
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Endocrinol. , 07 November 2023
Sec. Cellular Endocrinology
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1244300
This article is part of the Research Topic Hormonal Imbalance-Associated Oxidative Stress and Protective Benefits of Nutritional Antioxidants View all 13 articles
Sea buckthorn (Hippophae rhamnoides L.) is a flowering shrub, and its berries have been utilized for decades as a raw ingredient in cuisines and herbal remedies. This evidence-based study focuses on its key bioactive constituents, and mechanism of protective effects with a focus on female reproductive processes. Parts of the plant contain phenols, carotenoids (lycopene, carotene, lutein, and zeaxanthin), flavonoids (isorhamnetin, quercetin, glycosides, and kaempferol), tocopherols, sterols, polyunsaturated fatty acids, minerals, vitamins, omega 3, 6, 9 and rare omega 7 fatty acids etc. Key polyphenolic flavonoids such as isorhamnetin and quercetin are believed to be mainly responsible behind its health benefits (against cardiovascular diseases, metabolic syndrome, obesity etc.) through properties including anti-cancer, antioxidant, and anti-inflammatory activities. These sea buckthorn constituents appear to mediate healthy ovarian cell proliferation, death, and hormone release, as well as decrease ovarian cancer possibly through apoptosis, and hormonal (estrogen) release. Thus, sea buckthorn and its bioactive ingredients may have potential in the management of gynecological problems such as uterine inflammation, endometriosis, and easing symptoms of vulvovaginal atrophy in postmenopausal women (by targeting inflammatory cytokines and vascular endothelial growth factor – VEGF). Apigenin, myricetin, and luteolin have also been recommended as prospective ovarian cancer preventative and adjuvant therapy options as they can inhibit ovarian cancerogenesis by triggering apoptosis and halting the cell cycle in ovarian tumors. Furthermore, its oil (containing carotenoid, sterol, and hypericin) has been speculated as an alternative to estrogen replacement therapy for postmenopausal women particularly to improve vaginal epithelial integrity. However, it is uncertain whether steroid hormone receptors, reactive oxygen species (ROS), and inflammatory regulators are actually behind sea buckhorn’s actions. Sea buckthorn, and its compounds’ health promoting potential warrants further validation not just in vitro and in animal research, but also in clinical trials to identify and/or standardize optimal methods of delivery of biologically active molecules.
Nowadays, population diseases are becoming more and more widespread. Many factors are responsible for this phenomenon, be it stress, free radical production or lifestyle. The maintenance of the body’s redox status has been credited in large part to hormones (1). For example, estradiol has been proven to have a greater impact on the oxidant-antioxidant balance in numerous tissues. Although progesterone lacks the typical chemical structure of an antioxidant, it appears to lessen oxidative damage when present at high amounts (2, 3). Despite these findings, it is important to supplement dietary polyphenols and phytonutrients commonly found in plants. This evidence-based study focuses on the bioactive constituents, and mechanism(s) of protective effects of sea buckthorn (Hippophae rhamnoides L.) with a focus on female reproductive processes. Sea buckthorn is a flowering shrub belonging to the family Elaeagnaceae. It is native to cold temperate regions of Eurasia (4). This economically and ecologically important medicinal plant is a winter hardy, dioecious, wind-pollinated multipurpose shrub bearing yellow or orange berries with nitrogen-fixing ability. It grows widely in cold regions of the Indian Himalayas, China, Russia, and many other North American and European countries. Due to its enormous potential as a bioresource for land restoration, preventing soil erosion, and its variety of uses, it is frequently referred to as “cold desert gold” (5). Because of its usage in pharmaceutical and cosmetic compounds, as a source of energy, soil enhancer, and as rich nutritional content, sea buckthorn has a high economic worth (6). Almost all parts of the plant may be used as food, firewood, traditional medicine, and a fence. This plant includes many chemical compounds with a range of biological and medicinal effects (7). Sea buckthorn has been used for centuries as a medicinal and nutritional supplement across Asia and Europe (8). Its berries have been utilized for decades in many parts of the world as a raw ingredient in cuisines and herbal remedies. Berries’ therapeutic and/or nutritional properties make them an affordable source of raw material for the pharmaceutical industry, which benefits mankind (7). As herbal dietary supplements are used more often in many nations, it is crucial to regulate food items that include these ingredients. However, there is little information on the plant and its extracts’ safety assessment (8). Several medicinal benefits of this plant have been well documented, including antioxidant, antitumor, hepatoprotective, or immunomodulating activities (9, 10). Medicinal plants have widely been acknowledged to be the basis for active principles for both therapeutic and preventive measures. Recently, a range of pharmaceuticals have been reported for their antioxidant and anticancer potentials and regulation of hormonal levels to the advantage of management of several disease conditions. Alkaloids, phenols, and acetogenins isolated from graviola (Annona muricata) have not only shown promise as possible cancer-fighting agents but also in modulation of cellular proliferation and necrosis. This plant’s extract has been reported to downregulate anti-apoptotic genes involved in the pro-cancer metabolic pathways and decreasing the expression of proteins involved in cell invasion and metastasis while upregulating proapoptotic genes and genes involved in the destruction of cancer cells (11). Plant-derived polyphenols including resveratrol, curcumin, quercetin, green tea flavonoids, caffeic acid phenethyl ester, luteolin, xanthohumol, genistein, alpinetin, proanthocyanidins, anthocyanins, silymarin as well as phenolic substances such as thymol, alkaloids like berberine, storage polysaccharides like tamarind xyloglucan, and antioxidant hormones (e.g. melatonin) have been reported to target cellular signaling pathways to reduce intestinal inflammation occurring with inflammatory bowel disorder (1). Plant-based inhibitors of dipeptidyl peptidase-IV (an enzyme that triggers the catalysis of insulinotropic hormones by abating endogenous insulin levels and elevating glucose levels in blood plasma) such as alkaloids, phenolic acids, flavonoids, quercetin, and coumarin have recently been proposed as anti-diabetic by virtue of their hypoglycemic and antioxidative properties (12).
