
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 16 October 2023
Sec. Clinical Diabetes
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1283181
This article is part of the Research Topic Technologies for Diabetes, volume II View all 12 articles
 Alzbeta Santova1,2
Alzbeta Santova1,2 Lukas Plachy1
Lukas Plachy1 Vit Neuman1
Vit Neuman1 Marketa Pavlikova3
Marketa Pavlikova3 Lenka Petruzelkova1
Lenka Petruzelkova1 Petra Konecna4
Petra Konecna4 Petra Venhacova5
Petra Venhacova5 Jaroslav Skvor6
Jaroslav Skvor6 Renata Pomahacova7
Renata Pomahacova7 David Neumann8
David Neumann8 Jan Vosahlo9
Jan Vosahlo9 Jiri Strnadel10
Jiri Strnadel10 Kamila Kocourkova11
Kamila Kocourkova11 Barbora Obermannova1
Barbora Obermannova1 Stepanka Pruhova1
Stepanka Pruhova1 Ondrej Cinek1
Ondrej Cinek1 Zdenek Sumnik1* on behalf of the ČENDA Project Group
Zdenek Sumnik1* on behalf of the ČENDA Project GroupObjective: To compare parameters of glycemic control among three types of hybrid closed loop (HCL) systems in children with T1D (CwD) using population-wide data from the national pediatric diabetes registry ČENDA.
Methods: CwD aged <19 years treated with Medtronic MiniMed 780G (780G), Tandem t:slim X2 (Control-IQ) or do-it-yourself AndroidAPS (AAPS) systems for >12 months and monitored by CGM >70% of the time were included. HbA1c, times in glycemic ranges, and Glycemia Risk Index (GRI) were used for cross-sectional comparison between the HCL systems.
Results: Data from 512 CwD were analyzed. 780G, Control-IQ and AAPS were used by 217 (42.4%), 211 (41.2%), and 84 (16.4%) CwD, respectively. The lowest HbA1c value was observed in the AAPS group (44 mmol/mol; IQR 8.0, p<0.0001 vs any other group), followed by Control-IQ and 780G groups (48 (IQR 11) and 52 (IQR 10) mmol/mol, respectively). All of the systems met the recommended criteria for time in range (78% in AAPS, 76% in 780G, and 75% in Control-IQ users). CwD using AAPS spent significantly more time in hypoglycemia (5% vs 2% in 780G and 3% in Control-IQ) and scored the highest GRI (32, IQR 17). The lowest GRI (27, IQR 15) was seen in 780G users.
Conclusion: Although all HCL systems proved effective in maintaining recommended long-term glycemic control, we observed differences that illustrate strengths and weaknesses of particular systems. Our findings could help in individualizing the choice of HCL systems.
Recent advances in technology increased the chance of optimizing long-term glycemic control in people with type 1 diabetes (T1D). The latest breakthrough is represented by the hybrid closed-loop (HCL) algorithms that can modify blood glucose level based on automated insulin dose adjustment (1). Growing evidence shows that HCL represent a safe and effective tool for the overall improvement of glycemic outcomes (2–6). Several randomized trials showed superiority of HCL over any other treatment modality in children and adults with T1D (7–11).
To date, there are several HCL systems available. Among them, Tandem t:slim X2 with Control-IQ algorithm (Tandem Diabetes Care, San Diego, CA, USA) and Medtronic MiniMed 780G with SmartGuard algorithm (Medtronic Inc. Minneapolis, MN, USA) are the ones most widely used in Europe. In addition, AndroidAPS (AAPS), an unofficial do-it-yourself (DIY) HCL system, continues to maintain significant popularity (12). Although all HCL systems share the same principle of manual pre-prandial bolus administration and automated insulin dose adjustment in case of predicted hypo- or hyperglycemia, there are also several differences mainly related to glycemic targets, reaction to hyperglycemia and user adjustable settings. Moreover, the systems differ in the used algorithm: whereas Control-IQ and AAPS use manually fully adjustable algorithms, 780G uses a self-adjusting technology that limits the users ability to influence insulin dosage. Although there are proofs of the efficacy to improve glycemic outcomes in each of these systems individually (3, 13, 14), studies directly comparing different HCL systems head-to-head in real-life settings are limited.
The aim of this study is to compare the parameters of glycemic control among the three most common types of HCL algorithms used in Czechia (MiniMed 780G with SmartGuard, Tandem t:slim X2 with Control-IQ and AAPS) in children with T1D (CwD) using the population-wide data from the national pediatric diabetes registry ČENDA.
