
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 08 January 2024
Sec. Cancer Endocrinology
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1280221
This article is part of the Research TopicIntegrated Diagnostics and Biomarker Discovery in Endocrinology and Biomedical SciencesView all 17 articles
Background: Current research suggests that prostate cancer (PCa), one of the most common cancers in men, may be linked to insulin resistance (IR).Triglyceride-glucose index (TyG index) was made for a marker of insulin resistance. We investigated the relationship between the TyG index and the risk of PCa.
Objective: To assess the correlation and dose-response relationship between TyG index and prostate cancer.
Method: Retrospectively, 316 patients who required prostate biopsy puncture in the First Affiliated Hospital of Xinjiang Medical University from March 2017 to July 2021 were collected, and the relationship between factors such as the TyG index and prostate cancer was analyzed by Logistic regression model combined with a restricted cubic spline.
Results: (1) The differences in age, initial PSA and TyG index between the two groups were statistically significant; (2) Logistic regression results showed that the risk of prostate cancer in the highest quartile of the TyG index (Q4) was 3.387 times higher than that in the lowest quartile (Q1) (OR=3.387,95% CI [1.511,7.593], P=0.003); (3) The interaction results showed a significant interaction between the TyG index Q4 group and age with the risk of developing prostate cancer (P for interaction<0.001). (4) The results of the restricted cubic spline showed a linear dose-response relationship between the TyG index and the risk of prostate cancer; (5) The Receiver operating characteristic (ROC) curve results showed that the area under the curve (AUC) of the TyG index combined with initial PSA and age was 0.840, with a sensitivity and specificity of 62.5% and 93.3%, respectively.
Conclusion: TyG index and age are risk factors for prostate cancer, and the interaction between the TyG index and different risk factors may increase the risk of prostate cancer. TyG index has some predictive value for the risk of prostate cancer, and the risk of prostate cancer can be reduced by controlling the levels of blood lipids and blood glucose.
According to GLOBOCAN, in 2020 there will be approximately 1.4 million new cases and 375,000 deaths worldwide from prostate cancer (PCa), the second most common cancer in men and the fifth leading cause of cancer death worldwide (1). And in China, the problem of an ageing population is becoming increasingly serious (2, 3), Prostate cancer incidence and mortality rates are on a significant rise (4–9). Prostate cancer has become a common urological tumor in men, posing a serious risk to human health (1–12). Current factors that may influence prostate cancer are age, family history of tumors, genetic mutations, African ancestry, metabolic syndrome, and others (13–16). Metabolic syndrome is characterized by obesity, insulin resistance (IR), hypertension, and hyperlipidemia (17), and several current studies have demonstrated that insulin resistance is associated with prostate cancer, which can affect the development and progression of Pca through a variety of mechanisms, including the inflammatory pathway (Nuclear Factor Kappa B) (NF-κB) and cytokines, and increase the risk of developing prostate cancer (18–22). The current gold standard for the diagnosis of insulin resistance is Euglycemic-Hyperinsulinemic Clamp (23), but it is slightly cumbersome and expensive to use in practice (24). Studies have demonstrated the high sensitivity of the triglyceride-glucose index (TyG index) for identifying IR (25), the TyG index combines triglycerides (TG) and fasting plasma glucose (FPG), which are important in the diagnosis of IR, so the TyG index can be a reliable proxy for the diagnosis of insulin resistance (26, 27). Elevated TG and FPG have been shown to increase the risk of prostate cancer by Arthur R et al (28–30). And many recent studies have demonstrated that the TyG index is closely associated with the occurrence and development of cancer (31), TyG index can be used as a predictor of breast, colorectal, gastric,thyroid and non-small cell lung cancers (32–42). However, no study has been conducted to illustrate the relationship between TyG index and prostate cancer. Therefore, this study aims to illustrate the relationship between TyG index and prostate cancer, to investigate the dose-response relationship between TyG index and prostate cancer and the predictive value of TyG index in prostate cancer.
The examination results of 316 patients who underwent prostate biopsy punctures from March 2017 to July 2021 in the First Affiliated Hospital of Xinjiang Medical University were retrospectively collected and patients were used as study subjects. Patients diagnosed with benign prostatic hyperplasia (BPH) according to histopathology and immunohistochemistry in the Department of Pathology of the First Affiliated Hospital of Xinjiang Medical University were used as the control group, and patients diagnosed with prostate cancer were used as the case group.
① Those diagnosed with BPH between April 2017 and July 2021 based on histopathology and immunohistochemistry; ② Patients who meet the indications for prostate biopsy punctures; ③ First time prostate biopsy puncture performers; ④ People who are able to read, understand and provide consent.
① Patients with any type of cancer or previous history of cancer; ② Patients with a history of diabetes mellitus, use of glucose-lowering drugs, use of fenofibrate triglyceride-lowering drugs, and a history of hepatic, renal, or other diseases associated with disorders of lipid metabolism; ③ The data examined are incomplete.
