- 1Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine and Metabolic Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2Shanghai National Clinical Research Center for Metabolic Diseases, Key Laboratory for Endocrine and Metabolic Diseases of the National Health Commission of the PR China, Shanghai Key Laboratory for Endocrine Tumor, State Key Laboratory of Medical Genomics, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Aims: To examine the associations of sleep duration and changes in BMI with the onset of diabetic kidney disease (DKD).
Materials and methods: 2,959 participants with type 2 diabetes were divided into three groups based on sleep duration: short (<7 h/day), intermediate (7-9 h/day), or long (>9 h/day). Changes in BMI during follow-up were trisected into loss, stable, or gain groups. DKD was defined as either the urinary albumin/creatinine ratio (UACR) ≥ 3.39 mg/mmol or the estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73m², or both. Cox regression models were used to assess hazard ratios (HRs) and 95% confidence intervals (CIs).
Results: During a mean follow-up of 2.3 years, DKD occurred in 613 participants (20.7%). A J-shaped curve was observed between sleep duration and DKD. Compared to intermediate sleep duration, long sleep duration was associated with higher risks of DKD (HR 1.47; 95% CI: 1.19-1.81). In the joint analyses, compared to participants with intermediate sleep duration and stable BMI, long sleep duration with BMI gain had the highest risks of DKD (HR 2.04; 95% CI: 1.48-2.83). In contrast, short or intermediate sleep duration accompanied by decrease in BMI was associated with a reduced risk of DKD, with HRs of 0.50 (95% CI: 0.31-0.82) and 0.61 (95% CI:0.47-0.80), respectively.
Conclusions: Long sleep duration is significantly associated with an increased risk of DKD, which is further amplified by obesity or BMI gain. These findings suggest that both proper sleep duration and weight control are essential to preventing DKD.
Introduction
Diabetes affects 537 million adults globally in 2021 and is responsible for an estimated 2 million deaths, including those related to diabetic kidney disease (DKD) (1). It can lead to end-stage renal disease (ESRD) as kidney function deteriorates over time, ultimately necessitating interventions such as dialysis or kidney transplantation (2). The progression of DKD is heavily influenced by hyperglycemia, and common comorbidities such as hypertension and hyperlipidemia also play significant roles in the pathogenesis of DKD (3). These factors lead to glomerular endothelial dysfunction and podocyte damage, ultimately resulting in glomerulosclerosis and the formation of renal unit loss (4, 5). While the complex pathophysiological mechanisms involved are still being explored.
Sleep duration has been demonstrated as a critical factor influencing a variety of health outcomes like hyperglycemia, cardiovascular disease (CVD), and cognitive decline (6–8). The guidelines provided by the American Diabetes Association (ADA) indicate a correlation between inadequate or excessive sleep duration and elevated levels of glycated hemoglobin (HbA1c) (9). A previous cross-sectional study found both short and long sleep durations were associated with decreased estimated glomerular filtration rate (eGFR) as well as increased albuminuria (10). Another cross-sectional study suggested that longer daytime sleep among individuals with type 2 diabetes was linked to albuminuria (11). However, these cross-sectional studies cannot establish a causal relationship between sleep duration and DKD. Moreover, there is a notable scarcity of prospective cohort studies that have specifically investigated this association. Additionally, prior investigations have demonstrated a positive correlation between higher BMI and DKD, whereas a reduction in BMI has been linked to a decreased risk of DKD (12). This indicates that alterations in BMI contribute significantly to the onset of DKD. There is a growing recognition of the significance of comprehensive management of lifestyle and metabolic health status. Both of these aspects are incorporated as modifiable factors in the care goals for individuals with diabetes. It is highly meaningful to perform risk stratification for DKD by combining sleep duration and changes in BMI as it enhances the identification of high-risk populations and enables targeted interventions.
Therefore, the present study aimed to extend the existing knowledge by investigating the relationship between sleep duration and DKD, and exploring the potential influence of obesity and changes in BMI on this association.
Materials and methods
Study design and participants
This prospective cohort was part of a pilot and standard system, the National Metabolic Management Center (MMC) at Ruijin Hospital, Shanghai Jiao Tong University School of Medicine. The details of MMC have been previously described (13, 14). A total of 5,655 individuals aged 18 years or older, who had received a confirmed diagnosis of type 2 diabetes, were followed from June 2017 to December 2022. Out of these individuals, we excluded participants with a follow-up period of fewer than 6 months (n = 750), existing DKD at baseline (n = 1,327), or lacking information on sleep duration (n = 318), urinary albumin/creatinine ratio (UACR), or eGFR (n = 301). The final analysis comprised a total of 2,959 participants (the flowchart of participant inclusion appears in Supplementary Figure 1). Written informed consent was obtained from each participant, and the study protocol received ethical approval from the Institutional Review Board of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine.
General characteristics and outcomes
Participants underwent an extensive physical examination, blood sample collection, and completion of baseline questionnaires to obtain lifestyle-related information. The questionnaires included questions on night sleep duration, nap duration, and sleep quality. Participants’ total sleep duration of twenty-four hours was obtained by combining both nocturnal and midday sleep periods. Three self-reported options were available to assess sleep quality based on subjective evaluation: 1) good, 2) poor, and 3) requiring medication. The definition of ideal smoking status and alcohol consumption, as well as the standards for physical measurements, were detailed in our previously published studies (15, 16). Blood pressure was measured after a minimum of 5 minutes of rest. Blood samples were collected from each participant following an overnight fasting period to obtain HbA1c, fasting plasma glucose (FPG) and 2-hour post-load plasma glucose (PG), lipid profile, and other laboratory parameters. The UACR was determined by dividing the concentration of urinary albumin by the concentration of urinary creatinine. The eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation to assess renal function (17).
