
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 01 November 2023
Sec. Renal Endocrinology
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1265711
This article is part of the Research Topic Association between Diabetic Nephropathy and Diabetic Retinopathy or Non-diabetic Nephropathy View all 8 articles
Rationale & objective: A causal relationship concerning diabetic retinopathy (DR) and diabetic nephropathy (DN) has been studied in many epidemiological observational studies. We conducted a two-sample mendelian randomization study from the perspective of genetics to assess these associations.
Methods: 20 independent single nucleotide polymorphisms (SNPs) associated with diabetic retinopathy were selected from the FinnGen consortium. Summary-level data for diabetic nephropathy were obtained from the publicly available genome-wide association studies (GWAS) database, FinnGen and CKDGen consortium. Inverse variance weighted (IVW) was selected as the primary analysis. MR-Egger, weighted median (WM), simple mode and weighted mode were used as complementary methods to examine causality. Additionally, sensitivity analyses including Cochran’s Q test, MR-Egger, MR-Pleiotropy Residual Sum and Outlier (MR-PRESSO), and leave-one-out analyses were conducted to guarantee the accuracy and robustness of our MR analysis.
Results: Our current study demonstrated positive associations of genetically predicted diabetic retinopathy with diabetic nephropathy (OR=1.32; P=3.72E-11), type 1 diabetes with renal complications (OR=1.96; P= 7.11E-11), and type 2 diabetes with renal complications (OR=1.26, P=3.58E-04). Further subtype analysis and multivariate mendelian randomization (MVMR) also reached the same conclusion. A significant casualty with DN was demonstrated both in non-proliferative DR (OR=1.07, P=0.000396) and proliferative DR (OR=1.67, P=3.699068E-14). All the findings were robust across several sensitivity analyses.
Conclusion: Consistent with previous clinical studies, our findings revealed a positive correlation between DR and DN, providing genetic evidence for the non-invasive nature of DR in predicting DN.
Chronic kidney disease (CKD) is an irreversible change in renal structure and function caused by various causes, which can last for months or years. Recent studies on disease burden have revealed a significant global rise of 29.3% in all-age prevalence of CKD since 1990, contributing to 35.8 million disability-adjusted life years (DALYs) (1). Among these cases, diabetic nephropathy (DN) accounts for nearly one-third of DALYs, establishing CKD as a major worldwide public health concern (2). Diabetic nephropathy, a severe microvascular complication of diabetes, constitutes the primary cause of both CKD and end-stage renal disease (ESRD). Renal biopsy serves as the gold standard for diagnosing DN, providing valuable treatment guidance and prognostic indicators(3). However, renal biopsy is an invasive procedure, for patients with contraindications to not do, such as renal pyknosis, bleeding tendency, isolated kidney, uncontrolled hypertension, or severe anemia, should not be so as a way to monitor disease (4). In clinical practice, patients with type 2 diabetes often present with proteinuria, with or without DR. If a renal biopsy is not actively performed in such cases and treatment is solely based on diabetic kidney disease (DKD), it often delays the timing of intervention and leads to irreversible outcomes. So, it is crucial to develop noninvasive or minimally invasive and objective approaches to pathological assessment for the diagnosis and treatment of DN.
Diabetic retinopathy (DR) is a microvascular complication of diabetes mellitus that damages the endothelial cells of the retinal capillaries and causes abnormal vascular permeability, ischemia, and neovascularization. In clinical practice, it is usually divided into proliferative diabetic retinopathy and non-proliferative diabetic retinopathy according to the presence of retinal neovascularization (5), which can be diagnosed by ophthalmoscopy that is simpler and more non-invasive than renal biopsy. DR is the leading cause of blindness in adults aged 20 to 74, and almost all type 1 diabetes cases and 60% of type 2 diabetes cases will develop DR after 20 years (6).
The kidney bears a striking resemblance to the eye in terms of origin, development, and structure (7). For example, both the glomeruli and choroid have extensive vascular networks of similar structures; the inner retina and the glomerular filtration barrier share similar developmental pathways, and renin-angiotensin-aldosterone cascades have been found in both the kidney and retina (8). Park et al. also reported that about 26.7% of diabetic patients had both DN and DR (9). A plethora of investigations have substantiated the convergence between DN and DR in terms of their pathogenic mechanisms, as well as the similarities observed during their respective onset and progression (10–12). Glycation end products, a distinct set of compounds, play a pivotal role in this context. The process of non-enzymatic glycosylation, facilitated by hyperglycemia, expedites the generation of these compounds through protein reactions, consequently inducing escalated intracellular oxidative stress. Activation of NF-κB and free radicals can directly impact lipids and proteins, provoking vascular cellular impairment and inciting inflammation, ultimately culminating in renal and fundus vascular dysfunction (13, 14). However, it has been observed that 30% of DN patients with coexisting DR exhibit grade III or IV glomerulopathy, implying a correlation between the severity of glomerulopathy and the presence or absence of DR (15). Jia et al. conducted a national cross-sectional study in 2016 to investigate the prevalence of high levels of proteinuria in patients with T2DM and DR. Specifically, 47.8% of the 3,301 patients included had high levels of proteinuria, with the incidence of DR increasing as urine albumin levels rose (16).
The complications of diabetes presented a tendency of familial clustering. Several genes are shared between ocular and renal organogenesis, including Pax2, BMP7, and WT-1, and disruptions in these genes can give rise to a spectrum of diseases that manifest concomitant ocular and renal involvement (17). Genome-wide association study (GWAS)assume a pivotal role in identifying genes and pathways associated with diabetes complications. For example, porta et al. conducted a GWAS study of 3546 type 1 diabetic patients, resulting in the extraction of data of two thiamine transporter proteins (SLC19A2/3) and their transcription factors (SP1/2) to explore their association with severe retinopathy or nephropathy. They found a reduced incidence of severe retinopathy and a reduced combined phenotype of severe retinopathy and end-stage renal disease in a state of strong chain disequilibrium (18).
With the continuous advancement of sequencing technology, GWAS is widely used to study the genetic mechanism of complex diseases and identify a large number of genetic variants that are significantly associated with diabetes complications, such as diabetic nephropathy, diabetic retinopathy, diabetic cardiopathy, and diabetic painful neuropathy (19–21). Mendelian Randomization (MR) serves as an epidemiological analytical approach that utilizes genetic variations, specifically single nucleotide polymorphisms (SNPs), associated with exposure factors to elucidate the causal relationship between these exposures and subsequent outcomes. By virtue of the random assignment of alleles during conception, MR effectively mitigates biases encountered in clinical studies investigating disease etiology, including unmeasured and unknown confounding factors, as well as reverse causation (22, 23). In this study, we employed two-sample Mendelian randomization to investigate the causal relationship between diabetic retinopathy and diabetic nephropathy, subsequently estimating and comparing the magnitude of association across distinct stages of both conditions. Finally, multivariate Mendelian randomization analysis was conducted to adjust for potential confounding factors, including fasting glucose and HbA1c levels.
