- 1Institute of Applied Biology, Slovak University of Agriculture in Nitra, Nitra, Slovakia
- 2AgroBioTech Research Center, Slovak University of Agriculture in Nitra, Nitra, Slovakia
- 3Institute of Food Sciences, Slovak University of Agriculture in Nitra, Nitra, Slovakia
- 4Department of Zoology and Anthropology, Constantine the Philosopher University, Nitra, Slovakia
- 5Department of Life Science and Bioinformatics, Assam University, Silchar, India
Grapes are an economically important fruit crop, and their polyphenols (mainly phenolic acids, flavanols, flavonols, anthocyanins, proanthocyanidins, and stilbenes) can exert a wide range of health benefits as an interesting and valuable dietary supplement for natural complementary therapy. However, their potential physiological and therapeutic actions on reproductive processes have not been sufficiently elucidated. This evidence-based study presents current knowledge of grape extracts and polyphenols, as well as their properties and therapeutical actions in relation to female reproduction in a nutshell. Grape extract, and its polyphenols such as resveratrol, proanthocyanidin B2 or delphinidin may influence female reproductive physiology and pathology, as well as regulate multiple signaling pathways related to reproductive hormones, steroid hormones receptors, intracellular regulators of oxidative stress and subsequent inflammation, apoptosis, and proliferation. Their role in the management of ovarian cancer, age-related reproductive insufficiency, ovarian ischemia, PCOS, or menopausal syndrome has been indicated. In particular, the potential involvement of grapeseed extracts and/or proanthocyanidin B2 and delphinidin on ovarian steroidogenesis, oocyte maturation, and developmental capacity has been implicated, albeit at different regulatory levels. Grape polyphenols exert a wide range of health benefits posing grape extract as an interesting and valuable dietary supplement for natural complementary therapy. This evidence-based study focuses on the actions of grapeseed extract and grape polyphenols on female reproductive processes at various regulatory levels and multiple signalling pathways by regulating reproductive hormones (GnRH, gonadotropins, prolactin, steroid hormones, IGFBP), steroid receptors, markers of proliferation and apoptosis. However, lack of knowledge of standardized dosages so far limits their clinical application despite the wide range of their biological and therapeutic potentials.
1 Introduction
Grape is an economically important and one of the most grown fruits worldwide (1). Most of the production (about 80% of the yield) is used for wine making (2). An important by-product of grape processing – grape pomace is the most important residual after juice extraction or wine making and consists of peel, seed, stem, and pulp (3). Grape pomace is considered a rich source for the extraction of a wide range of valuable phytonutrients, which exhibit a variety of bioactivities, such as antioxidant, anti-inflammatory, cardioprotective, anti-aging, antimicrobial and anti-cancer properties (4–9). Bioactive substances including proanthocyanidins, anthocyanins, phenolic acids, stilbenes, and flavonols are abundant in grape by-products (10) that can help in prevention or management of several conditions such as inflammatory conditions characterized by bowel disruption and the involvement of the immune system and colorectal cancer. Grape by-products can promote remarkable effects in reducing pro-inflammatory, pro-oxidative, and proliferative actions in inflammatory bowel diseases and colorectal cancer both in vivo and in vitro (10). Moreover, bioactive substances, such as resveratrol (11), anthocyanidins like delphinidin (12) and procyanidin such as procyanidin B2 (13) are valuable in multiple industries, including pharmaceuticals, agri-food, or cosmetics (13–15). Another abundant by-product of winemaking is grapeseed oil, which is processed from grapeseeds and presents an excellent source of γ-tocotrienol, and α-tocopherol. It also contains fatty acids mainly linoleic, oleic, palmitic, and stearic acids, as well as polyunsaturated fatty acids (PUFAs), monounsaturated fatty acids (MUFAs) and saturated fatty acids (SFAs) (16). Furthermore. secondary plant metabolites such as polyphenols are produced by the grape berries during the growth in reaction to environmental stressors. They form significant components of red wines that enhance the sensory qualities and antioxidant capacity (17). Red wine polyphenols comprise newly generated ones during the winemaking process (such as highly polymerized polyphenols) in addition to those found in grapes as mentioned earlier many of which are recognized to possess beneficial impacts on health (17). Although several studies have summarized the most known physiological and therapeutic effects of grapes and their by-products (12, 15, 18–20), the action of grape extract and grape polyphenols on reproductive processes has not been sufficiently elucidated yet. The present evidence-based study summarizes the current knowledge concerning the provenance, properties, as well as physiological and therapeutic actions of grape extract and grape polyphenols on various cellular processes with a focus on female reproduction.
2 Provenance, bioactive substances and physiological actions
Grapes (Vitis vinifera L.) present an important source of phenolic compounds including phenolic acids, tannins, coumarins, flavonoids, flavones, and stilbenes (7). Grape pomace also contains neutral polysaccharides (30%), pectic substances (20%), and insoluble proanthocyanidins (15%) (21).
Among grape pomace compounds with high nutraceutical value, polyphenols (phenolic acids, flavanols, flavonols, anthocyanins, proanthocyanidins, and stilbenes) are the most interesting due to their bioactive properties (22–24). One of the most efficient bioactive compounds found in grape skin, seeds, and wine is stilbenoid resveratrol (25, 26), widely known primarily for its phytoestrogenic and antioxidant activities (27). Additionally, polyphenolic pigments anthocyanidins, including delphinidin, mainly extracted from grape skins, are responsible for many of the red-orange to blue-violet colors (28). Grapeseeds contain proanthocyanidins, which are composed of epicatechin and monomeric catechin, gallic acid, and polymeric and oligomeric proanthocyanidins (29). Interestingly, proanthocyanidins present more powerful free radical scavengers than vitamins C, E, or β-carotene (30). Monomeric and dimeric flavanols, as well as mono- and diglycosides have been identified in grapeseed extracts. Diglycosylated flavanol dimers have been detected in grape skin extracts, too. The concentration of the mono- and diglycosides depends largely on the grape variety and grape source (31). Major bioactive compounds present in different parts of grape and grape products are given in Table 1.
Grape polyphenols are effective inhibitors of enzymes linked with various ailments. Findings indicate an inverse relationship between the consumption of grapes or grape products and the development of age-related complications including cardiovascular disorders with an estimated 6–7% reduction in deaths from cardiovascular disorders (7). Studies demonstrated biological activities including antioxidant, cardioprotective, anti-cancer, anti-inflammatory, anti-aging, and antimicrobial properties exerted by grape polyphenols such as anthocyanins, flavanols, flavonols, and resveratrol. Chromatographic analysis confirmed the presence of 19 phytochemicals. The prominent compound was catechin followed by gallic acid, caftaric acid, and epicatechin (4–6, 48). Moreover, skin protection, antidiabetic, immunomodulatory and anti-neurodegenerative activities as well as hepatoprotective and neuroprotective effects using phenolic compounds gathered form grape ethanol extract have been reported (11, 15, 18, 48).
Oxidative stress has been associated with the pathogenesis of several chronic diseases and inflammatory processes. Polyphenols are strong antioxidants that act as a defense barrier against free radicals, as well as non-radical oxidants (49). Phenolic acids, stilbenoids, tannins, quinones, coumarins and flavonoids from grapes have the potential to enhance the oxidant capacity of cells stimulating enzymatic expression and reducing the reactive oxygen species (ROS) by either inhibiting their production or by directly scavenging them or via xenobiotic detoxification. For example, administration of Bordo grape juice to human test subjects, led to elevation of antioxidant activities and lowering of blood glucose (50). Grape polyphenols, particularly flavanols can maintain cellular protein homeostasis (proteostasis). Since impaired proteostasis is closely involved in all amyloid diseases, grapeseed extracts may be a valuable therapeutic agent for the prevention and/or management of neurodegenerative diseases (51). The antimicrobial activity against Gram-positive bacteria and antioxidant properties could be associated with phenolic compounds found in grape stems (52). Resveratrol isolated from grape stems was applied on hepatocellular carcinoma Hep-G2 (hepatoma G2) cells, breast adenocarcinoma MCF-7 cells, colon carcinoma HCT116 cells, and lymphoblastic leukemia cells (1301). After treatment, it was shown that resveratrol possesses anti-proliferative and apoptotic effects (53). Anthocyanidins have been found to possess anti-aging and anti- inflammatory properties (28). Lim and Song (12) described the possible use of delphinidin according to its effect on different types of cancers and various chronic diseases. For the study, they used ovarian adenocarcinoma cells (SKOV3) which were then treated with delphinidin alone or with various inhibitors of cell signaling proteins.
