- Department of Obstetrics and Gynecology, Fertility Center of CHA Gangnam Medical Center, CHA University School of Medicine, Seoul, Republic of Korea
Introduction: The global adoption of the “freeze-all strategy” has led to a continuous increase in utilization of single vitrified-warmed blastocyst embryo transfer (SVBT) owing to its clinical effectiveness. Accurate prediction of clinical pregnancy is crucial from a patient-centered perspective. However, this remains challenging, with inherent limitations due to the absence of precise and user-friendly prediction tools. Thus, this study primarily aimed to develop and assess a nomogram based on quantitative clinical data to optimize the efficacy of personalized prognosis assessment.
Materials and methods: We conducted a retrospective cohort analysis of ongoing pregnancy data from 658 patients with infertility who underwent SVBT at our center between October 17, 2017, and December 18, 2021. Patients were randomly assigned to the training (n=461) or validation (n=197) cohort for nomogram development and testing, respectively. A nomogram was constructed using the results of the multivariable logistic regression (MLR), which included clinical covariates that were assessed for their association with ongoing pregnancy.
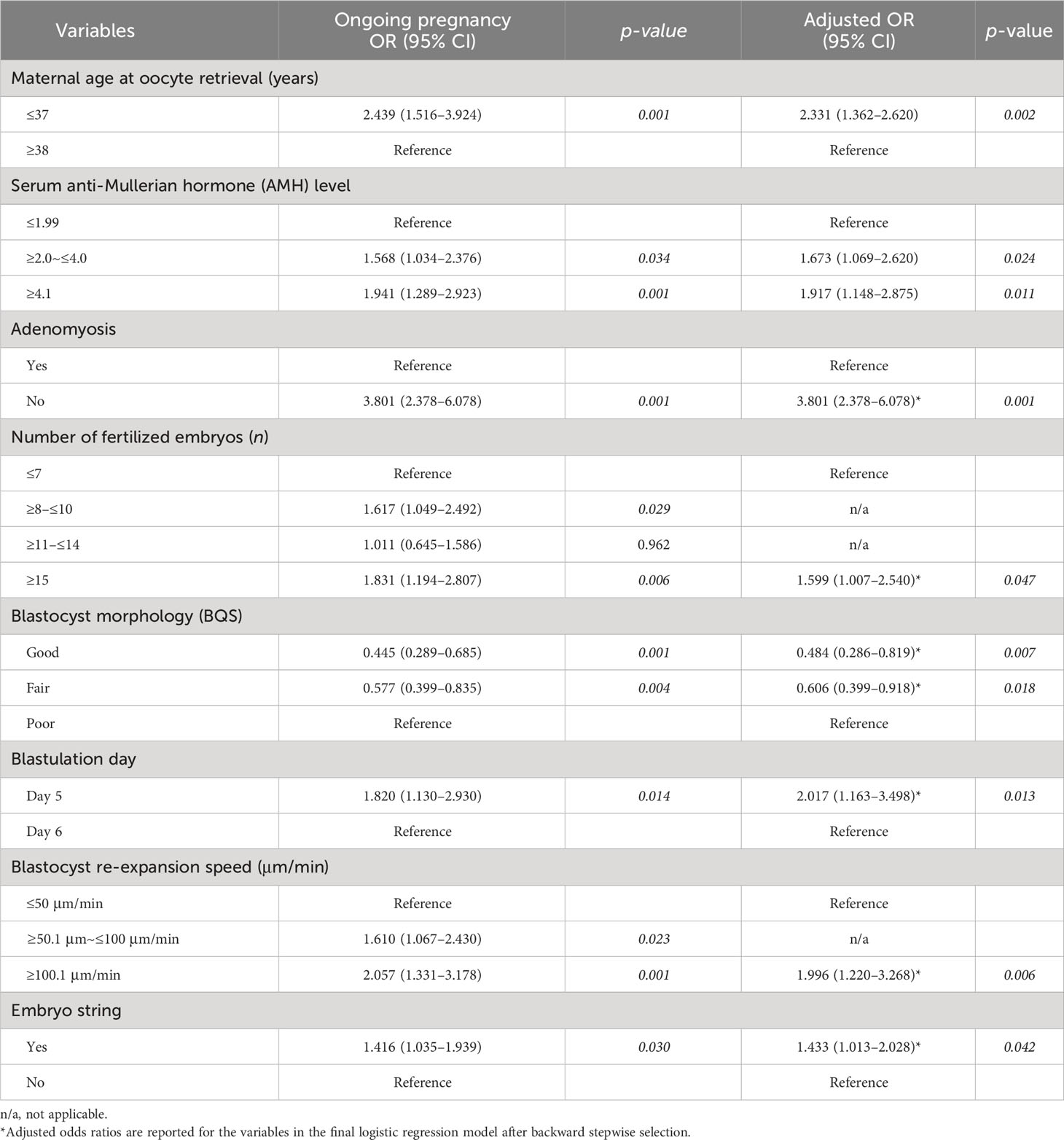
Results: The MLR identified eight significant variables that independently predicted ongoing pregnancy outcomes in the study population. These predictors encompassed maternal physiology, including maternal age at oocyte retrieval and serum anti-Müllerian hormone levels; uterine factors, such as adenomyosis; and various embryo assessment parameters, including the number of fertilized embryos, blastocyst morphology, blastulation day, blastocyst re-expansion speed, and presence of embryo string. The area under the receiver operating characteristic curve in our prediction model was 0.675 (95% confidence interval [CI], 0.622–0.729) and 0.656 (95% CI, 0.573–0.739) in the training and validation cohorts, respectively, indicating good discrimination performance in both cohorts.
Conclusions: Our individualized nomogram is a practical and user-friendly tool that can provide accurate and useful SVBT information for patients and clinicians. By offering this model to patients, clinical stakeholders can alleviate uncertainty and confusion about fertility treatment options and enhance patients’ confidence in making informed decisions.
1 Introduction
Vitrification has enhanced the efficiency and safety of embryo cryopreservation considerably, allowing for extended prolonged storage of embryos and improving the overall success rate of assisted reproductive technology (ART) procedures, including in vitro fertilization (IVF) (1). Furthermore, extended culture, which involves in vitro embryo development up to the blastocyst stage before uterine transfer, enables the selection of competent embryos for transfer, enhancing the prospects of achieving a successful pregnancy (2). Advancements in vitrification and extended culture are crucial in increasing the success rate of ART, particularly blastocyst transfer (3, 4). Frozen embryo transfer (FET) has advanced notably in recent years; however, conception can remain challenging for individuals experiencing infertility. In addition to medical considerations, such as success rates, patients may face financial burdens associated with ART procedures (5). These factors, coupled with the emotional stress inherent in the process, can contribute to the overall psychological burden of attempting conception and potentially strain relationships (6).
Shared decision-making is a collaborative process between healthcare professionals and patients to make informed decisions concerning healthcare treatments and interventions. During this process, healthcare professionals present patients with comprehensive information on available treatment options, including the associated risks, benefits, and probable outcomes. Patients are encouraged to ask questions, articulate their preferences and concerns, and provide input for the decision-making process. Ultimately, healthcare professionals and patients decide the most appropriate treatment option tailored to each patient’s circumstances (7, 8). Implementing a nomogram model can help healthcare professionals better inform couples with infertility about their chances of conceiving (9, 10). A nomogram is a visual tool that simplifies the creation of a prediction model for disease diagnosis, recurrence, or survival by considering various risk factors without requiring complicated calculations. Nomograms are useful for predicting outcomes in various medical scenarios (11–14).
Owing to the specificity of IVF treatment field, the proportion of patients participating in decision-making is higher than that in other medical fields; therefore, it is patient-centered. Effective communication of treatment plans, including the risks, benefits, and potential complications, is critical before initiating FET treatment (8, 15). However, current treatment strategies often lack concrete scientific evidence, making counseling challenging and frustrating for couples with infertility seeking individualized care (16). To address this, our study aimed to optimize treatment success by estimating personalized probabilities using a nomogram of embryonic (blastocyst assessment) predictors and patient physiology (maternal physiology and uterine factor) before treatment.
2 Materials and methods
2.1 Ethical approval
This retrospective study was approved by the Ethics Committee of the Institutional Review Board (IRB) of the CHA Gangnam Medical Center (IRB approval number: GCI 2022-06-008). The need for obtaining participant consent was waived due to the study’s retrospective nature and the use of medical records. The entire study was conducted in accordance with relevant guidelines and regulations.
2.2 Study design and patients
This retrospective cohort study was conducted at the CHA Fertility Center in Gangnam, South Korea, between October 17, 2017 and December 18, 2021. Overall, 1,919 single vitrified-warmed blastocyst embryo transfer (SVBT) cases were identified and analyzed. Demographic data and potential predictors were pre-selected based on a literature review and clinical experience (Supplementary Table 1). A detailed chart review confirmed embryo-related information. Exclusion criteria were double embryo transfer, cleavage-stage embryo transfer, in vitro maturation, oocyte donor cycle, uterine anomalies, endometrial thickness < 7 mm, not using the time lapse system (TLS), and significant data loss. The study flowchart (Figure 1) illustrates the selection process, resulting in the analysis of 658 SVBT cycles.
