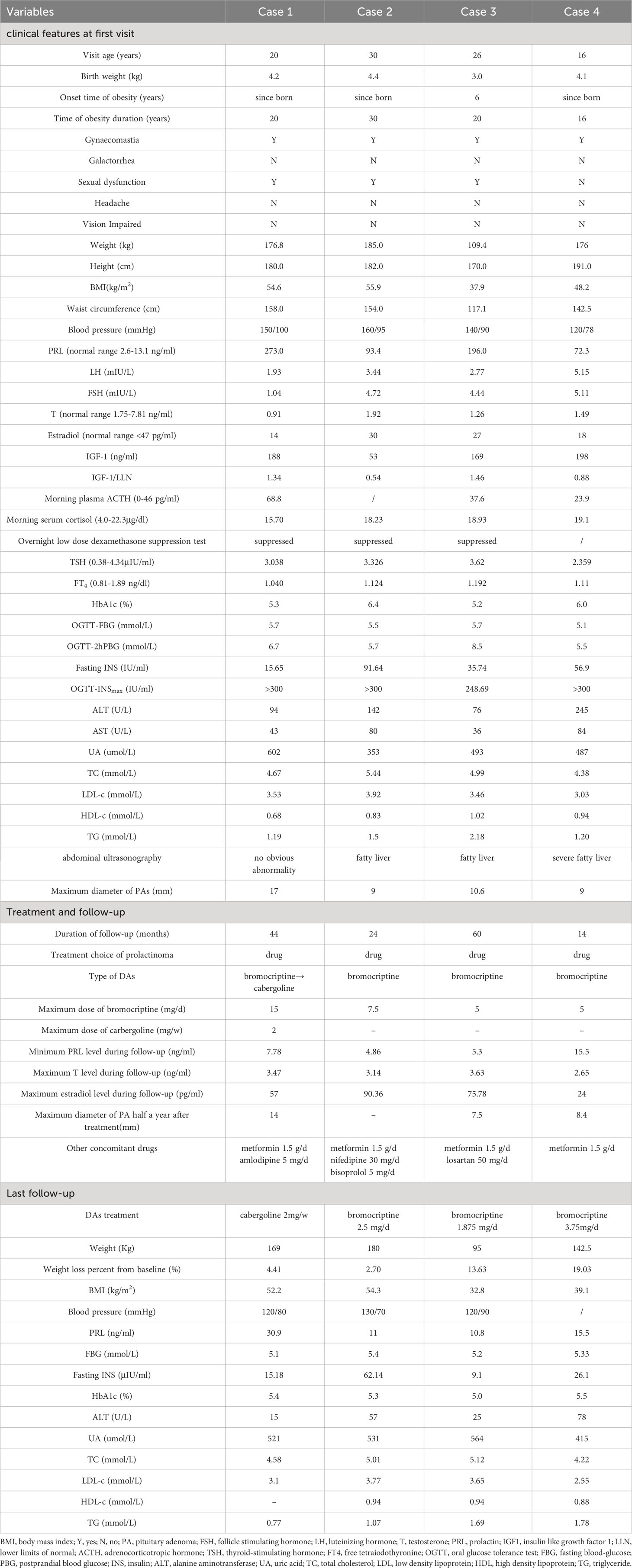
Table 1 The clinical features of 4 male prolactinoma patients mainly presenting with early-onset severe obesity.
All the patients were evaluated for obesity related complications: hyperinsulinemia was found in all of them and Case 3 had impaired glucose tolerance; 3 patients had dyslipidemia, including elevated low density lipoprotein (LDL-c) and triglyceride (TG), and decreased high density lipoprotein (HDL-c); All of them had abnormal liver function and fatty liver was found in 3 patients by abdominal ultrasound. 3 patients had hypertension; 3 patients had hyperuricemia.
Treatment and follow-up
The above patients were followed up for 14 ~ 44 months in our center.
