- China-Japan Union Hospital of Jilin University, Jilin University, Jilin, China
Purpose: Thyroid hormones sensitivity is a newly proposed clinical entity closely related with metabolic health. Prior studies have reported the cross-sectional relationship between thyroid hormones sensitivity and diabetes; however, the longitudinal association is unclear to date. We aimed to explore the relationship between impaired thyroid hormone sensitivity at baseline and diabetes onset using a cohort design.
Methods: This study enrolled 7283 euthyroid participants at the first visit between 2008 and 2009, and then annually followed until diabetes onset or 2019. Thyrotropin (TSH), free triiodothyronine (FT3) and free thyroxine (FT4) were measured to calculate thyroid hormone sensitivity by thyroid feedback quantile-based index (TFQI), Chinese-referenced parametric thyroid feedback quantile-based index (PTFQI), thyrotropin index (TSHI), thyrotroph thyroxine resistance index (TT4RI) and FT3/FT4 ratio. Cox proportional hazard model and cross-lagged panel analysis were used.
Results: The mean baseline age was 44.2 ± 11.9 years, including 4170 (57.3%) male. During a median follow-up of 5.2 years, 359 cases developed diabetes. There was no significant association between thyroid hormones sensitivity indices and diabetes onset, and adjusted hazard ratios per unit (95% CIs) were 0.89 (0.65-1.23) for TFQI, 0.91 (0.57-1.45) for PTFQI, 0.95 (0.70-1.29) for TSHI, 0.98 (0.70-1.01) for TT4RI and 2.12 (0.17-5.78) for FT3/FT4 ratio. Cross-lagged analysis supported the temporal association from fasting glucose to impaired thyroid hormones sensitivity indices.
Conclusions: Our findings could not demonstrate that thyroid hormones sensitivity status is a predictor of diabetes onset in the euthyroid population. Elevated fasting glucose (above 7.0 mmol/L) appeared to precede impaired sensitivity indices of thyroid hormones.
Introduction
By 2021 estimates, diabetes affects 536.6 million people globally, and the prevalence was estimated to rise from 10.5% to 12.2% (783.2 million) in 2045 (1). Approximately, 90-95% of diabetes are type 2 diabetes (2). The global cost of type 2 diabetes and its consequences are large and substantially increasing (3). Thyroid dysfunction and diabetes are closely linked, as the central and peripheral control of thyroid hormones has an impact on glucose homeostasis (4); and insulin sensitivity could modulate the feedback of thyroid hormones in turn (5). Prevalence of thyroid disorders in patients with diabetes is high and vice versa (6). Both hyperthyroidism and hypothyroidism are associated with the development of diabetes (7). From the population studies, evidence suggests that variations of thyroid function even within normal range could be associated with risk of diabetes under complex pathophysiologic mechanisms (8).
Thyroid hormones and thyrotropin (TSH) are inversely correlated under the negative feedback loop of hypothalamic-pituitary-thyroid axis (9). Normal thyroid hormones metabolism and action require adequate cellular receptors (10). The co-occurrence of high thyroid hormones and high TSH represents an acquired resistance to thyroid hormones in the general population (11). Thyroid hormones sensitivity is supposed to tract the metabolic health even in euthyroid population (12, 13). Previous studies have reported the cross-sectional association between thyroid hormones sensitivity and diabetes or prediabetes (12, 14, 15). However, the longitudinal association of thyroid hormones sensitivity with diabetes onset remains unknown to date. Considering the co-existence between thyroid dysfunction and diabetes, the bidirectional relationship needs further interpretation.
Therefore, this study aimed to explore the longitudinal association between thyroid hormones sensitivity indices and incident diabetes using a large cohort.
Method
Study population and design
Xiaotangshan Health Examination Cohort is a large-scale dynamic study investigating the risk factors and biomarkers of cardiovascular and metabolic diseases, which was initiated from 2008 at Beijing, China. The recruited participants were required to undertake annual health examinations, face-to-face questionnaire survey and blood sample collection. All plasma samples were tested in a fixed laboratory of Beijing Xiaotangshan Examination Hospital. This current study was a secondary analysis using data from Xiaotangshan Health Examination Cohort. This study included participants between 2008 and 2009 as baseline, and then followed until incident diabetes through repeated examination or censored at the last visit until 2019 following a previous study design (16). Those with pregnant women (n=20) or thyroid dysfunctions (n=1047) were excluded. Finally, a total of 7283 individuals of normal thyroid function were included in the final analysis.
The study was conducted according to the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Xiaotangshan Health Examination Center. All the participants gave an informed consent before participating.
Data collection and definition
The data of demographic characteristics, lifestyle, diseases histories and medication use were collected via a standard questionnaire by our well-trained staff. Educational levels were grouped into illiteracy or primary school (primary), middle school or high school (secondary) and bachelor’s degree or above (third). Active physical activity was defined as having moderate or intense activity at work or during leisure time more than 4 times and 80 minutes weekly. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared.
Systolic blood pressure and diastolic blood pressure were presented as the average of two measurements on the right arm using a sphygmomanometer after resting for at least 10 min. Hypertension status was defined as systolic pressure ≥140 mmHg or diastolic pressure ≥90 mmHg, self-reported diagnosis history of hypertension or use of antihypertensive medication (17). Dyslipidemia was defined as triglyceride ≥2.3 mmol/l, total cholesterol ≥6.2 mmol/l, low-density lipoprotein cholesterol ≥4.1 mmol/l, high-density lipoprotein cholesterol <1.0 mmol/l, self-reported diagnosis history of dyslipidemia or use of lipid-lowering medication (18). The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI 2009) serum creatinine equation (19). Concentration of fasting glucose were tested before breakfast after overnight fasting (no food, except drinking water, for at least 8 hours) by automatic biochemical analyzer Roche Cobas c 701 and SYSMEX HLC-723G8. The onset of diabetes was defined as a composite of fasting glucose ≥7.0 mmol/L or using any glucose-lowering medication or self-reported diagnosis history of diabetes (20).
Serum free triiodothyronine (FT3), free thyroxine (FT4) and thyrotropin (TSH) were measured on the day of sampling using electrochemiluminescence immunoassay (ECLIA) by the auto-analyzer Mindray CL-2000i. Samples were analyzed only when quality control met the acceptable criteria. The inter-assay and intra-assay coefficients of variation (CVs) were all <5.0% for FT3, FT4 and TSH according to the commercial kit instruction (Mindray, Shenzhen, China). The assay-specific reference ranges for FT3, FT4 and TSH were 2.76 to 6.45 pmol/L (to convert FT3 to ng/dL, divided by 15.361), 11.20 to 23.81 pmol/L (to convert FT4 to ng/dL, divided by 12.871) and 0.35 to 5.00 mIU/L. Normal thyroid function is defined as serum FT3, FT4 and TSH levels within the normal range and without use of thyroid hormone supplement.
