- Department of Minimally Invasive Gynecology, Beijing Obstetrics and Gynecology Hospital, Capital Medical University, Beijing Maternal and Child Health Care Hospital, Beijing, China
The junctional zone (JZ) is an important structure in the myometrium that maintains uterine fertility. Changes in the junctional zone are closely related to infertility and adenomyosis (ADS). As an increasing number of young women are affected by ADS, the disease is no longer considered typical of women over 40. With these changes, an increasing number of patients refuse hysterectomy and desire fertility preservation treatment. At the same time, ADS is a crucial factor causing female infertility. Therefore, the treatment of ADS-related infertility and preservation of reproductive function is one of the other major challenges facing clinicians. For these young patients, preserving fertility and even promoting reproduction has become a new challenge. Therefore, we searched and summarized these studies on PubMed and Google Scholar using keywords such as “adenomyosis”, “junctional zone”, and “infertility” to explore infertility causes, diagnosis, and treatment of ADS patients who wish to preserve their uterus or fertility and become pregnant, focusing on the junctional zone, to obtain a full appreciation of the new perspective on this disease.
1 Introduction
Adenomyosis is defined as the benign invasion of the endometrium into the myometrium, which microscopically exhibits endometrial glands and stroma surrounded by the hypertrophic and hyperplastic myometrium (1). It is characterized by abnormal uterine bleeding (AUB), pelvic pain symptoms, and infertility. However, the clinical presentation of adenomyosis is often mixed but can occasionally be asymptomatic (2). The disease is no longer considered typical of women over 40 years of age, as an increasing number of young women are affected by adenomyosis. Moreover, adenomyosis is diagnosed in 22% of infertile women under 40 years old undergoing treatment by assisted reproductive technology (ART) (3).
In 1983, shortly after magnetic resonance imaging (MRI) became available, the junctional zone (JZ) was described initially as a low-intensity band between the endometrium and the myometrium. The junctional zone is a distinct, hormone-dependent endometrial-myometrial interface, with a Müllerian origin, and the thickness changes during the menstrual cycle (4). In the nonpregnant uterus, highly specialized contraction waves originate exclusively from the JZ and participate in regulating diverse reproductive events, such as sperm transport, embryo implantation, and menstrual shedding (5).
Abnormal widening of the JZ is the consequence of uncoordinated inner myocyte proliferation called JZ hyperplasia (6). JZ hyperplasia and accompanying disruption could initiate endometrial mucosal penetration of endometrial glands into the myometrium (7). Alterations in JZ thickness and invasion of the endometrium into the inner myometrium could represent an early stage in the development of adenomyosis (8). The role of JZ in normal physiology and fertility suggests that abnormalities in this zone can cause disease and complications. Moreover, regular cyclical changes in myocytes in women with adenomyosis are absent, which may lead to many changes in ultrastructure (9).
Therefore, the focus on the JZ represents a new direction for patients with adenomyosis who wish to preserve or restore their fertility. Moreover, diagnosis and treatment of the JZ may be a solution to the plight of young women with adenomyosis. Previous articles have reviewed the function of the JZ in infertility (10, 11), but this article updates this part of the content of the latest literature and reviews the pathophysiological mechanism of JZ infertility, the role of imaging in diagnosis, and the influence of conservative treatment on infertility in adenomyosis. Impact of junctional zone on infertility.
The JZ consists of a three-dimensional (3D) mesh of irregular, mainly circular, and short muscular bundles and is part of the “archimetra” or “old uterus” seen in most vertebrate mammals. The two outer layers of the myometrium, the “neometra,” developed later in the evolutionary process to support childbirth, primarily due to the imbalance between the narrow pelvis and the fetal head (12). JZ contractility provides the main contractility of the unpregnant uterus. It plays a role in the fertility and pathogenesis of certain uterine diseases and symptoms through a hormone-dependent pattern. In the proliferative phase, retrograde contraction from the cervix to the fundus, and in the secretory phase, antegrade contraction from the fundus to the cervix play a role in sperm transport and early pregnancy preservation, respectively (5). The role of the JZ in normal physiology and fertility suggests that abnormalities in this zone can cause disease and complications. The structure and function of the JZ are lost during implantation and endometrial diseases, which can easily lead to the infiltration of trophoblasts or endometrial fragments in adenomyosis. Radiographic JZ thickening is a negative predictor of embryo implantation after IVF. Microenvironmental changes, such as abnormal contractions, hormonal changes, and immune abnormalities, can adversely affect the reproductive process and contribute particularly to excessive JZ peristalsis, which may lead to the development of uterine disease (13).
1.1 Effects of abnormal JZ thickness on fertility
The average thickness of the JZ has no clear standard. In early studies, the thickness of the JZ was defined as 5 mm to 8 mm. The thickness of the JZ increases in patients with adenomyosis, and the development of MRI technology provides the possibility for accurate measurement of JZ thickness. JZ MRI showed that a maximum thickness of the JZ ≥12 mm is a diagnostic criterion for adenomyosis (14). Currently, there is substantial research evidence indicating that increased JZ thickness can have adverse effects on reproduction and that there is a strong association between adenomyosis and reproductive impairment in women with a thickened JZ. Increased JZ thickness is considered a poor prognostic factor for implantation and is significantly associated with IVF implant failure. The average JZ is >7 mm, the maximal JZ is >10 mm, and the implantation failure rate is 95.8%. The IVF patient results show more than 74% failure. JZ thickening is an independent factor for embryo implantation failure, which is primarily unrelated to embryo quality or infertility subtype (15). Research has shown that the thinner the junctional area on the day of ovum pick up (OPU) is, the higher the implantation rate will be and that pregnant patients have a lower JZ thickness than nonpregnant patients, with an average JZ thickness of 0.27 cm (16). Compared with that of intrinsic type adenomyosis, the JZ area of adenomyosis in the extrinsic type is almost unchanged. At the same time, the extrinsic type shows a higher live birth rate and lower abortion rate. As the disease progresses, the miscarriage rate increases in the third trimester, which may be due to the involvement of impaired and deep placentation (14). In summary, JZ thickening is an important factor in reproductive disorders.
1.2 Effect of abnormal JZ contraction on fertility
Uterine peristalsis is a vital biomechanical activity for reproduction and fertility, and its dysfunction has a widespread impact on adenomyosis. Uterine peristalsis is spontaneous and coordinated contraction and relaxation in the nonpregnant uterus. JZ contraction varies in different periods, showing contraction from the cervix to the fundus in the nonpregnant period and contraction from the fundus to the cervix during pregnancy. Abnormal uterine contraction may be the basis of important diseases such as dysmenorrhea, infertility, endometriosis, implantation failure, spontaneous inflammation, abortion, and premature babies (17). Based on the various stages of the menstrual cycle, many studies have shown changes in the magnitude, frequency, and direction of contractions in the uterine JZ of normal uterus and ADS (18). Spontaneous peristalsis of the myometrium in the nonpregnant uterus plays a vital role in human reproduction. It involves menstrual blood discharge, sperm transport, and embryo implantation (19). Therefore, the JZ plays an essential role in normal reproduction, as shown in Table 1. There have been many related studies on the impact of structural and functional changes in the JZ on infertility management. The evolution of JZ contractility and infertility treatment can affect each other, often leading to poor treatment outcomes. In adenomyosis, the contraction of the JZ is disordered and irregular, and the enhancement of JZ activity will affect embryo implantation and sperm transportation during natural conception and assisted reproduction. Directional uterine contractility and integrity of uterine and fallopian tube transport function are necessary for sperm transport (24). Higher JZ activity and a corresponding increase in endometrial mobility may impair uterine receptivity and affect implantation. Contraction of the junctional area (JZ) during embryo transfer is associated with adverse outcomes, and factors that increase JZ contraction should be avoided. MRI shows interference of the sperm transport process by the excessive uterine peristalsis in adenomyosis, occurring mainly when diffuse adenomyosis affects the whole myometrium, and the sperm transport capacity is completely lost. Adenomyosis destroys the functional structure of the myometrium, which is responsible for directional sperm transport, thus affecting sperm transport and reducing the natural pregnancy rate (25). The study by Lesny P et al (22) evaluated the contraction of JZ during IVF and embryo transfer cycles in oocyte donors exposed to long-term ovarian stimulation protocols. When downregulated, no JZ shrinkage was observed. Seven days after superovulation, all patients displayed cervico-fundal, fundo-cervical, and random contractions. When injected with human chorionic gonadotropin-gonadotropin, the cervico-fundal waves dominated the image. However, the activity was most potent on the day of oocyte retrieval. All patients had JZ activity on the 2nd, 3rd, and 4th days after oocyte retrieval. Nevertheless, the regular wavy contractility gradually decreased, and only a single random movement was observed on the 4th day after oocyte retrieval. In summary, JZ activity was more significant throughout the IVF cycle than in the natural cycle. In another study by Biervliet FP et al. (23), patients with previous complex embryo transfers or complex mock embryo transfers received a transmyometrial embryo transfer (TMET). They found that TMET is a potent stimulus for JZ contractility and that increased contraction of the JZ results in abnormal implantation of embryos and ectopic pregnancy. In contrast, JZ contraction in the embryo transfer area is associated with adverse outcomes, and factors that increase JZ should be avoided. Lower endometrial activity during pregnancy is related to a higher pregnancy rate. The frequency of uterine peristalsis waves before embryo transfer negatively correlates with clinical pregnancy in fresh and frozen-thawed embryo transfer cycles. Compared with patients with a higher frequency, patients with uterine peristalsis waves < 3.0 waves/min before embryo transfer have higher chances of a successful pregnancy (26). Abnormal contraction of the JZ will also cause uterine pressure in the uterine cavity to rise, which will affect pregnancy. Smooth muscle contraction produces high and periodic intrauterine pressure on embryos, which is produced by uterine smooth muscle contraction and has the highest and most frequent occasional peak after implantation. Studies have shown that uterine smooth muscle contractions produce high and irregular intrauterine pressure. Improper contraction of uterine muscles leads to premature or delayed delivery (27).
