- 1Department of Ophthalmology, Fourth People’s Hospital of Shenyang, Shenyang, China
- 2Department of Endocrinology and Metabolism, First Affiliated Hospital of Soochow University, Suzhou, China
- 3Department of Ophthalmology, First Affiliated Hospital of Soochow University, Suzhou, China
Background: Diabetic retinopathy (DR) is a common complication of diabetes. The adipocytokines are closely associated with the occurrence and development of diabetes and its related complications. Literature confirms that the level of adiponectin in patients with DR is significantly higher; however, the relationship between other adipocytokines (leptin, chemerin, apelin, and omentin-1) and DR remains unclear.
Aim: This study aimed to systematically evaluate the association between adipocytokines (leptin, chemerin, apelin, and omentin-1) and DR.
Methods: The PubMed, Web of Science, Embase, EBSCO and Willy databases were used to search for potential studies with keywords such as “diabetic retinopathy” or “DR” in combination with the terms “leptin,” “chemerin”, “apelin” or “omentin-1” in the search titles or abstracts. Standardized mean differences (SMD) with corresponding 95% confidence intervals (CIs) were determined as the results of the meta-analysis.
Results: After screening, 18 articles were included in the meta-analysis including 750 DR cases and 993 controls. Leptin and chemerin levels in patients with DR were significantly higher than those in the control group (SMD: 0.68, 95% CI [0.1, 1.26]; SMD: 0.79, 95% CI [0.35, 1.23]). The omentin-1 levels in patients with DR were significantly lower than those in the controls (SMD: –0.85, 95% CI [–1.08, –0.62]).
Conclusions: To the best of our knowledge, this is the first meta-analysis to evaluate the leptin, chemerin, apelin, and omentin-1 levels in patients with DR. Further high-quality studies are warranted to support the association between these adipocytokines and DR.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=443770, identifier CRD42023443770.
Introduction
Diabetic retinopathy (DR) is a common complication of diabetes. A recently published meta-analysis involving 59 studies revealed that the global prevalence of DR in individuals with diabetes was 22.27% (1). By 2020, the estimated number of adults affected by DR worldwide was approximately 103.1 million, which is projected to increase to 160.5 million by 2045. Approximately 16 million individuals in the United States will reportedly experience DR by 2050 (2). In Europe, it is estimated that patients with DR will increase from 6.4 million in 2019 to 8.6 million by 2050, with 30% of the population requiring close monitoring or treatment (3). DR is clinically divided into non proliferative DR (NPDR) and proliferative DR (PDR) based on the presence or absence of retinal neovascularization. According to these findings, DR can be attributed to the detrimental effects of hyperglycemia on the retinal capillaries, resulting in edema, bleeding, and obstruction of the surrounding tissues (4). Concurrently, the elevated blood glucose levels induce retinal ischemia, hypoxia, and generation of neovascular proliferators, ultimately resulting in the onset of retinopathy. Furthermore, an extended duration of diabetes is positively correlated with an increased incidence of DR. The increase in the number of risk factors at target correlates with better cardiovascular-free survival in patients with type 2 diabetes at high cardiovascular risk (5). Thus, early intervention in the management of diabetes is of paramount significance (6).
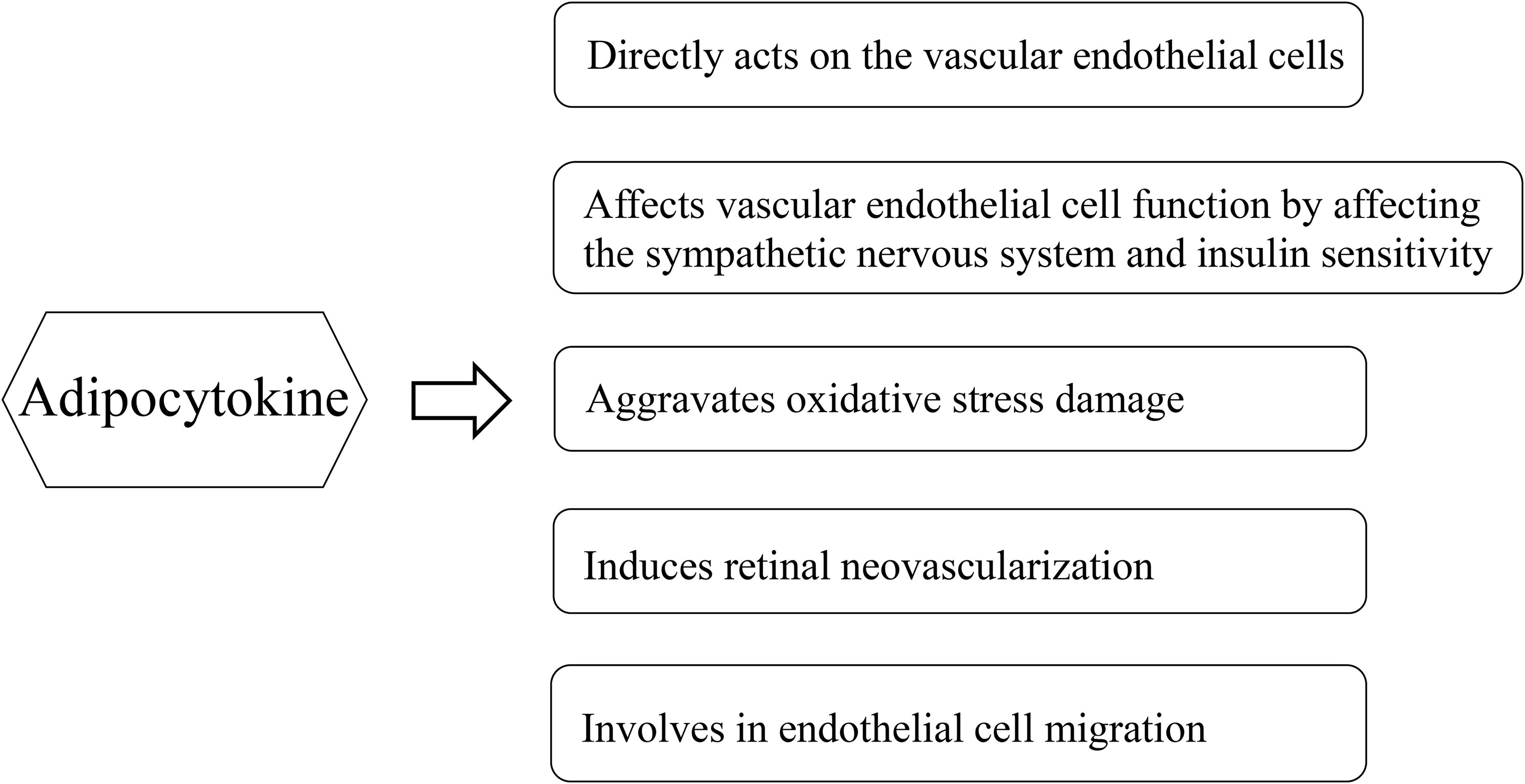
Adipose tissue has traditionally been considered a long-term energy storage organ, and researchers are now realizing its crucial role in metabolism. Adipose tissue is generally believed to be an endocrine organ that secretes various bioactive substances, and the factors secreted by the adipose tissue are collectively referred to as adipocytokines, which include adiponectin, leptin, apelin, chemerin, and omentin-1. These adipocytokines are closely associated with the occurrence and development of DR (Figure 1). Adipose factors can directly act on the vascular endothelial cells through the blood circulation. It can also indirectly affect vascular endothelial cell function by affecting the sympathetic nervous system and insulin sensitivity (7). Adipocytokines are mediators linking adipose tissue and inflammation, which in turn generate an unbalance in oxidative stress levels. In the development of diabetic retinopathy, as the retinal tissue is highly susceptible to damage mediated by oxidative stress due to its high concentration of polyunsaturated fats (8). Although literature has confirmed that the level of adiponectin in patients with DR is significantly high (9), the relationship between other adipocytokines and DR remains unclear. Leptin, which is a peptide-active factor specifically secreted by the human adipose tissue, plays important regulatory roles in human growth and development, immune function, cellular inflammatory responses, angiogenesis, platelet aggregation, epithelial cell proliferation, and migration (10). Some studies demonstrated that the leptin levels in the serum and aqueous humor of patients with DR were significantly increased compared to those of the control group (11, 12). However, Malik et al. demonstrated that the serum leptin levels of patients with DR were lower than those of the control group (13). Moreover, the relationship between chemerin, apelin, and omentin-1, and DR is controversial. Therefore, this study aimed to systematically evaluate the association between adipocytokines (leptin, chemerin, apelin, and omentin-1) and DR.