GWAS data pertaining to various stages of diabetic retinopathy and five distinct outcomes associated with diabetic nephropathy were obtained from the publicly accessible GWAS catalog, as well as the CKDGen Consortium (http://ckdgen.imbi.uni-freiburg.de/) and the FinnGen database (www.finngen.fi/fi). Given the re-analysis of previously summarized data, there was no need for additional ethical approval. Comprehensive information regarding the GWAS incorporated in the Mendelian randomization analysis is outlined in Table 1. Employing a two-sample Mendelian randomization approach, we initially ascertained the causal association between diabetic retinopathy and five related outcomes associated with diabetic nephropathy. Subsequently, we conducted an in-depth investigation to examine the dynamics of this relationship across distinct stages of diabetic retinopathy, specifically Non-proliferative and Proliferative diabetic retinopathy. Ultimately, potential associations with confounders were subsequently followed-up by multivariate mendelian randomization (MVMR) analyses to investigate the robustness of these associations to adjustment for fasting glucose and HbA1c. Summary statistics of hyperglycemia-related traits, encompassing fasting glucose and HbA1c, were sourced from the Meta-analyses of Glucose and Insulin-Related Traits Consortium (MAGIC), recognized as the most extensive publicly accessible meta-analytic endeavor conducted within non-diabetic populations (https://magicinvestigators.org). The omission of glycemic traits derived from diabetic patients stemmed from our recognition that the influence of anti-diabetic medications or exogenous insulin extends beyond genetic determinants, necessitating their exclusion from our analysis. The MR study relies on three fundamental assumptions (22, 24): (1) The selection of instrumental variables (IVs) must exhibit a significant and robust association with the exposure; (2) The selected instrumental variables (IVs) should be independent of any potential confounding factors that may influence the outcome.; and (3) The selected instrumental variables (IVs) exert a direct effect on the outcome through the exposure variable, without involvement of alternative pathways. The manuscript was meticulously prepared in accordance with the guidelines outlined in the STrengthening the Reporting of Observational Studies in Epidemiology-MR (STROBE-MR) checklist, specifically tailored for a comprehensive two-sample Mendelian randomization (MR) study (25). The schematic representation of our study’s overall workflow is visually presented in Figure 1. For the execution of two-sample MR and MVMR analyses, we employed R software (version 4.2.1) along with the Two-sample MR package (version 0.5.5).
Firstly, to identify SNPs that are highly related to diabetic retinopathy, we established the genome-wide significance criterion at p<5*10-8. Since relatively few SNPs were found for diabetic retinopathy when we constituted the exposure, a looser threshold (p<1*10-5) was selected (26). Secondly, considering the potential bias caused by solid linkage disequilibrium (LD), we clumped SNPs with LDr2< 0.001, kb = 10,000. Palindromic SNPs were excluded because it was not possible to determine if they were aligned for exposure and outcome in the GWASs of diabetic retinopathy. Subsequently, to avoid weak instrument bias, F statistics were calculated to assess instrument strength and the fraction of exposure variance was determined using the R2 value of each SNP. Finally, SNPs with F > 10 were extracted to ensure statistical efficiency (25, 27). The F-statistic for each SNP was calculated as follows:
R2 was calculated as follows:
R2: the cumulative explained variance of the selected IVs on exposure; MAF: the effect of allele frequency.
β: estimated effect of SNP; N: sample size of the GWAS.
We utilized PhenoScanner V2 (PhenoScanner.cam.ac.uk) to eliminate SNPs that were associated with potential confounding factors of DN (27).
Summary-level GWAS data pertaining to diabetic nephropathy were extracted from the Finn consortium, encompassing a cohort of 213,746 individuals of European ancestry (3,283 cases and 210,463 controls). Additionally, we gathered aggregated data on glomerular filtration rate in individuals with diabetes from a prior study, encompassing 144,935 participants of European (28). Summary statistics for urinary albumin-to-creatinine ratio with diabetes mellitus were extracted from CKDGen Consortium with 5,825 participants of European ancestry (29). To more accurately investigate the causal relationship between diabetic nephropathy and diabetic retinopathy, we acquired separate GWAS data for type 1 and type 2 diabetes respectively. Specifically, statistical summary data for type 2 diabetes with renal complications were obtained from the FinnGen database, encompassing 1,296 cases and 183,185 controls of European ancestry. For the construction of GWAS data relating to type 1 diabetes with renal disorders, summary-level GWAS data from FinnGen, comprising a total of 963 cases and 183,185 controls, were utilized.
We employed a comprehensive range of five analytical methods, namely inverse variance weighting (IVW), MR Egger, Weighted Median (WM), Simple Mode, and Weighted Mode, to investigate the causal relationship between diabetic retinopathy and diabetic nephropathy (29). The IVW method combines the estimates from individual IVs using inverse variance weights and provides a consistent and unbiased estimation of the causal effect under the assumption that all IVs are valid and there is no violation of the instrumental variable assumptions (30). MR Egger and Weighted Median (WM) are valuable tools in Mendelian randomization studies for addressing concerns about the presence of pleiotropy or violations of instrumental variable assumptions. It allows researchers to obtain causal effect estimates while accounting for pleiotropic effects and provides additional insights into the relationship between the exposure and outcome variables. However, the Weighted Median method’s non-parametric nature may lead to less precise estimates, at the same time, MR Egger relies on regression modeling and may have reduced statistical power (31). Therefore, the result of the IVW method is the most accurate and the primary method in our study (32), while the WM and MR-Egger methods were performed as additional tests. Here we adopted the random-effects IVW model for MR estimates considering heterogeneity among instrumental variables.
Several sensitivity tests were used, including Cochran’s Q-statistic, MR Egger, MR Pleiotropy RESidual Sum, and Outlier techniques (MR-PRESSO) to obtain robust MR estimates. Cochran’s Q test was employed to assess heterogeneity among instrumental variables, with a significance level of P< 0.05 indicating significant heterogeneity among SNPs (33). MR-Egger analysis was employed to assess the influence of horizontal pleiotropy on the MR estimates by estimating the average pleiotropic effect and evaluating its statistical its significance (P< 0.05) (34). Once there is horizontal pleiotropy, MR Pleiotropy RESidual Sum and Outlier (MR-PRESSO) method will be used to identify and address outliers induced by horizontal pleiotropy. By detecting and adjusting for these outliers, MR-PRESSO enhances the precision and reliability of causal effect estimates (34). Furthermore, to evaluate the robustness and influence of individual instrumental variables on the estimated causal effect, we utilize leave-one-out cross-validation or jackknife analysis (35).
We extracted 20 SNPs (single nucleotide polymorphisms) that are reliable (P< 1*10-5) and independent (r2< 0.001, kb = 10,000) of diabetic retinopathy from the FinnGen database, which included 206,234 participants of European ancestry and 16,380,446 SNPs. 5 SNPs (rs2476601, rs915894, rs3957146, rs2534659, rs689) were excluded with PhenoScannerV2 because these SNPs exhibited significant associations with established confounding factors (diabetes, HbA1c, and insulin resistance).2 SNPs (rs3957146, rs139334417) were detected as outliers for potential horizontal pleiotropy and discarded by MR-PRESSO. After Coordinating the allelic directions of Exposure-SNP and Outcome-SNP and eliminating palindromic SNP and incompatible SNP according to the size of EAF, we finally got a summary table of SNPs for background diabetic retinopathy. Refinement statistics are shown in Supplementary Table 1. The F-statistics of all the included SNPs were greater than 10, indicating the absence of weak instrument bias in Mendelian randomization (MR) analysis.
To further explore the causal relationship between DR and DN, summary-level GWAS data with non-proliferative background diabetic retinopathy and proliferative diabetic retinopathy were extracted.24 SNPs of non-proliferative background diabetic retinopathy were extracted as genetic instrumental variables.4 SNPs (rs6679677, rs9273401, rs9276710, rs17885785) were excluded with PhenoScannerV2 because these SNPs exhibited significant associations with established confounding factors (diabetes and insulin treatment). rs8192575 are identified as outliers by MR-PRESSO and addressed to minimize potential adverse effects on MR estimates. As for proliferative diabetic retinopathy,30 SNPs were selected as genetic instrumental variants while 7 SNPs (rs2476601, rs3957146, rs2855807, rs34337125, rs915894, rs7903146, rs689) were eliminated by PhenoScannerV2. Refinement statistics are shown in Supplementary Tables 2.1-4.5.