Definition of thyroid hormone sensitivity
We calculated thyroid feedback quantile-based index (TFQI) (12) as the cumulative distribution function (cdf) of FT4 and TSH representing the difference between FT4 quantile and the reversed TSH quantile as follows: TFQI = cdf FT4 - (1 - cdf TSH). Parametric TFQI (PTFQI) (21) uses the parameter standard cumulative distribution to improve the applicability to specific population as follows: PTFQI = Φ((FT4 − μFT4)/σFT4) − (1 − Φ((ln TSH − μln TSH)/σln TSH)), where μFT4 = 16.161, σFT4 = 2.131, μln TSH = 0.688, and σln TSH = 0.457 for this current Chinese population. Thyrotropin index (TSHI) (22) was calculated as ln TSH + 0.1345×FT4, and thyrotroph thyroxine resistance index (TT4RI) (23) was calculated as FT4×TSH. Values of TFQI and PTFQI ranges from -1 to 1, with negative and positive values representing good and impaired sensitivity to FT4. For THSI and TT4RI, higher values indicate poor thyroid hormones sensitivity. FT3/FT4 ratio, an index of conversion of thyroid hormones, was calculated as FT3 divided by FT4, and thus lower values indicate less thyroid hormones effect for the same rate of synthesis (24).
Statistical analysis
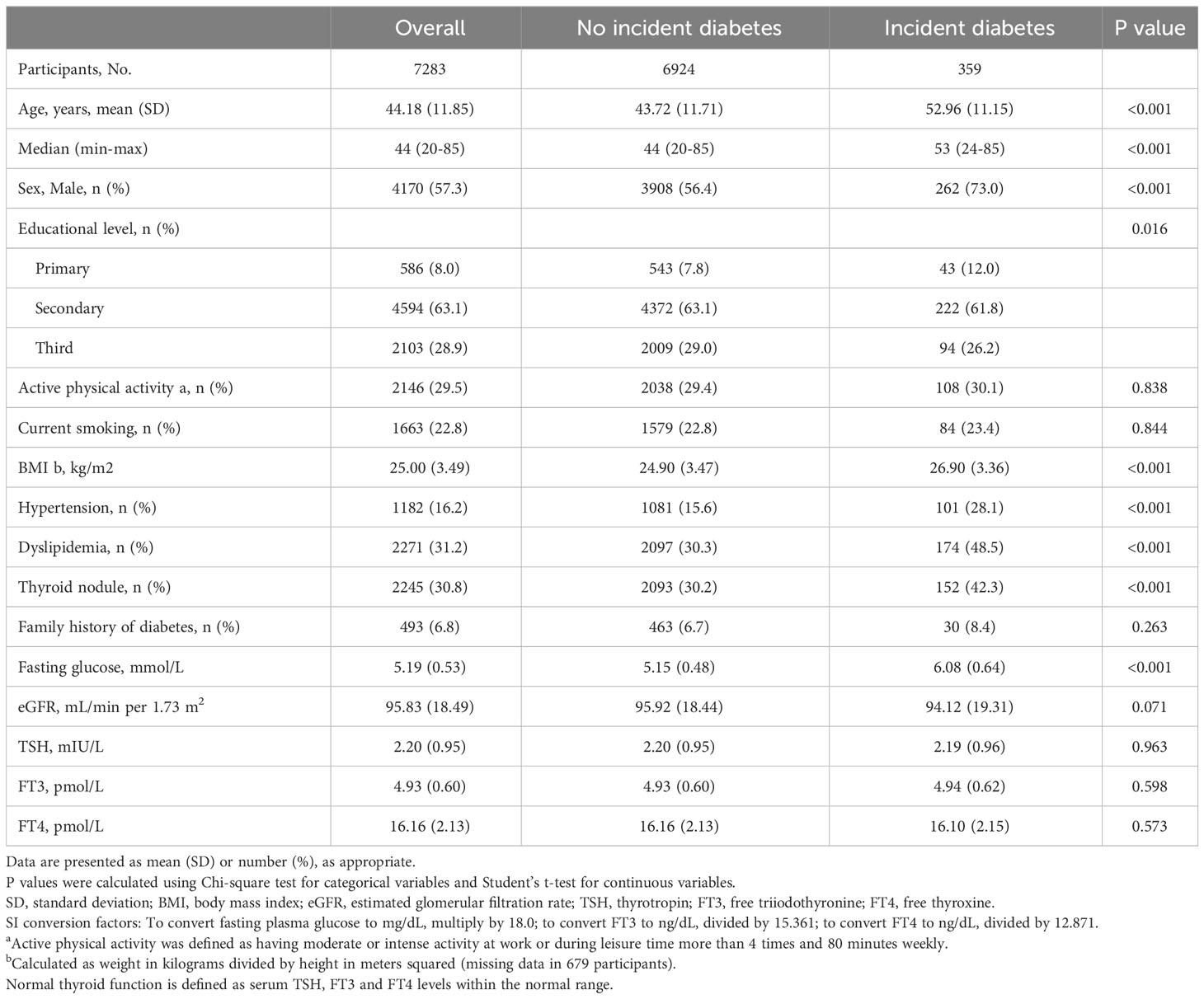
Baseline characteristics were described using mean (standard deviation, SD) and frequency (proportion), and the differences were compared by Student’s t-test and Chi-square test according to incident diabetes or not. Distribution of thyroid hormones sensitivity indices were presented using violin plot.
We used Kaplan-Meier curves and Cox proportional hazard model to explore the longitudinal association of baseline thyroid hormones sensitivity indices and incident diabetes. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated. Meanwhile, logistics model was used to calculate odds ratios (ORs) for investigating the cross-sectional association between thyroid hormones sensitivity indices and prevalent diabetes. The data of diabetic patients were extracted at the visit time of diagnosis and the data of non-diabetic participants were from the last visit during the follow-up for the cross-sectional analysis. Model 1 was primarily adjusted for age and sex; model 2 was further adjusted for education level, BMI, active physical activity, smoking, hypertension, dyslipidemia, eGFR and fasting glucose. Statistical power of the regression analysis was calculated.