① Patients diagnosed with prostate cancer based on histopathology and immunohistochemistry between April 2017 and July 2021; ② Patients who meet the indications for prostate biopsy punctures; ③ Patients undergoing their first prostate biopsy puncture with a first diagnosis of prostate cancer; ④ Patients with prostate cancer who were able to read, understand, and provide consent forms and complete medical records.
① Patients with a history of other types of cancer; ② Patients with a history of diabetes mellitus, use of glucose-lowering drugs, use of fenofibrate triglyceride-lowering drugs, and a history of hepatic, renal, or other diseases associated with disorders of lipid metabolism; ③ The data examined are incomplete.
① Patients with persistently elevated prostate-specific antigen (PSA) or greater than 4 ng/ml; ② A hard nodule of the patient’s prostate gland was found on physical examination of the prostate gland; ③ Transrectal prostate ultrasound hypoechoic nodules, prostate magnetic resonance abnormal signal nodules; ④ If the patient’s first puncture is negative, but with high-grade PIN, etc., and if the patient’s prostate-specific antigen is persistently elevated, another prostate puncture biopsy may be performed.
The study was a case-control study in which demographic data such as age, ethnicity, and education level of the study subjects were obtained based on hospital medical record information, and their family history of cancer was also asked; fasting vena cava blood was drawn from the study subjects to determine their initial serum PSA, fasting TG, FPG, blood calcium, testosterone, blood potassium, total cholesterol(TC), low-density lipoprotein (LDL), etc.
In both groups, blood specimens were collected from 8:00 to 9:00 am on the next day of admission in a fasting state, and were immediately sent for examination, and the results were kept. Patients who met the criteria for prostate biopsy were scheduled for transrectal prostate biopsy the day after the examination. Biopsies were performed by a number of senior urologists using the US BARD biopsy needles using the 12+X puncture method, in which 1-2 needles were punctured as the X-needle for suspicious areas suggested on nuclear magnetic resonance imaging or ultrasound, and the rest of the area was punctured by the systematic 12-needle puncture method (43, 44). Each needle of tissue was individually placed into a fixation vial containing 10% methanol aqueous solution, marked with the hospitalization number and date of puncture, and uniformly sent to the Department of Pathology of the First Affiliated Hospital of Xinjiang Medical University for examination, where the histopathological results were recorded and diagnosed by a senior pathologist.
TyG index was calculated from the formula: TyG = LN (fasting TG [mg/gl] * FPG [mg/gl]/2) (45). Body mass index (BMI) = weight (kg)/[height (m)]^2 (46). According to the World Health Organization’s criteria for smoking, divided into smokers and non-smokers. Alcohol consumption was also divided into drinkers and non-drinkers according to WHO criteria.
The data were analyzed using SPSS 26.0 and R 4.0.5 software. Use nonparametric tests, t-test, and restricted cubic spline plots to test the distribution of continuous data.
Normally distributed data are presented as means (standard deviations), while nonparametric data are expressed as median, minimum and maximum values. Categorical data were expressed as percentages, and the chi-square test was used for comparison between groups. The TyG index was divided into four quartiles according to the interquartile spacing method (‘Q1’ is<8.389, ‘Q2’ is 8.389-8.805, ‘Q3’ is 8.805-9.241, ‘Q4’ is>9.241), and the odds ratio (OR) of each quartile was calculated using the first quartile as a reference, and logistic regression was used to calculate the OR of the TyG index and prostate cancer. Logistic regression was used to develop correlation models to test the correlation between prostate cancer and the respective variables; model 1 was unadjusted, model 2 was adjusted for age and initial PSA, and model 3 was adjusted for age, initial PSA, smoking history, alcohol consumption, family history of cancer, BMI, TC, and LDL. Logistic regression was used to analyze the interaction between the TyG index and age and initial PSA. The test level α = 0.05. The linearity of the dose-response curves was assessed using restricted cubic spline plots and logistic regression models. The Receiver operating characteristic (ROC) curve were applied to assess the predictive value of the TyG index for prostate cancer.
A total of 316 individuals were included. Among them, 136 were in the case group with an age of (70.73 ± 9.80) years. The control group consisted of 180 individuals aged (65.10 ± 8.51) years. The differences in age, initial PSA and TyG index between the two groups were statistically significant (P<0.05) Table 1.
The results showed that in model 1, the OR (95% CI) for prostate cancer was 1.000,1.292 (0.679-2.460),1.238 (0.652-2.351), and 2.403 (1.266-4.564) with increasing quartiles of TyG index (P for trend=0.012). In model 2, factors associated with the risk of developing prostate cancer included age (OR=1.056,95% CI [1.020,1.094], P=0.002), initial PSA (OR=1.059,95% CI [1.031,1.088], P<0.001), and TyG index (P for trend=0.001), the risk of prostate cancer in the highest quartile of the TyG index (Q4) was 3.387 times higher than that in the lowest quartile (Q1) (OR=3.387,95% CI [1.511,7.593], P=0.003). In model 3, the OR (95% CI) for prostate cancer was 1.000,0.886 (0.350-2.241),2.065 (0.874-4.878), and 2.854 (1.200-6.790) as increasing quartiles of the TyG index (P for trend=0.002) Table 2.