This retrospective multicenter study is based on data from the national pediatric diabetes web-based registry ČENDA, described in detail elsewhere (15). In brief, the registry stores anonymized data about CwD aged <19 years who are followed in one of the participating pediatric diabetes outpatient clinics in the Czech Republic. The data in this study are based exclusively on the annual report from 2022. Forty-seven pediatric diabetes outpatient clinics participated in ČENDA in 2022. As of December 2022, the ČENDA registry included 4427 CwD which is estimated to be more than 95% of all pediatric diabetes cases in the Czech Republic. Participation in the registry is voluntary, all participants and/or their legal representatives signed a written informed consent. ČENDA registry is approved by the Ethical Committee of the Motol University Hospital and registered at the National Bureau for Personal Data Protection.
In ČENDA registry, collected data include basic demographic information, glycemic control status, data on acute or chronic complications and comorbidities and data on the type of treatment modality and continuous glucose monitoring use and their change. CGM usage is further categorized based on the proportion of time the child spent on CGM in the past year: no use,≤19%, 20%-39%, 40%-69%, 70%-89% and ≥90% category (16).
All children with T1D aged <19 years treated with one of the following HCLs - Medtronic MiniMed 780G (780G), Tandem t:slim X2 with Control-IQ algorithm (Control-IQ) or AAPS with Dana Diabecare RS (SOOIL Development, Seoul, Republic of Korea) or Accu-Chek Insight (Roche Diabetes Care, Mannheim, Germany) insulin pump for at least 12 months and monitored by CGM more than 70% of the time were included in the analysis. The study flowchart is shown in the Supplementary Figure 1. Before the initiation of HCL therapy, all children were educated about the proper configuration of the system and its appropriate utilization.
The median HbA1c, CGM-derived parameters and Glycemia Risk Index (GRI) from the last available visit were calculated and compared between the HCL groups. CGM-derived parameters included the following parameters: time in range – TIR (3.9-10.0 mmol/L; 70-180 mg/dL); time in hyperglycemia level 1 – TAR1 (10.1-13.9 mmol/L; 181-250 mg/dL); time in hyperglycemia level 2 – TAR2 (>13.9 mmol/L; >250 mg/dL); time in hypoglycemia level 1 – TBR1 (3.0-3.8 mmol/L; 54-69 mg/dL); time in hypoglycemia level 2 – TBR2 (<3.0 mmol/L; <54 mg/dL) (17). The median of the CGM-derived parameters were calculated from the last 14 days’ CGM records before the last outpatient visit. The Glycemia Risk Index was calculated using the standard formula: GRI = (3.0 × TBR <50 mg/dL) + (2.4 × TBR <70 mg/dL) + (1.6 × TAR >250 mg/dL) + (0.8 × TAR >180 mg/dL) (18). The occurrence of severe hypoglycemia (SH) and/or diabetic ketoacidosis (DKA) in 2022 was also collected and compared across the groups. A separate age-category analysis (0–5.99, 6–11.99, and 12-18.99 years) was performed for all of the above-mentioned parameters.
Data were summarized as means with standard deviation (SD) or medians with interquartile range where appropriate. The differences between HCL groups were assessed using ANOVA F-test or Kruskall-Wallis ANOVA. Categorical variables were summarized using absolute and relative frequencies and differences between the groups were tested using χ2-test. For better insight, cumulative distribution functions for HbA1c, TIR, and GRI were used to examine the relationship between the HCL groups.
To reduce the imbalance of baseline characteristics between the groups, we used the mnps function for multiple groups of the TWANG (The Toolkit for Weighting and Analysis of Nonequivalent Groups) library (19) to estimate the propensity score weights based on gender, current age, T1D duration, insulin dose, and BMI. This type of analysis differs from usual propensity score matching, in that it allows for multiple groups to be considered at once and keeps the original sample sizes. Weighted means/medians, 1st and 3rd quartiles were computed to reassess the differences. Comparisons between HCL groups were then carried out using weighted ANOVA regression models and Tukey post-hoc analysis for pairwise comparisons.
Data from 512 CwD (276 males and 236 females) who met the inclusion criteria were analyzed. 780G, Control-IQ and AAPS were used by 217 (42.4%), 211 (41.2%) and 84 (16.4%) children, respectively. The mean age of CwD in the study cohort was 12.8 ± 4.2 years, with the age category 12+ years the most represented (n = 323), followed by children aged 6–11.99 (n = 142) and <6 years (n = 46). The mean diabetes duration was 7.0 ± 3.6 years. We observed a statistically significant difference in age, T1D duration, duration of HCL therapy, daily insulin requirement and BMI-SDS between the users of studied HCL systems. The basic characteristics of the study group are summarized in the Supplementary Table 1 in detail.