Dopamine agonists (DAs) were used to treat their hyperprolactinemia. Case 1 was initially treated with bromocriptine. The drug dose was gradually increased to 15mg/d according to his prolactin (PRL) level. However, his prolactin level was still significantly increased at about 100ng/ml, suggesting the resistance for bromocriptine. Cabergoline was then used with the maximum dose of 2mg/w, resulting in a PRL reduction to below 30ng/ml. Cases 2 to 4 were treated with bromocriptine with the maximum dose of 5 to 7.5 mg/d, and their PRL level were successfully controlled within 20 ng/ml. In Case 1, 3 and 4, after 6 months of DAs treatment, the pituitary MRI reexamination showed that the pituitary adenomas had shrunk compared to before. During the follow-up, the testosterone levels of all patients were significantly higher than the baseline, and their erection dysfunction was improved. The monitoring of sex hormones revealed that the estradiol in Case 1 to 3 increased intermittently, with the maximum level of 57 to 90.36 pg/ml.
In addition, lifestyle guidance for obesity was given to all the patients. Metformin and antihypertensive drugs were administered according to the complications (Table 1). Their weight decreased in different degrees (2.70 to 19.03% lower than the baseline) during the following-up. At the same time, fasting insulin and liver function of all the patients were improved.
Discussion
Another 3 adult male prolactinoma patients with severe obesity were reported previously (3–5) (as shown in Table 2). Their age at diagnosis of prolactinomas was 24-39 years old, with their BMI of 64.4, 47.09 and 45.67 kg/m2, respectively. 2 of them mentioned their obesity onset age of 19 and 17 years old. Along with our patients, they also got weight loss and improvement of their metabolic abnormality after DAs, bariatric surgery and other treatments.
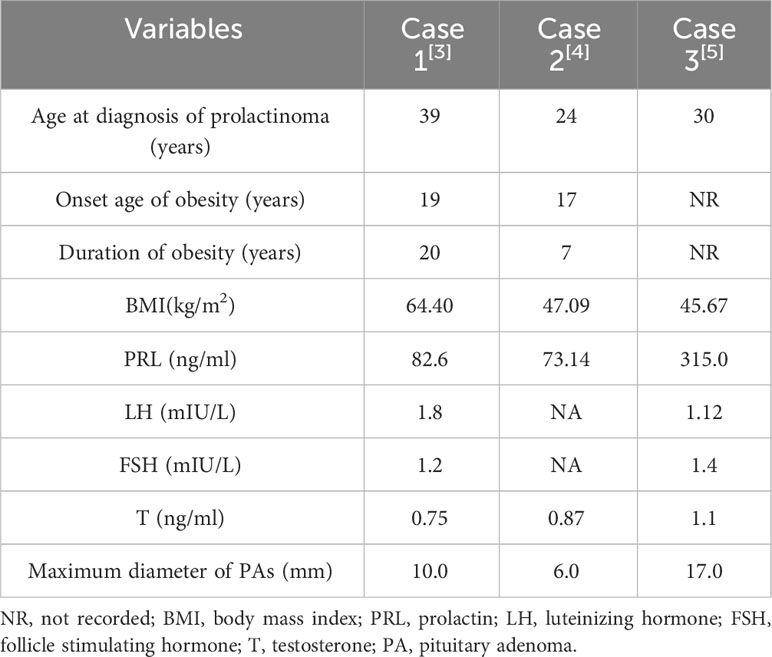
Table 2 Main clinical characteristics of 3 male prolactinoma patients with severe obesity in the literature.
PRL has been recognized as a regulatory factor of energy homeostasis during physiological and pathophysiological conditions, such as increasing leptin synthesis and secretion, permitting the circadian variation in lipogenic responsiveness (6–9). Previous animal and clinical studies have shown that hyperprolactinemia can cause obesity and related metabolic abnormalities. In the female mice lacking dopamine D2 receptors in lactotropes, long-term chronic hyperprolactinemia was found to increase the expression of the orexigenic genes, such as neuropeptide Y, in the hypothalamic arcuate nucleus and ventromedial nucleus, resulting in obvious weight gain and leptin resistance from the age of 5-10 months (10). Moreover, severe hyperprolactinemia was observed to promote brown adipose tissue whitening and exacerbate high-fat-diet-induced energy imbalance (11). On the contrary, in the mice lacking prolactin receptors, their beige differentiation of adipose depots was found to protect against high-fat-diet-induced obesity (12). In clinical studies, it was observed that prolactinoma patients had higher BMI than the general population, and the BMI of male patients increased more significantly (1). Additionally, the average BMI of patients with macroprolactinomas was significantly higher than that of patients with nonfunctioning pituitary macroadenomas (13).