The interaction results showed a significant interaction between the TyG index Q4 group and age with the risk of developing prostate cancer (P for interaction<0.001). Tests for the interaction between TyG index Q2 group (P for interaction=0.079), Q3 group (P for interaction=0.077), and age and risk of prostate cancer, as well as TyG index Q3 group (P for interaction=0.100) and initial PSA and risk of prostate cancer, were not significant Table 3.
After using restrictive cubic splines and adjusting for relevant confounders, there was a linear dose-response relationship between the TyG index and risk of prostate cancer prevalence (P overall<0.05, P non-linearity=0.412) Figure 1.
ROC curves for predicting prostate cancer were plotted based on the TyG index, initial PSA, and age. The results showed that the area under the curve (AUC) of TyG index, initial PSA, and age were 0.539, 0.801, and 0.680, respectively, while the AUC of TyG index combined with initial PSA and age was improved to 0.840, with a sensitivity and specificity of 62.5% and 93.3%, respectively. The accuracy of the TyG index combined with initial PSA and age in predicting the risk of prostate cancer was high. See Table 4 and Figure 2.
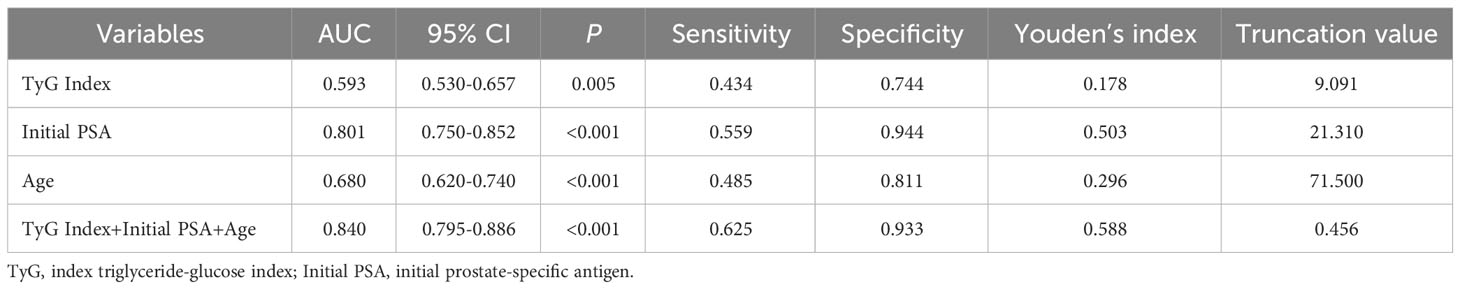
Table 4 ROC curve analysis of TyG index, age, initial PSA, and all three in combination with prostate cancer.
In this retrospective study, we investigated the effect of the TyG index on the risk of developing PCa and found for the first time that the TyG index predicts the risk of developing PCa. A meta-analysis showed a strong association between TyG index and cancer development (31),Panigoro S S’s study proved that there was a nonlinear dose-response relationship between TyG index and breast cancer (42), but so far no study has illustrated the dose-response relationship between TyG index and the risk of prostate cancer, and the restricted cubic spline model can intuitively describe the relationship between the independent variable and the dependent variable, therefore, the present study applies the restricted cubic spline model to analyze the relationship between the TyG index and the risk of prostate cancer. The results showed that there was a linear dose-response relationship between the TyG index and the risk of prostate cancer, and Logistic regression analysis showed that the risk of prostate cancer in the TyG index Q4 group was 3.387 times higher than that in the Q1 group, suggesting that the TyG index has an important effect on the occurrence and development of prostate cancer. We also used Logistic regression model to analyze the effect of interaction between age, initial PSA and TyG index groups on prostate cancer, and the results showed that there was an interaction between TyG index Q4 group and age and initial PSA, and the interaction between TyG index and different risk factors may increase the risk of prostate cancer. Therefore, we further analyzed the predictive value of TyG index and age on the risk of prostate cancer using ROC curves, and found that the AUCs of TyG index, initial PSA, and age were 0.539, 0.801, and 0.680, respectively. Whereas the AUC of TyG index combined with initial PSA and age was improved to 0.840, with a sensitivity and specificity of 62.5% and 93.3 percent. It indicates that TyG index combined with initial PSA and age has better accuracy than age, initial PSA and TyG index alone in predicting the risk of prostate cancer.