Definitions
Based on previous studies, the classification of sleep duration was as follows: intermediate (7-9 h/day), short (<7 h/day), and long (>9 h/day) (18). BMI was divided into three categories according to the criteria recommended by the National Health Commission of the People’s Republic of China: normal weight, <24 kg/m2; overweight, 24-27.9 kg/m2 and obese, ≥28 kg/m2. Central obesity was defined as waist circumference ≥90 cm for men and ≥85 cm for women (19). To perform the joint analysis, we categorized the study participants based on their changes in BMI from baseline to the final visit. Participants with the lowest one-third change in BMI were classified as the “loss” group; those with the middle one-third change were classified as the “stable” group; and the upper thirds were classified as the “gain” group (20). Considering the potential impact of baseline BMI status on DKD, we categorized participants into four groups based on changes in BMI status: “Remained normal BMI” for those with normal BMI at both baseline and the last examination, “Remained overweight or obese” for those who were overweight or obese at both examinations, “Became normal BMI” for those who were overweight or obese at baseline but had a normal BMI at the last examination, and “Became overweight or obese” for those with a normal BMI at baseline but were overweight or obese at the last examination. The details of the characteristics of BMI changes in the different groups, categorized based on changes in BMI or BMI status, have been provided in the Appendix (Table S1). The definition of diabetes was fasting plasma glucose ≥7.0 mmol/L, or 2-hour plasma glucose ≥11.1 mmol/L, or HbA1c ≥6.5%, or previously diagnosed by their healthcare provider (21). Albuminuria was defined as UACR ≥3.39 mg/mmol. The presence of albuminuria or eGFR <60 mL/min/1.73m2 was defined as the presence of DKD (22).
Statistical analyses
Continuous variables were presented as mean ± standard deviation or median with interquartile range, while categorical variables were presented as counts and proportions. To assess differences among groups, P-values were calculated using the ANOVA method for continuous variables and chi-square tests for categorical variables.
We employed Cox proportional hazards models to investigate the associations between sleep duration and the incidence of DKD. The Schoenfeld residuals method was utilized to evaluate the proportional hazards assumptions of the Cox models. According to previous studies on risk factors for kidney diseases, we selected potential confounding factors and adjusted for them in the multivariable model (23–25). Model 1 adjusted for age and sex, while in Model 2, additional adjustments were made for the duration of diabetes, HbA1c, smoking status, alcohol intake, BMI, eGFR, use of angiotensin-converting enzyme inhibitor (ACEI) or angiotensin receptor blockers (ARB); model 3 was further adjusted for history of hypertension, CVD and cancer, and sleep quality (good or poor/requiring medication) based on model 2. Restricted cubic splines with three knots placed at the 5th, 50th, and 95th percentiles were employed to examine the associations between sleep duration and DKD. We performed stratified analyses by age (<50 years, 50-60 years, or >60 years), sex, duration of diabetes (<5 years, or ≥5 years), glycemic control (HbA1c <7%, or ≥7%), BMI (<24 kg/m2, 24-27.9 kg/m2, or ≥28 kg/m2), changes in BMI (stable, loss, or gain), changes in BMI status (remained normal, remained overweight or obese, became normal, or became overweight or obese), and waist circumference (normal, or central obesity). Adjustments to the model were the same as in model 3 except for the stratification variables. The statistical significance of interactions was assessed using the likelihood ratio test, comparing models with and without cross-product terms between sleep duration categories (<7, 7-9, >9 h/day) and the stratification variables. To examine whether the association between sleep duration and DKD was modified by BMI, changes in BMI, or changes in BMI status, we conducted joint analyses. The model accounted for the covariates included in model 3, except for BMI.
Sensitivity analyses were conducted. Initially, the participants were categorized into three groups according to the percentage variation in BMI to test the robustness of the results: Individuals with a BMI reduction of 5.0% or higher were designated as the “BMI loss” group (n=636). This threshold aligns with the weight loss recommendation of ≥5% for individuals who are overweight or obese and diagnosed with type 2 diabetes, as routinely advocated by the American Diabetes Association (ADA) (26). As BMI decreased by an average of 1.3% for all participants during the follow-up period while the number of participants with a ≥ 5% increase in BMI was less than one in ten (n=286), the “BMI gain” group consisted of individuals who experienced a BMI increase of 3.0% or higher (n = 495). The participants in the “BMI stable” group had a BMI decrease of < 5.0% or an increase of < 3.0% (n=1805). Furthermore, we analyzed the effect of nighttime sleep on DKD with an additional adjustment for the duration of daytime naps.
The statistical analysis was conducted using R version 4.1.1 (R Foundation, Vienna, Austria). Two-tailed P-values were reported, and a significance level of 0.05 was used to determine statistical significance.
Results
Baseline characteristics
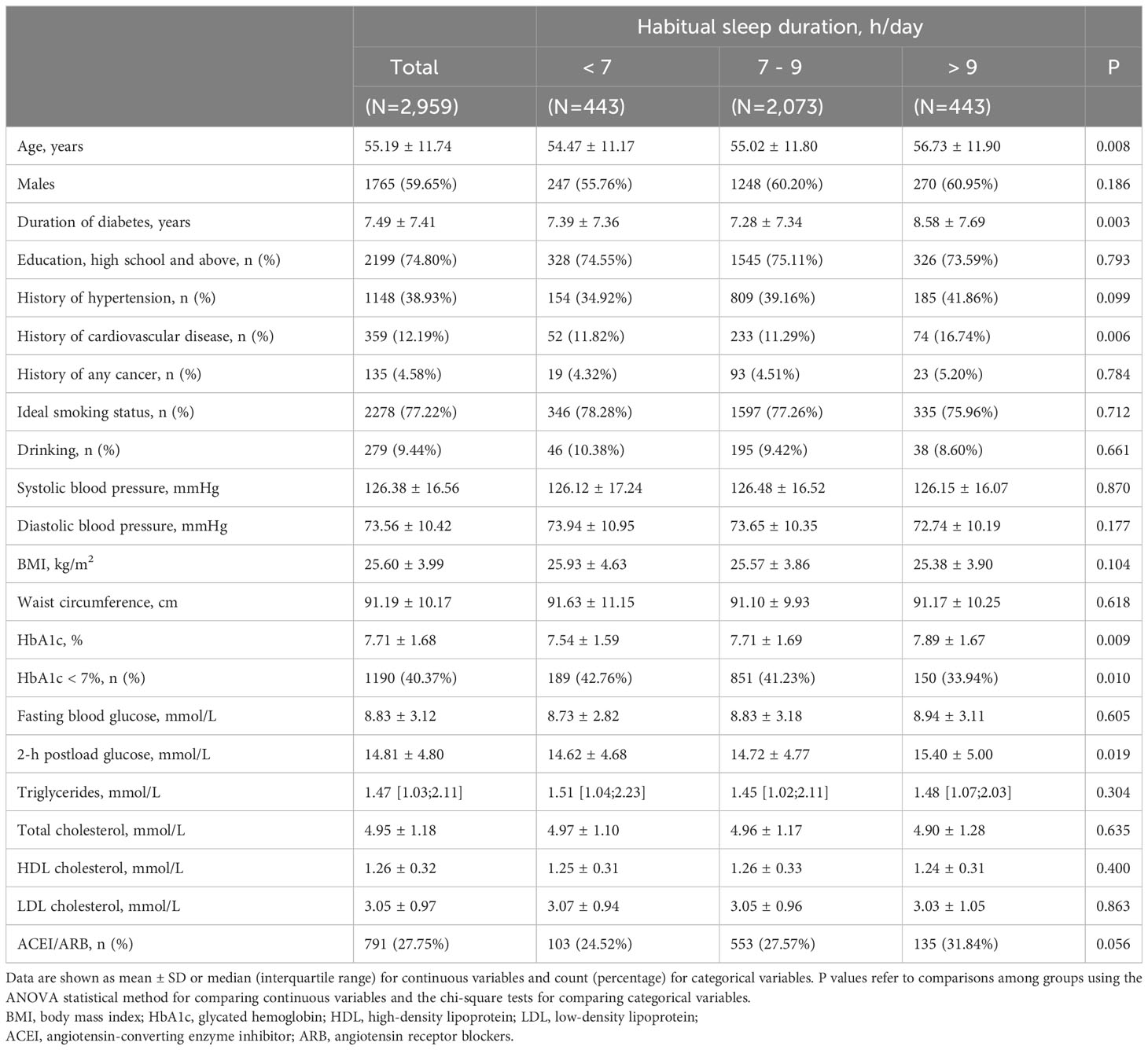
The baseline characteristics of the study participants according to sleep duration are presented in Table 1. The mean age of the participants was 55.2 (SD, 11.7) years, and 1,765 were males (59.7%). Of all the 2,959 participants involved, 15%, 70%, and 15% reported sleeping <7 hours, 7-9 hours, and >9 hours per day, respectively. Participants who reported sleeping >9 hours per day were slightly older with a longer duration of diabetes and poorer glycemic control compared to the other two groups (p < 0.05).