Methods
Literature search
Our meta-analysis was registered in PROSPERO under the accession number CRD42023443770. PubMed, Web of Science, Embase, EBSCO and Willy databases were used to search for potential studies. The following search terms were used for the title or abstract: “diabetic retinopathy” and “DR” in combination with the terms: “leptin,” “apelin,” “chemerin,” or “omentin-1”. The search period was from 1980 to 2023. We simultaneously traced the references of the collected relevant literature, searched for studies that met the inclusion criteria, and eliminated duplicate studies. This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines; the PRISMA list is provided in the Supplementary File (S1) (14).
Inclusion criteria
Only studies that met the following criteria were included in this meta-analysis: (1) studies with patients with DR, (2) studies reporting the correlation between the levels of adipocytokines (leptin, apelin, chemerin, and omentin-1) and DR, (3) studies with > 20 samples, and (4) studies written in English.
Exclusion criteria
(1) Reviews, case reports, comments, and animal experimental studies; (2) duplicate or repeat publications; and (3) literature with incomplete data.
Data extraction and risk of bias
Based on the inclusion and exclusion criteria, two researchers independently read the titles, abstracts, and full texts. In cases of disagreement, a third researcher was asked to intervene and make the final decision. Data were extracted, including the author, year, survey area, sample size, and indicators.
The Newcastle-Ottawa Scale (NOS) is a risk of a bias assessment tool for observational studies recommended by the Cochrane Collaboration (15). The quality of the included studies was evaluated according to the NOS. The NOS includes three aspects: the selection method of the case and control groups, comparability of the case and control groups, and evaluation method of exposure. The NOS ranged from zero to nine stars, and quality was based on star scores.
Statistical analysis