The median of HbA1c in the whole study group was 49 mmol/mol (6.6%). The ISPAD target of HbA1c <48 mmol/mol (<6.5%) (20) was reached by 76.2% of AAPS users, 49.8% of Control-IQ users, and 29.5% of 780G users. (Figure 1A). The lowest HbA1c value was seen in the AAPS users (44 mmol/mol; 6.2%; p<0.001 vs any other group), followed by the Control-IQ (48 mmol/mol, 6.5%) and 780G group (52 mmol/mol; 6.9%) (p<0.001 between the latter). (Supplementary Figure 2A). Similar results of HbA1c were observed after propensity score weighting recalculation (Figure 2A) and in all of the evaluated age groups (Supplementary Figure 3A; Supplementary Table 2).
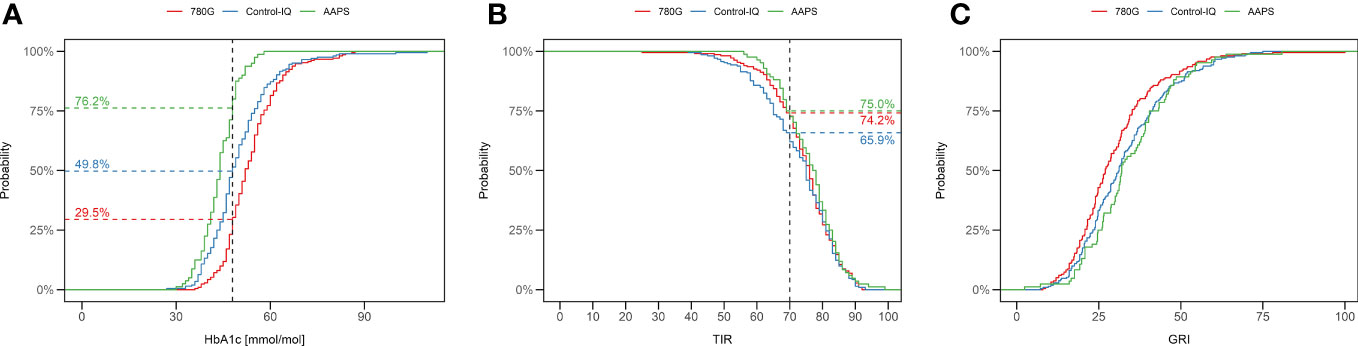
Figure 1 Percentage of children using 780G (red), Control-IQ (blue) and AAPS (green) achieving the ISPAD target of HbA1c 48mmol/mol (A), and TIR (70%) (B). The difference in proportions of CwD reaching a particular GRI value are depicted as C. (C) TIR, time in range; GRI, glycemia risk index.
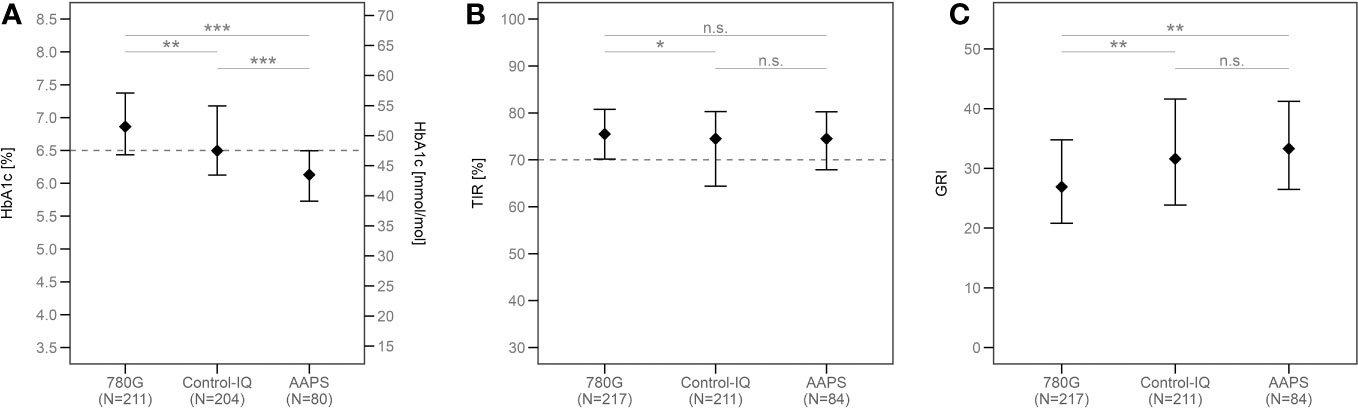
Figure 2 The medians of HbA1c (A), TIR (B) and GRI (C) in all groups according to the type of HCL system used after the propensity score weighting. ***p<0.001, **p<0.01, *p<0.05, ns, not significant. TIR, time in range; GRI, glycemia risk index; AAPS, AndroidAPS.