Grape pomace contains a high level of antioxidants with the ability to counteract chronic inflammatory symptoms which was demonstrated on colorectal adenocarcinoma-derived intestinal epithelial cell line Caco-2 after grape pomace ethanolic extract treatment (54). Additionally, grapeseeds contain several flavonoids and non-flavonoids which can exert antioxidant and anti-inflammatory activities. Beneficial effects of grapeseed extract in relation to oxidative stress and metabolic disorders such as insulin resistance have been associated with the modulation of plasma adipokines in mammals (55, 56). Grapeseed supplementation has the potential to scavenge oxygen free radicals in the egg yolk in mammals and chicken, as well as it can reduce oxidative damage in the liver in rats (57, 58). It has been reported that grapeseed proanthocyanidin extract possess anti-inflammatory and antioxidant activities (13, 59, 60), and can reduce cytotoxicity as well as genotoxicity (49), including decreasing oxidative damage induced by aflatoxins (61). Proanthocyanidin B2 found in grapeseed present one of the most valuable components of grapeseed extract and can be used due to its protective action against oxidative stress and development of cardiovascular diseases demonstrated on human umbilical vein endothelial cells (HUVEC) (62, 63). In this regard, grape extracts and their polyphenols exhibit protective effects against different toxins and a variety of mechanisms of their action, disturbing physiological homeostasis through increase in superoxide dismutase (SOD) levels and glutathione peroxidase activities, as well as decrease in malondialdehyde (MDA) levels or activation of the nuclear erythroid 2-related factor 2/ARE pathway demonstrated on PC12 rat cells (26). Possible physiological and therapeutic actions of grape polyphenols depending on their bioactive substances are presented in Table 2.
3 Effect on female reproductive processes
Reproductive dysfunctions can be indicated by a negative correlation between muscle growth and reproductive effectiveness (76, 77). Exposure to oxidative stress can lead to the inflammation initiation which is the trigger of multiple reproductive disorders, including ovarian cancer or multiple reproductive defects, such as oocyte mutation, polycystic ovary syndrome (PCOS), endometriosis, as well as can affect ovarian folliculogenesis, oocyte maturation and the release of sex hormones (78–80). Bioactive phytonutrients are known to impart several properties such as anti-inflammatory and antioxidant activities that may have a beneficial impact on reproductive functions (81, 82). Polyphenols can pass through various protective barriers in reproductive organs, which can possibly affect their physiological functions (11, 83, 84). Moreover, grape seed extract had positive impact on improving fertility in golden laying hens (85). Although few studies have been carried out on reproductive cells we have summarized the available information related to the effects of grape polyphenols on female reproductive organs and their (dys)functions in a nutshell. The action on female reproductive processes is presented in Figure 1.
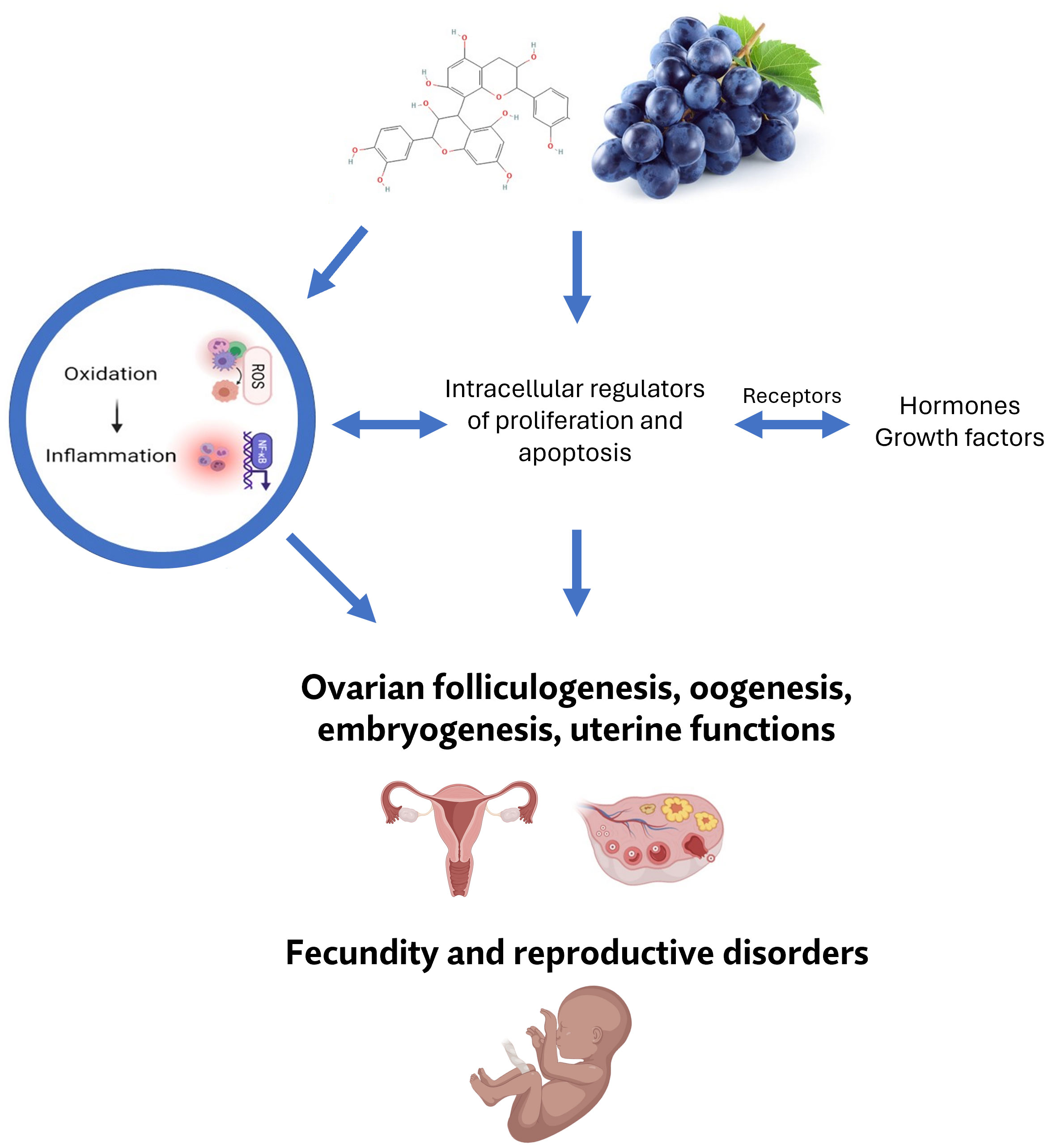
Figure 1 Possible involvement of bioactive constituents of grape (mainly its polyphenols) in female reproductive processes, and their pathological alterations.