1.3 Effects of estrogen receptors on fertility
Hormones are the primary regulators of female reproductive function, including ovulation, menstruation, embryo implantation, and pregnancy. At present, increasing evidence shows that hormone abnormalities can cause many diseases, which may lead to endocrine disorders among the endometrium, myometrium and cervix, and decidua and trophoblast, thus inducing pregnancy complications. Uterine dysfunction in women with adenomyosis may be due to normal peripheral estradiol levels and local hyper-estrogenism. However, it is still unclear exactly how estrogen influences the uterus. Some previous studies found that there were cyclical changes in estrogen receptor-α (ER-α) expression in the JZ in adenomyosis patients but that high expression was persistent (28) (Figure 1). As an estrogen-dependent disease, estrogen mediates JZ dysfunction through the constant high expression of ER, which plays a significant role in the pathogenesis of adenomyosis. Our study also demonstrated that estrogen could increase the intracellular free calcium of the JZ through a membrane receptor-dependent and nongenomic mechanism of action (29). This may lead to an abnormal contraction frequency and the JZ cycle, leading to disease progression and infertility in patients with ADS. However, this is far from enough to address the question. This suggests that, without a uterine specimen, the persistent high-level expression of ER on the JZ may be used as an indicator to assist in diagnosing adenomyosis. Thus, the pathological diagnosis was achieved under conservative treatment.
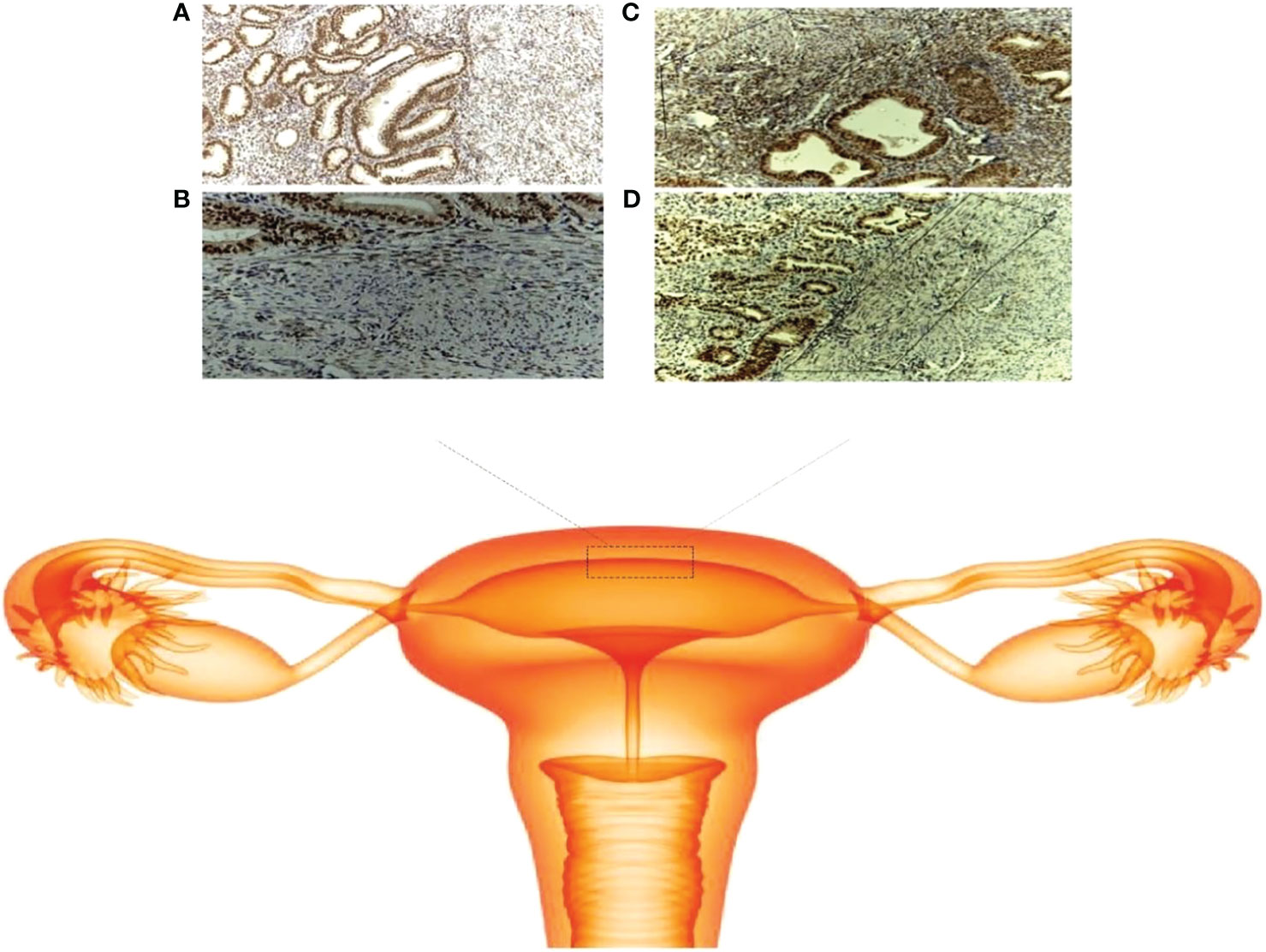
Figure 1 Schematic of the junctional zone. (A) ER expression in the adenomyosis group (× 100); (B) ER expression in the control group (× 100); (C) localization of the JZ in the adenomyosis group (× 100); (D) localization of the JZ in the control group (× 100).
1.4 Effects of oxytocin receptors on fertility
Oxytocin receptors are widely expressed in human uterine epithelial cells and smooth muscle cells, as well as in peritoneal endometriosis and ovarian endometriotic cysts. Adenomyosis is an estrogen-dependent disease; excessive estrogen may increase oxytocin-mediated uterine activity. In adenomyosis, the expression of oxytocin and its receptor increases, which causes spontaneous peristalsis of the myometrium. Because persistent local estrogen excess stimulates oxytocin through ER-α, it further contributes to uterine peristalsis disorder mediated by oxytocin and its receptor in the endometrium, resulting in JZ damage (30, 31). It is recognized that oxytocin can significantly increase the frequency of uterine peristalsis and is one of the most important mediators of uterine contraction, not only during pregnancy but also during the nonpregnant period. Real-time ultrasound has demonstrated that oxytocin-dependent peristaltic motion is limited to the endometrium and JZ in the nonpregnant uterus. Studies have shown that in myometrial smooth muscle cells, OTR expression levels are positively correlated with contraction amplitude (32). The expression pattern of oxytocin receptors in the JZ of adenomyosis patients is disrupted. The expression pattern of OTR is opposite to that in women with a normal JZ, showing that OTR expression in the proliferative isthmus is significantly lower than that in the fundus. The abnormal expression pattern of OTR may lead to the reversal of JZ contraction, thus interfering with sperm transport (18). Miaomaio et al. (33) studied the changes in oxytocin receptors in the JZ for women with endometriosis. They found that patients with endometriosis had higher serum oxytocin levels and higher uterine contraction rates. For the controls, the expression of OTR in the JZ of the proliferative uterus was significantly higher than that in the secretory phase in the cervical area and the uterine floor area. It was speculated that the JZ responded to the positive and negative effects of estrogen and prostaglandin activity, resulting in the synthesis and reduction of oxytocin receptors. OTR expression in the uterine junction of women with endometriosis appeared to have changed significantly and irregularly. Abnormal oxytocin receptor expression in the JZ in women with endometriosis may lead to abnormal uterine contractility, reduced fertility, and dysmenorrhea associated with endometriosis. Oxytocin increases Ca2+ signaling and uterine peristalsis to a greater extent in adenomyosis. These two changes indicate that uterine Ca2+ oscillation and peristalsis dysfunction may be pathogenic factors of adenomyosis and disrupt embryo implantation, leading to a decrease in fertility in adenomyosis (34).
