
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 09 January 2024
Sec. Reproduction
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1282056
Background: Observational studies have identified a strong association between polycystic ovary syndrome (PCOS) and hormone levels related to oral inflammatory diseases. To better understand the relationship between them, we conducted an analysis using a two-sample Mendelian randomization (MR) approach.
Methods: We gathered summary statistical data from previously published genome-wide association studies (GWAS) on PCOS and three sex hormones (AMH, Estradiol, LH) along with four oral inflammatory diseases (painful gums, loose teeth, mouth ulcers, and toothache). We selected single nucleotide polymorphisms (SNPs) as instrumental variables and employed four types of MR analysis methods to evaluate causal relationships between exposure and outcome. Finally, the robustness of our results was further validated through sensitivity tests and reverse MR.
Results: We observed that PCOS could increase the risk of mouth ulcers (ORIVW= 1.0013, 95%CI: 1.0001-1.0025, PIVW = 0.0278), painful gums (ORIVW= 1.0015, 95%CI:1.0003-1.0027, PIVW = 0.0163), and loose teeth (ORIVW= 1.0014, 95%CI: 1.0001-1.0027, PIVW = 0.0328). Moreover, LH was also found to increase the risk of mouth ulcers (ORIVW= 1.0031, 95%CI: 0.0001-1.0062, PIVW = 0.0457). MR-Egger regression, weighted mode, and WE indicated similar results. Additionally, we discovered no causal link between PCOS and toothache (PIVW>0.05), LH and painful gums, loose teeth, or toothache (PIVW>0.05), or AMH and Estradiol level with any of the four oral diseases (PIVW>0.05).
Conclusion: Our research provides new insights and references for exploring the effects of PCOS and related hormones on oral inflammatory lesions. For patients with PCOS, especially those with elevated LH levels, early intervention measures should be taken to prevent the occurrence of oral inflammatory diseases.
Polycystic ovary syndrome (PCOS) is the primary cause of anovulatory infertility in women, affecting 5-15% of women of reproductive age globally (1). It is the most common endocrine disorder in this demographic. PCOS is highly heterogeneous, characterized mainly by hormonal imbalances, polycystic ovarian changes, infrequent menstruation, insulin resistance, chronic inflammation, etc (2–4). Anti-Müllerian Hormone (AMH)、Estradiol and Luteinizing Hormone (LH) are vital sex hormones in women of reproductive age, with AMH levels in PCOS patients typically 2-4 times higher than in healthy women (5). Presently, the occurrence of PCOS is thought to be associated with genetics, environment, oxidative stress, inflammatory response, gut microbiome, and autophagy among other factors (6–8). Still, the specific cause remains unclear.
Oral health is closely related to various systemic diseases, and poor oral health often reflects the overall health status of an individual (9–15). Chronic inflammation can cause damage to oral mucosa and periodontal tissues, leading to mouth ulcers and recurring inflammation in teeth supporting structures, resulting in symptoms like painful gums and loose teeth, and eventually tooth loss. Mouth ulcers and periodontitis are the most common oral inflammatory diseases, posing significant challenges to public health (16). Their etiology may be associated with oral microbial dysbiosis, host susceptibility, endocrine, and metabolic disorders (17, 18).
The relationship between PCOS and sex hormones with common oral diseases is receiving increasing attention (19) and becoming a significant focus in reproductive endocrinology (20, 21). Research has proven a close relationship between PCOS and periodontal inflammation (20, 22). Some studies have found higher probing depths in the gums of PCOS patients, suggesting an increased risk of periodontal disease (23). Meta-analyses and retrospective cohort studies further indicate a significantly increased prevalence of periodontal disease in PCOS patients (24, 25). In a study by Mutlak et al. (26), it was discovered that patients with PCOS suffering from gingivitis had higher concentrations of AMH. They recommended that all doctors refer PCOS patients to dental clinics for comprehensive oral assessment and treatment.
Past studies on their relationship mainly centered on observational research and meta-analyses, methods prone to confounding factors, and cannot exclude the possibility of reverse causality. Randomized controlled trials are considered the gold standard for etiological research but can be influenced by various experimental conditions, making implementation challenging. Mendelian randomization analysis helps overcome some deficiencies of traditional observational research methods. Since gamete occurrence and zygote formation follow Mendelian genetic laws and are irreversible, MR can effectively avoid reverse causality and confounding factors. Parental genetic variations are randomly distributed to offspring, so MR analysis is somewhat equivalent to conducting a randomized controlled experiment.
In this study, we utilized large sample GWAS data and bidirectional MR methods to infer the causal relationships between PCOS, three related hormones, and common oral inflammatory diseases.
Our research project adopted a bidirectional Mendelian randomization approach, designating PCOS and three sex hormones as exposure variables and oral lesions as outcomes, to analyze the causal relationship between PCOS, sex hormones, and four types of oral lesions. Subsequently, we used the four types of oral lesions as exposures and PCOS with three sex hormones as outcomes to reverse analyze the causal relationships (See Figure 1).
Summary data for GWAS of PCOS and four types of oral inflammatory diseases were obtained from the Finnish database and included only European populations. The PCOS sample consisted of 118,870 individuals, with 642 cases and 118,228 controls, containing a total of 16,379,676 SNPs. The mouth ulcers sample totaled 336,138 cases, with 10,894,596 SNPs. The samples for painful gums, loose teeth, and toothache totaled 461,113 cases each, containing 9,851,867 SNPs. AMH summary data were sourced from a study by Verdiesen et al. (27), incorporating 7049 premenopausal women of European ancestry with 8,298,138 SNPs. Estradiol and LH summary data were sourced from research by Ruth et al. (28) and Sun et al. (29), containing 206,927 and 3,301 European samples, respectively, with corresponding SNP totals of 16,136,413 and 10,534,735.