The results of the systematic analysis are presented as standardized mean differences (SMDs) with corresponding 95% confidence intervals (CIs). The meta-analysis was conducted using Stata 12.0 software (College Station, TX, USA). First, a heterogeneity test was performed using I2. If I2 < 50%, a fixed effects model is used; If I2 ≥ 50%, heterogeneity is significant and a random effects model is used. The stability of the meta-analysis results was evaluated using a sensitivity analysis. Low-quality literature and the impact of a single study on the overall research results were excluded from each study. Publication bias was tested using Begg’s and Egger’s tests. A p value< 0.05 was considered statistically significant.
Results
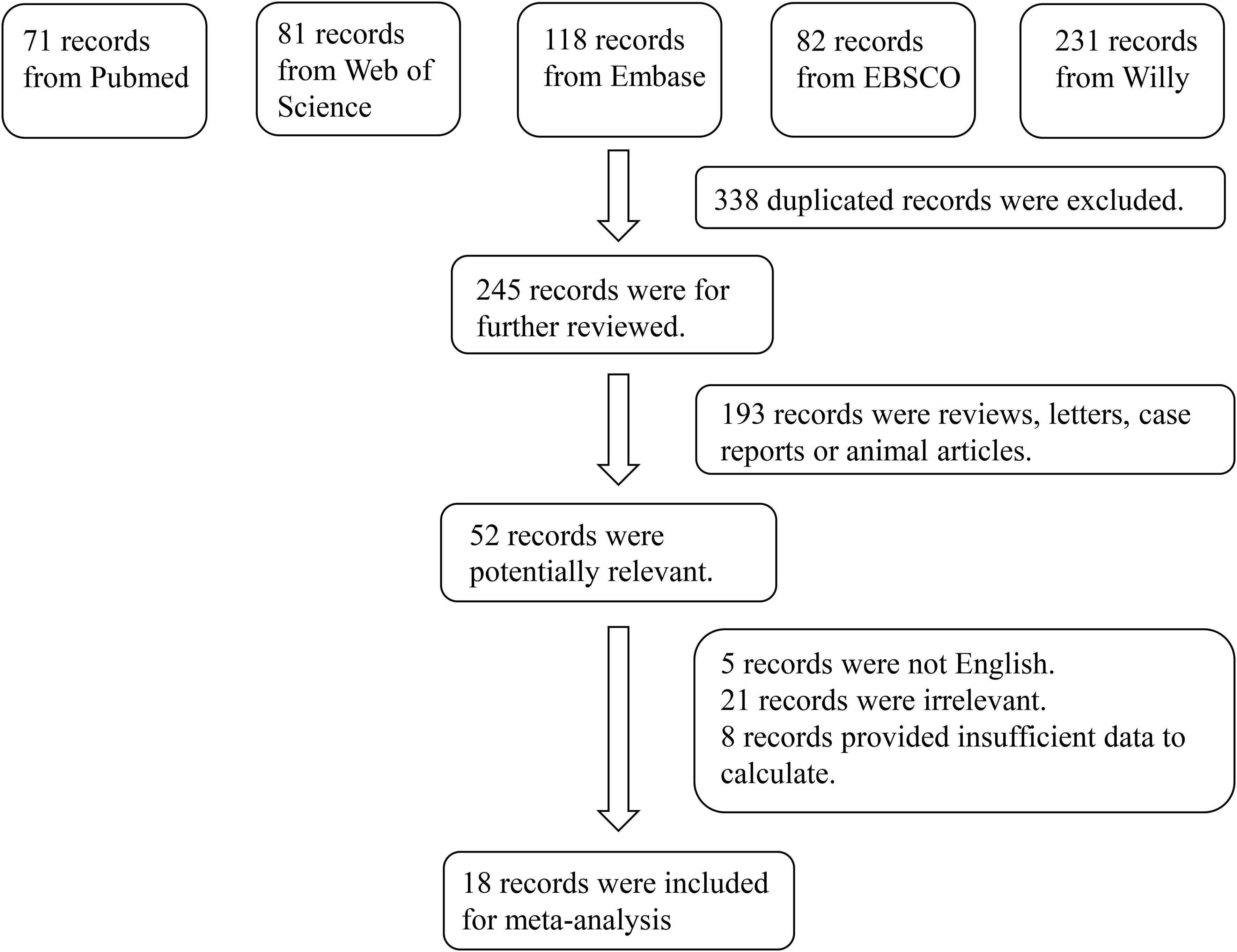
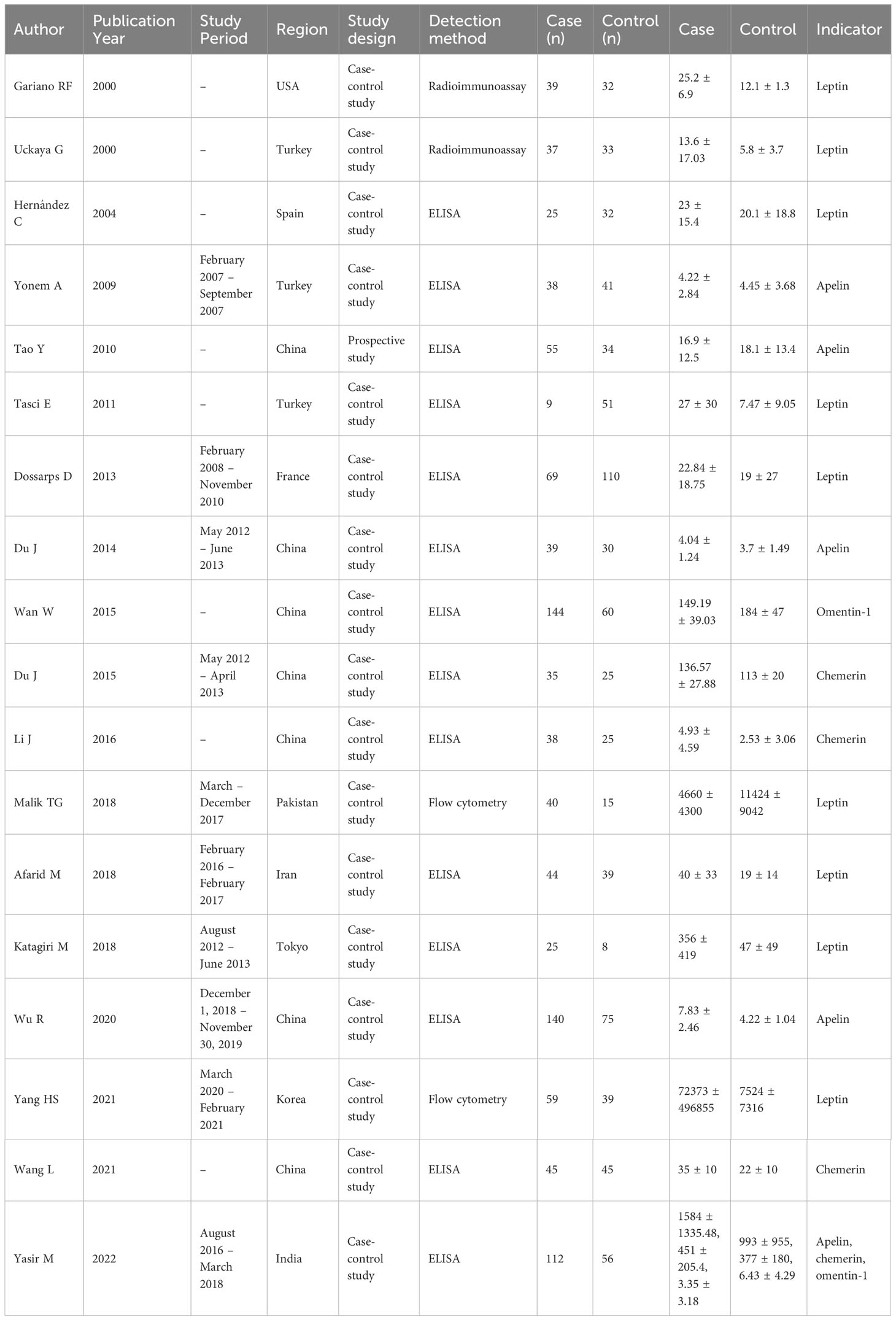
In total, 583 studies were retrieved from the PubMed, Web of Science, Embase, EBSCO and Willy. After screening, 18 articles were included in the meta-analysis (11–13, 16–30). The reasons for inclusion during the full-text selection are shown in Figure 2. Altogether, 18 articles included 750 DR cases and 993 controls. The characteristics of the selected studies are summarized in Table 1. Overall, in accordance with the suggested criteria for the Selection, Comparability, and Exposure categories of the Newcastle-Ottawa Scale, the studies included in this meta-analysis were of acceptable quality.
Results of the meta-analysis
The results of the meta-analysis demonstrated that the leptin and chemerin levels in patients with DR were significantly higher than those in non-DR patients (SMD: 0.68, 95% CI [0.1, 1.26]; SMD: 0.79, 95% CI [0.35, 1.23]); the forest funnel plots are presented in Figures 3, 4. The omentin-1 level in patients with DR was significantly lower than that in the controls (SMD: –0.85, 95% CI [–1.08, –0.62]) (Figure 5). However, the apelin levels in patients with DR were higher than those in the controls; however, no significant difference was observed (SMD:0.47, 95% CI [–0.25, 1.19]) (Figure 6). In addition, these associations were highly heterogeneous.