Independent association of sleep duration with DKD and albuminuria
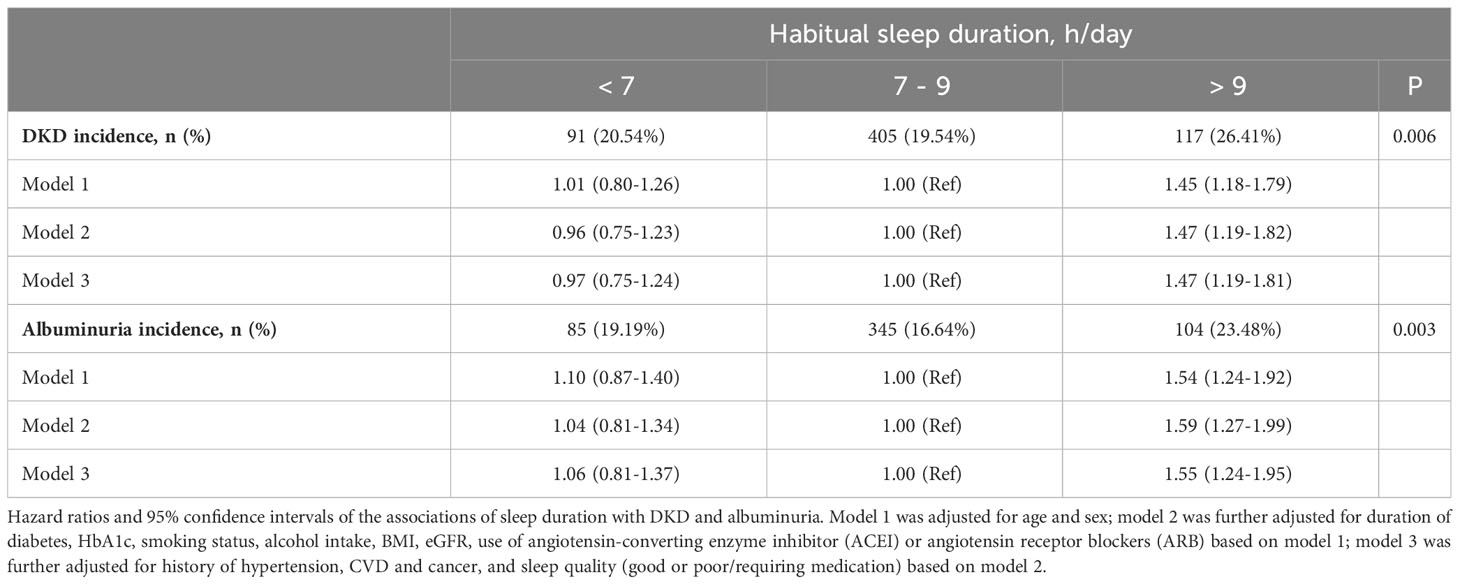
Throughout a mean ( ± SD) follow-up period of 2.3 (± 1.4) years, a total of 613 incidents (20.7%) of DKD were detected. Table 2 demonstrates a significant association between sleep duration and the risks of DKD. Participants with long sleep duration had higher risks of DKD (26.4%) compared to those with intermediate (19.5%) and short sleep duration (20.5%) (P = 0.006). Similarly, the incidence of albuminuria was higher in participants with long sleep duration, with 23.5% developing albuminuria compared to 16.6% in the reference group and 19.2% in the short sleep duration group (P = 0.003).
We did not detect any evidence of a violation of the proportional hazard assumption, employing a p-value threshold of 0.05 (Figure S2). After adjusting for age and gender, long sleep duration was associated with an increased risk of developing DKD (HR 1.45; 95% CI:1.18-1.79) compared with intermediate sleep duration (model 1). The association was prominent after additional adjustment for diabetes duration, smoking status, alcohol intake, BMI, eGFR, and use of ACEI/ARB (HR 1.47; 95% CI:1.19-1.82) (model 2). Furthermore, the observed association between longer sleep and risk of DKD remained significant after controlling for sleep quality and history of hypertension, CVD, and cancer (HR 1.47; 95% CI: 1.19-1.81) (model 3). Short sleep duration did not show an association with increased risks of DKD or albuminuria. The restricted cubic spline regression analysis confirmed a J-shaped curve, with >9 h/day of sleep being associated with a higher risk of DKD and albuminuria (Figure 1). The same covariates as in Model 3 were included in the analysis. In addition, consistent results were observed in subgroup analyses (all interaction P-values > 0.05) (see Figure S3).