However, a summary of sea buckthorn’s physiological and therapeutic effects on female reproductive systems and/or diseases is still lacking. The latest research on sea buckthorn’s components, characteristics, physiological effects, and therapeutic uses is reviewed together with their methods of action at multiple regulatory levels, with a focus on female reproductive systems.
The aim of this study was to review the progress in the research on sea buckthorn [regardless of the Latin name, Hippophae rhamnoides (including all subspecies)] and its potential application in female reproduction made from 2015 to 2023. Publications about the biological activity of sea buckthorn extracts and their constituents and the mechanism(s) of action have also been described. However, the number of available articles on pharmacological properties of different extracts or natural products from this plant is very large, hence the concerned section of the article only highlights some important aspects of research made during the last five years. The literature search was performed using Google Scholar, PubMed, and Scopus search engines, with a time limit from 2015 to 2023. Keywords “sea buckthorn” or ‘rhamnoides’ were combined with ‘flavonoids’, ‘isorhamnetin’, “phenolic compounds”, ‘quercetin’, ‘ovarian tumor’, ‘female reproduction’, “anti-inflammatory activity”, “anticancer activity”, “antiviral activity” etc. Finally, 84 original articles from this period were included in this review.
Together with leaves, sea buckthorn berries are rich in a variety of vitamins and other physiologically active components, including up to 106 nutraceutical and 74 bioactive chemicals (13) or even up to 190 bioactive compounds (4). Different parts of the plant contain phenols, carotenoids (lycopene, carotene, lutein, and zeaxanthin), flavonoids (isorhamnetin, quercetin, glycosides, and kaempferol), tocopherols, sterols (4, 13–15), polyunsaturated fatty acids, minerals, vitamins, omega 3, 6, 9 and rare omega 7 fatty acids (4), and dietary fibers (16). The oil derived from sea buckthorn seed is the only natural oil that contains a 1:1 ratio of omega 3 and omega 6 fatty acids (linolenic and linoleic acids) and has β-sitosterol as primary phytosterol (16). Berries are an excellent supply of vital polyunsaturated fatty acids, sugars, and tocopherols, while leaves are a good source of polyphenols (17, 18). H. rhamnoides L. subsp. yunnanensis (Yunnanensis), H. rhamnoides L. subsp. mongolica (Mongolica), H. rhamnoides L. subsp. turkestanica (Turkestanica) and H. rhamnoides L. subsp. sinensis are four different subspecies of sea buckthorn that have had their phytochemical compositions studied. H. rhamnoides L. subsp. yunnanensis has the largest cellular antioxidant and antiproliferative characteristics, whereas sinensis subspecies has the highest total phenolic content and related total antioxidant activity (19). Total flavonoid concentration of sea buckthorn is around 23 mg quercetin equivalent/g dried extract, and total polyphenol content is about 46 mg gallic acid equivalent/g dried extract (20). However, the bioactive content of berries is also affected by age, fruit size, climate, geographic location, and extraction process (21). Zheng et al. (22) found a variety of beneficial chemicals in these berries, including oleanolic acid, 19-alpha-hydroxy ursolic acid, succinic acid, ursolic acid, 5-hydroxymethyl-2-furancarbox-aldehyde, octacosanoic acid, palmitic acid, hippophae cerebroside, and 1-O-hexadecanolenin. Recently, a number of phytoprostanes, phytofurans, tocopherols, tocotrienols, carotenoids, and free amino acids have been detected in sea buckthorn berry juice (23). Berries contain high amounts of polysaccharides (18) and dietary fibers (7, 16). Some bioactive phenolic components, including quercetin-3-O-galactoside, quercetin-3-O-glucoside, kaempferol, and isorhamnetin (24, 25), as well as flavonol glycosides (di- and tri-glycosides) (15) have been detected in leaf extracts. Six compounds from sea buckthorn leaf extract have been isolated previously: kaempferol-3-O- β-α-(6’’-O-coumaryl) glycoside, 1-feruloyl-β-α-glucopyranoside, isorhamnetin-3-O-glucoside, quercetin-3-O-β-α-glucopyranoside, quercetin-3-O-β-α-glucopyranosyl-7-O-α-l-rhamnopyranoside, and isorhamnetin-3-O-rutinoside (26). Tannin fractions from leaves have been separated, and the main components are hydrolyzable gallo- and ellagi-tannins of the monomeric type: strictinin, isostrictinin, casuarinin, and casuarictin (25). Data show that sea buckthorn is a rich source of several biologically active compounds that may be helpful to health and effective in the prevention and treatment of a variety of illnesses (27). As mentioned above, key sea buckthorn polyphenolic flavonoids include isorhamnetin and quercetin (Figure 1).