Figure 2 displays the estimations regarding the causal impacts of background diabetic retinopathy on diabetic nephropathy, alongside forest plots showcasing the estimations for each outcome utilizing various MR methods. The funnel plot was symmetrical, guaranteeing the robustness of the MR analysis (Supplementary Figure 1). Cochran’s Q statistics and MR-Egger as well as MR-PRESSO indicated no significant heterogeneity was found in our study (Figure 3). In the IVW method, a significant causal relationship was both found in diabetic nephropathy (OR = 1.32, 95%CI = 1.21–1.43, P = 3.72E-11), type 1 diabetes with renal complications (OR = 1.96, 95%CI = 1.60–2.41, P = 7.11E-11) and type 2 diabetes with renal complications (OR = 1.26, 95%CI = 1.11–1.42, P = 3.58E-04). The MR-Egger and WM methods reached the same conclusion in diabetic nephropathy (MR-Egger: OR=1.37, 95% CI = 1.16–1.61, P = 2.61E-03 WM: OR = 1.34,95% CI = 1.20–1.50, P = 3.67E-07) and type 1 diabetes with renal complications (MR-Egger: OR=2.01, 95% CI = 1.35–2.99, P = 4.83E-03 WM: OR = 1.70,95% CI = 1.32–2.19, P = 3.65E-05). However, no significant causal association was found in the glomerular filtration rate (OR = 1.01, 95%CI = 0.98–1.04, P = 0.521). The leave-one-out analysis further confirmed the stability of our findings, demonstrating that the outcomes were robust and not substantially influenced by any individual SNP removal (Figure 4).
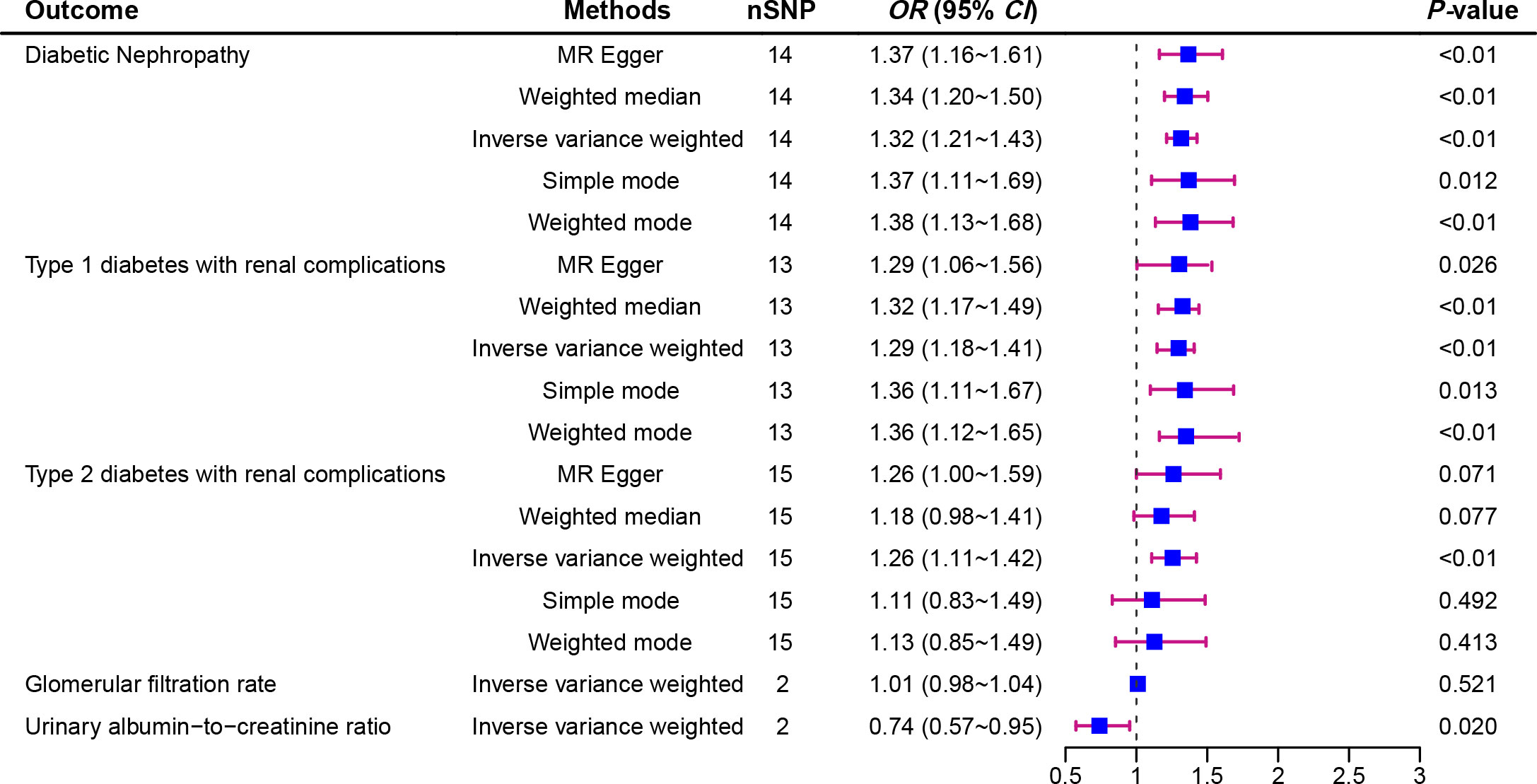
Figure 2 Forest plot of Mendelian randomization analyses showing the effect of diabetic retinopathy on the risk of diabetic nephropathy.