We used the cross-lagged panel to calculate the standard regression coefficients and 95% CIs of baseline thyroid hormones sensitivity indices on subsequent fasting glucose (β1), and the effect of baseline glucose parameters on subsequent thyroid hormones sensitivity indices (β2) after adjusting the auto-regressive effect and covariance. The statistical difference between β1 and β2 was examined using t test. This panel analysis was performed in R package ‘lavaan’. Given the unequal time of follow-up across participants, we performed the analysis adjusting for the follow-up time in model 1. Age, sex and BMI were additionally adjusted in model 2. As a sensitivity analysis, we also included the subgroup (n=1047) of thyroid dysfunctions at baseline and repeated the whole analysis.
The statistical power was calculated using PASS software (version 15). All statistical analyses were performed using R software (version 4.1.0), and a two-sided P value < 0.05 was considered statistically significant.
Results
Characteristics
Of 7283 individuals, the mean age was 44.2 (11.9) years, including 4170 (57.3%) males. During a median follow-up of 5.2 years, 359 (4.9%) cases developed diabetes. The detailed baseline characteristics are shown in Table 1. We calculated the thyroid hormones sensitivity indices both at baseline and follow-up. Table S1 shows the distribution of baseline thyroid hormones sensitivity indices stratified by age groups. Of note, there was no significant difference of baseline thyroid hormones sensitivity indices among people with and without incident diabetes during follow-up (Figure S1). However, the thyroid hormones sensitivity indices at follow-up were cross-sectionally correlated with prevalent diabetes (Figure S2). Table S2 shows the baseline characteristics after including 1047 participants with thyroid dysfunctions.
Cross-sectional and longitudinal association of thyroid hormones sensitivity indices with diabetes onset
Figure 1 presents the Kaplan-Meier curves of diabetes onset according to the quartile groups of baseline thyroid hormones sensitivity indices. There were no significant longitudinal associations between five thyroid hormones sensitivity indices and incident diabetes, and HRs (95% CIs) of per-unit were 0.89 (0.65-1.23) for TFQI, 0.91 (0.57-1.45) for PTFQI, 0.95 (0.70-1.29) for TSHI, 0.98 (0.70-1.01) for TT4RI and 2.12 (0.17-5.78) for FT3/FT4 ratio in the fully adjusted model (Table 2). Age, BMI, eGFR and fasting glucose were positively associated with diabetes onset, while people with higher education level had lower risk of incident diabetes (Table S3). No statistical significance was demonstrated in any strata in the subgroup analysis of age and sex (Figure S3). Following a previous study (12), it was assumed that people on the highest quartile of TFQI had 73.0% higher risk of diabetes. Given a 5.0% incidence rate of diabetes in our population, the current sample size of 7283 could achieve a statistical power of 0.992. Of note, there were significant cross-sectional associations between thyroid hormones sensitivity indices with prevalent diabetes at the end of follow-up as shown in Table S4. Results were consistent when repeating the analysis after including 1047 participants with thyroid dysfunctions at baseline Table S5.
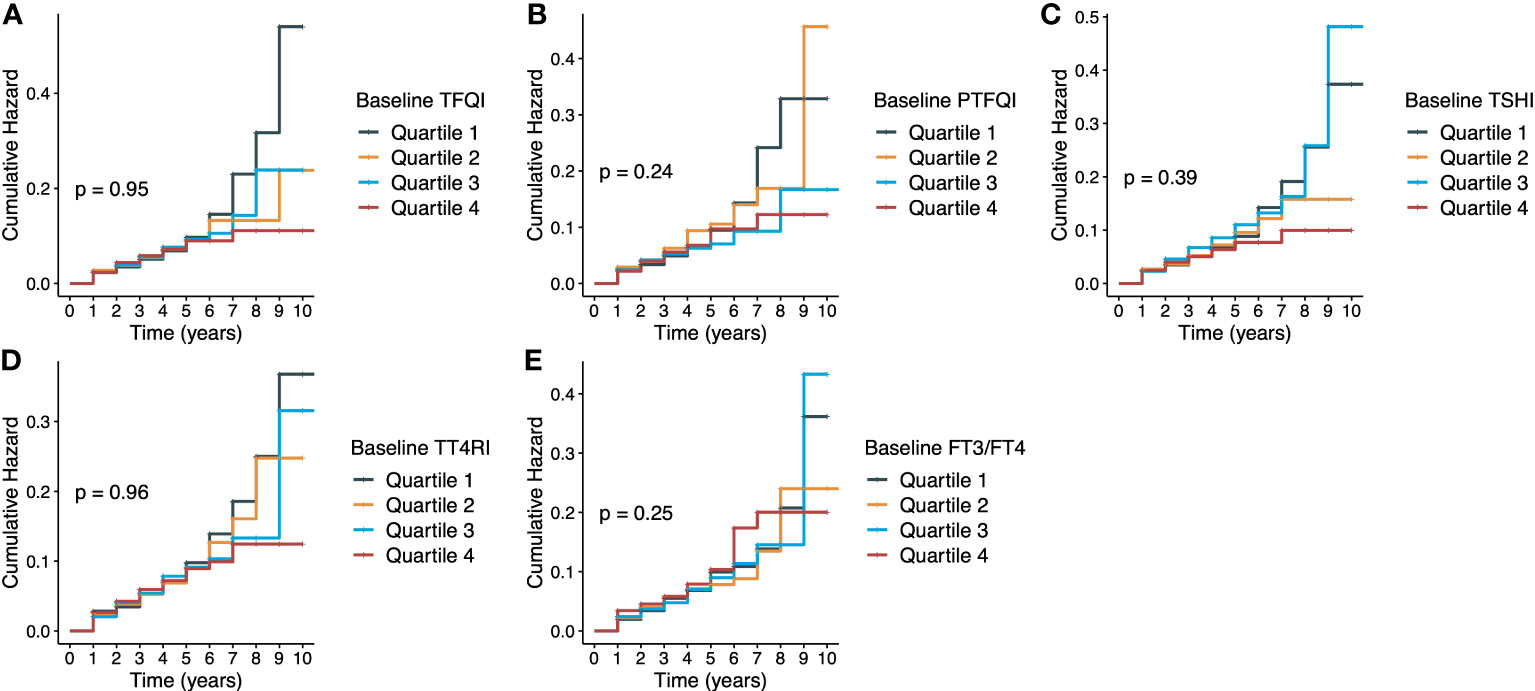
Figure 1 Kaplan-Meier curves of diabetes according to thyroid hormones sensitivity indices. (A) TFQI and diabetes; (B) PTFQI and diabetes; (C) TSHI and diabetes; (D) TT4RI and diabetes; (E) FT3/FT4 ratio and diabetes. TFQI, thyroid feedback quantile-based index; PTFQI, Chinese-referenced parametric thyroid feedback quantile-based index; TSHI, thyrotropin index; TT4RI, thyrotroph thyroxine resistance index; FT3, free triiodothyronine; FT4, free thyroxine.
