- Department of Endocrinology and Metabolism, Tianjin Medical University General Hospital, Tianjin, China
Objective: Sex steroid hormones are associated with the advancement of metabolic diseases such as dyslipidemia. This cross-sectional study aimed to investigate the relationship between dehydroepiandrosterone, dehydroepiandrosterone sulfate, androstenedione, and testosterone levels and the risk of dyslipidemia in people with type 2 diabetes mellitus.
Materials and Methods: The analysis included 1,927 patients with type 2 diabetes mellitus. Serum dehydroepiandrosterone, dehydroepiandrosterone sulfate, androstenedione, and testosterone levels were determined using lipid chromatography-tandem mass spectrometry. Multivariable analyses were performed to investigate the association between the variables and dyslipidemia.
Results: The multivariable-adjusted odds ratio (OR) and 95% confidence interval (CI) of dyslipidemia across DHEA tertiles were 0.39 and 0.24-0.64, respectively (p trend = 0.001). This relationship was still maintained when analyzed as a continuous variable (odds ratio, 0.96; 95% confidence interval, 0.92–0.99; P < 0.01). However, in males with type 2 diabetes mellitus, no significant correlations were found between rising levels of dehydroepiandrosterone sulfate, androstenedione, and total testosterone and the risk of dyslipidemia (all P > 0.05). Furthermore, there was no significant association between androgen precursors and total testosterone with regard to the risk of developing dyslipidemia (all P > 0.05).
Conclusions: Serum dehydroepiandrosterone levels were substantially and adversely correlated with dyslipidemia in adult men with T2DM. These results indicated that dehydroepiandrosterone may have an essential role in the development of dyslipidemia. More prospective research is required to validate this link.
Introduction
Dyslipidemia is a prevalent problem in people with type 2 diabetes mellitus (T2DM), presenting with varied manifestations depending on the amount and/or quality of lipoproteins. Primary and secondary dyslipidemia, which have strictly inherited origins, and dyslipidemia caused by other diseases or pathological conditions are frequently distinguished depending on the etiology. Genetic mutations are the most common cause of primary dyslipidemia. Secondary dyslipidemia is caused by other conditions (such as diabetes or obesity), with the latter being more common (1, 2). Patients with T2DM are more likely to develop coronary artery disease as a result of a type of secondary dyslipidemia known as diabetic dyslipidemia (3). Cardiovascular disease is the leading cause of morbidity and mortality in patients with diabetes, and it is highly influenced by dyslipidemia (4). Diabetic dyslipidemia is characterized by increasing low-density lipoprotein cholesterol (LDL-C) and triglyceride (TG) levels and decreased high-density lipoprotein cholesterol (HDL-C) levels (5).
Dehydroepiandrosterone (DHEA) and its sulfate ester dehydroepiandrosterone sulfate (DHEAS) are precursors of sex hormones secreted by the adrenal cortex (6, 7). In particular, DHEA is produced by the adrenocorticotropic hormone (ACTH) reaction and is converted into androstenedione, which is metabolized in the liver by dehydrogenation to testosterone or aromatization to estrogen (8). In the human body, the two most abundant cyclic steroid hormones are DHEA and DHEAS (9). DHEA and DHEAS blood concentrations in men and women reduce with age (10, 11). DHEA has been found to improve diabetes and obesity in animal models. DHEA may alleviate diabetes by increasing pancreatic insulin output as well as insulin sensitivity in the liver, fat, and muscle (12–14). Furthermore, by decreasing lipid synthesis and raising resting metabolic rate and calorie output, DHEA may have anti-obesity benefits (15, 16). However, the connection between DHEA and dyslipidemia remains unclear.
The purpose of this cross-sectional study was to investigate the link between DHEA, DHEAS, testosterone, and androstenedione levels and the risk of dyslipidemia in T2DM patients.
Materials and methods
Study participants
Medical records from 2,107 T2DM patients were evaluated between October 12, 2020, and June 30, 2022. All patients were treated at Tianjin Medical University General Hospital’s Department of Endocrinology and Metabolism, and their sex steroids hormone levels were assessed. When a person had numerous hospital records, only one of them was chosen. Patients under the age of 18 who did not have lipid data and pregnant women were excluded from the trial. Given that hormone usage can impact sex steroid hormone levels, this was also an exclusion criterion. Figure 1 depicts the patient-identifying method. Based on exclusion criteria, 1,927 T2DM individuals were enrolled in the final investigation.
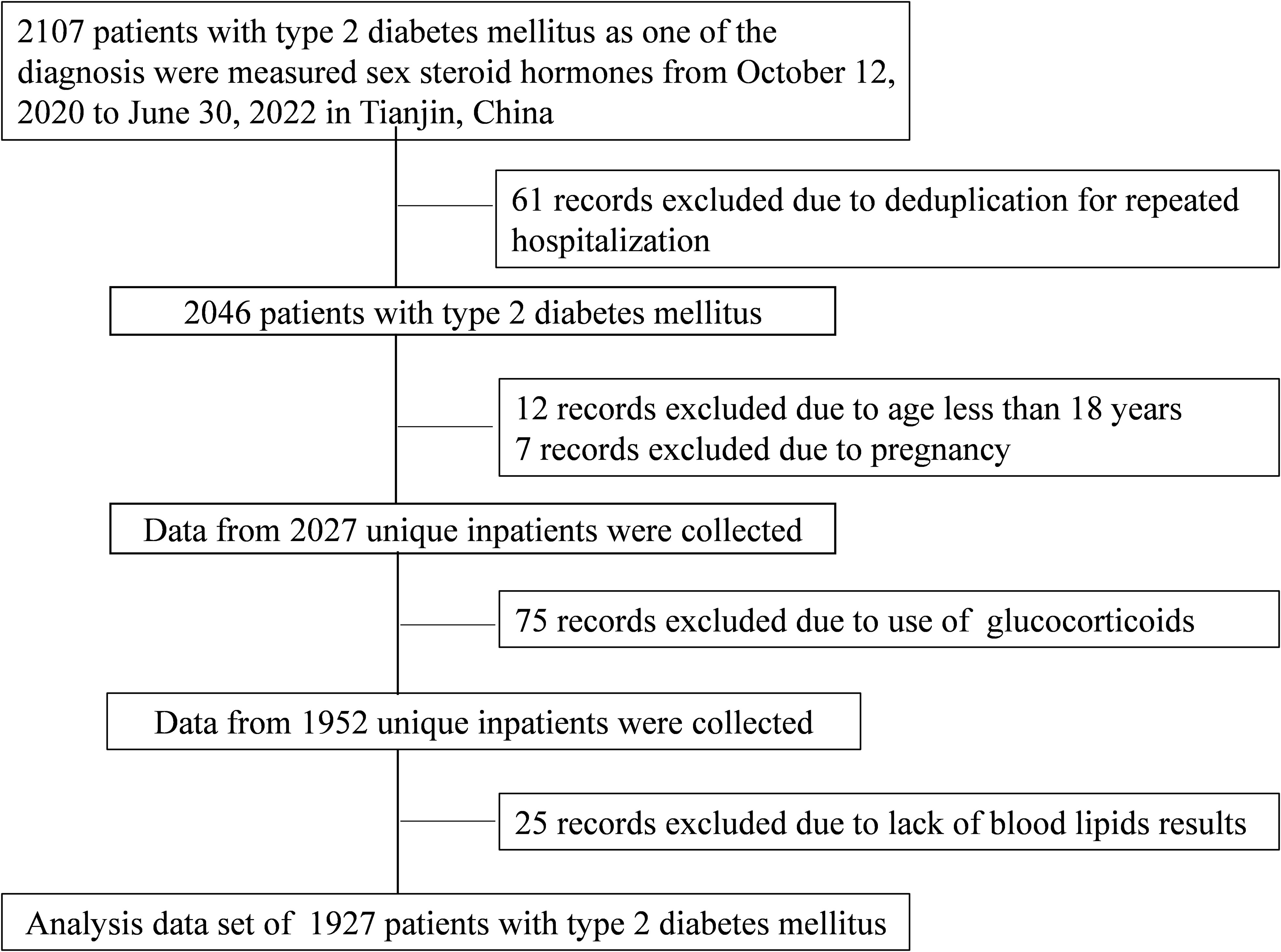
Figure 1 Flow chart of identification of study population. A total of 1,927 patients with T2DM were included in the final analysis based on the exclusion criteria. T2DM, type 2 diabetes mellitus.