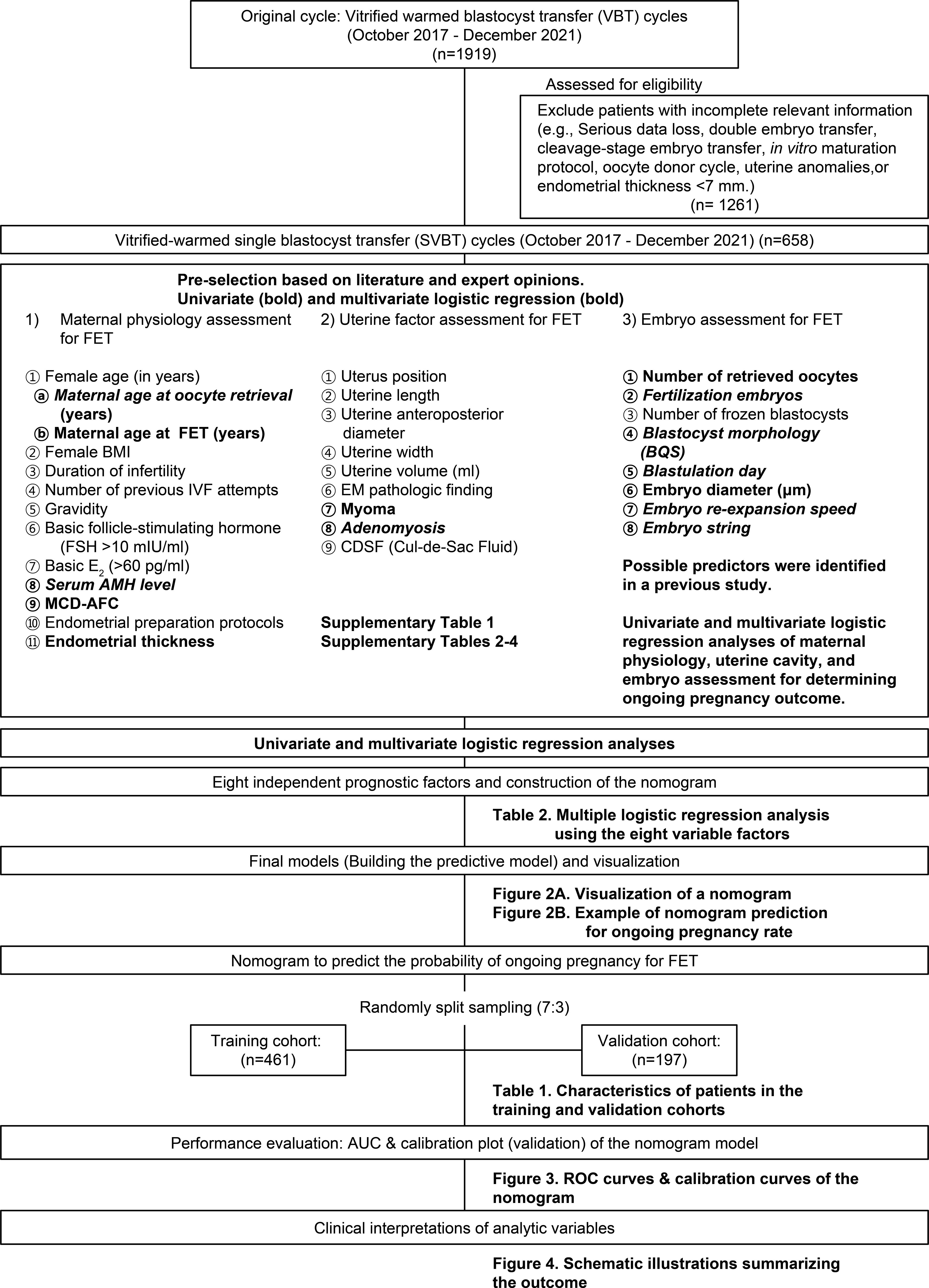
Figure 1 Flowchart of the experiment for predicting ongoing pregnancy after vitrified-warmed single embryo transfer cycles.
2.3 IVF and laboratory procedures
IVF and laboratory procedures were performed as previously described (17). The physician determined the dosage and duration of follicle-stimulating hormone (FSH) treatment based on patient information. Superovulation was induced using the gonadotropin-releasing hormone (GnRH) agonist long or antagonist protocol. The FSH dose was adjusted based on the growth of each follicle and serum estradiol (E2) concentration. Oocyte was retrieved under transvaginal ultrasound guidance when two or more follicles with a diameter of 17–18 mm or greater were identified following recombinant human chorionic gonadotropin administration. The retrieved oocytes were fertilized using conventional fertilization or intracytoplasmic sperm injection. A good-quality embryo was selected for transfer into the uterine cavity using a delivery catheter under abdominal ultrasound guidance. Surplus embryos, if available, were cryopreserved based on established protocols, considering criteria such as elevated progesterone or E2 levels or the risk of ovarian hyperstimulation syndrome. The vitrification and warming processes followed standardized methods as previously described (18, 19). Post-warming blastocyst survival was confirmed by assessing the morphological integrity using an inverted microscope (Vitrolife). Warmed blastocysts were cultured in TLS overnight, and their survival, re-expansion, and grading were evaluated based on the blastocyst quality score. Endometrial preparation for SVBT was conducted in natural or hormone replacement treatment cycles, as described in our previous study (20). In natural cycles, dominant ovarian follicle development was monitored, and SVBT was performed after ovulation. One day before embryo transfer. Vitrified blastocysts were warmed at 37°C. Upon confirmation of their survival and development, they were transferred to the uterine cavity under ultrasound guidance.
2.4 Development and validation of the model
Patient information was blinded, and they were assigned randomized numbers; a training (n=461) and a validation cohort (n=197) was established at a ratio of 7:3. Ongoing pregnancy, the endpoint of this study, was defined as the presence of a gestational sac with a heartbeat lasting for over 12 weeks. In the training cohort, 461 patients were included to develop a nomogram to predict the patient-specific probability of an ongoing pregnancy in women with SVBT. Backward stepwise conditional variable selection was performed to determine the independent covariates. A logistic regression model was used for multivariable analysis, including significant variables from the univariate analysis (p < 0.05). The variables entered into the nomogram development model were maternal age at oocyte retrieval, serum anti-Müllerian hormone (AMH) level, adenomyosis, number of fertilized embryos, blastocyst morphology score (BQS), vitrification day, blastocyst re-expansion speed (μm/min), and presence of an embryo string.
The values for each model covariate were mapped to points on a scale of 0–100, and the total points obtained for each model corresponded to the probability of an ongoing pregnancy. The performance of the model was assessed in the training cohort and internally validated by fitting it to the validation cohort using the same parameters. The predictive accuracy of the models was measured using the receiver operating characteristic (ROC) curve and the area under the curve (AUC). A calibration plot was used to plot the observed rates against the predicted probabilities to assess the agreement.
2.5 Statistical analysis
All statistical analyses were performed using IBM SPSS version 25.0 (IBM Corp., Armonk, NY, USA), R software (version 3.3.4, R Core Team, Vienna, Austria), and JAMOVI software (version 2.3, JAMOVI Project, Sydney, Australia). A p-value of < 0.05 was considered significant. Mean and standard deviation (SD) were used for normally distributed variables, chi-square for categorical variables, and t-test for continuous variables. First, univariate logistic regression analysis was performed on clinically important variables, and significant variables (p < 0.05) were potential predictors for the MLR. Step-by-step backward logistic regression analysis was conducted to obtain the final set of possible predictors. Two approaches were employed to assess the validity of the nomogram. First, discrimination or the ability of the nomogram to distinguish between ongoing pregnancy and non-pregnancy outcomes was quantified using the area under the receiver operating characteristic (AUROC) curve. The ROC curve provides a comprehensive evaluation of the nomogram’s predictive accuracy.
Second, calibration was assessed using a calibration plot. This plot compared the predicted probabilities from the nomogram with the observed probabilities of ongoing pregnancy, using the mean values of each group. A closer calibration plot line to the 45° angle corresponded with more accurate predictions. By evaluating discrimination and calibration, the utility and accuracy of the nomogram in predicting ongoing pregnancy could be determined.
Based on the initial analysis and previous studies, possible predictors were selected for the pregnancy prediction model. The characteristics were classified into three distinct phases: Phase 1, encompassing maternal physiology; Phase 2, centered on uterine factors; and Phase 3, revolving around embryo assessment. Elaborate details regarding these phases are in the Supplementary Materials and Methods.
