- 1School of Medical Information Engineering, Anhui University of Traditional Chinese Medicine, Hefei, Anhui, China
- 2Anhui Computer Application Research Institute of Chinese Medicine, China Academy of Chinese Medical Sciences, Hefei, Anhui, China
- 3School of Chinese Medicine, Anhui University of Traditional Chinese Medicine, Hefei, Anhui, China
Background: Systemic Immune-Inflammation Index (SII) has been reported to be associated with diabetes. We aimed to assess possible links between SII and diabetes.
Methods: Data were obtained from the 2017-2020 National Health and Nutrition Examination Survey (NHANES) database. After removing missing data for SII and diabetes, we examined patients older than 20 years. Simultaneously, the relationship between SII and diabetes was examined using weighted multivariate regression analysis, subgroup analysis, and smooth curve fitting.
Results: There were 7877 subjects in this study, the average SII was 524.91 ± 358.90, and the prevalence of diabetes was 16.07%. Weighted multivariate regression analysis found that SII was positively associated with diabetes, and in model 3, this positive association remained stable (OR = 1.04; 95% CI: 1.02–1.06; p = 0.0006), indicating that each additional unit of SII, the possibility of having diabetes increased by 4%. Gender, age, BMI, regular exercise, high blood pressure, and smoking did not significantly affect this positive link, according to the interaction test (p for trend>0.05).
Discussion: Additional prospective studies are required to examine the precise connection between higher SII levels and diabetes, which may be associated with higher SII levels.
1 Introduction
The chronic metabolic disorder known as diabetes is characterized by persistently high blood sugar levels (1). Worldwide, the prevalence of diabetes is rising each year and has emerged as a severe public health issue (2). More than 460 million people worldwide have diabetes, and type 2 diabetes affects more than 90% of these individuals. Diabetes harms patients’ health but also has a significant financial impact on families and society (3). Diabetic microangiopathy, one of the most prevalent early consequences of diabetes, is also a risk factor for heart and renal disease (4). Additionally, the study finds levels of systemic inflammation may explain the increased prevalence of diabetes (5).
Systemic Immunity-Inflammation Index (SII) is the platelet count multiplied by the neutrophil count divided by the lymphocyte count, which is considered to be a new and reliable indicator (6) for comprehensively measuring the systemic immunity and inflammation level of the subject (7). Inflammatory factor indicators have been linked to diabetes, according to an increasing number of research (8). Jie Wang et al. investigated the relationship between NLR and depression in patients with diabetes and found that NLR increased and the prevalence of depression increased (9). Bartosz Hudzik et al. find a link between PLR and diabetes (10). In diabetic patients, Dan Yu et al. discovered that LMR can be used as an index to predict the recurrence of rectal cancer (11). These indicators, which only represent two different types of immune cells, could not be very predictive. According to Jie Wang et al., SII is a risk factor for diabetes depression (12). It can be assumed that there may be a relationship between SII and diabetes since SII has been suggested as an index to detect depression in diabetes. However, no one has independently looked into the connection between SII and diabetes.
Liu Yongming et al. found that middle-aged and elderly diabetes caused by arteriosclerosis may be mediated by white blood cell count (13). Xiang Fang et al. Diabetes is related to the expression of inflammatory genes and white blood cell count, and blood glucose is positively correlated with inflammatory genes and white blood cell count (14). Francesco Zaccardi et al. found that compared with patients without diabetes, there was no difference in platelet count in patients with diabetes. However, this study still took into account the image of platelet count in the experiment and included it in the covariate to design the experiment (15).
The link between SII and diabetes will be examined in this article. We examine the association between SII and diabetes using data from the NHANES database for the years 2017 to 2020. We think that there may be a connection between the rise in SII and the rise in diabetes prevalence. Researchers and medical professionals offer references.
2 Materials and methods
2.1 Population research
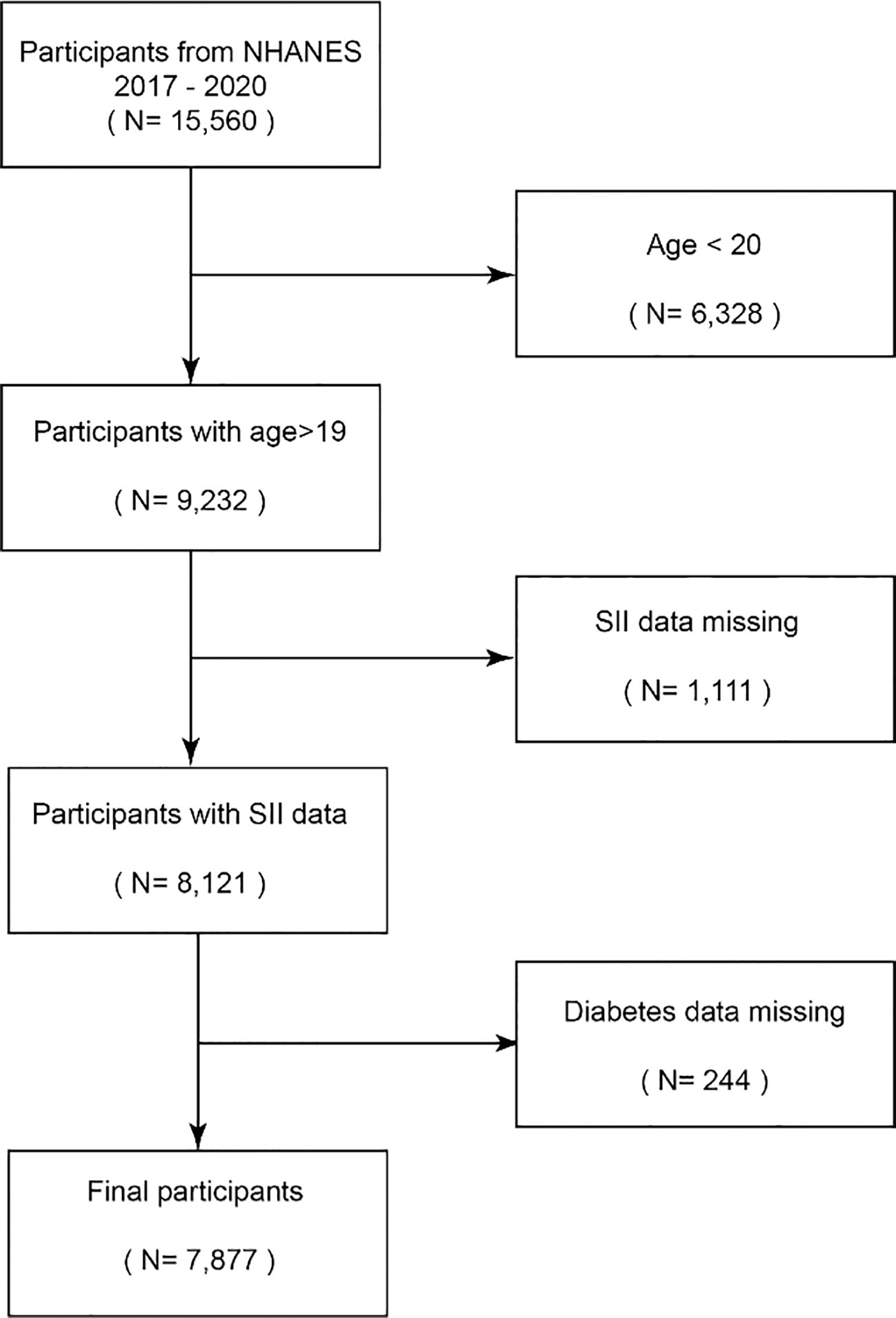
The National Health and Nutrition Examination Survey (NHANES) is a research program designed to assess adults’ and children’s health and nutritional status in the United States (16). The NHANES database contains population data, questionnaire data, laboratory data, and dietary data. This study is used to determine the prevalence and pathogenic factors of diseases, etc., and provides national standard references to help design sound public health policies (17). In total, 15,560 participants from the 2017–2018 and 2019–2020 research years were included in this study, whereas 6,328 participants under the age of 20 were omitted. Following the exclusion of 1111 people with missing SII data and 244 participants with missing diabetes data, 7877 participants were ultimately included in the study. Subjects were given the go-ahead to participate in the NHANES by the NCHS Ethics Review Board, and each participant gave their written informed consent (18) (Figure 1).