In recent years, research has shown that sea buckthorn can help with illness prevention and healing, including viral infections and cancer (15, 28) owing to its antioxidant (29, 30), anti-inflammatory (31), antiviral (32), antimicrobial (24, 30, 33), and antibacterial (34, 35) properties. Cardioprotective, anti-atherogenic, hepatoprotective, hypolipidemic (29, 36, 37), dermatological (4), antiproliferative (20), and anticancer (e.g., colon, liver, lung, cervical, ovarian, and breast cancer cells) effects have been reported, too (6, 15, 38). Proanthocyanidins, curcumin, and resveratrol have been demonstrated to have considerable advantages in cancer chemoprevention and radiotherapy (39). Similarly, kaempferol has been shown to suppress the growth of breast cancer (40). A higher dietary intake of phenolic substances, particularly flavonoids, and procyanidins, has been linked to a decreased risk of cancer (41). Aurolognans, bioactive constituents of sea buckthorn, have been linked with hepatoprotective, hypolipidemic, and anti-obesity effects (37). Moreover, anti-inflammatory action of this plant could be due to the presence of triterpenes – oleanolic, asiatic, and maslinic acids (42). Table 1 summarizes the physiological and therapeutic activities of sea buckthorn preparations through in vivo and in vitro experimentations on several experimental models.
The physicochemical and functional features of sea buckthorn berry pomace powder (PP) justify its usage as a fiber-rich dietary additive (16). PP had strong hydration qualities in addition to having a high protein content (21.09 g/100 g) such as 4.24 g/g and 9.98 mL/g of water-holding capacity and swelling capacity, respectively. The functional potential of the tested PP was determined by its in vitro hypoglycemic and hypolipidemic qualities, which were shown to be comparable to and, in some cases, superior to those of other dietary fiber powders made from by-products of the processing of fruits and vegetables. Berry PP had a cholesterol-binding capacity of 21.11 to 23.13 mg/g (16). Traditionally, sea buckthorn berries also serve as a Chinese medicine with multiple bioactivities (18). A recent bioassay-guided investigation applied to seek the hepatoprotective and hypolipidemic ingredients has been able to isolate three new (10 → 10’’)-biauronlignans (1–3), three new 10-(4’’-hydroxy-benzyl)-auronlignans (4–6), three new 10-O-β-D-glucopyranosyl-auronlignans (7–9), and eleven known auronlignan derivatives (10–20). Their structures have been established using lengthy and thorough infrared (IR), ultraviolet/visible (UV/Vis), nuclear magnetic resonance (NMR), and mass spectroscopy (MS) spectrum investigations, and these results have been compared with the published references. While compounds 2, 5, 8, and 12 displayed mild pancreatic lipase activity inhibition and reduced the moderately FFA-induced lipid accumulation in HepG2 liver cells, compounds 1, 4, 7, 11, 15, and 19 demonstrated moderate hepatoprotective activities against the damage in acetaminophen-induced HepG2 cells (37). In addition, structural data of a homogeneous polysaccharide from sea buckthorn (SBP-1-A) has recently been described, and it was discovered that SBP-1-A has a backbone of around 3,4).-β-l-Rhap-(1 → 4)-α-d-GalAp-(1 → with side chains made up of α-l-Araf, β-d-Galp, β-d-Glcp, and α-d-Glcp, of which the arabinose, glucose, and galactose residues have been identified as the primary monosaccharide compositions with a percentage surpassing 92%. Furthermore, the protein-free polysaccharide fraction (SBP-1) obtained after isolation of crude SBP showed an outstanding anti-obesity effect. According to the findings, consuming SBP-1 might increase the expression of peroxisome proliferator-activated receptor gamma coactivator 1 (PGC1α), uncoupling protein 1 (UCP-1), and PR domain containing 16 (PRDM 16) in adipocytes, activate brown adipocytes, and boost thermogenesis, which would prevent fat buildup and weight gain. It is crucial to remember that the type of preparation, its chemical composition, and its concentration all appear to have an impact on how sea buckthorn preparations affect hemostasis. The sea buckthorn preparations seem to be excellent regulators of hemostasis, particularly blood platelet function, due to their high phytochemical contents, notably phenolic components. Additionally, it is still uncertain how much of these preparations should be used for prophylaxis and therapy, and recommendations for using sea buckthorn preparations are frequently based on sparse clinical investigations. Thus, more randomized clinical studies with bigger samples are required, particularly those including healthy volunteers and those with the greatest cardiovascular risk factors. Additionally, the effects of several sea buckthorn components on hemostasis, including fibrinolysis and coagulation systems as well as blood platelet activities, should be studied in these trials. Since there is currently no reliable information about the anti-hemorrhagic effectiveness of sea buckthorn preparations in either people or animals, it is also crucial to investigate the role of various sea buckthorn products in the prevention and treatment of cardiovascular disorders (50). Recently, phytoprostanes in sea buckthorn juices have been discovered, and their quantities are highly connected with the capacity to reduce inflammation through inhibition of the 15-lipoxygenase enzyme (23). Due to the presence of possible inhibitors of α-amylase, α-glucosidase (tocopherols, tocotrienols, and certain amino acids), and pancreatic lipase (xanthophylls), sea buckthorn juice can be an intriguing anti-diabetic and anti-obesity diet. Juices act more effectively in lowering neurological alterations due to the presence of phytoprostanes, phytofurans, tocopherols, tocotrienols, and amino acids, making them possible anti-aging agents in the prevention of Alzheimer’s disease, the most prevalent kind of dementia. Juice from sea buckthorn may be crucial in the body’s fight against diseases brought on by free radical assault (23). Sea buckthorn insoluble dietary fiber (IDF) can be modified to increase its in vitro hypoglycemic capacity. Examples of these modifications include IDF, milled insoluble dietary fiber, and co-modified insoluble dietary fiber. Ball milling, as well as ball milling coupled with cellulose treatment reportedly enhanced the characteristics of IDF which provide a foundation for the extensive utilization of sea buckthorn resources (27).