3 Results
3.1 Description of the patient’s characteristics
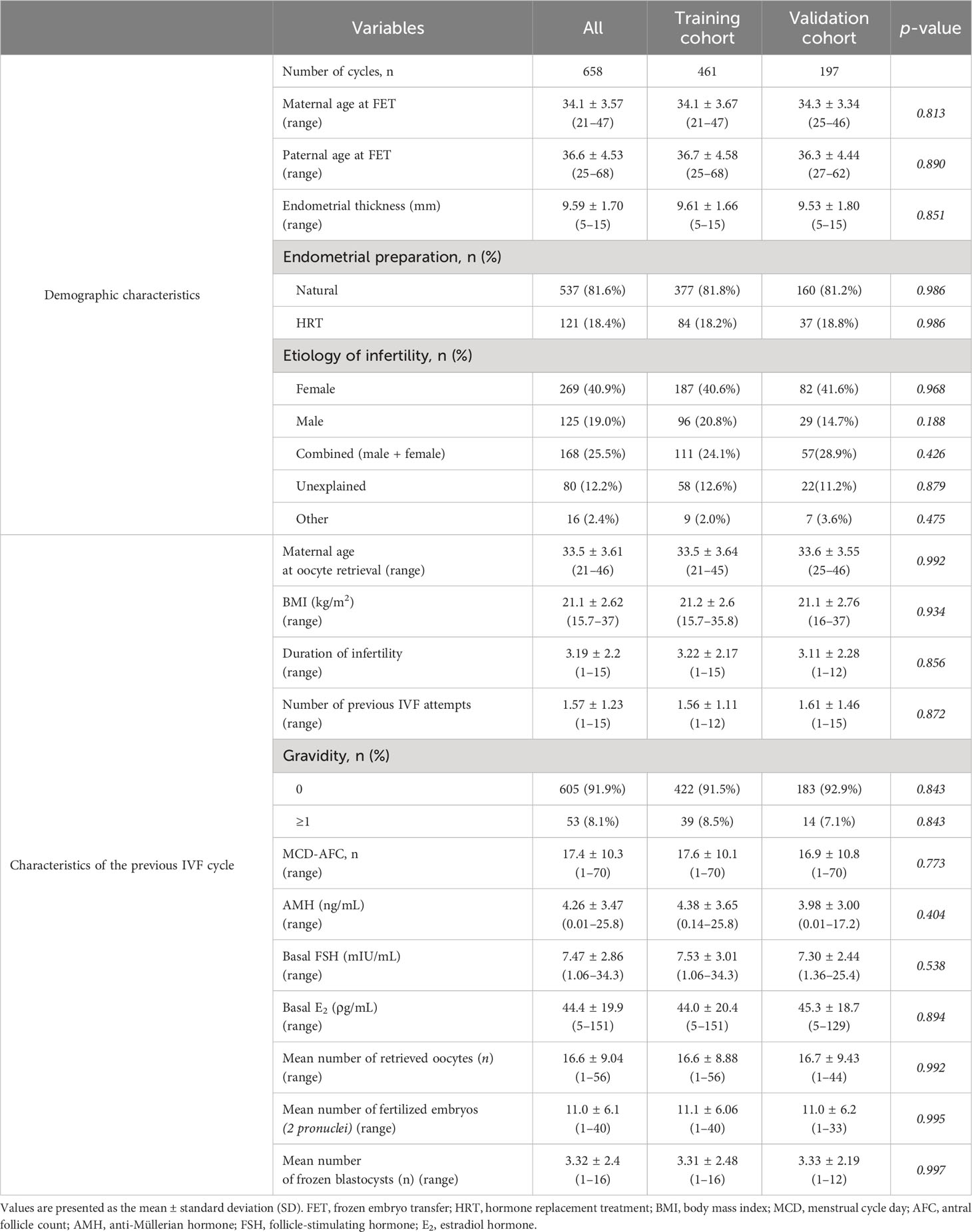
The baseline patient characteristics are summarized in Table 1. In total, 658 patients were analyzed, with 461 and 197 in the training and validation cohorts, respectively. The two cohorts had no significant differences in the demographic characteristics or previous IVF cycles. The mean maternal age at FET was 34.1 ± 3.57 years. No significant differences were observed in endometrial thickness (9.61 ± 1.66 vs. 9.53 ± 1.80 mm) or preparation method between the training and validation cohorts. Furthermore, no significant difference was observed in both cohorts for infertility etiology. AMH (4.38 ± 3.65 vs. 3.98 ± 3.00 ng/mL), basal FSH (7.53 ± 3.01 vs. 7.30 ± 2.44 mlU/mL), and E2 levels (44.0 ± 20.4 vs. 45.3 ± 18.7 ρg/mL) also did not differ significantly in previous IVF cycles between both cohorts. Moreover, no significant differences existed in the number of retrieved oocytes (16.6 ± 8.88 vs. 16.7 ± 9.43), the number of fertilized embryos (11.1 ± 6.06 vs. 11.0 ± 6.2), or the number of frozen blastocysts (3.31 ± 2.48 vs. 3.33 ± 2.19) between the two cohorts.
3.2 Association between eight predictor variables and ongoing pregnancy
First, independent variables associated with sustained pregnancy rates were evaluated in terms of maternal physiology, uterine factor, and embryo assessment. Regarding maternal physiology, univariate and multivariable logistic regression analyses revealed a significant association with maternal age at oocyte retrieval and serum AMH levels (Supplementary Table 2). However, only maternal age at FET, menstrual cycle day-antral follicle count (AFC), and endometrial thickness significantly correlated in the univariate logistic regression analysis. Female body mass index, duration of infertility, number of previous IVF attempts, gravidity, basal FSH level, basal E2 level, and endometrial preparation protocols were not associated with sustained pregnancy rates.
Similarly, the relationship between uterine factors and ongoing pregnancy was evaluated using univariate and multivariable logistic regression analyses. Adenomyosis was the only variable significantly associated with ongoing pregnancy (Supplementary Table 3). However, myoma only significantly correlated in the univariate logistic regression analysis. Uterine position, length, anteroposterior diameter, width, volume, endometrial (EM) pathology findings, and cul-de-sac fluid were not associated with sustained pregnancy rates.
Finally, we evaluated the relationship from the perspective of embryo assessment using univariate and multivariable logistic regression analyses. Of the 10 independent variables, the number of fertilized embryos, BQS, day of vitrification, re-expansion speed, and presence of embryo string were significantly correlated. The number of retrieved oocytes and embryo diameter were significant only in the univariate logistic regression analysis. The remaining variables, including fertilization method, number of vitrified blastocysts, and total vitrification, were not associated with sustained pregnancy rates (Supplementary Table 4).
The MLR results for the eight potential predictors of ongoing pregnancies are presented in Table 2. Eight predictors were significant: 1) maternal physiology (maternal age at oocyte retrieval and serum AMH level), 2) uterine factor (adenomyosis), and 3) embryo assessment (number of fertilized embryos, blastocyst morphology, blastulation day, blastocyst re-expansion speed, and presence of an embryo string).
3.3 Development of a nomogram for predicting ongoing pregnancy outcomes in SVBT cycles
We developed a nomogram to predict the probability of achieving ongoing pregnancy, as summarized in Figures 2A, B, which display the regression coefficients of the eight variables. Each factor is scored and represented by a line below the corresponding score. The length of each line is based on the magnitude of the regression coefficients. The total score represents the sum of all factor scores, and the corresponding probability values are shown. Adenomyosis had the greatest impact on ongoing pregnancy, as indicated by the longest line in the figure. An example of nomogram prediction for the ongoing pregnancy rate is illustrated in Figure 2B. For a couple undergoing ART treatment, the maternal age at oocyte retrieval was ≥38 years (0 points), the AMH level was 4.1 (44.72 points), the absence of adenomyosis was at 100 points, fertilization of ≤7 embryos was at 0 points, good blastocyst quality score was 36.33 points, vitrification on day 6 was at 0 points), blastocyst re-expansion speed of 100.1 was at 51.76 points, and the presence of embryo string was at 26.97 points. The cumulative score based on these various prediction indicators was 259.78, resulting in a predicted ongoing pregnancy rate of 0.9242 (92.42%).