Cross-lagged panel analyses
Figure 2 presents the cross-lagged panel analysis between thyroid hormones sensitivity indices and fasting glucose. The adjusted standardized correlation coefficients (95% CI) of baseline thyroid hormones sensitivity indices to follow-up fasting glucose (β1) was 0.012 (-0.007, 0.031) for TFQI, 0.016 (-0.003, 0.036) for PTFQI, 0.011 (-0.007, 0.032) for TSHI, 0.010 (-0.005, 0.029) for TT4RI and -0.013 (-0.027, 0.008) for FT3/FT4 ratio. The effects of baseline fasting glucose to subsequent thyroid hormones sensitivity indices (β2) were 0.032 (0.012-0.057) for TFQI, 0.034 (0.011, 0.056) for PTFQI, 0.030 (0.004, 0.050) for TSHI, 0.017 (0.011, 0.023) for TT4RI and -0.023 (-0.035, -0.011) for FT3/FT4 ratio (Table 3). After excluding those using anti-diabetic medication at the follow-up survey, the results remained almost consistent except that the adjusted standardized correlation coefficients between TT4RI and fasting glucose became insignificant, although the unadjusted coefficient from fasting glucose to TT4RI was still significant (Table S6).
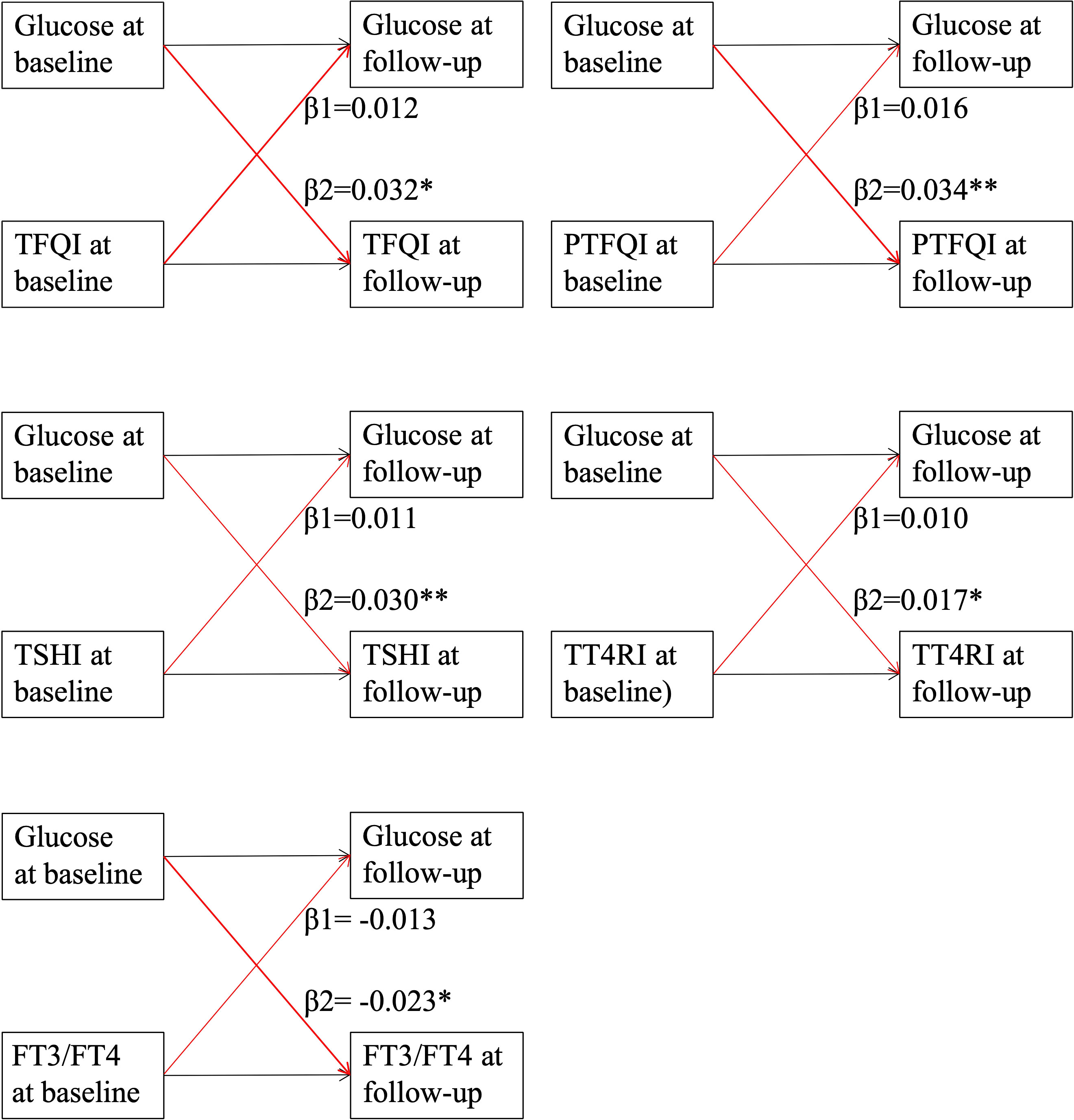
Figure 2 Cross-lagged panel analysis of thyroid hormones sensitivity indices with fasting glucose. Continuous levels of thyroid hormones sensitivity indices and fasting glucose at two time points (baseline and last follow-up) were used in the cross-lagged panel. TFQI, thyroid feedback quantile-based index; PTFQI, Chinese-referenced parametric thyroid feedback quantile-based index; TSHI, thyrotropin index; TT4RI, thyrotroph thyroxine resistance index; FT3, free triiodothyronine; FT4, free thyroxine. β1: thyroid hormones sensitivity index at baseline→ fasting glucose at follow-up; β2: fasting glucose at baseline→thyroid hormones sensitivity index at follow-up. ** indicates P value <0.01; * indicates P value <0.05.