3.1 Effect on ovaries
Oxidative stress plays an important role in ovarian aging and can lead to decline of fertility in animals and humans (71, 86). According to Shen et al. (87), apoptotic processes induced by oxidative stress in granulosa cells are considered a major cause of follicular atresia. It has been reported that polyphenols can improve the amount and quality of oocytes in mice and humans which was demonstrated after treating oocytes (71, 88). Beneficial effects of grapeseed extract on oocyte maturation and early development based on the mean numbers of cleavage, morula, and blastocyst rates have been observed in sheep (89). Grapeseed procyanidin B2 can positively affect oocyte viability in mice and promote their maturation and developmental capacity (74). The use of grapeseed extract could also be effective in the prevention or treatment of PCOS. Short-term grapeseed extract treatment provided a beneficial impact on PCOS positive women’s metabolic status (90). Furthermore, grapeseed extract can exert a positive impact on health in reproductive insufficiency and menopause and, also prevent negative morphological changes in ovaries due to reproductive ageing (71, 91). This effect could be due to the presence of proanthocyanidin B2, which has been observed in rat ovaries as a possible protection against age-dependent degenerative changes (11, 71). Some studies have described the protective role of proanthocyanidin B2 from grapeseed against damage to rat ovarian tissue induced by ischemia or ischemia/reperfusion (70, 71, 92). Grapeseed extract can affect resistance to chemotherapy and reduce human ovarian cancer cell growth (13). Delphinidin such as a member of the anthocyanidin family and a natural pigment in grapes may be a pivotal therapeutic target for the prevention of epithelial ovarian cancer (12). Grapeseed ethanol extract, as well as proanthocyanidin B2 can modulate human granulosa cell functions, including steroidogenesis, and can exert phytoestrogenic activity with a positive effect on steroid hormone production in human granulosa cells (93). Available data suggest the potential of using maternal diet supplemented with grapeseed extract in the improvement of egg quality in hens. Furthermore, grapeseed extract can ameliorate egg quality by decreasing the rate of double yolk eggs and by improving the size of normal eggs and the elasticity of the shell (60). In contrast, supplementation of dietary polyphenol resveratrol could not impact egg production and egg quality related to the shell, yolk, and albumen in quails (67). In hens, grapeseed proanthocyanidins have been reported to play an important role in the prevention of ovarian aging process by reducing oxidative stress (71).
3.2 Effect on uterus
Colitti et al. (94) described the possible impact of grapeseed extract on endometrial functions. In heifers, grapeseed extract (oral administration) affected the expression of several genes in the uterine endometrium. In addition, anti-inflammatory properties of resveratrol present in grapes can contribute to the prevention of endometriosis. This well-known phytonutrient has been considered a novel drug in endometriosis prevention and/or treatment (72, 95). Resveratrol has also been reported to modulate the response of endometrium to progesterone and estrogen during decidualization and reinforce hormone action during human endometrial stromal cell (ESC) differentiation, which could lead to improvement of women’s health (73). Another study provided evidence of promising chemopreventive properties of proanthocyanidins in grapeseeds against cervical cancer. Proanthocyanidin B2 can suppress cervical cancer proliferation and growth and induce apoptosis through the mitochondrial signaling pathway (68). Thus, available literature so far suggests the impact of grape polyphenols on uterine endometrium, decidualization, and their potential to prevent and/or treat endometriosis and cervical cancer. Physiological and therapeutic actions of grape polyphenols on female reproductive processes are presented in Table 3.
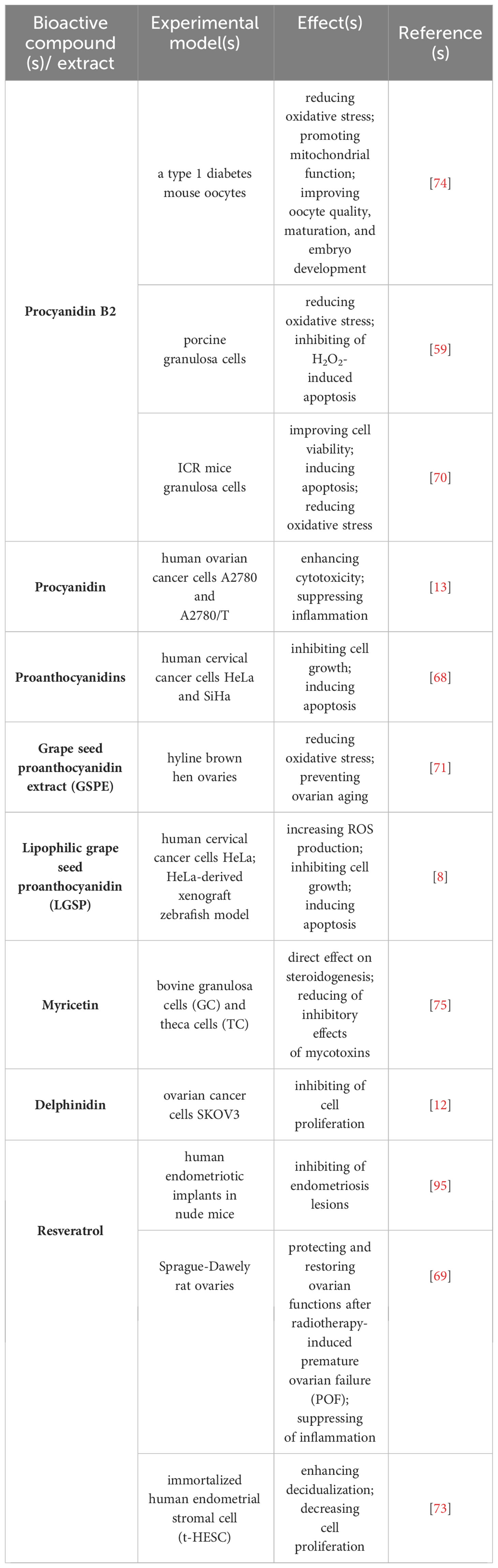
Table 3 Physiological and therapeutic actions of grape polyphenols on female reproductive processes.
4 Regulation of female reproductive processes
Grape, grape extract and their bioactive polyphenols can affect female reproductive processes via extracellular regulators and multiple intracellular signaling pathways. Their mechanism(s) of actions on female reproductive processes have been studied insufficiently, however, there are some studies describing the possible mechanism(s) of effect on female reproduction (11).
4.1 Hormonal regulation and steroidogenesis
Phenolic compounds present in grapes can affect the essential regulators of reproductive processes, including hypothalamic neurohormones (GnRH, oxytocin, LH and FSH), steroid hormones (estradiol, progesterone, testosterone) and prostaglandins (96). Furthermore, due to the chemical similarity of polyphenols to the structure of estrogens, they may exert hormone-like effects (estrogen-agonistic or antagonistic) by binding or activating estrogen receptors (ERα and ERß) (11, 97). A flavonol myricetin present in red wine can block insulin-like growth factor I (IGF-I)-induced progesterone production by granulosa cells and stimulate IGF-I induced estradiol production (75). Similarly, resveratrol can increase prolactin and IGF-I binding protein 1 (IGFBP1) release, which can result in enhanced decidualization of human embryonic stem cells (ESCs) in vitro (73). Grapeseed extract can influence insulin sensitivity by increasing insulin receptors expression and stimulation (98).
Regarding the effect on steroidogenesis, grape extracts, as well as grapeseed proanthocyanidin B2 improved progesterone and estradiol secretion and this was associated with a higher level of the cholesterol carriers, steroidogenic acute regulatory protein (StAR), cyclic adenosine monophosphate response element-binding protein (CREB), and mitogen-activated protein kinases extracellular signal-regulated kinases 1/2 (MAPK ERK1/2) phosphorylation in both primary luteinized human granulosa cells (hGC) and human tumor granulosa cells (KGN). Taken together, GSE and GSPB2 in vitro treatments decrease oxidative stress and increase steroidogenesis without affecting cell proliferation and viability in human granulosa cells (93).Another study described the ability of grapeseed extract to modulate an aromatase inhibitor in vitro as well as in vivo in aromatase-transfected MCF-7 (MCF-7aro) BC xenograft mice (99). Oral administration of grapeseed to heifers can alter progesterone release during estrous cycle after daily oral administration of grapeskin extract for 3 weeks (94). A flavonol myricetin present in red wine can directly affect ovarian function, including steroidogenesis in bovine granulosa cells and theca cells in vitro. These cells were gathered from non-pregnant beef cows. Moreover, myricetin has been able to reduce some of the inhibitory effects of mycotoxins on granulosa cell functions (75).