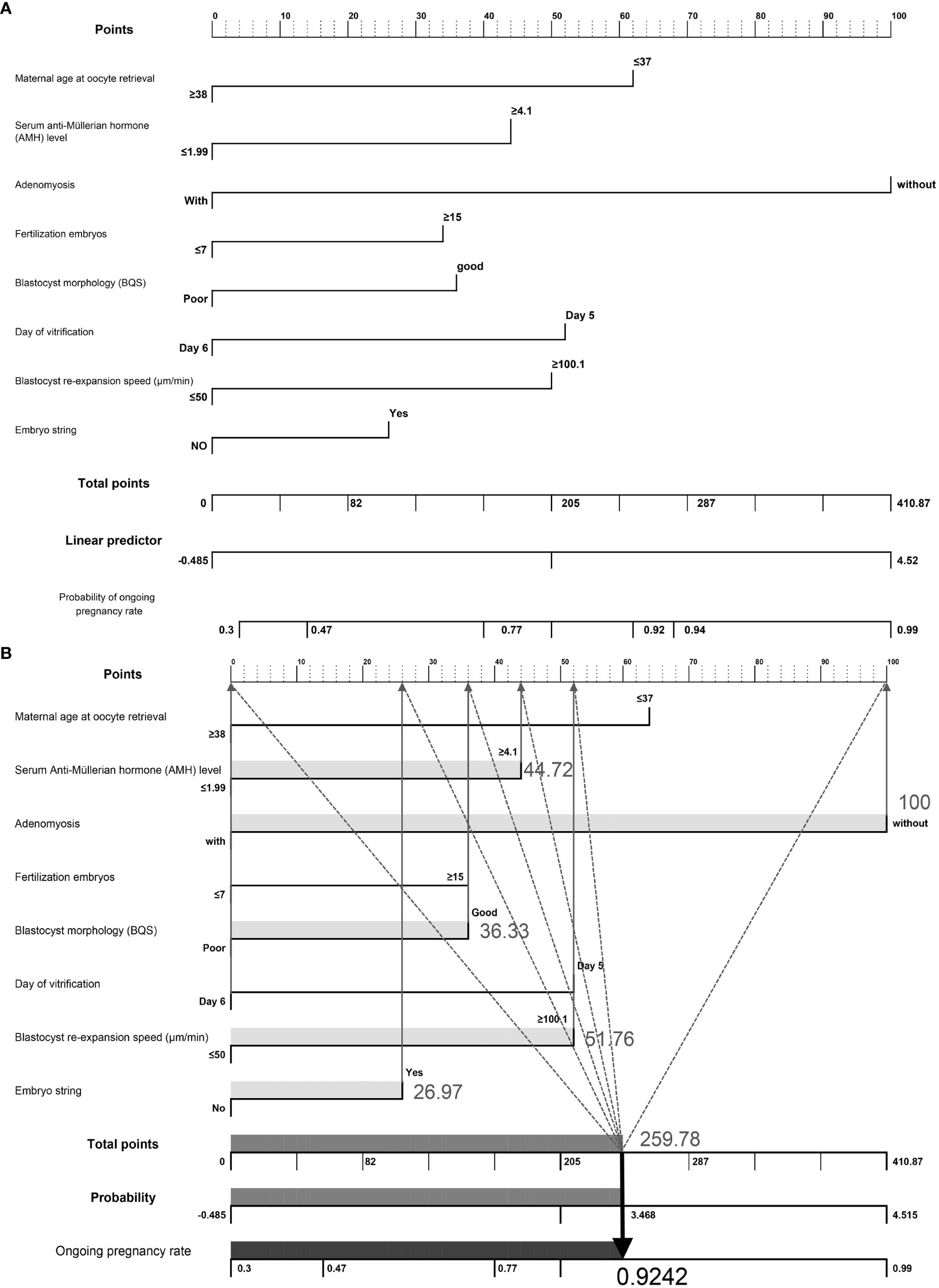
Figure 2 (A) Visualization of a nomogram to predict the probability of ongoing pregnancy in patients with infertility undergoing vitrified-warmed single embryo transfer. (B) Example of nomogram prediction for ongoing pregnancy rate. A couple with assisted reproductive technology treatment: maternal age at oocyte retrieval, ≥38 years (0 points); anti-Müllerian hormone level, 4.1 (44.72 points); without adenomyosis, (100 points); fertilized embryos, ≥ 7 (0 points); BQS, good (36.33 points); day of vitrification, day 6 (0 points); blastocyst re-expansion speed, 100.1 (51.76 points); presence of embryo string, (26.97 points). The cumulative score of the various predictive indicators was 44.72 + 100 + 36.33 + 51.76 + 26.97 = 259.78, and the corresponding predicted ongoing pregnancy rate was 0.9242 (92.42%).
3.4 Nomogram validation
The nomogram was validated using the AUROC and a calibration plot to determine the optimal threshold point. ROC curves were used to assess the nomogram prediction accuracy (Figure 3). In the training cohort, the AUROC was 0.675 (95% CI, 0.622–0.729), indicating good performance with a 79.85% sensitivity and 50.49% specificity (Figure 3A). The validation cohort had similar results, with an AUROC of 0.656 (95% CI, 0.573–0.739), a 91.79% sensitivity, and a 37.36% specificity (Figure 3B). The slopes of the calibration plots in the training and validation cohorts demonstrated good agreement between the predicted and ideal lines (Figures 3C, D).
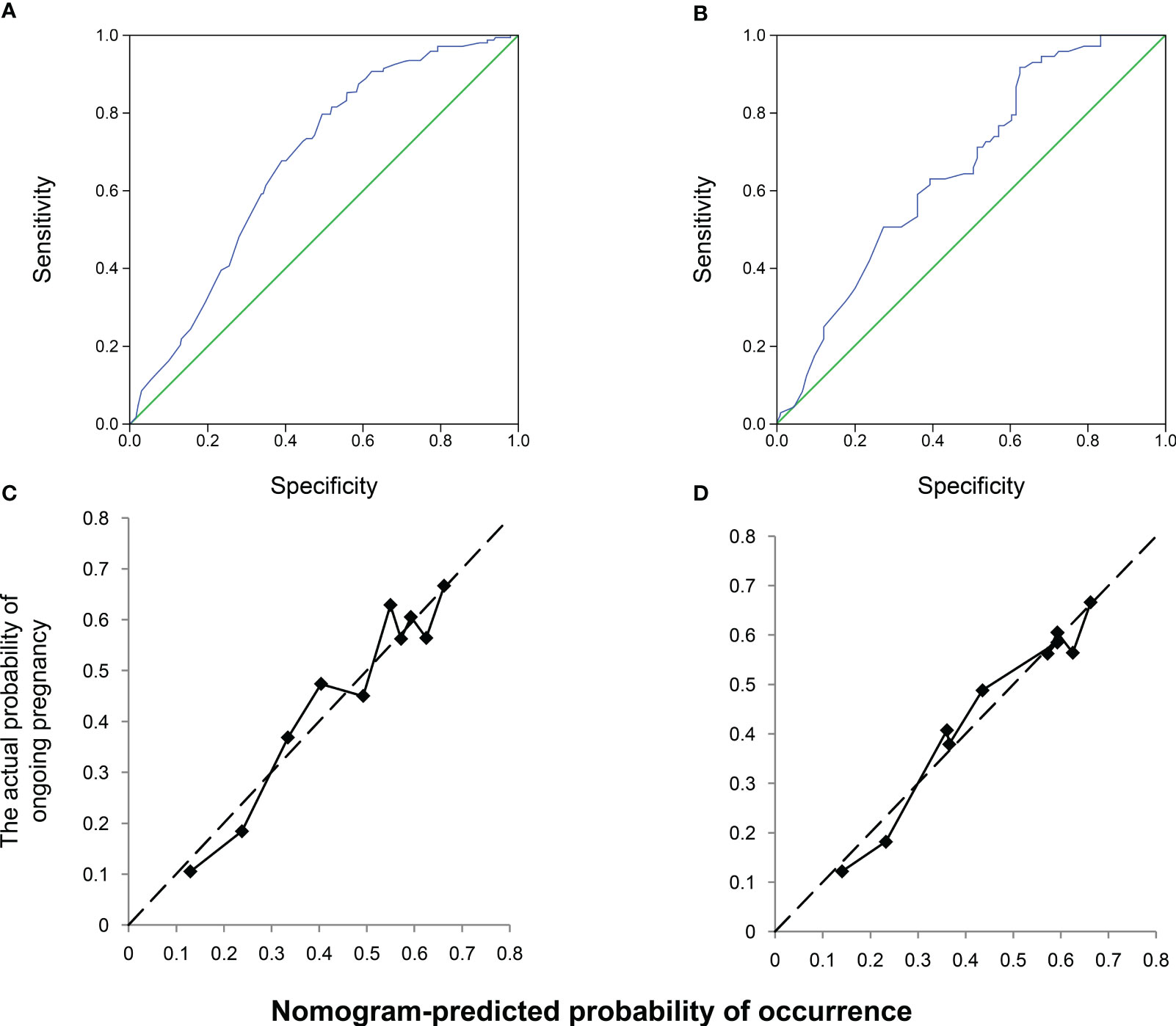
Figure 3 Receiver operating characteristic (ROC) and calibration curves of the nomogram. Discrimination between the training and validation cohorts. (A) ROC curve of the training modeling cohort: the area under the curve of the predictive model was 0.675 (95% confidence interval [CI], 0.622–0.729); (B) AUROC of the model, 0.656 (95% CI; 0.573–0.739); calibration curves of the nomogram in the (C) training and (D) validation groups.
4 Discussion
4.1 Principal findings
In our study, we conducted MLR analysis and identified eight significant variables that were independent predictors of ongoing pregnancy outcomes. These predictors included maternal physiology (maternal age at oocyte retrieval and serum AMH level), uterine factor (adenomyosis), and embryo assessment (number of fertilized embryos, blastocyst morphology, blastulation day, blastocyst re-expansion speed, and presence of embryo string). Using these predictors, we developed a prediction model that combined ongoing pregnancy outcome characteristics, which was validated in both training cohorts. This model demonstrated that ongoing pregnancy outcomes can be predicted by combining the key characteristics of maternal physiology, uterine factors, and major embryonic features. By estimating personalized probabilities and guiding potential treatment options for patients with infertility, our approach can improve the success of in vitro treatments. Our findings provide essential data for patient counseling, enabling attending physicians and embryo experts to generalize and provide clinically effective patient counseling.
