
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 29 November 2023
Sec. Thyroid Endocrinology
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1256208
This article is part of the Research Topic Direct or Indirect Endocrine and Metabolic Consequences of Malignancies View all 10 articles
Objective: The causal relationship between Rheumatoid arthritis (RA) and hypothyroidism/hyperthyroidism remains controversial due to the limitations of conventional observational research, such as confounding variables and reverse causality. We aimed to examine the potential causal relationship between RA and hypothyroidism/hyperthyroidism using Mendelian randomization (MR).
Method: We conducted a bidirectional two-sample univariable analysis to investigate the potential causal relationship between hypothyroidism/hyperthyroidism and RA. Furthermore, we performed a multivariate analysis to account for the impact of body mass index (BMI), smoking quantity, and alcohol intake frequency.
Results: The univariable analysis indicated that RA has a causative influence on hypothyroidism (odds ratio [OR]=1.07, 95% confidence interval [CI]=1.01–1.14, P=0.02) and hyperthyroidism (OR=1.32, 95% CI=1.15–1.52, P<0.001). When hypothyroidism/hyperthyroidism was considered as an exposure variable, we only observed a causal relationship between hypothyroidism (OR=1.21, 95% CI=1.05–1.40, P=0.01) and RA, whereas no such connection was found between hyperthyroidism (OR=0.91, 95% CI=0.83–1.01, P=0.07) and RA. In the multivariate MR analyses, after separately and jointly adjusting for the effects of daily smoking quantity, alcohol intake frequency, and BMI, the causal impact of RA on hypothyroidism/hyperthyroidism and hypothyroidism on RA remained robust. However, there is no evidence to suggest a causal effect of hyperthyroidism on the risk of RA (P >0.05).
Conclusion: Univariate and multivariate MR analyses have validated the causal association between RA and hypothyroidism/hyperthyroidism. Hypothyroidism confirmed a causal relationship with RA when employed as an exposure variable, whereas no such relationship was found between hyperthyroidism and RA.
Rheumatoid arthritis (RA) is a common autoimmune disease characterized by persistent joint pain and the degradation of joint cartilage and bone. Moreover, it causes varying degrees of harm to various extra-articular systems. In the general population, the prevalence of RA ranges from 0.5% to 1%, with a higher incidence in women than in men (1). RA leads to functional impairment, reduced work capacity, and a lower quality of life, substantially burdening individuals and society (2, 3). Furthermore, RA is associated with a significantly higher mortality rate than the general population, with approximately 40% of patients with RA succumbing to cardiovascular disease (4, 5). Thyroid dysfunction, encompassing hyperthyroidism, hypothyroidism, subclinical hyperthyroidism, and subclinical hypothyroidism, is a common endocrine disorder diagnosed primarily through biochemical indicators such as thyroid stimulating hormone (TSH), triiodothyronine (T3), thyroxine (T4), free triiodothyronine (FT3), and free thyroxine (FT4). The prevalence of hyperthyroidism ranges from 0.2% to 1.3% (6, 7), while that of hypothyroidism varies from 0.2 to 5.3% (6, 8). Hyperthyroidism and hypothyroidism could impact various bodily systems, including the integumentary, muscular, skeletal, cardiovascular, nervous, digestive, endocrine, and circulatory systems. While most studies have suggested a link between RA and thyroid dysfunction (9), one study found no significant difference in the incidence and prevalence of hypothyroidism between patients with and without RA (10).
Therefore, the potential causal relationship between RA and hypothyroidism/hyperthyroidism requires further investigation. Most of our insights into the relationship between hypothyroidism/hyperthyroidism and RA are derived from observational studies, which are susceptible to reverse causality, selective bias, and confounding variables. Therefore, further research employing innovative methodologies is warranted.
Mendelian randomization (MR) is one such technique that utilizes genetic variation as an instrumental variable to assess causal relationships between exposures and specific outcomes (11).
We acquired summary statistics for RA from the MRCIEU GWAS database available at https://gwas.mrcieu.ac.uk/. The pooled GWAS data, involving individuals of European ancestry, comprised a population of 58,284 for subsequent analysis (GWAS ID: ieu-a-832), with 14,361 cases and 42,923 control participants (12). We obtained summary data from the publicly available FinnGen Biobank for the GWAS datasets associated with hypothyroidism and hyperthyroidism. The dataset for hypothyroidism (GWAS ID: finn-b-E4_HYTHYNAS) comprised 26,306 cases and 187,684 controls, while hyperthyroidism (GWAS ID: finn-b-AUTOIMMUNE_HYPERTHYROIDISM) comprised 962 cases and 172,976 controls. In these datasets, hypothyroidism was defined as “Hypothyroidism, other/unspecified,” and hyperthyroidism as “Autoimmune hyperthyroidism.” Data regarding smoking quantity were extracted from the GWAS and Sequencing Consortium of Alcohol and Nicotine use (GSCAN) (13) (cigarettes per day: GWAS ID: ieu-b-25, sample size: 337,334). Summary-level data for alcohol intake frequency were obtained from the UK BioBank (GWAS ID: ukb-a-25, sample size: 336,965). Genetic instruments associated with BMI were sourced from a previously published GWAS study (14). We obtained these data from two consortia: the Genetic Investigation of Anthropometric Traits (GIANT) consortium and the Genetic Epidemiology of Adult Health and Aging Study (GERA) consortium (GWAS ID: ebi-a-GCST006368, sample size: 315,347).
To minimize bias due to population stratification, we focused exclusively on individuals of European ancestry. All datasets are available for download from the IEU GWAS database at this link: https://gwas.mrcieu.ac.uk/datasets/.
A detailed description of the data source is provided in Supplementary Table 1.
Since all the data were derived from publicly accessible studies, our research did not require patient consent or ethical clearance.