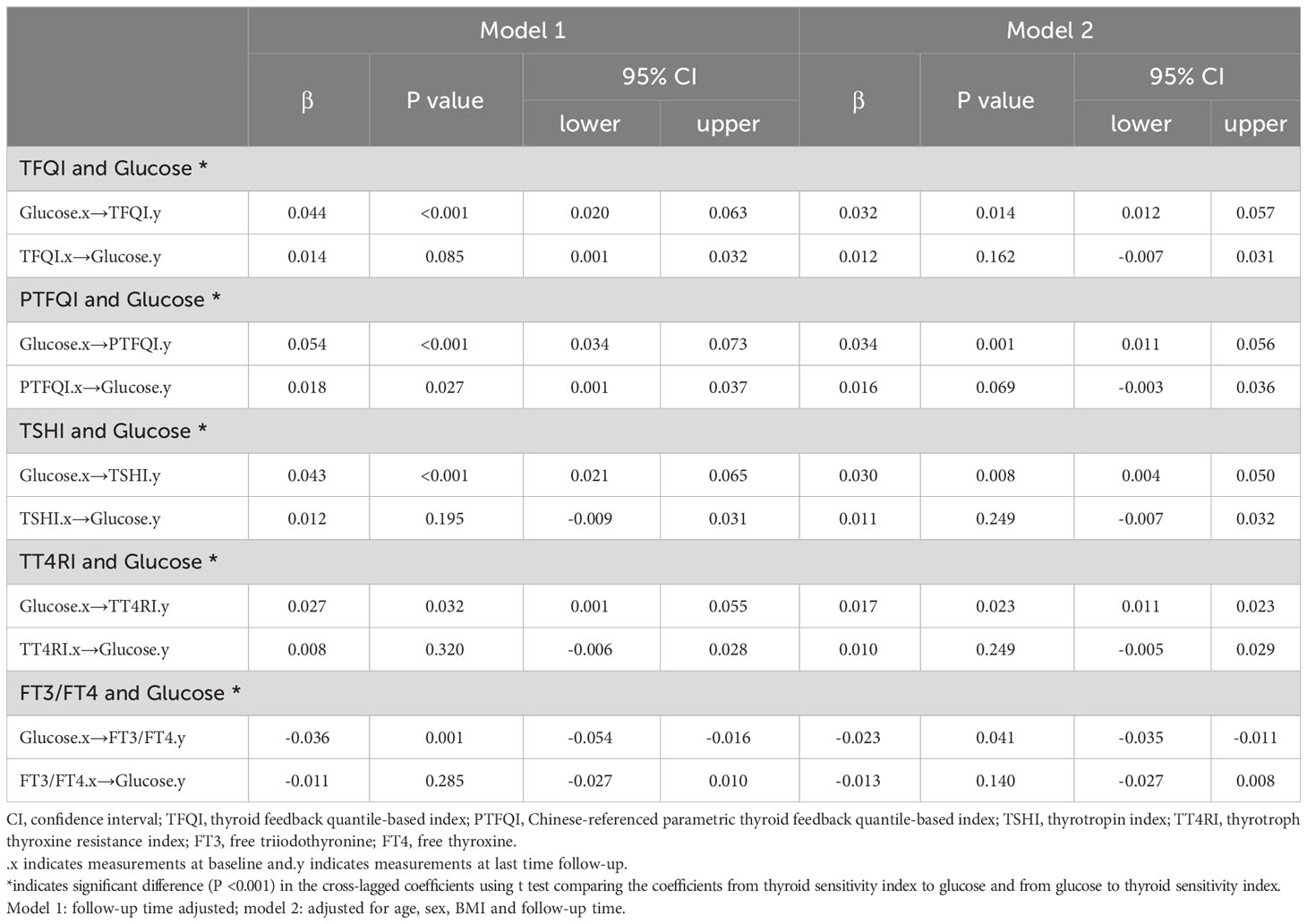
Table 3 Cross-lagged standard regression coefficient of thyroid hormones sensitivity indices with fasting glucose.
Discussion
In this study, we explored the longitudinal relationship between thyroid hormones sensitivity indices and diabetes onset using a large cohort. Our findings could not demonstrate that thyroid hormones sensitivity status is a predictor of diabetes onset. The cross-lagged panel analysis indicated that elevated fasting glucose level (above 7.0 mmol/L) precedes impaired sensitivity indices of thyroid hormones. The results remained consistent among multiple subgroups and sensitivity analyses.
The cross-sectional association between reduced sensitivity to thyroid hormones and metabolic abnormalities have been reported in previous studies. Laclaustra et al. proposed the TFQI index to indicate the resistance to thyroid hormones and found that TFQI index was associated with prevalent diabetes and metabolic syndrome (12). Mehran et al. reported that TFQI and PTFQI were related with high blood pressure and known diabetes among 5124 euthyroid subjects (14). Liu et al. found that decreased sensitivity to thyroid hormones is associated with lower risk of prediabetes (15). Yu et al. reported the significant association between impaired sensitivity to thyroid hormones and elevated blood glucose (above 7.0 mmol/L) among people with coronary heart diseases (25). Our study confirmed the cross-sectional association between thyroid hormones sensitivity and prevalent diabetes. However, the longitudinal and bidirectional association of thyroid hormones sensitivity with diabetes remains unknown to date. Using a cohort design, our findings could not demonstrate that thyroid hormones sensitivity indices are independently associated with the risk of diabetes onset. The insignificant relationship could also partially attribute to the fact that people developed diabetes trend to have more risk factors, such as being older, having higher BMI and lipids levels, having been diagnosed with hypertension (seen in Table 1). These coexisting factors could probably attenuate with effect of thyroid hormones sensitivity on diabetes. The opposite results between our cross-sectional and longitudinal analyses suggested reverse causation between thyroid hormones sensitivity and diabetes, which was supported by our cross-lagged panel analysis. Moreover, Mehran et al. also assumed that the interpretation for diabetes should be concerned with cautions, as there was no significant cross-sectional relationship between TFQI and new-onset diabetes (14). Of note, it has been recognized that TSH upper limit increases with normal ageing and the elderly may be adapted to the physiological increase (26). Our previous study also showed that the elderly seem not sensitive to high TSH regarding metabolic syndrome (27). In the subgroup analysis of age, we observed consistent results that thyroid hormones sensitivity indices are not independent risk factors of diabetes onset. Similarly, the cross-sectional associations of thyroid hormones sensitivity indices with other metabolic traits have been reported. We hypothesized that metabolic disorders including type 2 diabetes could lead to acquired resistance to thyroid hormones. The phenomenon of co-existing metabolic disorders and impaired thyroid hormones sensitivity warrants further research, especially for the bidirectional relationship between metabolic health and thyroid hormones sensitivity.