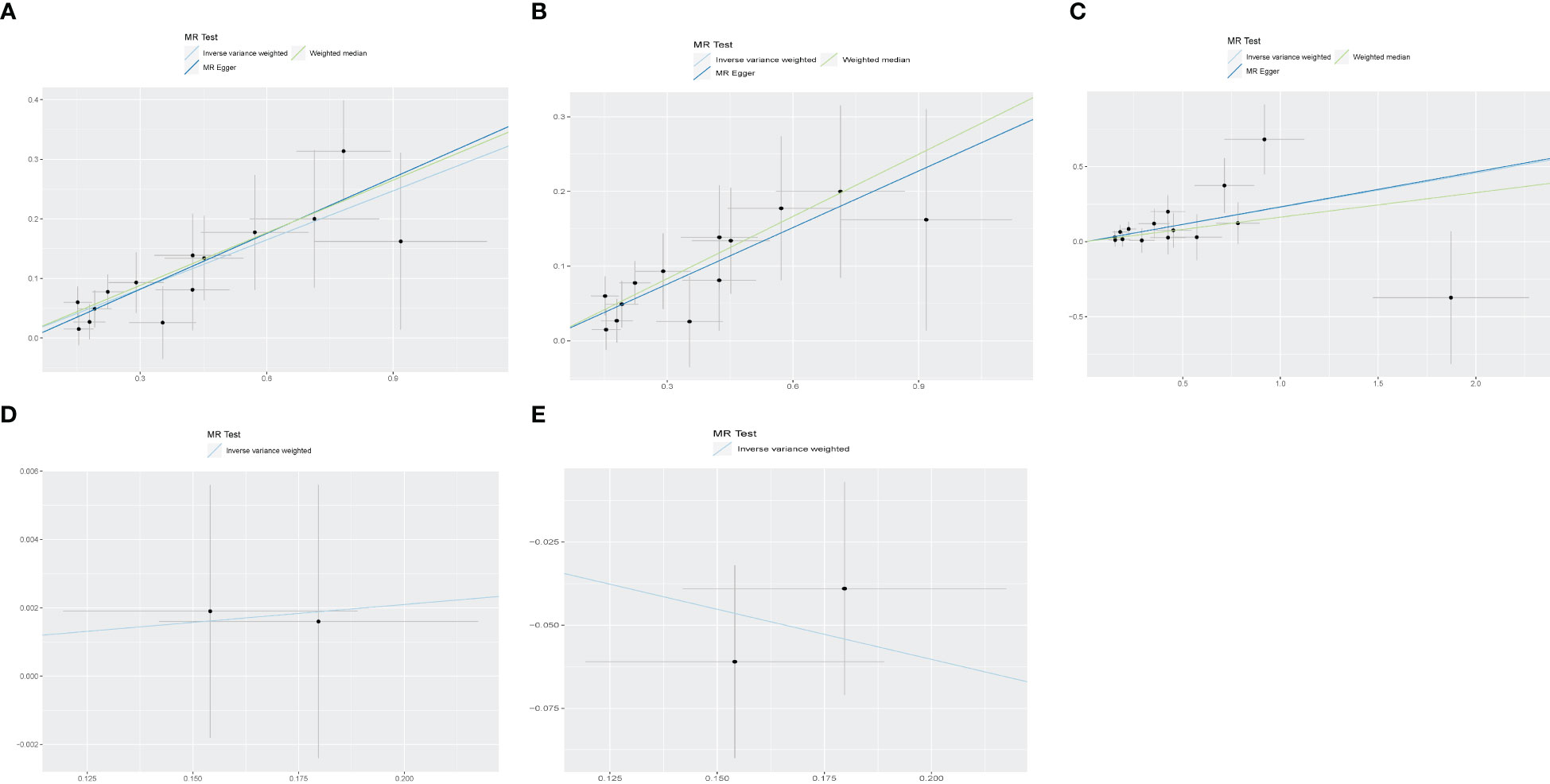
Figure 3 The scatter plot for MR analyses of causal associations between each diabetic retinopathy related SNP and Diabetic nephropathy (A), type 1 diabetes with renal complications (B), type2 diabetes with renal complications (C), glomerular filtration rate in diabetics (D), Glomerular filtration rate Urinary albumin−to−creatinine ratio (E).
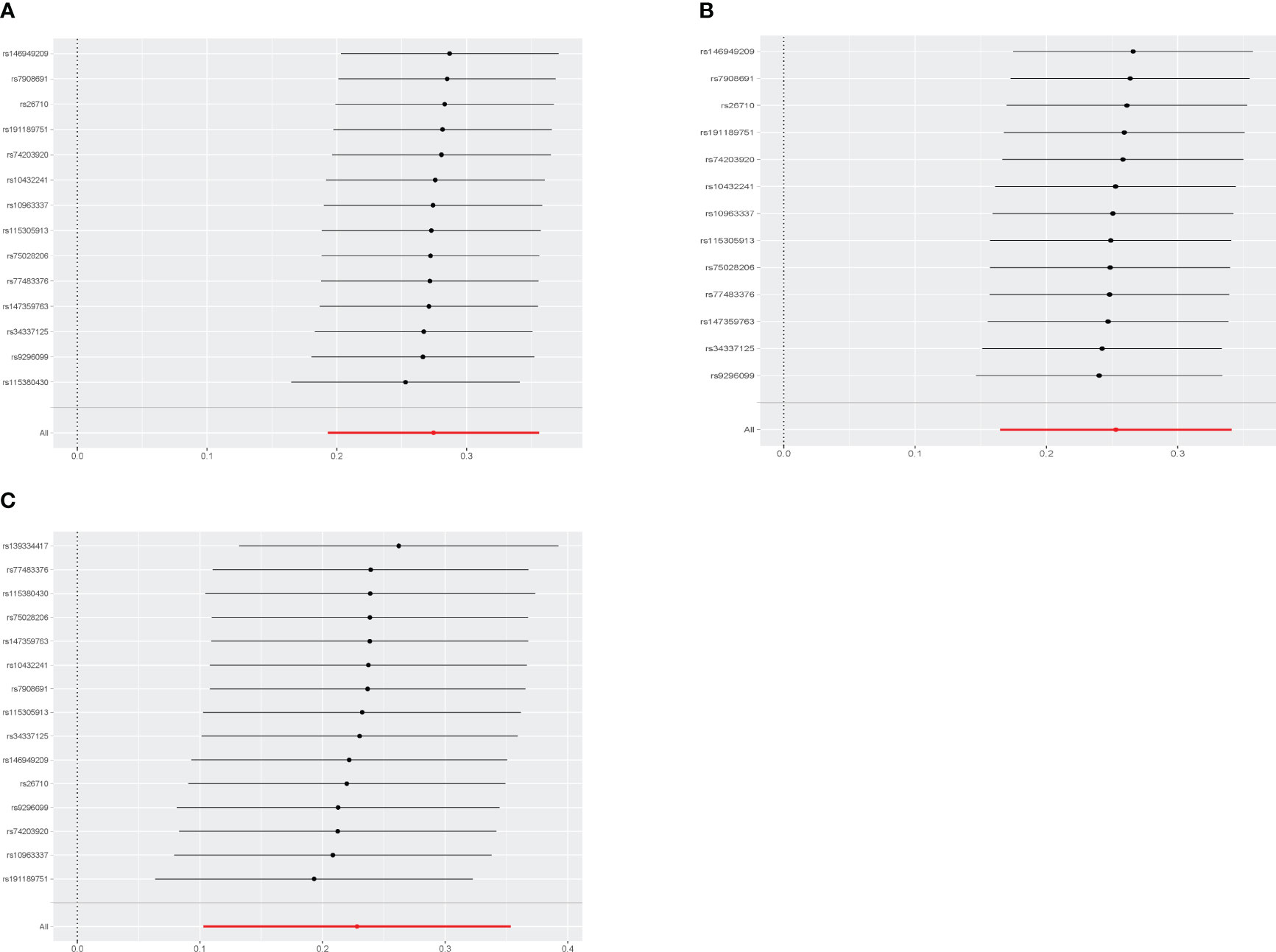
Figure 4 Leave-one-out sensitivity analyses of each diabetic retinopathy related SNP and Diabetic nephropathy (A), type 1 diabetes with renal complications (B), type2 diabetes with renal complications (C).
In the study of non-proliferative DR and DN, a noteworthy causal relationship was also found in almost outcomes (Figure 5). The odds ratio (OR) of IVW analysis of diabetic nephropathy was 1.07(95% confidence interval [CI], 1.03~1.11; P = 0.000396). As for type 2 diabetes with renal complications, the IVW method also indicated a clear causality (OR = 1.07, 95%CI = 1.01–1.14, P = 0.02285986). The result of IVW (OR= 5.257, P= 0.017), MR-Egger (OR= 5.257, P= 0.017) and Weight-Median (OR=2.818, 95% CI:1.145-6.936, P= 0.024) reached the same conclusion, indicating a significant positive causal connection were identified in coffee intake and diabetic nephropathy. However, no significant causal association was found in glomerular filtration rate (OR = 0.99, 95%CI = 0.98–1.01, P = 0.323) and urinary albumin (OR = 1.04, 95%CI = 0.91–1.19, P = 0.589). No indication of heterogeneity and horizontal pleiotropy was found in all five outcomes (Supplementary Figures 3, 4). These finding was all robust in the leave-one-out sensitivity analysis (Supplementary Figure 2).