4.2 Proliferation and apoptosis
Grape polyphenols may affect ovarian cell functions and physiological processes. Interestingly, it has been demonstrated that grapeseed proanthocyanidin B2 may play an important role in the regulation of apoptosis and proliferation in the ovaries (71). In addition, grapeseed proanthocyanidin B2 treatment can inhibit hydrogen peroxide (H2O2)-induced apoptosis in granulosa cells possibly via let-7a upregulation, resulting in protective effect and promotion of viability of porcine granulosa cells (59). Furthermore, grapeseed extracts can inhibit Akt phosphorylation, which can regulate multiple cellular processes such as cell proliferation, survival, and metabolism (100). Another grape constituent, delphinidin inhibits ovarian cancer cell proliferation via inactivation of PI3K/AKT and ERK1/2 mitogen-activated protein kinase signaling pathway, which could be a pivotal therapeutic target for the prevention of epithelial ovarian cancer (12).
Lipophilic grapeseed proanthocyanidin can exert an anti-proliferative effect on cervical cancer HeLa cells by increasing ROS production, resulting in the induction of cellular apoptosis, and cell cycle arrest in the G2/M phase. Proanthocyanidin can reduce mitochondrial membrane potential, upregulate Bax/Bcl-2 ratio, increase the release of cytochrome c, and activate caspase-3 and poly(ADP-Ribose)polymerase (PARP), and thus it can induce apoptotic processes in cervical cancer cells through the intrinsic mitochondrial/caspase-mediated pathway (8). Higher concentrations (50 to 100 μg/mL) of grapeseed extract and proanthocyanidin B2 can inhibit cell proliferation in a human ovarian granulosa-like tumor cell line KGN and hGCs, associated with decrease in cyclin D2 level and an increase in p21 and p27 levels to induce cell cycle arrest in G1 phase (93). While proanthocyanidin B2 did not influence nuclear and cytoplasmic apoptosis in porcine granulosa cells (59), it inhibited the ovarian cancer cell viability and enhanced the resistance to chemotherapy (13). Moreover, both grapeseed extract and proanthocyanidin B2 can increase the cleaved caspase-3 level and impair Bcl-2-associated death promoter protein (BAD) phosphorylation, resulting in cell death. Thus, both can inhibit the expression of intracellular markers (MAP kinase, cyclin D2, Akt phosphorylation), and promote the expression of proliferation inhibitors or apoptotic markers (p21, p27) in ovarian granulosa and ovarian cancer cells (13, 93, 101).
4.3 Oxidative stress
It is known that oxidative stress is a key promoter of reproductive alterations that can negatively affect ovarian functions through apoptosis induction. Moreover, it can dysregulate the expression of related genes (59, 87). ROS production can lead to oxidative stress affecting ovarian functions. Thanks to its protective properties against oxidative stress, grapeseed proanthocyanidin B2 can prevent ovarian aging by oxidative stress suppression in hens (71). In addition, proanthocyanidins can improve oocyte quality, viability, and maturation, as well as developmental capacity by inhibiting ROS production in murine oocytes (74). Dietary grapeseed extract supplementation can reduce ROS levels in egg yolk suggesting a reduction in both oxidative stress and lipid peroxidation in reproductive broiler hens (60). Additionally, a decrease in lipid peroxidation level and an increase in antioxidant capacity in egg yolk have been observed in laying hens, fed with grape pomace flour (102–104). Furthermore, grapeseed extract and grape polyphenols, such as resveratrol and proanthocyanidin B2 may suppress oxidative stress in non-cancerous and cancerous granulosa cells by promoting antioxidant enzymes (59, 93, 105). Moreover, grapeseed extract may exert a negative prooxidant or beneficial antioxidant effect through modulation of NOX actions (106) and possess the ability to regulate ROS production in human granulosa cells. At low concentrations (0.1 to 10 μg/mL), it can reduce oxidative stress by decreasing ROS content and NOX4 expression (93).
Hypothalamic–pituitary–adrenal axis is activated by stress, which can increase glucocorticoid secretion and disrupt the ovarian cycle (94). Maternal dietary supplementation of grapeseed extract can reduce plasma and tissue oxidative stress associated with the modulation of adipokines content in plasma and peripheral tissues in broiler hens (93).
Regarding the anti-inflammatory activity of grapes, a study indicated that grapeseed extract may reduce the expression of pro-inflammatory interleukins in rats suffering from PCOS (107). Furthermore, resveratrol acts in counteracting the inflammatory signaling pathway associated with radiotherapy-induced premature ovarian failure. Resveratrol has been reported to ameliorate cell damage in ovary induced by ionizing radiation and have a protective effect on endometriosis via downregulation of prostaglandins, interleukins, and stimulating inflammation transcription factor NF-κB (69, 72). Furthermore, resveratrol activates SIRT1 expression, resulting in the inhibition of poly(ADP-Ribose)polymerase-1 (PARP-1) and NF-κB expression-mediated inflammatory cytokines, as well as can restore ovarian function by increasing anti-Müllerian hormone (AMH) levels (69). Similarly, grapeseed procyanidin has demonstrated an inhibitory effect on NF-κB activity and MAPK/ERK pathway mediated YB-1 in ovarian cancer cells, suggesting its potential use as a chemo-sensitizer to overcome multidrug resistance in ovarian cancer patients (13).
Based on available reports, grape extract, and its polyphenols such as resveratrol, proanthocyanidin B2 or delphinidin may be considered to influence female reproductive physiological and pathological processes, as well as regulate multiple signaling pathways related to sex hormones, steroid receptors, intracellular regulators of proliferation, oxidative stress, inflammation, and apoptosis (11).
5 Possible application in reproductive biology and medicine
Utilization of grape by-products has attracted increasing attention for the availability of grape skins, their health benefits and pharmacological use. Grape polyphenols can play an important role in the prevention of reproductive disorders due to their ability to mitigate the negative impact of oxidative stress and inflammation on the reproductive processes. Moreover, the beneficial impact on oocyte maturation, cell viability, cell proliferation, as well as steroidogenesis has been reported. Resveratrol from grape stems may have a potential to prevent endometriosis and could serve as a novel dietary supplement. Furthermore, available data suggest the possible use of grapeseed extract to improve oocyte quality, as well as healthy gravidity, embryogenesis, and labour due to its beneficial effect on the endometrium. Moreover, the applicability of grapeseed extract including proanthocyanidin B2 in the prevention and/or management of endometriosis, age-related menopausal reproductive insufficiency and ovarian or cervical cancers has been mentioned. Therefore, grape extract and polyphenols present a promising biostimulator, which can be used as dietary supplement in the improvement of reproduction in the field of animal production, biotechnology, or assisted reproduction. Similarly, phytoestrogenic activity of grape might be used as a potential alternative tool to the hormonal treatment of disorders related to estrogen deficiency, such as menopausal syndrome, and osteoporosis. However, to our knowledge, such potential of grape extract or grape polyphenols has not been examined in depth yet.
6 Conclusions and possible directions of future studies
The present review sheds light on the potential health benefits of grape polyphenols while also emphasizing the need for further research and a more cautious interpretation of the findings. It is evident thatconfirmatory claims about the therapeutic effects of grape polyphenols cannot be made at this stage, given the intricacies of human physiology and the many variables at play. Grape polyphenols exert a wide range of health benefits posing grape extract as an interesting and valuable dietary supplement for natural complementary therapy. This evidence-based study focuses on the actions of grapeseed extract and grape polyphenols on female reproductive processes at various regulatory levels and multiple signalling pathways by regulating reproductive hormones (GnRH, gonadotropins, prolactin, steroid hormones, IGFBP), steroid receptors, markers of proliferation and apoptosis. Moreover, the role of grapes in various reproductive disorders, including reproductive insufficiency, PCOS, menopausal syndrome, ovarian cancer or ovarian ischemia has been indicated. Studies also demonstrate the impact of grapeseed extracts or their bioactive constituents (proanthocyanidin B2, resveratrol, delphinidin) on steroidogenesis, oocyte quality and maturation, and developmental capacity. However, lack of knowledge of standardized dosage limits the clinical applications of grapeseed extract despite the wide range of biological and therapeutic potential.