Studies by Albanes D et al. demonstrated that insulin resistance is associated with prostate cancer (20, 47), while Lebovitz HE et al. demonstrated that insulin resistance is associated with such as hyperinsulinemia (48), IGF levels (19, 20), and Phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) signaling pathways (49), which may play an important role in the development of prostate cancer. In patients with insulin resistance, insulin sensitivity is reduced, the efficiency of glucose uptake and utilization decreases, and the body compensatorily secretes excess insulin, inducing the development of hyperinsulinemia (50). Hyperinsulinemia has been shown to have a direct effect on the liver, inhibiting the production of insulin-like growth factor-binding proteins 1 and 2 (IGFBP-1,-2), while stimulating the production of insulin-like growth factor-1 (IGF-1) and increasing the bioavailability of IGF-1 (51–53). Whereas binding of IGF-1 to the IGF receptor activates the p21 ras/mitogen-activated protein kinase (MAPK) pathway and PI3K/Akt pathway (54). Activation of the MAPK pathway causes activated extracellular signal-regulated kinase (ERK) to translocate to the nucleus, reverse transcription activates transcription factors, alters gene expression, promotes cell growth, differentiation and mitosis, and facilitates PCa cell proliferation (55). Activation of the PI3K/Akt pathway promotes the phosphorylation of BAD that a pro-apoptotic Bcl2 family member in cells, phosphorylation of BAD inhibits the ability of BAD to bind and constrain the anti-apoptotic Bcl2 family members that BclxL and Bcl2, leading to apoptosis resistance in PCa cells, which in turn causes prostate cancer (54, 56). Increased bioavailability of IGF-1, increased binding of IGF-1 and IGF receptors, and increased intensity of IGF-1 action predispose to the induction of prostate cancer.
The TyG index, calculated from fasting triglycerides and blood glucose, was initially proposed as a biomarker of insulin resistance (25, 45), and subsequent studies by Tutunchi H and Lv L et al. have shown it to be associated with diabetes mellitus (57), hepatic fibrosis (58), cardiovascular disease (59), and erectile dysfunction (60). Blood glucose and lipids are important components of the TyG index, and some studies have reported a strong association between them and the development of prostate cancer (61–63). Aljada A et al. showed that hyperglycemia leads to an increase in intranuclear NF-κB in human subjects, that two of the target genes of NF-κB, such as cell cycle protein D1 and cMyc, play important roles in cell growth and proliferation, and that NF-κB itself regulates many genes involved in the process of cell proliferation, tumor formation, and metastasis, thereby influencing cancer development (61, 62). Elevation of body lipids causes enhanced lipid metabolism, which in turn causes enhanced adipocyte metabolism (63). Adipocytes produce inflammatory cytokines and form a protumorigenic environment (64), and periprostatic adipocytes also promote the extracapsular expansion of prostate cancer through chemokines, which affect the development of prostate cancer (65). In addition, increased fat cell metabolism also increases leptin production, a hormone secreted from fat cells that suppresses appetite and increases basal metabolism through metabolic signaling. It has been suggested that high leptin levels are associated with prostate, colon, breast, and endometrial cancers and that leptin may play a role in this through cell proliferation via MAPK signaling. On the other hand, it has also been suggested that leptin may stimulate angiogenesis, increase matrix metalloproteinase-2 expression, and lead to cancer metastasis (66). In conclusion, elevated blood glucose and lipids can affect the development of prostate cancer through a variety of mechanisms, and there is a correlation between TyG index and prostate cancer.
There are certain limitations in this study: First, this study is a single-center case-control study, there are some limitations in the selection of samples, only some of the influencing factors have been collected, and there is a lack of data on patients from different geographical regions, pending the expansion of the sample size at a later stage and the collection of more comprehensive factors for a multicenter study. Secondly, this study is a cross-sectional survey, only investigated a certain point in time indicators, did not take into account the influence of the trend of the indicators on the results, and the TyG index will be affected by diet-related factors, later we will use this study as a baseline, and carry out a cohort study to further observe the impact of changes in the TyG index on the risk of prostate cancer.
In conclusion, the TyG index is an important influencing factor for prostate cancer and has some predictive value for the risk of prostate cancer, which needs to be further determined in future prospective studies.
This study found that the TyG index is a risk factor for prostate cancer, and the interaction between the TyG index and different risk factors may increase the risk of prostate cancer. There is a linear dose-response relationship between the TyG index and the risk of prostate cancer, and the TyG index has a certain predictive value of the risk of prostate cancer, and the risk of prostate cancer can be reduced by controlling the levels of blood lipids and blood glucose. By controlling blood lipid and blood glucose levels, the risk of prostate cancer can be reduced.
