- 1Department of Clinical Pharmacology, Xiangya Hospital, Central South University, Changsha, Hunan, China
- 2Institute of Clinical Pharmacology, Central South University, Changsha, Hunan, China
- 3Engineering Research Center of Applied Technology of Pharmacogenomics, Ministry of Education, Changsha, Hunan, China
- 4National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Changsha, Hunan, China
- 5Department of General Surgery, The Second People’s Hospital of Hunan, Changsha, Hunan, China
- 6Department of Emergency Medicine, Trauma Center, The Second People’s Hospital of Hunan, Changsha, Hunan, China
Background: Human blood metabolites have demonstrated close associations with thyroid disorders in observational studies. However, it’s essential to determine whether these correlations imply causation. Mendelian Randomization (MR) offers a promising approach to investigate these patterns.
Aims: The primary aim of our investigation is to establish causality between blood metabolites and three thyroid disorders: TC, GD, and HT.
Methods: We employed a two-sample bidirectional MR analysis approach to assess the relationships between 452 blood metabolites and the three aforementioned thyroid disorders. Causal links were estimated using the IVW method, with sensitivity analyses conducted via MR-Egger, Weighted Median, and MR-PRESSO. We assessed potential heterogeneity and pleiotropy using MR-Egger intercept and Cochran’s Q statistic. Additionally, we conducted pathway analysis to identify potential metabolic pathways.
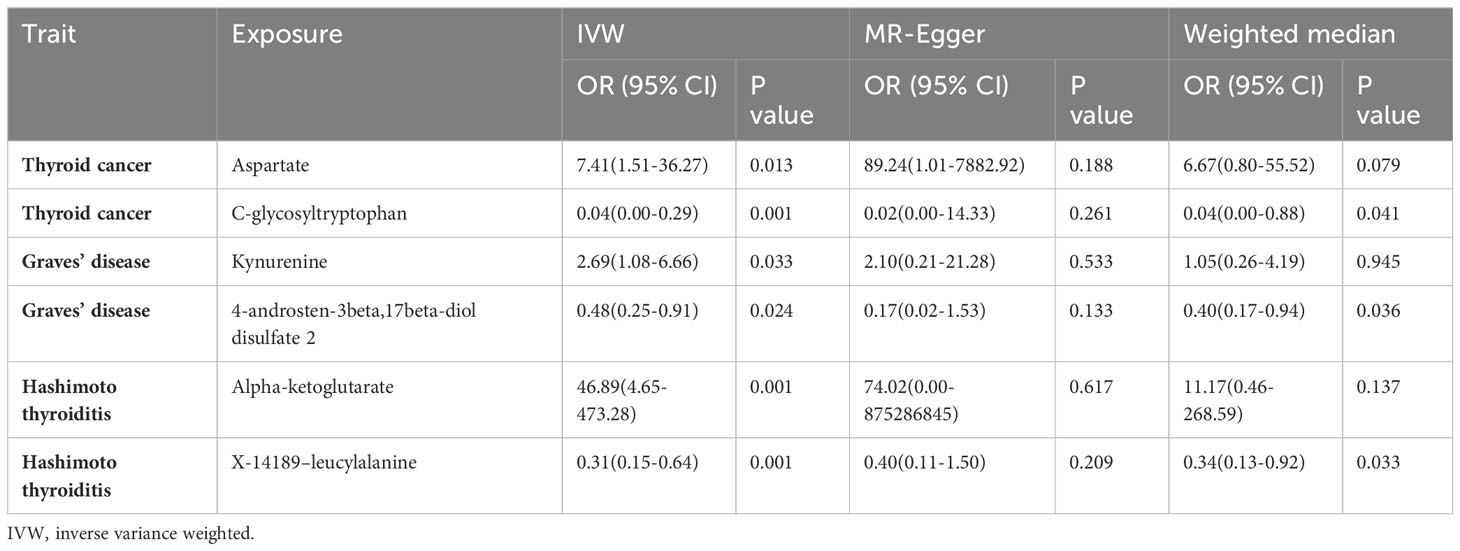
Results: We found 46 metabolites that showed suggestive associations with thyroid disease risk, especially Aspartate (ORIVW=7.41; 95%CI: 1.51-36.27; PIVW=0.013) and C-glycosyltryptophan (ORIVW=0.04; 95%CI: 0.00–0.29; PIVW=0.001) impacted TC, Kynurenine (ORIVW=2.69; 95%CI: 1.08–6.66; PIVW=0.032) and 4-androsten-3beta,17beta-diol disulfate 2 (ORIVW=0.78; 95%CI: 0.48–0.91; PIVW=0.024) significantly impacted GD, and Alpha-ketoglutarate (ORIVW=46.89; 95%CI: 4.65–473.28; PIVW=0.001) and X-14189–leucylalanine (ORIVW=0.31; 95%CI: 0.15–0.64 PIVW=0.001) significantly impacted HT. We also detected 23 metabolites influenced by TC and GD. Multiple metabolic pathways have been found to be involved in thyroid disease.
Conclusion: Our MR findings suggest that the identified metabolites and pathways can serve as biomarkers for clinical thyroid disorder screening and prevention, while also providing new insights for future mechanistic exploration and drug target selection.
Introduction
Thyroid diseases encompass a variety of disorders involving the thyroid gland, including thyroid cancer (TC), Graves’ disease (GD), and Hashimoto’s thyroiditis (HT). Thyroid cancer is the most prevalent malignant thyroid tumor (1), while Graves’ disease and Hashimoto’s thyroiditis are common organ-specific disorders (2), with Th17 cells playing a foundational role in these conditions (3). These three diseases notably affect both incidence rates and affected individuals’ quality of life. Thyroid hormones play a pivotal role in regulating numerous metabolic processes within the body, thereby influencing the overall metabolic status of individuals. The association between metabolic abnormalities and the incidence of thyroid disorders is of significance, as it directly impacts the quality of life for affected patients. Studying the relationship between metabolic issues and these thyroid diseases has garnered significant attention.
Observational research has shown a strong link between specific blood metabolites and the development and advancement of thyroid diseases. Regarding thyroid cancer, prior studies have established a correlation between blood thyroid hormone levels and thyroid cancer occurrence (4).. Additionally, some blood metabolites such as vitamin D, lipid metabolites, and carbohydrate metabolites have shown abnormal changes associated with thyroid cancer (5, 6). There is relatively less research on Graves’ disease and Hashimoto’s thyroiditis. Murdaca et al. discovered a certain correlation between the development of autoimmune thyroid diseases and factors such as vitamin D and the microbiome (7). In another study, there was a noticeable association between thyroid hormone levels in the blood of Graves’ disease patients and lipid metabolites (8, 9).. In studies on Hashimoto’s thyroiditis, the focus has been on the interaction between thyroid antibodies and immune-related metabolites in the blood (10, 11). Although prior research has explored the link between human blood metabolites and thyroid diseases, there remains a need for more extensive and systematic studies to fully determine the causal relationship between these diseases and metabolites.