A detailed overview of TIR in the study groups is shown in Table 1, the means of TIR are illustrated in Figure 3. The recommended target of TIR 70% (20) was achieved by 75% of CwD in the AAPS group, 74.2% of CwD in the 780G group, and 65.9% of CwD in the Control-IQ group. (Figure 1B) The highest median of TIR was achieved by AAPS users (78%), followed by 780G (76%), and Control-IQ users (75%). Only the difference between the AAPS and the Control-IQ group was assessed as statistically significant (p=0.035). On the other hand, the AAPS group spent the longest time in hypoglycemia with the mean of TBR1 5.2% (vs 2.9% for Control-IQ and 2.5% for 780G) and TBR2 1.5% (vs 0.8% for Control-IQ and 0.6% for 780G).
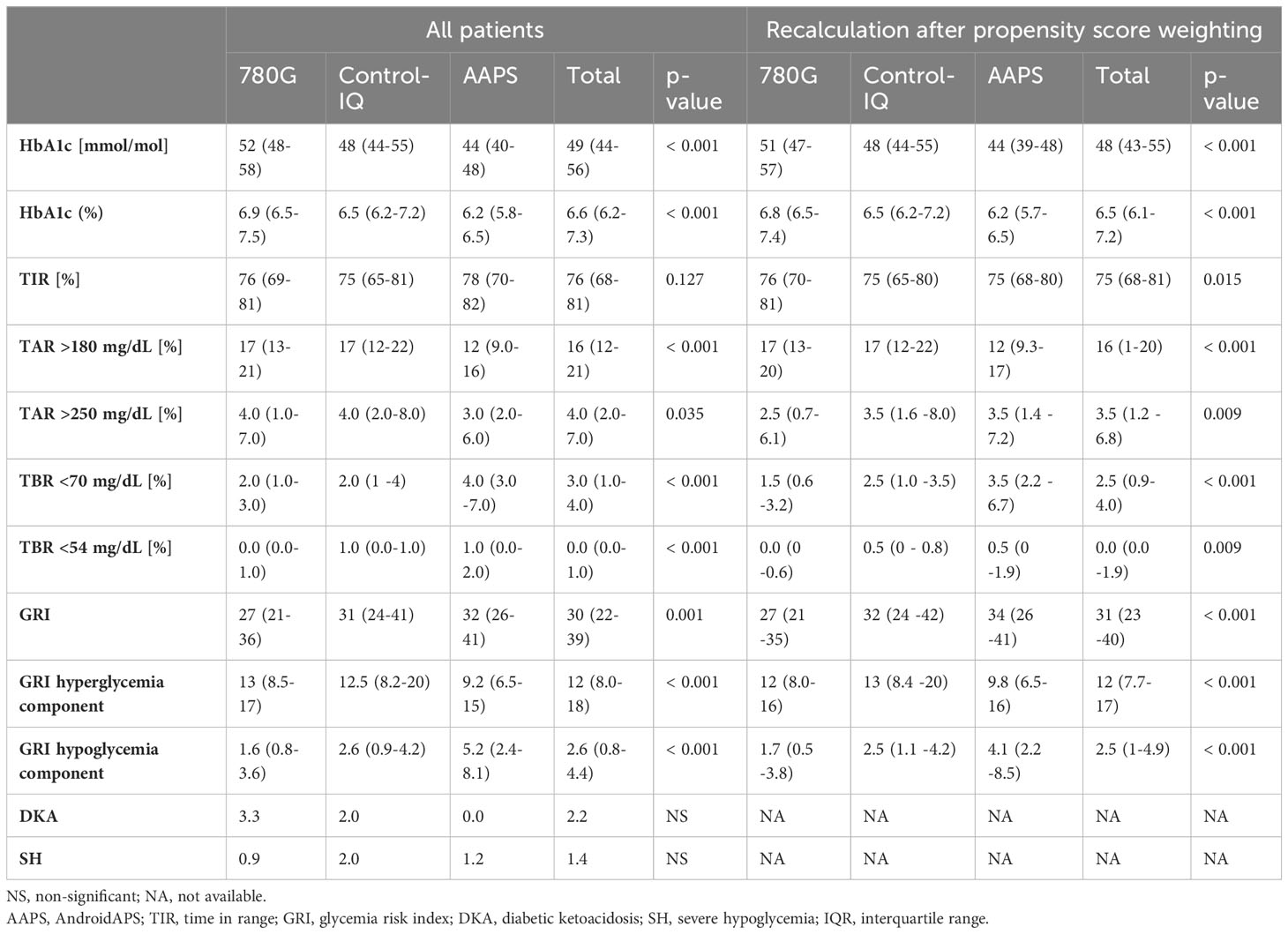
Table 1 Parameters of glycemic control by the type of HCL used. The results are shown as medians (IQR), and for DKA and SH the results are shown as events per 100-patient years.