Our study design comprised two primary steps. First, we performed a two-sample univariate analysis, using RA as the exposure variable, and examined its association with hypothyroidism/hyperthyroidism as the respective outcomes. Subsequently, we performed another two-sample univariate analysis, using hypothyroidism/hyperthyroidism as the exposure variables, and assessed their relationships with RA as the outcomes. In a second step, to ensure that any causal effects were not influenced by factors such as BMI, smoking quantity, and alcohol intake frequency, a multivariate analysis was conducted to account for the impact of these variables.
In MR studies, genetic variants frequently serve as instrumental variables (IVs). Three critical assumptions must be met to obtain reliable casual estimates in MR studies: 1) IVs should exhibit a strong association with the exposure; 2) IVs should not be associated with any potential confounders that might affect the relationship between the exposure and outcome; 3) IVs should exclusively influence the outcome through the exposure (15, 16). Figure 1 depicts a detailed description.
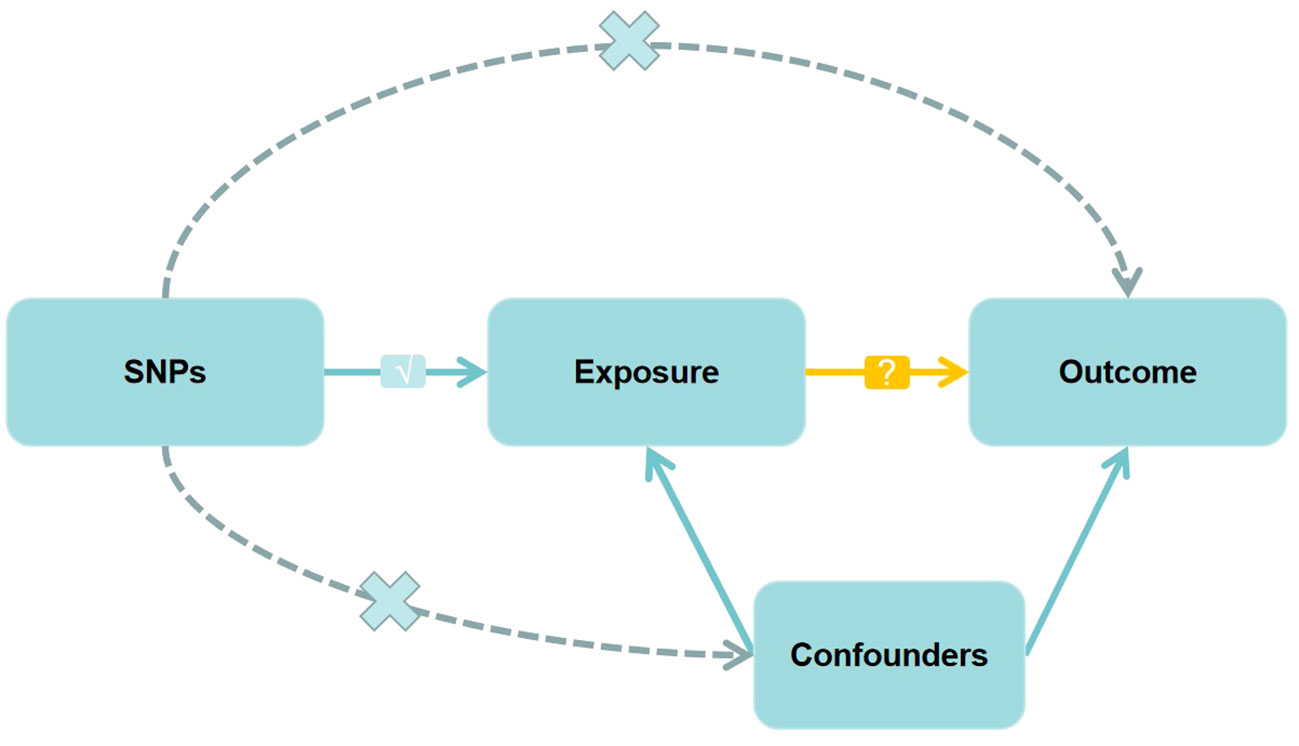
Figure 1 Directed acyclic graph of the Mendelian randomization (MR) framework investigating the causal relationship between exposure and outcome. The ‘×’ means that genetic variants are not associated with confounders or cannot be directly involved in outcome but via the exposure pathway. The ‘√’ means that genetic variants are highly correlated with exposure. SNPs, single-nucleotide polymorphisms.
Firstly, we identified independent single nucleotide polymorphisms (SNPs) significantly associated with the exposure (P<5×10-8). We conducted SNP clustering with a window size of 10,000 kb and an R2<0.001 threshold to remove linkage disequilibrium (LD). Subsequently, we checked all the exposure-related SNPs in the PhenoScanner database (http://www.phenoscanner.medschl.cam.ac.uk/) to identify any SNPs associated with potential confounders (BMI, smoking, alcohol consumption) and the outcome (P<5×10-8). We extracted SNP effects from the outcome GWAS dataset and harmonized the impact of the exposure and outcome. We excluded palindromic SNPs with ambiguous results (EAF >0.42). We employed the MR-Pleiotropy Residual Sum and Outlier (MR-PRESSO) method to identify and remove potential outliers (17). Finally, we assessed instrumental strength using the F-statistic (), following the procedures outlined by Burgess and Thompson (18).
For our MR analyses, we employed R (version 4.2.2) packages, namely “TwoSampleMR” (version 0.5.6) and “MR-PRESSO” (version 1.0).