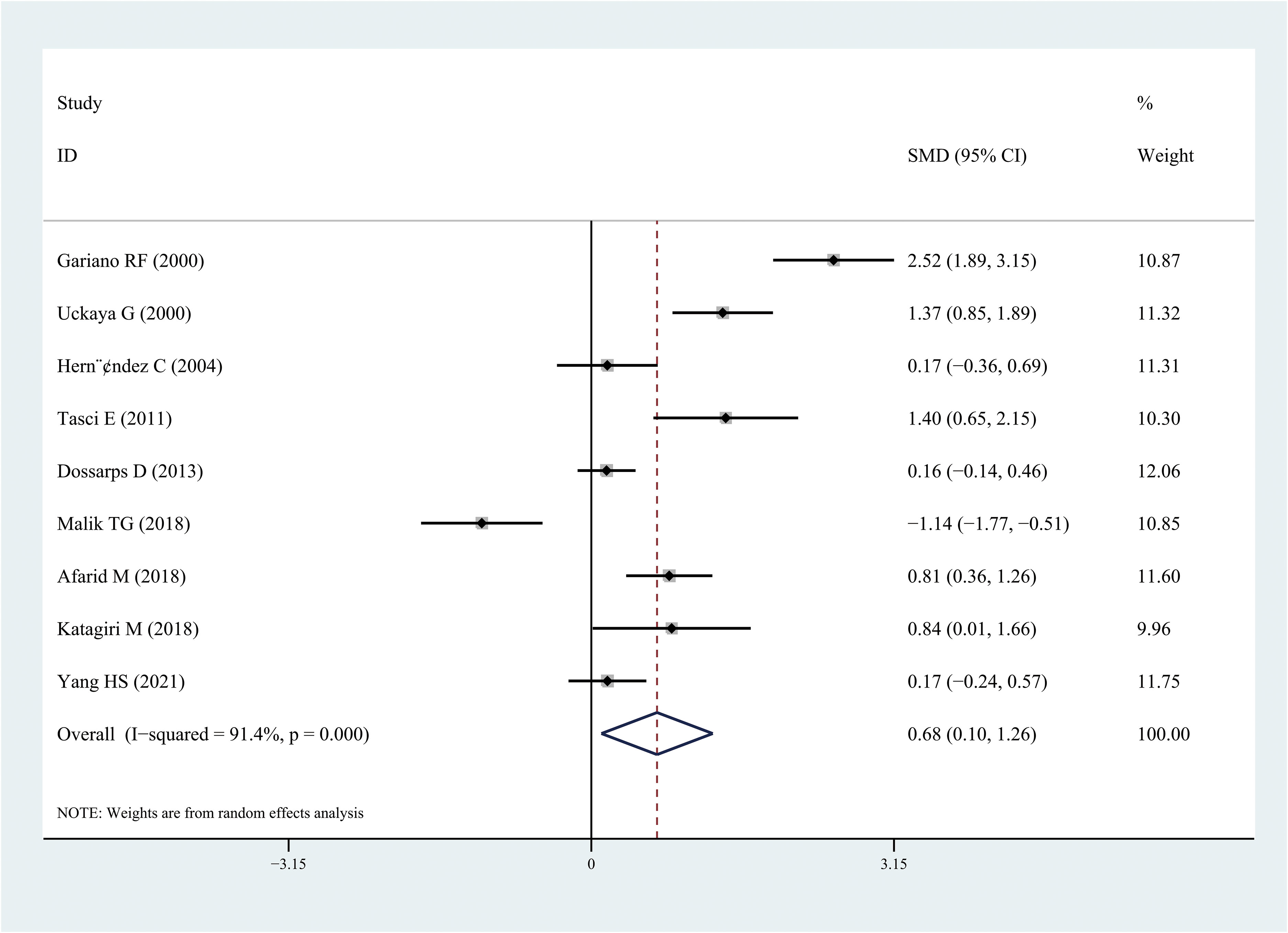
Figure 3 Forest plots of leptin level in patients with diabetic retinopathy compared with controls. Diamond represents the SMDs at 95% CI. SMD, standardized mean difference; CI, confidence interval.
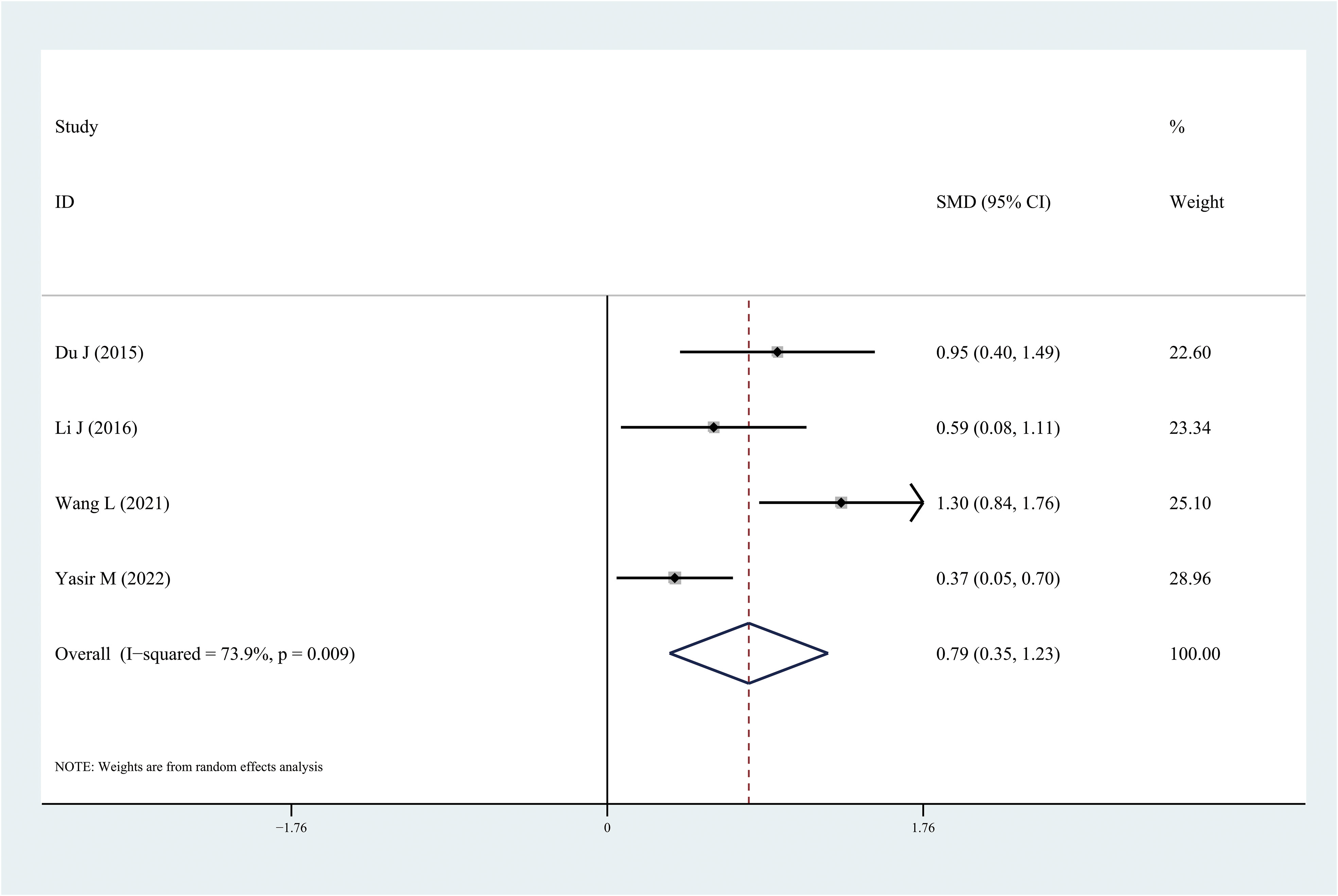
Figure 4 Forest plots of chemerin level in patients with diabetic retinopathy compared with controls. Diamond represents the pooled SMDs at 95% CI. SMD, standardized mean difference; CI, confidence interval.