The Tianjin Medical University General Hospital Institutional Review Board approved the project. The informed consent requirement (approval number: IRB2020-YX-027-01) was waived as the patient data were collected from the Department of Endocrinology and Metabolism’s electronic medical record and the patient’s identity was obscured.
Covariates
Age, sex, lifestyle factors, insurance, history of diabetes, hypertension, and dyslipidemia, height and weight, systolic and diastolic blood pressures (SBP, DBP), as well as blood levels for glycated hemoglobin (HbA1c), total cholesterol (TC), TG, LDL-C, HDL-C, uric acid (UA), ACTH, and C-reactive protein (CRP), were all taken from medical records. Body mass index (BMI) is computed by dividing a patient’s weight in kilograms by their height in meters squared. Cigarette smoking was defined as smoking more than one cigarette per day for at least one year, and participants were divided into three groups: never-smokers, prior smokers (those who had quit smoking for at least 6 months), and current smokers. Alcohol use was defined as consuming more than 400 mL alcohol/week > 1 year. Participants were divided into three groups: non-drinkers, former drinkers (characterized by temperance for at least 6 months), and current drinkers. The Endocrine and Metabolism Laboratory at Tianjin Medical University’s General Hospital used liquid chromatography-tandem mass spectrometry to evaluate the levels of androgen precursors. After the patients had fasted for at least 8 hours, venous blood samples were taken at 6 a.m. on the second day of admission. Our lab performed the steroid hormone assay as previously described (17–19).
Definitions
A fasting blood glucose (FBG) level of 7.0 mmol/L, a 2-hour plasma glucose level of 11.1 mmol/L, an HbA1c level of 6.5%, a self-identified diagnosis of diabetes, or usage of hypoglycemic drugs was considered diabetes (20). SBP of 140 mmHg, DBP of 90 mmHg, self-identified diagnosis of hypertension, or usage of antihypertensive medication were all considered hypertension (21). Dyslipidemia was defined as TC >6.20 mmol/L, LDL-C >4.13 mmol/L, TG >2.25 mmol/L or HDL-C <1.03 mmol/L, or usage of lipid-lowering drugs (22).
Statistical analyses
For continuous variables, standard deviations (SDs) and means are used to express normally distributed data, and the Student’s t-test was used to compare the groups. Interquartile ranges (IQRs) and medians were employed to represent non-normally distributed data, and the Mann–Whitney U test was employed to compare the groups. The chi-square test was used to compare results between the groups for categorical variables, which are expressed as numbers and frequencies.
The value and significance of the correlation coefficients of sex hormone concentrations in males and females were estimated using the Spearman’s correlations. To explore the influence of sex hormones on dyslipidemia, three models were adjusted for covariates. Model 1 was corrected for age; Model 2 was further adjusted for current alcohol and smoking use; and Model 3 was corrected for Model 2 plus BMI, type of insurance, T2DM duration, SBP, ACTH, and HbA1c. Testosterone, androstenedione, DHEAS, and DHEA levels were individually divided into tertile groups. The lowest tertile was utilized as the reference group for binary logistic regression analysis. After adjusting for age, BMI, current smoking, insurance type, current alcohol consumption, T2DM duration, SBP, and HbA1c, the dose–response relationship between sex hormones and dyslipidemia was analyzed with restrictive cubic splines. A p-value < 0.05 (two-tailed test) was considered significant for correlation intervals (CIs). IBM SPSS Statistics for Windows, version 25.0 (IBM Corporation, Armonk, NY, USA) was used for all analyses.
Results
Characteristics of the study participants
In the study’s male cohort of 1021, 786 (77%) of the participants had dyslipidemia. Table 1 shows that males with dyslipidemia had higher BMI, TG, FBG, UA, CRP, and creatinine (Cr) values, as well as a higher prevalence of hypertension than those in males with no dyslipidemia. However, the mean age, HDL-C, and duration of T2DM among males with dyslipidemia were lower than those in males with non-dyslipidemia (P < 0.05). In males with dyslipidemia, total testosterone (TT) and DHEA levels were decreased (P < 0.05).