Mendelian randomization (MR), an analytical approach, is vital for exploring the causal link between metabolites and thyroid diseases. Given the absence of viable randomized controlled trials or the practicality of initiating new ones, the MR method has emerged as a crucial alternative for evaluating the causal connection between metabolites and disease risk. Specifically, in the MR approach, single nucleotide polymorphisms (SNPs) are employed as instrumental variables (IVs) to represent the specific phenotype (12). The MR approach’s main strength is utilizing the naturally occurring genetic variations allocated randomly during fertilization. This mimics a randomized controlled trial, effectively minimizing biases from confounding factors like gender and age in causal analysis (13). Furthermore, genotype establishment happens before the onset of disease and remains mostly uninfluenced by disease advancement, thereby enabling the effective evaluation of the causal links between metabolites and thyroid diseases (14, 15).
To identify potential candidate metabolites associated with the etiology of thyroid diseases in a more exploratory manner, we performed bidirectional two-sample MR analysis using the latest and extensive genome-wide association study (GWAS) summary data available (16). The purpose of this analysis was to explore bidirectional causal associations between human blood metabolites and both TC and AITD (including GD and HT). This research approach enhances comprehension of thyroid disease pathogenesis and metabolic pathways, while also furnishing dependable evidence for devising viable strategies in thyroid screening and prevention within clinical settings.
Materials and methods
MR design
We employed a bidirectional two-sample MR to evaluate the causal link between 452 human blood metabolites and TC and AITD. Figure 1 provides a schematic overview of the study design and data sources. GWAS summary statistics were obtained to extract prominent single nucleotide polymorphisms [SNPs] serving as genetic instrumental variables for 452 human blood metabolites, TC, and AITD. Initially, we designated the 452 blood metabolites as the exposure and TC, along with AITD, as the outcomes to ascertain their potential roles in either inhibiting or fostering the onset of TC and AITD. TC and AITD were then used as the exposure and blood metabolites as the outcome to investigated changes in metabolites after the occurrence of disease. The summary-level data utilized in this study can be downloaded and has been obtained with ethical approval from the respective institutions overseeing each GWAS involved.

Figure 1 Schematic overview of the study design. (A) Mendelian randomization [MR] illustration. There are three principal assumptions in MR design: (1) IVs must be strongly correlated with exposure factors; (2) IVs was associated with outcomes only by exposure; (3) IVs cannot be associated with any confounding factors. (B) Bidirectional MR study of metabolites and thyroid disease TC, Thyroid cancer; GD, Graves' disease; HT, Hashimoto thyroiditis; IVW, Inverse variance weighted; WM, weighted median; LOO, leave-one-out.
Analyses were executed using R statistical software (version 4.2.3). MR analyses were conducted with the R-based tools “TwoSampleMR” and “MR-PRESSO,” and the meta-analysis employed the “meta” package (13).
Data sources
The data for this study were sourced from two GWASs: one focusing on metabolites and the other on thyroid disease. The dataset comprising 452 metabolites for the GWAS was amalgamated from a genome-wide association scan and high-throughput metabolic analysis study conducted by Shin et al. This study enrolled 7824 participants mainly from two European population cohorts, with screening conducted for approximately 21,000 SNPs (12, 17). The participant pool comprised 1,768 individuals from the KORA F4 study in Germany and 6,056 participants from the Twin study. Both studies received approval from local ethics committees, and all participants provided informed consent. This represents the most extensive investigation to date on the genetic impact on human serum metabolism. Following rigorous quality control, a total of 452 metabolites underwent genetic analysis in both cohorts. These encompassed 275 recognized metabolites and 177 unidentified ones. As detailed in the KEGG database, the 275 established metabolites are categorized into eight major metabolic groups: amino acids, carbohydrates, cofactors and vitamins, energy, lipids, nucleotides, peptides, and allogenic metabolism (18).
The GWAS summaries for TC, GD, and HT were acquired from the FINNGEN consortium (r9.finngen.fi). The FINNGEN consortium is a Finnish national meta-analysis of GWAS that analyzed 13 cohorts and biobanks. The GWAS summary data for TC included 288,920 samples (1783 individuals with TC and 287,137 without) with a dataset of 18,707,521 SNPS. The summary data for GD included 377,277 samples (2836 GD cases and 374,441 controls), encompassing a dataset of 18,709,621 SNPs. The GWAS summary data for HT contained 321,192 samples (489 Hashimoto’s thyroiditis and 320,703 controls) with a dataset of 18,708,398 SNPS. Supplementary Table S2 shows the detailed information.
Selection criteria for genetic variants
In the process of selecting instrumental variables (IVs) to signify potential exposure-outcome links, varied thresholds were established according to exposure variations. First, 452 metabolites were designated as the exposure. In this case, SNP with an association threshold of P<1×10-5 were extracted (19), mainly for MR Analysis, while a linkage disequilibrium parameter (r2) of <0.001 was set in the 10,000 kb window of the European 1000 Genome reference Panel to obtain top-level independent SNP. When the chosen SNPs from the exposure dataset were not present in the outcome dataset, proxy SNPs displaying substantial association with the selected variants (R2>0.8) were employed instead. Secondly, When TC and AITD were designated as exposures, IV significance adhered to the genome-wide statistical significance threshold (p<5×10-8) (20). Moreover, a linkage disequilibrium threshold of 0.001 and a clumping window of 10,000 kb were established. MR Steiger filters were employed to exclude SNPs with incorrect causal directions. Furthermore, R2 and F-statistic of the IVs were computed to identify potential weak IV bias (21). SNPs with F<10 were deemed weak instruments and removed to guarantee ample variance from all SNPs for the respective exposed group (22). The design formula is shown in Table S1, adhering to the common recommendation of using a threshold of F>10 for MR analysis. Then, exposure SNPs were isolated from outcome data, while excluding SNPs associated with the outcome. Following this, alignment of allele information for exposure and outcome SNPs was performed during data harmonization. Lastly, metabolites with a minimum of 2 SNPs were retained for MR analysis.
Primary analysis
This study employed bidirectional two-sample MR to estimate causal effects between metabolites and thyroid disease using inverse variance weighting (IVW) (23). IVW is a prominent method frequently employed in MR studies, effectively aggregating Wald ratios for each SNP to yield a consolidated estimate. The random effects model of IVW is utilized in cases of heterogeneity, while the fixed effects model is applied in its absence. We further applied a multiple-testing corrected threshold of P<0.05/275 (where 275 represents the count of known metabolites) using the Bonferroni correction to elucidate statistical significance (24). P-values ranging from 1.82×10-4 to 0.05 were regarded as possible associations.
Sensitivity analysis and direction validation
To validate that IV influences the outcome solely through the exposure, and to improve the robustness of the findings, sensitivity analysis is also required. Therefore, Different approaches such as weighted median, MR-Egger, MR-PRESSO, and leave-one-out were employed in sensitivity analysis to validate the stability of the significant estimates found (IVW P<0.05). Among them, The weighted median method enhances causal effect detection and reduces type I errors. MR-Egger identifies IV assumption violations and offers unaffected effect estimates. Concurrently, we examined the presence of horizontal pleiotropy using MR-Egger regression and the MR-PRESSO Global test (25). Horizontal pleiotropy suggests that IVs might be linked to outcomes through non-causal pathways, possibly yielding false positives (p<0.05). The leave-one-out method assesses whether individual SNPs influence the results (26). Furthermore, the Cochran Q test was employed to identify heterogeneity, with a resulting Cochran-Q derived p-value below 0.05 indicating its presence (27). Lastly, the MR Steiger directionality test was performed to validate if our findings aligned with our hypothesis (28).