In the cross-lagged panel analysis, the findings supported that fasting glucose appeared to precede the impaired thyroid hormones sensitivity, indicating elevated fasting glucose (above 7.0 mmol/L) may drive acquired resistance of thyroid hormones. Previous studies pointed that the prevalence of hyperthyroidism and hypothyroidism in patients with diabetes is higher than in the general population (28, 29). Diabetes could also aggravate the progression from subclinical to overt hypothyroidism (30). A review emphasized the urgent need to screen the onset of thyroid diseases in patients with diabetes (4). Our study suggested that diabetes potentially leads to the resistance of thyroid hormones in an euthyroid state. Consistently, studies have reported that the longer duration of diabetes is an important risk factor for the development of thyroid dysfunction (31, 32). The fact should be acknowledged is that the thyroid hormones sensitivity is defined by the mathematical interpretations of thyroid hormones, but not experimentally validated measurements. Both high FT4 and high TSH are present in the resistance to thyroid hormones. A mild acquired resistance to thyroid hormones might occur in the euthyroid population, of which the clinical significance and physiological importance still need more evidence to be addressed. All these resistance to thyroid hormone indices measure central sensitivity or resistance, i.e., the grade of pituitary gland inhibition by FT4 levels (12). Although the thyroid hormones sensitivity index may not be an independent risk predictor of diabetes, it could be a consequence of metabolic disorders reversely as observed in our study. Our results offer an explanation for thyroid profiles commonly found in patients with diabetes, that is at the population level, diabetes is associated with the subsequent resistance to thyroid hormones. It suggests that there might be other underlying mechanisms linking diabetes and resistance to thyroid hormone, which is also indicated in previous studies (33, 34). People of diabetes or other metabolic disorders should be aware of the thyroid function resistance and its consequences, including lipid pattern (35), renal and liver functions (13, 36), atherosclerosis (37), cognition performance (38) and mortality (12). Meanwhile, previous studies have demonstrated a close association between thyroid hormones sensitivity and metabolic disorders based on cross-sectional designs (39). The causal and temporal relationship needs more data to interpret the clinical relevance of thyroid hormones sensitivity in depth.
There are several potential mechanisms that could explain the relationship between glucose metabolism and thyroid hormones resistance. It has been indicated that only overt thyroid dysfunction leads to the disruption of glucose metabolism via insulin resistance (40, 41), which partially explains that thyroid hormones resistance under an euthyroid state could not predict diabetes onset. Conversely, our study indicated that hyperglycemia precedes thyroid hormones resistance, as hyperglycemia can control the conversion from thyroxine to triiodothyroxine in peripheral tissues and interfere the thyroid hormones (42, 43). In addition, inflammation may be involved in the pathogenesis of diabetes and thyroid hormones homeostasis. Studies have shown that people with abnormal glucose metabolism have high levels of inflammatory cytokines (44). The crosstalk between the cytokine IL-37 and thyroid hormones could be the key regulatory mechanism that justifies the glucose metabolic effects (45). The exact pathophysiological mechanism of glucose metabolism on thyroid hormones resistance needs further research.
The results should be interpreted in the context of limitations. First, this is an observational study design, and we were unable to claim the causal association between thyroid hormones sensitivity and diabetes. The observed results require further validation in other populations. Whether controlling glucose level could improve the thyroid hormones sensitivity status needs further clinical research. As an observational study, there are potential confounding factors that are not considered in this study, such as effect of specific medication use on TSH synthesis or secretion (glucocorticoid treatment, anticonvulsants and antidepressant medications). Second, this study is a large population study based on a health examination cohort, and the data of 2-hour postprandial blood glucose (2hPBG) and HbA1c were not available. The incidence of diabetes could be underestimated as 2hPBG and HbA1c can diagnose more people with diabetes compared with fasting glucose (1, 46), probably causing estimation deviation of the effect size. Further studies are needed to clarify the association between glucose dysregulation and thyroid hormones sensitivity in depth. Third, the follow-up period is relatively short, which is probably not enough to observe the effect of impaired thyroid hormones sensitivity on the risk of diabetes.
In summary, our findings could not demonstrate that thyroid hormones sensitivity status is a predictor of diabetes onset, suggesting diabetes may precede impaired sensitivity indices of thyroid hormones, which needs validation in further studies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Committee of Xiaotangshan Health Examination Center. All the participants gave an informed consent before participating. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
CC: Formal Analysis, Writing – original draft. HS: Writing – original draft. ZW: Methodology, Writing – review & editing. TZ: Writing – review & editing, Validation. JZ: Writing – review & editing, Resources. HY: Writing – review & editing, Formal Analysis. QL: Writing – review & editing, Data curation. ZM: Writing – review & editing, Supervision. LL: Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by the Science and Technology Development Project of JiLin Province (20210203062SF, 20200601007JC, YDZJ202201ZYTS093).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1267612/full#supplementary-material
Abbreviations
TSH, thyrotropin; FT3, free triiodothyronine; FT4, free thyroxine; TFQI, thyroid feedback quantile-based index; PTFQI, parametric thyroid feedback quantile-based index; TSHI, thyrotropin index; TT4RI, thyrotroph thyroxine resistance index.