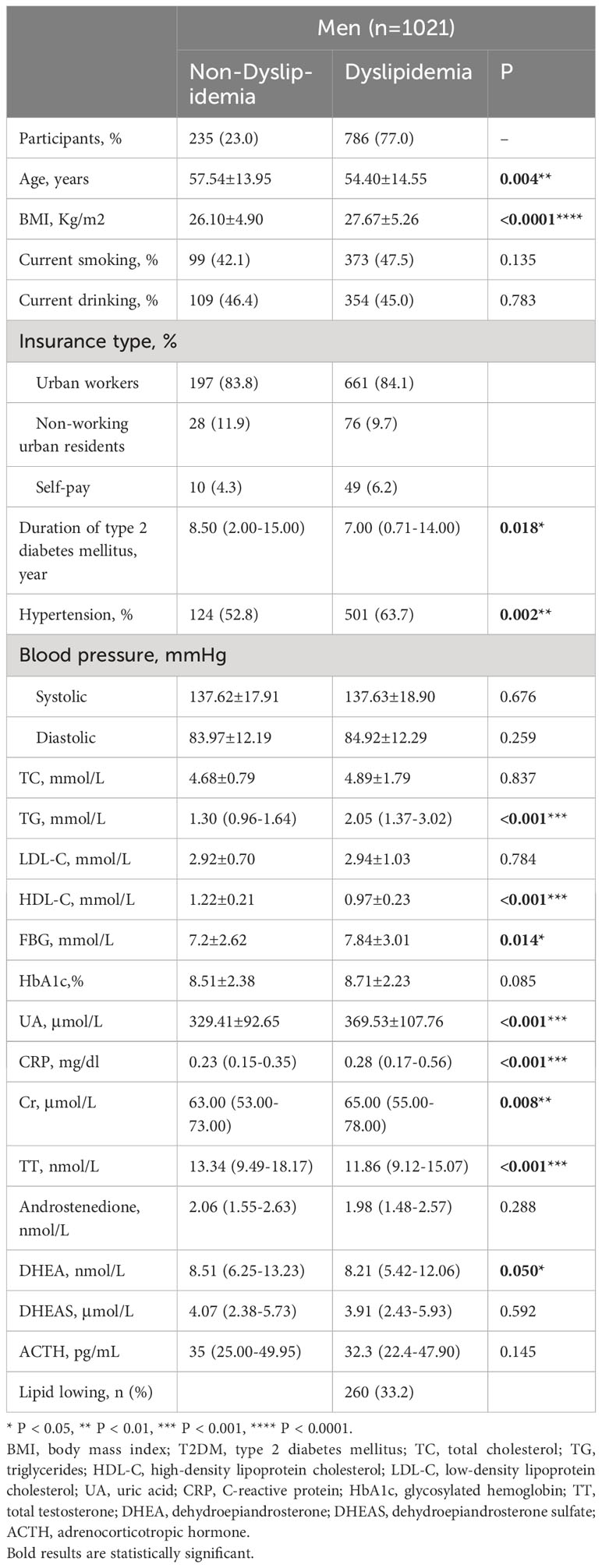
Table 1 Demographic characteristics and clinical parameters of patients with and without dyslipidemia (men, n=1021).
A total of 633 (69.9%) females met the criteria for dyslipidemia. Females with dyslipidemia had higher TC, TG, FBG, HbA1c, UA, and CRP levels but lower mean HDL-C levels than those of females without dyslipidemia (all P < 0.05). Similar to that of males, females with dyslipidemia had a higher prevalence of hypertension (all P < 0.05). Lower DHEA levels in females with dyslipidemia were also observed (P = 0.008; Table 2).
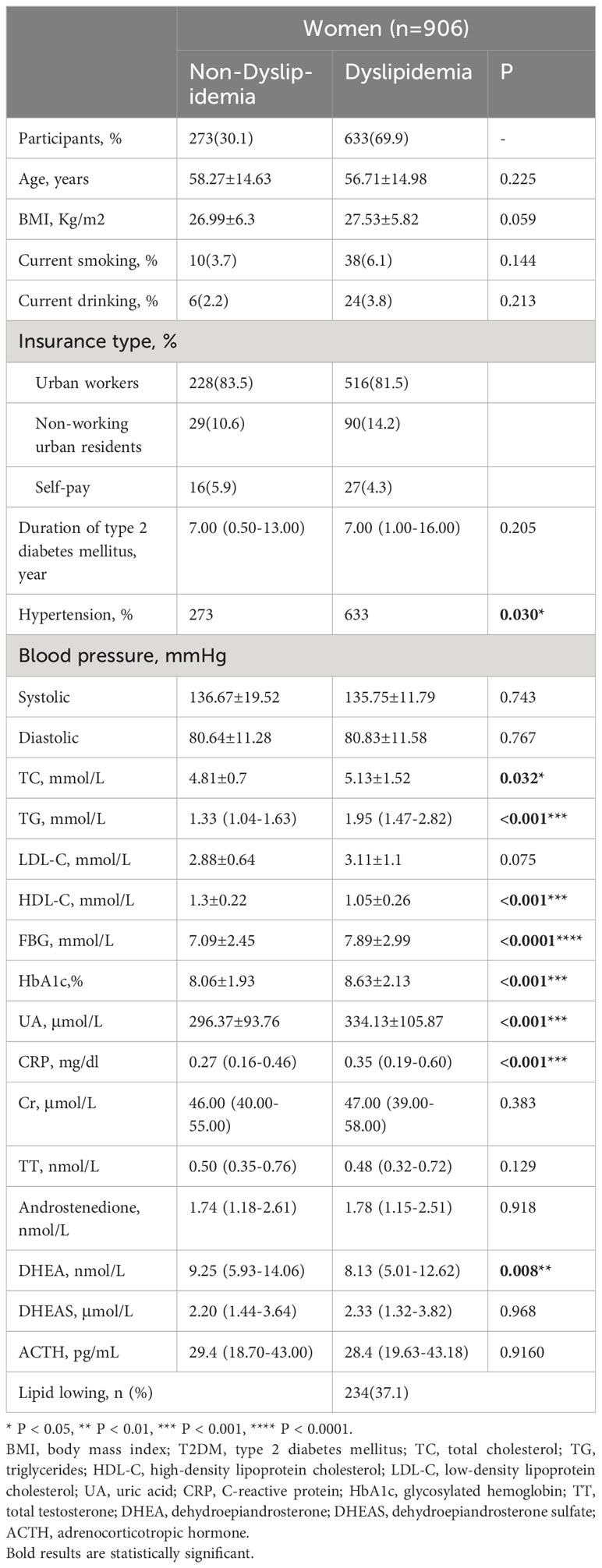
Table 2 Demographic characteristics and clinical parameters of patients with and without dyslipidemia (women, n=906).
Prevalence of dyslipidemia by tertiles of TT, androstenedione, DHEA, and DHEAS levels
Figure 2 shows the prevalence of dyslipidemia assessed in males and females in tertiles of serum DHEA, DHEAS, TT, and androstenedione levels. As the serum DHEA level of tertile increased, the percentage of males with dyslipidemia reduced considerably, with 81.8%, 75.1%, and 74.1% in tertiles 1–3, respectively (P < 0.05). Moreover, in males, the prevalence of dyslipidemia rose with increasing TT level tertiles, with 79.7%, 74.5%, and 69.7% in tertiles 1–3, respectively (P < 0.05). Dyslipidemia was more common in females as DHEA level tertiles increased, with 74.8%, 69.9%, and 64.9% in tertiles 1–3, respectively (P < 0.05).