Replication analysis
We replicated the IVW analysis using GWAS data for TC, GD, and HT from the IEU Open database (29), aiming to cross-validate the reliability of our findings. (Table S2)
Metabolic pathway analysis
The chosen metabolite metabolic pathways were investigated using the web-based tool Metaconflict 5.0 (30) https://www.metaboanalyst.ca/. MetaboAnalyst 5.0 serves as a user-friendly online tool for efficient metabolomics data analysis. In this study, only metabolites exceeding the advised threshold were analyzed for metabolic pathways (PIVW<0.05).
Results
Causal effects of the human blood metabolites on TC, GD, and HT
The metabolite instrumental variables ranged from 4 to 150, with a median count of 12. SNP F-statistics exceeded 10, indicating absence of weak instrumental variables. (Table S3). Based on these instrumental variables we performed the IVW MR Analysis for each pair of metabolites and TC and AITD. A total of 46 metabolites (PIVW<0.05) with significant associations were identified, including 22 known metabolites and 24 unknown metabolites. Among these, 10 (Figures S1-S4), 5(Figures S5-S8) and 7(Figures S9-S12) associations of known metabolites (Table 1) and 9, 4 and 11 unknown metabolite associations were identified with increased risk of TC, GD, and HT, respectively. Genetic variants elucidating metabolite associations with three thyroid diseases were presented in Tables S5-S7. Supplementary Figures displayed scatter plots, funnel plots, and leave-one-out sensitivity analysis.
Specifically, Aspartate (ORIVW=7.41; 95%CI: 1.51-36.27; PIVW=0.013), Kynurenine (ORIVW=2.69; 95%CI: 1.08–6.66; PIVW=0.032), Alpha-ketoglutarate (ORIVW=46.89; 95%CI: 4.65–473.28; PIVW=0.001) were the most notably risky metabolites for thyroid cancer, Graves’ disease and Hashimoto’s thyroiditis. On the contrary, C-glycosyltryptophan (ORIVW=0.04; 95%CI: 0.00–0.29; PIVW=0.001), 4-androsten-3beta,17beta-diol disulfate 2 (ORIVW=0.78; 95%CI: 0.48–0.91; PIVW=0.024), X-14189–leucylalanine (ORIVW=0.31; 95%CI: 0.15–0.64; PIVW=0.001) were factors with highest protective value for TC, GD and HT (Table 2). Table 1 shows the characteristics of all significant pathogenic relationships between known metabolites of different types of thyroid disease. Subsequently, Bonferroni correction was employed to identify causal association characteristics (P<1.82×10-4). The results showed that the P-values of the selected metabolites were all between 1.82×10-4 and 0.05, indicating that some metabolites were potentially associated with TC and ATD, but there was no clear causal relationship. Additional research is necessary to validate their relationship.
Because IVW methods are susceptible to weak instrumental bias, sensitivity analyses were conducted to ensure the robustness of the causal assessment. The MR-Egger, weighted mode, simple mode, and weighted median approaches yield consistent causal estimates in terms of both strength and direction. Directed pleiotropy was evaluated using MR-Egger intercept and Mendelian randomized pleiotropy residuals (MR-PRESSO), indicating no significant findings (all P > 0.05). Heterogeneity was assessed through IVW test and Cochran Q statistic in MR-Egger regression. There was also no indication of significant heterogeneity between instrument SNP effects. We further performed Steiger tests to verify the direction of effects from metabolites to TC, GD and HT. The Steiger P-value suggests no reverse causality bias in the identified causality (Table 1).
Causal effects of TC, GD, and HT on human blood metabolites
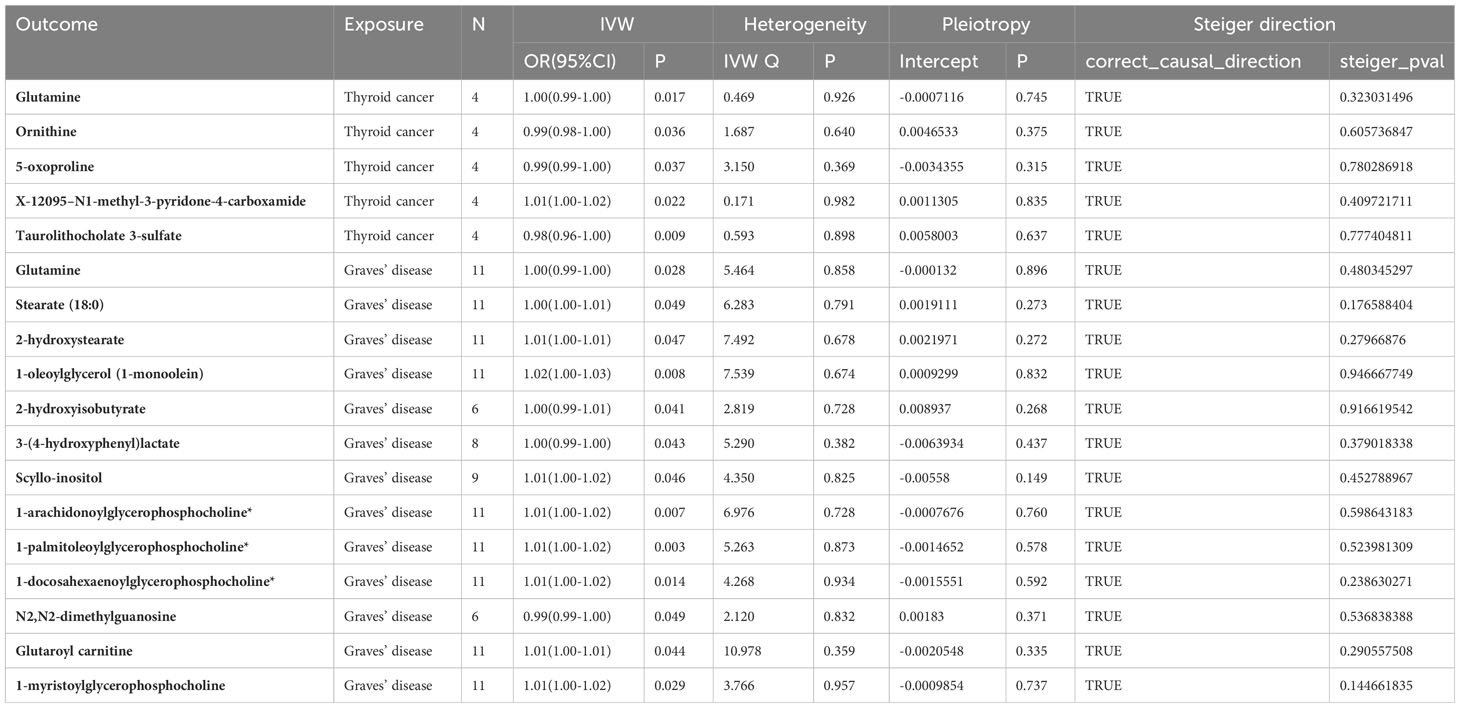
To assess any reverse causal effects, we performed a reverse MR Analysis using TC, GD and HT as exposures and 452 blood metabolites as results. Among them, the number of instrumental variables for each disease ranged from 4 to 11, and all IVs had F-statistics considerably > 10. (Table S4) Results suggested a causal effect of TC on Glutamine (ORIVW=1.00, 95%CI=0.99–1.00, PIVW = 0.016), Ornithine (ORIVW=0.99, 95%CI=0.98–1.00, PIVW = 0.036), 5-oxoproline (ORIVW=0.99, 95%CI = 0.99–1.00, PIVW= 0.037), X-12095–N1-methyl-3-pyridone-4-carboxamide (ORIVW=1.01, 95%CI = 1.00–1.02, PIVW= 0.021), and Taurolithocholate 3-sulfate (ORIVW=0.98, 95%CI = 0.96–1.00, PIVW = 0.009) (Figures S13-S16). GD has potential causal relationship with 13 metabolites such as Glutamine (ORIVW=1.00, 95% CI = 0.99–1.00, PIVW= 0.027) (Table 3; Figures S17-S20). The Q statistic of Cochran showed no heterogeneity (P>0.05). An MR-Egger intercept revealed no horizontal pleiotropy (P>0.05), while Cochran’s Q statistics indicated no heterogeneity (P>0.05). By conducting MR Steiger’s directionality test, SNPs with wrong directionality were excluded, and the results showed that HT had no causal relationship with metabolites. Tables S8-S9 provide the genetic variants responsible for the association between the three thyroid diseases and identified metabolites.