2.2 Exposure variables and outcome variables
SII is a composite index made up of platelet count, neutrophil count, and lymphocyte count that is used as an exposure variable and is assessed using an automated hematology analysis instrument (CoulterDxH 800 analyzer). calculated by dividing the lymphocyte count by the platelet count, and then multiplying by the neutrophil count (19).
Did your doctor inform you that you had diabetes? Are these other questions asked by a professional interviewer utilizing a computer-assisted personal interview (CAPI) system at home? The subject was deemed to have diabetes if his response was yes. Having diabetes diagnosed by a doctor was intended to be an outcome variable (20).
2.3 Covariates
We summarized potential covariates that might confound the association between SII and diabetes. The covariates selected for this study were: age(year), Albumin refrigerated serum, White blood cell count, Platelet count, blood urea nitrogen content, chloride content, dietary protein intake, carbohydrate intake, total sugar intake, total fat intake, cholesterol intake, gender, race, education level (divided into no high school education, high school education, high school education or above), marital status (married with a partner, divorced and separated, widowed, never married), poverty-income ratio (0-1.5, 1.5-3.5, >3.5), BMI (0-25, 25-30, >30), whether you have high blood pressure, whether you smoke (21), whether you exercise regularly (22). Among them, the BMI classification corresponds to three groups of normal weight, overweight, and obese. Refrigerated serum albumin is measured in g/L, and albumin concentration is measured using the dye bromocreol violet (BCP). The PH value binds to albumin in the PH range of 5.2-6.8, and the color change is measured at 600nm, with secondary measurements at 700nm. Albumin value measurement is used in the diagnosis and treatment of certain liver and heart diseases as well as diabetes (23). Both the White blood cell count (24) and the Platelet count (25) are measured in 1000 cells/uL. A complete blood count on a blood specimen is measured at a mobile testing center using a Beckman Coulter DxH 800 instrument. The VCS (volume, conductivity, and dispersion) method was used to determine the leucocytes. Blood urea nitrogen was measured at 340nm using a coupled enzyme reaction in the unit of mmol/L (26). The chloride content was measured in mmol/L, and the serum electrolyte concentration was determined by the indirect ion selective electrode (ISE) method. Sample dilution 1:31 (27). Dietary protein intake, carbohydrate intake, total sugar intake, total fat intake, and cholesterol intake, these data are from the dietary section of the database. Dietary recall interviews were collected at a mobile test center (MEC), and participants’ total nutrient intake on day one was used in this study (28). High blood pressure was determined by the source of the questionnaire part, whether or not the doctor considered having high blood pressure (29). Whether you smoke or not, according to the part of the questionnaire, whether you have smoked 100 cigarettes so far (30).
2.4 Statistical methods
Continuous variables were expressed as means with standard deviations in the description of the study population, while categorical variables were expressed as percentages. The association between participants in the SII/100 quartile group and the presence of diabetes was assessed using the t-test for continuous variables and the chi-square test for categorical variables. To investigate the relationship between SII and the prevalence of diabetes, multivariate logistic regression was performed. Model 1 had no covariates, model 2 added covariates age, sex, and race, and model 3 adjusted variables: age, sex, race, urine albumin content (31), refrigerated serum content, blood urea nitrogen content (32), chloride content, dietary protein intake, carbohydrate intake, total sugar intake, total fat intake, cholesterol intake, education Degree (divided into no high school education, high school education, high school education or above), marital status (married with a partner, divorced and separated, widowed, never married), poverty-income ratio (0-1.5, 1.5-3.5, >3.5), BMI (0-25, 25-30, >30), whether you have high blood pressure (33), whether you smoke, whether you exercise regularly. SII and diabetes were assessed in the model using odds ratios (ORs) and 95% confidence intervals (CIs). Create a multivariate test by controlling variables and fitting smooth curves to three models. The relationship and inflection point between SII and diabetes were examined using a threshold effect analysis model. R Studio (version 4.2.2) and empowered stats (version 2.0) were used to do the statistical study. 0.05 was the threshold for significance. We employ a weighting approach to lower the dataset’s volatility.
3 Results
3.1 Baseline characteristics of the study population
A total of 7877 subjects were included in this study, of which 48.14% were male and 51.86% were female, with an average age of 50.87 ± 17.56. The mean value of SII was 524.91 ± 358.90, and the prevalence of diabetes was 16.07%.
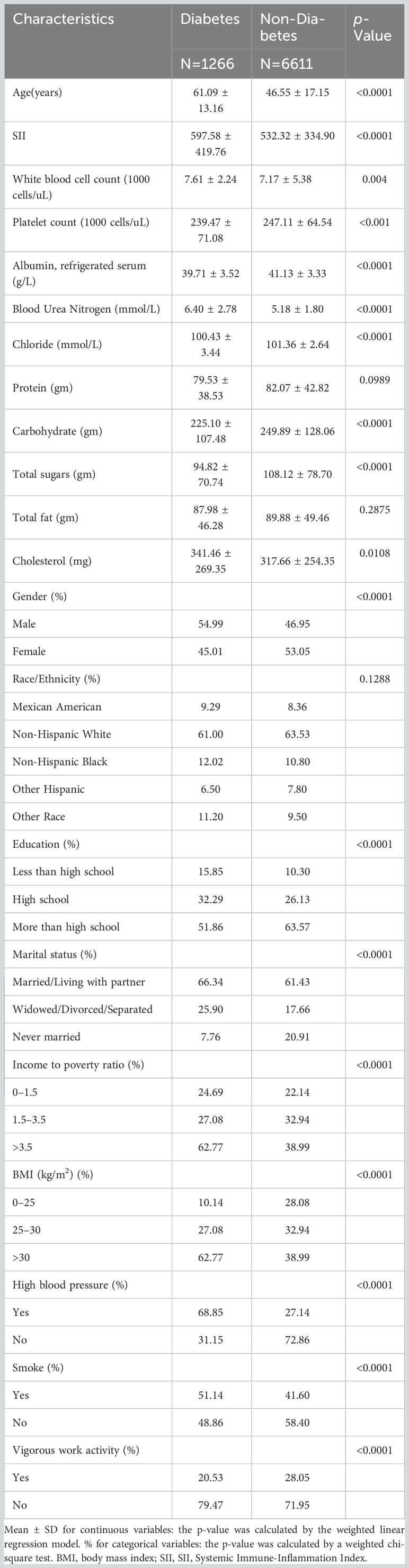
Table 1 is a table of diabetes-based weighted demographic baseline characteristics. The average age of subjects with diabetes was 61.09 ± 13.16, and the average age of subjects without diabetes was 46.55 ± 17.15. The average age of subjects with diabetes was higher than that of subjects without diabetes. Diabetes is used as a stratified variable. The presence or absence of diabetes is related to age, gender, education level, marital status, poverty-income ratio, BMI, hypertension, smoking or not, regular exercise, SII, albumin content, blood urea nitrogen content, Chloride content, dietary carbohydrate intake, dietary total sugar intake, and dietary cholesterol intake were significantly correlated (p<0.05). Compared with people without diabetes, people with diabetes tended to be older, high school educated, married or in a partner, had higher SII, higher albumin, higher blood urea nitrogen, lower chloride, carbohydrate non-Hispanic white males with lower intake, lower sugar intake, higher cholesterol intake, poverty-income ratio >3.5, BMI >30 kg/m2, hypertension, smoking, and no regular exercise. People with diabetes have higher white blood cell counts and lower platelet counts than those without diabetes.