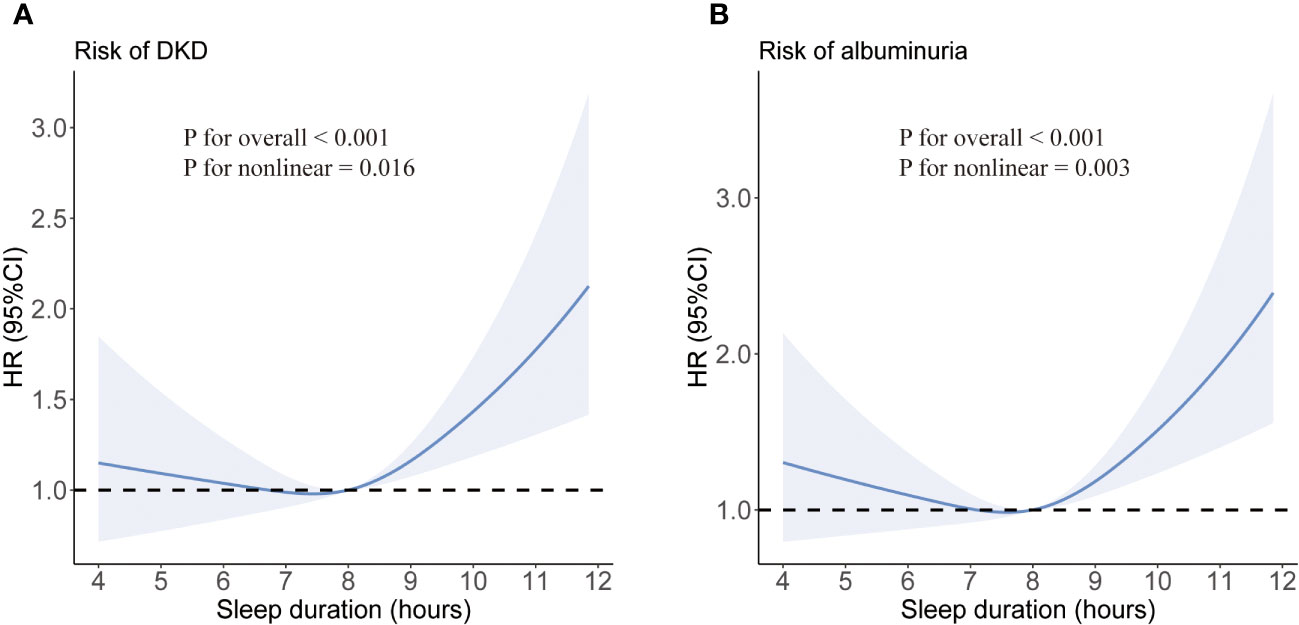
Figure 1 Multivariate-adjusted spline curves for associations of sleep duration with DKD (A) and albuminuria (B). Sleep duration was fitted as a smooth term using a restricted cubic spline with 3 knots. Shading indicates 95% confidence intervals. The model was adjusted for age, sex, duration of diabetes, HbA1c, smoking status, alcohol intake, BMI, eGFR, use of ACEI/ARB, history of hypertension, CVD and cancer, and sleep quality.
Joint association of sleep duration and BMI with DKD and albuminuria
Figures 2A, B; Table S2 show the joint association of sleep duration and BMI with DKD and albuminuria. Individuals with long sleep duration and overweight had higher risks of DKD (HR 2.12; 95% CI: 1.52-2.94) and albuminuria (HR 2.45; 95% CI: 1.72-3.48) compared to the reference group, which consists of individuals with intermediate sleep duration and BMI < 24kg/m2. Similarly, individuals with long sleep duration and obesity were at higher risk of DKD (HR 1.83; 95% CI: 1.17-2.86) and albuminuria (HR 2.06; 95% CI: 1.27-3.35) compared to the reference group. Additionally, participants with short or intermediate sleep duration and obesity were also associated with an elevated risk of DKD and albuminuria.
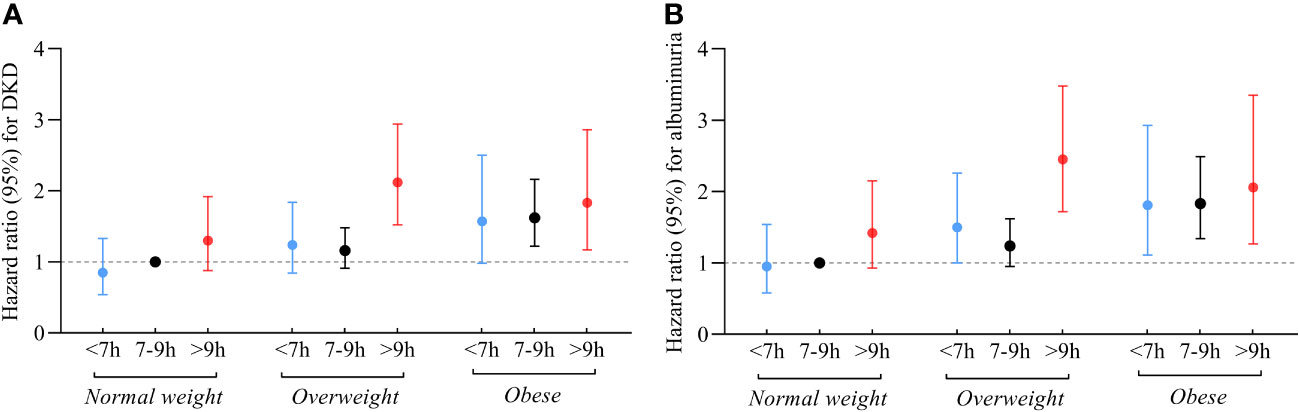
Figure 2 Relationship between sleep duration and risk of DKD (A) and albuminuria (B) among participants with varying BMI. Those who slept for 7-9 hours/day and had a BMI < 24 kg/m2 were referenced. All models were adjusted for age, sex, duration of diabetes, HbA1c, smoking status, alcohol intake, eGFR, use of ACEI/ARB, history of hypertension, CVD and cancer, and sleep quality.
Joint association of sleep duration and changes in BMI with DKD and albuminuria
Figures 3A, B; Table S3 illustrate the joint association of sleep duration and changes in BMI with DKD and albuminuria. Participants who had long sleep duration and experienced BMI gain faced the highest risks of DKD (HR 2.04; 95% CI: 1.48-2.83) and albuminuria (HR 2.09; 95% CI: 1.48-2.93) compared to the reference group, comprising individuals with intermediate sleep duration and stable BMI. In contrast, participants with short sleep patterns and experiencing BMI loss were found to have lower risks of DKD (HR 0.50; 95% CI: 0.31-0.82) and albuminuria (HR 0.55; 95% CI: 0.34-0.91) compared to the reference group. Similarly, participants with intermediate sleep patterns and BMI loss also had a protective effect on the development of DKD (HR 0.61; 95% CI: 0.47-0.80) and albuminuria (HR 0.57; 95% CI: 0.43-0.77).