4.2 Clinical interpretations of analytic variables
4.2.1 Maternal age at oocyte retrieval
Maternal age, a widely recognized factor, negatively affects pregnancy outcomes in numerous prior studies (10, 21, 22). In a recent study, maternal aging significantly affected embryonic development from oocyte maturation to blastocyst formation (21). Additionally, a meta-analysis indicated that as the women advance in age at the time of ART, the pregnancy rate decreases by 6% (23). Our findings are consistent with those of previous studies demonstrating that maternal age is crucial for pregnancy outcomes, which, according to Ottosen et al., is unsurprising (24). Therefore, promoting childbirth at a younger age is recommended to enhance the likelihood of successful childbirth and mitigate the adverse effects of age-related decline in fertility. Additionally, future research should prioritize advancements to tackle this challenge and instigate a comprehensive reassessment of prevailing methodologies.
4.2.2 Serum AMH level
According to a systematic review by Broer et al., AMH was proposed as the best predictor of poor response after IVF to at least the same extent as AFC (25). Furthermore, Tremellen et al. reported that serum AMH levels correlate with the ovarian response to gonadotropin stimulation during IVF, making it a widely used ovarian reserve marker (26). Balachandren N’s study also revealed a positive correlation between higher AMH levels (22.1 ρmol/L vs. 10.5 ρmol/L) and cumulative live birth after one cycle of IVF and the transfer of all frozen embryos (27). Consistent with previous studies, our findings indicated that higher serum AMH levels increase the probability of ongoing pregnancy. Conversely, lower levels of AMH, <1.99 ng/mL, negatively affected ongoing pregnancy rates. However, as emphasized in a recent review article by Kotlyar et al., the predictive value of AMH as a qualitative marker for oocyte and pregnancy outcomes remains unclear (28). Therefore, further research is required to investigate the role of AMH as a qualitative marker and its impact on pregnancy outcomes.
4.2.3 Uterine factor
This study identified an association between uterine factors, specifically adenomyosis, and pregnancy outcomes. Recently, Wu et al. developed a validated model for predicting live births in patients with adenomyosis undergoing FET, where a uterine volume > 102.02 cm³ was a significant predictor (29). Similarly, Li et al. reported a uterine volume cut-off of > 98.81 cm³ as a predictor of lowered live birth rate in patients with adenomyosis undergoing FET (30). Another study reported a low success rate and a significantly higher miscarriage rate following IVF in women with adenomyosis than in those without adenomyosis (31). Adenomyosis causes adverse pregnancy outcomes by affecting uterine contractility, endometrial function, and receptivity (32). Previous studies have revealed that an optimal uterine length is associated with increased pregnancy rates, while an excessively large uterus with high volume decreases the clinical pregnancy rate (33, 34). However, our study identified a significant difference in the ongoing pregnancy rate based on the presence of adenomyosis only. In contrast, other factors, such as uterine length and volume, did not significantly correlate. These different and partly conflicting study results may be attributed to varying study designs and population sizes. Further research using more reliable measurement techniques is required to explore the racial and regional correlations of uterine factors in greater detail.
4.2.4 Number of fertilized embryos
This study’s results suggest that an increased number of fertilized embryos is associated with a higher rate of ongoing pregnancies. A greater number of oocytes and fertilized embryos leads to the development of high-quality blastocysts (35, 36). Moreover, large-scale national data (SART data) have demonstrated that up to five blastocysts increased the chances of pregnancy and childbirth. In contrast, subsequent increases were related to a decreasing trend in pregnancy and childbirth rates (37). Additionally, one study proposed that a goal of 10 oocytes is reasonable for maintaining a balance between patient safety and efficiency (38). This study focused only on patients who underwent FET; nonetheless, the results partly concur with those of previous studies. No proportional relationship was observed between the number of retrieved eggs and blastocysts produced; however, more fertilized embryos led to an improved pregnancy rate. Opportunities for conception and childbirth differ significantly across institutions and locations, depending on various ART factors. Therefore, a comprehensive examination of the relationships among the patient, culture, and transplant conditions, and other factors is necessary.
4.2.5 Blastocyst morphology
Over the past few decades, our understanding of embryonic development, conception, and pregnancy has significantly increased. Furthermore, many studies have highlighted the fundamental and crucial role of morphological characteristics as predictors of embryo selection (39–41). Previous studies have suggested that top-quality embryos with high-quality characteristics maintained after warming increase the probability of pregnancy while maintaining their morphological features (42–44). Thus, high-quality embryo transfer is the most critical factor in live birth rates. Our previous study also demonstrated that good-quality embryos exhibit high clinical characteristics, and their importance has been continually emphasized (17–19). However, recent studies have revealed that embryo selection based solely on morphological characteristics is limited in predicting the possibility of pregnancy (45). Therefore, future research should focus on selecting good embryos to provide more objective and quantitative measurements, resulting in greater insights into evaluating their usefulness in improving clinical outcomes.
4.2.6 Blastulation day
With advancements in culture technology, blastocyst transfer is now commonly performed in many IVF centers (46). Regarding development, some embryos reach the blastocyst stage on day 5 of the culture, while others develop into blastocysts on day 6 or 7 (47, 48). Each embryo has characteristics that make its development dynamic. Studies have compared the IVF outcomes of day 5 and day 6 embryo transfer to determine the more efficient embryo (49, 50). However, the optimal approach remains elusive and unclear (48, 51–53). Some studies have suggested that the development time of the blastocyst stage is crucial, whereas others have emphasized the quality of the blastocyst stage over the vitrification time. Our results revealed that day 5 frozen embryos were more efficient than day 6 embryos. However, a binary comparison between freezing time and embryo quality criteria is insufficient. Further research is needed to identify other factors that may influence blastocyst development and analyze their sub-correlations more thoroughly. Therefore, in-depth studies on the relationship between freezing time and quality are required.
4.2.7 Blastocyst re-expansion speed
Recently, studies have focused on identifying key factors that affect the morphological parameters of embryos during the early developmental dynamic processes using practical analyses based on time-lapse imaging. Traditionally, in many studies predicting embryonic characteristics post-warming, re-expansion speed has been highlighted as a crucial parameter, and Ahlstrom et al. identified it as the most important parameter for prioritizing embryo transfer, as embryos with >60% viability within 2–4 h should be transferred first (39). In addition, Shu et al. reported that 78% of embryos displayed re-expansion within 3–4 h of thawing (54). Similarly, studies have uncovered a strong correlation between the maximum and minimum cross-sectional axes measurements and re-expansion outcomes (55–58). Consistent with previous studies, our use of TLS imaging revealed that embryos with faster developmental speeds, as indicated by the quantitative measurement of re-expansion speed, yielded better results.
4.2.8 Cytoplasmic (blastocyst) string
The function of cytoplasmic strings (CS) during early embryonic development remains poorly understood. They generally consist of long, thin projections (0.1–0.3 mm) that connect the inner cell mass and trophectoderm during blastocyst formation (59). Some studies have suggested the importance of CS in human blastocysts; however, current literature indicates that its significance may be limited, and other studies have observed no negative effects (60). In mice, CS promotes cell expansion and migration and is rich in actin (59). Ebner et al. observed a possible association between CS and blastocoel collapse and morphological features in human studies but uncovered no adverse effects on live birth rates or neonatal outcomes (60). Conversely, Eastick et al. proposed a possible relationship between the presence of CS in human blastocysts and the cumulative pregnancy rate (61, 62). Our results revealed that one or more CS were observed in good-quality embryos, with greater early- and mid-stage observations, which disappeared as expansion progressed. The shape and width of the CS vary with the degree of expansion. Our findings are consistent with those of Eastick et al., indicating that CS appear dynamically in the most potentially viable embryos within a short period during the early stages.
4.3 Highlight and interpretation of findings
Recently, infertility treatments have increasingly relied on ART and personalized procedures, reflecting the evolution of the medical paradigm toward evidence-based medicine. Emotional understanding between medical professionals and patients is essential to ensure safety, efficiency, and a reduced time to achieving pregnancy. The commonly applied one-size-fits-all IVF treatment may not be suitable for women with infertility who desire to conceive because of the complex interactions between each individual’s endocrine function, embryo status, and uterine factors. Our research proposes a personalized approach that comprehensively considers three factors, maternal physiology, intrauterine conditions, and embryo evaluation, to provide evidence-based and comprehensive information that meets the needs of patients and fosters a trusting relationship. Pregnancy is a continuous process; therefore, continuous collection and investigation of factors affecting pregnancy outcomes is essential. More rigorous personalized tools will assist more patients in establishing healthy families through IVF in the future, thereby increasing the overall success and utilization rates of ART.