Table 2 is a table of SII-based weighted demographic baseline characteristics. SII quartile subjects have significant differences in age, albumin content, chloride content, protein content, gender, race, education level, income-poverty ratio, BMI, hypertension, diabetes, etc. (p<0.05). Subjects in the fourth quartile of the SII quartile tend to be older, have lower protein intake, lower carbohydrate intake, high school education or higher, poverty-income ratio>3.5, BMI>30 kg/m2, have Hypertensive, non-Hispanic white females without diabetes. SII was also significantly different in the quartile group from white blood cell count and platelet count, with the fourth quartile having the highest count.
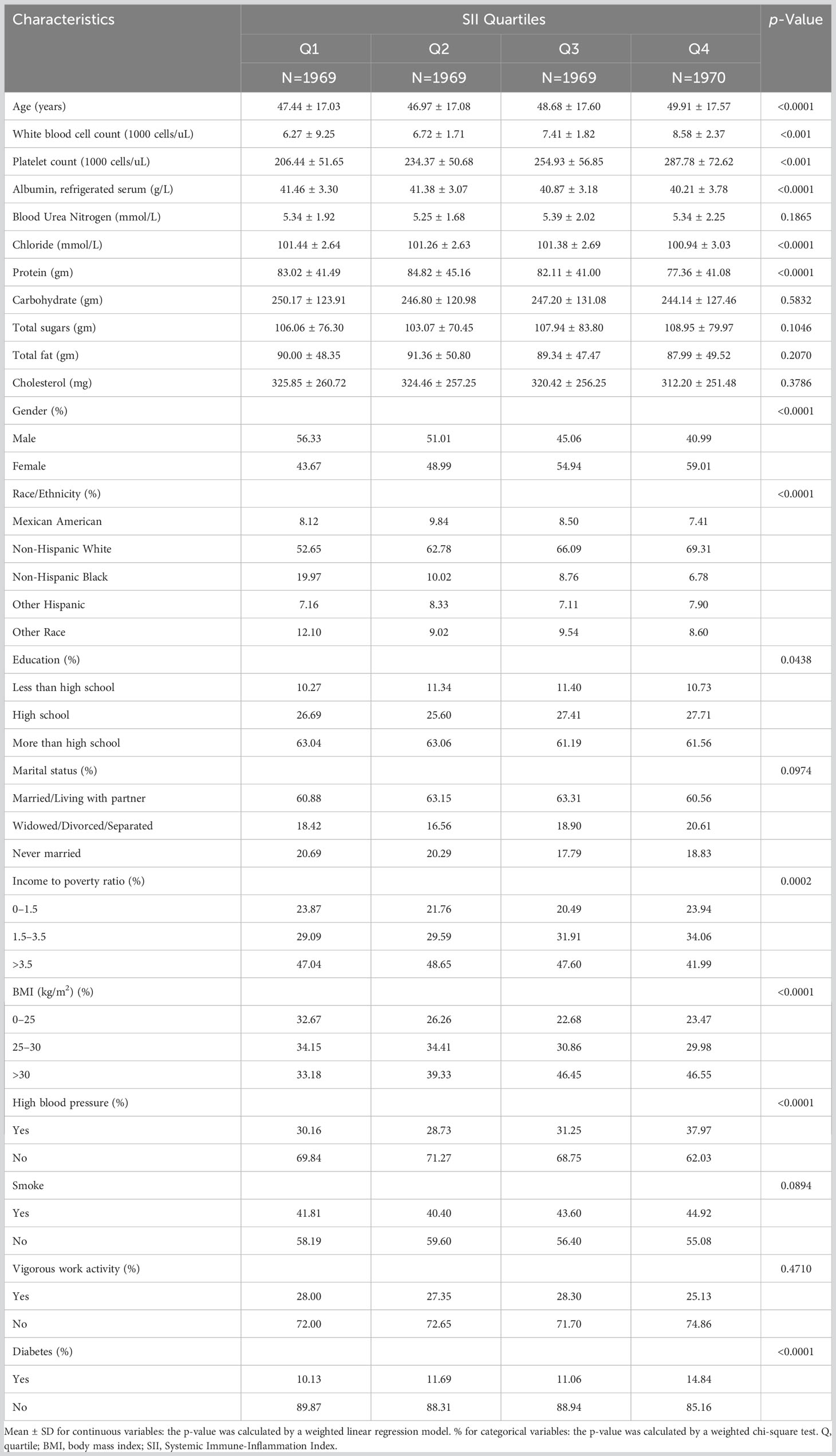
Table 2 Baseline characteristics of study population according to Systemic Immune-Inflammation Index quartiles, weighted.
3.2 Relationship between SII and diabetes
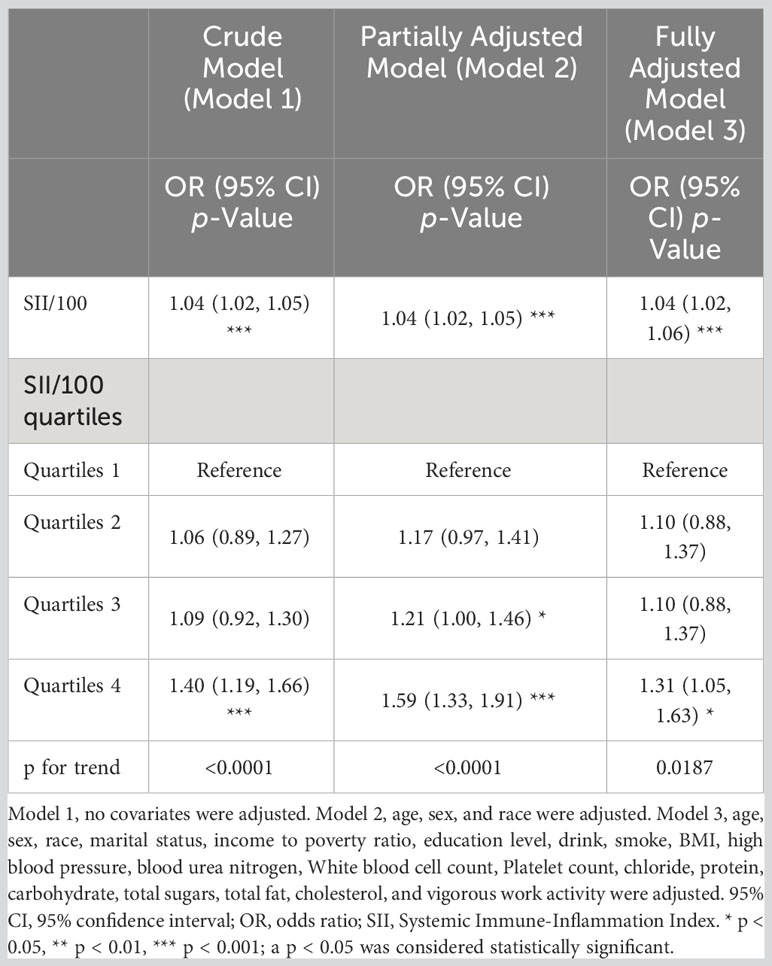
Since the effect size was not obvious, we magnified the value of SII by 100 times to compare the relationship between SII/100 and diabetes. Table 3 shows the multivariate regression analysis between SII/100 and diabetes. For diabetes, a positive association between SII/100 and diabetes was observed. In model 3, this positive association remained stable (OR = 1.04; 95% CI: 1.02–1.06; p = 0.0006), indicating that each unit increase in SII/100 increased the likelihood of having diabetes by 4%. In a sensitivity analysis, a fully adjusted model for the SII/100 quartile (OR = 1.31; 95% CI: 1.05-1.63; p = 0.0187) indicated a stable relationship between elevated SII and increased odds of developing diabetes. Compared with the first quartile, participants in quartile 4 had a 31% increased risk of developing diabetes. At the same time, the p for trend of the three models were all <0.05, which was statistically significant.