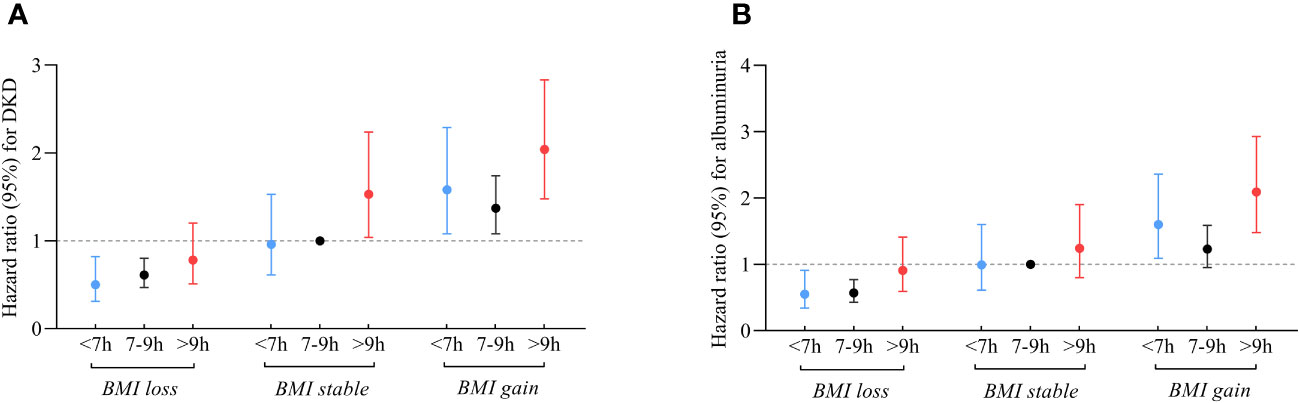
Figure 3 Relationship between sleep duration and risk of DKD (A) and albuminuria (B) among participants with varying changes in BMI. Those who slept 7-9 hours/day and had stable BMI during follow-up were referenced. All models were adjusted for age, sex, duration of diabetes, HbA1c, smoking status, alcohol intake, eGFR, use of ACEI/ARB, history of hypertension, CVD and cancer, and sleep quality.
Joint association of sleep duration and changes in BMI status with DKD and albuminuria
Figures 4A, B; Table S4 depict the joint association of sleep duration and changes in BMI status with DKD and albuminuria. Participants who had long sleep duration and became overweight or obese faced the highest risks of DKD (HR 2.49; 95% CI:1.14-5.40), in contrast to the reference group consisting of individuals with intermediate sleep durations and remained BMI normal. Similarly, participants who had long sleep durations and remained overweight or obese were also at an increased risk of DKD (HR 2.46; 95% CI: 1.79-3.39). Conversely, participants with intermediate sleep patterns who transitioned to a normal BMI exhibited reduced risks of DKD compared to the reference group (HR 0.58; 95%CI: 0.34-0.97). Furthermore, the group with short sleep patterns that transitioned to a normal BMI had no participants who developed DKD.
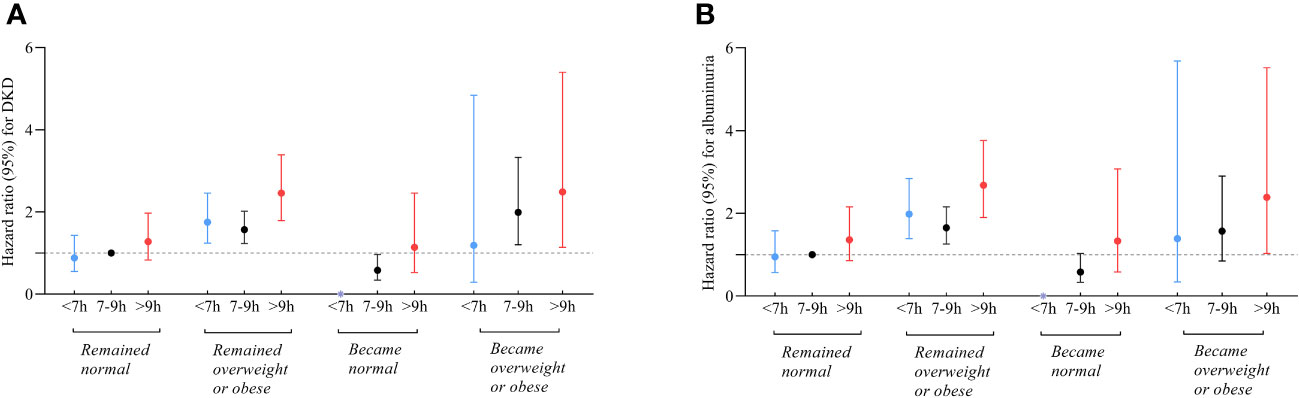
Figure 4 Relationship between sleep duration and risk of DKD (A) and albuminuria (B) among participants with varying changes in BMI status. Those who slept 7-9 hours/day and remained normal BMI during follow-up were referenced. All models were adjusted for age, sex, duration of diabetes, HbA1c, smoking status, alcohol intake, eGFR, use of ACEI/ARB, history of hypertension, CVD and cancer, and sleep quality. * The group with short sleep patterns that transitioned to a normal BMI had no participants who developed DKD.
Sensitivity analyses
The results remained largely consistent in all sensitivity analyses (Figures S4-5; Table S5).
Discussion
This prospective cohort study examined the relationship between sleep duration and the occurrence of DKD in individuals diagnosed with T2DM. Our findings revealed a significant link between long sleep duration (>9 h/day) and an elevated risk of DKD, which remained after adjusting for potential confounding variables. We identified a nonlinear relationship between sleep duration and the onset of DKD, characterized by a J-shaped curve, which was also present in the occurrence of albuminuria. Furthermore, we found that participants with both long sleep duration and BMI gain faced the greatest risks of DKD compared to individuals with intermediate sleep duration and stable BMI. To our knowledge, this is the first prospective cohort to report that long sleep duration was associated with a higher risk of DKD when compared to those who slept 7-9 hours per day. Moreover, our results emphasize the influence of BMI changes on this association, suggesting that the combination of longer sleep and BMI gain may synergistically contribute to the development of DKD.