Maslinic acid functions via the nuclear factor kappa light chain enhancer of activated B cells (NF-κB) and erythroid 2-related factor 2 (Nrf2) signaling pathways, whereas oleanolic and asiatic acids act via the NF-κB, mitogen-activated protein kinase (MAPK), and Nrf2 signaling pathways to exert anti-inflammatory effects on macrophages. These three substances can be employed as natural anti-inflammatory dietary supplements because they exhibited specific inhibitory effects on the LPS-induced inflammatory response in vitro. However, more research is necessary, including in vivo investigations, to encourage the usage of sea buckthorn-derived products (42). The active components of sea buckthorn that are responsible for the biological effects haven’t yet been fully identified. The flavonoids quercetin and isorhamnetin, a 3′-O-methylated metabolite of quercetin, are thought to be principally in charge. There is proof that isorhamnetin can prevent cells from proliferating (42), promote apoptosis and mitigate tumor development (51–53), suppress inflammatory processes (33, 42, 54), improve cognitive functions (54), and affect numerous metabolic processes (33). The anti-cancer (29, 55), cardioprotective (56), and anti-obesity (57) effects of quercetin have been reported, too. Presence of several flavonoids including isorhamnetin (54, 58), auronlignan (18), polysaccharides (37, 59), and dietary fibers (16, 27) indicate towards the anti-cholesterol and anti-obesity effects of sea buckthorn. Thus, a number of physiological and pathological processes can be targeted by sea buckthorn and its bioactive compounds. Nevertheless, a majority of the studies on sea buckhorn action were performed for medicinal purposes in pathological conditions, including on cancer cells. Therefore, obtained information could potentially be helpful for management of tumors, but the biological value of the information is limited due to the fact that whether and how the dietary consumption of sea buckwheat could affect healthy organism and its cells. Even the applicability of sea buckthorn for treatment of cancer is yet to be sufficiently demonstrated by clinical trials. Furthermore, sea buckthorn constituents responsible for the effect of the whole plant remains to be identified. The molecules, which might be responsible for sea buckhorn effect have been hypothesized on the basis of their presence in the plant and the similarity of their as well as whole plant effects. But there isn’t a single thorough experiment that has compared these impacts. Furthermore, there is no conclusive evidence of a functional connection from the similarities of the effects. Therefore, extensive research is needed to identify the components of sea buckthorn that are responsible for its biological and therapeutic effects.
Although the evidence for each of these processes is weak and the interactions between these mechanisms are poorly understood, extracellular and intracellular modes of action of sea buckthorn and its constituents on cells have been postulated. Nevertheless, it has been proposed that the ability of sea buckthorn to prevent and to treat infections and cancer (15) as mentioned previously can mainly be due to its antioxidant (29), anti-inflammatory (31), antiviral (32), antimicrobial (24, 33), and antibacterial (34, 35) properties. Numerous clinical disorders, including allergies, cancer, and many others, are mostly driven by inflammation. By stimulating Nrf2-dependent pathways, sea buckthorn’s anti-inflammatory effects may be mediated (60). An effective anti-inflammatory target, the heme oxygenase-1 (HO-1) axis, is known to be regulated in part by Nrf2. Nrf2 is essential for regulating the production of antioxidant genes, which in turn have anti-inflammatory effects (60). The protective action of sea buckthorn polysaccharide is linked to the activation of the Nrf-2/HO-1-SOD-2 signaling pathway (36). Recent investigations revealed a relationship between the production of additional inflammatory mediators including the NF-κB pathway and macrophage metabolism and the Nrf2/antioxidant response element system (60). It’s interesting to note that a sea buckthorn polysaccharide has been shown to protect the liver against acetaminophen (APAP)-induced liver damage in rats. Enzymes like alanine aminotransferase (ALT) and aspartate aminotransferase (AST) have been able to be reduced by it (36). The antioxidant qualities of sea buckthorn’s components have been used to describe a variety of its therapeutic benefits. In the liver, brain, and plasma, for instance, sea buckthorn supplementation elevated glutathione (GSH) and GSH-Px levels and