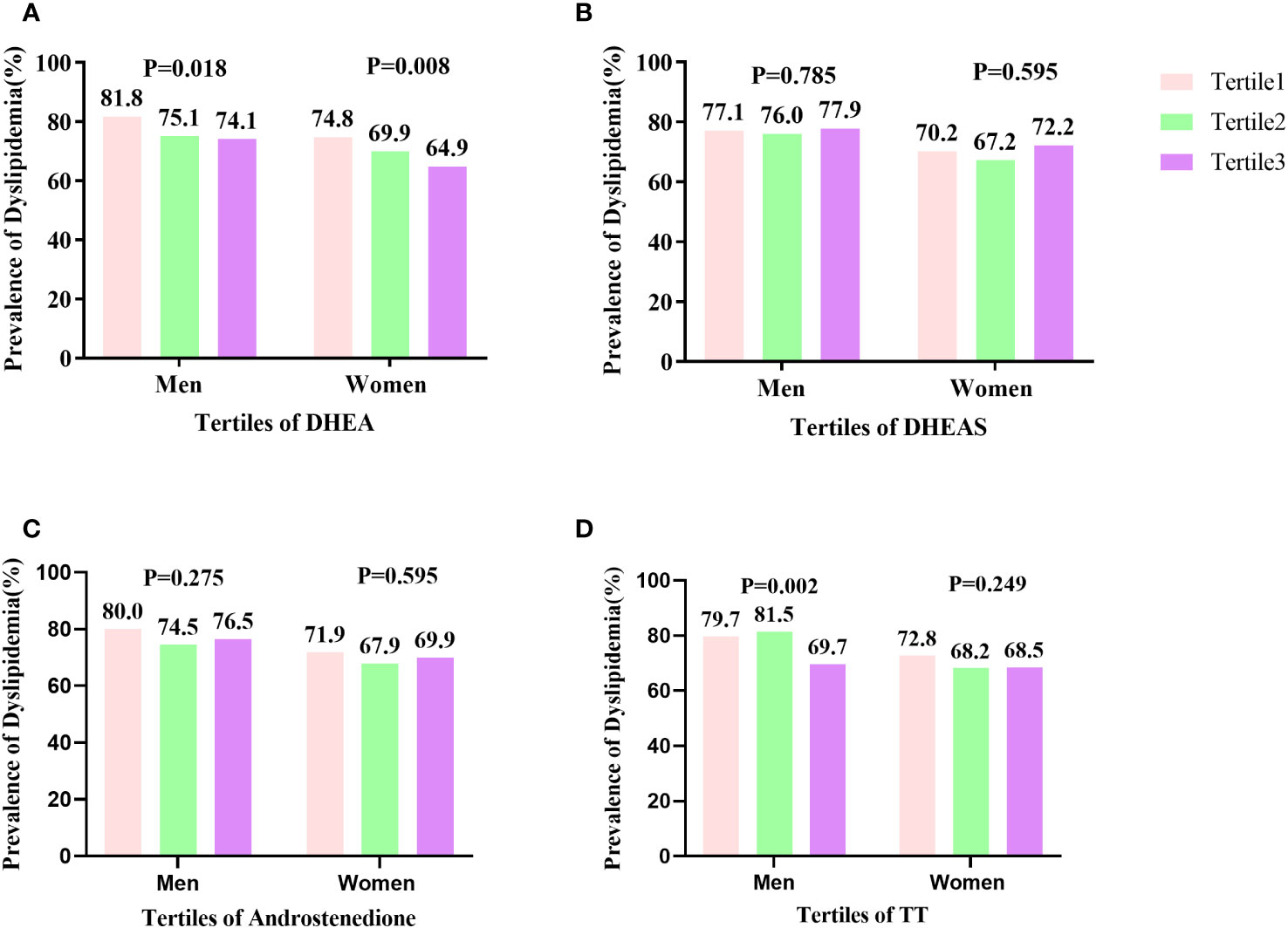
Figure 2 Prevalence of dyslipidemia by tertiles of TT, androstenedione, DHEA, and DHEAS levels: (A) prevalence of dyslipidemia by DHEA level; (B) prevalence of dyslipidemia by DHEAS level; (C) prevalence of dyslipidemia by androstenedione level; (D) prevalence of dyslipidemia by TT level. The prevalence of dyslipidemia significantly decreased in line with increasing tertiles of serum DHEA levels in females (P = 0.008) and (P = 0.018) in males. The percentage of males with dyslipidemia significantly decreased in accordance with increasing tertiles of serum TT level (P = 0.002).