We assessed the association between hypothyroidism/hyperthyroidism and RA using three methods: inverse variance weighted (IVW), weighted median (WM), and Mendelian randomization-Egger (MR-Egger) methods. IVW method offers consistent estimates when all genetic variants are valid IVs (19). Conversely, MR-Egger regression provides consistent estimates, notably when all considered genetic variants are incorrect IVs. The weighted median approach generates consistent appraisals, requiring at least half of the weights to be derived from accurate IVs (20). Our primary result was based on the IVW method, while MR-Egger and WM approaches were used to assess the reliability and stability of the results.
We used the MR-Egger intercept test to detect horizontal pleiotropy, with a P-intercept >0.05 indicating the absence of such pleiotropy. We further employed the IVW method and Egger regression to evaluate heterogeneity, with P<0.05 indicating its presence. Cochran’s Q statistic was used to assess heterogeneity (21). Moreover, we conducted a leave-one-out analysis to investigate whether a single SNP was driving the causal association.
A rigorous screening process included removing specific SNPs, including rs6679677, rs13426947, rs3087243, rs34046593, rs2561477, rs2844456, rs6936656, rs1571878, rs12764378, rs706778, rs8032939, and rs34536443 for their associations with confounders or outcomes. Furthermore, rs1042169, rs13330176, rs225433, rs2661798, rs3799963, and rs4452313 were excluded as they failed to harmonize. Subsequently, we employed 26 valid IVs for MR estimation of RA’s impact on hypothyroidism/hyperthyroidism (Supplementary Tables 2, 3). For the MR estimation of hypothyroidism’s impact on RA, we considered and utilized 31 valid IVs (Supplementary Table 5), following the removal of specific SNPs (rs6679677, rs4853458, rs11571297, rs932036, rs9277542, rs707937, rs7902146, rs4409785, and rs7310615) due to their associations with confounders or outcomes, and rs9265890, which failed to harmonize. We considered and utilized three valid IVs for the MR estimation of hyperthyroidism’s impact on RA (Supplementary Table 5). These were retained after excluding specific SNPs (rs6679677 and rs9275576) due to their associations with confounders or outcomes and rs9265890, which failed to harmonize.
In the initial step of the univariable MR analysis, we found a significant causal effect of RA on hypothyroidism (IVW: odds ratio [OR] = 1.07, 95% confidence interval [CI] = 1.01–1.14, P = 0.02). The results obtained from the WM method aligned with those from the IVW method (P = 0.02). However, the MR-Egger regression method indicated a similar causal effect direction; however, they did not reach significance (P = 0.63). A significant causal effect of RA on hyperthyroidism was observed (IVW OR = 1.32, 95% CI = 1.15–1.52, P< 0.001). The WM method results were consistent with the IVW method (P = 0.03); however, the MR-Egger regression method results were insignificant (P = 0.21). Conversely, in the inverse analysis, hypothyroidism exhibited a significant causal effect on RA (IVW OR = 1.21, 95% CI = 1.05–1.40, P = 0.01). However, neither reached significance, while the WM method (P = 0.06) and MR-Egger regression (P = 0.89) indicated causal effects in the same direction. However, there was no estimated causal effect of hyperthyroidism on RA in the IVW (P = 0.07) and MR-Egger regression methods (P = 0.27). Although, the WM method (P = 0.02) showed a causal effect of hyperthyroidism on RA, the focus was on the results of the IVW method, as shown in Table 1 and Figure 2A. All instrumental variables used in this study exhibited F-statistic values exceeding 10, indicating the robustness of the selected IVs (Supplementary Tables 2-5).
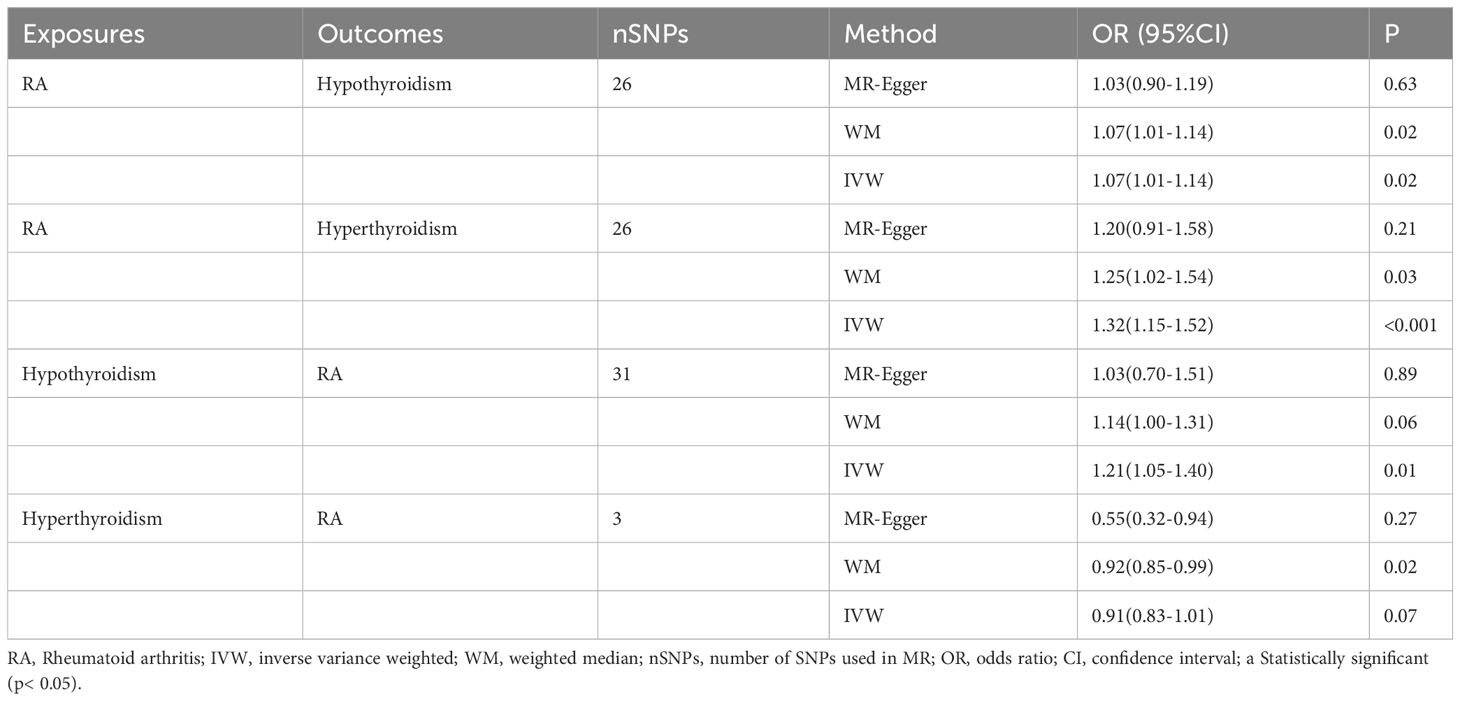
Table 1 MR Results of RA on Risk of hypothyroidism/hyperthyroidism, and hypothyroidism/hyperthyroidism on Risk of RA.