the production of nitric oxide (NO) and the inducible nitric oxide synthase (iNOS), which was linked to lessened liver damage (36) and oxidative and nitrosative stress in liver and brain of rats (61). Antioxidant chemicals, especially phenolic components such as flavonoids kaempferol, isorhamnetin, and quercetin, are responsible for sea buckthorn’s antitumor action. These flavonoids defend against oxidative stress, which can cause cancer and genetic alterations in cells (50, 62). The formation of ROS was reduced along with the reduction in glioma cell viability after sea buckthorn extract treatment, at the very least (26). Masoodi et al. (63) has proposed that sea buckthorn reduces the production of a certain antigen and inhibits cellular growth in prostate cancer cells. Additionally, rat glioma cells’ fast multiplication was suppressed by sea buckthorn leaf extract, which is thought to have done so via causing the first stages of cell death. The increased expression of B-cell lymphoma 2/Bcl-2-associated X protein (Bcl-2/Bax) and acetaminophen-induced inhibition of c-Jun N-terminal kinase phosphorylation are further signs of sea buckthorn’s impact on cytoplasmic apoptosis (36). The pro-apoptotic Bax gene expression was increased by sea buckthorn extracts, and its localization, accumulation, and translocation in the nuclei were all encouraged (26). However, as demonstrated in human retinoblastoma cells, the quercetin-induced rise in cytochrome c levels together with the activation of caspase-3 and caspase-9 results in apoptosis in cancer cells (64). Additionally, it was shown previously that buckthorn procyanidins might cause cell death in a dose-dependent way (65). It is possible that these procyanidins might block intracellular fatty acid synthase (FAS) activity and cause human breast cancer cells to undergo apoptosis. At least, sea buckthorn procyanidins were discovered to restrict the proliferation of cancer cells, and FAS is a critical enzyme for de novo long-chain fatty acid production, which is present in high amounts in cancer cells (65). Several flavonoids (especially isorhamnetin) affecting a number of enzymes regulating fat synthesis and metabolism can be responsible for their hypolipidemic, cholesterol-lowering, and anti-obesity effects (58). In addition, the anti-obesity effects of sea buckthorn polysaccharides could be due to their stimulatory action on brown adipose tissue and thermogenesis inducing its “burning” (37). Finally, sea buckthorn’s anti-diabetic and anti-obesity effects can be attributed to the ability of its dietary fibers to reduce glucose production and metabolism via suppression of glucose adsorption, glucose diffusion inhibition, starch digestion inhibition, starch pasting interference, and α-amylase activity (27). Androgen receptors are the next potential mediator of sea buckthorn’s actions on tissues that are dependent on hormones. These receptors control the expression of androgen-responsive genes through ligand-dependent transcription factors. The target androgen responsive genes cannot be activated, and prostate cancer growth cannot be stopped if the androgen receptor is somehow kept in the cytoplasm and its shuttling into the nucleus is blocked. Prostate cancer cells’ androgen receptors have been shown to be affected by the administration of sea buckthorn leaf extracts, which was correlated with the suppression of genes that respond to androgens, cellular growth, and survival of these cells (63). The beneficial effects of sea buckthorn on spermatogenesis may be due to its effect on androgen receptors. By increasing spermatogonia proliferation, stem cell survival, and lowering sperm abnormalities, sea buckthorn therapy enhanced spermatogenesis and had a protective effect against the negative effects of gamma radiation (66). Available literature demonstrates a number of signaling molecules and mechanisms mediating sea buckthorn’s actions on various targets in the organism and/or their pathologies at a cellular level. Some of their mediators could be specific for particular target cells or organs (e.g., steroid hormones for steroid-dependent processes). Other mediators could be more pervasive, such as those that influence cell proliferation, apoptosis, and oxidative processes. Additionally, although they haven’t been fully explored yet, functional hierarchy linkages between the mediators of sea buckthorn’s effects are feasible. Last but not least, studies of the effects of sea buckthorn and its mediators have primarily used cell cultures. As a result, appropriate in vivo research should be used to confirm the findings gained using such models.