A subgroup analysis of the association between SII and diabetes is shown in Figure 2. SII was significantly correlated with male sex, age less than 60, 0<BMI<25, and irregular exercise (p<0.05). The interaction test showed that there was no statistical difference in the relationship between SII and diabetes in each category, and gender, age, BMI, regular exercise, hypertension, and smoking had no significant impact on this positive relationship (p for trend>0.05).
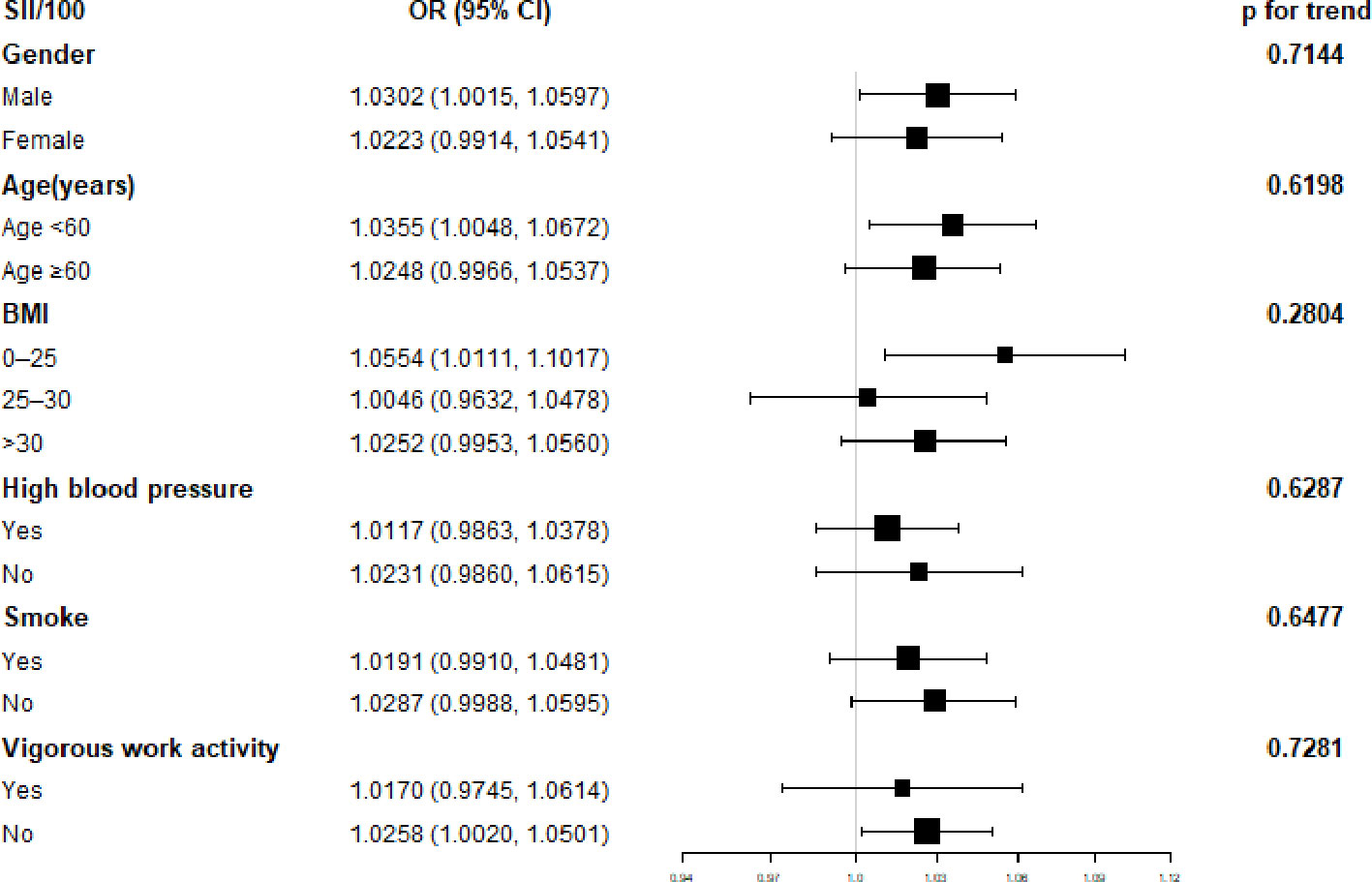
Figure 2 Subgroup analysis for the association between Systemic Immune-Inflammation Index and Diabetes.