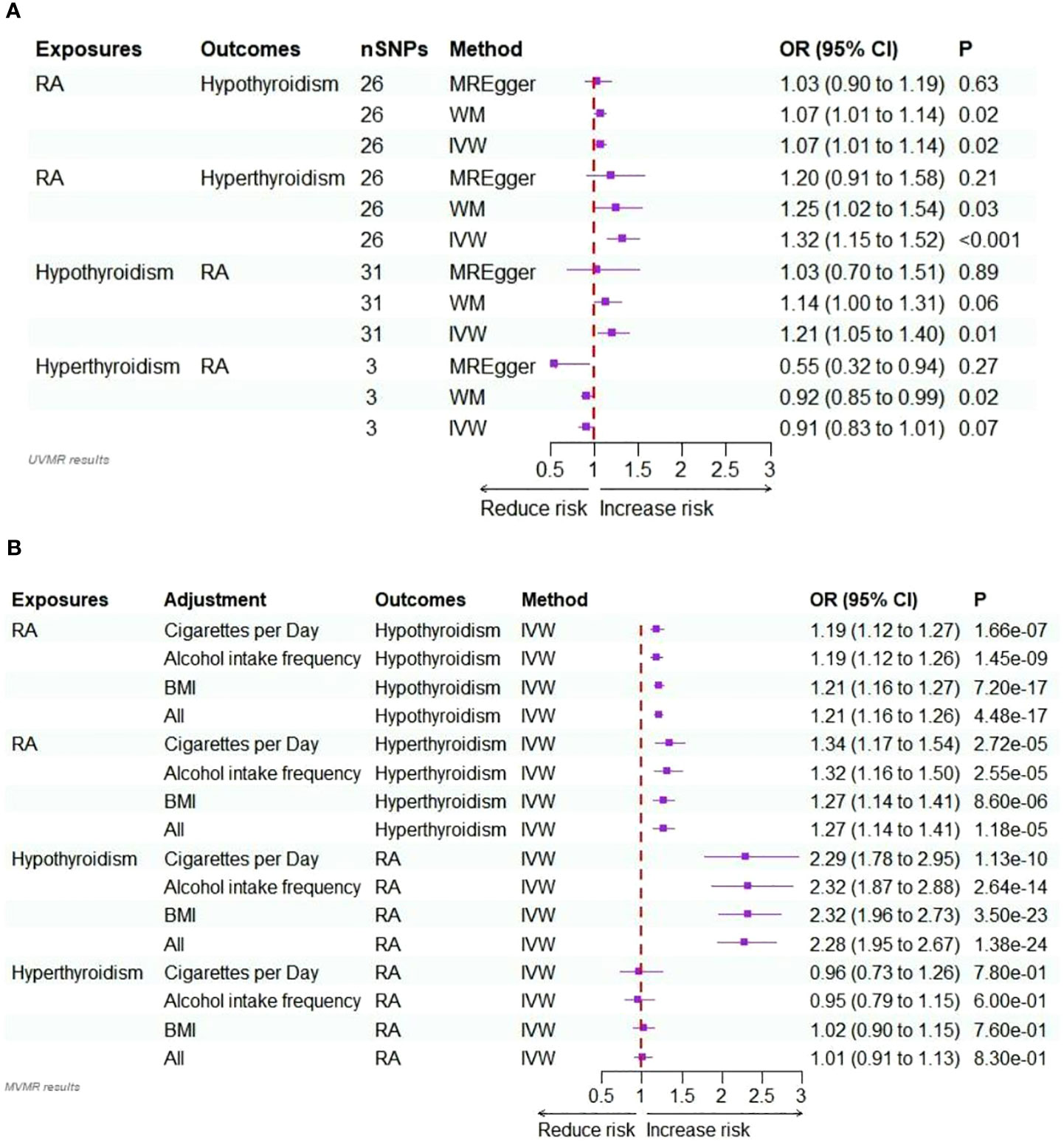
Figure 2 (A) Forest plots of the bidirectional two-sample univariable Mendelian randomization analysis of the relationship for RA on hypothyroidism/hyperthyroidism, and hypothyroidism/hyperthyroidism on RA. (B) Forest plots of the bidirectional two-sample multivariable Mendelian randomization analysis of the relationship for RA on hypothyroidism/hyperthyroidism, and hypothyroidism/hyperthyroidism on RA.