After the recalculation using the propensity score weighting, the similar results were observed in all of the groups, with the TIR of 76% scored by 780G users, 75% by AAPS users and 75% by Control-IQ users. While the TIR of the 780G group did not differ significantly from the AAPS group (p=0.99), there was a statistical difference between the 780G and the Control-IQ group (p=0.02, Figure 2B). The medians of TIR in all age groups are shown in detail in Supplementary Table 2.
The median GRI of all CwD included in the study was 30. The lowest GRI value was achieved by the users of 780G (27), followed by Control-IQ (31) and AAPS (32) (Table 1). The difference in GRI between 780G users and the other two assessed HCL systems was significant (p<0.05), whereas no significant difference was found between the Control-IQ and AAPS groups (p=0.72). An overview of GRI results is shown in Table 1 and Supplementary Figure 2C. The cumulative distribution of GRI by HCL systems is shown in Figure 1C.
The lowest GRI in the 780G group (p<0.005 vs both other groups) as well as no significant difference between AAPS and Control-IQ users (p=0.53) was consistently observed also in the matched cohort (Figure 2C) and all age categories (Supplementary Figure 3C; Supplementary Table 2).
There was no statistically significant difference observed in the occurrence of diabetic ketoacidosis nor severe hypoglycemia events between the groups over the observed study period (Table 1).
This population-based study compared the parameters of glycemic control in CwD treated by one of the HCL systems (MiniMed 780G, Control-IQ, AAPS) for at least one year. The results revealed that all three systems are effective in achieving the international recommended goals of T1D control. Nevertheless, there are clearly discernible differences that illustrate the strengths and weaknesses of the systems assessed.
The results of well-powered pediatric studies testing the HCL systems individually are in line with our data. Arrieta et al. demonstrated a mean of TIR 73.9% in a cohort of 3211 CwD treated with 780G (21). Similarly Breton et al. described TIR 73.5% in a group of 9451 children using Control-IQ (13). These data are comparable with our findings as in our cohort the mean TIR values of 74.5% and 72.9% were recorded for 780G and Control-IQ, respectively.
To date, similarly focused studies are characterized by small number of participants and limited spectrum of outcomes. The 1-month real-life observational study of 31 CwD did not reveal any significant differences in CGM-derived parameters between Control-IQ and 780G (mean TIR 70.5% vs 70.1%) (22). In contrast, Bassi et al. compared these two systems retrospectively in a 1-year follow-up study comprising 74 children and adults with type 1 diabetes and observed a significant superiority of the 780G system in terms of time in range (71% vs 68%, p=0.001), time above range (p=0.001), average glucose levels (p=0.001) and standard deviation of glycemia (p=0.031) (23). The DIY AAPS system has not been subjected to a comparison in similar studies yet.
Our study revealed some differences in the parameters of glucose control between the HCL systems. Generally, AAPS users achieved the lowest HbA1c, however, they also presented with the highest hypoglycemia rates. In contrast, CwD using 780G were characterized by the lowest time spent in hypoglycemia and consequently scored the lowest GRI. The explanation for these differences might lie in the system settings and the algorithms used by the systems. 780G uses a self-adjusting technology and only allows users to set the insulin-to-carbohydrate ratio, target glycemia, and insulin activity. This setting significantly reduces the potential for insulin overdose when hyperglycemia is corrected by the user. This might explain the lowest hypoglycemia rate in the 780G group and consequently, the highest HbA1c value since hypoglycemia is one of the main factors contributing to the HbA1c value (24). Additionally, the 780G scored the lowest GRI underlining the fact that this index is preferentially driven by hypoglycemia rather than hyperglycemia (18).The position of AAPS is on the opposite side of the spectrum as this system enables the user to individualize and adjust any of the settings. Moreover, AAPS is a DIY system that requires the user to initially download and set it up possibly biasing this group with more motivated and tech-savvy CwD and/or their parents/guardians. Given the flexibility of AAPS input settings and the potentially higher motivation of AAPS users to achieve the lowest possible HbA1c, these users may be prone to overcorrect hyperglycemia with a subsequent risk of hypoglycemia. The Control-IQ algorithm represents a kind of middle ground between these systems. Most settings can be adjusted by the user but some functionalities (i.e. target glycemia) can only be changed to a limited extent. Thus, it scores mostly in the middle between 780G and AAPS in the evaluated parameters.