One of the most potent active components in sea buckthorn berries is isorhamnetin, a 3′-O-methylated metabolite of quercetin that has a wide range of pharmacological effects, including anti-cancer ones. The modulation of PI3K/AKT/PKB, NF-κB, MAPK, and other signaling pathways, as well as the production of associated cytokines and protein kinases involved in controlling cell apoptosis and proliferation, are all part of the mechanisms of action (53, 54). Through the activation of apoptotic genes and apoptosis and the downregulation of oncogenes, isorhamnetin can inhibit the development of cancer. Isorhamnetin has also been demonstrated to inhibit the PI3K-AKT-mTOR pathway (phosphatidylinositol 3-kinase, protein kinase B, and the mammalian target of rapamycin), which in turn inhibits the proliferation of cancer cells by inducing cell cycle arrest at the G2/M phase. Additionally, isorhamnetin can increase the production of cyclin B1 protein while decreasing the phosphorylation levels of AKT, phosph-p70S6 kinase, and phosph-4E-BP1 proteins (52). Additionally, isorhamnetin can enhance liver and kidney functioning by lowering blood levels of urea nitrogen, AST, and ALT. Additionally, by preventing the dimerization of the toll-like receptor 4, isorhamnetin can reduce infection-induced liver and kidney inflammation as well as inflammation-induced cell death (33). Some physiological actions of isorhamnetin could be mediated by an interplay of several intracellular signaling pathways. For example, isorhamnetin can mitigate the adverse effect of obesity on cognitive functions by suppression of neuroinflammation via downregulation of MAPK- and NFkB-dependent pathways (54). Quercetin is another ingredient that may contribute to the benefits of sea buckthorn. Quercetin’s ability to influence intracellular signaling pathways that regulate cell proliferation and apoptosis may be the cause of its anti-cancer effects (29, 55, 67). It can cause cell cycle arrest through suppression of its promoters cyclin B1 and MAPK/ERK1/2 and activation of transcription factor p53. It can also prolong DNA repair and promote apoptosis through inhibition of survivin, activation of transforming growth factor-β (TGF-β), PI3K/AKT/mTOR, Wnt/-catenin, NOTCH, sonic hedgehog signaling pathway (SHH), Janus kinas Additionally, quercetin can inhibit tumorigenesis by controlling VEGF and its receptors, which are the factors that stimulate tumor vascularization (55). The presence of quercetin, tannins, and other polyphenolic flavonoids in sea buckthorn extract, as well as their radical scavenging and anti-inflammatory properties, may be responsible for the protection of spermatozoa (66), and cardiomyocytes (56, 57). Some possible mechanisms of action of sea buckthorn and its bioactive components on cancer cells are summarized in Figure 2. In fine, the literature concerning the mechanisms and/or mediators of action of sea buckhorn compounds isorhamnetin and quercetin indicate an existence of multiple pathways of these molecules on target cells. The key mechanisms of their action (e.g., related to cell proliferation, apoptosis, or oxidative stress) are like mechanisms of sea buckthorn whole plant’s effects. Nevertheless, it remains to be investigated whether the effects of the whole plant and its mechanisms
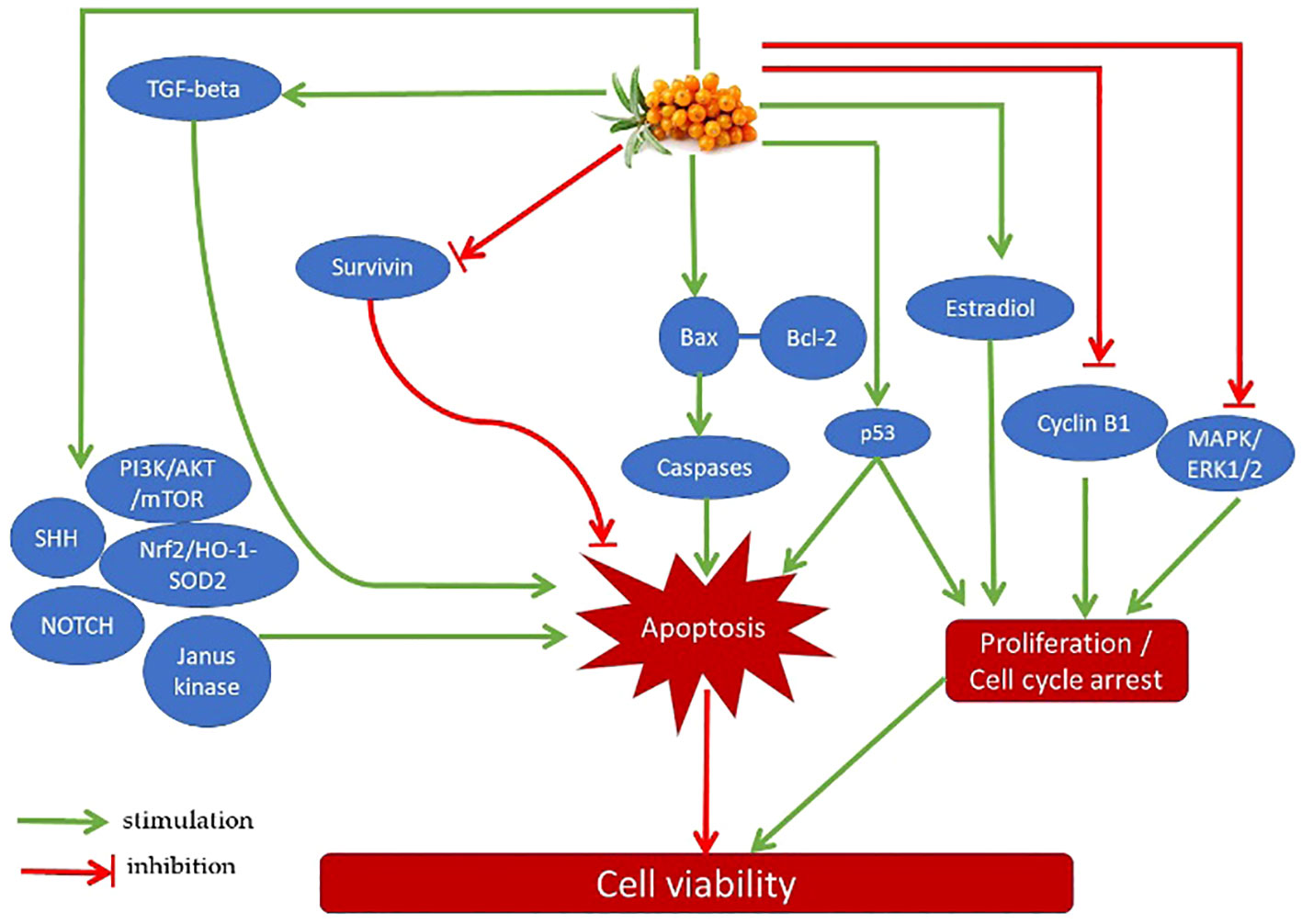
Figure 2 Possible mechanisms of action of sea buckthorn and its bioactive components on cancer cells.
of action could be explained by the presence of only isorhamnetin and quercetin, or other constituents and their mechanisms of action could be involved in mediating sea buckthorn’s effects. Furthermore, the data concerning these mediators, which were obtained predominantly by in vitro experiments, require verification by corresponding in vivo studies.