References
1. Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract (2022) 183:109119. doi: 10.1016/j.diabres.2021.109119
2. Huebschmann AG, Huxley RR, Kohrt WM, Zeitler P, Regensteiner JG, Reusch JEB. Sex differences in the burden of type 2 diabetes and cardiovascular risk across the life course. Diabetologia (2019) 62(10):1761–72. doi: 10.1007/s00125-019-4939-5
3. Bommer C, Sagalova V, Heesemann E, Manne-Goehler J, Atun R, Bärnighausen T, et al. Global economic burden of diabetes in adults: projections from 2015 to 2030. Diabetes Care (2018) 41(5):963–70. doi: 10.2337/dc17-1962
4. Biondi B, Kahaly GJ, Robertson RP. Thyroid dysfunction and diabetes mellitus: two closely associated disorders. Endocr Rev (2019) 40(3):789–824. doi: 10.1210/er.2018-00163
5. Duntas LH, Orgiazzi J, Brabant G. The interface between thyroid and diabetes mellitus. Clin Endocrinol (Oxf) (2011) 75(1):1–9. doi: 10.1111/j.1365-2265.2011.04029.x
6. Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab (2002) 87(2):489–99. doi: 10.1210/jcem.87.2.8182
7. Gauthier BR, Sola-García A, Cáliz-Molina M, Lorenzo PI, Cobo-Vuilleumier N, Capilla-González V, et al. Thyroid hormones in diabetes, cancer, and aging. Aging Cell (2020) 19(11):e13260. doi: 10.1111/acel.13260
8. Chaker L, Ligthart S, Korevaar TI, Hofman A, Franco OH, Peeters RP, et al. Thyroid function and risk of type 2 diabetes: a population-based prospective cohort study. BMC Med (2016) 14(1):150. doi: 10.1186/s12916-016-0693-4
9. Ortiga-Carvalho TM, Sidhaye AR, Wondisford FE. Thyroid hormone receptors and resistance to thyroid hormone disorders. Nat Rev Endocrinol (2014) 10(10):582–91. doi: 10.1038/nrendo.2014.143
10. Visser WE, van Mullem AA, Visser TJ, Peeters RP. Different causes of reduced sensitivity to thyroid hormone: diagnosis and clinical management. Clin Endocrinol (Oxf) (2013) 79(5):595–605. doi: 10.1111/cen.12281
11. Tjørve E, Tjørve KM, Olsen JO, Senum R, Oftebro H. On commonness and rarity of thyroid hormone resistance: a discussion based on mechanisms of reduced sensitivity in peripheral tissues. Med Hypotheses (2007) 69(4):913–21. doi: 10.1016/j.mehy.2006.12.056
12. Laclaustra M, Moreno-Franco B, Lou-Bonafonte JM, Mateo-Gallego R, Casasnovas JA, Guallar-Castillon P, et al. Impaired sensitivity to thyroid hormones is associated with diabetes and metabolic syndrome. Diabetes Care (2019) 42(2):303–10. doi: 10.2337/dc18-1410
13. Ding X, Wang Y, Liu J, Wang G. Impaired sensitivity to thyroid hormones is associated with elevated homocysteine levels in the euthyroid population. J Clin Endocrinol Metab (2022) 107(9):e3731–7. doi: 10.1210/clinem/dgac371
14. Mehran L, Delbari N, Amouzegar A, Hasheminia M, Tohidi M, Azizi F. Reduced sensitivity to thyroid hormone is associated with diabetes and hypertension. J Clin Endocrinol Metab (2022) 107(1):167–76. doi: 10.1210/clinem/dgab646
15. Liu B, Wang Z, Fu J, Guan H, Lyu Z, Wang W. Sensitivity to thyroid hormones and risk of prediabetes: A cross-sectional study. Front Endocrinol (Lausanne) (2021) 12:657114. doi: 10.3389/fendo.2021.657114
16. Zhang X, Wu H, Fan B, Shi M, Lau ESH, Yang A, et al. Lifetime risk of developing diabetes in Chinese people with normoglycemia or prediabetes: A modeling study. PLoS Med (2022) 19(7):e1004045. doi: 10.1371/journal.pmed.1004045
17. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr., et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA (2003) 289(19):2560–72. doi: 10.1001/jama.289.19.2560
18. [2016 Chinese guideline for the management of dyslipidemia in adults]. Zhonghua Xin Xue Guan Bing Za Zhi (2016) 44(10):833–53. doi: 10.3760/cma.j.issn.0253-3758.2016.10.005
19. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med (2009) 150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
20. Standards of medical care in diabetes-2020 abridged for primary care providers. Clin Diabetes (2020) 38(1):10–38. doi: 10.2337/cd20-as01
21. Alonso-Ventura V, Civeira F, Alvarado-Rosas A, Lou-Bonafonte JM, Calmarza P, Moreno-Franco B, et al. A cross-sectional study examining the parametric thyroid feedback quantile index and its relationship with metabolic and cardiovascular diseases. Thyroid (2022). doi: 10.1089/thy.2022.0025
22. Jostel A, Ryder WD, Shalet SM. The use of thyroid function tests in the diagnosis of hypopituitarism: definition and evaluation of the TSH Index. Clin Endocrinol (Oxf) (2009) 71(4):529–34. doi: 10.1111/j.1365-2265.2009.03534.x
23. Yagi H, Pohlenz J, Hayashi Y, Sakurai A, Refetoff S. Resistance to thyroid hormone caused by two mutant thyroid hormone receptors beta, R243Q and R243W, with marked impairment of function that cannot be explained by altered in vitro 3,5,3'-triiodothyroinine binding affinity. J Clin Endocrinol Metab (1997) 82(5):1608–14. doi: 10.1210/jcem.82.5.3945
24. Chen X, Zhou Y, Zhou M, Yin Q, Wang S. Diagnostic values of free triiodothyronine and free thyroxine and the ratio of free triiodothyronine to free thyroxine in thyrotoxicosis. Int J Endocrinol (2018) 2018:4836736. doi: 10.1155/2018/4836736
25. Yu L, Li Z, Yang R, Pan G, Cheng Q, He Y, et al. Impaired sensitivity to thyroid hormones is associated with elevated blood glucose in coronary heart disease. Front Endocrinol (Lausanne) (2022) 13:895843. doi: 10.3389/fendo.2022.895843
26. Goichot B, Raverot V, Klein M, Vija Racaru L, Abeillon-du Payrat J, Lairez O, et al. Management of thyroid dysfunctions in the elderly. French Endocrine Society consensus 2019 guidelines. Short version. Ann Endocrinol (Paris) (2020) 81(5):511–5. doi: 10.1016/j.ando.2020.05.002
27. Wu Z, Jiang Y, Zhou D, Chen S, Zhao Y, Zhang H, et al. Sex-specific association of subclinical hypothyroidism with incident metabolic syndrome: A population-based cohort study. J Clin Endocrinol Metab (2022) 107(6):e2365–72. doi: 10.1210/clinem/dgac110