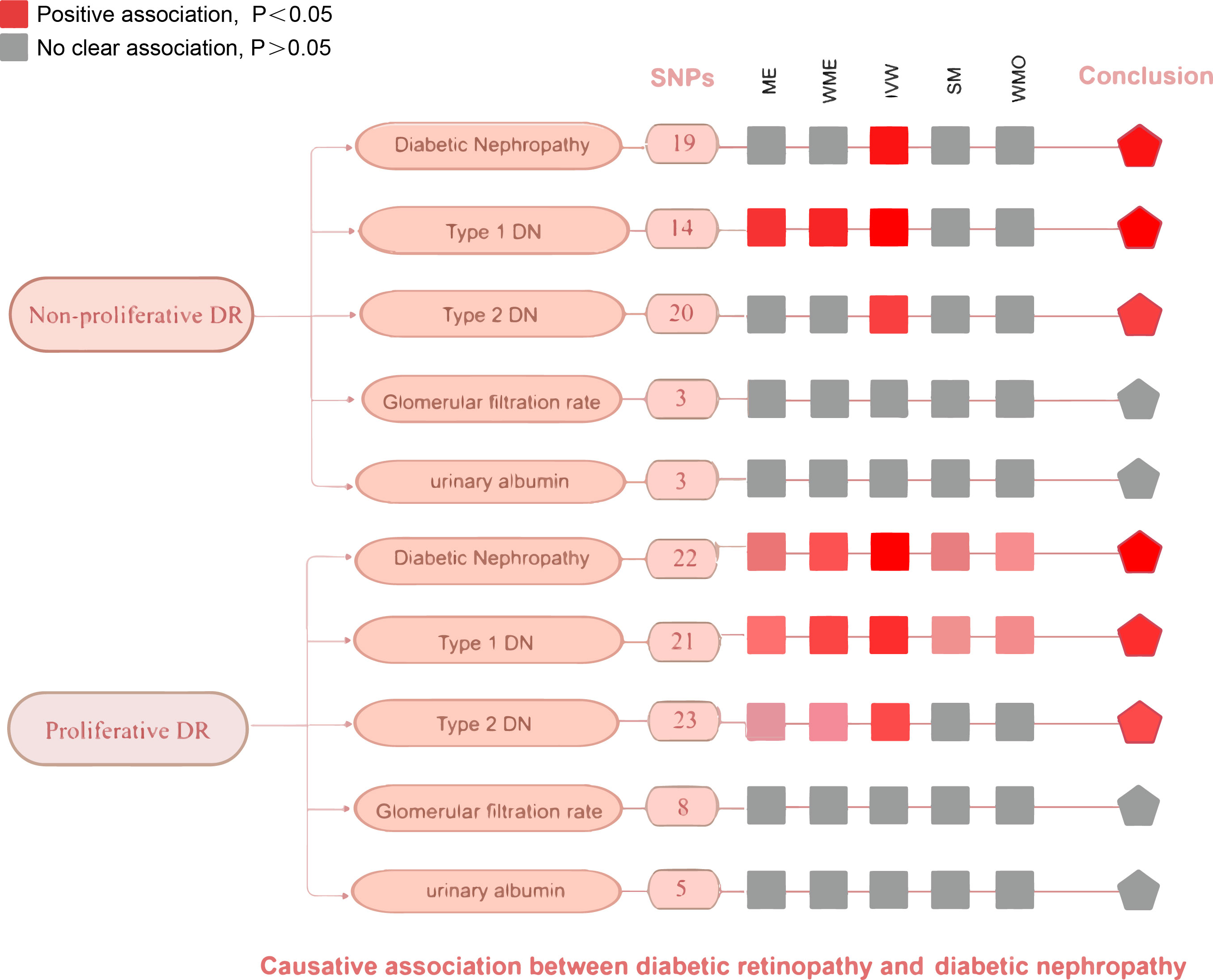
Figure 5 An overview of primary results from the two-sample MR studies showing the effect of non-proliferative DR and proliferative DR on the risk of diabetic nephropathy. Full data for all results depicted in the figure can be found in the Supplementary Table S5. IVM, inverse variance weighted method; WM, weighted median estimator; ME, MR Egger; SM, Simple mode; MO, Weighted mode.
We conducted the same analysis for the database about proliferative DR and DN and reached a similar conclusion eventually (Figure 5). The IVW method indicates a significant causal relationship both in diabetic nephropathy (OR = 1.67, 95%CI = 1.46–1.90, P = 3.699068E-14), type 1 diabetes with renal complications (OR = 2.99, 95%CI = 2.23–4.00, P=1.945534E-13), type 2 diabetes with renal complications (OR = 1.83, 95%CI = 1.46–2.29, P = 1.807069E-07). Similar to the results of IVW analyses, the MR-Egger (OR=1.90, 95% CI: 1.38-2.63, P=8.623717E-04) and WM analyses (OR=1.70, 95% CI: 1.39-2.09, P=2.967800E-07) suggested a relationship of a distinctive character between proliferative DR and diabetic nephropathy. When it comes to type 1/2 diabetes with renal complications, the MR-Egger and WM analyses also yielded identical positive outcomes. The P value of Cochran’s Q test was 0.5113727, 0.1362339, and 0.2482583 respectively, indicating that the heterogeneity was low and insignificant. The pleiotropy analysis using the MR-Egger intercept test and MR-PRESSO showed that all genetic variants used had no substantial pleiotrophin in diabetic nephropathy (Egger intercept = -0.01609629, P = 0.3885723; global test P=0.6417), type 1 diabetes with renal complications y (Egger intercept =-0.06371645, P =0.1453258; global test P=0.23) and type 2 diabetes with renal complications (Egger intercept =-0.005553915, P = 0.8617735; global test P= 0.3243333). The sensitivity analysis with the leave-one-out approach found that the pooled effect estimates changed insignificantly, ensuring the robustness and reliability of the outcomes (Supplementary Figure 5). However, random effects IVW estimates indicated that more severity of proliferative DR is not causally associated with a higher risk of glomerular filtration rate in diabetics (OR= 1.00, 95% CI: 0. 96-1.04, P=0.960) or urinary albumin (OR= 1.12, 95% CI: 0.77-1.62 P=0.553). Sensitivity tests suggest neither heterogeneity nor polymorphism in our MR analysis.
Considering DR and DN are microvascular complications of diabetes caused by chronic hyperglycaemia, we embarked on a comprehensive investigation by incorporating two prevalent risk factors, fasting glucose and HbA1c, into our MVMR analysis. The utilization of the MVMR methodology serves the purpose of elucidating the intricate interplay among these risk factors, thus enhancing the robustness and credibility of our study. Within the framework of the MVMR analysis, while meticulously controlling for HbA1c, a direct causal link between background diabetic retinopathy and the heightened susceptibility to both DN (IVW OR = 1.57, 95% CI = 1.47-1.68, P = 2.98E-43) and Type 2 diabetes accompanied by renal complications (IVW OR = 1.22, 95% CI = 1.11-1.34, P = 3.87E-05) was affirmed. Furthermore, subsequent adjustments for both HbA1c and fasting glucose unveiled substantial support for a direct causal effect of proliferative DR on the risk of DN (HbA1c: IVW OR = 2.13, 95% CI= 1.96-2.31, P< 0.001; fasting glucose: IVW OR = 2.16, 95% CI= 1.70-2.73, P< 0.001) (Supplementary Table 8).