1.5 Effect of the JZ on immunity and of local inflammation on fertility
Immune changes significantly impact the occurrence and development of ADS and decidualization of the endometrium. The endometrial lumen epithelium is generally not adhesive, and the receptive phenotype must be acquired transitorily to allow the attachment and invasion of blastocysts. The “implantation window” starts approximately six days after ovulation and lasts 2 to 4 days, which coincides with the decidualization of uterine tissue. Although the term “decidualization” refers primarily to the transformation of endometrial stromal cells into specialized decidual cells, this differentiation process also involves specialized immune cells, including uterine NK (uNK) cells, macrophages, and changes in smooth muscle cells (35). Therefore, immune changes in the JZ also affect women’s fertility. An altered immune response, which might be the primary abnormality in the pathogenesis of adenomyosis, was reported recently. This results in the disturbance of the JZ. This change may promote the invasion of the endometrium into the myometrium through the interface in adenomyosis. This conclusion may also be supported by the high expression of the IL-18 system in eutopic endometrial tissue in the JZ (36). Activation of inflammatory pathways may be associated with immune and vascular dysfunction in the placenta/decidua interaction, leading to obstetric complications such as fetal growth restriction (FGR), preeclampsia (PE), and preterm birth (PTB) (37). During the process of female pregnancy, embryos and trophoblast cells are successfully implanted into the maternal decidua; trophoblast invasion and spiral artery remodeling are essential steps for a successful pregnancy. The structural and functional impairment of the JZ zone is the cause of pregnancy failure. Obstetric complications are the basis of the disease. During pregnancy, notable vascular changes occur first in the endometrium and then in the uterine JZ, and at the same time, trophoblastic invasion causes the decidualization of maternal tissues. The JZ widely represents the inner third of the myometrium and participates in the placenta along with the endometrium. Therefore, the leading site of vascular pathology in pregnancy is not in the placenta or decidua but in the JZ (38). The placenta is characterized by interstitial and endovascular trophoblast cells invading the uterine JZ with changes in spiral arteries. When endovascular trophoblast cells invade and damage the tissue, they fail to reshape the JZ segment of the spiral route, which leads to different degrees of uterine and placental ischemia, oxidative injury, cell death, and necrosis. Defective deep placentation can cause pregnancy complications, such as abortion, in the second trimester, placental abruption, preterm birth, FGR, and preeclampsia. Defective deep placentation in patients with adenomyosis is due to defective remodeling of the spiral arteries (5). During pregnancy, decidual transformation is insufficient, endovascular trophoblast cells are blocked due to arrest at the level of the JZ, and spiral artery access to the muscular bundles is obstructed, explaining the vascular resistance in pPROM and PTB (39). Defective endovascular trophoblast invasion disorder may be secondary to the absence of natural killer cells in the thickened JZ that are involved in the depth of trophoblast invasion (37). The disruption of JZ before pregnancy can impact placental formation and pregnancy outcomes (16). Continued research in this area may provide new methods for diagnosing adenomyosis.
2 Role of imaging in the diagnosis and fertility of the junctional zone
The JZ area changes with age, and the structure and function of the JZ in patients with adenomyosis change, which may be the reason for the increased risk of adverse pregnancy outcomes. However, molecular research on the particular location of the JZ region is not extensive at present. The traditional method of diagnosing adenomyosis is pathologically performed after hysterectomy. Recently, with the enhanced comprehension of the JZ, evaluation by means of various modalities, such as imaging technology, to accurately diagnose adenomyosis has become possible, and imaging technology can also be used to examine its structure and function. The imaging evaluation of the JZ before pregnancy may be helpful for the diagnosis of adenomyosis, the identification of infertility factors, and the risk of obstetric complications.
2.1 MRI in the diagnosis and fertility of the junctional zone
In healthy women of reproductive age, the JZ appears as a band of low intensity between the endometrium of high intensity and the outer myometrium of intermediate signal intensity visualized by magnetic resonance imaging (MRI). Many studies have shown that the thickness of the JZ in patients with adenomyosis is significantly different from that on average (Table 2). As Figure 2 shows, a threshold ≥ 12 mm for JZ thickness was described as a critical marker of adenomyosis (46). MRI was reported to have 78% sensitivity and 88% specificity for the diagnosis of adenomyosis because of its ability to visualize the JZ (47). Meanwhile, when coming to less than 8 mm, the thickness could be used as a negative index to exclude the diagnosis of adenomyosis (42). However, according to Tina Tellum et al (48). For the diagnosis of adenomyosis in young women, JZ irregularities might be a more accurate indicator, with a sensitivity of 74% and a specificity of 83%. At the same time, adenomyosis often appears as a local thickening, while diffuse thickening may be physiological, and this characteristic may prevent misjudgment. Although the diagnosis of adenomyosis by means of the JZ is relatively accurate, there are also some flaws in this method. The most obvious problem is that 20% of premenopausal women lack a definable JZ. Fast breath-hold T2-weighted MRI might be helpful to improve performance in measuring the JZ to diagnose uterine adenomyosis (49). MRI can be more accurate in the classification of adenomyosis, although there is no uniform standard for the type of adenomyosis at present. Adenomyosis of the outer myometrium (external adenomyosis), which is not connected with the JZ. The inner myometrium (internal adenomyosis) is characterized by destruction of the JZ accompanied by diffuse growth of the endometrium in the myometrium (50). One study focusing on prepregnancy uterine ultrasound and MR images found that patients with adenomyosis had a 1.84-fold increased risk of spontaneous preterm delivery and a 1.98-fold increased risk of preterm premature rupture of membranes (PPROM) (51). In one study, adenomyosis was diagnosed based on MRI. The IVF/ICSI fresh and frozen-thawed ET were compared according to different classifications. The extrinsic group had a lower abortion rate and a higher live birth rate than the advanced group, which had a higher proportion of miscarriages at or after 12 weeks (52). Classification of adenomyosis by MRI is essential to evaluate patients’ fertility. In focal adenomyosis, sperm transport is mainly hyperperistaltic, while diffuse adenomyosis often shows the loss of sperm transport capacity. Natural conception is complex in this group of women (25). Therefore, MRI examination is essential in infertile patients.
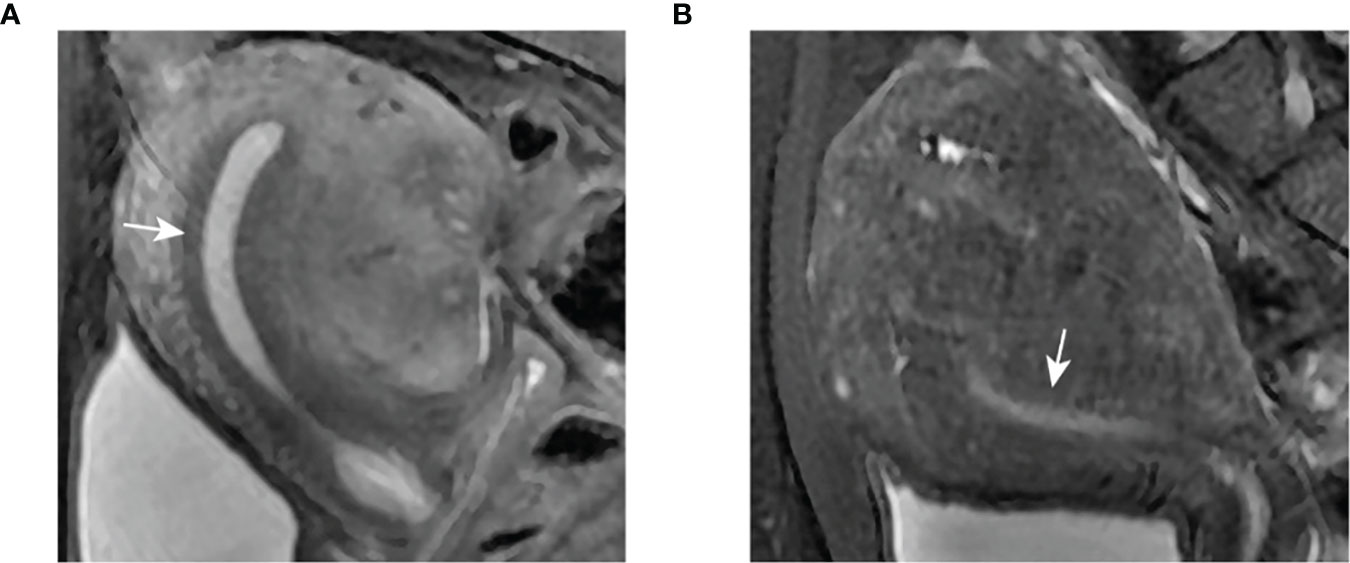
Figure 2 Clear demarcation of JZ appearance on MRI. (A) Thickened JZ in a patient with focal adenomyosis. (B) Thickened JZ in a patient with diffuse adenomyosis.