4.4 Strengths and weaknesses of the study
This study has several strengths, including the comprehensive evaluation of the combined impact of clinical and biological characteristics on the ongoing pregnancy rate in women undergoing SVBT, which has been challenging to identify in previous ART studies. Furthermore, this study highlights the patterns and significance of multiple variables for prediction, confirming a positive association between maternal physiology, uterine factors, embryo assessment, and pregnancy success. This study revealed that these variables are basic and practical factors that can be easily obtained in the laboratory, making them user-friendly combinations of clinical and embryonic morphological characteristics.
The results of studies conducted in the UK and Italy were similar (63, 64). The model developed and validated in our study demonstrated some discriminatory ability; however, it did not demonstrate substantial superiority over other validation models. Numerous factors can influence pregnancy outcomes, and our study may have limitations in capturing all of them, particularly male sperm and genetic influences. In other words, this limitation could be attributed to the unpredictable nature of cycle characteristics and the limitations of retrospective data. Therefore, prospective studies are necessary for reliable validation. Additionally, the nomogram was developed based on data obtained from a single center and internally validated; thus, further external validation at multiple centers is required. Furthermore, innovative approaches and models should be tested using routine measures in clinical practice, and new variables and treatment approaches should be individualized.
5 Conclusion
Eight critical predictors were identified from a pool of 28 candidate variables, with clinical perspective being critical in the models. The resulting nomogram is a user-friendly tool that integrates readily available clinical and biological characteristics, such as maternal physiology, uterine factor, and embryo assessment, to predict ongoing pregnancy outcomes in infertile patients undergoing SVBT. Moreover, it offers more informative predictions than continuous pre-cycle pregnancy rates in routine practice. Sharing this model with patients can alleviate uncertainty and confusion regarding fertility treatment options, thereby increasing confidence in making informed decisions. The findings of this study are summarized in Figure 4.
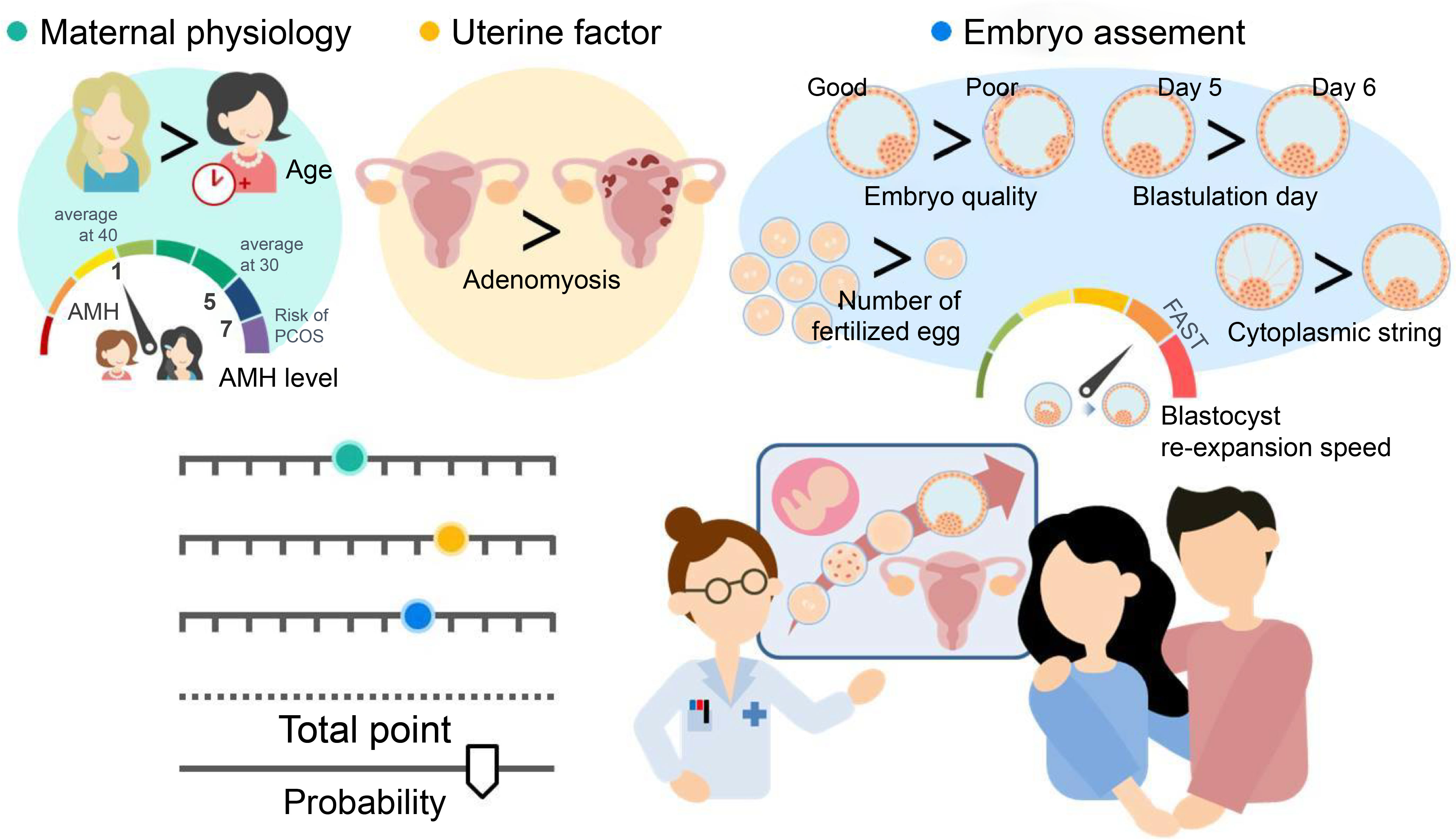
Figure 4 The concept derived from all the data obtained. Our results reveal that the nomogram is a user-friendly tool that combines readily available clinical and biological characteristics, such as maternal physiology (maternal age at oocyte retrieval and serum anti-Müllerian hormone [AMH] level), uterine factors (adenomyosis), and embryo assessment (number of fertilized embryos, blastocyst morphology, blastulation day, blastocyst re-expansion speed, and presence of embryo string). Shared decision-making involves exchanging information between patients and physicians based on objective data to facilitate understanding.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Institutional Review Board (IRB) of the CHA Gangnam Medical Center (GCI 2022-06-008-002). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JKP: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Validation, Writing – original draft, Writing – review & editing. JEP: Conceptualization, Data curation, Writing – original draft, Writing – review & editing, Validation, Formal Analysis. SB: Writing – review & editing, Investigation, Validation. HJJ: Writing – review & editing, Formal Analysis. JWK: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing. WSL: Conceptualization, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by a grant from the Korean Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HI21C1560020021).
Acknowledgments
The authors sincerely thank Jun Hee Choe for providing statistical support for this manuscript. They would also like to thank the embryologists and physicians at the CHA Fertility Center Gangnam, who contributed significantly to this project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1257764/full#supplementary-material
Abbreviations
AMH, Anti-Müllerian hormone; AFC, Antral follicle count; AUC, Area under the curve; ART, Assisted reproductive technology; BQS, Blastocyst morphology score; CS, Cytoplasmic strings; FSH, Follicle-stimulating hormone; FET, Frozen embryo transfer; IRB, Institutional Review Board; IVF, In vitro fertilization; MLR, Multivariable logistic regression; FOC, Receiver operating characteristic; E2, Serum estradiol; SVBT, Single vitrified-warmed blastocyst embryo transfer; SD, Standard deviation; TLS, Time lapse study.