In the sensitivity analysis, Cochran’s Q test (P<0.05) revealed heterogeneity in the valid IVs used to estimate the effect of RA on hypothyroidism and hypothyroidism on RA. Therefore, a random effects model was employed. Conversely, the valid IVs used to assess the impact of RA on hyperthyroidism and hyperthyroidism on RA did not exhibit heterogeneity in Cochran’s Q test (P >0.05). Furthermore, there was no evidence of horizontal pleiotropy (P >0.05 for MR-Egger intercept) in any of the univariate MR analyses (Supplementary Table 6). The leave-one-out analysis results (Supplementary Figure 3) indicated no individual SNP significantly affected the causal effects. Scatter and funnel plots are presented in Supplementary Figures 1 and 2.
In multivariable MR analysis, we adjusted individually for smoking quantity, alcohol intake frequency, and BMI; strong evidence supported a direct causal effect of RA on the risk of hypothyroidism. This was observed when adjusting for smoking quantity (IVW: OR = 1.19, P<0.001), alcohol intake frequency (IVW: OR = 1.19, P<0.001), and BMI (IVW: OR = 1.21, P<0.001). In multivariable MR analysis jointly adjusted for these factors, the effect size of the association slightly increased (IVW: OR = 1.21, P<0.001).
Similarly, in multivariable MR analysis, when individually adjusting for smoking quantity, alcohol intake frequency, and BMI, strong evidence supported a direct causal effect of RA on the risk of hyperthyroidism. This was observed when adjusting for smoking quantity (IVW: OR = 1.34, P<0.001), alcohol intake frequency (IVW: OR = 1.32, P<0.001), and BMI (IVW: OR = 1.27, P<0.001). In multivariable MR analysis jointly adjusted for these factors, the effect size of the association was slightly reduced (IVW: OR = 1.27, P<0.001).
In the multivariable MR analysis, where we individually adjusted for smoking quantity, alcohol intake frequency, and BMI, there was compelling evidence of a direct causal effect of hypothyroidism on the risk of RA. Specifically, when adjusted for smoking quantity, the IVW OR was 2.29 with P<0.001. Similarly, the IVW OR was 2.32 (P<0.001) when adjusted for alcohol intake frequency and 2.32 (P<0.001) when adjusted for BMI. The association remained strong even in the multivariable MR analysis, simultaneously adjusted for all these factors (IVW: OR = 2.28, P<0.001).
However, there was no discernible evidence of a causal effect of hyperthyroidism on the risk of RA in the multivariate MR analyses, whether adjusted for smoking quantity, alcohol intake frequency, and BMI individually or collectively (P >0.05). Detailed results are provided in Supplementary Table 7, Figure 2B.
In our study, we performed bidirectional two-sample univariable and multivariable MR analyses to investigate the potential causal relationship between RA and hypothyroidism/hyperthyroidism. The results of the univariable analysis indicated a causal relationship between RA and hypothyroidism/hyperthyroidism. However, when we considered hypothyroidism/hyperthyroidism as exposure factors, we only identified a causal relationship between hypothyroidism and RA and not between hyperthyroidism and RA. In the multivariate MR analyses, after adjusting for smoking quantity, alcohol intake frequency, and BMI, the causal association between RA and hypothyroidism/hyperthyroidism and that between hypothyroidism and RA remained robust.
RA is an autoimmune disease characterized by symmetrical synovial inflammation resulting from a complex interplay of genetic and environmental factors that disrupt immune tolerance. Extra-articular clinical manifestations occur in approximately 40% of patients with RA (22). Thyroid dysfunction represents one of the most common chronic endocrine disorders. Patients with thyroid dysfunction frequently exhibit clinical manifestations similar to those seen in patients with RA, such as fatigue, muscle weakness, joint pain, and swelling. Furthermore, both conditions share similar pathogenic mechanisms involving autoimmune, inflammatory, genetic, and environmental factors. This study focuses explicitly on hyperthyroidism and hypothyroidism in thyroid dysfunction. Thyroid dysfunction, with or without autoimmune thyroid disease (AITD), is observed in 6% to 33.8% of patients with RA (23). The results of a meta-analysis highlight an increased risk of thyroid dysfunction in patients with RA, with a more significant association with hypothyroidism (OR= 2.25, 95% CI=1.78–2.84) than hyperthyroidism (OR 1.65, 95% CI 1.24–2.19), consistent with our findings (9). The predominant causes of hyperthyroidism and hypothyroidism are Graves’ disease and Hashimoto’s thyroiditis, respectively. In a study involving 2791 cases of Graves’ disease and 495 cases of Hashimoto’s thyroiditis, 3.15% of individuals with Graves’ disease and 4.24% of individuals with Hashimoto’s thyroiditis had RA (24). Furthermore, research by Nisihara et al. revealed positive antinuclear antibodies (ANA) in 17.5% of patients with AITD (excluding rheumatic diseases), with rheumatoid factor (RF) detected in 7.7% of such patients (25). In a study by Elnady et al., significant differences were observed with ANA (50.8%), RF (34.4%), and anti-cyclic citrullinated peptide (anti-CCP) (19.7%) (26). Conversely, in a cohort study of 800 patients with RA, 9.8% had AITD, 37.8% tested positive for anti-thyroid peroxidase (TPO) antibodies, and 20.8% were positive for anti-thyroglobulin (TG) antibodies (27). This evidence suggests an association between RA and hypothyroidism/hyperthyroidism, warranting further investigation into the underlying mechanisms that link these conditions.