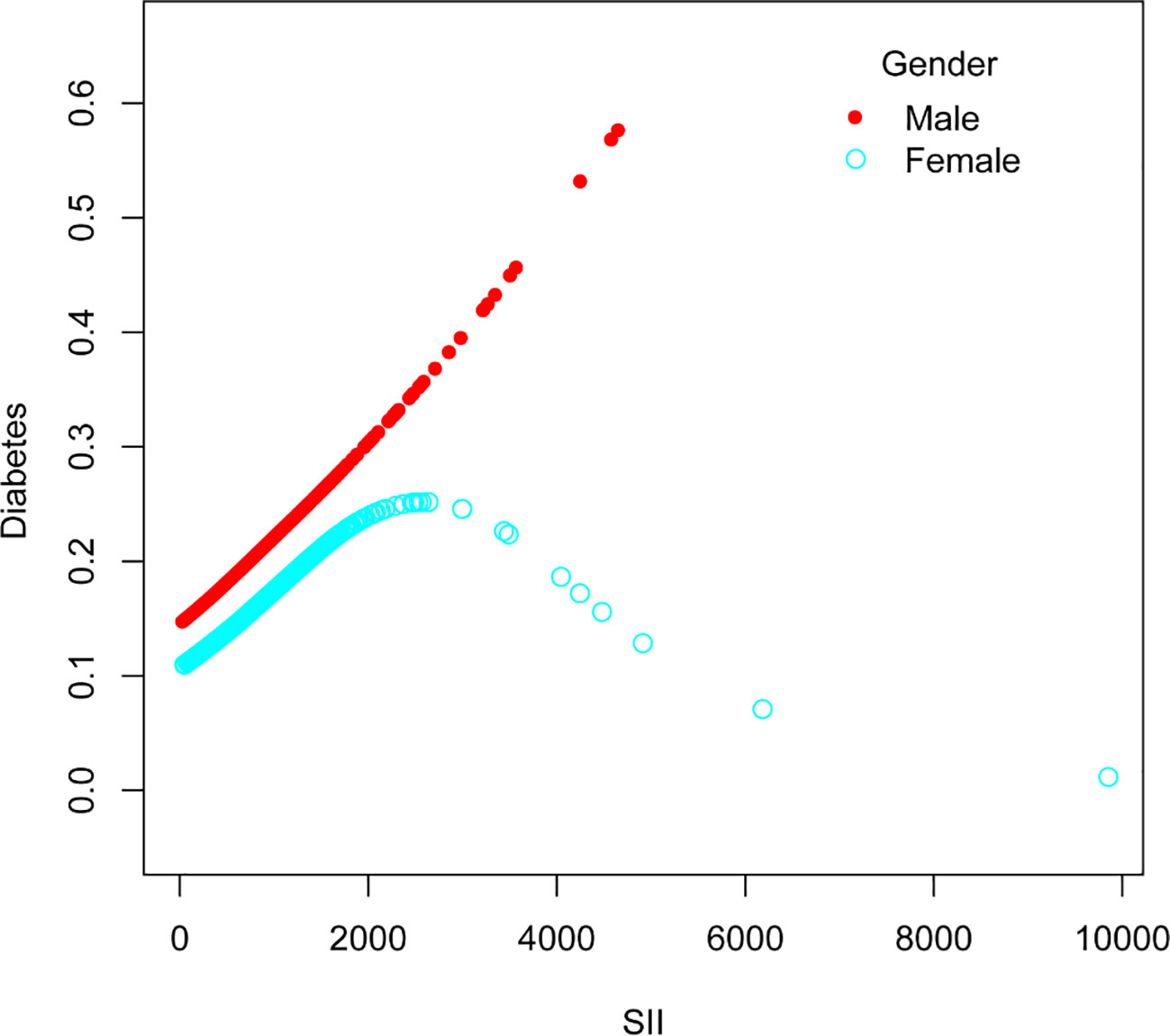
The nonlinear association between SII and diabetes was then described using smooth curve fitting (Figures 3, 4). Adjusted variables: age, sex, education, marital status, poverty-income ratio, BMI, hypertension, smoking, regular exercise, albumin level, blood urea nitrogen level, chloride level, dietary carbohydrate intake, dietary total sugar intake, and dietary cholesterol intake, and high blood pressure. Interaction test showed that there was no statistically significant difference in the association between SII and diabetes among different stratification groups, and all interactions were p>0.05, indicating that age, sex, BMI, sampling, regular exercise, and hypertension had no significant dependence on this positive association. After stratified analysis by sex, it was found that the female stratification presents an inverted U-shaped curve.
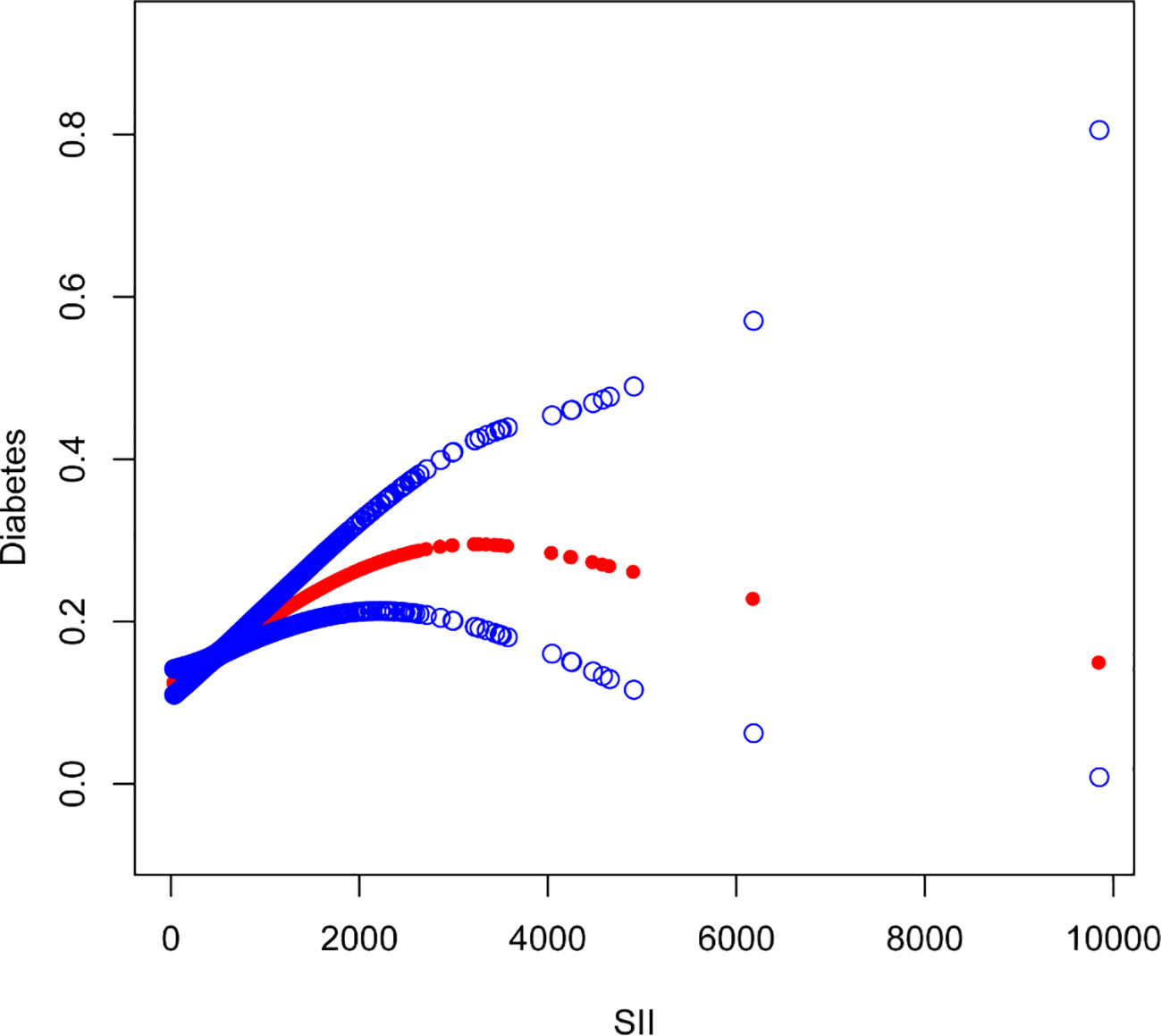
Figure 3 The association between Systemic Immune-Inflammation Index and Diabetes. The solid red line represents the smooth curve fit between variables. Blue bands represent the 95% confidence interval from the fit.
4 Discussion
Our research revealed an association between increased SII and a higher prevalence of diabetes. After stratified analysis by gender, it was discovered that female stratification likewise exhibits an inverted U-shaped curve in the connection between SII and diabetes.