The SNPs chosen as instrumental variables needed to fulfill three assumptions of Mendelian randomization: a correlation with exposure; independence, free from other confounding influences; and an effect on the outcome only through exposure. We set a threshold of P< 5 × 10–6 for SNP selection, simultaneously calculating the F-value to test the strength of IV, and choosing strongly correlated instrumental variables with F >10 (30). The linkage disequilibrium coefficient was set to r2<0.001, with R2-values for linkage disequilibrium distance at 10000 kb (31). Finally, we conducted multiple sensitivity analyses, including MR-Egger regression, to exclude pleiotropy interference and manually removed SNPs associated with confounding factors.
We employed four methods for two-sample MR analysis, with inverse variance-weighted (IVW) considered the primary research method. The IVW involves calculating the Wald ratio for each SNP and then combining them using the inverse of the variance as weights (32), resulting in a more accurate overall effect estimate. In addition, MR-Egger regression (33), weighted median method, and weighted mode were used as supplementary methods to analyze exposure-outcome correlations.
Sensitivity checks were conducted to validate the robustness of MR results. We assessed heterogeneity between the two samples using Cochran’s Q test. By calculating the MR-Egger intercept’s relationship with 0, we determined whether there was horizontal pleiotropy among SNPs. If the intercept was greater than 0, horizontal pleiotropy was present, meaning outcomes would still exist without exposure interference. We used the MR-PRESSO global test to exclude IVs with horizontal pleiotropy and outliers (34). The leave-one-out method was employed, sequentially excluding each SNP and testing the remaining ones for robustness (35).