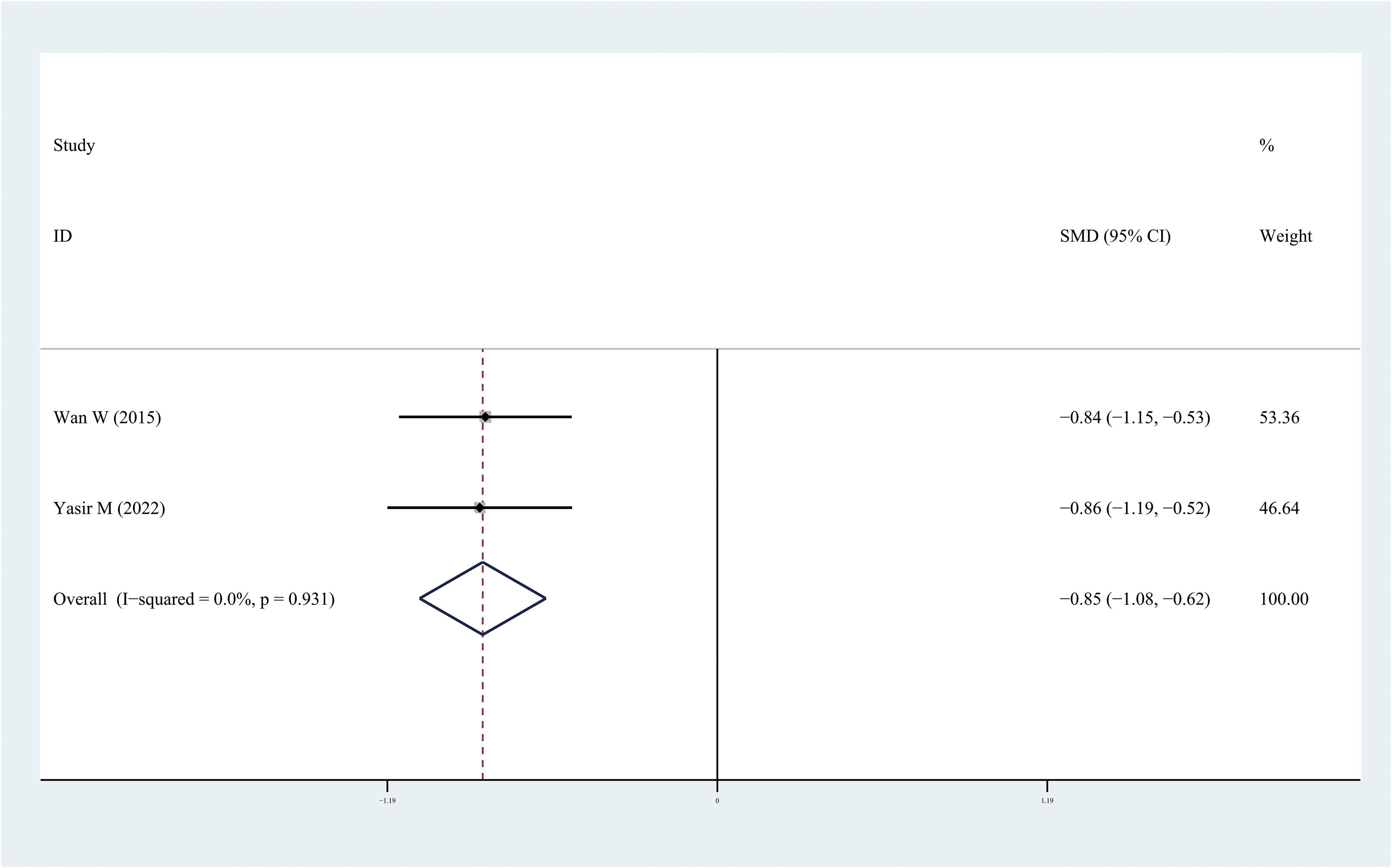
Figure 5 Forest plots of omentin-1level in patients with diabetic retinopathy compared with controls. Diamond represents the pooled SMDs at 95% CI. SMD, standardized mean difference; CI, confidence interval.
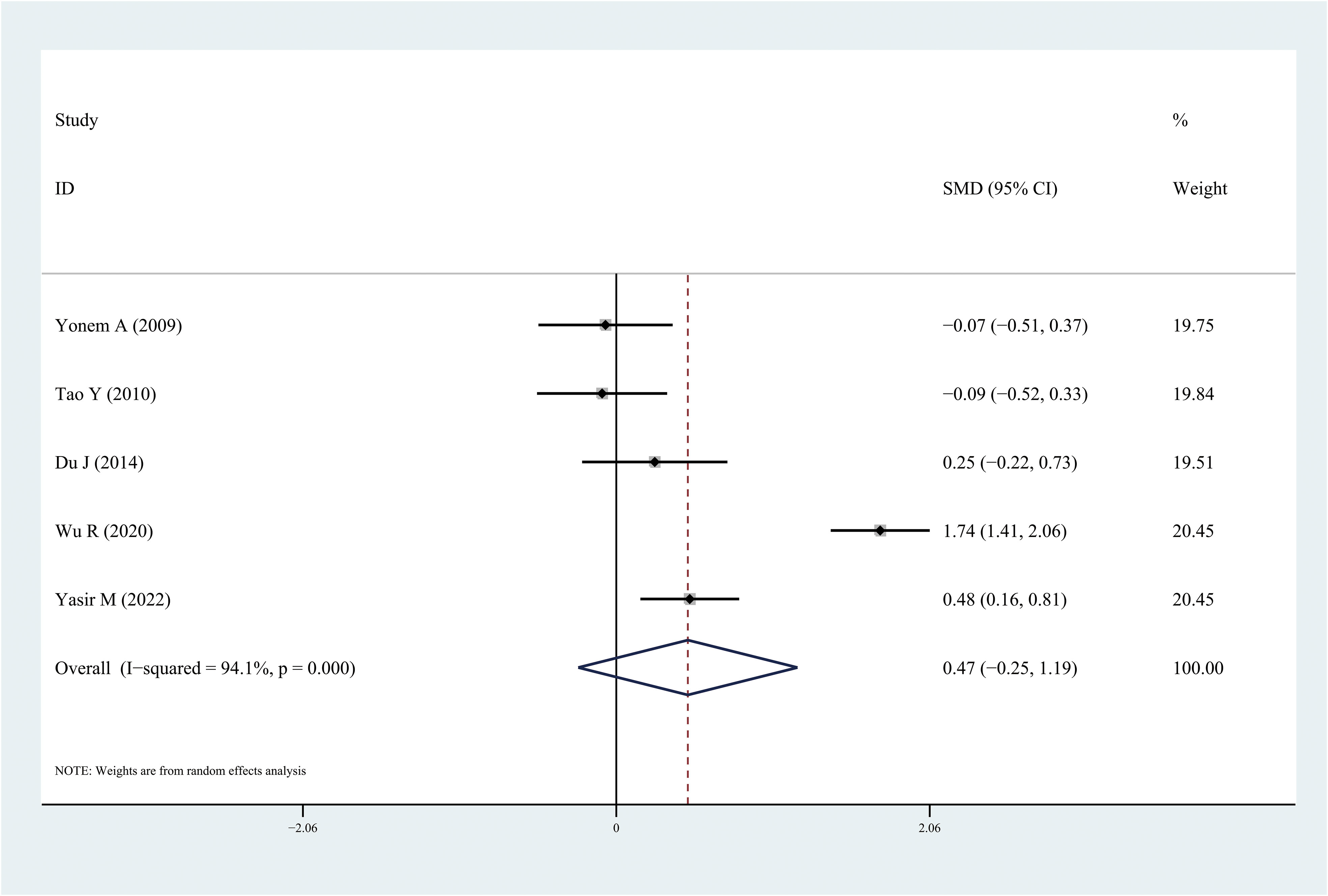
Figure 6 Forest plots of apelin level in patients with diabetic retinopathy compared with controls. Diamond represents the pooled SMDs at 95% CI. SMD, standardized mean difference; CI, confidence interval.