The study involving human participants. The human data used in this study were obtained and applied in accordance with the Declaration of Helsinki. The study was reviewed and approved by the Ethics Committee of First Affiliated Hospital of Xinjiang Medical University (approval number: 20220308-166). Subjects provided written informed consent for participation in this study.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by Ethics committee of First Affiliated Hospital of Xinjiang Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
YZ: Methodology, Software, Writing – original draft, Writing – review & editing. TL: Formal analysis, Methodology, Project administration, Validation, Writing – review & editing. GM: Conceptualization, Data curation, Investigation, Writing – review & editing. YD: Investigation, Methodology, Software, Validation, Visualization, Writing – review & editing. SX: Formal analysis, Methodology, Supervision, Visualization, Writing – review & editing. YG: Conceptualization, Project administration, Resources, Writing – review & editing. NT: Data curation, Formal analysis, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing. HA: Conceptualization, Funding acquisition, Investigation, Resources, Software, Visualization, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Xinjiang Uygur Autonomous Region Natural Science Outstanding Youth Programme (Number: 2023D01E05), the Key Project of Natural Science Foundation of Xinjiang Uygur Autonomous Region (Number: 2022D01D39), Xinjiang Uygur Autonomous Region Tianshan Talent Youth Top-notch Project (Number: 2022TSYCCX0026), National Science and Nature Fund (Number: 82360476) and Xinjiang Medical University’s 17th College Students’ Innovative Training Program Project (Number: S202210760061).
The authors thank all participants and investigators.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1280221/full#supplementary-material
PCa, Prostate cancer; BPH, Benign prostatic hyperplasia; TyG index, Triglyceride-glucose index; PSA, Prostate-specific antigen; FPG, Fasting plasma glucose; TG, Triglyceride; TC, Total cholesterol; ALP, Alkaline phosphatase; LDL, Low-density lipoprotein; BMI, Body mass index; ROC, Receiver operating characteristic; AUC, Area under the curve; CI, Confidence interval; IGF, Insulin-likegrowth factors; IR, Insulin resistance; PI3K, Phosphatidylinositol 3-kinase; Akt, Protein kinase B; NF-κB, Nuclear Factor Kappa B; ERK, Extracellular signal-regulated kinase.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca-a Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Jiang QB, Feng QS. Aging and health in China. Front Public Health (2022) 10:998769. doi: 10.3389/fpubh.2022.998769
3. Zhang WL, Liu SF, Du JL, Liu LY, Guo XJ, Xu ZR. A framework of grey prediction models on China’s population aging under the perspective of regional differences. J Grey System (2022) 34:1–14.
4. He J, Chen WQ, Li N, Cao W, Ye DW, Ma JH, et al. China guideline for the screening and early detection of prostate cancer (2022, Beijing). Chin J Oncol (2022) 44:29–53. doi: 10.3760/cma.j.cn112152-20211226-00975
5. Gu XY, Zheng RS, Zhang SW, Zeng HM, Sun KX, Zou XN, et al. Analysis of the incidence trend and age change of prostate cancer in China’s tumor registration areas from 2000 to 2014. Chin J Prev Med (2018) 52:586–92. doi: 10.3760/cma.j.issn.0253-9624.2018.06.006
6. Liu JZ, Dong L, Zhu YJ, Dong BJ, Sha JJ, Zhu HH, et al. Prostate cancer treatment-China’s perspective. Cancer Lett (2022) 550. doi: 10.1016/j.canlet.2022.215927
7. He J, Chen WQ, Li N, et al. Guidelines for screening and early diagnosis and treatment of prostate cancer in China (2022 Beijing). Chin Tumor (2022) 31:1–30. doi: 10.3760/cma.j.cn112152-20211226-00975
8. Li X, Zeng XY. Advances in epidemiology of prostate cancer in China. Cancer Prev Res (2021) 48:98–102. doi: 10.3971/j.issn.1000-8578.2021.20.0370
9. Chinese expert consensus on prostate cancer screening (2021 edition). Chin J Cancer (2021) 31:435–40. doi: 10.19401/j.cnki.1007-3639.2021.05.010
10. Nguyen-Nielsen M, Borre M. Diagnostic and therapeutic strategies for prostate cancer. Semin Nucl Med (2016) 46:484–90. doi: 10.1053/j.semnuclmed.2016.07.002
11. Groeben C, Wirth MP. Prostate cancer: Basics on clinical appearance, diagnostics and treatment. Med Monatsschr Pharm (2017) 40:192–201.