All data analyses were performed using R software 4.2.1, including the TwoSampleMR package (version 0.5.6), MR-PRESSO package, and MendelianRandomization package.
To further explore whether there exists a reverse causal relationship between PCOS, three related sex hormones, and four types of oral lesions, we conducted a reverse Mendelian randomization analysis, using the four oral lesions as exposure variables and PCOS with three sex hormones as outcomes.
When PCOS is considered as the exposure variable, at the significance level of P<5 , 10 SNPs were identified as instrumental variables. When considering AMH and Estradiol as exposure variables at the P<5 significance level, 46 and 14 SNPs, respectively, were selected as conforming instrumental variables. As for LH, when it’s taken as an exposure variable at P<5 , 9 SNPs were chosen as suitable instrumental variables. All of the instrumental variables had an F-value greater than 10, thus eliminating the impact of weak instruments (See Supplementary Table S1).
Our findings reveal that PCOS is associated with an increased risk of Mouth ulcers (ORIVW= 1.0013, 95%CI: 1.0001-1.0025, PIVW = 0.0278), painful gums (ORIVW= 1.0015, 95%CI:1.0003-1.0027, PIVW = 0.0163), and Loose teeth (ORIVW= 1.0014, 95%CI: 1.0001-1.0027, PIVW = 0.0328). MR-Egger regression, weighted mode, and WE indicated similar results (See Figures 2, 3). Moreover, we found no causal association between PCOS and Toothache (PIVW>0.05) (See Supplementary Table S5).
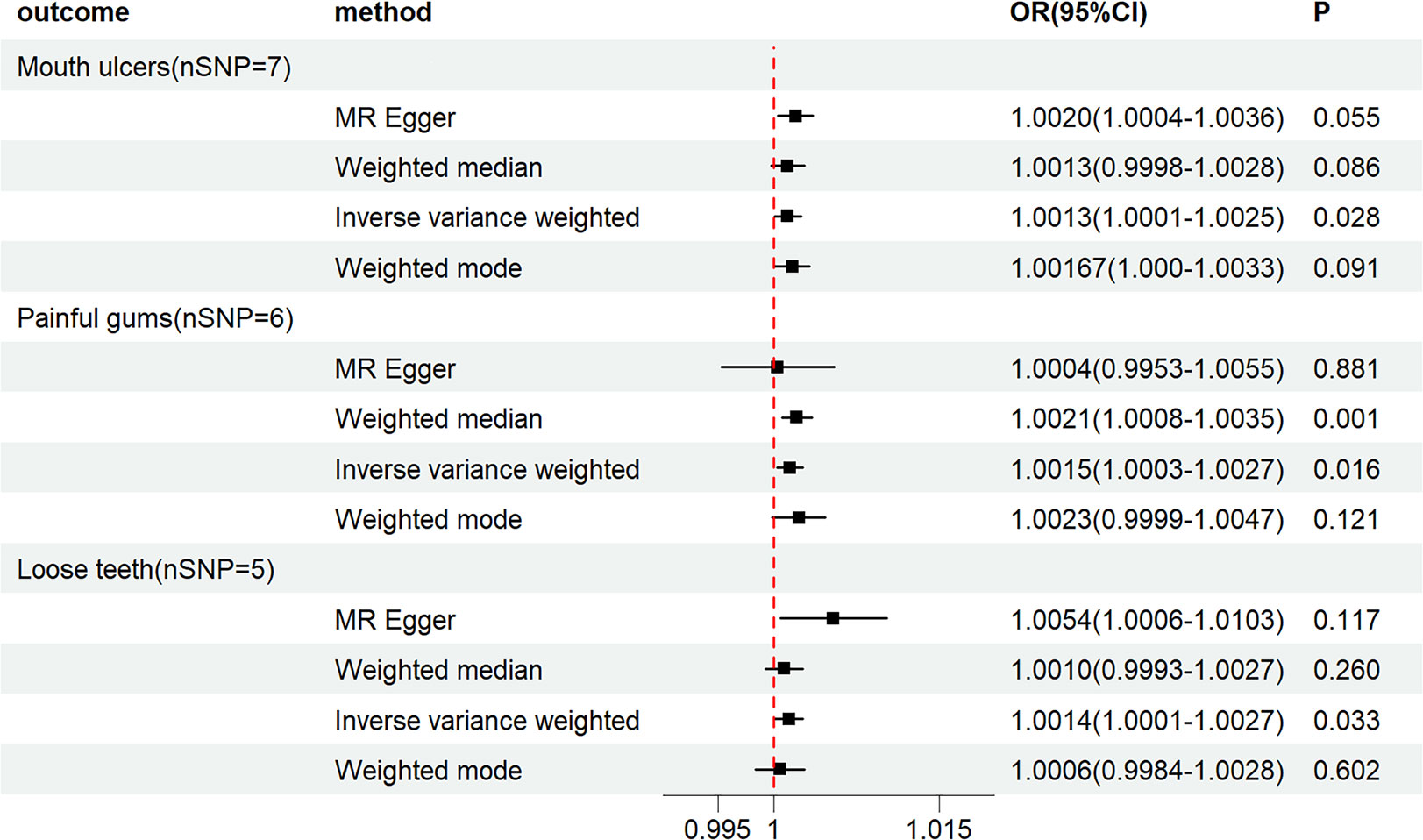
Figure 2 Forest plot of MR analysis results between PCOS and Mouth ulcers, painful gums, Loose teeth.
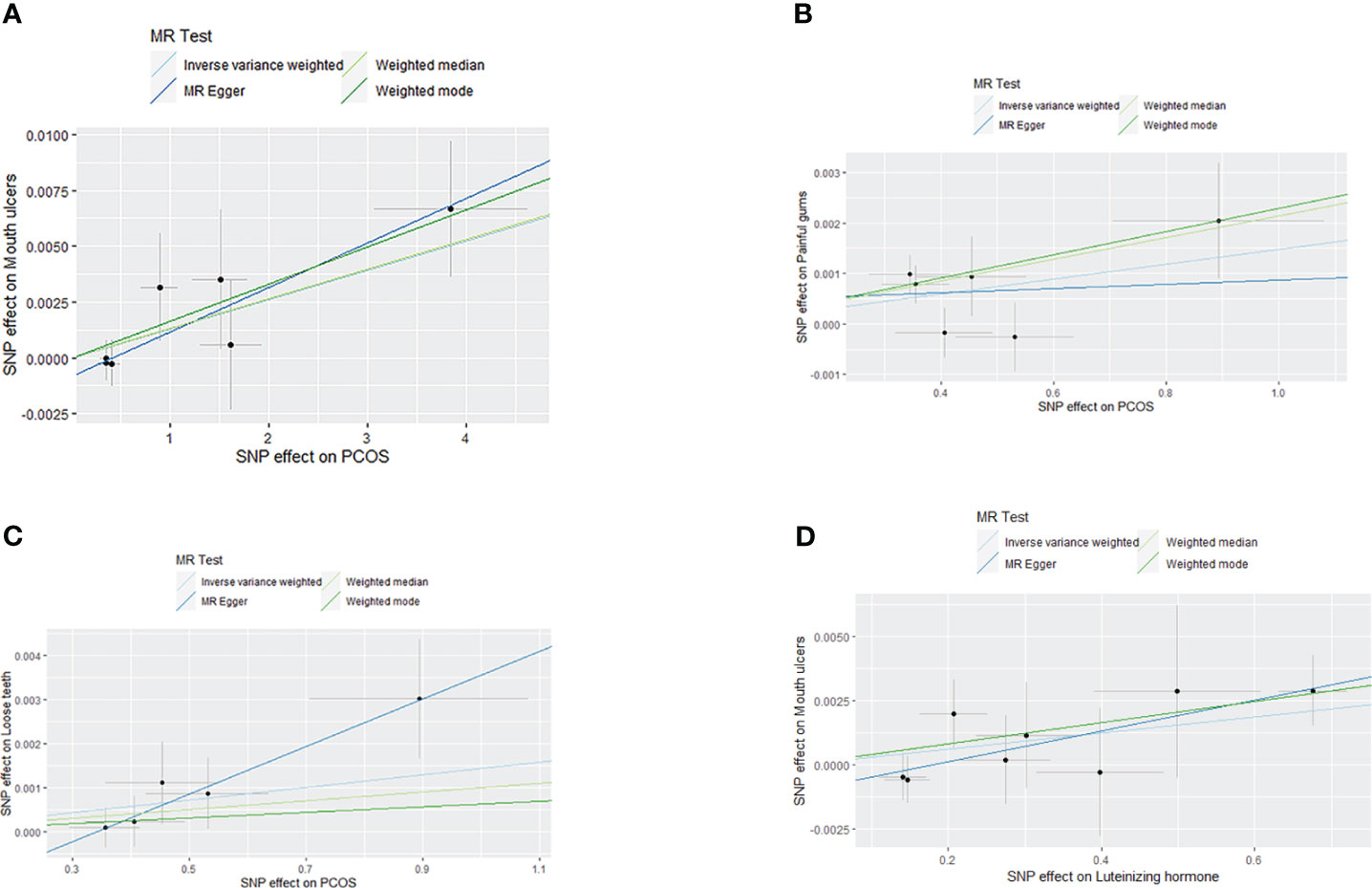
Figure 3 Scatter plot of MR analysis results: (A) scatter plot of MR analysis results between PCOS and Mouth ulcers; (B) scatter plot of MR analysis results between PCOS and painful gums; (C) scatter plot of MR analysis results between PCOS and Loose teeth; (D) scatter plot of MR analysis results between LH and Mouth ulcers.