Obesity can lead to dysfunction of dopamine related pathways: obesity was found to affect the availability of dopamine transporter in the midbrain striatum (14), lower forebrain dopamine levels (15, 16); Additionally, the level of dopamine D2 receptor in obese patients was lower, and its availability was also decreased (17–19).
Till now, DA is still selected as the first-line treatment for most prolactinomas (20). Mirjana Doknic,et al had reported that bromocriptine, by increasing dopaminergic tone, could influence body weight and likely body composition by mechanisms in addition to reducing hyperprolactinemia in prolatinoma patients (21).Ezrokhi M et al. revealed that timed daily DA treatment improved hypothalamic and neuroendocrine pathologies associated with metabolic syndrome in SHR rats, which coupled to a transformation of liver metabolism potentiating a reduction of elevated lipogenic and gluconeogenic capacity (22). Therefore, DAs therapy for the prolactinoma patients might bring additional metabolic benefits beyond simply reducing hyperprolactinemia.
In conclusion, this paper describes the clinical characteristics of 4 male prolactinoma patients with severe obesity as the main clinical manifestation. Hyperprolactinemia/prolactinoma can cause and aggravate obesity through a variety of ways. Serum prolactin level should be screened in obese patients, especially those with long-term and severe obesity, in order to avoid miss-diagnosis of hyperprolactinemia.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
LW: writing – original draft. XW: writing – review & editing. FG: writing – review & editing. HP: writing – review & editing. ZH: writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by National High Level Hospital Clinical Research Funding (2022-PUMCH-A-064) and National key clinical specialty improvement project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Al Sabie F, Tariq Z, Erickson D, Donegan D. Association between prolactinoma and body mass index. Endocr Pract (2021) 27(4):312–7. doi: 10.1016/j.eprac.2020.09.001
2. Leite F, Ribeiro L. Dopaminergic pathways in obesity-associated inflammation. J Neuroimmune Pharmacol (2020) 15(1):93–113. doi: 10.1007/s11481-019-09863-0
3. Mollar Puchades MA, Gómez RC, del Olmo García MI, Marco JL, Portolés RS, Galiana PA, et al. Hypogonadotropic hypogonadism in a patient with morbid obesity. Obes Surg (2007) 17(8):1127–31. doi: 10.1007/s11695-007-9190-3
4. Yan JP, Han ZP, Li TT, Yang XL, Hu Y. Severe obesity caused by prolactinoma related to the abnormal insulin metabolism-A case report. Obes Res Clin Pract (2021) 15(5):506–8. doi: 10.1016/j.orcp.2021.07.004
5. Ali M, Mirza L. Morbid obesity due to prolactinoma and significant weight loss after dopamine agonist treatment. AACE Clin Case Rep (2021) 7(3):204–6. doi: 10.1016/j.aace.2021.01.004
6. Carré N, Binart N. Prolactin and adipose tissue. Biochimie (2014) 97:16–21. doi: 10.1016/j.biochi.2013.09.023
7. Cincotta AH, Meier AH. Prolactin permits the expression of a circadian variation in lipogenic responsiveness to insulin in hepatocytes of the golden hamster (Mesocricetus auratus). J Endocrinol (1985) 106:173–6. doi: 10.1677/joe.0.1060173
8. Gualillo Lago O, García F, Menéndez M, Señarís C, Casanueva FF, et al. Prolactin stimulates leptin secretion by rat white adipose tissue. Endocrinology (1999) 140:5149–53. doi: 10.1210/endo.140.11.7147
9. Pirchio R, Graziadio C, Colao A, Pivonello R, Auriemma RS. Metabolic effects of prolactin. Front Endocrinol (Lausanne). (2022) 13:1015520. doi: 10.3389/fendo.2022.1015520