Replication analysis
To further validate our findings, we conducted replication analyses using GWAS data from IEU for TC, GD, and HT. The results demonstrated that the three metabolites, 1-linoleoylglycerol(1-monolinolein), Gamma-tocopherol, and 2-hydroxyisobutyrate, all passed the replication test based on IVW analysis. Subsequent combined analyses of FINNGEN and IEU Open GWAS datasets further confirmed that higher levels of 1-linoleoylglycerol (1-monolinolein) and Gamma-tocopherol were associated with reduced risk of TC and HT in individuals with genetic susceptibility. Additionally, fluctuations in 2-hydroxyisobutyrate levels were observed following the occurrence of GD (Figure 2).
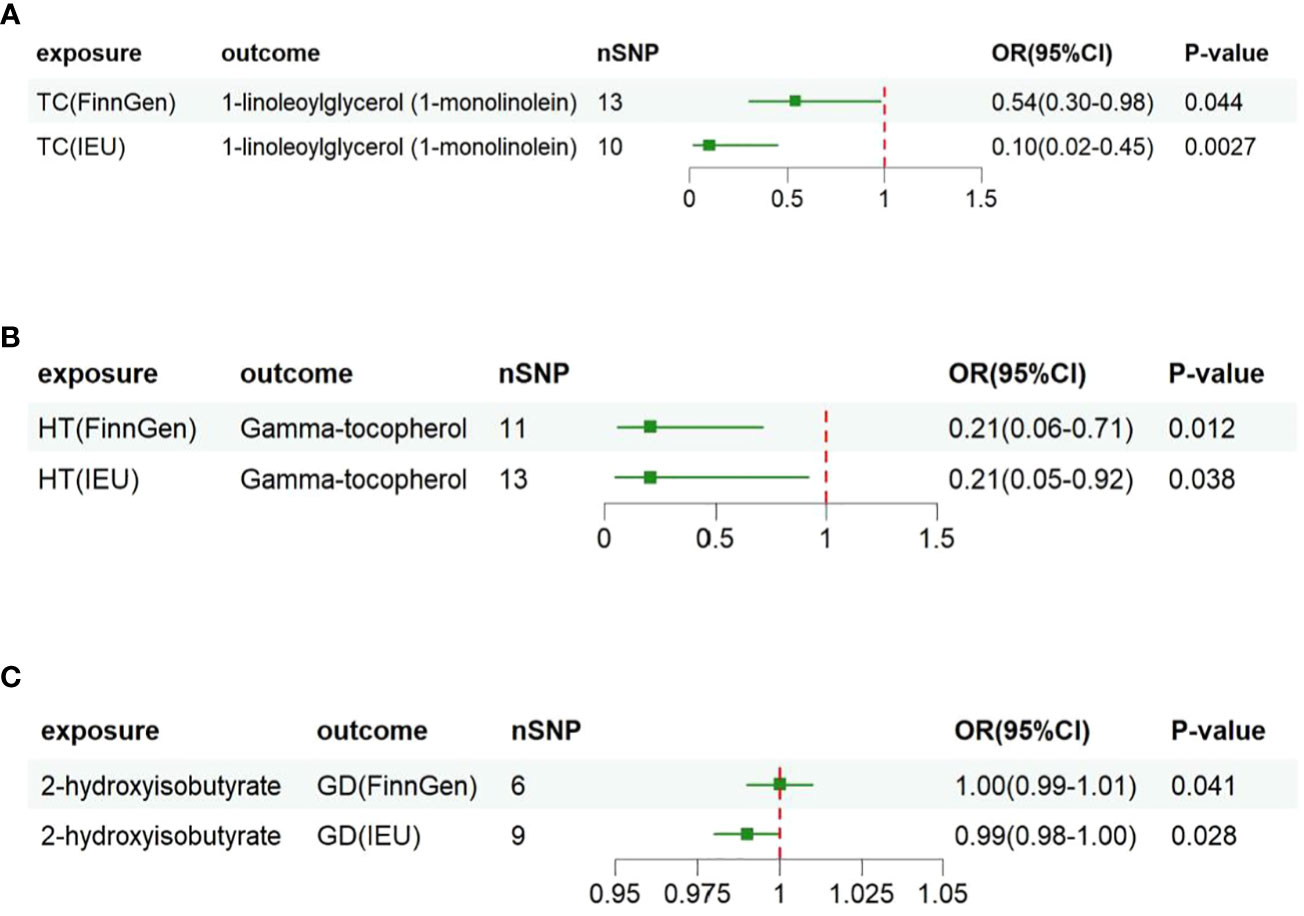
Figure 2 Associations of genetically predicted three metabolites with risk of thyroid disorders using IVW method with different GWAS datasets. OR, odds ratio; CI, confidence. (A) 1-linoleoylglycerol (1-monolinolein) shows potential causal association in two different thyroid cancer datasets (FinnGen Consortium and IEU Open GWAS); (B) Gamma-tocopherol shows potential causal association in two different Hashimoto's thyroiditis datasets; (C) Potential causal effects of 2-hydroxyisobutyrate in two different Graves' disease datasets.