28. Perros P, McCrimmon RJ, Shaw G, Frier BM. Frequency of thyroid dysfunction in diabetic patients: value of annual screening. Diabetes Med (1995) 12(7):622–7. doi: 10.1111/j.1464-5491.1995.tb00553.x
29. Chubb SA, Davis WA, Inman Z, Davis TM. Prevalence and progression of subclinical hypothyroidism in women with type 2 diabetes: the Fremantle Diabetes Study. Clin Endocrinol (Oxf) (2005) 62(4):480–6. doi: 10.1111/j.1365-2265.2005.02246.x
30. Gray RS, Borsey DQ, Irvine WJ, Seth J, Clarke BF. Natural history of thyroid function in diabetics with impaired thyroid reserve: a four year controlled study. Clin Endocrinol (Oxf) (1983) 19(4):445–51. doi: 10.1111/j.1365-2265.1983.tb00018.x
31. Mehalingam V, Sahoo J, Bobby Z, Vinod KV. Thyroid dysfunction in patients with type 2 diabetes mellitus and its association with diabetic complications. J Family Med Prim Care (2020) 9(8):4277–81. doi: 10.4103/jfmpc.jfmpc_838_20
32. Al-Geffari M, Ahmad NA, Al-Sharqawi AH, Youssef AM, Alnaqeb D, Al-Rubeaan K. Risk factors for thyroid dysfunction among type 2 diabetic patients in a highly diabetes mellitus prevalent society. Int J Endocrinol (2013) 2013:417920. doi: 10.1155/2013/417920
33. Koulouri O, Moran C, Halsall D, Chatterjee K, Gurnell M. Pitfalls in the measurement and interpretation of thyroid function tests. Best Pract Res Clin Endocrinol Metab (2013) 27(6):745–62. doi: 10.1016/j.beem.2013.10.003
34. Lambadiari V, Mitrou P, Maratou E, Raptis AE, Tountas N, Raptis SA, et al. Thyroid hormones are positively associated with insulin resistance early in the development of type 2 diabetes. Endocrine (2011) 39(1):28–32. doi: 10.1007/s12020-010-9408-3
35. Sun H, Zhu W, Liu J, An Y, Wang Y, Wang G. Reduced sensitivity to thyroid hormones is associated with high remnant cholesterol levels in chinese euthyroid adults. J Clin Endocrinol Metab (2022) 108(1):166–74. doi: 10.1210/clinem/dgac523
36. Lai S, Li J, Wang Z, Wang W, Guan H. Sensitivity to thyroid hormone indices are closely associated with NAFLD. Front Endocrinol (Lausanne) (2021) 12:766419. doi: 10.3389/fendo.2021.766419
37. Liu Y, Li Z, Yang T, Li L, Yu L, Liu F, et al. Impaired sensitivity to thyroid hormones and carotid plaque in patients with coronary heart disease: A RCSCD-TCM study in China. Front Endocrinol (Lausanne) (2022) 13:940633. doi: 10.3389/fendo.2022.940633
38. Yu ZW, Pu SD, Sun XT, Wang XC, Gao XY, Shan ZY. Impaired sensitivity to thyroid hormones is associated with mild cognitive impairment in euthyroid patients with type 2 diabetes. Clin Interv Aging (2023) 18:1263–74. doi: 10.2147/CIA.S413584
39. Wu Z, Jiang Y, Li P, Wang Y, Zhang H, Li Z, et al. Association of impaired sensitivity to thyroid hormones with hyperuricemia through obesity in the euthyroid population. J Transl Med (2023) 21(1):436. doi: 10.1186/s12967-023-04276-3
40. Brent GA. Mechanisms of thyroid hormone action. J Clin Invest (2012) 122(9):3035–43. doi: 10.1172/JCI60047
41. Yavuz DG, Yüksel M, Deyneli O, Ozen Y, Aydin H, Akalin S. Association of serum paraoxonase activity with insulin sensitivity and oxidative stress in hyperthyroid and TSH-suppressed nodular goitre patients. Clin Endocrinol (Oxf) (2004) 61(4):515–21. doi: 10.1111/j.1365-2265.2004.02123.x
42. Nair A, Jayakumari C, Jabbar PK, Jayakumar RV, Raizada N, Gopi A, et al. Prevalence and associations of hypothyroidism in Indian patients with type 2 diabetes mellitus. J Thyroid Res (2018) 2018:5386129. doi: 10.1155/2018/5386129
43. Kalra S, Aggarwal S, Khandelwal D. Thyroid dysfunction and type 2 diabetes mellitus: screening strategies and implications for management. Diabetes Ther (2019) 10(6):2035–44. doi: 10.1007/s13300-019-00700-4
44. Mirza S, Hossain M, Mathews C, Martinez P, Pino P, Gay JL, et al. Type 2-diabetes is associated with elevated levels of TNF-alpha, IL-6 and adiponectin and low levels of leptin in a population of Mexican Americans: a cross-sectional study. Cytokine (2012) 57(1):136–42. doi: 10.1016/j.cyto.2011.09.029
45. Majnarić LT, Bosnić Z, Štefanić M, Wittlinger T. Cross-talk between the cytokine IL-37 and thyroid hormones in modulating chronic inflammation associated with target organ damage in age-related metabolic and vascular conditions. Int J Mol Sci (2022) 23(12):6456. doi: 10.3390/ijms23126456
46. Davies MJ, D'Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the american diabetes association (ADA) and the european association for the study of diabetes (EASD). Diabetes Care (2018) 41(12):2669–701. doi: 10.2337/dci18-0033
Keywords: thyroid hormones sensitivity, type 2 diabetes onset, cross-lagged analysis, TSH (thyroid stimulating hormone), free triiodothyronine (FT3), free thyroxine
Citation: Cui C, Sui H, Wang Z, Zhang T, Zheng J, Yan H, Li Q, Mo Z and Liu L (2023) Thyroid hormone sensitivity and diabetes onset: a longitudinal cross-lagged cohort. Front. Endocrinol. 14:1267612. doi: 10.3389/fendo.2023.1267612
Received: 26 July 2023; Accepted: 03 October 2023;
Published: 16 October 2023.
Edited by:
Joseph V. Martin, Rutgers University Camden, United StatesReviewed by:
Kenji Nagao, Ajinomoto, JapanCristian Serafinceanu, National Institute for Diabetes, Nutrition and Metabolic Diseases, Romania
Jose De Jesus Garduno Garcia, Universidad Autónoma del Estado de México, Mexico
Copyright © 2023 Cui, Sui, Wang, Zhang, Zheng, Yan, Li, Mo and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin Liu, bGl1bGluOTlAamx1LmVkdS5jbg==; Zhanhao Mo, bW96aGFuaGFvQGpsdS5lZHUuY24=
 Cancan Cui
Cancan Cui He Sui
He Sui Zhijia Wang
Zhijia Wang Zhanhao Mo
Zhanhao Mo Lin Liu
Lin Liu