2.2 Ultrasound in the diagnosis of the junctional zone
Ultrasound research is essential for the comprehension of JZ, and the performance of JZ under ultrasound has a complex relationship with structural organization and biochemical characteristics. Contraction of the JZ under nongravity conditions, which occurs approximately 3-5 times/minute, can be observed by ultrasound (9). The advantages of ultrasound diagnostics are the ease of use, low price, and good exploration quality. With improvements to the technique, ultrasound has gradually become known for its high diagnostic accuracy in detecting the site and position of adenomyosis. As the diagnostic accuracy of 3D-TVUS over the course of JZ thickness maturation has been affirmed and efforts have recently been made to reach an agreement upon radiological criteria in adenomyosis diagnosis, the position of the JZ in the non-invasive diagnosis of adenomyosis has gradually increased (53) (Table 3). Reinhold et al. (40). showed that 2D-TVUS and MRI had the same accuracy in diagnosing adenomyosis. With the introduction of 3D-TVUS, high-frequency probes, and more advanced modes, JZ becomes easier to see in the US. 3D-TVUS can be used to evaluate the side and bottom of the JZ and clearly show the endometrium protruding to the myometrium. Unlike traditional ultrasound, 3D-TVUS coronal sectioning can enable a parameter evaluation similar to that of MRI with a higher accuracy, at 84% sensitivity and 84% specificity for the diagnosis of adenomyosis (47). This enables clinicians to evaluate the effect of endometrial ablation and medical therapy through the appearance of JZ alterations, as shown in Figure 3. Ultrasound can be used to exclude other complications, enhance the diagnosis of adenomyosis, and give full play to its discrimination of fertility. TVS can also be used to evaluate damage to the JZ in adenomyosis and judge fertility potential. Exacoustos et al. reported that infertility and abortion in adenomyosis at the JZ junction are high (57). Ultrasonic evidence of adenomyosis was found in many infertile women prior to embryo transfer and hurt IVF/ICSI results. Marvelous et al. evaluated the influence of ultrasonic manifestations of adenomyosis on the success rate of IVF. The ultrasonic signs of any adenomyosis are related to the success rate of IVF and the abnormal degree of morphological features indicated by ultrasound (58). Similarly, Dean and others found that the woman’s risk of infertility increased with the increasing number of ultrasound signs of adenomyosis (52). Recent studies have shown that sonographic markers in asymptomatic adenomyosis may not be associated with changes in pregnancy outcomes after transplantation of a single thawed euploid blastocyst. Routine screening for asymptomatic adenomyosis in an unselected population of infertile patients undergoing frozen embryo transfer may not be necessary (59).
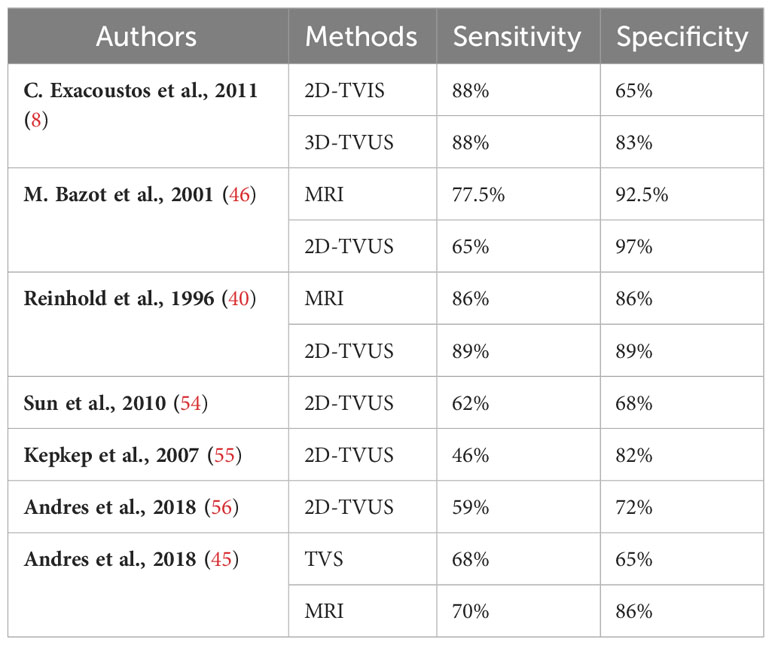
Table 3 Comparison of the sensitivity and specificity of different methods for detecting the JZ in diagnosing adenomyosis.
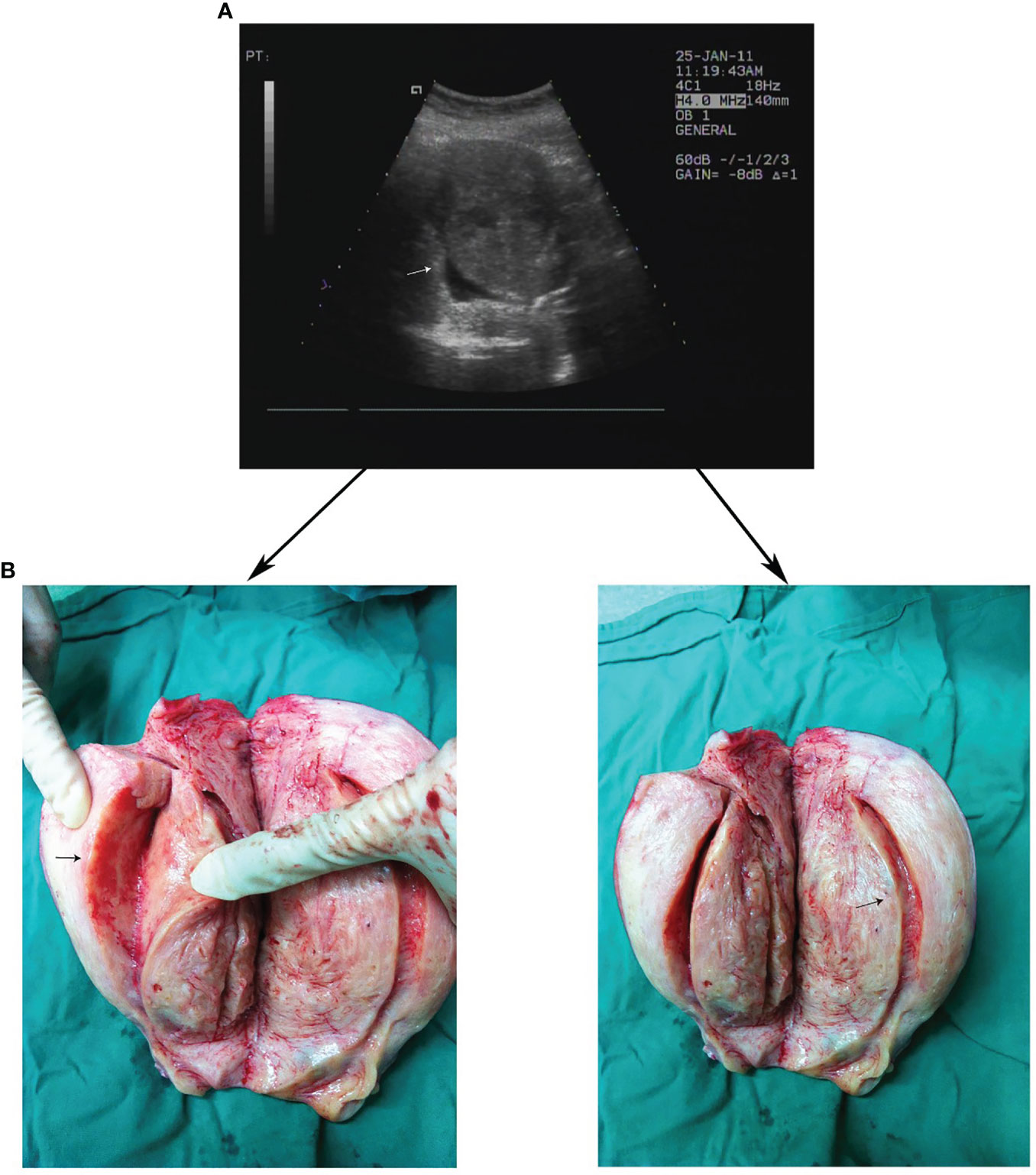
Figure 3 Clear demarcation of JZ appearance on TVUS and gross surgical specimens. (A) Thickened JZ in a patient with severe adenomyosis on TVUS. (B) Corresponding gross surgical specimens.
3 Junctional zone in conservative therapy and reproductive management
Although few randomized double-blind clinical studies focusing on medical treatment for adenomyosis have been performed, medical therapy currently shows increasing efficacy in patients requiring control of symptoms or fertility treatments. The rationale for using medical treatment is based on the pathogenetic mechanisms of adenomyosis. Given the critical role of the JZ in adenomyosis, there are a few types of medicine that have a positive effect on the JZ in the treatment of adenomyosis (Figure 4). The effectiveness of HIFU on the JZ in the treatment of adenomyosis has also been shown.