Metabolic pathway analysis
We further carried out the metabolite pathway analysis using all metabolites discovered through the IVW approach (P<0.05). Through forward MR Analysis, we detected six potential metabolic pathways for thyroid cancer and four for Hashimoto’s thyroiditis. (Table S7). The results show that “Aminoacyl-tRNA biosynthesis” (P=0.002), “Phenylalanine, tyrosine and tryptophan biosynthesis” (P=0.007) pathway might be participated in the genesis of TC (Figure 3A). “D-Glutamine and D-glutamate metabolism” (P=0.01) and “Butanoate metabolism” (P=0.03) pathway might be associated with HT (Figure 3B). Among them, there exists a common metabolic pathway (i.e., “Arginine biosynthesis” and “Histidine metabolism”) shared by TC (P=0.02, P=0.03) and HT (P=0.03, P=0.04).
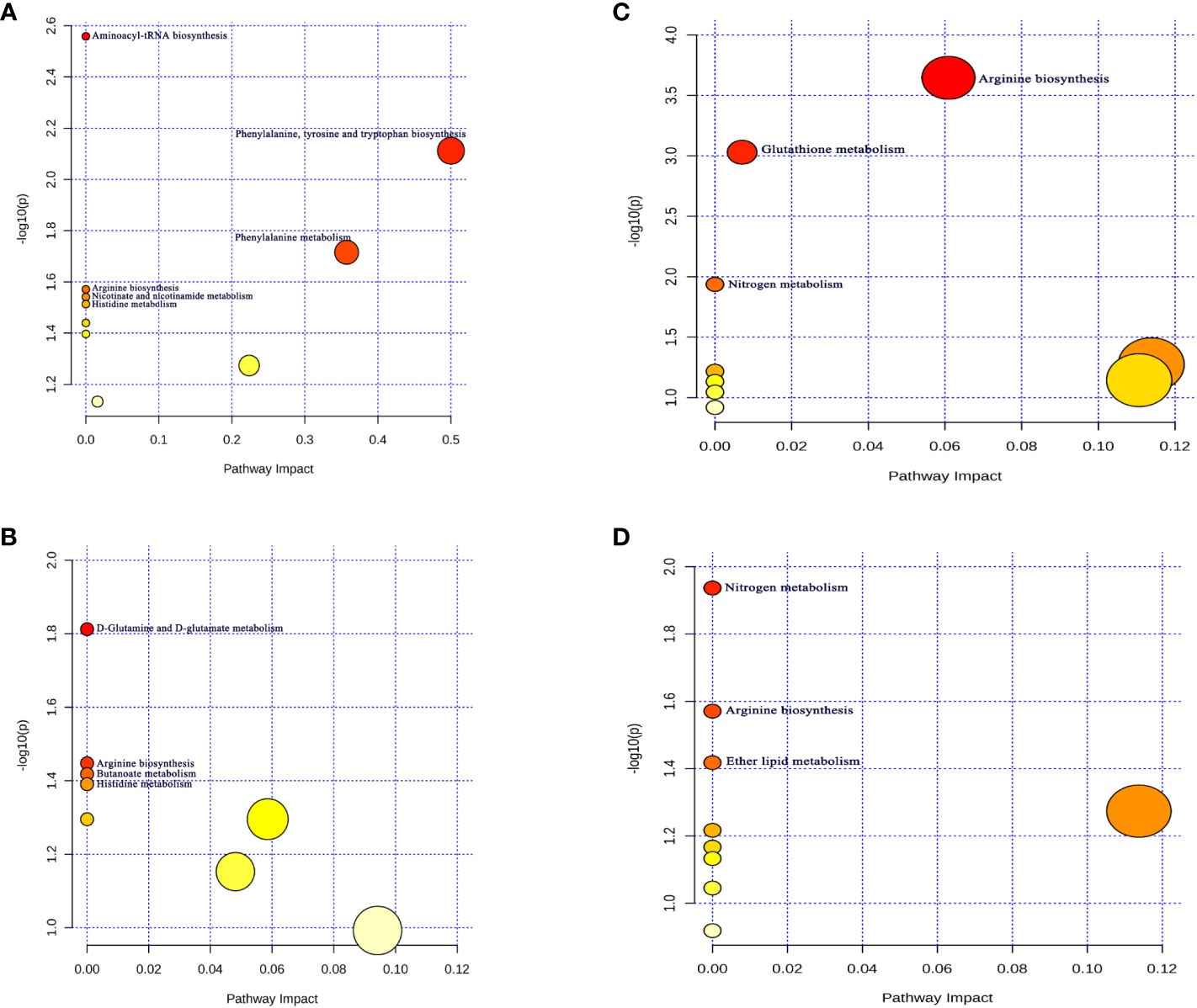
Figure 3 Potential metabolic pathways associated with thyroid disease. (A) Potential metabolic pathways involved in the pathogenesis of thyroid cancer in positive MR Analysis; (B) Hashimoto thyroiditis (The positive MR Analysis); related to Additional File 2 Table S7 (C) Thyroid cancer (The reverse MR Analysis); (D) Graves' disease (The reverse MR Analysis). related to Additional File 2 Table S8 Pathway analysis based on "Kyoto Encyclopedia of Genes and Genomes" (KEGG). The color and size of each circle is based on p-values (yellow: higher p-values and red: lower p-values) and pathway impact values (the larger the circle the higher the impact score) calculated from the topological analysis, respectively. Pathways were considered significantly enriched if p < 0.05, impact >0.1 and number of metabolite hits in the pathway >1.
In reverse MR Analysis, we observed that TC (Figure 3C) and GD (Figure 3D) may increase or decrease the levels of related metabolites in vivo through four potential metabolic pathways. Among them, “arginine biosynthesis”, “D-glutamine and D-glutamate metabolism”, and “nitrogen metabolism” were three shared metabolic pathways present in both TC and GD (P < 0.05) (Table S8).
Discussion
Observational studies have reported associations between metabolites and certain types of thyroid disorders. In this study, we conducted a two-sample bidirectional Mendelian Randomization (MR) analysis using publicly available GWAS summary statistics to investigate the causal relationships between human blood metabolites and various thyroid disorders, including Graves’ disease, Hashimoto’s thyroiditis, and thyroid cancer. Our findings revealed intricate interactions between metabolites and thyroid diseases. To the best of our knowledge, this represents the inaugural MR investigation into establishing causality between blood metabolites and thyroid disorders. In the forward MR Analysis, we found a total of 46 suggestive associations. Thyroid disease is linked to risk factors like Aspartate and protective factors like C-glycosyltryptophan. In the replication analysis, the results revealed that each of the three thyroid disorders had one overlapping metabolite in different datasets. Extensive sensitivity analyses showed that these associations were robust to the pleipotency of the MR Methods and tools used, using MR-PRESSO and leave-one analyses, and showed consistent findings.