References
1. Rienzi L, Gracia C, Maggiulli R, LaBarbera AR, Kaser DJ, Ubaldi FM, et al. Oocyte, embryo and blastocyst cryopreservation in ART: systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum Reprod Update (2017) 23:139–55. doi: 10.1093/humupd/dmw038
2. He Y, Chen S, Liu J, Kang X, Liu H. Effect of blastocyst morphology and developmental speed on transfer strategy for grade "C" blastocyst in vitrified-warmed cycles. J Ovarian Res (2021) 14:51. doi: 10.1186/s13048-021-00798-w
3. Nagy ZP, Shapiro D, Chang CC. Vitrification of the human embryo: a more efficient and safer in vitro fertilization treatment. Fertil Steril (2020) 113:241–7. doi: 10.1016/j.fertnstert.2019.12.009
4. Bourdon M, Maignien C, Pocate-Cheriet K, Plu Bureau G, Marcellin L, Patrat C, et al. The freeze-all strategy after IVF: which indications? Reprod BioMed Online (2021) 42:529–45. doi: 10.1016/j.rbmo.2020.11.013
5. Wu AK, Elliott P, Katz PP, Smith JF. Time costs of fertility care: the hidden hardship of building a family. Fertil Steril (2013) 99:2025–30. doi: 10.1016/j.fertnstert.2013.01.145
6. Rockliff HE, Lightman SL, Rhidian E, Buchanan H, Gordon U, Vedhara K. A systematic review of psychosocial factors associated with emotional adjustment in in vitro fertilization patients. Hum Reprod Update (2014) 20:594–613. doi: 10.1093/humupd/dmu010
7. Légaré F, Ratté S, Stacey D, Kryworuchko J, Gravel K, Graham ID, et al. Interventions for improving the adoption of shared decision making by healthcare professionals. Cochrane Database Syst Rev (2010) 12(5):Cd006732. doi: 10.1002/14651858.CD006732.pub2
8. van der Steeg JW, Steures P, Eijkemans MJ, Habbema JD, Bossuyt PM, Hompes PG, et al. Do clinical prediction models improve concordance of treatment decisions in reproductive medicine? Int J Obst Gynecol (2006) 113:825–31. doi: 10.1111/j.1471-0528.2006.00992.x
9. Eastham JA, Kattan MW, Scardino PT. Nomograms as predictive models. Urol Oncol: Semin Orig (2002) 20:108–15. doi: 10.1053/suro.2002.32936
10. Qu P, Chen L, Zhao D, Shi W, Shi J. Nomogram for the cumulative live birth in women undergoing the first IVF cycle: Based on 26, 689 patients in China. Front Endocrinol (2022) 13:900829. doi: 10.3389/fendo.2022.900829
11. Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol (2008) 26:1364–70. doi: 10.1200/JCO.2007.12.9791
12. Dessolle L, Fréour T, Barrière P, Daraï E, Ravel C, Jean M, et al. A cycle-based model to predict blastocyst transfer cancellation. Hum Reprod (2010) 25:598–604. doi: 10.1093/humrep/dep439
13. Guo Z, Chen W, Wang Y, Chu R, Xu X, Zhang L, et al. Nomogram to predict an endometrial thickness above 7.5 mm in the frozen embryo transfer cycle of women with a thin endometrium. Reprod BioMed Online (2022) 44:324–32. doi: 10.1016/j.rbmo.2021.10.022
14. Zou Y, Zhang Y, Yin Z, Wei L, Lv B, Wu Y. Establishment of a nomogram model to predict macrosomia in pregnant women with gestational diabetes mellitus. BMC Pregnancy Childbirth (2021) 21:581. doi: 10.1186/s12884-021-04049-0
15. Meijerink AM, Cissen M, Mochtar MH, Fleischer K, Thoonen I, de Melker AA, et al. Prediction model for live birth in ICSI using testicular extracted sperm. Hum Reprod (2016) 31:1942–51. doi: 10.1093/humrep/dew146
16. Choi B, Bosch E, Lannon BM, Leveille MC, Wong WH, Leader A, et al. Personalized prediction of first-cycle in vitro fertilization success. Fertil Steril (2013) 99:1905–11. doi: 10.1016/j.fertnstert.2013.02.016
17. Kim HJ, Park JK, Eum JH, Song H, Lee WS, Lyu SW. Embryo selection based on morphological parameters in a single vitrified-warmed blastocyst transfer cycle. Reprod Sci (2021) 28:1060–8. doi: 10.1007/s43032-020-00349-6
18. Park JK, Ahn SY, Seok SH. Clinical usability of embryo development using a combined qualitative and quantitative approach in a single vitrified-warmed blastocyst transfer: assessment of pre-vitrified blastocyst diameter and post-warmed blastocyst re-expansion speed. J Clin Med (2022) 11(23):7085. doi: 10.3390/jcm11237085
19. Park JK, Ahn SY. Does post-warming extended culture duration affect the clinical and obstetric outcomes of patients of advanced maternal age? A single-center study. J Korean Med Sci (2022) 37(12):e96. doi: 10.3346/jkms.2022.37.e96
20. Lee HN, Park JK, Paek SK, Byun JH, Song H, Lee HJ, et al. Does duration of cryostorage affect survival rate, pregnancy, and neonatal outcomes? Large-scale single-center study of slush nitrogen (SN(2)) vitrified-warmed blastocysts. Int J Gynaecol Obstet (2021) 152:351–7. doi: 10.1002/ijgo.13381
21. Ezoe K, Miki T, Akaike H, Shimazaki K, Takahashi T, Tanimura Y, et al. Maternal age affects pronuclear and chromatin dynamics, morula compaction and cell polarity, and blastulation of human embryos. Hum Reprod (2023) 38:387–99. doi: 10.1093/humrep/dead001
22. Xiong F, Sun Q. A nomogram to assist blastocyst selection in vitrified-warmed embryo transfer cycles. J Obstet Gynaecol Res (2022) 48:1816–28. doi: 10.1111/jog.15138
23. Broer SL, van Disseldorp J, Broeze KA, Dolleman M, Opmeer BC, Bossuyt P, et al. Added value of ovarian reserve testing on patient characteristics in the prediction of ovarian response and ongoing pregnancy: an individual patient data approach. Hum Reprod Update (2013) 19:26–36. doi: 10.1093/humupd/dms041
24. Ottosen LD, Kesmodel U, Hindkjaer J, Ingerslev HJ. Pregnancy prediction models and eSET criteria for IVF patients–do we need more information? J Assist Reprod Genet (2007) 24:29–36. doi: 10.1007/s10815-006-9082-9
25. Broer SL, Mol BW, Hendriks D, Broekmans FJ. The role of antimullerian hormone in prediction of outcome after IVF: comparison with the antral follicle count. Fertil Steril (2009) 91:705–14. doi: 10.1016/j.fertnstert.2007.12.013
26. Tremellen KP, Kolo M, Gilmore A, Lekamge DN. Anti-mullerian hormone as a marker of ovarian reserve. Aust N Z J Obstet Gynaecol (2005) 45:20–4. doi: 10.1111/j.1479-828X.2005.00332.x
27. Balachandren N, Salman M, Diu NL, Schwab S, Rajah K, Mavrelos D. Ovarian reserve as a predictor of cumulative live birth. Eur J Obstet Gynecol Reprod Biol (2020) 252:273–7. doi: 10.1016/j.ejogrb.2020.06.063
28. Kotlyar A, Seifer DB. Anti-Müllerian hormone as a qualitative marker - or just quantity? Curr Opin Obstet Gynecol (2020) 32:219–26. doi: 10.1097/GCO.0000000000000623
29. Wu Y, Yang R, Lin H, Cao C, Jiao X, Zhang Q. A validated model for individualized prediction of live birth in patients with adenomyosis undergoing frozen-thawed embryo transfer. Front Endocrinol (2022) 13:902083. doi: 10.3389/fendo.2022.902083
30. Li X, Pan N, Zhang W, Wang Y, Ge Y, Wei H, et al. Association between uterine volume and pregnancy outcomes in adenomyosis patients undergoing frozen-thawed embryo transfer. Reprod BioMed Online (2021) 42:384–9. doi: 10.1016/j.rbmo.2020.10.002
31. Salim R, Riris S, Saab W, Abramov B, Khadum I, Serhal P. Adenomyosis reduces pregnancy rates in infertile women undergoing IVF. Reprod BioMed Online (2012) 25:273–7. doi: 10.1016/j.rbmo.2012.05.003
32. Harada T, Khine YM, Kaponis A, Nikellis T, Decavalas G, Taniguchi F. The impact of adenomyosis on women's fertility. Obstet Gynecol Surv (2016) 71:557–68. doi: 10.1097/OGX.0000000000000346
33. Hawkins LK, Correia KF, Srouji SS, Hornstein MD, Missmer SA. Uterine length and fertility outcomes: a cohort study in the IVF population. Hum Reprod (2013) 28:3000–6. doi: 10.1093/humrep/det344
34. Gao H, Liu DE, Li Y, Tang J, Hu S, Wu X, et al. Uterine size and volume are associated with a higher clinical pregnancy rate in patients undergoing assisted reproduction technology: A longitudinal study (A STROBE-compliant article). Med (Baltimore) (2019) 98:e14366. doi: 10.1097/MD.0000000000014366
35. Hariton E, Kim K, Mumford SL, Palmor M, Bortoletto P, Cardozo ER, et al. Total number of oocytes and zygotes are predictive of live birth pregnancy in fresh donor oocyte in vitro fertilization cycles. Fertil Steril (2017) 108:262–8. doi: 10.1016/j.fertnstert.2017.05.021
36. Scaravelli G, Zacà C, Levi Setti PE, Livi C, Ubaldi FM, Villani MT, et al. Fertilization rate as a novel indicator for cumulative live birth rate: a multicenter retrospective cohort study of 9,394 complete in vitro fertilization cycles. Fertil Steril (2021) 116:766–73. doi: 10.1016/j.fertnstert.2021.04.006