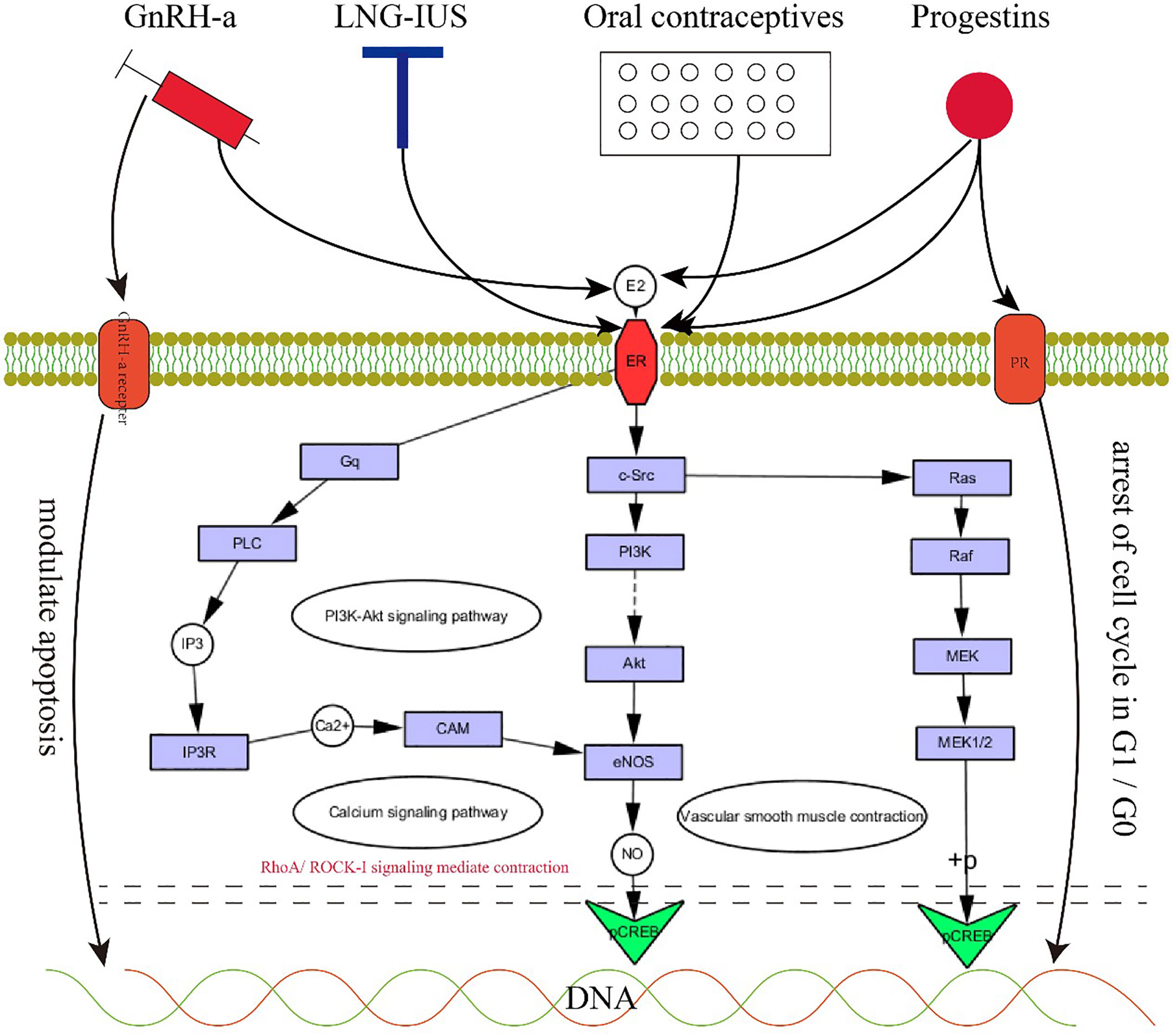
Figure 4 The rationale for medical therapy. 4. GnRH-a, LNG-IUS, oral contraceptives and progestins exert positive effects on JZ by acting on progesterone receptors, estrogen receptors, and GnRH-a receptors to regulate the PI3K-Akt signaling pathway, calcium signaling pathway and vascular smooth muscle contraction.
3.1 GnRH analogues
GnRH-a relieves pain, causes amenorrhea, and significantly reduces menstrual bleeding. For premenopausal women, regardless of the size of the uterus, the use of GnRH-a has achieved excellent clinical results. The rationale for using GnRH analogues for the medical treatment of adenomyosis is the direct antiproliferative effect within the myometrium through actions on the GnRH receptors expressed by adenomyotic lesions with a systemic and local hypoestrogenic effect through a central downregulation and a profound suppression of gonadotropin secretion. GnRH-a can normalize local estrogen metabolism in the eutopic endometrium of women with adenomyosis by decreasing the expression of aromatase cytochrome P450 (60). When GnRH-a suppresses estrogen for a sufficient amount of time, adenomyotic lesions regress. Uterine size decreases, and symptoms are relieved due to the recovery of the structure and function of the JZ in adenomyosis. On the other hand, GnRH-a appears to directly affect endometrial cells by increasing the percentage of apoptotic cells and reducing the release of cytokines such as IL-1β and VEGF, the key molecules in the invagination of the endometrium into the JZ (61). Due to the outstanding efficacy of GnRH-a, it is often used as the first choice for conservative treatment of patients with a large uterus or anemia. The main side effects of GnRH-a are menopausal symptoms caused by low estrogen and the possibility of bone loss with long-term use. There is no consensus on the best treatment for subfertility in adenomyosis. The improved downregulation scheme can improve the clinical pregnancy rate of moderate and severe adenomyosis patients undergoing FTET through the influence of the endometrial inflammatory response and myometrial contractility and its impact on uterine receptivity (62). During IVF/ICSI, the pregnancy outcome of women with adenomyosis who used the ultralong GnRH-a protocol was improved, and the early abortion rate was reduced. In patients with diffuse adenomyosis in particular, the pregnancy and live birth rates have been improved (63). Moreover, an observational cohort study showed that during IVF, patients with adenomyosis had a better clinical pregnancy rate, implantation rate, and live birth rate after treatment with an ultralong GnRH agonist (64). Overall, GnRH-a treatment may benefit the pregnancy outcome of women with adenomyosis, but the exact therapeutic effect still needs more research.
3.2 Levonorgestrel-releasing intrauterine system
The levonorgestrel-releasing intrauterine system (LNG-IUS) has been successfully used to treat adenomyosis to reduce menstrual blood loss and pain by reducing the thickness of the myometrial JZ and total thickness of uterine volume. It is recommended as a therapy for patients with hypermenorrhea. Several mechanisms may explain the role of the LNG-IUS in adenomyosis. First, after insertion of the LNG-IUS, the high local concentration of LNG on the endometrium induces endometrial inactivity to estrogen via downregulation of estrogen receptors, resulting in glandular atrophy stromal deciduation on both eutopic and ectopic endometrium that produces a marked reduction in menstrual blood loss. Progestin also acts directly on adenomyotic foci through absorption within the myometrium. In addition, endometrial inactivity can inhibit the activity of aromatase and Cox-2, further decreasing the production of estrogen and prostaglandin, leading to a reduction in JZ hypertrophy and hyperplasia and finally reducing the thickness of the JZ and improving uterine contraction. Bragheto observed a significant decrease of 24.2% in JZ thickness after insertion of the LNG-IUS, and a reduction in pain and abnormal bleeding associated with adenomyosis was also documented. Uterine artery blood flow decreases obviously, along with shrinkage of the uterine volume (65). The LNG-IUS can improve the implantation rates and clinical pregnancy rates of women with adenomyosis who receive IVF. The odds ratio (OR) of ongoing pregnancy increases significantly with the use of the LNG-IUS (66).
3.3 Oral contraceptives
The rationale for using oral contraceptives (OCs) in adenomyosis is related to the induced decidualization and subsequent atrophy of the endometrium, reducing pain and AUB. Some studies have reported a significantly thinner JZ in the posterior uterine wall in women with adenomyosis who were treated with OCs compared with women who were not (67, 68). Additionally, a MRI study of nulliparous women showed that the JZ thickness is significantly affected by hormonal contraception (69). Moreover, according to the study by Antoine et al. (15), compared to that of nonusers, the front and back walls of the middle body and fundus of the contraceptive users had significantly thinner connection areas.
3.4 Progestins
Progestins are used to treat adenomyosis by inhibiting the secretion of pituitary gonadotropin. It leads to an aperiodic state of low estrogen, giving rise to endometrial decidualization and pseudopregnancy, which appear as amenorrhea. Recent studies showed a correlation between serum progesterone levels and the apparent diffusion coefficient (ADC) of the JZ, which could assess the extent of myometrial invasion of the endometrium (70).
Progestins can relieve dysmenorrhea and reduce menstrual bleeding. However, it is rarely used as a clinical prescription for adenomyosis therapy. Dienogest listed in Japan and Europe has beneficial therapeutic effects. Dienogest is the world’s first specific prescription drug for adenomyosis, and a phase III clinical trial of dienogest was completed in Japan in 2017. This trial demonstrated the good performance of dienogest in relieving dysmenorrhea symptoms of adenomyosis (71). Dienogest can inhibit the proliferation of ectopic endometrial stromal cells by arresting the cell cycle in G1/G0, and P can control E2-induced cell proliferation and increase p27 protein expression in endometrial glandular cells (72). Kazuaki Neriishi et al. (73) conducted a retrospective cohort study to make long-term observations of dienogest. The main side effect is irregular uterine bleeding, which causes 20% of people to stop taking the drug.
3.5 Anti-platelet treatment
In addition to the abovementioned changes in the JZ caused by cell cycle and pathway regulation, microtrauma of the myometrium is also an important reason cause of endometrial fragment invasion into the myometrium and eventual adenomyosis. The increase in myofibroblasts in the JZ often indicates the occurrence of microtrauma and simultaneously causes hyperperistalsis and microdehiscences in the JZ, which facilitate the development of adenomyosis (74, 75). Some evidence has shown that platelets might play an important role in adenomyosis pathogenesis because platelets induce epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation, ultimately leading to fibrosis (76). A study in a mouse model of adenomyosis demonstrated that antiplatelet treatment (thromboxane A2 synthesis inhibitor) was effective in suppressing myometrial infiltration, improving generalized hyperalgesia, and reducing both uterine hyperactivity and systemic corticosterone levels. In addition, decreased expression of some proteins involved in adenomyosis fibrogenesis was demonstrated, supporting the promising role of antiplatelet therapy in adenomyosis (77). However, to date, no studies have been published or registered on the use of agents targeting platelets.