Sensitivity analysis and publication bias
Using sensitivity analysis by excluding individual studies one by one, the results showed little difference, suggesting that the results of this study were relatively credible. A comprehensive search of the articles obtained from the databases was performed. Begg’s and Egger’s tests were also performed and the results revealed that the possibility of publication bias was small.
Discussion
This meta-analysis is the first to evaluate the leptin, apelin, chemerin, and omentin-1 levels in patients with DR. Some studies have examined the levels of these adipocytokines in patients with DR; however, owing to inconsistent results, determining the relationship between these adipocytokines and DR is challenging. In this meta-analysis, 18 independent studies were included and analyzed. The results demonstrated that the leptin and chemerin levels in patients with DR were significantly higher than those in the controls (SMD: 0.68, 95% CI [0.1, 1.26]; SMD: 0.79, 95% CI [0.35, 1.23]). The omentin-1 levels in patients with DR were significantly lower than those in the controls (SMD: –0.85, 95% CI [–1.08, –0.62]). However, the apelin levels in patients with DR were higher than those in the controls; however, no significant difference was observed.
Leptin, which is a single-chain protein molecule containing 167 amino acid residues, is synthesized, and secreted by the adipose tissue. The main function of leptin is to regulating energy metabolism via the central nervous system. It regulates appetite through a negative feedback system and completes processes, such as reducing appetite, inhibiting energy intake, promoting energy consumption, and reducing fat synthesis, by activating receptors in target cells (10). Leptin can inhibit the secretion of insulin in patients with diabetes, thereby, affecting the insulin-mediated vascular endothelial dilation function (VEDF). Evidence suggests that the leptin levels in the vitreous body are associated with neovascular ophthalmopathies (11). Leptin can stimulate Signal transducer and activator of transcription 3 (STAT3) in the retinal endothelial cells, thereby increasing STAT3 phosphorylation and vascular endothelial growth factor (VEGF) mRNA expression in the vascular endothelium, which may be the molecular mechanism that induces retinal neovascularization (31). Leptin receptors are present in the fibrovascular tissue outside the retina of patients with diabetes. Kimura et al. demonstrated that leptin can increase the level of nitric oxide in the blood (32). Leptin induces endothelial cell proliferation by promoting endothelin secretion and mitosis. Park et al. suggested that leptin could regulate the reorganization of the vascular matrix by upregulating the expression of matrix metalloproteinases and promoting mitosis of vascular endothelial cells, thus participating in the formation of new blood vessels (33).
Chemerin, also known as retinoic acid receptor response protein-2, was first identified as a coding product of tazarotene-induced gene-2 in psoriatic lesions (34). In 2007, Bozaoglu et al. determined chemerin as a new adipose factor that is highly expressed in adipocytes and associated with obesity and metabolic syndrome (35). Increasing evidence suggests that chemerin is frequently involved in neovascularization. Patients with PDR had significantly higher serum chemerin levels, and a positive correlation exists between chemerin and VEGF. The chemerin and CMKLR1 systems are implicated in mediating cellular migration following inflammation. Accumulating evidence has revealed that chemerin, similar to VEGF, inhibits angiogenesis and the formation of new blood vessels in human endothelial cells (36).
In 2003, omentin-1 was first isolated and identified from the visceral omental adipose tissue by Yang et al., who demonstrated that treatment with recombinant omentin-1 can enhance insulin-stimulated glucose transport, indicating that omentin-1 can improve insulin sensitivity and resistance (37). Omentin-1, which is a newly discovered secreted protein with insulin sensitivity, is associated with obesity and obesity-related diseases, such as insulin resistance and diabetes. There are two highly homologous omentins: omentin-1 and omentin-2. Among them, omentin-1 is the main circulating form in human plasma. According to reports, the omentin-1 levels are decreased in individuals with type 2 diabetes mellitus, metabolic syndrome, and obesity. DR is associated with angiogenesis. Endothelial cell migration and angiogenesis induced by VEGF are significantly reduced by omentin-1 (38, 39). Omentin-1 may serve as an important protective factor against the development of DR by acting as an antiangiogenic mediator. One study found that omentin inhibited the inflammatory response induced by tumor necrosis factor (TNF) in human endothelial cells and smooth muscle cells of the vascular system. Inflammatory cytokines, such as interleukin-6 and C-reactive protein, are inversely associated with serum omentin-1 levels. Inflammation may reportedly contribute to DR and omentin-1 may play an inhibitory role in the inflammatory pathways in DR (40, 41).
Apelin is a G protein-coupled receptor, which was discovered in 1993. It is homologous to the angiotensin II type 1 receptor and is called angiotensin receptor-like protein J (APJ). The APJ gene is located on 11q12, and the APJ protein is composed of 380 amino acids (42). Apelin plays an important role in the regulation of T2DM. Apelin can promote glucose and lipid metabolism, increase insulin sensitivity, reduce blood sugar levels, improve diabetes, and reduce the occurrence of diabetes by regulating cardiovascular function and reducing food intake (43). In this study, although an increase in the apelin levels was observed in patients with DR, no statistically significant differences were found.