12. Vietri MT, D’Elia G, Caliendo G, Resse M, Casamassimi A, Passariello L, et al. Hereditary prostate cancer: genes related, target therapy and prevention. Int J Mol Sci (2021) 22. doi: 10.3390/ijms22073753
13. Pagadala M, Lui A, Lynch J, Panizzon M, Carter H, Hauger R, et al. Healthy lifestyle and Agent Orange exposure modulate inherited prostate cancer risk: An analysis of the Million Veteran Program. (2022). doi: 10.1101/2022.07.09.22277437
14. Fragkoulis C, Glykas I, Tzelves L, Stasinopoulos K, Lazarou L, Kaoukis A, et al. Association of metabolic syndrome with prostate cancer diagnosis and aggressiveness in patients undergoing transrectal prostate biopsy. Arch Ital Urol Androl (2021) 93:291–5. doi: 10.4081/aiua.2021.3.291
15. Gomez-Gomez E, Carrasco-Valiente J, Campos-Hernandez JP, Blanca-Pedregosa AM, Jimenez-Vacas JM, Ruiz-Garcia J, et al. Clinical association of metabolic syndrome, C-reactive protein and testosterone levels with clinically significant prostate cancer. J Cell Mol Med (2019) 23:934–42. doi: 10.1111/jcmm.13994
16. Zhang JQ, Geng H, Ma M, Nan XY, Sheng BW. Metabolic syndrome components are associated with increased prostate cancer risk. Med Sci Monitor (2015) 21:2387–96. doi: 10.12659/msm.893442
17. Lifshitz K, Ber Y, Margel D. Role of metabolic syndrome in prostate cancer development. Eur Urol Focus (2021) 7:508–12. doi: 10.1016/j.euf.2021.04.022
18. Li Q, Kuriyama S, Kakizaki M, et al. History of diabetes mellitus and the risk of prostate cancer: the Ohsaki Cohort Study. Cancer Causes Control (2010) 21:1025–32. doi: 10.1007/s10552-010-9530-9
19. Burton AJ, Tilling KM, Holly JM, Hamdy FC, Rowlands M-AE, Donovan JL, et al. Metabolic imbalance and prostate cancer progression. Int J Mol Epidemiol Genet (2010) 1:248–71.
20. Hsing AW, Gao Y-T, Chua S Jr., Deng J, Stanczyk FZ. Insulin resistance and prostate cancer risk. J Natl Cancer Institute (2003) 95:67–71. doi: 10.1093/jnci/95.1.67
21. Mendonça FM, de Sousa FR, Barbosa AL, et al. Metabolic syndrome and risk of cancer: which link? Metabolism (2015) 64:182–9. doi: 10.1016/j.metabol.2014.10.008
22. Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science (1993) 259:87–91. doi: 10.1126/science.7678183
23. Tam CS, Xie VT, Johnson WD, Cefalu WT, Redman LM. Ravussin E.Defining insulin resistance from hyperinsulinemic-euglycemic clamps. Diabetes Care (2012) 35:1605–10. doi: 10.2337/dc11-2339
24. Pranata R, Huang IA, Irvan, Lim MA, Vania R. The association between triglyceride-glucose index and the incidence of type 2 diabetes mellitus-a systematic review and dose-response meta-analysis of cohort studies. Endocrine (2021) 74:254–62. doi: 10.1007/s12020-021-02780-4
25. Simental-Mendía LE, Rodríguez-Morán M. Guerrero-Romero F.The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord (2008) 6:299–304. doi: 10.1089/met.2008.0034
26. Guerrero-Romero F, Simental-Mendia LE, Gonzalez-Ortiz M, Martinez-Abundis E, Ramos-Zavala MG, Hernandez-Gonzalez SO, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab (2010) 95:3347–51. doi: 10.1210/jc.2010-0288
27. Sanchez-Garcia A, Rodriguez-Gutierrez R, Mancillas-Adame L, Gonzalez-Nava V, Gonzalez-Colmenero AD, Solis RC, et al. Diagnostic accuracy of the triglyceride and glucose index for insulin resistance: A systematic review. Int J Endocrinol (2020):4678526. doi: 10.1155/2020/4678526
28. Arthur R, Moller H, Garmo H, Holmberg L, Stattin P, Malmstrom H, et al. Association between baseline serum glucose, triglycerides and total cholesterol, and prostate cancer risk categories. Cancer Med (2016) 5:1307–18. doi: 10.1002/cam4.665
29. Murtola TJ, Salli SM, Talala K, Taari K, TammelaL TLJ, Auvinen A. Blood glucose, glucose balance, and disease-specific survival after prostate cancer diagnosis in the Finnish Randomized Study of Screening for Prostate Cancer. Prostate Cancer Prostatic Dis (2019) 22:453–60. doi: 10.1038/s41391-018-0123-0
30. Van Hemelrijck M, Garmo H, Holmberg L, Walldius G, Jungner I, Hammar N, et al. Prostate cancer risk in the swedish AMORIS study the interplay among triglycerides, total cholesterol, and glucose. Cancer (2011) 117:2086–95. doi: 10.1002/cncr.25758
31. Wang H, Yan FF, Cui YN, Chen FN, Wang GX, Cui WW. Association between triglyceride glucose index and risk of cancer: A meta-analysis. Front Endocrinol (2023) 13:1098492. doi: 10.3389/fendo.2022.1098492
32. Okamura T, Hashimoto Y, Hamaguchi M, Obora A, Kojima T, Fukui M. Triglyceride-glucose index (TyG index) is a predictor of incident colorectal cancer: a population-based longitudinal study. BMC Endocrine Disord (2020) 20. doi: 10.1186/s12902-020-00581-w
33. Liu T, Zhang QS, Wang YM, Ma XM, Zhang Q, Song MM, et al. Association between the TyG index and TG/HDL-C ratio as insulin resistance markers and the risk of colorectal cancer. BMC Cancer (2022) 22. doi: 10.1186/s12885-022-10100-w
34. Shi H, Zhou L, Yang S, Zhou H. The relationship between Triglyceride and glycose (TyG) index and the risk of gynaecologic and breast cancers. Clin Nutr ESPEN (2022) 51:345–52. doi: 10.1016/j.clnesp.2022.08.004
35. Karadag I, Karakaya S, Akkan T, Demir B, Alkurt EG, Dogan M. The potential prognostic marker tyG index predicts time to brain metastasis at HER2 positive breast cancer. Cancer Manage Res (2023) 15:311–7. doi: 10.2147/cmar.S403445
36. Alkurt EG, Ozkan MB, Turhan VB. Predictive value of triglyceride/glucose index (TyG) in predicting breast cancer in patients with breast mass. Eur Rev Med Pharmacol Sci (2022) 26:4671–6.