Autoimmune thyroid disease significantly contributes to thyroid dysfunction. Shared physiopathologic mechanisms exist among autoimmune diseases (AD), including RA and AITD (28, 29). These conditions share common susceptibility genes and environmental factors, which could account for the higher incidence of autoimmune diseases in affected individuals. Some susceptibility genes associated with the development of RA include HLA-DRB1, PTPN22, AFF3, CD28, CD40, CTLA4, IL2RA, IL2, IL21, PRKCQ, STAT4, TAGAP, REL, TNFAIP3, TRAF1, BLK, CCL21, FCGR2A, PADI4, and PRDM1 (30). Furthermore, susceptibility genes for AITD include TSHR, TG, HLA, CTLA4, PTPN22, CD40, CD25, ARID 5B, BT61, FCRL3, IL2RA, and FOXP3 (31). Among these, PTPN22, CTLA4, HLA_DRB1, FCRL3, and IL2RA are common susceptibility genes between both conditions, while CD40 is exclusively associated with Graves’ disease and RA (32). Regarding environmental factors, smoking significantly increases the risk of RA (33) and Graves’ disease (34). Surprisingly, smoking has been found to reduce the risk of hypothyroidism, although this protective effect diminishes in the years following smoking cessation (35–37). Alcohol intake frequency is protective against RA (38, 39). Meanwhile, Carlé demonstrated that moderate alcohol intake frequency has diminished the risk of developing Hashimoto’s thyroiditis and Graves’ disease (40). Furthermore, gut flora dysbiosis has been proposed as an essential environmental factor in developing RA and hypothyroidism/hyperthyroidism (41–43). Chen et al. found that anti-tumor necrosis factor treatment (anti-TNFα) in mice reduced the expression of pro-inflammatory cytokines in the thyroid gland, thereby reducing inflammation (44). Hennie et al. showed that in individuals with RA and hypothyroidism, anti-TNFα therapy was associated with improved thyroid function (45). Moreover, patients receiving anti-TNFα treatment had a lower incidence of thyroid disease than those who did not (46). Elevated levels of interleukin-1 (IL-1) and IL-6 have been associated with the severity of RA and joint damage (47, 48). In summary, our study associates RA with hypothyroidism/hyperthyroidism, confirming that RA increases the risk of hypothyroidism/hyperthyroidism, and conversely, hypothyroidism elevates the risk of RA. However, we did not identify a causal relationship between hyperthyroidism and the risk of developing RA. We propose that hyperthyroidism might have a protective effect against RA by potentially increasing T regulatory cells, countering the adverse effects of hyperthyroidism on autoimmunity. However, further experimental validation with larger sample sizes is essential.
The strengths of our study include the utilization of bidirectional two-sample univariable and multivariable MR analyses to explore the potential causal relationship between RA and hypothyroidism/hyperthyroidism. Furthermore, using exposure and outcome datasets from distinct consortiums minimizes the impact of sample overlap.
However, this research has several limitations. Firstly, despite employing an MR design and excluding known confounders, the unaccounted potential confounders could still affect the results. Secondly, this research exclusively focused on individuals of European ancestry, which could limit the generalizability of the findings to individuals of other ancestral backgrounds. Therefore, caution is warranted when interpreting the implications of our findings for broader populations. Thirdly, autoimmune diseases generally exhibit a higher susceptibility in women than men. However, due to limitations in the available data from the original GWAS, we could not stratify the study by sex. Furthermore, our respective indicators for smoking and alcohol consumption, namely “ cigarettes per day” and “alcohol intake frequency” provide a limited perspective as they only reflect the quantity and frequency of smoking and drinking without capturing the full biological effects. Comprehensive indicators are available, such as the duration, frequency, and type of smoking, and the description of drinking includes the number of units per drink, the total weekly alcohol consumption, and the type of alcohol consumed. Therefore, further analysis is needed to comprehensively explore the effects of smoking and drinking. Finally, hypothyroidism and hyperthyroidism have complex etiologies with multiple subtypes, and the lack of consideration for these subtypes is a limitation in our MR analysis.
Our study utilized bidirectional two-sample univariable and multivariable MR analytical techniques to investigate a potential causal relationship between RA and hypothyroidism/hyperthyroidism. We confirmed the causal relationship between RA and hypothyroidism/hyperthyroidism. When hypothyroidism/hyperthyroidism were considered as exposure factors, we identified a causal relationship between hypothyroidism and RA; however, no such association was identified between hyperthyroidism and RA.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethical review and approval were not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the participant’s legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and institutional requirements.
RL: Conceptualization, Data curation, Formal Analysis, Methodology, Writing – original draft. XD: Data curation, Visualization, Writing – original draft. XL: Visualization, Writing – original draft. QL: Data curation, Visualization, Writing – original draft. KZ: Data curation, Visualization, Writing – original draft. DP: Supervision, Writing – review & editing.