Based on our results, we propose that 780G might be an advantageous option for CwD with recurrent hypoglycemia episodes or CwD with a fear or impaired awareness of hypoglycemia. On the other hand, higher time in hypoglycemia found in the AAPS group suggests that clinicians should preferentially focus on addressing this in CwD treated with this system, possibly adjusting the settings accordingly and emphasizing the risks of hypoglycemia and its prevention.
Our study has several strengths, which encompass a representativeness of the study population (including children younger than 6 years), unique data on AAPS, and a broad spectrum of parameters (including first data on GRI in HCL systems).
There are several limitations of our study. Firstly, there were pre-existing differences between the groups in diabetes duration, age, insulin dose, and BMI-SD. The number of CwD using a specific HCL also differed. To this end, we used propensity score weighting to minimize the bias and enable a meaningful comparison. The results remained similar even after propensity score weighting which might give our findings more credence. However, despite the use of propensity score weighting, we were unable to eliminate the bias stemming from differences in individual device settings, bolus timing, and the correct use of automatic mode by the participants (25). On the other hand, all of the subjects underwent similar standardized education during the introduction of HCL which should minimize this bias. As this is a cross-sectional observational study, we cannot exclude selection bias at the level of individual diabetologists preference of one of the HCL systems. A large number of children included and the propensity score weighting analysis nonetheless mitigates this risk. Additionally, we were not able to include some relevant information that were not collected in the ČENDA database in 2022 such as average glycemia and glycemic variability.
Although all of the tested HCL systems proved effective in maintaining recommended long-term glycemic control, we observed differences that might illustrate strengths and weaknesses of particular systems. Our findings could help individualizing the choice of HCL systems.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Ethics committee of the Motol University Hospital, Prague, Czechia. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
AS: Conceptualization, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. LuP: Conceptualization, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. VN: Conceptualization, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. MP: Data curation, Formal Analysis, Methodology, Visualization, Writing – original draft, Writing – review & editing. LeP: Conceptualization, Writing – original draft, Writing – review & editing. PK: Investigation, Writing – original draft, Writing – review & editing. PV: Investigation, Writing – original draft, Writing – review & editing. JaS: Investigation, Writing – review & editing. RP: Investigation, Writing – review & editing. DN: Investigation, Writing – review & editing. JV: Investigation, Writing – review & editing. KK: Investigation, Writing – review & editing. BO: Investigation, Writing – review & editing. SP: Conceptualization, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. OC: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. ZS: Conceptualization, Funding acquisition, Investigation, Methodology, Supervision, Visualization, Writing – original draft, Writing – review & editing. JiS: Investigation, Writing - review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by grants from the Czech Diabetes Society and the Czech Ministry of Health (conceptual support project to research organization 00064203 -FN Motol).
The authors thank the remaining members of the ČENDA Study Group (in alphabetic order of cities) – Drs: J. Vyžrálková, M. Pejchlová (Brno), L. Kocinová (Česká Lípa), I. Röchlová (Frýdek-Místek), A. Kudličková (Hodonín), A. Benešová (Chomutov), J. Češek (Chrudim), M. Adam (Jablonec), P. Kracíková (Jičín), P. Vlachý, M. Svojsík (Jihlava), M. Jiřičková (Jilemnice), K. Poločková (Karviná), J. Kytnarová (Prague-VFN), K. Dimová (Kladno), S. Fialová (Kroměříž), M. Vracovská (Klatovy), J. Sivíčková, P. Pelcová (Karlovy Vary), E. Hlaďáková (Kyjov), J. Bartošová (Liberec), M. Kulinová (Mladá Boleslav), N. Filáková (Ostrava – City Hospital), M. Romanová (Prague-FNKV), M. Šnajderová, S. Koloušková (Prague – Motol), L. Týce (Náchod), E. Farkaš (Nový Jičín), Z. Ježová (Nové Město na Moravě), M. Honková (Opava), B. Červíčková (Pardubice, Trutnov), T. Farová (Písek), K. Fiklík (Plzeň), J. Malý (Sokolov), M. Gregora (Strakonice), J. Malý (Svitavy), J. Chocholová (Tábor), P. Eichl (Teplice), O. Michálková (Třebíč), M. Struminský (Třinec), M. Procházka (Ústí nad Orlicí), H. Vávrová (Vsetín), P. Gogelová (Zlín), P. Mikyška (Znojmo). Marie Kajprová and Jakub Šumník are gratefully acknowledged for help with data entry.