Over the past decade, continuous discoveries of metabolic biomarkers related to thyroid disorders have emerged in the field of metabolomics research. Tissues, blood, urine, and feces are typical sample sources for metabolomic profiling. Among these, blood serves as a rich reservoir of readily accessible metabolites, making it valuable for identifying circulating biomarkers in thyroid disease screening. In metabolomic studies pertaining to plasma/serum, alterations in the metabolic profiles of three thyroid disorders have been observed, with the most common metabolic categories being amino acids, fatty acids, and lipids. For instance, in a study by Zhao et al. (31), metabolic differences were compared between thyroid cancer patients and healthy controls. This analysis revealed a significant increase in serum amino acid metabolite levels in thyroid cancer patients, while levels of lipid and choline metabolites were comparatively lower. Our research indicates that Aspartate, Stearoylcarnitine, and Gamma-glutamylglutamine are associated with a higher risk of thyroid cancer. Furthermore, after the onset of TC and GD, there is a decrease in glutamine levels. In TC patients, Taurolithocholate 3-sulfate increases, while GD patients exhibit higher levels of N2,N2-dimethylguanosine. Similar results were reported by Liu et al. (9) in their serum metabolomics pattern analysis of autoimmune thyroid disease patients, revealing differences in amino acids, fatty acids, and lipid-related metabolites among all subjects. Conversely, the occurrence of thyroid disorders also leads to fluctuations in metabolite levels. Patients with thyroid dysfunction exhibit more significant variations in metabolite levels. In our reverse MR analysis, we identified 23 metabolites with suggestive associations, suggesting that the onset of TC and GD alters the composition of metabolites. These findings may have implications for public health interventions aimed at reducing the risk of thyroid disease.
This MR analysis additionally recognized specific metabolites, some of which had been previously reported in other studies. C-glycosyltryptophan is a blood metabolite in the tryptophan pathway, and as a strong protective factor of TC, it has been shown to have a causal association with chronic kidney disease (CKD) in Cheng et al. (32). In our study, phenylalanine increased the risk of TC and kynurenine increased the risk of GD. This echoes prior discoveries. For instance, in line with metabolomics, heightened phenylalanine levels were observed in TC patients, and seven metabolites, including kynurenine, showed marked elevation in GD patients when compared to healthy controls (33). Nonetheless, the sample sizes in these investigations span from dozens to hundreds, which might not adequately represent the broader population. The metabolite and thyroid disease data in this study included a larger sample size, making this study more representative. Carnitine is a quaternary ammonium compound synthesized from phosphatidylcholine, as well as the amino acids lysine and methionine. It has been studied as a therapy or protective agent for many diseases (34). Marie-Josée et al. discovered (35) that carnitine can enhance the in vivo bioavailability of butyrate. When combined, butyrate—a potential anticancer compound—can inhibit proliferation and induce apoptosis in human colon cancer cells. Another study (36) confirmed that carnitine reduced the effect of butyrate as an HDAC inhibitor and restrained the induction of H3 acetylation by butyrate in colorectal cancer cells. Our results confirm that carnitine is a protective factor for TC, further research is needed to explore the potential mechanisms underlying the combined impact of carnitine and butyrate in thyroid cancer. γ-tocopherol has demonstrated a protective effect against HT in two different GWAS databases. Previous analyses have reported unique antioxidant and anti-inflammatory activities associated with γ-tocopherol, suggesting its potential role in preventing thyroid disorders through the regulation of inflammation-related mechanisms (37). Future functional analysis is necessary, substantial experimental endeavors are still required in later stages to establish a more precise assessment of the initial speculation.
In this study, we identified ten significant metabolic pathways associated with TC and HT through pathway enrichment analysis. Among these pathways, “Arginine Biosynthesis” and “Histidine Metabolism” were found to be linked to both TC and HT. Medullary thyroid carcinoma is a malignant tumor originating from the C-cells of the thyroid, which synthesize and secrete calcitonin (38, 39), with arginine serving as a precursor for calcitonin synthesis (40). Patients with this condition typically require lifelong arginine supplementation to maintain normal arginine levels in their bodies. Additionally, thyroid hormone synthesis depends on both arginine and histidine (41), with tyrosine in the histidine metabolism pathway being a precursor for thyroid hormone synthesis (42). In individuals with Hashimoto’s thyroiditis, the production of anti-thyroid peroxidase antibodies (anti-TPO antibodies) and anti-thyroglobulin antibodies can lead to attacks on thyroid tissue, affecting thyroid hormone synthesis and causing inflammation and damage (43). Furthermore, we also identified “Aminoacyl-tRNA Biosynthesis” as the most strongly associated pathway with TC, while “D-Glutamine and D-Glutamate Metabolism” pathway was primarily linked to GD and HT. Aminoacyl-tRNA is synthesized through esterification of tRNA with the appropriate amino acid at its 3’-end. Oxidative stress induces the rapid translocation of TyrRS from the cytoplasm to the nucleus to prevent DNA damage (44). Inhibiting tRNA aminoacylation has proven to be an effective antibacterial strategy, impeding a critical step in protein synthesis. Furthermore, Yu et al. (45) observed microbial changes in TCs that led to alterations in aminoacyl-tRNA biosynthesis. In humans (46), autosomal recessive mutations in glutamate-pyruvate transaminase 2 (GPT2) cause a neurological syndrome characterized by intellectual disability, microcephaly, and progressive motor symptoms. Thyroid dysfunction is a major contributor to muscle weakness. Thyroid hormones (TH) serve as crucial metabolic regulators that coordinate short-term and long-term energy demands (47). Related research has shown that THs regulate glutamine and glucose metabolism through GPT2, coupling glycolysis with the TCA cycle to maintain muscle mass. In summary, these findings suggest that aminoacyl-tRNA biosynthesis and glutamine metabolism may play vital roles in the biological mechanisms of thyroid disorders. Our research findings provide a profound perspective in understanding the relationship between metabolites and thyroid disorders, revealing strong associations between various metabolites and thyroid diseases. These insights pave the way for future investigations to enhance the diagnosis, prevention, and treatment of thyroid disorders.
This study boasts several key strengths. Firstly, it stands as the most comprehensive MR investigation concerning the metabolite-thyroid disease association, boasting the largest sample size to date. Secondly, we meticulously opted for GWAS data from the FINNGEN Consortium as a primary analysis, and corroborated our findings using IEU Open GWAS datasets, markedly enhancing result reliability. Thirdly, both reverse MR Analysis and sensitivity analysis indicated the absence of pleiotropy or heterogeneity, underscoring the statistical robustness of our outcomes.
Nonetheless, certain limitations should be acknowledged in this study. Initially, owing to the constrained count of SNPs achieving genome-wide significance, we opted for a relaxed P threshold—a prevalent practice employed in similar contexts. Furthermore, the veracity of SNPs with relaxed P-values is corroborated by the genuine direction ascertained from the Steiger test. Secondly, while it’s generally advisable to employ sizable GWAS sample sizes for MR Studies, our research employed a relatively modest metabolite GWAS sample size, potentially impacting the robustness of our MR findings. Another limitation pertains to the predominantly European ancestry of our participants. Caution must be exercised when generalizing these findings to other ethnic groups, necessitating further investigation. Fourthly, metabolite levels exhibit variation across diverse cell and tissue types. Our study solely examines the causal link between blood-measured metabolites and thyroid disease, failing to address the relevance of metabolite levels in more biologically pertinent tissues, such as the thyroid. Finally, post Bonferroni correction, we didn’t ascertain a distinct causal connection between metabolites and thyroid disease, implying the requirement for additional research to corroborate this relationship.