The present study demonstrated a significant association between genetically predicted diabetic retinopathy and an increased risk of diabetic nephropathy. Furthermore, the results of two MR study confirmed that there was indeed a causal association both in non-proliferative DR and proliferative DR. The observed correlations between DR and DN encompass shared pathogenic mechanisms, pathological outcomes, and clinical manifestations. The utilization of artificial intelligence and machine learning in quantifying retinal vascular geometric parameters holds promise in providing new insights for the diagnosis and differentiation of DN. Our study provides a genetic basis for the diagnosis of diabetic nephropathy in diabetic retinopathy.
This finding was in line with the outcomes of a prospective population-based study in 2020, in which bilateral fundic photographs of 91 Chinese type 2 diabetic patients with biopsy-confirmed DN were obtained to estimate the hazard ratio for the effect of the severity of diabetic retinopathy on ESRD. The experiment results show that the severity of retinopathy at the time of biopsy was a prognostic factor for progression to ESRD (HR 2.18, 95% confidence interval 1.05 to 4.53, P = 0.04) (15). Likewise, a systematic review by Pearce et al. demonstrated a strong association between DR and DN. The presence of DR increases the risk of developing nephropathy and serves as a predictive indicator for the progression of both microalbuminuria and macroalbuminuria (36). Another meta-analysis revealed that the sensitivity of DR in distinguishing between DN nondiabeticetic renal disease (NDRD) in type 2 diabetes patients is 0.65, with a specificity of 0.75. Importantly, it emphasized the high specificity of DR diagnosis for PDR in identifying DN (37). Yamanouchi et al. observed that with increasing severity of DR (from no DR, non-proliferative DR, to proliferative DR), the grading of DN based on renal pathological changes such as glomerular injury, interstitial fibrosis and tubular atrophy (IFTA) scores, as well as diffuse lesion scores, progressively increased. This suggests that the severity of DR could serve as a clinical indicator for predicting the severity of renal pathology (38). However, in another study, the correlation between DR and Grade IIb and III glomerular lesions was significantly higher than that with Grade IV lesions, possibly due to differences in the mechanisms of glomerular nodular sclerosis and global glomerulosclerosis. The specific reasons require further investigation (39). In retinal fundus photographs, calculations of the central artery and retinal central vein dimensions, as well as the arteriovenous ratio (AVR), can provide indications of cardiovascular risk (40). Similarly, several studies have attempted to predict renal pathological changes through fundus examination. Multiple cross-sectional studies have demonstrated an association between the enlargement of retinal vein diameter and the severity of both DR and DN (41). Erdogmus et al. reported that DR has a sensitivity of 75%, specificity of 91%, positive predictive value of 88%, and negative predictive value of 81% in predicting DN. Multivariable regression analysis suggested that DR is an independent predictor of DN (42). However, other studies have demonstrated inconsistencies between DN and DR manifestations. Christensen et al. reported that albuminuria patients with type 2 diabetes without DR have a prevalence of biopsies with normal glomerular structure or nondiabetic kidney diseases of approximately 30% (43). These contradictory findings observed may be attributed to inherent biases or confounders intrinsic in observational studies, including limitations stemming from small sample sizes, heterogeneity in demographic characteristics, variations in study designs, reverse causation, and selection bias, such as differences in methods used to assess DR. It is important to note that observational studies primarily analyze correlations rather than establishing causation. To mitigate the impact of potential bias and confounders, MR analysis was conducted to investigate the causal association between DR and DN from a genetic standpoint. In our study, there was remarkable evidence supporting the fact that DR was causally associated with DN.
Several potential pathways have been proposed to elucidate the mechanisms underlying the causality between DR and DN. Advanced glycation end products (AGEs), a heterogeneous group of molecules that form through a series of non-enzymatic reactions between reducing sugars and proteins, lipids, or nucleic acids, once bound to the receptor for AGEs (RAGE), can initiate multiple signaling pathways that contribute to microvascular damage. The AGE-RAGE complex can activate signaling pathways such as reactive oxygen species (ROS) and mitogen-activated protein kinase (MAPK) in various cells. This activation leads to increased inflammation and proliferation of mesangial cells in the kidneys, ultimately resulting in apoptosis (44). In DR, the AGE-RAGE complex induces oxidative stress, osteogenic differentiation, calcification in pericytes, and also triggers apoptosis (45). On top of the AGE-RAGE component with excess glucose in the cell, DAG is formed, which subsequently triggers the activation of protein kinase C (PKC) (46). Lin et al. demonstrated that activated protein kinase C (aPKC) can induce the release of endothelin and VEGF, leading to increased vascular permeability in animal models of retinal inflammation (47). Wang et al. also mentioned LY333531, a PKCβ inhibitor, can promote the degradation of type IV collagen and fibronectin while downregulate the expression of the pro-apoptotic protein swiprosin-1, attenuating glomerular endothelial cell apoptosis (48). Additionally, SHP-1 is a class of protein tyrosine phosphatases with an SH2 domain that controls intracellular phosphorylation levels of tyrosine (49). High glucose levels can activate PKCδ/p38α MAPK, leading to independent activation of downstream NFκB and activation of SHP-1, ultimately resulting in the development of DR (50). Likewise, elevated glucose levels were also reported to activate SHP-1 activity and inhibit VEGF in podocytes, thereby contributing to glomerular disease progression in diabetes (51).
Nowadays, an increasing body of clinical and fundamental research has accumulated compelling evidence supporting a significant correlation between DN and DR. To the best of our knowledge, this study represents the first large-scale Mendelian randomization investigation focusing on the relationship between DR and DN. Our study possesses several distinct advantages. Firstly, the utilization of MR methodology, considered as a natural randomized controlled trial approach, provides more robust evidence compared to previous observational studies. Secondly, our study exclusively focuses on individuals of European descent, thereby minimizing potential biases arising from population stratification. Moreover, we conducted a staging study of disease progression aiming to explore the causal relationship between DR and DN precisely. A comprehensive set of sensitivity analyses, including MR Egger, Cochran’s Q test, MR-PRESSO, and leave-one-out analysis, consistently demonstrated the robustness of the findings.
This study has several limitations that should be acknowledged. Firstly, despite utilizing the largest available sample size and the most recent GWAS dataset for MR analysis, it is important to recognize that our study had a relatively smaller sample size and event count when compared to population-based observational studies. Secondly, meeting three assumptions is crucial. Of particular difficulty is fulfilling the second assumption, which necessitates that instrumental variables should not be associated with confounding factors. To address this concern, we employed PhenoScanner to manually exclude SNPs associated with known confounders, thereby striving to ensure reliable and plausible results. However, it is impractical to account for all potential confounding factors exhaustively. Thirdly, Systems biology contains complex interactions between genes, proteins, and other molecules, as well as the influence of environmental factors, thus incorporating data on developmental compensation and epigenetic phenomena would provide a more comprehensive and accurate understanding of causality.
In conclusion, our MR study confirms a significant correlation between DR and DN based on genetic evidence, highlighting the non-invasive nature and relatively accurate diagnostic capability of diabetic retinopathy in predicting diabetic nephropathy. Future research efforts should involve long-term extensive multicenter cohort studies to validate biomarkers and mechanistic studies to confirm the association between DR and DN in terms of severity and disease progression, which will provide more effective assistance in the early non-invasive detection and real-time monitoring of DN and in slowing down its progression.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
JF: Writing – original draft. CL: Writing – original draft. DZ: Writing – original draft. QH: Writing – review & editing. LL: Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by General Project Funds from the Health Department of Zhejiang Province (No. 2020KY439).