Relationships between DHEA, DHEAS, androstenedione, and TT levels with dyslipidemia risk
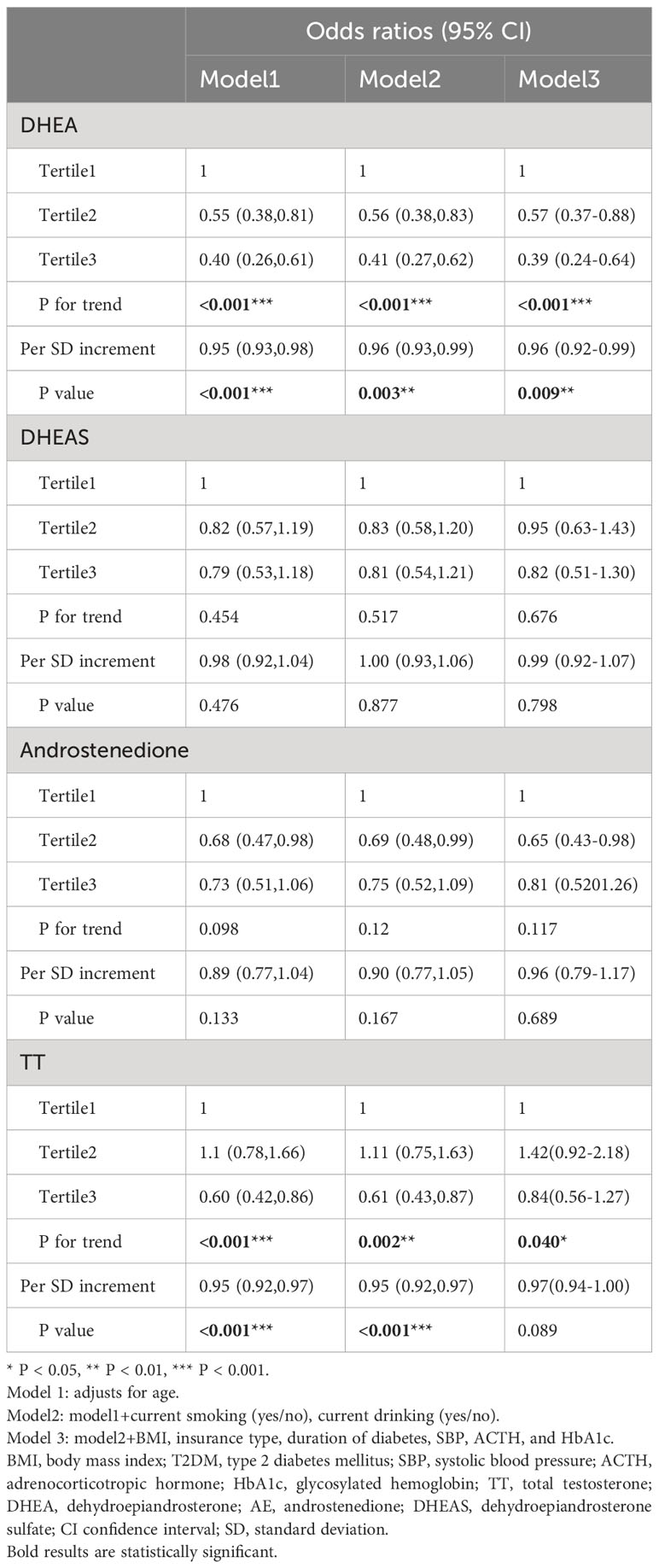
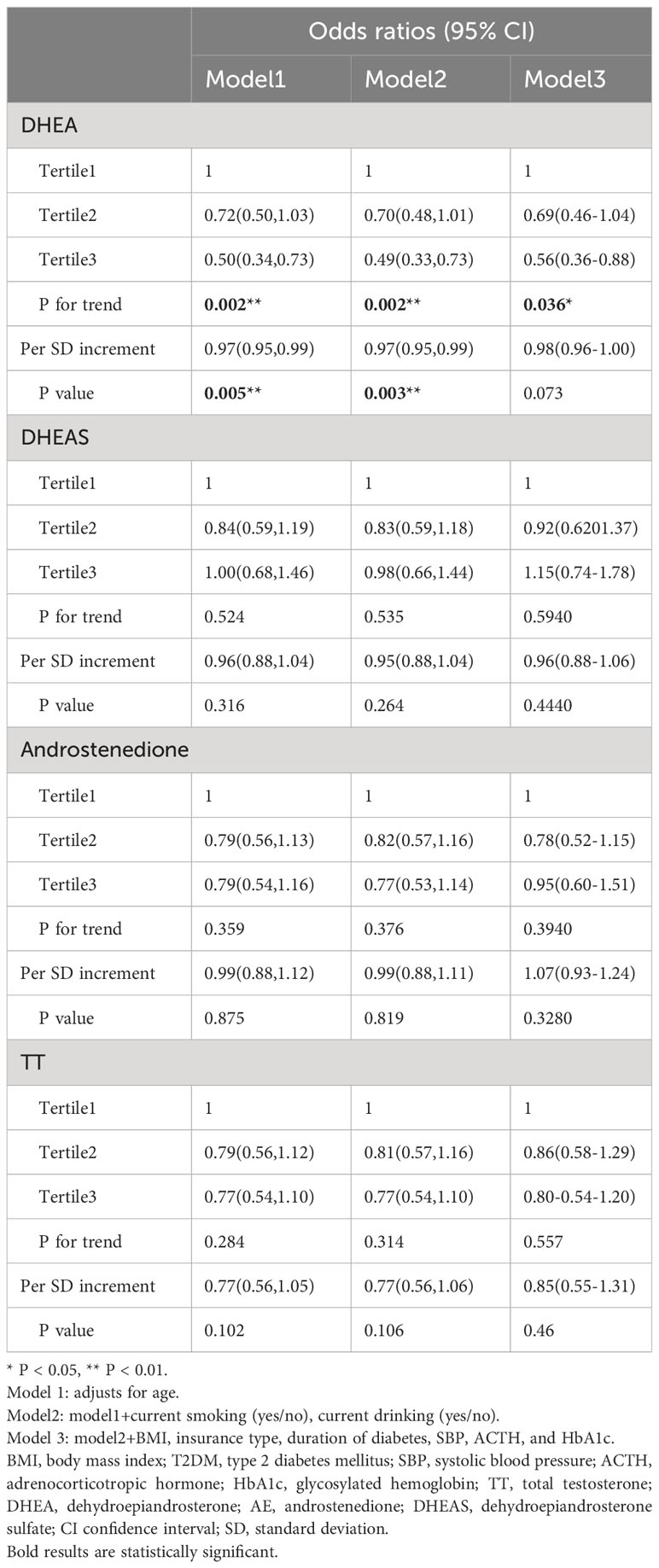
Table 3 shows the associations between the risk of dyslipidemia in males after correcting for DHEA, androstenedione, DHEAS, and testosterone levels, as well as confounding factors, in logistic regression analysis. After adjusting for relevant variables (age, current smoking, BMI, current drinking, T2DM duration, insurance type, SBP, ACTH, and HbA1c) in model 3, the risk of dyslipidemia was reduced with increasing serum DHEA levels (OR, 0.39, tertile 3 vs. tertile 1; 95% CI, 0.24–0.64, P < 0.001 for trend). Moreover, when DHEA level was measured as a continuous variable, each SD increase was related to a decrease of 4% in the odds of developing dyslipidemia in model 3 (OR, 0.96; 95% CI, 0.92-0.99; P = 0.009). Higher concentrations of serum androstenedione, TT, and DHEAS were not correlated with a higher risk of dyslipidemia (all P > 0.05).
Table 4 illustrates that lower serum DHEA levels in females with T2DM were related to a higher OR of dyslipidemia in Models 1 (OR, 0.50; 95% CI, 0.34–0.73; P < 0.05) and 2 (OR, 0.49; 95% CI, 0.33–0.73; P < 0.05). When DHEA level was analyzed as a continuous variable, a 1-SD increase in serum DHEA level was associated with a 3% reduction in dyslipidemia risk in females in Models 1 (OR, 0.97; 95% CI, 0.95–0.99; P = 0.005) and 2 (OR, 0.97; 95% CI, 0.95–0.99; P = 0.003). In the fully corrected mode, however, this link was not statistically significant (OR, 0.98; 95% CI, 0.96–1.00; P > 0.05). There were no statistically significant connections between higher DHEA, androstenedione, and TT levels and the hazard ratio of dyslipidemia. (P > 0.05).