Our study shows that LH increases the risk of Mouth ulcers (ORIVW= 1.0031, 95%CI: 0.0001-1.0062, PIVW = 0.0457), with MR-Egger regression, weighted mode, and WE suggesting similar outcomes (See Figures 4, 3). No causal relationships were found between LH and painful gums, Loose teeth, or Toothache (PIVW>0.05) (See Supplementary Table S8). Simultaneously, neither AMH nor Estradiol was found to have a causal relationship with the four types of oral diseases (PIVW>0.05) (See Supplementary Tables S6, S7).
Initially, heterogeneity tests were performed on the IV using IVW and MR-Egger methods, revealing no heterogeneity (P>0.05) (See Supplementary Table S2). The MR-Egger regression’s intercept test and MR-PRESSO global test (P>0.05) indicated that our selected instrumental variables did not exhibit horizontal pleiotropy, thereby excluding the influence of confounding factors (See Supplementary Tables S3, S4). The Leave-one-out test indicated that the MR results were robust (See Figure 5).
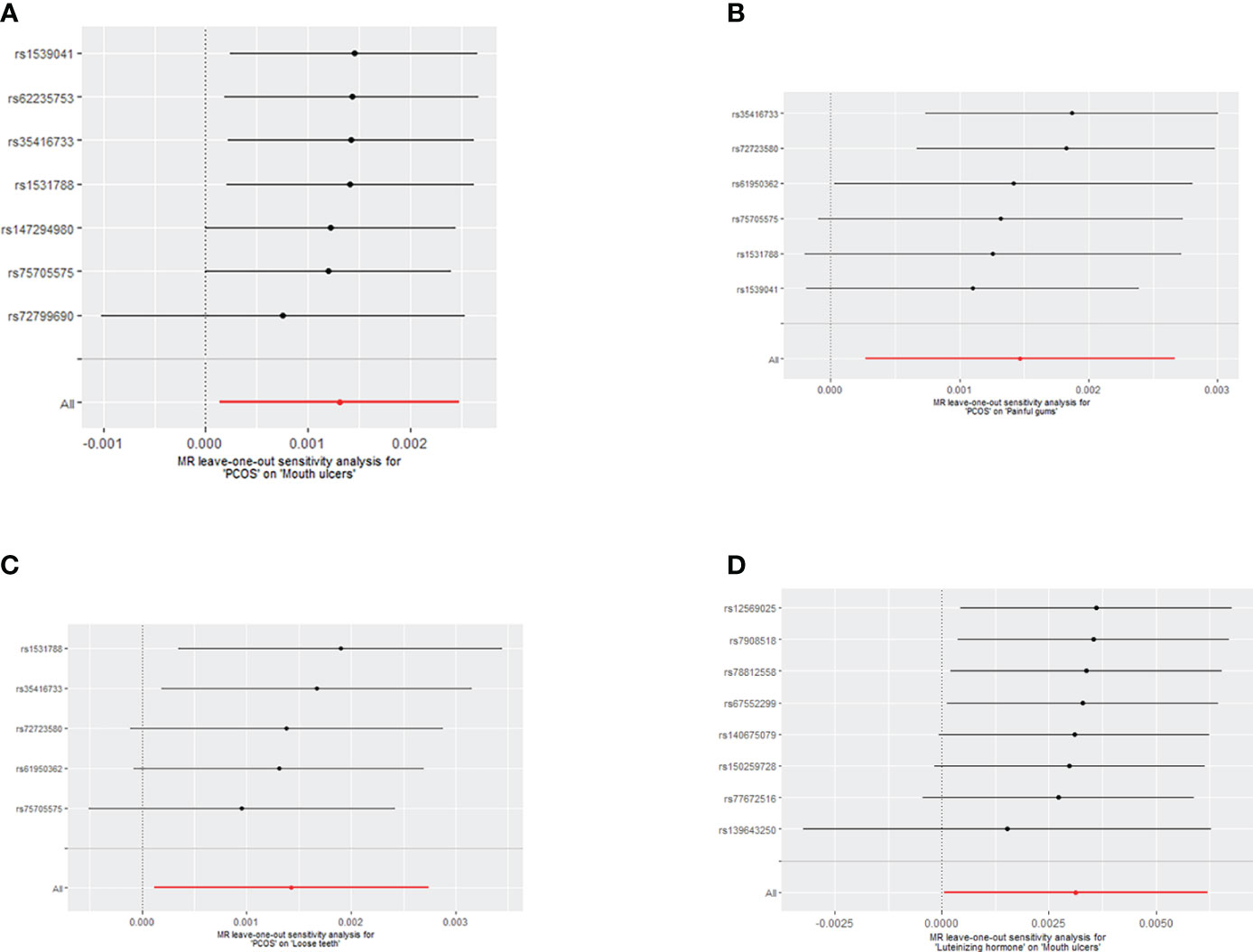
Figure 5 (A) Leave-one-out analysis of the effect of individual SNPs on the association between PCOS and the risk of Mouth ulcers; (B) Leave-one-out analysis of the effect of individual SNPs on the association between PCOS and the risk of painful gums; (C) Leave-one-out analysis of the effect of individual SNPs on the association between PCOS and the risk of Loose teeth; (D) Leave-one-out analysis of the effect of individual SNPs on the association between LH and the risk of Mouth ulcers.