To our knowledge, few studies have individually assessed the association between SII and diabetes. Ahmet Elbeyli et al. found that SII can be used as a diagnostic marker for diabetic macular edema and improve diabetic retinopathy (34). Jie Wang et al. found that SII can be used as a diagnostic marker for diabetic depression (12). Wencong Guo et al. found that higher SII levels were significantly associated with diabetic nephropathy (35). Kübra Özata Gündoğdu et al. studied the association of diabetic macular edema with serous macular detachment and SII, and elevated SII levels may increase the incidence of serous macular detachment (36). Safak Ozer Balin et al. reported that SII can be used as a predictive marker for diabetic foot osteomyelitis (37). Yohanes Andy Rias et al. surveyed 294 Indonesian diabetic patients and found that low levels of SII can regulate psychological problems in diabetic patients (38). In contrast, Yohanes Andy Rias et al.’s research results showed a benign effect of low-level SII, which is different from the general high-level SII. Therefore, it is found that the level of SII may have different effects on the judgment of diabetes, and it is meaningful for us to study the relationship between SII and diabetes. Research by Jingxin Zhou et al. suggested that SII may be a potential marker for the treatment of diabetic macular edema (39). We hypothesize that there may be a relationship between SII and diabetes, which may be a good prospective marker, in light of the studies’ confirmation of SII’s predictive power. We discovered through our research that there is currently no research on the relationship between SII and diabetes alone, but there is research on the relationship between SII and diabetes-related diseases like the aforementioned diabetic macular edema, diabetic nephropathy, and diabetic depression. The studies mentioned above utilized several survey techniques and study populations at the same time. Our investigation discovered a potential link between greater SII levels and a higher chance of developing diabetes, which is consistent with the majority of studies.
Despite being a novel inflammatory marker, diabetes has not been examined with SII alone. However, diabetes has been linked in clinical studies with several traditional inflammatory indicators. Zhao-tong Jia et al. measured serum IMA and hs-CRP concentrations in patients with diabetic retinopathy by the rate-nephelometric method. There may be a positive correlation between hs-CRP concentration and the prevalence of diabetic retinopathy (r = 0.617, P < 0.01) (40). Hai-hang Liu et al. believed that serum hs-CRP concentration can predict the incidence of diabetes, and inflammatory factors play an important role in diabetes research (41). Klisic et al. studied the association of type 2 diabetes with some inflammatory factors, platelet-to-neutrophil ratio (PNR), monocyte/granulocyte-to-lymphocyte ratio (M/GLR), derived neutrophils to lymphocytes Cell ratio (dNLR), all three indicators are independently associated with type 2 diabetes (42). Klisic’s study affirmed the role of traditional inflammatory factors in the prediction of diabetes but did not specifically explore the correlation between the two, so in-depth research in this study is necessary. Si-Yang Wang et al. demonstrated that the ratio of neutrophils to lymphocytes can be used as a marker for predicting diabetes (43). Si-Yang Wang’s research also affirmed the outstanding work of inflammatory factors in the diagnosis of diabetes but did not conduct specific correlation studies. Mohamad Akbari’s group (44) and Dan Qu’s group (45) both considered elevated levels of interleukin 6 (IL-6) to be an independent predictor of diabetes. Compared with traditional inflammatory factors, SII binds three types of immune cells, reflects the inflammatory state well comprehensively, and has shown better prognostic value in several studies (46). For example, Afiat Berbudi et al. studied the effect of SII, neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR), and monocyte/lymphocyte ratio (MLR) in predicting the impact of type 2 diabetes on the immune system. Their ROC curve analysis confirmed that among these markers, SII was more effective and accurate in predicting the impact of T2DM on the immune system (47). Huaping Huang et al. found that SII can predict the postoperative survival rate of patients with cervical cancer, and it is more accurate and effective than other inflammatory factors (7). Hongmei Zhang et al. studied the relationship between leukocytes, centriocytes, lymphocytes, and diabetes, and the P values were all <0.001, and the final experiment found that the increase in leukocyte level was related to hyperglycemia. The research conclusion of Hongmei Zhang et al. is the same as ours (48). Saori Kashima et al., using the data from Yuport Medical Examination Center, also found that increased white blood cell count levels may increase the probability of diabetes, which is also consistent with our conclusion (49). Jin-Young Hwang et al. found that the prevalence of diabetes may increase with the increase of platelet count (50). This is consistent with our results. Yuqin Qian et al. found that platelet count may be related to diabetic peripheral neuropathy and is a potential risk marker (51). The SII selected in this study included platelet count, and the experimental results could truly reflect the burden of inflammation and accurately reflect the relationship between SII and diabetes.
The mechanisms underlying the positive association between inflammation and diabetes are unclear. Hitomi Usui Kataoka et al. suggested that endoplasmic reticulum stress may affect the pathogenesis of diabetes (52). Haichen Zhang et al. found epigenetic abnormalities in diabetic patients, which may provide information for target drug prediction (53). Mina Wang et al. summarized two causes of diabetes mellitus: impaired insulin action and impaired insulin secretion or a combination of both factors (54).
Our research has some advantages. The sample size is sufficient to be representative, and the years chosen are the most recent two data sets. We also adjusted for confounding factors to produce robust results. For example, previous studies have mentioned that dietary intake (55), physical activity (56) and protein intake (57) increase the prevalence of diabetes. As a result, we added protein intake and physical activity as factors to the fully adjusted model, which strengthened our findings. Our study does, however, have certain drawbacks. There is no causal association because it is a cross-sectional study, hence several prospective studies are required to explain the causative relationship. Confounding effects cannot be ruled out, even though we accounted for covariates to lessen their impact on the results.
5 Conclusion
More research is required to confirm our findings, which showed that SII levels are strongly related to diabetes.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
In 2003, the NHANES Institutional Review Board (IRB) changed its name to the NCHS Research Ethics Review Board (ERB). In 2018, the name was changed from NCHS Research Ethics Review Board to NCHS Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
Conceptualization: YN and HZ. Methodology: YN and HZ. Software: YN and HZ. Validation: YN, HK, and HZ. Formal analysis: YN and HZ. Investigation: YN, HK, and HZ. Resources: YN, JW, HK, and HZ. Data curation: YN, HK, and HZ. Writing—original draft preparation: YN, and HZ. Writing—review and editing: HK. Visualization: YN. Supervision: YN. Project administration: YN, and HZ. Funding acquisition: YN, and JW. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the 2022 Anhui New Era Education Quality Engineering Project (2022cxcysj13), Xinan Medical Education Department Key Practice Room Open Fund Items(2020xayx07) and 2022 Anhui University of Traditional Chinese Medicine Innovation and Entrepreneurship Project (Provincial-level project) (s202210369006).