On the other hand, it should be remembered that in vitro and in vivo studies have been performed with far greater quantities of polyphenols than those frequently found in human diets. Hence, the extent of grape polyphenols consumed on a regular basis is an open question and needs to be addressed in future studies. Determining suitable doses for therapeutic applications remains a critical challenge, as highlighted in the previous sections. The appropriate dosage of grape polyphenols is a key factor in achieving the desired health outcomes, and future research should focus on defining these optimal dosage ranges and accounting for potential variations in individual responses. Moreover, the studies have mainly been performed in vitro or in vivo, whilst clinical studies are lacking and the efficacy of all grape phytosubstances on reproductive processes has not been tested properly yet.
Author contributions
Conceptualization: SR, AK; writing – original draft preparation: LK, AK; writing – review and editing: SB, MM, LB, AS, SR; supervision: AK. All authors contributed to the article and approved the submitted version.
Funding
The work was supported by the Ministry of Education, Science, Research and Sport of the Slovak Republic projects APVV-18-0312, APVV-21-0206 VEGA 1/0266/20, and KEGA 033SPU-4/2021.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ma ZY, Nie ZL, Ren C, Liu XQ, Zimmer EA, Wen J. Phylogenomic relationships and character evolution of the grape family (Vitaceae). Mol Phylogenet Evol (2021) 154:106948. doi: 10.1016/j.ympev.2020.106948
2. Olejar KJ, Ricci A, Swift S, Zujovic Z, Gordon KC, Fedrizzi B, et al. Characterization of an antioxidant and antimicrobial extract from cool climate, white grape marc. Antioxidants (2019) 8(7):232. doi: 10.3390/antiox8070232
3. Averilla JN, Oh J, Kim HJ, Kim JS, Kim JS. Potential health benefits of phenolic compounds in grape processing by-products. Food Sci Biotechnol (2019) 28:1607–15. doi: 10.1007/s10068-019-00628-2
4. Faria A, Calhau C, de Freitas V, Mateus N. Procyanidins as antioxidants and tumor cell growth modulators. J Agric Food Chem (2006) 54(6):2392–7. doi: 10.1021/jf0526487
5. Mantena SK, Baliga MS, Katiyar SK. Grape seed proanthocyanidins induce apoptosis and inhibit metastasis of highly metastatic breast carcinoma cells. Carcinogenesis (2006) 27(8):1682–91. doi: 10.1093/carcin/bgl030
6. Xia EQ, Deng GF, Guo YJ, Li HB. Biological activities of polyphenols from grapes. Int J Mol Sci (2010) 11(2):622–46. doi: 10.3390/ijms11020622
7. Dwibedi V, Jain S, Singhal D, Mittal A, Rath SK, Saxena S. Inhibitory activities of grape bioactive compounds against enzymes linked with human diseases. Appl J Microbiol Biotechnol (2022) 106(4):1399–417. doi: 10.1007/s00253-022-11801-9
8. Li C, Zhang L, Liu C, He X, Chen M, Chen J. Lipophilic grape seed proanthocyanidin exerts anti-Cervical cancer effects in hela cells and a hela-Derived xenograft zebrafish model. Antioxidants (2022) 11:2. doi: 10.3390/antiox11020422
9. Yang C, Han Y, Tian X, Sajid M, Mehmood S, Wang H, et al. Phenolic composition of grape pomace and its metabolism. Crit Rev Food Sci Nutr (2022) 1:17. doi: 10.1080/10408398.2022.2146048
10. Laurindo LF, Direito R, Bueno Otoboni AMM, Goulart RA, Quesada K, Barbalho SM. Grape processing waste: effects on inflammatory bowel disease and colorectal cancer. Food Rev Int (2023) 0(0):1–34. doi: 10.1080/87559129.2023.2168281
11. Sirotkin AV, Kolesarova A. Environmental contaminants and medicinal plants action on female reproduction. London: Academic Press (2022).
12. Lim W, Song G. Inhibitory effects of delphinidin on the proliferation of ovarian cancer cells via PI3K/AKT and ERK 1/2 MAPK signal transduction. Oncol Lett (2017) 14(1):810–8. doi: 10.3892/ol.2017.6232
13. Zhao BX, Sun YB, Wang SQ, Duan L, Huo QL, Ren F, et al. Grape seed procyanidin reversal of p-glycoprotein associated multi-drug resistance via down-regulation of NF-κB and MAPK/ERK mediated YB-1 activity in A2780/T cells. PloS One (2013) 8(8):e71071. doi: 10.1371/journal.pone.0071071
14. Glampedaki P, Dutschk V. Stability studies of cosmetic emulsions prepared from natural products such as wine, grape seed oil and mastic resin. Colloids Surf A Physicochem Eng Asp (2014) 460:306–11. doi: 10.1016/j.colsurfa.2014.02.048
15. Nassiri-Asl M, Hosseinzadeh H. Review of the pharmacological effects of Vitis vinifera (Grape) and its bioactive constituents: an update. Phytother Res (2016) 30(9):1392–403. doi: 10.1002/ptr.5644
16. Fernandes L, Casal S, Cruz R, Pereira JA, Ramalhosa E. Seed oils of ten traditional Portuguese grape varieties with interesting chemical and antioxidant properties. Food Res Int (2013) 50(1):161–6. doi: 10.1016/j.foodres.2012.09.039
17. Buljeta I, Pichler A, Šimunović J, Kopjar M. Beneficial effects of red wine polyphenols on human health: comprehensive review. Curr Issues Mol Biol (2023) 45(2):782–798. doi: 10.3390/cimb45020052
18. Teixeira A, Baenas N, Dominguez-Perles R, Barros A, Rosa E, Moreno DA, et al. Natural bioactive compounds from winery by-products as health promoters: a review. Int J Mol Sci (2014) 15(9):15638–78. doi: 10.3390/ijms150915638
19. Huang Q, Liu X, Zhao G, Hu T, Wang Y. Potential and challenges of tannins as an alternative to in-feed antibiotics for farm animal production. Anim Nutr (2018) 4(2):137–50. doi: 10.1016/j.aninu.2017.09.004
20. Gouvinhas I, Pinto R, Santos R, Saavedra MJ, Barros AI. Enhanced phytochemical composition and biological activities of grape (Vitis vinifera L.) Stems growing in low altitude regions. Sci Hortic (2020) 265:109248. doi: 10.1016/j.scienta.2020.109248
21. Spinei M, Oroian M. The potential of grape pomace varieties as a dietary source of pectic substances. Foods (2021) 10:4. doi: 10.3390/foods10040867
22. Georgiev V, Ananga A, Tsolova V. Recent advances and uses of grape flavonoids as nutraceuticals. Nutrients (2014) 6(1):391–415. doi: 10.3390/nu6010391
23. Gerardi C, D’amico L, Migoni D, Santino A, Salomone A, Carluccio MA, et al. Strategies for reuse of skins separated from grape pomace as ingredient of functional beverages. Front Bioeng Biotechnol (2020) 8:645. doi: 10.3389/fbioe.2020.00645
24. Gerardi C, Pinto L, Baruzzi F, Giovinazzo G. Comparison of antibacterial and antioxidant properties of red (cv. Negramaro) and white (cv. Fiano) skin pomace extracts. Molecules (2021) 26(19):5918. doi: 10.3390/molecules26195918
25. Kuršvietienė L, Stanevičienė I, Mongirdienė A, Bernatonienė J. Multiplicity of effects and health benefits of resveratrol. Medicina (2016) 52(3):148–55. doi: 10.1016/j.medici.2016.03.003
26. Tabeshpour J, Mehri S, Shaebani Behbahani F, Hosseinzadeh H. Protective effects of Vitis vinifera (grapes) and one of its biologically active constituents, resveratrol, against natural and chemical toxicities: A comprehensive review. Phytother Res (2018) 32(11):2164–90. doi: 10.1002/ptr.6168
27. Nashine S, Nesburn AB, Kuppermann BD, Kenney MC. Role of resveratrol in transmitochondrial AMD RPE cells. Nutrients (2020) 12(1):159. doi: 10.3390/nu12010159
29. Bagchi D, Bagchi M, Stohs SJ, Das DK, Ray SD, Kuszynski CA, et al. Free radicals and grape seed proanthocyanidin extract: importance in human health and disease prevention. Toxicology (2000) 148:187–97. doi: 10.1016/s0300-483x(00)00210-9