To validate whether a bidirectional causal relationship exists between PCOS and the related three hormones and oral lesions, we considered Mouth ulcers, painful gums, Loose teeth, and Toothache as exposure variables and PCOS, AMH、Estradiol and LH as outcomes. We extracted instrumental variables for two-sample MR analysis at the significance level of P<5 . The results from all four MR analysis methods found no significant causal effects (P>0.05), indicating that there is no reverse causal relationship between PCOS and the three related hormones (AMH, Estradiol, and LH) and the four oral lesions (See Supplementary Table S9).
This research is the first to utilize Mendelian randomization methods to investigate PCOS and associated hormones (AMH, Estradiol and LH) with four oral inflammatory lesions (mouth ulcers, painful gums, loose teeth, and toothache). Four types of MR analysis methods were applied for bidirectional MR analysis. The results indicate a positive correlation between PCOS and three types of oral lesions (mouth ulcers, painful gums, loose teeth), and a positive correlation between LH and the risk of oral ulcers.
In the selection of exposure variables for our study, we chose LH, E2 and AMH due to their direct involvement in ovarian function and their established roles in the pathophysiology of PCOS. While testosterone and other androgens, along with insulin levels, are indeed central to many of the disease’s consequences, our focus on LH, E2, and AMH was guided by their critical roles in ovarian follicular development and their utility as markers for ovarian reserve and function. LH and E2 are pivotal in the regulation of the menstrual cycle and are often dysregulated in PCOS, providing insight into the anovulatory aspect of the syndrome. AMH, which is typically elevated in PCOS, serves as an indicator of the granulosa cell mass and is reflective of the increased follicular count characteristic of the condition. Our aim was to elucidate aspects of PCOS pathophysiology that are directly related to ovarian dysfunction, and these hormones provide a clear window into these mechanisms.
Painful gums, loose teeth, and toothache are primary symptoms of periodontitis, resulting from toxins secreted by bacteria within dental plaque on the gums and nearby surfaces, eventually leading to tooth loss (36). Studies have reported that, compared to a healthy population, the plaque index in the oral cavity of PCOS patients is higher (37), with an increased susceptibility to soft tissue infections and worse periodontal conditions (38–40). This leads to a close association between PCOS and mouth ulcers, as well as inflammatory periodontal diseases. Further research has revealed that the risk of moderate periodontitis in untreated PCOS patients (OR = 5.64, 95% CI: 2.09-15.24) was reduced to one-fifth (OR = 2.88, 95% CI: 1.18-6.98) in those treated with contraceptives and metformin (41).
In light of previous research, we recognize that the relationship between PCOS and periodontal inflammation has been widely discussed, but no study has identified the specific connection between PCOS and the symptoms of inflammatory periodontal disease. Also, the association between PCOS and other inflammatory oral conditions, such as mucosal lesions, remains unreported. Past studies on PCOS and oral inflammatory diseases were primarily observational, involving only one instance of genetic variation research. In 2021, Wu et al. (42) conducted a bidirectional MR analysis on the causal connection between PCOS and periodontitis in the European population, finding no evidence of causality. Our study builds on this, further examining the relationship between PCOS and three typical symptoms of periodontitis, exploring the causal relationship between PCOS and oral inflammatory diseases.
The mechanisms through which PCOS leads to oral inflammatory diseases may involve changes in oral microbiota, oxidative stress (22, 43), inflammatory responses, bone metabolism abnormalities, and associations with hormonal imbalances and insulin resistance within PCOS patients (44). Notably, the quantity of Capnocytophaga gingivalis in the subgingival microbial community shows a positive correlation with estrogen levels and is closely related to PCOS oral inflammation (45). Researchers have also discovered significant reductions in actinomycetes and increased spirochete quantities in the oral microbiome of PCOS patients, which could destabilize the oral microecology and cause chronic inflammation (46–49). Actinomycetes are a vital group of microorganisms that maintain the stability of the oral microecosystem (50). A reduction in their quantity can disrupt this delicate microecological balance, leading to an increased migration of immune cells from the gingival sulcus. This, in turn, further activates the body’s immune system, thereby triggering the onset of persistent chronic inflammation.Furthermore, the reduction in estrogen levels in PCOS patients can increase pro-inflammatory factors, thereby enhancing the risk of oral inflammatory diseases (51). PCOS can also affect bone metabolism and absorption, leading to loose teeth and eventual tooth loss, possibly related to reduced vitamin D levels. Additionally, insulin resistance, a common comorbidity in PCOS, can induce RANKL expression, increasing bone absorption (52).
This study, utilizing bidirectional MR analysis, can eliminate confounding factors and reverse causation interference, yet some limitations remain. Firstly, our research focuses on the European population, possibly leading to certain biases. Secondly, the biological significance of SNPs as instrumental variables (IVs) is complex, and relying solely on genome-wide significance cannot ensure the validity of genetic variations. Lastly, the classification of original data precluded further subtype analysis based on ICD standards, allowing only a holistic analysis of PCOS. While the results of reverse MR analysis were not significant, they still require cautious interpretation, and future validation with a larger sample size is necessary.