37. Jung MH, Yi SW, An SJ, Yi JJ, Ihm SH, Han S, et al. Associations between the triglyceride-glucose index and cardiovascular disease in over 150,000 cancer survivors: a population-based cohort study. Cardiovasc Diabetol (2022) 21. doi: 10.1186/s12933-022-01490-z
38. Yan X, Gao YJ, Tong JZ, Tian M, Dai JH, Zhuang Y. Association between triglyceride glucose index and non-small cell lung cancer risk in Chinese population. Front Oncol (2021) 11:585388. doi: 10.3389/fonc.2021.585388
39. Kim YM, Kim JH, Park JS, Baik SJ, Chun J, Youn YH, et al. Association between triglyceride-glucose index and gastric carcinogenesis: a health checkup cohort study. Gastric Cancer (2022) 25:33–41. doi: 10.1007/s10120-021-01222-4
40. Alkurt EG, Sahin F, Tutan B, Canal K, Turhan VB. The relationship between papillary thyroid cancer and triglyceride/glucose index, which is an indicator of insulin resistance. Eur Rev Med Pharmacol Sci (2022) 26:6114–20.
41. Jiang J, Teng L. Comment on: “Association between triglyceride-glucose index and gastric carcinogenesis: a health checkup cohort study. Gastric Cancer (2021) 24:1370–1. doi: 10.1007/s10120-021-01232-2
42. Panigoro SS, Sutandyo N, Witjaksono F, Siregar NC, Ramli R, Hariani R, et al. The association between triglyceride-glucose index as a marker of insulin resistance and the risk of breast cancer. Front Endocrinol (2021) 12:745236. doi: 10.3389/fendo.2021.745236
43. van der Leest M, Cornel E, Israel B, Hendriks R, Padhani AR, Hoogenboom M, et al. Head-to-head comparison of transrectal ultrasound-guided prostate biopsy versus multiparametric prostate resonance imaging with subsequent magnetic resonance-guided biopsy in biopsy-naive men with elevated prostate-specific antigen: A large prospective multicenter clinical study. Eur Urol (2019) 75:570–8. doi: 10.1016/j.eururo.2018.11.023
44. Huang C, Huang YH, Pu JX, Xi QL, Wei XD, Qiu F, et al. Comparison of MRI/US fusion targeted biopsy and systematic biopsy in biopsy-naive prostate patients with elevated prostate-specific antigen: A diagnostic study. Cancer Manage Res (2022) 14:1395–407. doi: 10.2147/cmar.S350701
45. Du T, Yuan G, Zhang MX, Zhou XR, Sun XX, Yu XF. Clinical usefulness of lipid ratios, visceral adiposity indicators, and the triglycerides and glucose index as risk markers of insulin resistance. Cardiovasc Diabetol (2014) 13. doi: 10.1186/s12933-014-0146-3
46. Robinson K, Muir S, Newbury A, Santos-Merx L, Appleton KM. Perceptions of body weight that vary by body mass index:Clear associations with perceptions based on personal control and responsibility. J Health Psychol (2022) 27:147–65. doi: 10.1177/1359105320916540
47. Albanes D, Weinstein SJ, Wright ME, Mannisto S, Limburg PJ, Snyder K, et al. Serum insulin, glucose, indices of insulin resistance, and risk of prostate cancer. J Natl Cancer Institute (2009) 101:1272–9. doi: 10.1093/jnci/djp260
48. Lebovitz HE. Insulin resistance: definition and consequences. Exp Clin Endocrinol Diabetes (2001) 109 Suppl 2:S135–48. doi: 10.1055/s-2001-18576
49. Ramasubbu K, Devi Rajeswari V. Impairment of insulin signaling pathway PI3K/Akt/mTOR and insulin resistance induced AGEs on diabetes mellitus and neurodegenerative diseases: a perspective review. Mol Cell Biochem (2023) 478:1307–24. doi: 10.1007/s11010-022-04587-x
50. Kolb H, Kempf K, Röhling M, Martin S. Insulin: too much of a good thing is bad. BMC Med (2020) 18:224. doi: 10.1186/s12916-020-01688-6
51. Uzunlulu M, Telci Caklili O, Oguz A. Association between metabolic syndrome and cancer. Ann Nutr Metab (2016) 68:173–9. doi: 10.1159/000443743