There is no evidence accessible in scientific databases about the effects of entire sea buckthorn on ovarian functions. Dietary sea buckthorn oil did not influence bovine ovarian folliculogenesis, oocyte quality, or embryo developmental ability (68). Nonetheless, some physiologically active sea buckthorn elements have been shown to influence female reproductive processes. However, the direct impact of quercetin on fundamental ovarian cell processes (proliferation, apoptosis, and hormone release) may vary depending on the species (69). Quercetin also inhibits the development of human metastatic ovarian cancer cells and affects the intrinsic apoptotic mechanism (70–73). Isorhamnetin, another sea buckthorn component, can stimulate ovarian cell proliferation and estrogen release (52, 70), and suppress estrogen-dependent ovarian cancer development (70, 73). Other physiologically active components of sea buckhorn have also been proven to be advantageous to ovarian cancer cells. Apigenin, myricetin, and luteolin have been shown to cause apoptosis, decrease cell proliferation, limit cell invasion, and stop the cell cycle of ovarian cancer. Furthermore, apigenin, myricetin, and luteolin have been recommended as prospective ovarian cancer preventative and adjuvant therapy options (70, 74). Another sea buckthorn ingredient that can inhibit ovarian cancerogenesis is kaempherol. In cultivated ovarian cancer cells, it can at least trigger apoptosis and halt the cell cycle (70, 73, 75–77). Furthermore, kaempherol can inhibit angiogenesis in ovarian tumors (76). As a result, sea buckthorn components may influence ovarian cell proliferation, death, and hormone release. In ovarian cancer cells, on the other hand, sea buckthorn components can cause apoptosis, decrease cell proliferation, and stop the cell cycle. Nonetheless, the relevance of the collected data is restricted by the fact that the claimed effects on ovarian functions are primarily the product of in vitro investigations, with majority of these tests being done on ovarian cancer cells rather than healthy cells. There is a need for more information on the entire sea buckthorn’s activity on ovarian functions (including dysfunctions and malignant transformation) both in vitro and in vivo.
Traditionally, sea buckthorn has been used to treat gynecological diseases such as uterine inflammation and endometriosis. Its oil helps reduce the symptoms of endometriosis and uterine inflammation. These effects might be linked to the carotenoid, sterol, and hypericin content of the plant (55). Consumption of sea buckthorn extract or oil may also be beneficial in the avoidance of vaginal difficulties during menopause, which is associated with vaginal atrophy and the thinning and drying of the vaginal mucosa. Menopausal women who used sea buckthorn oil had better vaginal epithelial integrity and a higher vaginal health score. It has been proposed as an alternative to estrogen replacement therapy for postmenopausal women’s vaginal health (78). Furthermore, a novel vaginal gel containing sea buckthorn oil (Meclon Idra Alfasigma) has recently been registered. It appears to be a viable option as a local agent for alleviating symptoms of vulvovaginal atrophy (vaginal dryness, itching, and burning feeling) and enhancing sexual function in postmenopausal women (79). Sea buckthorn also has a high concentration of vitamins C and E. Infertile or subfertile women undergoing controlled ovarian stimulation may benefit from vitamin C and E supplementation in terms of uterine features, endometrial thickness, and endometrial blood flow. Furthermore, the antioxidant and anticoagulant properties of vitamins C and E are assumed to be responsible for the increase in fertility (80). In contrast to its effect on the ovary, sea buckthorn, and its components have been shown to have a therapeutic effect on the management of gynecological problems such as uterine inflammation, endometriosis, and signs of vulvovaginal atrophy in postmenopausal women. We summarized the important effects in Table 2. However, the effect of sea buckthorn on the healthy vagina and uterus has not been thoroughly established. Furthermore, the components of sea buckthorn that affect these organs are mainly unknown. Even the role of vitamins in mediating the benefits of sea buckthorn is more or less theoretical and requires scientific validation.