Our bidirectional MR study indicates a potential association between PCOS and certain oral inflammatory diseases, including mouth ulcers, painful gums, and loose teeth. Notably, the findings, particularly those derived from the IVW method, warrant cautious interpretation due to the observed variability in statistical significance across different methods and the proximity of odds ratios to unity. While our study adds to the growing body of evidence on this subject, the relatively modest effect sizes suggest that these associations, although statistically significant, may have limited clinical impact. Consequently, our results should be viewed as preliminary until further corroborated by additional research. The study underscores the importance of considering a broad range of methodological approaches and highlights the need for continued investigation into the complex interplay between PCOS and oral health.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
QM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. YC: Formal analysis, Investigation, Supervision, Writing – review & editing. HG: Data curation, Funding acquisition, Project administration, Writing – review & editing. QG: Formal analysis, Investigation, Methodology, Writing – original draft. XZ: Formal analysis, Investigation, Methodology, Writing – original draft. MX: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by the National Natural Science Foundation of China (82104913),Traditional Chinese Medicine bureau of Guangdong Province (20221176) , National Famous Elderly Chinese Medicine Expert Huang Jianling Inheritance Workshop, National Chinese Medicine Personnel Education Letter [2022] No.75 and National Administration of Traditional Chinese Medicine National Renowned TCM Master Studio Construction Project (1199ws02).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1282056/full#supplementary-material
1. Barrea L, Frias-Toral E, Verde L, Ceriani F, Cucalón G, Garcia-Velasquez E, et al. PCOS and nutritional approaches: Differences between lean and obese phenotype. Metab Open (2021) 12(2589-9368):100123. doi: 10.1016/j.metop.2021.100123
2. Glueck CJ, Goldenberg NJM. Characteristics of obesity in polycystic ovary syndrome: Etiology, treatment, and genetics. Metabolism (2019) 92:108–20. doi: 10.1016/j.metabol.2018.11.002
3. Kakoly N, Khomami M, Joham A, Cooray S, Misso M, Norman R, et al. Ethnicity, obesity and the prevalence of impaired glucose tolerance and type 2 diabetes in PCOS: a systematic review and meta-regression. Hum reproduction (2018) 24(4):455–67. doi: 10.1093/humupd/dmy007
4. Dumesic DA, Abbott DH, Sanchita S, Chazenbalk GD. Endocrine–metabolic dysfunction in polycystic ovary syndrome: An evolutionary perspective. Curr Opin Endocrine Metab Res (2020) 12:41–8. doi: 10.1016/j.coemr.2020.02.013
5. Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril (2009) 91(2):456–88. doi: 10.1016/j.fertnstert.2008.06.035
6. Parker J, O’Brien C, Hawrelak J. A narrative review of the role of gastrointestinal dysbiosis in the pathogenesis of polycystic ovary syndrome. Obstetrics Gynecology Science (2022) 65(1):14–28. doi: 10.5468/ogs.21185
7. Sabbadin C, Andrisani A, Ambrosini G, Bordin L, Donà G, Manso J, et al. Aldosterone in gynecology and its involvement on the risk of hypertension in pregnancy. Front Endocrinol (2019) 10:575. doi: 10.3389/fendo.2019.00575
8. Aboeldalyl S, James C, Seyam E, Ibrahim EM, Shawki HE-D, Amer S. The role of chronic inflammation in polycystic ovarian syndrome—a systematic review and meta-analysis. Int J Mol Sci (2021) 22(5):2734. doi: 10.3390/ijms22052734
9. King S, Chow CK, Eberhard J. Oral health and cardiometabolic disease: understanding the relationship. Internal Med J (2022) 52(2):198–205. doi: 10.1111/imj.15685
10. Albuquerque-Souza E, Sahingur SE. Periodontitis, chronic liver diseases, and the emerging oral-gut-liver axis. Periodontology 2000. (2022) 89(1):125–41. doi: 10.1111/prd.12427
11. Ptasiewicz M, Grywalska E, Mertowska P, Korona-Głowniak I, Poniewierska-Baran A, Niedźwiedzka-Rystwej P, et al. Armed to the teeth—the oral mucosa immunity system and microbiota. Int J Mol Sci (2022) 23(2):882. doi: 10.3390/ijms23020882
12. Burxonova Zarafruz Qobilovna BMA. Manifestation of symptoms in the oral cavity in patients with tuberculosis infection. Web Of Sci (2022) 3(11):402–7. doi: 10.17605/OSF.IO/TJXCS
13. Neupane SP, Virtej A, Myhren LE, Bull VH. Behavior,, Immunity-Health. Biomarkers common for inflammatory periodontal disease and depression: A systematic review. Brain behavior immunity-health (2022) 21:100450. doi: 10.1016/j.bbih.2022.100450