This work was supported by Sichuan Provincial Administration of Traditional Chinese Medicine (2021MS090);
The authors thank the studies or consortiums referenced and included in the present analysis for providing public datasets.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1256208/full#supplementary-material
1. Silman AJ, Pearson JE. Epidemiology and genetics of rheumatoid arthritis. Arthritis Res (2002) 4 Suppl 3(Suppl 3):S265–72. doi: 10.1186/ar578
2. Cross M, Smith E, Hoy D, Carmona L, Wolfe F, Vos T, et al. The global burden of rheumatoid arthritis: estimates from the global burden of disease 2010 study. Ann Rheumatic Dis (2014) 73(7):1316–22. doi: 10.1136/annrheumdis-2013-204627
3. Sokka T, Kautiainen H, Pincus T, Verstappen SMM, Aggarwal A, Alten R, et al. Work disability remains a major problem in rheumatoid arthritis in the 2000s: data from 32 countries in the QUEST-RA study. Arthritis Res Ther (2010) 12(2):R42. doi: 10.1186/ar2951
4. Symmons DPM, Gabriel SE. Epidemiology of CVD in rheumatic disease, with a focus on RA and SLE. Nat Rev Rheumatol (2011) 7(7):399–408. doi: 10.1038/nrrheum.2011.75
5. Holmqvist M, Gränsmark E, Mantel A, Alfredsson L, Jacobsson LTH, Wallberg-Jonsson S, et al. Occurrence and relative risk of stroke in incident and prevalent contemporary rheumatoid arthritis. Ann Rheumatic Dis (2013) 72(4):541–6. doi: 10.1136/annrheumdis-2012-201387
6. Garmendia Madariaga A, Santos Palacios S, Guillén-Grima F, Galofré JC. The incidence and prevalence of thyroid dysfunction in Europe: a meta-analysis. J Clin Endocrinol Metab (2014) 99(3):923–31. doi: 10.1210/jc.2013-2409
7. Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab (2002) 87(2):489–99. doi: 10.1210/jcem.87.2.8182
8. Rosario PW. Subclinical hypothyroidism. N Engl J Med (2017) 377(14):1404. doi: 10.1056/NEJMc1709853
9. Liu Y-J, Miao H-B, Lin S, Chen Z. Association between rheumatoid arthritis and thyroid dysfunction: A meta-analysis and systematic review. Front Endocrinol (2022) 13:1015516. doi: 10.3389/fendo.2022.1015516
10. McCoy SS, Crowson CS, Gabriel SE, Matteson EL. Hypothyroidism as a risk factor for development of cardiovascular disease in patients with rheumatoid arthritis. J Rheumatol (2012) 39(5):954–8. doi: 10.3899/jrheum.111076
11. Davey Smith G, Ebrahim S. What can mendelian randomisation tell us about modifiable behavioural and environmental exposures? BMJ (Clinical Res ed.) (2005) 330(7499):1076–9. doi: 10.1136/bmj.330.7499.1076
12. Okada Y, Wu D, Trynka G, Raj T, Terao C, Ikari K, et al. Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature (2014) 506(7488):376–81. doi: 10.1038/nature12873
13. Liu M, Jiang Y, Wedow R, Li Y, Brazel DM, Chen F, et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet (2019) 51(2):237–44. doi: 10.1038/s41588-018-0307-5
14. Hoffmann TJ, Choquet H, Yin J, Banda Y, Kvale MN, Glymour M, et al. A large multiethnic genome-wide association study of adult body mass index identifies novel loci. Genetics (2018) 210(2):499–515. doi: 10.1534/genetics.118.301479
15. Smith GD, Ebrahim S. 'Mendelian randomization': can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol (2003) 32(1):1-22. doi: 10.1093/ije/dyg070
16. Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet (2014) 23(R1):R89–98. doi: 10.1093/hmg/ddu328
17. Verbanck M, Chen C-Y, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet (2018) 50(5):693–8. doi: 10.1038/s41588-018-0099-7
18. Burgess S, Thompson SG. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol (2011) 40(3):755–64. doi: 10.1093/ije/dyr036
19. Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol (2013) 37(7):658–65. doi: 10.1002/gepi.21758
20. Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan NA, Thompson JR. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: the role of the I2 statistic. Int J Epidemiol (2016) 45(6):1961–74. doi: 10.1093/ije/dyw220
21. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol (2016) 40(4):304–14. doi: 10.1002/gepi.21965
22. Guellec D, Cozien S, Ruyssen-Witrand A, Dieudé P, Saraux A. Prevalence and clinical significance of extra-articular manifestations at diagnosis in the ESPOIR cohort with recent-onset arthritis. Semin In Arthritis Rheumatism (2020) 50(3):409–13. doi: 10.1016/j.semarthrit.2020.01.004
23. Przygodzka M, Filipowicz-Sosnowska A. Prevalence of thyroid diseases and antithyroid antibodies in women with rheumatoid arthritis. Polskie Archiwum Medycyny Wewnetrznej (2009) 119(1-2):39–43. doi: 10.20452/pamw.600
24. Boelaert K, Newby PR, Simmonds MJ, Holder RL, Carr-Smith JD, Heward JM, et al. Prevalence and relative risk of other autoimmune diseases in subjects with autoimmune thyroid disease. Am J Med (2010) 123(2):183.e1–9. doi: 10.1016/j.amjmed.2009.06.030
25. Nisihara R, Pigosso YG, Prado N, Utiyama SRR, De Carvalho GA, Skare TL, et al. Rheumatic disease autoantibodies in patients with autoimmune thyroid diseases. Med Principles Pract Int J Kuwait University Health Sci Centre (2018) 27(4):332–6. doi: 10.1159/000490569
26. Elnady BM, Kamal NM, Shaker RHM, Soliman AF, Hasan WA, Alghamdi HA, et al. Prevalence and clinical significance of nonorgan specific antibodies in patients with autoimmune thyroiditis as predictor markers for rheumatic diseases. Medicine (2016) 95(38):e4336. doi: 10.1097/MD.0000000000004336
27. Cárdenas Roldán J, Amaya-Amaya J, Castellanos-de la Hoz J, Giraldo-Villamil J, Montoya-Ortiz G, Cruz-Tapias P, et al. Autoimmune thyroid disease in rheumatoid arthritis: a global perspective. Arthritis (2012) 2012:864907. doi: 10.1155/2012/864907