Conclusion
In summary, this study establishes a bidirectional causal link between human blood metabolites and thyroid disease. Our forward MR Analysis pinpointed 46 metabolites potentially influencing thyroid disease progression, while reverse MR Analysis identified 23 metabolites possibly influenced by thyroid disease development. Additionally, potential metabolic pathways underlying metabolite-thyroid disease association were identified. Yet, comprehensive clinical investigations are required to unveil the precise metabolite-thyroid disease relationship and unravel the mechanistic underpinnings further.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
ZL: Funding acquisition, Project administration, Resources, Writing – review & editing. XZ: Writing – original draft, Writing – review & editing. JZ: Conceptualization, Investigation, Software, Writing – review & editing. ZX: Data curation, Investigation, Methodology, Writing – review & editing. XL: Data curation, Formal Analysis, Project administration. JH: Writing – review & editing, Data curation, Formal Analysis, Project administration. HH: Writing – review & editing, Project administration, Validation, Visualization.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article. This work was financially supported by the Natural Science Foundation of Hunan Province, China Grant [No. 2020JJ4888].
Acknowledgments
The authors express gratitude to the participants of the included GWAS cohorts and acknowledge the contributions of the FINNGEN consortium, IEU Open GWAS project, and Metabolomics GWAS for generously sharing their GWAS summary statistics.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1270336/full#supplementary-material
Abbreviations
TC, Thyroid cancer; GD, Graves’ disease; HT, Hashimoto thyroiditis; AITD, Autoimmune thyroid diseases; MR, Mendelian randomization; SNPs, Single nucleotide polymorphisms; IVs, Instrumental variables; MR-PRESSO, MR pleiotropy residual sum and outlier; GWAS, Genome-wide association study; IVW, Inverse variance weighted; WM, Weighted median; LOO, Leave-one-out; KEGG, Kyoto encyclopedia of genes and genomes.
References
1. Cabanillas ME, McFadden DG, Durante C. Thyroid cancer. Lancet (2016) 388(10061):2783–95. doi: 10.1016/S0140-6736(16)30172-6
2. Antonelli A, Ferrari SM, Corrado A, Di Domenicantonio A, Fallahi P. Autoimmune thyroid disorders. Autoimmun Rev (2015) 14(2):174–80. doi: 10.1016/j.autrev.2014.10.016
3. Murdaca G, Colombo BM, Puppo F. The role of Th17 lymphocytes in the autoimmune and chronic inflammatory diseases. Intern Emerg Med (2011) 6(6):487–95. doi: 10.1007/s11739-011-0517-7
4. Alkurt EG, Şahin F, Tutan B, Canal K, Turhan VB. The relationship between papillary thyroid cancer and triglyceride/glucose index, which is an indicator of insulin resistance. Eur Rev Med Pharmacol Sci (2022) 26(17):6114–20. doi: 10.26355/eurrev_202209_29629
5. Nagayama Y, Hamada K. Reprogramming of cellular metabolism and its therapeutic applications in thyroid cancer. Metabolites (2022) 12(12):1214. doi: 10.3390/metabo12121214
6. Farrokhi YR, Rezaie Tavirani M, Arefi Oskouie A, Mohajeri-Tehrani MR, Soroush AR. The metabolomics and lipidomics window into thyroid cancer research. Biomarkers (2017) 22(7):595–603. doi: 10.1080/1354750X.2016.1256429
7. Murdaca G, Tonacci A, Negrini S, Greco M, Borro M, Puppo F, et al. Emerging role of vitamin D in autoimmune diseases: An update on evidence and therapeutic implications. Autoimmun Rev (2019) 18(9):102350. doi: 10.1016/j.autrev.2019.102350
8. Al-Majdoub M, Lantz and P. Spegel M. Treatment of Swedish patients with graves' Hyperthyroidism is associated with changes in acylcarnitine levels. Thyroid (2017) 27(9):1109–17. doi: 10.1089/thy.2017.0218
9. Liu J, Fu J, Jia Y, Yang N, Li J, Wang G. SERUM METABOLOMIC PATTERNS IN PATIENTS WITH AUTOIMMUNE THYROID DISEASE. Endocr Pract (2020) 26(1):82–96. doi: 10.4158/EP-2019-0162
10. Cayres LCF, de Salis LVV, Rodrigues GSP, Lengert AVH, Biondi APC, Sargentini LDB, et al. Detection of alterations in the gut microbiota and intestinal permeability in patients with hashimoto thyroiditis. Front Immunol (2021) 12:579140. doi: 10.3389/fimmu.2021.579140
11. Jiang W, Lu G, Gao D, Lv Z, Li D. The relationships between the gut microbiota and its metabolites with thyroid diseases. Front Endocrinol (Lausanne) (2022) 13:943408. doi: 10.3389/fendo.2022.943408
12. Krumsiek J, Suhre K, Evans AM, Mitchell MW, Mohney RP, Milburn MV, et al. Mining the unknown: a systems approach to metabolite identification combining genetic and metabolic information. PloS Genet (2012) 8(10):e1003005. doi: 10.1371/journal.pgen.1003005
13. Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife (2018) 7:e34408. doi: 10.7554/eLife.34408
14. Zeng H, Ge J, Xu W, Ma H, Chen L, Xia M, et al. Type 2 diabetes is causally associated with reduced serum osteocalcin: A genomewide association and mendelian randomization study. J Bone Miner Res (2021) 36(9):1694–707. doi: 10.1002/jbmr.4330
15. Wang J, Liu D, Tian E, Guo ZQ, Chen JY, Kong WJ, et al. Is hearing impairment causally associated with falls? Evidence from a two-sample mendelian randomization study. Front Neurol (2022) 13:876165. doi: 10.3389/fneur.2022.876165
16. Johnson CH, Ivanisevic J, Benton HP, Siuzdak G. Bioinformatics: the next frontier of metabolomics. Anal Chem (2015) 87(1):147–56. doi: 10.1021/ac5040693
17. Shin SY, Fauman EB, Petersen AK, Krumsiek J, Santos R, Huang J, et al. An atlas of genetic influences on human blood metabolites. Nat Genet (2014) 46(6):543–50. doi: 10.1038/ng.2982
18. Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res (2000) 28(1):27–30. doi: 10.1093/nar/28.1.27
19. Cao J, Wang N, Luo Y, Ma C, Chen Z, Chenzhao C, et al. A cause-effect relationship between Graves' disease and the gut microbiome contributes to the thyroid-gut axis: A bidirectional two-sample Mendelian randomization study. Front Immunol (2023) 14:977587. doi: 10.3389/fimmu.2023.977587
20. Howard DM, Adams MJ, Shirali M, Clarke TK, Marioni RE, Davies G, et al. Addendum: Genome-wide association study of depression phenotypes in UK Biobank identifies variants in excitatory synaptic pathways. Nat Commun (2018) 9(1):3578. doi: 10.1038/s41467-018-05310-5
21. Pierce BL, Ahsan H, Vanderweele TJ. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int J Epidemiol (2011) 40(3):740–52. doi: 10.1093/ije/dyq151
22. Holsinger KE, Weir BS. Genetics in geographically structured populations: defining, estimating and interpreting F(ST). Nat Rev Genet (2009) 10(9):639–50. doi: 10.1038/nrg2611
23. Pierce BL, Burgess S. Efficient design for Mendelian randomization studies: subsample and 2-sample instrumental variable estimators. Am J Epidemiol (2013) 178(7):1177–84. doi: 10.1093/aje/kwt084
24. Sedgwick P. Multiple hypothesis testing and Bonferroni's correction. BMJ (2014) 349:g6284. doi: 10.1136/bmj.g6284
25. Hemani G, Bowden J, Davey SG. Evaluating the potential role of pleiotropy in Mendelian randomization studies. Hum Mol Genet (2018) 27(R2):R195–208. doi: 10.1093/hmg/ddy163
26. Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol (2017) 32(5):377–89. doi: 10.1007/s10654-017-0255-x
27. Cohen JF, Chalumeau M, Cohen R, Korevaar DA, Khoshnood B, Bossuyt PM. Cochran's Q test was useful to assess heterogeneity in likelihood ratios in studies of diagnostic accuracy. J Clin Epidemiol (2015) 68(3):299–306. doi: 10.1016/j.jclinepi.2014.09.005
28. Hemani G, Tilling K, Davey SG. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PloS Genet (2017) 13(11):e1007081. doi: 10.1371/journal.pgen.1007081
29. Ishigaki K, Akiyama M, Kanai M, Takahashi A, Kawakami E, Sugishita H, et al. Large-scale genome-wide association study in a Japanese population identifies novel susceptibility loci across different diseases. Nat Genet (2020) 52(7):669–79. doi: 10.1038/s41588-020-0640-3
30. Pang Z, Chong J, Zhou G, de Lima Morais DA, Chang L, Barrette M, et al. MetaboAnalyst 5.0: narrowing the gap between raw spectra and functional insights. Nucleic Acids Res (2021) 49(W1):W388–96. doi: 10.1093/nar/gkab382
31. Zhao WX, Wang B, Zhang LY, Yan SY, Yang YH. Analysis on the metabolite composition of serum samples from patients with papillary thyroid carcinoma using nuclear magnetic resonance. Int J Clin Exp Med (2015) 8(10):18013–22.