ZS, LP and SP reported speakers’ honoraria from Medtronic, Abbott and A-Import. VN reported speakers’ honoraria from Medtronic.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1283181/full#supplementary-material
1. Boughton CK, Hovorka R. New closed-loop insulin systems. Diabetologia (2021) 64:1007–15. doi: 10.1007/s00125-021-05391-w
2. Carlson AL, Sherr JL, Shulman DI, Garg SK, Pop-Busui R, Bode BW, et al. Safety and glycemic outcomes during the miniMedTM advanced hybrid closed-loop system pivotal trial in adolescents and adults with type 1 diabetes. Diabetes Technol Ther (2022) 24:178–89. doi: 10.1089/dia.2021.0319
3. Da Silva J, Lepore G, Battelino T, Arrieta A, Castañeda J, Grossman B, et al. Real-world performance of the miniMedTM 780G system: first report of outcomes from 4120 users. Diabetes Technol Ther (2022) 24:113–9. doi: 10.1089/dia.2021.0203
4. Pinsker JE, Müller L, Constantin A, Leas S, Manning M, McElwee Malloy M, et al. Real-world patient-reported outcomes and glycemic results with initiation of control-IQ technology. Diabetes Technol Ther (2021) 23:120–7. doi: 10.1089/dia.2020.0388
5. Sumnik Z, Pavlikova M, Neuman V, Petruzelkova L, Konecna P, Venhacova P, et al. Glycemic control by treatment modalities: national registry-based population data in children and adolescents with type 1 diabetes. Horm Res Paediatr (2023). doi: 10.1159/000530833
6. Braune K, O’Donnell S, Cleal B, Lewis D, Tappe A, Willaing I, et al. Real-world use of do-it-yourself artificial pancreas systems in children and adolescents with type 1 diabetes: online survey and analysis of self-reported clinical outcomes. JMIR Mhealth Uhealth (2019) 7:e14087. doi: 10.2196/14087
7. Collyns OJ, Meier RA, Betts ZL, Chan DSH, Frampton C, Frewen CM, et al. Improved glycemic outcomes with medtronic miniMed advanced hybrid closed-loop delivery: results from a randomized crossover trial comparing automated insulin delivery with predictive low glucose suspend in people with type 1 diabetes. Diabetes Care (2021) 44:969–75. doi: 10.2337/dc20-2250
8. Breton MD, Kanapka LG, Beck RW, Ekhlaspour L, Forlenza GP, Cengiz E, et al. A randomized trial of closed-loop control in children with type 1 diabetes. N Engl J Med (2020) 383:836–45. doi: 10.1056/NEJMoa2004736
9. Wadwa RP, Reed ZW, Buckingham BA, DeBoer MD, Ekhlaspour L, Forlenza GP, et al. Trial of hybrid closed-loop control in young children with type 1 diabetes. N Engl J Med (2023) 388:991–1001. doi: 10.1056/NEJMoa2210834
10. Choudhary P, Kolassa R, Keuthage W, Kroeger J, Thivolet C, Evans M, et al. Advanced hybrid closed loop therapy versus conventional treatment in adults with type 1 diabetes (ADAPT): a randomised controlled study. Lancet Diabetes Endocrinol (2022) 10:720–31. doi: 10.1016/S2213-8587(22)00212-1
11. Petruzelkova L, Jiranova P, Soupal J, Kozak M, Plachy L, Neuman V, et al. Pre-school and school-aged children benefit from the switch from a sensor-augmented pump to an AndroidAPS hybrid closed loop: A retrospective analysis. Pediatr Diabetes (2021) 22:594–604. doi: 10.1111/pedi.13190
12. Braune K, Lal RA, Petruželková L, Scheiner G, Winterdijk P, Schmidt S, et al. Open-source automated insulin delivery: international consensus statement and practical guidance for health-care professionals. Lancet Diabetes Endocrinol (2022) 10:58–74. doi: 10.1016/S2213-8587(21)00267-9
13. Breton MD, Kovatchev BP. One year real-world use of the control-IQ advanced hybrid closed-loop technology. Diabetes Technol Ther (2021) 23:601–8. doi: 10.1089/dia.2021.0097
14. Gawrecki A, Zozulinska-Ziolkiewicz D, Michalak MA, Adamska A, Michalak M, Frackowiak U, et al. Safety and glycemic outcomes of do-it-yourself AndroidAPS hybrid closed-loop system in adults with type 1 diabetes. PLoS One (2021) 16:e0248965. doi: 10.1371/journal.pone.0248965