10. Lopez-Vicchi F, Ladyman SR, Ornstein AM, Gustafson P, Knowles P, Luque GM, et al. Chronic high prolactin levels impact on gene expression at discrete hypothalamic nuclei involved in food intake. FASEB J (2020) 34(3):3902–14. doi: 10.1096/fj.201902357R
11. Lopez-Vicchi F, De Winne C, Ornstein AM, Sorianello E, Toneatto J, Becu-Villalobos D. Severe hyperprolactinemia promotes brown adipose tissue whitening and aggravates high fat diet induced metabolic imbalance. Front Endocrinol (Lausanne). (2022) 13:883092. doi: 10.3389/fendo.2022.883092
12. Auffret J, Viengchareun S, Carré N, Denis RG, Magnan C, Marie PY, et al. Beige differentiation of adipose depots in mice lacking prolactin receptor protects against high-fat-diet-induced obesity. FASEB J (2012) 26(9):3728–37. doi: 10.1096/fj.12-204958
13. Schmid C, Goede DL, Hauser RS, Brändle M. Increased prevalence of high Body Mass Index in patients presenting with pituitary tumours: severe obesity in patients with macroprolactinoma. Swiss Med Wkly (2006) 136(15-16):254–8. doi: 2006/15/smw-10955
14. Nam SB, Kim K, Kim BS, Im HJ, Lee SH, Kim SJ, et al. The effect of obesity on the availabilities of dopamine and serotonin transporters. Sci Rep (2018) 8(1):4924. doi: 10.1038/s41598-018-22814-8
15. Hansen HH, Jensen MM, Overgaard A, Weikop P, Mikkelsen JD. Tesofensine induces appetite suppression and weight loss with reversal of low forebrain dopamine levels in the diet-induced obese rat. Pharmacol Biochem Behav (2013) 110:265–71. doi: 10.1016/j.pbb.2013.07.018
16. Zhang C, Wei NL, Wang Y, Wang X, Zhang JG, Zhang K. Deep brain stimulation of the nucleus accumbens shell induces anti-obesity effects in obese rats with alteration of dopamine neurotransmission. Neurosci Lett (2015) 589:1–6. doi: 10.1016/j.neulet.2015.01.019
17. Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, et al. Brain dopamine and obesity. Lancet (2001) 357(9253):354–7. doi: 10.1016/S0140-6736(00)03643-6
18. Volkow ND, Wang GJ, Telang F, Fowler JS, Thanos PK, Logan J, et al. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. Neuroimage (2008) 42(4):1537–43. doi: 10.1016/j.neuroimage.2008.06.002
19. de Weijer BA, van de Giessen E, van Amelsvoort TA, Boot E, Braak B, Janssen IM, et al. Lower striatal dopamine D2/3 receptor availability in obese compared with non-obese subjects. EJNMMI Res (2011) 1(1):37. doi: 10.1186/2191-219X-1-37
20. Petersenn S, Fleseriu M, Casanueva FF, Giustina A, Biermasz N, Biller B, et al. Diagnosis and management of prolactin-secreting pituitary adenomas: a Pituitary Society international Consensus Statement. Nat Rev Endocrinol (2023) 19:722–740. doi: 10.1038/s41574-023-00916-2
21. Doknic M, Pekic S, Zarkovic M, Medic-Stojanoska M, Dieguez C, Casanueva F, et al. Dopaminergic tone and obesity: an insight from prolactinomas treated with bromocriptine. Eur J Endocrinol (2002) 147:77–84. doi: 10.1530/eje.0.1470077
Keywords: Prolactinoma, obesity, male, dopamine agonist, metabolic response
Citation: Wang L, Wang X, Gong F, Pan H and Zhu H (2023) Clinical characteristics of male prolactinoma patients mainly presenting with severe obesity and the metabolic response to dopamine agonist therapy. Front. Endocrinol. 14:1285477. doi: 10.3389/fendo.2023.1285477
Received: 30 August 2023; Accepted: 08 November 2023;
Published: 29 November 2023.
Edited by:
Xiang’En Shi, Capital Medical University, ChinaReviewed by:
Mirjana Doknic, University of Belgrade, SerbiaAnthony Cincotta, VeroScience LLC, United States
Copyright © 2023 Wang, Wang, Gong, Pan and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huijuan Zhu, c2hlbmd4aW4yMDA0QDE2My5jb20=
 Linjie Wang
Linjie Wang Xiaojing Wang1,2
Xiaojing Wang1,2 Fengying Gong
Fengying Gong Hui Pan
Hui Pan Huijuan Zhu
Huijuan Zhu