However, this study has certain limitations. Owing to the lack of large sample sizes, most studies included in this meta-analysis had small sample sizes. Furthermore, many isomers of adipocytokines, such as apelin-12 and apelin-36, exist; some studies did not indicate which isomer was used. Different detection methods of adipocytokines levels have been used in these studies. These factors may have affected the results. Therefore, the results obtained herein should be interpreted with caution as further research is needed.
Conclusion
To the best of our knowledge, this meta-analysis is the first to evaluate the leptin, chemerin, apelin, and omentin-1 levels in patients with DR. Our findings suggest that leptin, chemerin, and omentin-1 levels are significantly altered in patients with DR. Further high-quality studies are warranted to confirm the association between adipocytokines and DR.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
YJ: investigation, software, writing – original draft. HF: writing – original draft, formal analysis, project administration. JX: writing – original draft, resources, visualization. YX: writing – original draft, data curation. XS: conceptualization, writing – original draft.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article. This research was financially supported by the Natural Science Foundation of Jiangsu Province (grant No. BK20200204).
Acknowledgments
We thank Editage for English-language editing.
Conflict of interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1271027/full#supplementary-material
Supplementary File S1 | Preferred reporting items for systematic review and meta-analyses (PRISMA) checklist.
References
1. Teo ZL, Tham YC, Yu M, Chee ML, Rim TH, Cheung N, et al. Global prevalence of diabetic retinopathy and projection of burden through 2045: systematic review and meta-analysis. Ophthalmology (2021) 128:1580–91. doi: 10.1016/j.ophtha.2021.04.027
2. Nagda D, Mitchell W, Zebardast N. The functional burden of diabetic retinopathy in the United States. Graefes Arch Clin Exp Ophthalmol (2021) 259:2977–86. doi: 10.1007/s00417-021-05210-3
3. Li JQ, Welchowski T, Schmid M, Letow J, Wolpers C, Pascual-Camps I, et al. Prevalence, incidence and future projection of diabetic eye disease in Europe: a systematic review and meta-analysis. Eur J Epidemiol (2020) 35:11–23. doi: 10.1007/s10654-019-00560-z
4. Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet (2010) 376(9735):124–36. doi: 10.1016/S0140-6736(09)62124-3
5. Sasso FC, Simeon V, Galiero R, Caturano A, De Nicola L, Chiodini P, et al. The number of risk factors not at target is associated with cardiovascular risk in a type 2 diabetic population with albuminuria in primary cardiovascular prevention. Post-hoc analysis of the NID-2 trial. Cardiovasc Diabetol (2022) 21(1):235. doi: 10.1186/s12933-022-01674-7
6. Wang W, Lo A. Diabetic retinopathy: pathophysiology and treatments. Int J Mol Sci (2018) 19(6):1816. doi: 10.3390/ijms19061816
7. Unamuno X, Gomez-Ambrosi J, Rodriguez A, Becerril S, Fruhbeck G, Catalan V. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur J Clin Invest (2018) 48(9):e12997. doi: 10.1111/eci.12997
8. Caturano A, D'Angelo M, Mormone A, Russo V, Mollica MP, Salvatore T, et al. Oxidative stress in type 2 diabetes: impacts from pathogenesis to lifestyle modifications. Curr Issues Mol Biol (2023) 45(8):6651–66. doi: 10.3390/cimb45080420
9. Fan X, Wu Q, Li Y, Hao Y, Ning N, Kang Z, et al. Association between adiponectin concentrations and diabetic retinopathy in patients with type 2 diabetes:a meta analysis. Chin Med J-Peking (2014) 127(4):765–71. doi: 10.3760/cma.j.issn.0366-6999.20132507
10. Zhang Y, Chua SJ. Leptin function and regulation. Compr Physiol (2017) 8(1):351–69. doi: 10.1002/cphy.c160041
11. Gariano RF, Nath AK, D'Amico DJ, Lee T, Sierra-Honigmann MR. Elevation of vitreous leptin in diabetic retinopathy and retinal detachment. Invest Ophthalmol Vis Sci (2000) 41(11):3576–81.
12. Uckaya G, Ozata M, Bayraktar Z, Erten V, Bingol N, Ozdemir IC. Is leptin associated with diabetic retinopathy? Diabetes Care (2000) 23(3):371–6. doi: 10.2337/diacare.23.3.371
13. Malik TG, Ahmed SS, Gul R, Ayesha E. Comparative analysis of serum proangiogenic biomarkers between those with and without diabetic retinopathy. J Coll Physicians Surg Pak (2018) 28(9):686–9. doi: 10.29271/jcpsp.2018.09.686
14. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (2014) (Accessed 2014 Aug).
15. Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-analyses (2014) (Accessed 2014 Aug 5).
16. Hernández C, Lecube A, Castellanos JM, Segura RM, Garat M, García-Arumí J, et al. Intravitreous leptin concentrations in patients with proliferative diabetic retinopathy. Retina (Philadelphia Pa.) (2004) 24(1):30–5. doi: 10.1097/00006982-200402000-00005
17. Yonem A, Duran C, Unal M, Ipcioglu OM, Ozcan O. Plasma apelin and asymmetric dimethylarginine levels in type 2 diabetic patients with diabetic retinopathy. Diabetes Res Clin PR (2009) 84(3):219–23. doi: 10.1016/j.diabres.2009.03.001
18. Tao Y, Lu Q, Jiang YR, Qian J, Wang JY, Gao L, et al. Apelin in plasma and vitreous and in fibrovascular retinal membranes of patients with proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci (2010) 51(8):4237–42. doi: 10.1167/iovs.09-4466
19. Tasci E, Ozbek MN, Onenli Mungan N, Temiz F, Topaloglu AK, Yuksel B. Low serum adiponectin levels in children and adolescents with diabetic retinopathy. Eurasian J Med (2011) 43(1):18–22. doi: 10.5152/eajm.2011.04
20. Dossarps D, Petit JM, Guiu B, Cercueil JP, Duvillard L, Bron AM, et al. Body fat distribution and adipokine secretion are not associated with diabetic retinopathy in patients with type 2 diabetes mellitus. Ophthalmic Res (2013) 51(1):42–5. doi: 10.1159/000355323
21. Du J, Li X, Li R, Xu L, Ma R, Liu S, et al. Elevation of serum apelin-13 associated with proliferative diabetic retinopathy in type 2 diabetic patients. Int J Ophthalmol-Chi (2014) 7(6):968–73. doi: 10.3980/j.issn.2222-3959.2014.06.10
22. Wan W, Li Q, Zhang F, Zheng G, Lv Y, Wan G, et al. Serum and vitreous concentrations of omentin-1 in diabetic retinopathy. Dis Markers (2015) 2015:1–4. doi: 10.1155/2015/754312
23. Du J, Li R, Xu L, Ma R, Liu J, Cheng J, et al. Increased serum chemerin levels in diabetic retinopathy of type 2 diabetic patients. Curr Eye Res (2015) 41(1):114–20. doi: 10.3109/02713683.2015.1004718
24. Li J, Hu W, Song H, Lin J, Tang X. Increased vitreous chemerin levels are associated with proliferative diabetic retinopathy. Ophthalmologica (2016) 236(2):61–6. doi: 10.1159/000447752
25. Katagiri M, Shoji J, Inada N, Kato S, Kitano S, Uchigata Y. Evaluation of vitreous levels of advanced glycation end products and angiogenic factors as biomarkers for severity of diabetic retinopathy. Int Ophthalmol (2018) 38(2):607–15. doi: 10.1007/s10792-017-0499-1
26. Afarid M, Attarzadeh A, Farvardin M, Ashraf H. The association of serum leptin level and anthropometric measures with the severity of diabetic retinopathy in type 2 diabetes mellitus. Med hypothesis Discovery Innovation Ophthalmol (2018) 7(4):156–62.