30. Bagchi D, Swaroop A, Preuss HG, Bagchi M. Free radical scavenging, antioxidant and cancer chemoprevention by grape seed proanthocyanidin: an overview. Mutat Res-Fund Mol M (2014) 768:69–73. doi: 10.1016/j.mrfmmm.2014.04.004
31. Zerbib M, Cazals G, Enjalbal C, Saucier C. Identification and quantification of flavanol glycosides in Vitis vinifera grape seeds and skins during ripening. Molecules (2018) 23(11):2745. doi: 10.3390/molecules23112745
32. Pastrana-Bonilla E, Akoh CC, Sellappan S, Krewer G. Phenolic content and antioxidant capacity of muscadine grapes. J Agric Food Chem (2003) 51(18):5497–4503. doi: 10.1021/jf030113c
33. Hernandez-Jimenez A, Gomez-Plaza E, Martinez-Cutillas A, Kennedy JA. Grape skin and seed proanthocyanidins from Monastrell× Syrah grapes. J Agric Food Chem (2009) 57(22):10798–803. doi: 10.1021/jf903465p
34. Anna Malinowska M, Billet K, Drouet S, Munsch T, Unlubayir M, Tungmunnithum D, et al. Grape cane extracts as multifunctional rejuvenating cosmetic ingredient: Evaluation of sirtuin activity, tyrosinase inhibition and bioavailability potential. Molecules (2020) 25(9):2203. doi: 10.3390/molecules25092203
35. Yu F, Li BY, Yin M, Lu WD, Li XL, Cheng M, et al. Proteomic analysis of liver mitochondria of db/db mice treated with grape seed procyanidin B2. J Food Biochem (2020) 44(11):e13443. doi: 10.1111/jfbc.13443
36. Suc L, Rigou P, Mouls L. Detection and identification of oxidation markers of the reaction of grape tannins with volatile thiols commonly found in wine. J Agric Food Chem (2021) 69(10):3199–208. doi: 10.1021/acs.jafc.0c07163
37. Yilmazer-Musa M, Griffith AM, Michels AJ, Schneider E, Frei B. Grape seed and tea extracts and catechin 3-gallates are potent inhibitors of α-amylase and α-glucosidase activity. J Agric Food Chem (2012) 0:36. doi: 10.1021/jf301147n
38. Luo L, Cui Y, Zhang S, Li L, Li Y, Zhou P, et al. Preparative separation of grape skin polyphenols by high-speed counter-current chromatography. Food Chem (2016) 212:712–21. doi: 10.1016/j.foodchem.2016.06.009
39. Fia G, Bucalossi G, Gori C, Borghini F, Zanoni B. Recovery of bioactive compounds from unripe red grapes (cv. Sangiovese) through a green extraction. Foods (2020) 9:5. doi: 10.3390/foods9050566
40. Viana-Mattioli S, Cinegaglia N, Bertozzi-Matheus M, Bueno-Pereira TO, Caldeira-Dias M, Cavalli RC, et al. SIRT1-dependent effects of resveratrol and grape juice in an in vitro model of preeclampsia. Biomed Pharmacother (2020) 131:110659. doi: 10.1016/j.biopha.2020.110659
41. Gašić U, Ćirić I, Pejčić T, Radenković D, Djordjević V, Radulović S, et al. Polyphenols as possible agents for pancreatic diseases. Antioxidants (2020) 9:6. doi: 10.3390/antiox9060547
42. Makris DP, Boskou G, Andrikopoulos NK, Kefalas P. Characterization of certain major polyphenolic antioxidants in grape (Vitis vinifera cv. Roditis) stems by liquid chromatography-mass spectrometry. Eur Food Res Technol (2008) 226:1075–9. doi: 10.1007/s00217-007-0633-9
43. Ostberg-Potthof JJ, Berger K, Richling E, Winterhalter P. Activity-guided fractionation of red fruit extracts for the identification of compounds influencing glucose metabolism. Nutrients (2019) 11:5. doi: 10.3390/nu11051166
44. Karadeniz F, Durst RW, Wrolstad RE. Polyphenolic composition of raisins. J Agric Food Chem (2000) 48(11):5343–50. doi: 10.1021/jf0009753
45. Zhang BW, Xing Y, Wen C, Yu XX, Sun WL, Xiu ZL, et al. Pentacyclic triterpenes as α-glucosidase and α-amylase inhibitors: structure-activity relationships and the synergism with acarbose. Bioorg Med Chem Lett (2017) 27(22):5065–70. doi: 10.1016/j.bmcl.2017.09.027
46. Rivero-Pérez MD, Muniz P, González-Sanjosé ML. Contribution of anthocyanin fraction to the antioxidant properties of wine. Food Chem Toxicol (2008) 46(8):2815–22. doi: 10.1016/j.fct.2008.05.014
47. Zdunić G, Gođevac D, Šavikin K, Krivokuća D, Mihailović M, Pržić Z, et al. Grape seed polyphenols and fatty acids of autochthonous Prokupac vine variety from Serbia. Chem Biodivers (2019) 16:7. doi: 10.1002/cbdv.20190.0053
48. Recinella L, Chiavaroli A, Veschi S, Cama A, Acquaviva A, Libero ML, et al. A grape (Vitis vinifera L.) pomace water extract modulates inflammatory and immune response in SW-480 cells and isolated mouse colon. Phytother Res (2022) 36:12. doi: 10.1002/ptr.7581
49. Mancini M, Cerny MEV, Cardoso NS, Verissimo G, Maluf SW. Grape seed components as protectors of inflammation, DNA damage, and cancer. Curr Nutr Rep (2023) 12:141–150. doi: 10.1007/s13668-023-00460-5
50. Copetti C, Franco FW, MaChado EDR, Soquetta MB, Quatrin A, Ramos VDM, et al. Acute consumption of bordo grape juice and wine improves serum antioxidant status in healthy individuals and inhibits reactive oxygen species production in human neuron-like cells. J Nutr Metab (2018) 2018:4384012. doi: 10.1155/2018/4384012
51. Mahdipour R, Ebrahimzadeh-Bideskan A, Hosseini M, Shahba S, Lombardi G, Malvandi AM, et al. The benefits of grape seed extract in neurological disorders and brain aging. Nutr Neurosci (2022) 26(5):369–83. doi: 10.1080/1028415X.2022.2051954
52. Leal C, Santos RA, Pinto R, Queiroz M, Rodrigues M, Saavedra MJ, et al. Recovery of bioactive compounds from white grape (Vitis vinifera L.) stems as potential antimicrobial agents for human health. Saudi J Biol Sci (2020) 27(4):1009–15. doi: 10.1016/j.sjbs.2020.02.013
53. Elgizawy HA, Ali AA, Hussein MA. Resveratrol: isolation, and its nanostructured lipid carriers, inhibits cell proliferation, induces cell apoptosis in certain human cell lines carcinoma and exerts protective effect against paraquat-induced hepatotoxicity. J Med Food (2021) 24(1):89–100. doi: 10.1089/jmf.2019.0286
54. Calabriso N, Massaro M, Scoditti E, Verri T, Barca A, Gerardi C, et al. Grape pomace extract attenuates inflammatory response in intestinal epithelial and endothelial cells: Potential health-promoting properties in bowel inflammation. Nutrients (2022) 14(6):1175. doi: 10.3390/nu14061175
55. Decorde K, Agne A, Lacan D, Ramos J, Fouret G, Ventura E, et al. Preventive effect of a melon extract rich in superoxide scavenging activity on abdominal and liver fat and adipokine imbalance in high-fat-fed hamsters. J Agric Food Chem (2009) 57(14):6461–7. doi: 10.1021/jf900504g
56. González-Abuín N, Martínez-Micaelo N, Margalef M, Blay M, Arola-Arnal A, Muguerza B, et al. A grape seed extract increases active glucagon-like peptide-1 levels after an oral glucose load in rats. Food Funct (2014) 5(9):2357–64. doi: 10.1039/c4fo00447g
57. Dulundu E, Ozel Y, Topaloglu U, Toklu H, Ercan F, Gedik N, et al. Grape seed extract reduces oxidative stress and fibrosis in experimental biliary obstruction. J Gastroenterol Hepatol (2007) 22(6):885–92. doi: 10.1111/j.1440-1746.2007.04875.x
58. Choi SK, Zhang XH, Seo JS. Suppression of oxidative stress by grape seed supplementation in rats. Nutr Res Pract (2012) 6(1):3–8. doi: 10.4162/nrp.2012.6.1.3
59. Zhang JQ, Wang XW, Chen JF, Ren QL, Wang J, Gao BW, et al. Grape seed procyanidin B2 protects porcine ovarian granulosa cells against oxidative stress-induced apoptosis by upregulating let-7a expression. Oxid Med Cell Longev (2019) 2019:1076512. doi: 10.1155/2019/1076512