3.6 Focused ultrasound surgery
Focused ultrasound therapy is a minimally invasive treatment for adenomyosis that focuses high-energy ultrasound on focal lesions. The treatment method can be oriented and monitored by ultrasound (HIFU) or MRI (MRgFUS). The advantages of HIFU include its non-invasiveness, lack of radiation and reproducibility. Especially for women with fertility requirements, HIFU has more advantages in maintaining the physiological structure of the uterus and preserving the integrity of the uterus. The disadvantage is that the selection criteria for patients are more stringent. This treatment is recommended only for premenopausal women with adenomyosis without suspicious pelvic adhesions, no history of lower abdominal surgery, abdominal wall thickness <5 cm, and lesion diameter between 3-10 cm (78). A retrospective study evaluated the symptoms of dysmenorrhea and menstrual volume in 350 patients with adenomyosis. This trial showed that the ablation rate of HIFU for adenomyosis was above 70% and that the rate of alleviation of dysmenorrhea was over 80%. Two hundred twenty-four of the patients completed a two-year follow-up, and the rate of symptom relief was over 80%. In one trial, the JZ thickness of 18 patients with adenomyosis who underwent MRgFUS was measured before and one year after surgery. Symptoms resolved in 18 patients one year after treatment, and there was no relapse. The JZ thickness was also significantly lower than that before treatment (44). Therefore, the JZ may be used to predict the prognosis of adenomyosis. Compared with traditional laparoscopy, patients treated with HIFU had higher pregnancy and natural conception rates. Laparoscopic lesionectomy may remove a large amount of myometrium and may reduce myometrial volume, leading to a lack of sensitivity to uterine growth during pregnancy and an increased risk of uterine scarring resulting in uterine rupture (79). The postoperative pregnancy rate of diffuse adenomyotic lesions was significantly lower than that of focal adenomyosis (80).
One clinical study showed that the treatment effect of HIFU combined with LNG-IUS was significantly higher than that of HIFU and HIFU combined with GnRH-a from the perspective of dysmenorrhea degree and menstrual volume (81). However, the treatment of this combination therapy for adenomyosis combined with infertility needs further study.
4 Conclusion
In summary, according to current research, changes in the junctional zone affect the occurrence and development of adenomyosis and affect the fertility of women of reproductive age. Therefore, it has gradually become the central research link for ADS patients with infertility. Structural and functional defects of the JZ play an integral role in the development of adenomyosis. Although many conservative treatments have effectively alleviated adenomyosis symptoms, current research on the part of medicine in the JZ lacks a deeper understanding of specific molecular mechanisms. There is still a lack of research on reproductive outcomes after drug treatment. In addition, the changes in the JZ have a significant impact on women’s fertility, but there has been no long-term follow-up study for further observation and confirmation.
Moreover, the treatments mentioned above each have unique associated risks and benefits, and most of them have been studied only for short-term use in small study populations. The efficacy of long-term treatment still needs well-conducted randomized controlled trials. Treatment should be tailored to the individual patient’s specific symptoms or unique request; the same sign may have different implications for different women.
Author contributions
SW and HD designed the study, retrieved the data, and wrote, revised, and reviewed the manuscript. All the authors have read and approved the final manuscript. All authors contributed to the article.
Funding
This work was supported by the Natural Science Foundation of China (30872744 and 81270680) and Beijing Obstetrics and Gynecology Hospital, Capital Medical University (FCYY201920).
Acknowledgments
We are grateful to Beijing Obstetrics and Gynecology Hospital, Capital Medical University, for encouraging and supporting this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bird CC, McElin TW, Manalo-Estrella P. The elusive adenomyosis of the uterus–revisited. Am J Obstet Gynecol (1972) 112(5):583–93. doi: 10.1016/0002-9378(72)90781-8
2. Peric H, Fraser IS. The symptomatology of adenomyosis. Best Pract Res Clin Obstet Gynaecol (2006) 20(4):547–55. doi: 10.1016/j.bpobgyn.2006.01.006
3. Puente JM, Fabris A, Patel J, Patel A, Cerrillo M, Requena A, et al. Adenomyosis in infertile women: prevalence and the role of 3d ultrasound as a marker of severity of the disease. Reprod Biol Endocrinol (2016) 14(1):60. doi: 10.1186/s12958-016-0185-6
4. He YL, Ding N, Qi YF, Li Y, Xiang Y, Qian TY, et al. Visualising the boundary sharpness of uterine zonal structures using high-resolution T2-weighted images during the menstrual cycle. Clin Radiol (2019) 74(1):81.e19–24. doi: 10.1016/j.crad.2018.09.008
5. Brosens I, Derwig I, Brosens J, Fusi L, Benagiano G, Pijnenborg R. The enigmatic uterine junctional zone: the missing link between reproductive disorders and major obstetrical disorders? Hum Reprod (2010) 25(3):569–74. doi: 10.1093/humrep/dep474
6. Larsen SB, Lundorf E, Forman A, Dueholm M. Adenomyosis and junctional zone changes in patients with endometriosis. Eur J Obstet Gynecol Reprod Biol (2011) 157(2):206–11. doi: 10.1016/j.ejogrb.2011.03.003
7. Brosens JJ, de Souza NM, Barker FG. Uterine junctional zone: function and disease. Lancet (1995) 346(8974):558–60. doi: 10.1016/S0140-6736(95)91387-4
8. Exacoustos C, Brienza L, Di Giovanni A, Szabolcs B, Romanini ME, Zupi E, et al. Adenomyosis: three-dimensional sonographic findings of the junctional zone and correlation with histology. Ultrasound Obstet Gynecol (2011) 37(4):471–9. doi: 10.1002/uog.8900
9. Zhang Y, Zhou L, Li TC, Duan H, Yu P, Wang HY. Ultrastructural features of endometrial-myometrial interface and its alteration in adenomyosis. Int J Clin Exp Pathol (2014) 7(4):1469–77.
10. Tanos V, Lingwood L, Balami S. The importance of the junctional zone of the endometrium in human reproduction. Hum Fertil (Camb) (2022) 25(1):4–12. doi: 10.1080/14647273.2020.1720316
11. Barbanti C, Centini G, Lazzeri L, Habib N, Labanca L, Zupi E, et al. Adenomyosis and infertility: the role of the junctional zone. Gynecol Endocrinol (2021) 37(7):577–83. doi: 10.1080/09513590.2021.1878131
12. Noe M, Kunz G, Herbertz M, Mall G, Leyendecker G. The cyclic pattern of the immunocytochemical expression of oestrogen and progesterone receptors in human myometrial and endometrial layers: characterization of the endometrial-subendometrial unit. Hum Reprod (1999) 14(1):190–7. doi: 10.1093/humrep/14.1.190
13. Harmsen MJ, Trommelen LM, de Leeuw RA, Tellum T, Juffermans LJM, Griffioen AW, et al. Uterine junctional zone and adenomyosis: comparison of Mri, transvaginal ultrasound and histology. Ultrasound Obstet Gynecol (2023) 62(1):42–60. doi: 10.1002/uog.26117
14. Bazot M, Daraï E. Role of transvaginal sonography and magnetic resonance imaging in the diagnosis of uterine adenomyosis. Fertil Steril (2018) 109(3):389–97. doi: 10.1016/j.fertnstert.2018.01.024
15. Maubon A, Faury A, Kapella M, Pouquet M, Piver P. Uterine junctional zone at magnetic resonance imaging: A predictor of in vitro fertilization implantation failure. J Obstet Gynaecol Res (2010) 36(3):611–8. doi: 10.1111/j.1447-0756.2010.01189.x
16. Maged AM, Ramzy A-M, Ghar MA, El Shenoufy H, Gad Allah SH, Wahba AH, et al. 3d ultrasound assessment of endometrial junctional zone anatomy as a predictor of the outcome of Icsi cycles. Eur J Obstet Gynecol Reprod Biol (2017) 212:160–5. doi: 10.1016/j.ejogrb.2017.03.035
17. Pang W-J, Feng X, Wang X, Wang L, Sun N-X. Analysis of the effect of phloroglucinol on pregnancy outcomes involving frozen embryo transfers in patients with endometriosis: A retrospective case-control study. Front Surg (2022) 9:994775. doi: 10.3389/fsurg.2022.994775
18. Zhang Y, Yu P, Sun F, Li TC, Cheng Jm, Duan H. Expression of oxytocin receptors in the uterine junctional zone in women with adenomyosis. Acta Obstet Gynecol Scand (2015) 94(4):412–8. doi: 10.1111/aogs.12595
19. Shaked S, Jaffa AJ, Grisaru D, Elad D. Uterine peristalsis-induced stresses within the uterine wall may sprout adenomyosis. Biomech Model Mechanobiol (2015) 14(3):437–44. doi: 10.1007/s10237-014-0614-4
20. Meylaerts LJ, Wijnen L, Ombelet W, Bazot M, Vandersteen M. Uterine junctional zone thickness in infertile women evaluated by Mri. J Magn Reson Imaging (2017) 45(3):926–36. doi: 10.1002/jmri.25422
21. Kunz G, Beil D. Characterization of the uterine junctional zone prior to Ivf/Icsi: an observational study. Arch Gynecol Obstet (2010) 281(5):945–53. doi: 10.1007/s00404-009-1285-8
22. Lesny P, Killick SR, Tetlow RL, Robinson J, Maguiness SD. Uterine junctional zone contractions during assisted reproduction cycles. Hum Reprod Update (1998) 4(4):440–5. doi: 10.1093/humupd/4.4.440
23. Biervliet FP, Lesny P, Maguiness SD, Robinson J, Killick SR. Transmyometrial embryo transfer and junctional zone contractions. Hum Reprod (2002) 17(2):347–50. doi: 10.1093/humrep/17.2.347
24. Kissler S, Siebzehnruebl E, Kohl J, Mueller A, Hamscho N, Gaetje R, et al. Uterine contractility and directed sperm transport assessed by hysterosalpingoscintigraphy (Hssg) and intrauterine pressure (Iup) measurement. Acta Obstet Gynecol Scand (2004) 83(4):369–74. doi: 10.1111/j.0001-6349.2004.00412.x
25. Kissler S, Zangos S, Wiegratz I, Kohl J, Rody A, Gaetje R, et al. Utero-tubal sperm transport and its impairment in endometriosis and adenomyosis. Ann N Y Acad Sci (2007) 1101:38–48. doi: 10.1196/annals.1389.036
26. Zhu L, Che HS, Xiao L, Li YP. Uterine peristalsis before embryo transfer affects the chance of clinical pregnancy in fresh and frozen-thawed embryo transfer cycles. Hum Reprod (2014) 29(6):1238–43. doi: 10.1093/humrep/deu058
27. Arman BM, Binder NK, de Alwis N, Kaitu'u-Lino T, Hannan NJ. Repurposing existing drugs as a therapeutic approach for the prevention of preterm birth. Reproduction (2023) 165(1):R9–R23. doi: 10.1530/REP-22-0226
28. Wang J, Zhang H-h, Duan H. [Expression of erα in endometrial-myometria interface of human adenomyosis]. Zhonghua Yi Xue Za Zhi (2010) 90(27):1914–7.