Discussion
In this cross-sectional study, we present evidence for the connection of DHEA with the risk of developing dyslipidemia in T2DM patients. After correcting for potential confounders such as age, smoking, alcohol use, BMI, T2DM duration, insurance type, SBP, ACTH, and HbA1c levels, we found that lowered serum DHEA levels in males with T2DM are independently associated with the risk of dyslipidemia. In females with T2DM, DHEA levels and the risk of dyslipidemia had no statistically significant connection. Moreover, there was little association between androstenedione, TT, and DHEA levels and the incidence of dyslipidemia in males and females with T2DM.
DHEA secretion in humans is age-related (23). DHEA is the primary byproduct of prenatal adrenal production, resulting in high circulating DHEA levels at birth. Throughout the primary year of life, serum DHEA concentrations fall to nearly inconspicuous levels, which corresponds to the postnatal development of the fetal adrenal gland. DHEAS levels continue little until the sixth to the tenth year of life when they gradually increase due to increasing DHEA secretion from the adrenal reticular zone, a condition known as adrenal primordium (24, 25).
DHEA has been demonstrated to have a positive effect on the progression of T2DM. Yamaguchi et al. discovered that serum DHEA levels were considerably less in patients with T2DM having high blood insulin levels compared with those in controls and those with T2DM without high serum insulin levels (26). Previous research has linked raised serum DHEA levels to an elevated risk of coronary heart disease (CHD) in men. Lower blood DHEA concentrations were connected with the occurrence of CHD, regardless of established risk factors, according to prospective research enrolling males between the ages of 40 and 70 (27). Low-dose DHEA treatment enhanced vascular endothelial functionality and insulin sensitivity while decreasing plasminogen activator inhibitor type 1 concentration in a randomized, double-blind investigation of men with hypercholesterolemia (28). In a 1-year randomized controlled trial, a healthy population aged 60-79 years was administered 50 mg/day of taking DHEA, and their serum DHEAS levels were restored to young adult levels after 6 months. DHEA treatment enhanced the density of bone minerals in women, primarily at the femoral neck, Ward’s triangle, and upper radius. In men, no effect on bone turnover was observed (29). According to a 4-month cross over controlled study involving patients with adrenal insufficiency randomized to receive DHEA or placebo, DHEA significantly reduced TC and HDL-C levels and increased overall well-being, compared to placebo (30). In addition, among 270 postmenopausal women who participated in the observational Women’s Ischemia Syndrome Evaluation (WISE) study, decreased DHEAS levels were found to be linked with a higher probability of cardiovascular disease and all-cause death (31).
However, findings regarding the link between DHEA and dyslipidemia are inconsistent. In a previous meta-analysis, DHEA supplementation was administered to both older men and postmenopausal women. DHEA supplementation decreased fat mass in older men (32). When compared to a placebo group, DHEA did not affect glycemia, insulin, or TC. This action depends on the conversion of DHEA metabolites into androgens or estrogens. In another study, DHEA treatment had no impact on TC, HDL-C, LDL, TG, serum glucose, weight, or BMI in postpartum women (33). In a crossover of double-blinding placebo-controlled research, DHEA supplementation had no consequence on serum insulin, glucose, or lipid profiles in aged men (34). DHEA treatment increased the concentrations of TC, phospholipids, LDL-C, TG, and apo-B in rabbits with hyperlipidemia fed with an atherogenic diet. DHEA, a weakened androgenic and probable predecessor of androgens and estrogens, might affect sex hormone levels as well as serum lipid concentrations indirectly by altering the hormone environment (35).
Findings from animal studies indicate that DHEA supplementation may be effective in reducing chronic complications of diabetes, however, the mechanism is unknown. The db/db mouse was used as a T2DM animal model. Coleman et al. discovered that DHEA therapy decreased hyperglycemia, increased insulin sensitivity, and retained islet function and β-cell structure in db/db mice (36). Kimura et al. found that DHEA reduced serum TNF levels in Zucker fatty rats (37), whereas Aoki et al. discovered that DHEA suppressed increased glucose-6-phosphatase and fructokinase levels in C57BL/Ksj-db/db mice (38). Campbell et al. DHEA activates the PI3-kinase/AKT pathway in the liver and the PI3K/atypical PKCzeta/lambda pathway in muscle (39). These researchers concluded that DHEA supplementation could help prevent insulin resistance. As a result, we hypothesized that DHEA’s alleviated insulin resistance effect explains certain aspects of the inverse connection between DHEA and diabetic dyslipidemia. Furthermore, a hepatic androgen receptor deficiency decreases fatty acid oxidation and increases hepatic de novo lipogenesis by decreasing PPARα expression, which causes hepatic steatosis and insulin resistance (40). This may also explain the link between low serum DHEA levels and dyslipidemia. The lack of a significant relationship between DHEA and dyslipidemia in women may be because of estrogenic protective mechanisms that improve lipid metabolism by preventing the accumulation of white adipose tissue by decreasing fatty acid and triglyceride production and lipogenesis (41).
There are some limitations to the current investigation. The association of DHEA, DHEAS, testosterone, and androstenedione with dyslipidemia does not establish a causal association due to the cross-sectional methodology. Second, we did not collect data on all the characteristics associated with dyslipidemia, such as eating habits, which may have limited our multivariable analysis. Third, the sample size was limited. As a result, future studies should rely on larger sample sizes. Fourth, because the study’s population was drawn from hospitalized patients, the results may not fully reflect the general community, which should be included as a control in future research. Finally, because this trial enrolled patients with T2DM, the results might not accurately reflect the general diabetic community.