28. Deshmukh HA, Maiti AK, Kim-Howard XR, Rojas-Villarraga A, Guthridge JM, Anaya J-M, et al. Evaluation of 19 autoimmune disease-associated loci with rheumatoid arthritis in a Colombian population: evidence for replication and gene-gene interaction. J Rheumatol (2011) 38(9):1866–70. doi: 10.3899/jrheum.110199
29. Anaya J-M, Rojas-Villarraga A, García-Carrasco M. The autoimmune tautology: from polyautoimmunity and familial autoimmunity to the autoimmune genes. Autoimmune Dis 2012 (2012) p:297193. doi: 10.1155/2012/297193
30. Zhernakova A, Withoff S, Wijmenga C. Clinical implications of shared genetics and pathogenesis in autoimmune diseases. Nat Rev Endocrinol (2013) 9(11):646–59. doi: 10.1038/nrendo.2013.161
31. Effraimidis G, Wiersinga WM. Mechanisms in endocrinology: autoimmune thyroid disease: old and new players. Eur J Endocrinol (2014) 170(6):R241–52. doi: 10.1530/EJE-14-0047
32. Huber A, Menconi F, Corathers S, Jacobson EM, Tomer Y. Joint genetic susceptibility to type 1 diabetes and autoimmune thyroiditis: from epidemiology to mechanisms. Endocrine Rev (2008) 29(6):697–725. doi: 10.1210/er.2008-0015
33. Meng W, Zhu Z, Jiang X, Too CL, Uebe S, Jagodic M, et al. DNA methylation mediates genotype and smoking interaction in the development of anti-citrullinated peptide antibody-positive rheumatoid arthritis. Arthritis Res Ther (2017) 19(1):71. doi: 10.1186/s13075-017-1276-2
34. Ferrari SM, Fallahi P, Antonelli A, Benvenga S. Environmental issues in thyroid diseases. Front In Endocrinol (2017) 8:50. doi: 10.3389/fendo.2017.00050
35. Belin RM, Astor BC, Powe NR, Ladenson PW. Smoke exposure is associated with a lower prevalence of serum thyroid autoantibodies and thyrotropin concentration elevation and a higher prevalence of mild thyrotropin concentration suppression in the third National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab (2004) 89(12):6077–86. doi: 10.1210/jc.2004-0431
36. Andersen SL, Olsen J, Wu CS, Laurberg P. Smoking reduces the risk of hypothyroidism and increases the risk of hyperthyroidism: evidence from 450,842 mothers giving birth in Denmark. Clin Endocrinol (2014) 80(2):307–14. doi: 10.1111/cen.12279
37. Carlé A, Bülow Pedersen I, Knudsen N, Perrild H, Ovesen L, Banke Rasmussen L, et al. Smoking cessation is followed by a sharp but transient rise in the incidence of overt autoimmune hypothyroidism - a population-based, case-control study. Clin Endocrinol (2012) 77(5):764–72. doi: 10.1111/j.1365-2265.2012.04455.x
38. Azizov V, Dietel K, Steffen F, Dürholz K, Meidenbauer J, Lucas S, et al. Ethanol consumption inhibits TFH cell responses and the development of autoimmune arthritis. Nat Commun (2020) 11(1):1998. doi: 10.1038/s41467-020-15855-z
39. Scott IC, Tan R, Stahl D, Steer S, Lewis CM, Cope AP. The protective effect of alcohol on developing rheumatoid arthritis: a systematic review and meta-analysis. Rheumatol (Oxford England) (2013) 52(5):856–67. doi: 10.1093/rheumatology/kes376
40. Carlé A, Bülow Pedersen I, Knudsen N, Perrild H, Ovesen L, Rasmussen LB, et al. Graves' hyperthyroidism and moderate alcohol consumption: evidence for disease prevention. Clin Endocrinol (2013) 79(1):111–9. doi: 10.1111/cen.12106
41. Yan H-X, An W-C, Chen F, An B, Pan Y, Jin J, et al. Intestinal microbiota changes in Graves' disease: a prospective clinical study. Bioscience Rep (2020) 40(9):BSR20191242. doi: 10.1042/BSR20191242
42. Knezevic J, Starchl C, Tmava Berisha A, Amrein K. Thyroid-gut-axis: how does the microbiota influence thyroid function? Nutrients (2020) 12(6):1769. doi: 10.3390/nu12061769
43. Chen Y, Ma C, Liu L, He J, Zhu C, Zheng F, et al. Analysis of gut microbiota and metabolites in patients with rheumatoid arthritis and identification of potential biomarkers. Aging (2021) 13(20):23689–701. doi: 10.18632/aging.203641
44. Chen K, Wei Y, Sharp GC, Braley-Mullen H. Decreasing TNF-alpha results in less fibrosis and earlier resolution of granulomatous experimental autoimmune thyroiditis. J Leukocyte Biol (2007) 81(1):306–14. doi: 10.1189/jlb.0606402
45. Raterman HG, Jamnitski A, Lems WF, Voskuyl AE, Dijkmans BAC, Bos WH, et al. Improvement of thyroid function in hypothyroid patients with rheumatoid arthritis after 6 months of adalimumab treatment: a pilot study. J Rheumatol (2011) 38(2):247–51. doi: 10.3899/jrheum.100488
46. Tarhan F, Orük G, Niflioğlu O, Ozer S. Thyroid involvement in ankylosing spondylitis and relationship of thyroid dysfunction with anti-TNF α treatment. Rheumatol Int (2013) 33(4):853–7. doi: 10.1007/s00296-012-2438-9
47. Srirangan S, Choy EH. The role of interleukin 6 in the pathophysiology of rheumatoid arthritis. Ther Adv In Musculoskeletal Dis (2010) 2(5):247–56. doi: 10.1177/1759720X10378372
Keywords: Mendelian randomization, rheumatoid arthritis, hypothyroidism, hyperthyroidism, causal relationship
Citation: Lai R, Deng X, Lv X, Liu Q, Zhou K and Peng D (2023) Causal relationship between rheumatoid arthritis and hypothyroidism or hyperthyroidism: a bidirectional two-sample univariable and multivariable Mendelian randomization study. Front. Endocrinol. 14:1256208. doi: 10.3389/fendo.2023.1256208
Received: 10 July 2023; Accepted: 08 November 2023;
Published: 29 November 2023.
Edited by:
Terry Francis Davies, Icahn School of Medicine at Mount Sinai, United StatesReviewed by:
Sudhanshu Kumar Bharti, Patna University, IndiaCopyright © 2023 Lai, Deng, Lv, Liu, Zhou and Peng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dezhong Peng, MTU4MjgwNTU2OThAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.