29. Wang S, Duan H. Rapid Effects of Estrogen on Intracellular Ca2+ Regulation in Junctional Myometrium through the Menstrual Cycle in Uteri with and without Adenomyosis. J Minim Invasive Gynecol (2015) 22(6S):S173. doi: 10.1016/j.jmig.2015.08.642
30. Orazov M, Radzinsky V, Sharapova O, Kostin I, Chitanava Y. Oxytocinergic regulation in pathogenesis of pelvic pain caused by adenomyosis. Gynecol Endocrinol (2020) 36(sup1):20–3. doi: 10.1080/09513590.2020.1816723
31. García-Solares J, Donnez J, Donnez O, Dolmans M-M. Pathogenesis of uterine adenomyosis: invagination or metaplasia? Fertil Steril (2018) 109(3):371–9. doi: 10.1016/j.fertnstert.2017.12.030
32. Guo S-W, Mao X, Ma Q, Liu X. Dysmenorrhea and its severity are associated with increased uterine contractility and overexpression of oxytocin receptor (Otr) in women with symptomatic adenomyosis. Fertil Steril (2013) 99(1):231–40. doi: 10.1016/j.fertnstert.2012.08.038
33. Huang M, Li X, Guo P, Yu Z, Xu Y, Wei Z. The abnormal expression of oxytocin receptors in the uterine junctional zone in women with endometriosis. Reprod Biol Endocrinol (2017) 15(1):1. doi: 10.1186/s12958-016-0220-7
34. Qu M, Lu P, Bellve K, Lifshitz LM, ZhuGe R. Mode switch of Ca2 + Oscillation-mediated uterine peristalsis and associated embryo implantation impairments in mouse adenomyosis. Front Physiol (2021) 12:744745. doi: 10.3389/fphys.2021.744745
35. Dietl J, Hönig A, Kämmerer U, Rieger L. Natural killer cells and dendritic cells at the human feto-maternal interface: an effective cooperation? Placenta (2006) 27(4-5):341–7. doi: 10.1016/j.placenta.2005.05.001
36. Huang H-Y, Yu H-T, Chan S-H, Lee C-L, Wang H-S, Soong Y-K. Eutopic endometrial interleukin-18 system Mrna and protein expression at the level of endometrial-myometrial interface in adenomyosis patients. Fertil Steril (2010) 94(1):33–9. doi: 10.1016/j.fertnstert.2009.01.132
37. Vannuccini S, Clifton VL, Fraser IS, Taylor HS, Critchley H, Giudice LC, et al. Infertility and reproductive disorders: impact of hormonal and inflammatory mechanisms on pregnancy outcome. Hum Reprod Update (2016) 22(1):104–15. doi: 10.1093/humupd/dmv044
38. Tanos V, Lingwood L, Balami S. Junctional zone endometrium morphological characteristics and functionality: review of the literature. Gynecol Obstet Invest (2020) 85(2):107–17. doi: 10.1159/000505650
39. Brosens I, Pijnenborg R, Benagiano G. Defective myometrial spiral artery remodelling as a cause of major obstetrical syndromes in endometriosis and adenomyosis. Placenta (2013) 34(2):100–5. doi: 10.1016/j.placenta.2012.11.017
40. Reinhold C, McCarthy S, Bret PM, Mehio A, Atri M, Zakarian R, et al. Diffuse adenomyosis: comparison of endovaginal us and Mr imaging with histopathologic correlation. Radiology (1996) 199(1):151–8. doi: 10.1148/radiology.199.1.8633139
41. Byun JY, Kim SE, Choi BG, Ko GY, Jung SE, Choi KH. Diffuse and focal adenomyosis: Mr imaging findings. Radiographics (1999) 19:S161–S70. doi: 10.1148/radiographics.19.suppl_1.g99oc03s161
42. Sofic A, Husic-Selimovic A, Carovac A, Jahic E, Smailbegovic V, Kupusovic J. The significance of Mri evaluation of the uterine junctional zone in the early diagnosis of adenomyosis. Acta Inform Med (2016) 24(2):103–6. doi: 10.5455/aim.2016.24.103-106
43. Dashottar S, Singh AK, Debnath J, Muralidharan CG, Singh RK, Kumar S. Comparative analysis of changes in Mr imaging of pre and post intrauterine progesterone implants in adenomyosis cases. Med J Armed Forces India (2015) 71(2):145–51. doi: 10.1016/j.mjafi.2015.01.008
44. Ferrari F, Arrigoni F, Miccoli A, Mascaretti S, Fascetti E, Mascaretti G, et al. Effectiveness of magnetic resonance-guided focused ultrasound surgery (Mrgfus) in the uterine adenomyosis treatment: technical approach and Mri evaluation. Radiol Med (2016) 121(2):153–61. doi: 10.1007/s11547-015-0580-7
45. Dueholm M, Lundorf E, Hansen ES, Sørensen JS, Ledertoug S, Olesen F. Magnetic resonance imaging and transvaginal ultrasonography for the diagnosis of adenomyosis. Fertil Steril (2001) 76(3):588–94. doi: 10.1016/S0015-0282(01)01962-8
46. Bazot M, Cortez A, Darai E, Rouger J, Chopier J, Antoine JM, et al. Ultrasonography compared with magnetic resonance imaging for the diagnosis of adenomyosis: correlation with histopathology. Hum Reprod (2001) 16(11):2427–33. doi: 10.1093/humrep/16.11.2427
47. Tellum T, Nygaard S, Lieng M. Noninvasive diagnosis of adenomyosis: A structured review and meta-analysis of diagnostic accuracy in imaging. J Minim Invasive Gynecol (2020) 27(2):408–18.e3. doi: 10.1016/j.jmig.2019.11.001
48. Tellum T, Matic GV, Dormagen JB, Nygaard S, Viktil E, Qvigstad E, et al. Diagnosing adenomyosis with Mri: A prospective study revisiting the junctional zone thickness cutoff of 12 mm as a diagnostic marker. Eur Radiol (2019) 29(12):6971–81. doi: 10.1007/s00330-019-06308-3
49. Bazot M, Daraï E, Clément de Givry S, Boudghène F, Uzan S, Le Blanche AF. Fast breath-hold T2-weighted Mr imaging reduces interobserver variability in the diagnosis of adenomyosis. AJR Am J Roentgenol (2003) 180(5):1291–6. doi: 10.2214/ajr.180.5.1801291
50. Bourdon M, Oliveira J, Marcellin L, Santulli P, Bordonne C, Maitrot Mantelet L, et al. Adenomyosis of the inner and outer myometrium are associated with different clinical profiles. Hum Reprod (2021) 36(2):349–57. doi: 10.1093/humrep/deaa307
51. Juang CM, Chou P, Yen MS, Twu NF, Horng HC, Hsu WL. Adenomyosis and risk of preterm delivery. BJOG (2007) 114(2):165–9. doi: 10.1111/j.1471-0528.2006.01186.x
52. Eisenberg VH, Arbib N, Schiff E, Goldenberg M, Seidman DS, Soriano D. Sonographic signs of adenomyosis are prevalent in women undergoing surgery for endometriosis and may suggest a higher risk of infertility. BioMed Res Int (2017) 2017:8967803. doi: 10.1155/2017/8967803
53. Tanos V, Balami S, Lingwood L. Junctional zone endometrium alterations in gynecological and obstetrical disorders and impact on diagnosis, prognosis and treatment. Curr Opin Obstet Gynecol (2019) 31(6):418–27. doi: 10.1097/GCO.0000000000000572
54. Sun Y-L, Wang C-B, Lee C-Y, Wun T-H, Lin P, Lin Y-H, et al. Transvaginal sonographic criteria for the diagnosis of adenomyosis based on histopathologic correlation. Taiwan J Obstet Gynecol (2010) 49(1):40–4. doi: 10.1016/S1028-4559(10)60007-1
55. Kepkep K, Tuncay YA, Göynümer G, Tutal E. Transvaginal sonography in the diagnosis of adenomyosis: which findings are most accurate? Ultrasound Obstet Gynecol (2007) 30(3):341–5. doi: 10.1002/uog.3985
56. Andres MP, Borrelli GM, Ribeiro J, Baracat EC, Abrão MS, Kho RM. Transvaginal ultrasound for the diagnosis of adenomyosis: systematic review and meta-analysis. J Minim Invasive Gynecol (2018) 25(2):257–64. doi: 10.1016/j.jmig.2017.08.653