Acknowledgments
Thanks to Jing Zhang (Shanghai Tongren Hospital) for his work on the NHANES database. Thank you Nie for half a year of hard work. Thanks to every reviewer and editor who reviewed this article, put forward good suggestions and made a lot of efforts for the improvement of the research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Crawford JD, Bode HH. Diabetes and the amplifier hypothesis. N Engl J Med (1970) 282:1266–7. doi: 10.1056/NEJM197005282822211
2. Xu NY, Nguyen KT, DuBord AY, Pickup J, Sherr JL, Teymourian H, et al. Diabetes technology meeting 2021. J Diabetes Sci Technol (2022) 16:1016–56. doi: 10.1177/19322968221090279
3. Elliott TL, Pfotenhauer KM. Classification and diagnosis of diabetes. Primary Care: Clinics Office Pract (2022) 49:191–200. doi: 10.1016/j.pop.2021.11.011
4. Wang D, Li J, Luo G, Zhou J, Wang N, Wang S, et al. Nox4 as a novel therapeutic target for diabetic vascular complications. Redox Biol (2023) 64:102781. doi: 10.1016/j.redox.2023.102781
5. Jung CH, Lee MJ, Kang YM, Jang JE, Leem J, Hwang JY, et al. The risk of incident type 2 diabetes in a korean metabolically healthy obese population: the role of systemic inflammation. J Clin Endocrinol Metab (2015) 100:934–41. doi: 10.1210/jc.2014-3885
6. Hu B, Yang X-R, Xu Y, Sun Y-F, Sun C, Guo W, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res (2014) 20:6212–22. doi: 10.1158/1078-0432.CCR-14-0442
7. Huang H, Liu Q, Zhu L, Zhang Y, Lu X, Wu Y, et al. Prognostic value of preoperative systemic immune-inflammation index in patients with cervical cancer. Sci Rep (2019) 9:3284. doi: 10.1038/s41598-019-39150-0
8. Shi J, Tao Y, Wang L, Chen S, Zhou Z, Meng L, et al. Combined effect of diabetes and frailty on mortality among Chinese older adults: A follow-up study. Front Endocrinol (2023) 13:1105957. doi: 10.3389/fendo.2022.1105957
9. Wang J, Zhou D, Li X. The association between neutrophil-to-lymphocyte ratio and diabetic depression in U.S. Adults with diabetes: findings from the 2009-2016 national health and nutrition examination survey (NHANES). BioMed Res Int (2020) 2020:8297628. doi: 10.1155/2020/8297628
10. Atak B, Aktas G, Duman TT, Erkus E, Kocak MZ, Savli H. Diabetes control could through platelet-to-lymphocyte ratio in hemograms. Rev Assoc Med Bras (2019) 65:38–42. doi: 10.1590/1806-9282.65.1.38
11. Yu D, An G, Yao J. Lymphocyte-to-monocyte ratio combined with CA19-9 for predicting postoperative recurrence of colorectal cancer in patients with diabetes. J Clin Lab Anal (2021) 35:e23944. doi: 10.1002/jcla.23944
12. Wang J, Zhou D, Dai Z, Li X. Association between systemic immune-inflammation index and diabetic depression. CIA (2021) 16:97–105. doi: 10.2147/CIA.S285000
13. Liu Y, Lai X, Guo W, Ma L, Li W, Fang Q, et al. Total white blood cell count mediated the association between increased arterial stiffness and risk of type 2 diabetes mellitus in chinese adults. Arterioscler Thromb Vasc Biol (2020) 40:1009–15. doi: 10.1161/ATVBAHA.119.313880
14. Fang X, Dorcely B, Ding X-P, Yin S, Son N-H, Hu S-L, et al. Glycemic reduction alters white blood cell counts and inflammatory gene expression in diabetes. J Diabetes Complications (2018) 32:1027–34. doi: 10.1016/j.jdiacomp.2018.08.003
15. Zaccardi F, Rocca B, Pitocco D, Tanese L, Rizzi A, Ghirlanda G. Platelet mean volume, distribution width, and count in type 2 diabetes, impaired fasting glucose, and metabolic syndrome: a meta-analysis. Diabetes Metab Res Rev (2015) 31:402–10. doi: 10.1002/dmrr.2625
16. Curtin LR, Mohadjer LK, Dohrmann SM, Kruszon-Moran D, Mirel LB, Carroll MD, et al. National Health and Nutrition Examination Survey: sample design, 2007-2010. Vital Health Stat (2013) 2:1–23.
17. Mirel LB, Mohadjer LK, Dohrmann SM, Clark J, Burt VL, Johnson CL, et al. National Health and Nutrition Examination Survey: estimation procedures, 2007-2010. Vital Health Stat (2013) 2:1–17.
18. Curtin LR, Mohadjer LK, Dohrmann SM, Montaquila JM, Kruszan-Moran D, Mirel LB, et al. The national health and nutrition examination survey: sample design, 1999-2006. Vital Health Stat (2012) 2:1–39.
19. Hu B, Yang X-R, Xu Y, Sun Y-F, Sun C, Guo W, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res (2014) 20:6212–22. doi: 10.1158/1078-0432.CCR-14-0442
20. Neves JS, Leitão L, Magriço R, Bigotte Vieira M, Viegas Dias C, Oliveira A, et al. Caffeine consumption and mortality in diabetes: an analysis of NHANES 1999–2010. Front Endocrinol (2018) 9:547. doi: 10.3389/fendo.2018.00547
21. Li X, Huan J, Lin L, Hu Y. Association of systemic inflammatory biomarkers with depression risk: Results from National Health and Nutrition Examination Survey 2005-2018 analyses. Front Psychiatry (2023) 14:1097196. doi: 10.3389/fpsyt.2023.1097196
22. Luo Y, Zhang J, Liu T, Yin Z, Jin Y, Han J, et al. The systemic-immune-inflammation index predicts the recurrence of atrial fibrillation after cryomaze concomitant with mitral valve surgery. BMC Cardiovasc Disord (2022) 22:45. doi: 10.1186/s12872-022-02494-z
23. Serum albumin concentration and incident type 2 diabetes risk: new findings from a population-based cohort study . SpringerLink (Accessed September 14, 2023).
24. Russell CD, Parajuli A, Gale HJ, Bulteel NS, Schuetz P, de Jager CPC, et al. The utility of peripheral blood leucocyte ratios as biomarkers in infectious diseases: A systematic review and meta-analysis. J Infect (2019) 78:339–48. doi: 10.1016/j.jinf.2019.02.006
25. Kohlmorgen C, Gerfer S, Feldmann K, Twarock S, Hartwig S, Lehr S, et al. Dapagliflozin reduces thrombin generation and platelet activation: implications for cardiovascular risk reduction in type 2 diabetes mellitus. Diabetologia (2021) 64:1834–49. doi: 10.1007/s00125-021-05498-0
26. Feng P, Wang G, Yu Q, Zhu W, Zhong C. First-trimester blood urea nitrogen and risk of gestational diabetes mellitus. J Cell Mol Med (2020) 24:2416–22. doi: 10.1111/jcmm.14924
27. Anton IC, Mititelu-Tartau L, Popa EG, Poroch M, Poroch V, Pelin A-M, et al. Zinc chloride enhances the antioxidant status, improving the functional and structural organic disturbances in streptozotocin-induced diabetes in rats. Medicina (Kaunas) (2022) 58:1620. doi: 10.3390/medicina58111620
28. Hawley JA, Sassone-Corsi P, Zierath JR. Chrono-nutrition for the prevention and treatment of obesity and type 2 diabetes: from mice to men. Diabetologia (2020) 63:2253–9. doi: 10.1007/s00125-020-05238-w
29. Cryer MJ, Horani T, DiPette DJ. Diabetes and hypertension: A comparative review of current guidelines. J Clin Hypertens (Greenwich) (2016) 18:95–100. doi: 10.1111/jch.12638