14. Kim J, Kim HJ, Jeon J, Song T-J. Association between oral health and cardiovascular outcomes in patients with hypertension: a nationwide cohort study. J Hypertension (2022) 40(2):374–81. doi: 10.1097/HJH.0000000000003022
15. Sebring D, Buhlin K, Norhammar A, Rydén L, Jonasson P, EndoReCo, et al. Endodontic inflammatory disease: A risk indicator for a first myocardial infarction. Int Endodontic J (2022) 55(1):6–17. doi: 10.1111/iej.13634
16. Eke P, Borgnakke W, Genco R. Recent epidemiologic trends in periodontitis in the USA. Periodontology 2000 (2020) 82(1):257–67. doi: 10.1111/prd.12323
17. Liu J, Liu Z, Huang J, Tao R. Effect of probiotics on gingival inflammation and oral microbiota: A meta-analysis. Oral Diseases (2022) 28(4):1058–67. doi: 10.1111/odi.13861
18. Qi X, Lin W, Wu Y, Li Q, Zhou X, Li H, et al. Cbd promotes oral ulcer healing via inhibiting cmpk2-mediated inflammasome. J Dental Res (2022) 101(2):206–15. doi: 10.1177/00220345211024528
19. Kellesarian S, Malignaggi V, Kellesarian T, Al-Kheraif A, Alwageet M, Malmstrom H, et al. Association between periodontal disease and polycystic ovary syndrome: a systematic review. Int J impotence Res (2017) 29(3):89–95. doi: 10.1038/ijir.2017.7
20. Tanguturi SC, Nagarakanti S. Polycystic ovary syndrome and periodontal disease: underlying links-a review. Indian J Endocrinol Metab (2018) 22(2):267–73. doi: 10.4103/ijem.IJEM_577_17
21. MaChado V, Escalda C, Proença L, Mendes JJ, Botelho J. Is there a bidirectional association between polycystic ovarian syndrome and periodontitis? A systematic review and meta-analysis. J Clin Med (2020) 9(6):1961. doi: 10.3390/jcm9061961
22. Márquez-Arrico CF, Silvestre-Rangil J, Gutiérrez-Castillo L, Martinez-Herrera M, Silvestre FJ, Rocha M. Association between periodontal diseases and polycystic ovary syndrome: a systematic review. Journal of clinical medicine (2020) 9(5):1586. doi: 10.3390/jcm9051586
23. Işık Y, Telatar GY, Neşelioğlu S, Biçer C, Gürlek B. Evaluation of periodontal status in different phenotypes of polycystic ovary syndrome in untreated patients of early reproductive age: a case–control study. J Obstetrics Gynaecol Res (2020) 46(3):459–65. doi: 10.1111/jog.14179
24. MaChado V, Escalda C, Proença L, Mendes JJ, Botelho J. Is there a bidirectional association between polycystic ovarian syndrome and periodontitis? A systematic Rev meta-analysis (2020) 9(6):1961. doi: 10.3390/jcm9061961
25. Tong C, Wang Y-H, Yu H-C, Chang Y-C. Increased risk of polycystic ovary syndrome in Taiwanese women with chronic periodontitis: a nationwide population-based retrospective cohort study. J Women's Health (2019) 28(10):1436–41. doi: 10.1089/jwh.2018.7648
26. Mutlak S, Abdulkareem E. Effect gingivitis on anti-mullierian hormone in females with polycystic ovary syndrome. Int J Biosci (2018) 13:165–72. doi: 10.12692/ijb/13.3.165-172
27. Verdiesen RMG, van der Schouw YT, van Gils CH, Verschuren WMM, Broekmans FJM, Borges MC, et al. Genome-wide association study meta-analysis identifies three novel loci for circulating anti-Müllerian hormone levels in women. Hum Reprod (Oxford England) (2022) 37(5):1069–82. doi: 10.1093/humrep/deac028
28. Ruth KS, Day FR, Tyrrell J, Thompson DJ, Wood AR, Mahajan A, et al. Using human genetics to understand the disease impacts of testosterone in men and women. Nat Med (2020) 26(2):252–8. doi: 10.1038/s41591-020-0751-5
29. Sun BB, Maranville JC, Peters JE, Stacey D, Staley JR, Blackshaw J, et al. Genomic atlas of the human plasma proteome. Nature (2018) 558(7708):73–9. doi: 10.1038/s41586-018-0175-2
30. Burgess S, Small DS, Thompson SG. A review of instrumental variable estimators for Mendelian randomization. Statistical methods in medical research. Statistical methods in medical research (2017) 26(5):2333–55. doi: 10.1177/0962280215597579
31. Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet (2014) 23(R1):R89–98. doi: 10.1093/hmg/ddu328
32. Ye T, Shao J, Kang H. Debiased inverse-variance weighted estimator in two-sample summary-data Mendelian randomization. Ann statistics (2021) 49(4):2079–100. doi: 10.1214/20-AOS2027
33. Minelli C, Del Greco MF, van der Plaat DA, Bowden J, Sheehan NA, Thompson J. The use of two-sample methods for Mendelian randomization analyses on single large datasets. Int J Epidemiol (2021) 50(5):1651–9. doi: 10.1093/ije/dyab084
34. Ong JS, MacGregor S. Implementing MR-PRESSO and GCTA-GSMR for pleiotropy assessment in Mendelian randomization studies from a practitioner's perspective. Genet Epidemiol (2019) 43(6):609–16. doi: 10.1002/gepi.22207
35. Vehtari A, Gelman A, Gabry J. Practical Bayesian model evaluation using leave-one-out cross-validation and WAIC. Stat computing (2017) 27:1413–32. doi: 10.1007/s11222-016-9696-4