We sincerely thank all GWAS participants and investigators for sharing the summary statistics data publicly available. We highly appreciate the free drawing support for Figures 1, 3 provided by the Figdraw platform.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1265711/full#supplementary-material
1. Bikbov B, Purcell CA, Levey AS, Smith M, Abdoli A, Abebe M, et al. Global, regional, and national burden of chronic kidney disease 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet (London England) (2020) 395:709–33. doi: 10.1016/S0140-6736(20)30045-3
2. Xiong Y, Zhou L. The signaling of cellular senescence in diabetic nephropathy. Oxid Med Cell Longevity (2019) 2019:7495629. doi: 10.1155/2019/7495629
3. Galardi A, Colletti M, Di Paolo V, Vitullo P, Antonetti L, Russo I, et al. Exosomal miRNAs in pediatric cancers. Int J Mol Sci (2019) 20(18):1–22. doi: 10.3390/ijms20184600
4. Li H, Shen Y, Yu Z, Huang Y, He T, Xiao T, et al. Potential role of the renal arterial resistance index in the differential diagnosis of diabetic kidney disease. Front Endocrinol (2021) 12:731187. doi: 10.3389/fendo.2021.731187
5. Wu L, Gao L, Cao Y, Chen F, Sun T, Liu Y. Analysis of the protective mechanism of liraglutide on retinopathy based on diabetic mouse model. Saudi J Biol Sci (2019) 26:2096–101. doi: 10.1016/j.sjbs.2019.09.032
6. Fouda AY, Xu Z, Suwanpradid J, Rojas M, Shosha E, Lemtalsi T, et al. Targeting proliferative retinopathy: Arginase 1 limits vitreoretinal neovascularization and promotes angiogenic repair. Cell Death Dis (2022) 13:745. doi: 10.1038/s41419-022-05196-8
7. Wong CW, Wong TY, Cheng CY, Sabanayagam C. Kidney and eye diseases: common risk factors, etiological mechanisms, and pathways. Kidney Int (2014) 85:1290–302. doi: 10.1038/ki.2013.491
8. Wilkinson-Berka JL, Agrotis A, Deliyanti D. The retinal renin-angiotensin system: roles of angiotensin II and aldosterone. Peptides (2012) 36:142–50. doi: 10.1016/j.peptides.2012.04.008
9. Park HC, Lee YK, Cho A, Han CH, Noh JW, Shin YJ, et al. Diabetic retinopathy is a prognostic factor for progression of chronic kidney disease in the patients with type 2 diabetes mellitus. PloS One (2019) 14:e0220506. doi: 10.1371/journal.pone.0220506
10. Duprez DA, Forbang NI, Allison MA, Peralta CA, Shea S and Jacobs DR Jr. Association of C2, a derivative of the radial artery pressure waveform, with new onset of type 2 diabetes mellitus: the MESA study. Cardiovasc Diabetol (2019) 18:62. doi: 10.1186/s12933-019-0868-3
11. Ribeiro de Carvalho G, Loduca Lima V, da Veiga GL, Adami F, da Costa Aguiar Alves B, Cristiano Pereira E, et al. Effects of intravitreal bevacizumab therapy in patients with proliferative diabetic retinopathy. Diabetes Metab Syndrome Obes Targets Ther (2020) 13:3149–55. doi: 10.2147/DMSO.S243873
12. Cho CH, Yoo G, Kim M, Kurniawati UD, Choi IW, Lee SH. Dieckol, derived from the edible brown algae ecklonia cava, attenuates methylglyoxal-associated diabetic nephropathy by suppressing AGE-RAGE interaction. Antioxid (Basel Switzerland) (2023) 12(3):1–18. doi: 10.3390/antiox12030593
13. Moran EP, Wang Z, Chen J, Sapieha P, Smith LE, Ma JX. Neurovascular cross talk in diabetic retinopathy: Pathophysiological roles and therapeutic implications. Am J Physiol Heart Circulatory Physiol (2016) 311:H738–749. doi: 10.1152/ajpheart.00005.2016
14. Paul S, Ali A, Katare R. Molecular complexities underlying the vascular complications of diabetes mellitus - A comprehensive review. J Diabetes Its Complications (2020) 34:107613. doi: 10.1016/j.jdiacomp.2020.107613
15. Zhao L, Ren H, Zhang J, Cao Y, Wang Y, Meng D, et al. Diabetic retinopathy, classified using the lesion-aware deep learning system, predicts diabetic end-stage renal disease in Chinese patients. Endocrine Practice: Off J Am Coll Endocrinol Am Assoc Clin Endocrinologists (2020) 26:429–43. doi: 10.4158/EP-2019-0512
16. Guo K, Zhang L, Zhao F, Lu J, Pan P, Yu H, et al. Prevalence of chronic kidney disease and associated factors in Chinese individuals with type 2 diabetes: Cross-sectional study. J Diabetes Its Complications (2016) 30:803–10. doi: 10.1016/j.jdiacomp.2016.03.020
17. Izzedine H, Bodaghi B, Launay-Vacher V, Deray G. Eye and kidney: from clinical findings to genetic explanations. J Am Soc Nephrol JASN (2003) 14:516–29. doi: 10.1097/01.ASN.0000051705.97966.AD
18. Porta M, Toppila I, Sandholm N, Hosseini SM, Forsblom C, Hietala K, et al. Variation in SLC19A3 and protection from microvascular damage in type 1 diabetes. Diabetes (2016) 65:1022–30. doi: 10.2337/db15-1247
19. Chen P, Takeuchi F, Lee JY, Li H, Wu JY, Liang J, et al. Multiple nonglycemic genomic loci are newly associated with blood level of glycated hemoglobin in East Asians. Diabetes (2014) 63:2551–62. doi: 10.2337/db13-1815
20. Chang YC, Chang EY, Chuang LM. Recent progress in the genetics of diabetic microvascular complications. World J Diabetes (2015) 6:715–25. doi: 10.4239/wjd.v6.i5.715
21. Wen Z, Zou X, Xie X, Zheng S, Chen X, Zhu K, et al. Association of polymorphisms in miRNA processing genes with type 2 diabetes mellitus and its vascular complications in a southern Chinese population. Front Endocrinol (2019) 10:461. doi: 10.3389/fendo.2019.00461
22. Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ (Clin Res ed) (2018) 362:k601. doi: 10.1136/bmj.k601
23. Si S, Hou L, Chen X, Li W, Liu X, Liu C, et al. Exploring the causal roles of circulating remnant lipid profile on cardiovascular and cerebrovascular diseases: mendelian randomization study. J Epidemiol (2022) 32:205–14. doi: 10.2188/jea.JE20200305
24. Zheng J, Baird D, Borges MC, Bowden J, Hemani G, Haycock P, et al. Recent developments in mendelian randomization studies. Curr Epidemiol Rep (2017) 4:330–45. doi: 10.1007/s40471-017-0128-6
25. Skrivankova VW, Richmond RC, Woolf BAR, Yarmolinsky J, Davies NM, Swanson SA, et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomization: the STROBE-MR statement. Jama (2021) 326:1614–21. doi: 10.1001/jama.2021.18236
26. Shi H, Zhao T, Geng R, Sun L, Fan H. The associations between gut microbiota and chronic respiratory diseases: a Mendelian randomization study. Front Microbiol (2023) 14:1200937. doi: 10.3389/fmicb.2023.1200937