57. Exacoustos C, Morosetti G, Conway F, Camilli S, Martire FG, Lazzeri L, et al. New sonographic classification of adenomyosis: do type and degree of adenomyosis correlate to severity of symptoms? J Minim Invasive Gynecol (2020) 27(6):1308–15. doi: 10.1016/j.jmig.2019.09.788
58. Mavrelos D, Holland TK, O'Donovan O, Khalil M, Ploumpidis G, Jurkovic D, et al. The impact of adenomyosis on the outcome of Ivf-embryo transfer. Reprod BioMed Online (2017) 35(5):549–54. doi: 10.1016/j.rbmo.2017.06.026
59. Neal S, Morin S, Werner M, Gueye NA, Pirtea P, Patounakis G, et al. Three-dimensional ultrasound diagnosis of adenomyosis is not associated with adverse pregnancy outcome following single thawed euploid blastocyst transfer: prospective cohort study. Ultrasound Obstet Gynecol (2020) 56(4):611–7. doi: 10.1002/uog.22065
60. Ishihara H, Kitawaki J, Kado N, Koshiba H, Fushiki S, Honjo H. Gonadotropin-releasing hormone agonist and danazol normalize aromatase cytochrome P450 expression in eutopic endometrium from women with endometriosis, adenomyosis, or leiomyomas. Fertil Steril (2003) 79 Suppl 1:735–42. doi: 10.1016/S0015-0282(02)04813-6
61. Meresman GF, Bilotas MA, Lombardi E, Tesone M, Sueldo C, Barañao RI. Effect of Gnrh analogues on apoptosis and release of interleukin-1beta and vascular endothelial growth factor in endometrial cell cultures from patients with endometriosis. Hum Reprod (2003) 18(9):1767–71. doi: 10.1093/humrep/deg356
62. Latif S, Wattar BHA, Balachandren N, Lukaszewski T, Saridogan E, Yasmin E, et al. Effectiveness of modified downregulation for women with moderate and severe adenomyosis of the uterus prior to frozen thawed embryo transfer (Moda) study protocol: A pragmatic randomised-controlled trial. BMJ Open (2021) 11(10):e050248. doi: 10.1136/bmjopen-2021-050248
63. Lan J, Wu Y, Wu Z, Wu Y, Yang R, Liu Y, et al. Ultra-long Gnrh agonist protocol during Ivf/Icsi improves pregnancy outcomes in women with adenomyosis: A retrospective cohort study. Front Endocrinol (Lausanne) (2021) 12:609771. doi: 10.3389/fendo.2021.609771
64. Hou X, Xing J, Shan H, Mei J, Sun Y, Yan G, et al. The effect of adenomyosis on ivf after long or ultra-long Gnrh agonist treatment. Reprod BioMed Online (2020) 41(5):845–53. doi: 10.1016/j.rbmo.2020.07.027
65. Bragheto AM, Caserta N, Bahamondes L, Petta CA. Effectiveness of the levonorgestrel-releasing intrauterine system in the treatment of adenomyosis diagnosed and monitored by magnetic resonance imaging. Contraception (2007) 76(3):195–9. doi: 10.1016/j.contraception.2007.05.091
66. Liang Z, Yin M, Ma M, Wang Y, Kuang Y. Effect of pretreatment with a levonorgestrel-releasing intrauterine system on Ivf and vitrified-warmed embryo transfer outcomes in women with adenomyosis. Reprod BioMed Online (2019) 39(1):111–8. doi: 10.1016/j.rbmo.2019.03.101
67. Kido A, Togashi K, Nakai A, Kataoka ML, Koyama T, Fujii S. Oral contraceptives and uterine peristalsis: evaluation with Mri. J Magn Reson Imaging (2005) 22(2):265–70. doi: 10.1002/jmri.20384
68. McCarthy S, Tauber C, Gore J. Female pelvic anatomy: Mr assessment of variations during the menstrual cycle and with use of oral contraceptives. Radiology (1986) 160(1):119–23. doi: 10.1148/radiology.160.1.3715022
69. Meylaerts LJ, Wijnen L, Grieten M, Palmers Y, Ombelet W, Vandersteen M. Junctional zone thickness in young nulliparous women according to menstrual cycle and hormonal contraception use. Reprod BioMed Online (2017) 34(2):212–20. doi: 10.1016/j.rbmo.2016.10.013
70. He YL, Ding N, Li Y, Li Z, Xiang Y, Jin ZY, et al. Cyclic changes of the junctional zone on 3 T Mri images in young and middle-aged females during the menstrual cycle. Clin Radiol (2016) 71(4):341–8. doi: 10.1016/j.crad.2015.12.005
71. Osuga Y, Fujimoto-Okabe H, Hagino A. Evaluation of the efficacy and safety of dienogest in the treatment of painful symptoms in patients with adenomyosis: A randomized, double-blind, multicenter, placebo-controlled study. Fertil Steril (2017) 108(4):673–8. doi: 10.1016/j.fertnstert.2017.07.021
72. Fu L, Osuga Y, Morimoto C, Hirata T, Hirota Y, Yano T, et al. Dienogest inhibits brdu uptake with G0/G1 arrest in cultured endometriotic stromal cells. Fertil Steril (2008) 89(5 Suppl):1344–7. doi: 10.1016/j.fertnstert.2007.03.042
73. Neriishi K, Hirata T, Fukuda S, Izumi G, Nakazawa A, Yamamoto N, et al. Long-term dienogest administration in patients with symptomatic adenomyosis. J Obstet Gynaecol Res (2018) 44(8):1439–44. doi: 10.1111/jog.13674
74. Ibrahim MG, Sillem M, Plendl J, Chiantera V, Sehouli J, Mechsner S. Myofibroblasts are evidence of chronic tissue microtrauma at the endometrial-myometrial junctional zone in uteri with adenomyosis. Reprod Sci (2017) 24(10):1410–8. doi: 10.1177/1933719116687855
75. Ibrahim MG, Chiantera V, Frangini S, Younes S, Köhler C, Taube ET, et al. Ultramicro-trauma in the endometrial-myometrial junctional zone and pale cell migration in adenomyosis. Fertil Steril (2015) 104(6):1475–83.e1-3. doi: 10.1016/j.fertnstert.2015.09.002
76. Shen M, Liu X, Zhang H, Guo S-W. Transforming growth factor B1 signaling coincides with epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation in the development of adenomyosis in mice. Hum Reprod (2016) 31(2):355–69. doi: 10.1093/humrep/dev314
77. Zhu B, Chen Y, Shen X, Liu X, Guo S-W. Anti-platelet therapy holds promises in treating adenomyosis: experimental evidence. Reprod Biol Endocrinol (2016) 14(1):66. doi: 10.1186/s12958-016-0198-1
78. Cheung VYT. Current status of high-intensity focused ultrasound for the management of uterine adenomyosis. Ultrasonography (2017) 36(2):95–102. doi: 10.14366/usg.16040
79. Szubert M, Koziróg E, Olszak O, Krygier-Kurz K, Kazmierczak J, Wilczynski J. Adenomyosis and infertility-review of medical and surgical approaches. Int J Environ Res Public Health (2021) 18(3):1235. doi: 10.3390/ijerph18031235
80. Huang YF, Deng J, Wei XL, Sun X, Xue M, Zhu XG, et al. A comparison of reproductive outcomes of patients with adenomyosis and infertility treated with high-intensity focused ultrasound and laparoscopic excision. Int J Hyperthermia (2020) 37(1):301–7. doi: 10.1080/02656736.2020.1742390
Keywords: adenomyosis, junctional zone, infertility, diagnosis, therapy
Citation: Wang S and Duan H (2023) The role of the junctional zone in the management of adenomyosis with infertility. Front. Endocrinol. 14:1246819. doi: 10.3389/fendo.2023.1246819
Received: 24 June 2023; Accepted: 25 September 2023;
Published: 10 October 2023.
Edited by:
Lokesh Kumar, Genus ABS Global, United StatesReviewed by:
Tae Hoon Kim, University of Missouri, United StatesMonika Dwivedi, Birla Institute of Technology, India
Srinivas Reddy Boreddy, Jackson Laboratory, United States
Copyright © 2023 Wang and Duan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hua Duan, ZHVhbmh1YUBjY211LmVkdS5jbg==
 Sha Wang
Sha Wang Hua Duan
Hua Duan