27. Wu R, Zhu Z, Zhou D. VEGF, apelin and HO-1 in diabetic patients with retinopathy: a correlation analysis. BMC Ophthalmol (2020) 20(1):326. doi: 10.1186/s12886-020-01593-9
28. Yang HS, Choi YJ, Han HY, Kim HS, Park SH, Lee KS, et al. Serum and aqueous humor adiponectin levels correlate with diabetic retinopathy development and progression. PloS One (2021) 16(11):e259683. doi: 10.1371/journal.pone.0259683
29. Wang L, Zhang Y, Guo Y, Ding W, Chang A, Wei J, et al. Chemerin/CMKLR1 axis promotes the progression of proliferative diabetic retinopathy. Int J Endocrinol (2021) 2021:1–8. doi: 10.1155/2021/4468625
30. Yasir M, Senthilkumar GP, Jayashree K, Ramesh BK, Vadivelan M, Palanivel C. Association of serum omentin-1, apelin and chemerin concentrations with the presence and severity of diabetic retinopathy in type 2 diabetes mellitus patients. Arch Physiol Biochem (2022) 128:313–20. doi: 10.1080/13813455.2019.1680698
31. Suganami E, Takagi H, Ohashi H. Leptin stimulates ischemia-induced retinal neovascularization: possible role of vascular endothelial growth factor expressed in retinal endothelial cells. Diabetes (2004) 53:2443. doi: 10.2337/diabetes.53.9.2443
32. Kimura K, Tsuda K, Baba A, Kawabe T, Boh-oka S, Ibata M, et al. Involvement of nitric oxide in endothelium-dependent arterial relaxation by leptin. Biochem Biophys Res Common (2000) 273:745–9. doi: 10.1006/bbrc.2000.3005
33. Park HY, Kwon HM, Lim HJ, Hong BK, Lee JY, Park BE, et al. Potential role of leptin in angiogenesis: leptin induces endothelial cell proliferation and expression of matrix metalloproteinases in vivo and in vitro. Yonsei Med J (2000) 41:68. doi: 10.1038/emm.2001.17
34. Bozaoglu K, Curran JE, Stocker CJ, Zaibi MS, Segal D, Konstantopoulos N, et al. Chemerin, a novel adipokine in the regulation of angiogenesis. J Clin Endocrinol Metab (2010) 95:2476–85. doi: 10.1210/jc.2010-0042
35. Sato K, Yoshizawa H, Seki T, Shirai R, Yamashita T, Okano T, et al. Chemerin-9, a potent agonist of chemerin receptor (ChemR23), prevents atherogenesis. Clin Sci (Lond) (2019) 133:1779–96. doi: 10.1042/CS20190336
36. Parolini S, Santoro A, Marcenaro E, Luini W, Massardi L, Facchetti F, et al. The role of chemerin in the colocalization of NK and dendritic cell subsets into inflamed tissues. Blood (2007) 109:3625–32. doi: 10.1182/blood-2006-08-038844
37. Moreno-Navarrete JM, Ortega F, Castro A, Sabater M, Ricart W, Fernandez-Real JM. Circulating omentin as a novel biomarker of endothelial dysfunction. Obes (Silver Spring) (2011) 19(8):1552–9. doi: 10.1038/oby.2010.351
38. Tan BK, Adya R, Farhatullah S, Chen J, Lehnert H, Randeva HS. Metformin treatment may increase omentin-1 levels in women with polycystic ovary syndrome. Diabetes (2010) 59(12):3023–31. doi: 10.2337/db10-0124
39. Yamawaki H, Kuramoto J, Kameshima S, Usui T, Okada M, Hara Y. Omentin, a novel adipocytokine inhibits TNF-induced vascular inflammation in human endothelial cells. Biochem Biophys Res Commun (2011) 408(2):339–43. doi: 10.1016/j.bbrc.2011.04.039
40. Kazama K, Usui T, Okada M, Hara Y, Yamawaki H. Omentin plays an anti-inflammatory role through inhibition of TNF-alpha-induced superoxide production in vascular smooth muscle cells. Eur J Pharmacol (2012) 686(1-3):116–23. doi: 10.1016/j.ejphar.2012.04.033
41. Pan HY, Guo L, Li Q. Changes of serum omentin-1 levels in normal subjects and in patients with impaired glucose regulation and with newly diagnosed and untreated type 2 diabetes. Diabetes Res Clin Pract (2010) 88(1):29–33. doi: 10.1016/j.diabres.2010.01.013
42. Antushevich H, Wojcik M. Review apelin in disease. Clin Chim Acta (2018) 483:241–8. doi: 10.1016/j.cca.2018.05.012
Keywords: adipocytokines, diabetic retinopathy, DR, leptin, chemerin
Citation: Jiang Y, Fan H, Xie J, Xu Y and Sun X (2023) Association between adipocytokines and diabetic retinopathy: a systematic review and meta-analysis. Front. Endocrinol. 14:1271027. doi: 10.3389/fendo.2023.1271027
Received: 01 August 2023; Accepted: 21 September 2023;
Published: 06 October 2023.
Edited by:
Ferdinando Carlo Sasso, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Raffaele Galiero, University of Campania Luigi Vanvitelli, ItalyAlfredo Caturano, University of Campania Luigi Vanvitelli, Italy
Copyright © 2023 Jiang, Fan, Xie, Xu and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Sun, c3VueGluNzdAMTI2LmNvbQ==; Yao Xu, NjA0MDYyMDQwQHFxLmNvbQ==
 Yanhua Jiang1
Yanhua Jiang1 Yao Xu
Yao Xu Xin Sun
Xin Sun