27. Palmer TM, Lawlor DA, Harbord RM, Sheehan NA, Tobias JH, Timpson NJ, et al. Using multiple genetic variants as instrumental variables for modifiable risk factors. Stat Methods Med Res (2012) 21:223–42. doi: 10.1177/0962280210394459
28. Pattaro C, Teumer A, Gorski M, Chu AY, Li M, Mijatovic V, et al. Genetic associations at 53 loci highlight cell types and biological pathways relevant for kidney function. Nat Commun (2016) 7:10023. doi: 10.1038/ncomms10023
29. Teumer A, Tin A, Sorice R, Gorski M, Yeo NC, Chu AY, et al. Genome-wide association studies identify genetic loci associated with albuminuria in diabetes. Diabetes (2016) 65:803–17. doi: 10.2337/db15-1313
30. Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol (2017) 46:1985–98. doi: 10.1093/ije/dyx102
31. van Kippersluis H, Rietveld CA. Pleiotropy-robust mendelian randomization. Int J Epidemiol (2018) 47:1279–88. doi: 10.1093/ije/dyx002
32. Lee CH, Cook S, Lee JS, Han B. Comparison of two meta-analysis methods: inverse-variance-weighted average and weighted sum of Z-scores. Genomics Inf (2016) 14:173–80. doi: 10.5808/GI.2016.14.4.173
33. Kulinskaya E, Dollinger MB, Bjørkestøl K. On the moments of Cochran's Q statistic under the null hypothesis, with application to the meta-analysis of risk difference. Res Synthesis Methods (2020) 11:920. doi: 10.1002/jrsm.1446
34. Amar D, Sinnott-Armstrong N, Ashley EA, Rivas MA. Graphical analysis for phenome-wide causal discovery in genotyped population-scale biobanks. Nat Commun (2021) 12:350. doi: 10.1038/s41467-020-20516-2
35. Morais CLM, Santos MCD, Lima KMG, Martin FL. Improving data splitting for classification applications in spectrochemical analyses employing a random-mutation Kennard-Stone algorithm approach. Bioinf (Oxford England) (2019) 35:5257–63. doi: 10.1093/bioinformatics/btz421
36. Pearce I, Simó R, Lövestam-Adrian M, Wong DT, Evans M. Association between diabetic eye disease and other complications of diabetes: Implications for care. A systematic review. Diabetes Obes Metab (2019) 21:467–78. doi: 10.1111/dom.13550
37. He F, Xia X, Wu XF, Yu XQ, Huang FX. Diabetic retinopathy in predicting diabetic nephropathy in patients with type 2 diabetes and renal disease: a meta-analysis. Diabetologia (2013) 56:457–66. doi: 10.1007/s00125-012-2796-6
38. Yamanouchi M, Mori M, Hoshino J, Kinowaki K, Fujii T, Ohashi K, et al. Retinopathy progression and the risk of end-stage kidney disease: results from a longitudinal Japanese cohort of 232 patients with type 2 diabetes and biopsy-proven diabetic kidney disease. BMJ Open Diabetes Res Care (2019) 7:e000726. doi: 10.1136/bmjdrc-2019-000726
39. Zhang J, Wang Y, Li L, Zhang R, Guo R, Li H, et al. Diabetic retinopathy may predict the renal outcomes of patients with diabetic nephropathy. Renal Failure (2018) 40:243–51. doi: 10.1080/0886022X.2018.1456453
40. Wong TY, Knudtson MD, Klein R, Klein BE, Hubbard LD. A prospective cohort study of retinal arteriolar narrowing and mortality. Am J Epidemiol (2004) 159:819–25. doi: 10.1093/aje/kwh119
41. Tziomalos K, Athyros VG. Diabetic nephropathy: new risk factors and improvements in diagnosis. Rev Diabetic Stud RDS (2015) 12:110–8. doi: 10.1900/RDS.2015.12.110
42. Erdogmus S, Kiremitci S, Celebi ZK, Akturk S, Duman N, Ates K, et al. Non-diabetic kidney disease in type 2 diabetic patients: prevalence, clinical predictors and outcomes. Kidney Blood Pressure Res (2017) 42:886–93. doi: 10.1159/000484538
43. Christensen PK, Larsen S, Horn T, Olsen S, Parving HH. Causes of albuminuria in patients with type 2 diabetes without diabetic retinopathy. Kidney Int (2000) 58:1719–31. doi: 10.1046/j.1523-1755.2000.00333.x
44. Yamagishi S, Matsui T. Advanced glycation end products, oxidative stress and diabetic nephropathy. Oxid Med Cell Longevity (2010) 3:101–8. doi: 10.4161/oxim.3.2.11148
45. Mao YX, Cai WJ, Sun XY, Dai PP, Li XM, Wang Q, et al. RAGE-dependent mitochondria pathway: a novel target of silibinin against apoptosis of osteoblastic cells induced by advanced glycation end products. Cell Death Dis (2018) 9:674. doi: 10.1038/s41419-018-0718-3
46. Ferlita S, Yegiazaryan A, Noori N, Lal G, Nguyen T, To K, et al. Type 2 diabetes mellitus and altered immune system leading to susceptibility to pathogens, especially mycobacterium tuberculosis. J Clin Med (2019) 8(12):1–11. doi: 10.3390/jcm8122219
47. Lin CM, Titchenell PM, Keil JM, Garcia-Ocaña A, Bolinger MT, Abcouwer SF, et al. Inhibition of atypical protein kinase C reduces inflammation-induced retinal vascular permeability. Am J Pathol (2018) 188:2392–405. doi: 10.1016/j.ajpath.2018.06.020
48. Wang ZB, Zhang S, Li Y, Wang RM, Tong LC, Wang Y, et al. LY333531, a PKCβ inhibitor, attenuates glomerular endothelial cell apoptosis in the early stage of mouse diabetic nephropathy via down-regulating swiprosin-1. Acta Pharmacol Sin (2017) 38:1009–23. doi: 10.1038/aps.2016.172
49. Liu J, Qin X, Pan D, Zhang B, Jin F. Amino acid-mediated metabolism: A new power to influence properties of stem cells. Stem Cells Int (2019) 2019:6919463. doi: 10.1155/2019/6919463
50. Geraldes P, Hiraoka-Yamamoto J, Matsumoto M, Clermont A, Leitges M, Marette A, et al. Activation of PKC-delta and SHP-1 by hyperglycemia causes vascular cell apoptosis and diabetic retinopathy. Nat Med (2009) 15:1298–306. doi: 10.1038/nm.2052
Keywords: Mendelian randomization, diabetic retinopathy, diabetic nephropathy, causality, proliferative diabetic retinopathy
Citation: Fang J, Luo C, Zhang D, He Q and Liu L (2023) Correlation between diabetic retinopathy and diabetic nephropathy: a two-sample Mendelian randomization study. Front. Endocrinol. 14:1265711. doi: 10.3389/fendo.2023.1265711
Received: 23 July 2023; Accepted: 18 October 2023;
Published: 01 November 2023.
Edited by:
Xiaoyong Yu, Shaanxi Provincial Hospital of Traditional Chinese Medicine, ChinaReviewed by:
Godfrey Mutashambara Rwegerera, University of Botswana, BotswanaCopyright © 2023 Fang, Luo, Zhang, He and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin Liu, bGl1bGluQGhtYy5lZHUuY24=; Qiang He, cWlhbmdoZTE5NzNAMTI2LmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.