Although the association between long sleep duration and the risk of DKD has been proposed, relevant studies published thus far are sparse (10, 11). Several studies have examined the connection between sleep duration and CKD in the general population; however, the findings have yielded inconsistent results. A previous investigation identified a U-shaped relationship between both insufficient sleep duration (≤4 hours) and excessive sleep duration (>10 hours) and CKD in middle-aged and older people (27), while the Nurses’ Health Study (NHS) did not observe a correlation between longer sleep (≥9 hours) and rapid decline in eGFR (decrease of more than 30%) (28). Furthermore, research conducted in Japan discovered that longer sleep duration (>8 hours) was an important predictor of end-stage kidney disease (ESKD) (29). The disparities between our findings and the conclusions of previous research may be attributed, in part, to variations in race, geographical locations, underlying disease, and baseline renal function among these study populations.
The mechanisms involved in the negative effects of long sleep duration on the onset of DKD have not been sufficiently appreciated. To begin with, prolonged periods of sleep may trigger an upsurge in high-sensitivity C-reactive protein (hs-CRP) levels and stimulate an inflammatory response, which further leads to cellular injury, glomerular endothelial dysfunction, proliferation of mesangial cells, and increased vascular permeability (30, 31). These factors can ultimately contribute to the development of albuminuria and DKD (32). Second, longer sleep duration is associated with several metabolic abnormalities, including hypertension, dyslipidemia, and insulin resistance (33, 34), all of which are closely associated with the onset of DKD (35). Furthermore, numerous studies have reported that individuals with long sleep durations often have other unhealthy daily behaviors, such as sedentary behavior and lack of physical activity, which are well-established risk factors for diabetes complications, including DKD (36–38).
Consistent with previous studies suggesting that obesity is an independent risk factor for DKD (39), our study extends this understanding through joint analyses of sleep duration and variability in BMI and BMI status. In our study, the effect of long sleep duration on the incidence of DKD was more pronounced among those who experienced BMI gain, transitioned to overweight or obese, or remained overweight or obese. The biological mechanisms underlying the joint effects are still unclear. On one hand, previous studies have indicated that prolonged sleep has been associated with reduced physical activity, decreased energy expenditure, and subsequent weight gain (40). On the other hand, evidence suggests that obese individuals tend to have a higher risk of obstructive sleep apnea (OSA), a condition that adversely affects the quality of sleep and contributes to excessive daytime sleepiness (41, 42). This bidirectional association between sleep and obesity forms a detrimental cycle that exacerbates the risk of DKD. In addition, both long sleep duration and obesity are related to risk factors of DKD, like insulin resistance, hypertension, dyslipidemia, and inflammatory response (43, 44). On the contrary, participants with short or intermediate sleep duration and a decrease in BMI during follow-up were observed to be related to a reduction in the risk of DKD. The finding suggests that maintaining appropriate sleep duration and achieving weight loss may have a protective effect against DKD. A previous randomized controlled trial has demonstrated that weight loss ameliorates insulin resistance and results in lower HbA1c and systolic blood pressure in individuals with obesity, thereby delaying the onset of the microvascular complications of diabetes (45). This finding coincides with the outcomes of our analysis. Further exploration is needed to understand the underlying mechanisms of this relationship. Additionally, further research is warranted to establish specific and effective strategies targeting sleep duration and weight management to prevent or delay the onset and progression of DKD.
The strengths of our study include its large sample size and the implementation of a longitudinal study design, which provides a more robust approach compared to cross-sectional studies. Our finding extends previous research by exploring the combined effects of sleep duration and changes in BMI on the occurrence of DKD. Additionally, we fully adjusted for potential confounding factors to ensure the reliability of the outcomes. In particular, we adjusted for sleep quality to assess the independent risk of sleep duration for DKD, which has rarely been considered in previous studies.
Despite the novel insights provided by this study, several potential limitations should also be acknowledged. Firstly, although the association between sleep duration and BMI changes and DKD incidence reached statistical significance, our conclusions still need to be validated in populations of other genetic backgrounds. Secondly, our assessment of sleep duration is self-reported while those measured using polysomnography are more objective. However, self-reported questionnaires have been widely utilized as a more feasible form of epidemiological investigation in large-scale population studies.
Conclusion
Long sleep duration was significantly associated with increased risks of DKD. Notably, compared to participants with intermediate sleep duration and stable BMI, long sleep duration with BMI gain had higher risks of DKD. Conversely, short or intermediate sleep duration with loss of BMI was associated with decreased risks of DKD, indicating that both appropriate sleep duration and weight control are required to prevent the development of DKD. Future investigations are warranted to elucidate the underlying mechanisms of this association and develop better intervention strategies for sleep habits and weight management.
STROBE statement
This study was reported in accordance with STROBE guidelines for cohort studies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Institutional Review Board of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
CL: Conceptualization, Data curation, Visualization, Writing – original draft. JZ: Methodology, Validation, Writing – original draft. XW: Data curation, Methodology, Visualization, Writing – original draft. JS: Formal Analysis, Methodology, Writing – review & editing. QF: Conceptualization, Software, Writing – original draft. WZ: Data curation, Validation, Writing – review & editing. LS: Data curation, Investigation, Software. ZH: Data curation, Investigation, Validation. JH: Writing – review & editing. WG: Formal Analysis, Validation, Writing – review & editing. WW: Funding acquisition, Resources. YP: Supervision, Writing – review & editing. YZ: Funding acquisition, Supervision, Project administration.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from the National Natural Science Foundation of China (grant no. 82270896 to YZ), “Management strategy of the tertiary prevention and treatment of diabetes based on DIP system” supported by China Health Promotion Foundation (to WW), the Capacity building for multidisciplinary cooperation in diagnosis and treatment of major metabolic diseases (grant no. Z155080000004 to WW), the Shanghai Medical and Health Development Foundation (grant no. DMRFP_II_01 to YZ, grant no. DMRFP_II_02 to WZ), the Program for Shanghai Outstanding Medical Academic Leader (grant no. 2019LJ07 to YZ), and Innovative research team of high-level local universities in Shanghai.