15. Šumník Z, Venháčová J, Škvor J, Pomahačová R, Konečná P, Neumann D, et al. Five years of improving diabetes control in Czech children after the establishment of the population-based childhood diabetes register ČENDA. Pediatr Diabetes (2020) 21:77–87. doi: 10.1111/pedi.12929
16. Šumník Z, Pavlíková M, Pomahačová R, Venháčová P, Petruželková L, Škvor J, et al. Use of continuous glucose monitoring and its association with type 1 diabetes control in children over the first 3 years of reimbursement approval: Population data from the ČENDA registry. Pediatr Diabetes (2021) 22:439–47. doi: 10.1111/pedi.13184
17. Battelino T, Danne T, Bergenstal RM, Amiel SA, Beck R, Biester T, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care (2019) 42:1593–603. doi: 10.2337/dci19-0028
18. Klonoff DC, Wang J, Rodbard D, Kohn MA, Li C, Liepmann D, et al. A glycemia risk index (GRI) of hypoglycemia and hyperglycemia for continuous glucose monitoring validated by clinician ratings. J Diabetes Sci Technol (2022) 193229682210852. doi: 10.1177/19322968221085273
19. Cefalu M, Ridgeway G, Mccaffrey D, Morral A, Griffin AB, Burgette L. Toolkit for weighting and analysis of nonequivalent groups. R Package (2006).
20. Sundberg F, deBeaufort C, Krogvold L, Patton S, Piloya T, Smart C, et al. ISPAD Clinical Practice Consensus Guidelines 2022: Managing diabetes in preschoolers. Pediatr Diabetes (2022) 23:1496–511. doi: 10.1111/pedi.13427
21. Arrieta A, Battelino T, Scaramuzza AE, Da Silva J, Castañeda J, Cordero TL, et al. Comparison of MiniMed 780G system performance in users aged younger and older than 15 years: Evidence from 12 870 real-world users. Diabetes Obes Metab (2022) 24:1370–9. doi: 10.1111/dom.14714
22. Schiaffini R, Deodati A, Nicoletti MC, Carducci C, Ciampalini P, Lorubbio A, et al. Comparison of two advanced hybrid closed loop in a pediatric population with type 1 diabetes: a real-life observational study. Acta Diabetol (2022) 59:959–64. doi: 10.1007/s00592-022-01886-z
23. Bassi M, Patti L, Silvestrini I, Strati MF, Ponzano M, Minuto N, et al. One-year follow-up comparison of two hybrid closed-loop systems in Italian children and adults with type 1 diabetes. Front Endocrinol (Lausanne) (2023) 14:1099024. doi: 10.3389/fendo.2023.1099024
24. Tsujino D, Nishimura R, Onda Y, Seo C, Ando K, Morimoto A, et al. The relationship between HbA1c values and the occurrence of hypoglycemia as assessed by continuous glucose monitoring in patients with type 1 diabetes. Diabetol Metab Syndr (2016) 8:53. doi: 10.1186/s13098-016-0167-z
Keywords: type 1 diabetes, pediatrics, hybrid closed loop, AndroidAPS, registry
Citation: Santova A, Plachy L, Neuman V, Pavlikova M, Petruzelkova L, Konecna P, Venhacova P, Skvor J, Pomahacova R, Neumann D, Vosahlo J, Strnadel J, Kocourkova K, Obermannova B, Pruhova S, Cinek O and Sumnik Z (2023) Are all HCL systems the same? long term outcomes of three HCL systems in children with type 1 diabetes: real-life registry-based study. Front. Endocrinol. 14:1283181. doi: 10.3389/fendo.2023.1283181
Received: 25 August 2023; Accepted: 26 September 2023;
Published: 16 October 2023.
Edited by:
Maurizio Delvecchio, Giovanni XXIII Children’s Hospital, ItalyReviewed by:
Marco Marigliano, Integrated University Hospital Verona, ItalyCopyright © 2023 Santova, Plachy, Neuman, Pavlikova, Petruzelkova, Konecna, Venhacova, Skvor, Pomahacova, Neumann, Vosahlo, Strnadel, Kocourkova, Obermannova, Pruhova, Cinek and Sumnik. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zdenek Sumnik, emRlbmVrLnN1bW5pa0BsZm1vdG9sLmN1bmkuY3o=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.