32. Cheng Y, Li Y, Benkowitz P, Lamina C, Köttgen A, Sekula P. The relationship between blood metabolites of the tryptophan pathway and kidney function: a bidirectional Mendelian randomization analysis. Sci Rep (2020) 10(1):12675. doi: 10.1038/s41598-020-69559-x
33. Du Y, Fan P, Zou L, Jiang Y, Gu X, Yu J, et al. Serum metabolomics study of papillary thyroid carcinoma based on HPLC-Q-TOF-MS/MS. Front Cell Dev Biol (2021) 9:593510. doi: 10.3389/fcell.2021.593510
34. Ueland HO, Ulvik A, Løvås K, Wolff ASB, Breivik LE, Stokland AM, et al. Systemic activation of the kynurenine pathway in graves disease with and without ophthalmopathy. J Clin Endocrinol Metab (2023) 108(6):1290–7. doi: 10.1210/clinem/dgad004
35. Roy MJ, Dionne S, Marx G, Qureshi I, Sarma D, Levy E, et al. In vitro studies on the inhibition of colon cancer by butyrate and carnitine. Nutrition (2009) 25(11-12):1193–201. doi: 10.1016/j.nut.2009.04.008
36. Han A, Bennett N, MacDonald A, Johnstone M, Whelan J, Donohoe DR. Cellular metabolism and dose reveal carnitine-dependent and -independent mechanisms of butyrate oxidation in colorectal cancer cells. J Cell Physiol (2016) 231(8):1804–13. doi: 10.1002/jcp.25287
37. Jiang Q, Im S, Wagner JG, Hernandez ML, Peden DB. Gamma-tocopherol, a major form of vitamin E in diets: Insights into antioxidant and anti-inflammatory effects, mechanisms, and roles in disease management. Free Radic Biol Med (2022) 178:347–59. doi: 10.1016/j.freeradbiomed.2021.12.012
38. Matrone A, Gambale C, Prete A, Elisei R. Sporadic medullary thyroid carcinoma: towards a precision medicine. Front Endocrinol (Lausanne) (2022) 13:864253. doi: 10.3389/fendo.2022.864253
39. Wong A, Nabata K, Wiseman SM. Medullary thyroid carcinoma: a narrative historical review. Expert Rev Anticancer Ther (2022) 22(8):823–34. doi: 10.1080/14737140.2022.2089118
40. Hou Y, Zeng H, Li Z, Feng N, Meng F, Xu Y, et al. Structural mechanisms of calmodulin activation of Shigella effector OspC3 to ADP-riboxanate caspase-4/11 and block pyroptosis. Nat Struct Mol Biol (2023) 30(3):261–72. doi: 10.1038/s41594-022-00888-3
41. Marti ILA, Reith W. Arginine-dependent immune responses. Cell Mol Life Sci (2021) 78(13):5303–24. doi: 10.1007/s00018-021-03828-4
42. Rivlin R, Asper SP. Tyrosine and the thyroid hormones. Am J Med (1966) 40(6):823–7. doi: 10.1016/0002-9343(66)90198-7
43. Umar H, Muallima N, Adam JM, Sanusi H. Hashimoto's thyroiditis following Graves' disease. Acta Med Indones (2010) 42(1):31–5.
44. Wei N, Shi Y, Truong LN, Fisch KM, Xu T, Gardiner E, et al. Oxidative stress diverts tRNA synthetase to nucleus for protection against DNA damage. Mol Cell (2014) 56(2):323–32. doi: 10.1016/j.molcel.2014.09.006
45. Ho JM, Bakkalbasi E, Söll D, Miller CA. Drugging tRNA aminoacylation. RNA Biol (2018) 15(4-5):667–77. doi: 10.1080/15476286.2018.1429879
46. Yu X, Jiang W, Kosik RO, Song Y, Luo Q, Qiao T, et al. Gut microbiota changes and its potential relations with thyroid carcinoma. J Adv Res (2022) 35:61–70. doi: 10.1016/j.jare.2021.04.001
Keywords: metabolites, Mendelian randomization, bidirectional, thyroid cancer, autoimmune thyroid disease
Citation: Zhang X, Zhou J, Xie Z, Li X, Hu J, He H and Li Z (2023) Exploring blood metabolites and thyroid disorders: a bidirectional mendelian randomization study. Front. Endocrinol. 14:1270336. doi: 10.3389/fendo.2023.1270336
Received: 31 July 2023; Accepted: 26 September 2023;
Published: 09 October 2023.
Edited by:
Jadwiga Maria Furmaniak, Independent Researcher, Cardiff, United KingdomReviewed by:
Marcos Edgar Herkenhoff, University of São Paulo, BrazilGiuseppe Murdaca, University of Genoa, Italy
Copyright © 2023 Zhang, Zhou, Xie, Li, Hu, He and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhi Li, ODAxOTU4QGNzdS5lZHUuY24=
 Xuan Zhang1,2,3,4,5
Xuan Zhang1,2,3,4,5 Jiating Zhou
Jiating Zhou Xi Li
Xi Li Zhi Li
Zhi Li