Acknowledgments
We would like to thank the participants for their willingness to take part in the management of MMC.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1278665/full#supplementary-material
References
1. Xie J, Wang M, Long Z, Ning H, Li J, Cao Y, et al. Global burden of type 2 diabetes in adolescents and young adults, 1990-2019: systematic analysis of the global burden of disease study 2019. BMJ (2022) 379:e072385. doi: 10.1136/bmj-2022-072385
2. Tuttle KR, Bakris GL, Bilous RW, Chiang JL, de Boer IH, Goldstein-Fuchs J, et al. Diabetic kidney disease: A report from an ada consensus conference. Diabetes Care (2014) 37(10):2864–83. doi: 10.2337/dc14-1296
3. Porrini E, Ruggenenti P, Mogensen CE, Barlovic DP, Praga M, Cruzado JM, et al. Non-proteinuric pathways in loss of renal function in patients with type 2 diabetes. Lancet Diabetes Endocrinol (2015) 3(5):382–91. doi: 10.1016/S2213-8587(15)00094-7
4. Anders HJ, Huber TB, Isermann B, Schiffer M. Ckd in diabetes: diabetic kidney disease versus nondiabetic kidney disease. Nat Rev Nephrol (2018) 14(6):361–77. doi: 10.1038/s41581-018-0001-y
5. Cole JB, Florez JC. Genetics of diabetes mellitus and diabetes complications. Nat Rev Nephrol (2020) 16(7):377–90. doi: 10.1038/s41581-020-0278-5
6. Mokhlesi B, Temple KA, Tjaden AH, Edelstein SL, Utzschneider KM, Nadeau KJ, et al. Association of self-reported sleep and circadian measures with glycemia in adults with prediabetes or recently diagnosed untreated type 2 diabetes. Diabetes Care (2019) 42(7):1326–32. doi: 10.2337/dc19-0298
7. Wang CS, Bangdiwala SI, Rangarajan S, Lear SA, AlHabib KF, Mohan V, et al. Association of estimated sleep duration and naps with mortality and cardiovascular events: A study of 116 632 people from 21 countries. Eur Heart J (2019) 40(20):1620–9. doi: 10.1093/eurheartj/ehy695
8. Sabia S, Fayosse A, Dumurgier J, van Hees VT, Paquet C, Sommerlad A, et al. Association of sleep duration in middle and old age with incidence of dementia. Nat Commun (2021) 12(1):2289. doi: 10.1038/s41467-021-22354-2
9. American Diabetes Association Professional Practice C. 4. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes-2022. Diabetes Care (2022) 45(Suppl 1):S46–59. doi: 10.2337/dc22-S004
10. Tan NYQ, Chan J, Cheng CY, Wong TY, Sabanayagam C. Sleep duration and diabetic kidney disease. Front Endocrinol (Lausanne) (2018) 9:808. doi: 10.3389/fendo.2018.00808
11. Li X, Chattopadhyay K, Qian X, Yu J, Xu M, Li L, et al. Association between sleep duration and albuminuria in patients with type 2 diabetes: A cross-sectional study in Ningbo, China. Diabetes Metab Syndr Obes (2022) 15:1667–75. doi: 10.2147/DMSO.S366064
12. Polemiti E, Baudry J, Kuxhaus O, Jager S, Bergmann MM, Weikert C, et al. Bmi and bmi change following incident type 2 diabetes and risk of microvascular and macrovascular complications: the epic-potsdam study. Diabetologia (2021) 64(4):814–25. doi: 10.1007/s00125-020-05362-7
13. Zhang Y, Wang W, Ning G. Metabolic management center: an innovation project for the management of metabolic diseases and complications in China. J Diabetes (2019) 11(1):11–3. doi: 10.1111/1753-0407.12847
14. Zhang Y, Shi J, Peng Y, Zhao Z, Zheng Q, Wang Z, et al. Artificial intelligence-enabled screening for diabetic retinopathy: A real-world, multicenter and prospective study. BMJ Open Diabetes Res Care (2020) 8(1):e001596. doi: 10.1136/bmjdrc-2020-001596
15. Wang S, Shi J, Peng Y, Fang Q, Mu Q, Gu W, et al. Stronger association of triglyceride glucose index than the homa-ir with arterial stiffness in patients with type 2 diabetes: A real-world single-centre study. Cardiovasc Diabetol (2021) 20(1):82. doi: 10.1186/s12933-021-01274-x
16. Fang Q, Xiang M, Shi J, Zhou Y, Peng Y, Wang S, et al. Subclinical atherosclerosis associates with diabetic retinopathy incidence: A prospective study. Acta Diabetol (2022) 59(8):1041–52. doi: 10.1007/s00592-022-01897-w
17. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med (2009) 150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
18. Han H, Wang Y, Li T, Feng C, Kaliszewski C, Su Y, et al. Sleep duration and risks of incident cardiovascular disease and mortality among people with type 2 diabetes. Diabetes Care (2023) 46(1):101–10. doi: 10.2337/dc22-1127
19. People’s republic of China national health and family planning commission Ws/T 428-2013 criteria of weight for adults. Chinese standard (2013). Available at: https://www.chinesestandard.net/PDF/English.aspx/WST428-2013.