60. Barbe A, Mellouk N, Ramé C, Grandhaye J, Anger K, Chahnamian M, et al. A grape seed extract maternal dietary supplementation improves egg quality and reduces ovarian steroidogenesis without affecting fertility parameters in reproductive hens. PloS One (2020) 15(5):e0233169. doi: 10.1371/journal.pone.0233169
61. Rajput SA, Sun L, Zhang NY, Khalil MM, Ling Z, Chong L, et al. Grape seed proanthocyanidin extract alleviates aflatoxinB1-induced immunotoxicity and oxidative stress via modulation of NF-κB and Nrf2 signaling pathways in broilers. Toxins (2019) 11:1. doi: 10.3390/toxins11010023
62. Yu F, Li BY, Li XL, Cai Q, Zhang Z, Cheng M, et al. Proteomic analysis of aorta and protective effects of grape seed procyanidin B2 in db/db mice reveal a critical role of milk fat globule epidermal growth factor-8 in diabetic arterial damage. PloS One (2012) 7(12):e52541. doi: 10.1371/journal.pone.0052541.e52541
63. Yin W, Li B, Li X, Yu F, Cai Q, Zhang Z, et al. Critical role of prohibition in endothelial cell apoptosis caused by glycated low-density lipoproteins and protective effects of grape seed procyanidin B2. J Cardiovasc Pharmacol (2015) 65(1):13–21. doi: 10.1097/fjc.0000000000000157
64. Qian YP, Cai YJ, Fan GJ, Wei QY, Yang J, Zheng LF, et al. Antioxidant-based lead discovery for cancer chemoprevention: the case of resveratrol. J Med Chem (2009) 52(7):1963–74. doi: 10.1021/jm8015415
65. Yilmaz Y, Toledo RT. Major flavonoids in grape seeds and skins: antioxidant capacity of catechin, epicatechin, and gallic acid. J Agric Food Chem (2004) 52(2):255–60. doi: 10.1021/jf030117h
66. Anastasiadi M, Chorianopoulos NG, Nychas GJE, Haroutounian SA. Antilisterial activities of polyphenol-rich extracts of grapes and vinification byproducts. J Agric Food Chem (2009) 57(2):457–63. doi: 10.1021/jf8024979
67. Sahin K, Akdemir FATİ. H., Orhan C, Tuzcu M, Hayirli A, Sahin N. Effects of dietary resveratrol supplementation on egg production and antioxidant status. Poult Sci (2010) 89(6):1190–8. doi: 10.3382/ps.2010-00635
68. Chen Q, Liu XF, Zheng PS. Grape seed proanthocyanidins (GSPs) inhibit the growth of cervical cancer by inducing apoptosis mediated by the mitochondrial pathway. PloS One (2014) 9(9):e107045. doi: 10.1371/journal.pone.0107045
69. Said RS, El-Demerdash E, Nada AS, Kamal MM. Resveratrol inhibits inflammatory signaling implicated in ionizing radiation-induced premature ovarian failure through antagonistic crosstalk between silencing information regulator 1 (SIRT1) and poly (ADP-ribose) polymerase 1 (PARP-1). Biochem Pharmacol (2016) 103:140–50. doi: 10.1016/j.bcp.2016.01.019
70. Zhang JQ, Gao BW, Wang J, Ren QL, Chen JF, Ma Q, et al. Critical role of FoxO1 in granulosa cell apoptosis caused by oxidative stress and protective effects of grape seed procyanidin B2. Oxid Med Cell Longev (2016) 2016:16. doi: 10.1155/2016/6147345.6147345
71. Liu X, Lin X, Mi Y, Li J, Zhang C. Grape seed proanthocyanidin extract prevents ovarian aging by inhibiting oxidative stress in the hens. Oxid Med Cell Longev (2018) 2018:16. doi: 10.1155/2018/9390810.9390810z
72. Dull AM, Moga MA, Dimienescu OG, Sechel G, Burtea V, Anastasiu CV. Therapeutic approaches of resveratrol on endometriosis via anti-inflammatory and anti-angiogenic pathways. Molecules (2019) 24(4):667. doi: 10.3390/molecules24040667
73. Citrinovitz ACM, Langer L, Strowitzki T, Germeyer A. Resveratrol enhances decidualization of human endometrial stromal cells. Reproduction (2020) 159(4):453–63. doi: 10.1530/REP-19-0425
74. Luo Y, Zhuan Q, Li J, Du X, Huang Z, Hou Y, et al. Procyanidin B2 improves oocyte maturation and subsequent development in type 1 diabetic mice by promoting mitochondrial function. Reprod Sci (2020) 27:2211–22. doi: 10.1007/s43032-020-00241-3
75. Spicer LJ, Schütz LF. Effects of grape phenolics, myricetin and piceatannol, on bovine granulosa and theca cell proliferation and steroid production in vitro. Food Chem Toxicol (2022) 167:113288. doi: 10.1016/j.fct.2022.113288
76. Chen SE, McMurtry JP, Walzem RL. Overfeeding-induced ovarian dysfunction in broiler breeder hens is associated with lipotoxicity. Poult Sci (2006) 85(1):70–81. doi: 10.1093/ps/85.1.70
77. Richards MP, Proszkowiec-Weglarz M. Mechanisms regulating feed intake, energy expenditure, and body weight in poultry. Poult Sci (2007) 86(7):1478–90. doi: 10.1093/ps/86.7.1478
78. Predescu DV, Crețoiu SM, Crețoiu D, Alexandra Pavelescu L, Suciu N, Radu BM, et al. G protein-coupled receptors (GPCRs)-mediated calcium signaling in ovarian cancer: Focus on GPCRs activated by neurotransmitters and inflammation-associated molecules. Int J Mol Sci (2019) 20(22):5568. doi: 10.3390/ijms20225568
79. Snider AP, Wood JR. Obesity induces ovarian inflammation and reduces oocyte quality. Reproduction (2019) 158(3):R79–90. doi: 10.1530/REP-18-0583
80. Wang Y, Nicholes K, Shih IM. The origin and pathogenesis of endometriosis. Annu Rev Pathol (2020) 15:71–95. doi: 10.1146/annurev-pathmechdis-012419-032654
81. Atanasov AG, Waltenberger B, Pferschy-Wenzig EM, Linder T, Wawrosch C, Uhrin P, et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol Adv (2015) 33(8):1582–614. doi: 10.1016/j.bioteChadv.2015.08.001
82. Forni C, Facchiano F, Bartoli M, Pieretti S, Facchiano A, D’Arcangelo D, et al. Beneficial role of phytochemicals on oxidative stress and age-related diseases. BioMed Res Int (2019) 2019:8748253. doi: 10.1155/2019/8748253
83. Wocławek-Potocka I, Mannelli C, Boruszewska D, Kowalczyk-Zieba I, Waśniewski T, Skarżyński DJ. Diverse effects of phytoestrogens on the reproductive performance: cow as a model. Int J Endocrinol (2013) 2013:650984. doi: 10.1155/2013/650984
84. Ly C, Yockell-Lelievre J, Ferraro ZM, Arnason JT, Ferrier J, Gruslin A. The effects of dietary polyphenols on reproductive health and early development. Hum Reprod (2015) 21(2):228–48. doi: 10.1093/humupd/dmu058
85. Olaku OO, Ojukwu MO, Zia FZ, White JD. The role of grape seed extract in the treatment of chemo/radiotherapy induced toxicity: a systematic review of preclinical studies. Nutr Cancer (2015) 67(5):730–40. doi: 10.1080/01635581.2015.1029639
86. Barbe A, Bongrani A, Mellouk N, Estienne A, Kurowska P, Grandhaye J, et al. Mechanisms of adiponectin action in fertility: an overview from gametogenesis to gestation in humans and animal models in normal and pathological conditions. Int J Mol Sci (2019) 20(7):1526. doi: 10.3390/ijms20071526
87. Shen M, Lin F, Zhang J, Tang Y, Chen WK, Liu H. Involvement of the up-regulated FoxO1 expression in follicular granulosa cell apoptosis induced by oxidative stress. J Biol Chem (2012) 287(31):25727–40. doi: 10.1074/jbc.M112.349902
88. Sun YL, Tang SB, Shen W, Yin S, Sun QY. Roles of resveratrol in improving the quality of postovulatory aging oocytes in vitro. Cells (2019) 8(10):1132. doi: 10.3390/cells8101132
89. Karimian M, Zandi M, Sanjabi MR, Masoumian M, Ofoghi H. Effects of grape seed extract, quercetin and vitamin C on ovine oocyte maturation and subsequent embryonic development. Cell Mol Biol (2018) 64(4):98–102. doi: 10.14715/cmb/2018.64.4.16
90. Sedighi P, Helli B, Sharhani A, Vatanpur A. Effects of grape seed extract supplementation on fasting blood glucose, insulin resistance, and lipid profile in women with polycystic ovary syndrome. Anat Sci J (2020) 17(2):73–82.