52. Barnard RJ, Aronson WJ, Tymchuk CN, Ngo TH. Prostate cancer: another aspect of the insulin-resistance syndrome? Obes Rev (2002) 3:303–8. doi: 10.1046/j.1467-789x.2002.00081.x
53. Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer (2004) 4:579–91. doi: 10.1038/nrc1408
54. Ibrahim YH, Yee D. Insulin-like growth factor-I and cancer risk. Growth Horm IGF Res (2004) 14:261–9. doi: 10.1016/j.ghir.2004.01.005
55. Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res (2002) 12:9–18. doi: 10.1038/sj.cr.7290105
56. Chen H, Zhou L, Wu X, et al. The PI3K/AKT pathway in the pathogenesis of prostate cancer. Front Biosci (2016) 21:1084–91. doi: 10.2741/4443
57. Selvi NMK, Nandhini S, Sakthivadivel V, Lokesh S, Srinivasan AR, Sumathi S. Association of triglyceride-glucose index (TyG index) with hbA1c and insulin resistance in type 2 diabetes mellitus. Maedica (Bucur) (2021) 16:375–81. doi: 10.26574/maedica.2021.16.3.375
58. Tutunchi H, Naeini F, Mobasseri M, Ostadrahimi A. Triglyceride glucose (TyG) index and the progression of liver fibrosis: A cross-sectional study. Clin Nutr ESPEN (2021) 44:483–7. doi: 10.1016/j.clnesp.2021.04.025
59. Tao LC, Xu JN, Wang TT, Hua F, Li JJ. Triglyceride-glucose index as a marker in cardiovascular diseases: landscape and limitations. Cardiovasc Diabetol (2022) 21:68. doi: 10.1186/s12933-022-01511-x
60. Yilmaz M, Karaaslan M, Tonyali S, Celik M, Toprak T, Odabas O. Triglyceride-Glucose Index (TyG) is associated with erectile dysfunction: A cross-sectional study. Andrology (2021) 9:238–44. doi: 10.1111/andr.12904
61. Aljada A, Friedman J, Ghanim H, et al. Glucose ingestion induces an increase in intranuclear nuclear factor kappaB, a fall in cellular inhibitor kappaB, and an increase in tumor necrosis factor alpha messenger RNA by mononuclear cells in healthy human subjects. Metabolism (2006) 55:1177–85. doi: 10.1016/j.metabol.2006.04.016
62. Chen C, Edelstein LC, Gélinas C. The Rel/NF-kappaB family directly activates expression of the apoptosis inhibitor Bcl-x(L). Mol Cell Biol (2000) 20:2687–95. doi: 10.1128/MCB.20.8.2687-2695.2000
63. Zhou C, Yao L. Biochemistry and Molecular Biology. Ninth. Beijing: People’s Health Publishing House (2018) p. 152–7.
64. Esposito K, Chiodini P, Colao A, Lenzi A, Giugliano D. Metabolic syndrome and risk of cancer: a systematic review and meta-analysis. Diabetes Care (2012) 35:2402–11. doi: 10.2337/dc12-0336
65. Kaiser A, Haskins C, Siddiqui MM, Hussain A, D’Adamo C. The evolving role of diet in prostate cancer risk and progression. Curr Opin Oncol (2019) 31:222–9. doi: 10.1097/CCO.0000000000000519
Keywords: prostate cancer, triglyceride-glucose index, insulin resistance, endocrinology, urology
Citation: Zhou Y, Li T, Muheiyati G, Duan Y, Xiao S, Gao Y, Tao N and An H (2024) Triglyceride-glucose index is a predictor of the risk of prostate cancer: a retrospective study based on a transprostatic aspiration biopsy population. Front. Endocrinol. 14:1280221. doi: 10.3389/fendo.2023.1280221
Received: 19 August 2023; Accepted: 15 December 2023;
Published: 08 January 2024.
Edited by:
Sijung Yun, Predictiv Care, Inc., United StatesReviewed by:
Cesar Miguel Momesso Santos, Faculty Enau, BrazilCopyright © 2024 Zhou, Li, Muheiyati, Duan, Xiao, Gao, Tao and An. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hengqing An, Mzg1MTg0MTJAeGptdS5lZHUuY24=; Ning Tao, Mzg1MTg0MTJAcXEuY29t
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.