37. Smeltzer S, Acharya K, Truong T, Pieper C, Muasher S. Clinical pregnancy and live birth increase significantly with every additional blastocyst up to five and decline after that: an analysis of 16,666 first fresh single-blastocyst transfers from the Society for Assisted Reproductive Technology registry. Fertil Steril (2019) 112:866–873.e1. doi: 10.1016/j.fertnstert.2019.06.030
38. Li Y, Duan Y, Yuan X, Cai B, Xu Y, Yuan Y. A novel nomogram for individualized gonadotropin starting dose in GnRH antagonist protocol. Front Endocrinol (2021) 12:688654. doi: 10.3389/fendo.2021.688654
39. Ahlström A, Westin C, Wikland M, Hardarson T. Prediction of live birth in frozen-thawed single blastocyst transfer cycles by pre-freeze and post-thaw morphology. Hum Reprod (2013) 28:1199–209. doi: 10.1093/humrep/det054
40. Allen M, Hale L, Lantsberg D, Kieu V, Stevens J, Stern C, et al. Post-warming embryo morphology is associated with live birth: a cohort study of single vitrified-warmed blastocyst transfer cycles. J Assist Reprod Genet (2022) 39:417–25. doi: 10.1007/s10815-021-02390-z
41. Minasi MG, Colasante A, Riccio T, Ruberti A, Casciani V, Scarselli F, et al. Correlation between aneuploidy, standard morphology evaluation and morphokinetic development in 1730 biopsied blastocysts: a consecutive case series study. Hum Reprod (2016) 31:2245–54. doi: 10.1093/humrep/dew183
42. Xiong F, Sun Q, Li G, Yao Z, Chen P, Wan C, et al. Association between the number of top-quality blastocysts and live births after single blastocyst transfer in the first fresh or vitrified-warmed IVF/ICSI cycle. Reprod BioMed Online (2020) 40:530–7. doi: 10.1016/j.rbmo.2020.01.005
43. Irani M, Reichman D, Robles A, Melnick A, Davis O, Zaninovic N, et al. Morphologic grading of euploid blastocysts influences implantation and ongoing pregnancy rates. Fertil Steril (2017) 107:664–70. doi: 10.1016/j.fertnstert.2016.11.012
44. Kim MK, Park JK, Jeon Y, Seok SH, Chang EM, Lee WS. Effects of paternal age on human embryo development in in vitro fertilization with preimplantation genetic screening. Clin Exp Reprod Med (2019) 46:22–9. doi: 10.5653/cerm.2019.46.1.22
45. Kirkegaard K, Ahlström A, Ingerslev HJ, Hardarson T. Choosing the best embryo by time lapse versus standard morphology. Fertil Steril (2015) 103:323–32. doi: 10.1016/j.fertnstert.2014.11.003
46. Langley MT, Marek DM, Gardner DK, Doody KM, Doody KJ. Extended embryo culture in human assisted reproduction treatments. Hum Reprod (2001) 16:902–8. doi: 10.1093/humrep/16.5.902
47. Ueno S, Uchiyama K, Kuroda T, Okimura T, Yabuuchi A, Kobayashi T, et al. Establishment of day 7 blastocyst freezing criteria using blastocyst diameter for single vitrified-warmed blastocyst transfer from live birth outcomes: a single-center, large cohort, retrospectively matched study. J Assist Reprod Genet (2020) 37:2327–35. doi: 10.1007/s10815-020-01882-8
48. Tannus S, Cohen Y, Henderson S, Al Ma'mari N, Shavit T, Son WY, et al. Fresh transfer of Day 5 slow-growing embryos versus deferred transfer of vitrified, fully expanded day 6 blastocysts: which is the optimal approach? Hum Reprod (2019) 34:44–51. doi: 10.1093/humrep/dey351
49. Park DS, Kim JW, Chang EM, Lee WS, Yoon TK, Lyu SW. Obstetric, neonatal, and clinical outcomes of day 6 vs. day 5 vitrified-warmed blastocyst transfers: retrospective cohort study with propensity score matching. Front Endocrinol (2020) 11:499. doi: 10.3389/fendo.2020.00499
50. He Y, Tang Y, Liu H, Liu J, Mao Y. No advantage of single day 6 good-quality blastocyst transfer versus single day 5 poor-quality blastocyst transfer in frozen-thawed cycles stratified by age: a retrospective study. BMC Pregnancy Childbirth (2023) 23:79. doi: 10.1186/s12884-023-05387-x
51. Shi W, Zhou H, Chen L, Xue X, Shi J. Live birth rate following frozen-thawed blastocyst transfer is higher in high-grade day 6 blastocysts than in low-grade day 5 blastocysts. Front Endocrinol (2022) 13:1066757. doi: 10.3389/fendo.2022.1066757
52. Jiang Y, Song G, Zhang XH, Miao SB, Wu XH. Frozen blastocysts: assessing the importance of day 5/day 6 blastocysts or blastocyst quality. Exp Ther Med (2022) 23:333. doi: 10.3892/etm.2022.11262
53. Lane SL, Reed L, Schoolcraft WB, Katz-Jaffe MG. Euploid day 7 blastocysts of infertility patients with only slow embryo development have reduced implantation potential. Reprod BioMed Online (2022) 44:858–65. doi: 10.1016/j.rbmo.2021.08.027
54. Shu Y, Watt J, Gebhardt J, Dasig J, Appling J, Behr B. The value of fast blastocoele re-expansion in the selection of a viable thawed blastocyst for transfer. Fertil Steril (2009) 91:401–6. doi: 10.1016/j.fertnstert.2007.11.083
55. Sciorio R, Thong D, Thong KJ, Pickering SJ. Clinical pregnancy is significantly associated with the blastocyst width and area: a time-lapse study. J Assist Reprod Genet (2021) 38:847–55. doi: 10.1007/s10815-021-02071-x
56. Sciorio R, Meseguer M. Focus on time-lapse analysis: blastocyst collapse and morphometric assessment as new features of embryo viability. Reprod BioMed Online (2021) 43:821–32. doi: 10.1016/j.rbmo.2021.08.008
57. Huang TT, Huang DH, Ahn HJ, Arnett C, Huang CT. Early blastocyst expansion in euploid and aneuploid human embryos: evidence for a non-invasive and quantitative marker for embryo selection. Reprod BioMed Online (2019) 39:27–39. doi: 10.1016/j.rbmo.2019.01.010
58. van Marion ES, Chavli EA, Laven JSE, Steegers-Theunissen RPM, Koster MPH, Baart EB. Longitudinal surface measurements of human blastocysts show that the dynamics of blastocoel expansion are associated with fertilization method and ongoing pregnancy. Reprod Biol Endocrinol: RB&E (2022) 20:53. doi: 10.1186/s12958-022-00917-2
59. Salas-Vidal E, Lomelí H. Imaging filopodia dynamics in the mouse blastocyst. Dev Biol (2004) 265:75–89. doi: 10.1016/j.ydbio.2003.09.012
60. Ebner T, Sesli Ö, Kresic S, Enengl S, Stoiber B, Reiter E, et al. Time-lapse imaging of cytoplasmic strings at the blastocyst stage suggests their association with spontaneous blastocoel collapse. Reprod BioMed Online (2020) 40:191–9. doi: 10.1016/j.rbmo.2019.11.004
61. Eastick J, Venetis C, Cooke S, Chapman M. The presence of cytoplasmic strings in human blastocysts is associated with the probability of clinical pregnancy with fetal heart. J Assist Reprod Genet (2021) 38:2139–49. doi: 10.1007/s10815-021-02213-1
62. Ma BX, Yang L, Tian Y, Jin L, Huang B. Cytoplasmic strings between ICM and mTE are a positive predictor of clinical pregnancy and live birth outcomes: A time-lapse study. Front Med (2022) 9:934327. doi: 10.3389/fmed.2022.934327
63. Smith AD, Tilling K, Lawlor DA, Nelson SM. External validation and calibration of IVFpredict: a national prospective cohort study of 130,960 in vitro fertilisation cycles. PloS One (2015) 10:e0121357. doi: 10.1371/journal.pone.0121357
Keywords: nomogram, prediction model, ongoing pregnancy outcome, single vitrified-warmed blastocyst embryo transfer, in vitro fertilization
Citation: Park JK, Park JE, Bang S, Jeon HJ, Kim JW and Lee WS (2023) Development and validation of a nomogram for predicting ongoing pregnancy in single vitrified-warmed blastocyst embryo transfer cycles. Front. Endocrinol. 14:1257764. doi: 10.3389/fendo.2023.1257764
Received: 12 July 2023; Accepted: 10 October 2023;
Published: 23 November 2023.
Edited by:
Johannes Ott, Medical University of Vienna, AustriaReviewed by:
Sofia Amjad, Ziauddin University, PakistanMarlene Hager, Medical University of Vienna, Austria
Copyright © 2023 Park, Park, Bang, Jeon, Kim and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ji Won Kim, aGFwcHlqaXdvbkBjaGFtYy5jby5rcg==; Woo Sik Lee, d29vc2xlZV9kZUBjaGFtYy5jby5rcg==
†These authors have contributed equally to this work and share first authorship
‡ORCID: Jae Kyun Park, orcid.org/0000-0002-5333-4990
Ji Eun Park, orcid.org/0009-0002-9878-5813
Soyoung Bang, orcid.org/0000-0002-0286-4131
Haeng Jun Jeon, orcid.org/0000-0003-1816-5789
Ji Won Kim, orcid.org/0000-0001-5066-1593
Woo Sik Lee, orcid.org/0000-0002-2329-1774
 Jae Kyun Park
Jae Kyun Park Ji Eun Park
Ji Eun Park Soyoung Bang‡
Soyoung Bang‡