91. Cutts JK, Peavy TR, Moore DR, Prasain J, Barnes S, Kim H. Ovariectomy lowers urine levels of unconjugated (+)-catechin,(–)-epicatechin, and their methylated metabolites in rats fed grape seed extract. Horm Mol Biol Clin Investig (2013) 16(3):129–38. doi: 10.1515/hmbci-2013-0044
92. Yıldırım Ş., Topaloğlu N, Tekin M, Küçük A, Erdem H, Erbaş M, et al. Protective role of Proanthocyanidin in experimental ovarian torsion. Med J Islam Repub Iran (2015) 29:185.
93. Barbe A, Ramé C, Mellouk N, Estienne A, Bongrani A, Brossaud A, et al. Effects of grape seed extract and proanthocyanidin B2 on in vitro proliferation, viability, steroidogenesis, oxidative stress, and cell signaling in human granulosa cells. Int J Mol Sci (2019) 20(17):4215. doi: 10.3390/ijms20174215
94. Colitti M, Sgorlon S, Stradaioli G, Farinacci M, Gabai G, Stefanon B. Grape polyphenols affect mRNA expression of PGHS-2, TIS11b and FOXO3 in endometrium of heifers under ACTH-induced stress. Theriogenology (2007) 68(7):1022–30. doi: 10.1016/j.theriogenology.2007.07.018
95. Bruner-Tran KL, Osteen KG, Taylor HS, Sokalska A, Haines K, Duleba AJ. Resveratrol inhibits development of experimental endometriosis in vivo and reduces endometrial stromal cell invasiveness in vitro. Biol Reprod (2011) 84(1):106–12. doi: 10.1095/biolreprod.110.086744
96. Hashem NM, Gonzalez-Bulnes A, Simal-Gandara J. Polyphenols in farm animals: source of reproductive gain or waste? Antioxidants (2020) 9(10):1023. doi: 10.3390/antiox9101023
97. Yildiz HB, Kiralp S, Toppare L, Yagci Y. Immobilization of tyrosinase in poly (ethyleneoxide) electrodes and determination of phenolics in red wines. React Funct Polym (2005) 63(2):155–61. doi: 10.1016/j.reactfunctpolym.2005.02.016
98. Meeprom A, Sompong W, Suwannaphet W, Yibchok-anun S, Adisakwattana S. Grape seed extract supplementation prevents high-fructose diet-induced insulin resistance in rats by improving insulin and adiponectin signaling pathways. Br J Nutr (2011) 106(8):1173–81. doi: 10.1017/S0007114511001589
99. Eng ET, Ye J, Williams D, Phung S, Moore RE, Young MK, et al. Suppression of estrogen biosynthesis by procyanidin dimers in red wine and grape seeds. Cancer Res (2003) 63(23):8516–22.
100. Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell (2007) 129(7):1261–74. doi: 10.1016/j.cell.2007.06.009
101. Homayoun M, Targhi RG, Soleimani M. Anti-proliferative and anti-apoptotic effects of grape seed extract on chemo-resistant OVCAR-3 ovarian cancer cells. Res Pharm Sci (2020) 15(4):390. doi: 10.4103/1735-5362.293517
102. Kara K, Kocaoğlu Güçlü B, Baytok E, Şentürk M. Effects of grape pomace supplementation to laying hen diet on performance, egg quality, egg lipid peroxidation and some biochemical parameters. J Appl Anim Res (2016) 44(1):303–10. doi: 10.1080/09712119.2015.1031785
103. Galli GM, Da Silva AS, Biazus AH, Reis JH, Boiago MM, Topazio JP, et al. Feed addition of curcumin to laying hens showed anticoccidial effect, and improved egg quality and animal health. Res Vet Sci (2018) 118:101–6. doi: 10.1016/j.rvsc.2018.01.022
104. Reis JH, Gebert RR, Barreta M, Boiago MM, Souza CF, Baldissera MD, et al. Addition of grape pomace flour in the diet on laying hens in heat stress: Impacts on health and performance as well as the fatty acid profile and total antioxidant capacity in the egg. J Therm Biol (2019) 80:141–9. doi: 10.1016/j.jtherbio.2019.01.003
105. Mikuła-Pietrasik J, Sosińska P, Murias M, Wierzchowski M, Brewińska-Olchowik M, Piwocka K, et al. High potency of a novel resveratrol derivative, 3, 3′, 4, 4′-tetrahydroxy-trans-stilbene, against ovarian cancer is associated with an oxidative stress-mediated imbalance between DNA damage accumulation and repair. Oxid Med Cell Longev (2015) 2015:135691. doi: 10.1155/2015/135691
106. Tousson E, Elgharabawy RM, Elmasry TA. Grape seed proanthocyanidin ameliorates cardiac toxicity induced by boldenone undecylenate through inhibition of NADPH oxidase and reduction in the expression of NOX2 and NOX4. Oxid Med Cell Longev (2018) 2018:9434385. doi: 10.1155/2018/9434385
Keywords: grapeseed extract, phenolic compounds, proanthocyanidin, resveratrol, delphinidin, female reproduction, steroid hormones, proliferation
Citation: Kohut L, Baldovska S, Mihal M, Belej L, Sirotkin AV, Roychoudhury S and Kolesarova A (2024) The multiple actions of grape and its polyphenols on female reproductive processes with an emphasis on cell signalling. Front. Endocrinol. 14:1245512. doi: 10.3389/fendo.2023.1245512
Received: 23 June 2023; Accepted: 11 December 2023;
Published: 04 January 2024.
Edited by:
Luna Samanta, Ravenshaw University, IndiaReviewed by:
Chris Scott, Charles Sturt University, AustraliaSudhanshu Kumar Bharti, Patna University, India
Copyright © 2024 Kohut, Baldovska, Mihal, Belej, Sirotkin, Roychoudhury and Kolesarova. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adriana Kolesarova, YWRyaWFuYS5rb2xlc2Fyb3ZhQHVuaWFnLnNr
 Ladislav Kohut1
Ladislav Kohut1 Simona Baldovska
Simona Baldovska Alexander V. Sirotkin
Alexander V. Sirotkin Shubhadeep Roychoudhury
Shubhadeep Roychoudhury Adriana Kolesarova
Adriana Kolesarova