20. Lee DC, Sui X, Artero EG, Lee IM, Church TS, McAuley PA, et al. Long-term effects of changes in cardiorespiratory fitness and body mass index on all-cause and cardiovascular disease mortality in men: the aerobics center longitudinal study. Circulation (2011) 124(23):2483–90. doi: 10.1161/CIRCULATIONAHA.111.038422
21. American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care (2010) 33 Suppl 1(Suppl 1):S62–9. doi: 10.2337/dc10-S062
22. Kdoqi. Kdoqi clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis (2007) 49(2 Suppl 2):S12–154. doi: 10.1053/j.ajkd.2006.12.005
23. Bjornstad P, Laffel L, Lynch J, El Ghormli L, Weinstock RS, Tollefsen SE, et al. Elevated serum uric acid is associated with greater risk for hypertension and diabetic kidney diseases in obese adolescents with type 2 diabetes: an observational analysis from the treatment options for type 2 diabetes in adolescents and youth (Today) study. Diabetes Care (2019) 42(6):1120–8. doi: 10.2337/dc18-2147
24. Geng T, Li X, Ma H, Heianza Y, Qi L. Adherence to a healthy sleep pattern and risk of chronic kidney disease: the Uk biobank study. Mayo Clin Proc (2022) 97(1):68–77. doi: 10.1016/j.mayocp.2021.08.028
25. Li C, Shang S. Relationship between sleep and hypertension: findings from the nhanes (2007-2014). Int J Environ Res Public Health (2021) 18(15):7867. doi: 10.3390/ijerph18157867
26. ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 5. Facilitating positive health behaviors and well-being to improve health outcomes: standards of care in diabetes-2023. Diabetes Care (2023) 46(Supple 1):S68–96. doi: 10.2337/dc23-S005
27. Sun H, Qin K, Zou C, Wang HH, Lu C, Chen W, et al. The association of nighttime sleep duration and quality with chronic kidney disease in middle-aged and older Chinese: A cohort study. Sleep Med (2021) 86:25–31. doi: 10.1016/j.sleep.2021.08.007
28. McMullan CJ, Curhan GC, Forman JP. Association of short sleep duration and rapid decline in renal function. Kidney Int (2016) 89(6):1324–30. doi: 10.1016/j.kint.2015.12.048
29. Yamamoto R, Shinzawa M, Isaka Y, Yamakoshi E, Imai E, Ohashi Y, et al. Sleep quality and sleep duration with Ckd are associated with progression to Eskd. Clin J Am Soc Nephrol (2018) 13(12):1825–32. doi: 10.2215/CJN.01340118
30. Syauqy A, Hsu CY, Rau HH, Kurniawan AL, Chao JC. Association of sleep duration and insomnia symptoms with components of metabolic syndrome and inflammation in middle-aged and older adults with metabolic syndrome in Taiwan. Nutrients (2019) 11(8):1848. doi: 10.3390/nu11081848
31. Sinha SK, Nicholas SB, Sung JH, Correa A, Rajavashisth TB, Norris KC, et al. Hs-Crp is associated with incident diabetic nephropathy: findings from the Jackson heart study. Diabetes Care (2019) 42(11):2083–9. doi: 10.2337/dc18-2563
32. Sinha SK, Shaheen M, Rajavashisth TB, Pan D, Norris KC, Nicholas SB. Association of race/ethnicity, inflammation, and albuminuria in patients with diabetes and early chronic kidney disease. Diabetes Care (2014) 37(4):1060–8. doi: 10.2337/dc13-0013
33. Wang S, Wu Y, Ungvari GS, Ng CH, Forester BP, Gatchel JR, et al. Sleep duration and its association with demographics, lifestyle factors, poor mental health and chronic diseases in older Chinese adults. Psychiatry Res (2017) 257:212–8. doi: 10.1016/j.psychres.2017.07.036
34. Brady EM, Bodicoat DH, Hall AP, Khunti K, Yates T, Edwardson C, et al. Sleep duration, obesity and insulin resistance in a multi-ethnic Uk population at high risk of diabetes. Diabetes Res Clin Pract (2018) 139:195–202. doi: 10.1016/j.diabres.2018.03.010
35. Group TS. Effects of metabolic factors, race-ethnicity, and sex on the development of nephropathy in adolescents and young adults with type 2 diabetes: results from the today study. Diabetes Care (2021) 45(5):1056–64. doi: 10.2337/dc21-1085
36. Lakerveld J, Mackenbach JD, Horvath E, Rutters F, Compernolle S, Bardos H, et al. The relation between sleep duration and sedentary behaviours in European adults. Obes Rev (2016) 17 Suppl 1(S1):62–7. doi: 10.1111/obr.12381
37. Geng T, Zhu K, Lu Q, Wan Z, Chen X, Liu L, et al. Healthy lifestyle behaviors, mediating biomarkers, and risk of microvascular complications among individuals with type 2 diabetes: A cohort study. PloS Med (2023) 20(1):e1004135. doi: 10.1371/journal.pmed.1004135
38. Liu G, Li Y, Pan A, Hu Y, Chen S, Qian F, et al. Adherence to a healthy lifestyle in association with microvascular complications among adults with type 2 diabetes. JAMA Netw Open (2023) 6(1):e2252239. doi: 10.1001/jamanetworkopen.2022.52239
39. Wang L, Xu X, Zhang M, Hu C, Zhang X, Li C, et al. Prevalence of chronic kidney disease in China: results from the sixth China chronic disease and risk factor surveillance. JAMA Intern Med (2023) 183(4):298–310. doi: 10.1001/jamainternmed.2022.6817
40. Cespedes EM, Bhupathiraju SN, Li Y, Rosner B, Redline S, Hu FB. Long-term changes in sleep duration, energy balance and risk of type 2 diabetes. Diabetologia (2016) 59(1):101–9. doi: 10.1007/s00125-015-3775-5
41. Tuomilehto H, Seppa J, Uusitupa M. Obesity and obstructive sleep apnea–clinical significance of weight loss. Sleep Med Rev (2013) 17(5):321–9. doi: 10.1016/j.smrv.2012.08.002
42. Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet (2014) 383(9918):736–47. doi: 10.1016/S0140-6736(13)60734-5
43. Wang D, Chen J, Zhou Y, Ma J, Zhou M, Xiao L, et al. Association between sleep duration, sleep quality and hyperlipidemia in middle-aged and older chinese: the Dongfeng-Tongji cohort study. Eur J Prev Cardiol (2019) 26(12):1288–97. doi: 10.1177/2047487319843068
44. Gonzalez-Muniesa P, Martinez-Gonzalez MA, Hu FB, Despres JP, Matsuzawa Y, Loos RJF, et al. Obesity. Nat Rev Dis Primers (2017) 3:17034. doi: 10.1038/nrdp.2017.34
Keywords: sleep duration, obesity, changes in BMI, type 2 diabetes, diabetic kidney disease
Citation: Liu C, Zhang J, Wei X, Shi J, Fang Q, Zhou W, Sun L, Hu Z, Hong J, Gu W, Wang W, Peng Y and Zhang Y (2023) Effects of sleep duration and changes in body mass index on diabetic kidney disease: a prospective cohort study. Front. Endocrinol. 14:1278665. doi: 10.3389/fendo.2023.1278665
Received: 16 August 2023; Accepted: 06 October 2023;
Published: 26 October 2023.
Edited by:
Md Abdul Hye Khan, University of Missouri, United StatesReviewed by:
Yongfu Yu, Fudan University, ChinaYijun Lin, Xiamen University Affiliated Cardiovascular Hospital, China
Copyright © 2023 Liu, Zhang, Wei, Shi, Fang, Zhou, Sun, Hu, Hong, Gu, Wang, Peng and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Peng, cGVubnlwcHBAMTI2LmNvbQ==; Yifei Zhang, ZmVpZmVpLWFAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Cong Liu1,2†
Cong Liu1,2† Qianhua Fang
Qianhua Fang Lin Sun
Lin Sun Zhuomeng Hu
Zhuomeng Hu Jie Hong
Jie Hong Weiqiong Gu
Weiqiong Gu Yifei Zhang
Yifei Zhang