As a result, after controlling for risk variables, lower serum DHEA levels in males with T2DM were found to be correlated with the risk of dyslipidemia. Interestingly, no associations were identified among DHEAS, androstenedione, testosterone, and the incidence of dyslipidemia in either males or females. According to our data, DHEA may play a part in the progression of dyslipidemia in males with T2DM. More prospective research is required to validate this link.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The Tianjin Medical University General Hospital Institutional Review Board approval number: IRB2020-YX-027-01. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because as the patient data were collected from the Department of Endocrinology and Metabolism’s electronic medical record and the patient’s identity was obscured. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
ML: Writing – review & editing. SC: Writing – original draft. SL: Data curation, Writing – review & editing. YF: Writing – review & editing. XZ: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Key R&D Program of China 2019YFA0802502 and 2022YFE0131400; we acknowledge the support of the National Natural Science Foundation of China (81830025, 82220108014), and Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-030A) and the Tianjin Education Commission Research Program Project (2020KJ147) and the Special Research Fund for Central Universities, Peking Union Medical College (3332021083).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kolovou PMKGD, Anagnostopoulou KK, Bilianou H, Mikhailidis DP. Primary and secondary hypertriglyceridaemia. Curr Drug Targets (2009) 10(4):336–43. doi: 10.2174/138945009787846452
2. Pirillo A, Casula M, Olmastroni E, Norata GD, Catapano AL. Global epidemiology of dyslipidaemias. Nat Rev Cardiol (2021) 18:689–700. doi: 10.1038/s41569-021-00541-4
3. Scicali R, Di Pino A, Ferrara V, Urbano F, Piro S, Rabuazzo AM, et al. New treatment options for lipid-lowering therapy in subjects with type 2 diabetes. Acta Diabetol (2018) 55:209–18. doi: 10.1007/s00592-017-1089-4
4. Nelson AJ, Rochelau SK, Nicholls SJ. Managing dyslipidemia in type 2 diabetes. Endocrinol Metab Clin North Am (2018) 47:153–73. doi: 10.1016/j.ecl.2017.10.004
5. Wu L, Parhofer KG. Diabetic dyslipidemia. Metabolism (2014) 63:1469–79. doi: 10.1016/j.metabol.2014.08.010
6. Shackleton ERCHL, Phillips A, Chang T. Androstanediol and 5-androstenediol profiling for detecting exogenously administered dihydrotestosterone, epitestosterone, and dehydroepiandrosterone: potential use in gas chromatography isotope ratio mass spectrometry. Steroids (1997) 62(10):665–73. doi: 10.1016/S0039-128X(97)00065-2
7. K.M. VA, Eberling MD P. Physiological importance of dehydroepiandrosterone. Lancet (London England) (1994) 343(8911):1479–81. doi: 10.1016/S0140-6736(94)92587-9
8. H.A. R, Tait JF. In vivo conversion of dehydroisoandrosterone to plasma androstenedione and testosterone in man. J Clin Endocrinol Metab (1967) 27(1):79–88. doi: 10.1210/jcem-27-1-79
9. Rutkowski K, Sowa P, Rutkowska-Talipska J, Kuryliszyn-Moskal A, Rutkowski R. Dehydroepiandrosterone (DHEA): hypes and hopes. Drugs (2014) 74:1195–207. doi: 10.1007/s40265-014-0259-8
10. Kushnir MM, Blamires T, Rockwood AL, Roberts WL, Yue B, Erdogan E, et al. Liquid chromatography-tandem mass spectrometry assay for androstenedione, dehydroepiandrosterone, and testosterone with pediatric and adult reference intervals. Clin Chem (2010) 56:1138–47. doi: 10.1373/clinchem.2010.143222
11. L. GA, Liu CH, Fischer UG, Yen SSC. Marked attenuation of ultradian and circadian rhythms of dehydroepiandrosterone in postmenopausal women:evidence for a reduced 17,20-desmolase enzymatic activity. J Clin Endocrinol Metab (1990) 71:900–6. doi: 10.1210/jcem-71-4-900
12. L. EH, Coleman DL, Applezweig N. Therapeutic effects of dehydroepiandrosterone metabolites in diabetes mutant mice (C57BL/ksJ-db/db). Endocrinology (1984) 115(1):239–43. doi: 10.1210/endo-115-1-239
13. Aoki K, Terauchi Y. Effect of dehydroepiandrosterone (DHEA) on diabetes mellitus and obesity. Vitam Horm (2018) 108:355–65. doi: 10.1016/bs.vh.2018.01.008
14. S. RW, Coleman DL, Leiter EH. Effect of genetic background on the therapeutic effects of dehydroepiandrosterone (DHEA) in diabetes-obesity mutants and in aged normal mice. Diabetes (1984) 33(1):26–32. doi: 10.2337/diabetes.33.1.26
15. Han DH, Hansen PA, Chen MM, Holloszy JO. DHEA treatment reduces fat accumulation and protects against insulin resistance in male rats. The journals of gerontology. Ser A Biol Sci Med Sci (1998) 53:B19–24. doi: 10.1093/gerona/53A.1.B19
16. Villareal DT HJ. Effect of DHEA on abdominal fat and insulin action in elderly women and men: a randomized controlled trial. JAMA (2004) 292:2243–8. doi: 10.1001/jama.292.18.2243
17. Zhang X, Xiao J, Liu Q, Ye Y, Guo W, Cui J, et al. Low serum total testosterone is associated with non-alcoholic fatty liver disease in men but not in women with type 2 diabetes mellitus. Int J Endocrinol (2022) 2022:8509204. doi: 10.1155/2022/8509204
18. Zhang X, Xiao J, Liu T, He Q, Cui J, Tang S, et al. Low serum dehydroepiandrosterone and dehydroepiandrosterone sulfate are associated with coronary heart disease in men with type 2 diabetes mellitus. Front Endocrinol (Lausanne) (2022) 13:890029. doi: 10.3389/fendo.2022.890029
19. Zhang X, Huang Y, Xu N, Feng W, Qiao J, Liu M. Low serum dehydroepiandrosterone levels are associated with diabetic retinopathy in patients with type 2 diabetes mellitus. J Diabetes Investig (2023) 14(5):675–85. doi: 10.1111/jdi.13997
20. Zhu D, Society C. Guideline for the prevention and treatment of type 2 diabetes mellitus in China (2020 edition). Chin J Endocrinol Metab (2021) 37:311–98. doi: 10.3760/cma.j.cn311282-20210304-00142
21. Joint Committee for Guideline R. 2018 Chinese guidelines for prevention and treatment of hypertension-A report of the revision committee of Chinese guidelines for prevention and treatment of hypertension. J Geriatr Cardiol (2019) 16:182–241. doi: 10.11909/j.issn.1671-5411.2019.03.014