30. Durlach V, Vergès B, Al-Salameh A, Bahougne T, Benzerouk F, Berlin I, et al. Smoking and diabetes interplay: A comprehensive review and joint statement. Diabetes Metab (2022) 48:101370. doi: 10.1016/j.diabet.2022.101370
31. Wang K, Mao Y, Lu M, Liu X, Sun Y, Li Z, et al. Association between serum Klotho levels and the prevalence of diabetes among adults in the United States. Front Endocrinol (2022) 13:1005553. doi: 10.3389/fendo.2022.1005553
32. Li B, Yuan Z, Zhang Y, Li F, Huang L, Yang Z, et al. Exploring the role of uterine fibroids in promotion of cardiovascular diseases by diabetes exposure: Findings from national health and nutrition examination survey 1999–2006. Front Cardiovasc Med (2022) 9:975920. doi: 10.3389/fcvm.2022.975920
33. Xia F, Li Q, Luo X, Wu J. Identification for heavy metals exposure on osteoarthritis among aging people and Machine learning for prediction: A study based on NHANES 2011-2020. Front Public Health (2022) 10:906774. doi: 10.3389/fpubh.2022.906774
34. Elbeyli A, Kurtul BE, Ozcan SC, Ozarslan Ozcan D. The diagnostic value of systemic immune-inflammation index in diabetic macular oedema. Clin Exp Optometry (2022) 105:831–5. doi: 10.1080/08164622.2021.1994337
35. Guo W, Song Y, Sun Y, Du H, Cai Y, You Q, et al. Systemic immune-inflammation index is associated with diabetic kidney disease in Type 2 diabetes mellitus patients: Evidence from NHANES 2011-2018. Front Endocrinol (2022) 13:1071465. doi: 10.3389/fendo.2022.1071465
36. Özata Gündoğdu K, Doğan E, Çelik E, Alagöz G. Serum inflammatory marker levels in serous macular detachment secondary to diabetic macular edema. Eur J Ophthalmol (2022) 32:3637–43. doi: 10.1177/11206721221083465
37. Ozer Balin S, Ozcan EC, Uğur K. A new inflammatory marker of clinical and diagnostic importance in diabetic foot infection: systemic immune-inflammation index. Int J Lower Extremity Wounds (2022), 15347346221130817. doi: 10.1177/15347346221130817
38. Rias YA, Tsai HT, Thato R, Apriyanto BS, Chou KR, Ho SC, et al. Synergistic interactions of insufficient physical activity and a high systemic immune-inflammation index on psychological problems in Indonesians with type 2 diabetes mellitus. Biol Res For Nurs (2023), 10998004231162050. doi: 10.1177/10998004231162050
39. Zhou J, Song S, Zhang Y, Jin K, Ye J. OCT-based biomarkers are associated with systemic inflammation in patients with treatment-naïve diabetic macular edema. Ophthalmol Ther (2022) 11:2153–67. doi: 10.1007/s40123-022-00576-x
40. Jia Z, Liu C, Li H. [Changes of the concentration of serum ischemia modified albumin and high sensitivity C-reactive protein in type 2 diabetic patients with retinopathy]. Zhonghua Yan Ke Za Zhi (2009) 45:805–8.
41. Liu H, Zhao D, Wang W, Qin L, Liu J, Sun J, et al. [Association between high sensitivity C-reactive protein levels in serum and the 5-year-accumulative-risk of diabetes]. Zhonghua Liu Xing Bing Xue Za Zhi (2011) 32:1–4.
42. Camerlingo C. Novel leukocyte and thrombocyte indexes in patients with prediabetes and type 2 diabetes mellitus. Eur Rev (2022).
43. Wang S-Y, Shen T-T, Xi B-L, Shen Z, Zhang X. Vitamin D affects the neutrophil-to-lymphocyte ratio in patients with type 2 diabetes mellitus. J Diabetes Invest (2021) 12:254–65. doi: 10.1111/jdi.13338
44. Akbari M, Hassan-Zadeh V. IL-6 signalling pathways and the development of type 2 diabetes. Inflammopharmacol (2018) 26:685–98. doi: 10.1007/s10787-018-0458-0
45. Qu D, Liu J, Lau CW, Huang Y. IL-6 in diabetes and cardiovascular complications. Br J Pharmacol (2014) 171:3595–603. doi: 10.1111/bph.12713
46. Wang J-W, Hu K, Qian H-P, Yuan Q, Liu Q, Ma C, et al. Systemic immune-inflammation landscape in brain metastasis needing neurosurgical resection: Analysis of 230 consecutive cases in a single center. Immun Inflamm Dis (2022) 10:e694. doi: 10.1002/iid3.694
47. Berbudi A, Rahmadika N, Tjahjadi AI, Ruslami R. Type 2 diabetes and its impact on the immune system. Curr Diabetes Rev (2020) 16:442–9. doi: 10.2174/1573399815666191024085838
48. Zhang H, Yang Z, Zhang W, Niu Y, Li X, Qin L, et al. White blood cell subtypes and risk of type 2 diabetes. J Diabetes Complications (2017) 31:31–7. doi: 10.1016/j.jdiacomp.2016.10.029
49. Kashima S, Inoue K, Matsumoto M, Akimoto K. White blood cell count and C-reactive protein independently predicted incident diabetes: yuport medical checkup center study. Endocr Res (2019) 44:127–37. doi: 10.1080/07435800.2019.1589494
50. Hwang J-Y, Kwon Y-J, Choi W-J, Jung D-H. Platelet count and 8-year incidence of diabetes: The Korean Genome and Epidemiology Study. Diabetes Res Clin Pract (2018) 143:301–9. doi: 10.1016/j.diabres.2018.07.033
51. Qian Y, Zeng Y, Lin Q, Huang H, Zhang W, Yu H, et al. Association of platelet count and plateletcrit with nerve conduction function and peripheral neuropathy in patients with type 2 diabetes mellitus. J Diabetes Investig (2021) 12:1835–44. doi: 10.1111/jdi.13535
52. Kataoka HU, Noguchi H. and β-cell pathogenesis of type 1 and type 2 diabetes and islet transplantation. Cell Med (2013) 5:53–7. doi: 10.3727/215517913X666512
54. Wang M, Tan Y, Shi Y, Wang X, Liao Z, Wei P. Diabetes and sarcopenic obesity: pathogenesis, diagnosis, and treatments. Front Endocrinol (2020), 11. doi: 10.3389/fendo.2020.00568
55. Xie J, Wang Z, Zhang X, Wang J, Feng W, Hu Y, et al. Association between daily eating frequency and mortality in people with diabetes: Findings from NHANES 1999–2014. Front Nutr (2023) 10:937771. doi: 10.3389/fnut.2023.937771
56. Pivovarov JA, Taplin CE, Riddell MC. Current perspectives on physical activity and exercise for youth with diabetes. Pediatr Diabetes (2015) 16:242–55. doi: 10.1111/pedi.12272
57. Gutierrez-Mariscal FM, Alcalá-Diaz JF, Quintana-Navarro GM, de la Cruz-Ares S, Torres-Peña JD, Cardelo MP, et al. Changes in quantity plant-based protein intake on type 2 diabetes remission in coronary heart disease patients: from the CORDIOPREV study. Eur J Nutr (2023) 62:1903–13. doi: 10.1007/s00394-022-03080-x
Keywords: systemic immune-inflammation index, diabetes, NHANES, cross-sectional study, population-based study
Citation: Nie Y, Zhou H, Wang J and Kan H (2023) Association between systemic immune-inflammation index and diabetes: a population-based study from the NHANES. Front. Endocrinol. 14:1245199. doi: 10.3389/fendo.2023.1245199
Received: 23 June 2023; Accepted: 13 October 2023;
Published: 31 October 2023.
Edited by:
Md Abdul Hye Khan, University of Missouri, United StatesReviewed by:
Leena Chacko, Meso Scale Discovery, United StatesJayanta Gupta, Florida Gulf Coast University, United States
Copyright © 2023 Nie, Zhou, Wang and Kan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Wang, d2FuZ2ppbmcyMTYxQDEyNi5jb20=
 Yiqi Nie
Yiqi Nie Haiting Zhou3
Haiting Zhou3