36. Kassebaum N, Bernabé E, Dahiya M, Bhandari B, Murray C, Marcenes W. Global burden of severe periodontitis in 1990-2010: a systematic review and meta-regression. J Dental Res (2014) 93(11):1045–53. doi: 10.1177/0022034514552491
37. Zia A, Hakim S, Khan A, Bey A, Ateeq H, Parveen S, et al. Bone markers and bone mineral density associates with periodontitis in females with poly-cystic ovarian syndrome. J Bone Mineral Metab (2022) 40(3):487–97. doi: 10.1007/s00774-021-01302-6
38. Akcalı A, Bostanci N, Özçaka Ö, Öztürk-Ceyhan B, Gümüş P, Tervahartiala T, et al. Elevated matrix metalloproteinase-8 in saliva and serum in polycystic ovary syndrome and association with gingival inflammation. Innate immunity (2015) 21(6):619–25. doi: 10.1177/1753425915572172
39. Rahiminejad ME, Moaddab A, Zaryoun H, Rabiee S, Moaddab A, Khodadoustan A. Comparison of prevalence of periodontal disease in women with polycystic ovary syndrome and healthy controls. Dental research journal (2015) 12(6):507. doi: 10.4103/1735-3327.170547
40. Varadan M, Gopalkrishna P, Bhat PV, Kamath SU, Kumar S. Influence of polycystic ovary syndrome on the periodontal health of Indian women visiting a secondary health care centre. Clin Oral investigations (2019) 23:3249–55. doi: 10.1007/s00784-018-2741-2
41. Porwal S, Tewari S, Sharma RK, Singhal SR, Narula S. Periodontal status and high-sensitivity C-reactive protein levels in polycystic ovary syndrome with and without medical treatment. J periodontol (2014) 85(10):1380–9. doi: 10.1902/jop.2014.130756
42. Wu P, Zhang X, Zhou P, Zhang W, Li D, Lv M, et al. Assessment of bidirectional relationships between polycystic ovary syndrome and periodontitis: Insights from a Mendelian randomization analysis. Front Genet (2021) 12:644101. doi: 10.3389/fgene.2021.644101
43. Saglam E, Canakci CF, Sebin SO, Saruhan N, Ingec M, Canakci H, et al. Evaluation of oxidative status in patients with chronic periodontitis and polycystic ovary syndrome: A cross-sectional study. J Periodontol (2018) 89(1):76–84. doi: 10.1902/jop.2017.170129
44. Zhao F, Dong T, Yuan K-Y, Wang N-J, Xia F-Z, Liu D, et al. Shifts in the bacterial community of supragingival plaque associated with metabolic-associated fatty liver disease. Front Cell Infection Microbiol (2020) 10:581888. doi: 10.3389/fcimb.2020.581888
45. Wendland N, Opydo-Szymaczek J, Mizgier M, Jarząbek-Bielecka G. Subgingival microflora in adolescent females with polycystic ovary syndrome and its association with oral hygiene. gingivitis selected Metab hormonal parameters (2021) 25:1485–96. doi: 10.1007/s00784-020-03456-5
46. Li N, Li Y, Qian C, Liu Q, Cao W, Ma M, et al. Dysbiosis of the saliva microbiome in patients with polycystic ovary syndrome. Front Cell Infection Microbiol (2021) 10:624504. doi: 10.3389/fcimb.2020.624504
47. Lindheim L, Bashir M, Münzker J, Trummer C, Zachhuber V, Pieber TR, et al. The salivary microbiome in polycystic ovary syndrome (PCOS) and its association with disease-related parameters: a pilot study. Front Microbiol (2016) 7:1270. doi: 10.3389/fmicb.2016.01270
48. Wendland N, Opydo-Szymaczek J, Mizgier M, Jarząbek-Bielecka GJCOI. Subgingival microflora in adolescent females with polycystic ovary syndrome and its association with oral hygiene, gingivitis, and selected metabolic and hormonal parameters. Clin Oral Investigations (2021) 25:1485–96. doi: 10.1007/s00784-020-03456-5
49. Akcalı A, Bostanci N, Özçaka Ö, Öztürk-Ceyhan B, Gümüş P, Buduneli N, et al. Association between polycystic ovary syndrome, oral microbiota and systemic antibody responses. PLoS One (2014) 9(9):e108074. doi: 10.1371/journal.pone.0108074
50. Ko Y, Lee E-M, Park JC, Gu MB, Bak S, SJJoP Ji, et al. Salivary microbiota in periodontal health and disease and their changes following nonsurgical periodontal treatment. J Periodontal Implant Science (2020) 50(3):171. doi: 10.5051/jpis.2020.50.3.171
51. Jafri Z, Bhardwaj A, Sawai M, Sultan N. Influence of female sex hormones on periodontium: A case series. J Natural science biol Med (2015) 6(Suppl 1):S146. doi: 10.4103/0976-9668.166124
Keywords: Mendelian randomization, PCOS, mouth ulcers, painful gums, periodontitis, SNP, AMH
Citation: Min Q, Chen Y, Geng H, Gao Q, Zhang X and Xu M (2024) Causal relationship between PCOS and related sex hormones with oral inflammatory diseases: a bidirectional Mendelian randomization study. Front. Endocrinol. 14:1282056. doi: 10.3389/fendo.2023.1282056
Received: 23 August 2023; Accepted: 01 December 2023;
Published: 09 January 2024.
Edited by:
Johannes Ott, Medical University of Vienna, AustriaCopyright © 2024 Min, Chen, Geng, Gao, Zhang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min Xu, eHVtaW4zMzMzQGd6dWNtLmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.