22. Jun-Ren ZHU R-LG, Shui-Ping ZHAO, Guo-Ping LU, Dong ZHAO, Jian-Jun LI. 2016 Chinese guidelines for the management of dyslipidemia in adults. J Geriatr Cardiol (2018) 15:1–29. doi: 10.11909/j.issn.1671-5411.2018.01.011
23. Orentreich N, Brind JL, Rizer RL, Vogelman JH. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab (1984) 59:551–5. doi: 10.1210/jcem-59-3-551
24. Reiter EO, Fuldauer VG, Root AW. Secretion of the adrenal androgen, dehydroepiandrosterone sulfate, during normal infancy, childhood, and adolescence, in sick infants, and in children with endocrinologic abnormalities. J Pediatr (1977) 90:766–70. doi: 10.1016/S0022-3476(77)81244-4
25. Sklar CA, Kaplan SL, Grumbach MM. Evidence for dissociation between adrenarche and gonadarche: studies in patients with idiopathic precocious puberty, gonadal dysgenesis, isolated gonadotropin deficiency, and constitutionally delayed growth and adolescence. J Clin Endocrinol Metab (1980) 51:548–56. doi: 10.1210/jcem-51-3-548
26. T. S-i, Yamaguchi Y, Yamakawa T, Kimura M, Ukawa K, Yamada Y, et al. Reduced serum dehydroepiandrosterone levels in diabetic patients with hyperinsulinaemia. Clin Endocrinol (2002) 52:727–33. doi: 10.1046/j.1365-2265.1998.00533.x
27. Qin Y, H OS, Khani V, Tan SC, Zhi Y. Effects of dehydroepiandrosterone (DHEA) supplementation on the lipid profile: A systematic review and dose-response meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis (2020) 30:1465–75. doi: 10.1016/j.numecd.2020.05.015
28. Kawano H, Yasue H, Kitagawa A, Hirai N, Yoshida T, Soejima H, et al. Dehydroepiandrosterone supplementation improves endothelial function and insulin sensitivity in men. J Clin Endocrinol Metab (2003) 88:3190–5. doi: 10.1210/jc.2002-021603
29. Baulieu EE, Thomas G, Legrain S, Lahlou N, Roger M, Debuire B, et al. Dehydroepiandrosterone (DHEA), DHEA sulfate, and aging contribution of the DHEAge Study to a sociobiomedical issue. Proc Natl Acad Sci United States America (2000) 97:4279–84. doi: 10.1073/pnas.97.8.4279
30. Gurnell EM, Hunt PJ, Curran SE, Conway CL, Pullenayegum EM, Huppert FA, et al. Long-term DHEA replacement in primary adrenal insufficiency: a randomized, controlled trial. J Clin Endocrinol Metab (2008) 93:400–9. doi: 10.1210/jc.2007-1134
31. Shufelt C, Bretsky P, Almeida CM, Johnson BD, Shaw LJ, Azziz R, et al. DHEA-S levels and cardiovascular disease mortality in postmenopausal women: results from the National Institutes of Health–National Heart, Lung, and Blood Institute (NHLBI)-sponsored Women's Ischemia Syndrome Evaluation (WISE). J Clin Endocrinol Metab (2010) 95:4985–92. doi: 10.1210/jc.2010-0143
32. Corona G, Rastrelli G, Giagulli VA, Sila A, Sforza A, Forti G, et al. Dehydroepiandrosterone supplementation in elderly men: a meta-analysis study of placebo-controlled trials. J Clin Endocrinol Metab (2013) 98:3615–26. doi: 10.1210/jc.2013-1358
33. Elraiyah T, Sonbol MB, Wang Z, Khairalseed T, Asi N, Undavalli C, et al. Clinical review: The benefits and harms of systemic dehydroepiandrosterone (DHEA) in postmenopausal women with normal adrenal function: a systematic review and meta-analysis. J Clin Endocrinol Metab (2014) 99:3536–42. doi: 10.1210/jc.2014-2261
34. Jedrzejuk D, Medras M, Milewicz A, Demissie M. Dehydroepiandrosterone replacement in healthy men with age-related decline of DHEA-S: effects on fat distribution, insulin sensitivity and lipid metabolism. Aging Male (2009) 6:151–6. doi: 10.1080/tam.6.3.151.156
35. M. A, Bednarek-Tupikowska G, Kossowska B, Bohdanowicz-Pawlak A, Sciborski R. The influence of DHEA on serum lipids, insulin and sex hormone levels in rabbits with induced hypercholesterolemia. Off J Int Soc Gynecological Endocrinol (1995) 9(1):23–28. doi: 10.3109/09513599509160187
36. L. EH, Coleman DL, Schwizer RW. Therapeutic effects of dehydroepiandrosterone (DHEA) in diabetic mice. Diabetes (1982) 31(9):830–3. doi: 10.2337/diabetes.31.9.830
37. Kimura M, Tanaka S, Yamada Y, Kiuchi Y, Yamakawa T, Sekihara H. Dehydroepiandrosterone decreases serum tumor necrosis factor-alpha and restores insulin sensitivity: independent effect from secondary weight reduction in genetically obese Zucker fatty rats. Endocrinology (1998) 139:3249–53. doi: 10.1210/endo.139.7.6118
38. Aoki K, Kikuchi T, Mukasa K, Ito S, Nakajima A, Satoh S, et al. Dehydroepiandrosterone suppresses elevated hepatic glucose-6-phosphatase mRNA level in C57BL/KsJ-db/db mice: comparison with troglitazone. Endocrine J (2000) 47:799–804. doi: 10.1507/endocrj.47.799
39. Campbell CSG, Caperuto LC, Hirata AE, Araujo EP, Velloso LA, Saad MJ, et al. The phosphatidylinositol/AKT/atypical PKC pathway is involved in the improved insulin sensitivity by DHEA in muscle and liver of rats in vivo. Life Sci (2004) 76:57–70. doi: 10.1016/j.lfs.2004.06.017
40. Lin H-Y, Yu IC, Wang R-S, Chen Y-T, Liu N-C, Altuwaijri S, et al. Increased hepatic steatosis and insulin resistance in mice lacking hepatic androgen receptor. Hepatology (2008) 47:1924–35. doi: 10.1002/hep.22252
Keywords: dehydroepiandrosterone, dehydroepiandrosterone sulfate, dyslipidemia, diabetes mellitus, type 2, androgen
Citation: Chen S, Li S, Zhang X, Fan Y and Liu M (2023) Low serum dehydroepiandrosterone is associated with diabetic dyslipidemia risk in males with type 2 diabetes. Front. Endocrinol. 14:1272797. doi: 10.3389/fendo.2023.1272797
Received: 04 August 2023; Accepted: 07 November 2023;
Published: 24 November 2023.
Edited by:
Qi Pan, Peking University, ChinaReviewed by:
Widya Wasityastuti, Gadjah Mada University, IndonesiaAndrea Carranza, National Scientific and Technical Research Council (CONICET), Argentina
Copyright © 2023 Chen, Li, Zhang, Fan and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming Liu, bWluZ2xpdUB0bXUuZWR1LmNu; Yuxin Fan, ZmFuMzA4NDExODU0QDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
 Shanshan Chen†
Shanshan Chen† Yuxin Fan
Yuxin Fan Ming Liu
Ming Liu