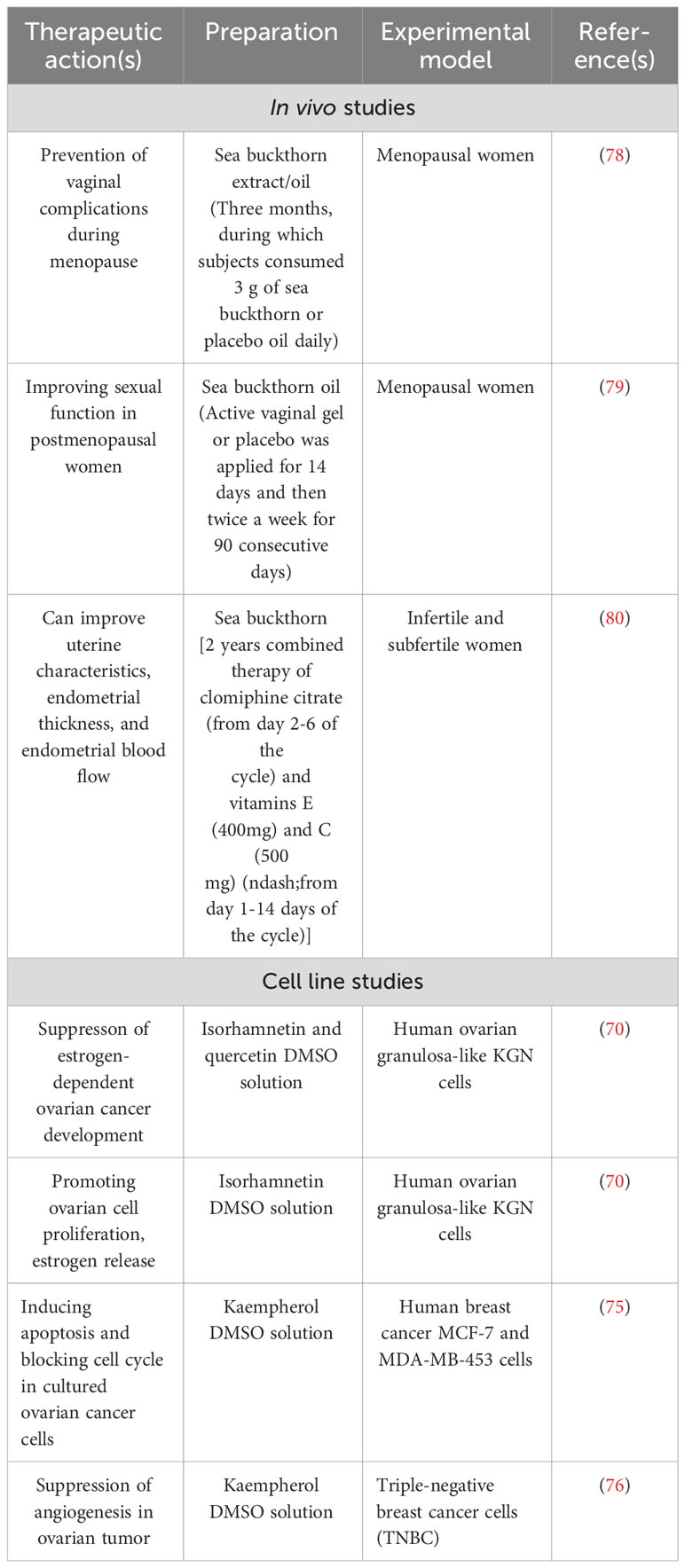
Table 2 Physiological and therapeutic actions of sea buckthorn and its constituencies relating to female reproductive processes.
There is inadequate information to support the mechanism of sea buckthorn’s impacts on female reproductive systems. Nonetheless, existing evidence allows us to sketch some of the processes and mediators of sea buckthorn or its active ingredients in the female reproductive system. Sea buckthorn oil has been shown to reverse endometriosis in rat. The therapy lowered the levels of inflammatory cytokines (inflammation markers and promoters) and VEGF (angiogenesis markers and promoters) (55). Therefore, cytokines and VEGF could be extracellular mediators of the curative action of sea buckthorn on endometriosis. Imran et al. (76) also hypothesized that kaempferol would reduce tumor development and angiogenesis by lowering VEGF expression via hypoxia-inducible factor 1α (HIF-1α), a physiological activator of VEGF synthesis. Some sea buckthorn flavonoids have also been shown to operate on ovarian cells via intracellular regulators of proliferation and death. For example, quercetin promotes caspase-3 expression, which may result in DNA fragmentation and apoptosis. In addition, quercetin has been demonstrated to reduce the expression of anti-apoptotic proteins while increasing the synthesis of pro-apoptotic proteins in a variety of cancer cell lines, including ovarian cancer (71, 72). Isorhamnetin has the potential to influence ovarian cancer cell proliferation and apoptosis by targeting intracellular PI3K/Akt signaling pathway promoters of the cell cycle (cyclins) and apoptosis (Bax, Bcl, and cytochrome) (52, 77). Kampherol has been shown to enhance the production of morphological indications of apoptosis (membrane blebbing) and the accumulation of apoptotic intracellular markers and promoters (caspases 3, 8, and 9, as well as Bax) while decreasing the expression of anti-apoptotic Bcl-2. Furthermore, kaempferol caused cell cycle arrest at the G0/G1 checkpoint, as well as inhibition of cyclin B1 and Cdc2 expression (75). Imran et al. (76) suggested that kaempferol can induce apoptosis and cell cycle arrest at the G2/M phase via upregulation of checkpoint kinase 2/cell division cycle 25C/cyclin-dependent kinase 2 (Chk2/Cdc25C/Cdc2), receptors DR5 and DR4, c-Jun N-terminal kinase (JNK), C/EBP homologous protein (CHOP), p38, p21, the extracellular signal-regulated kinase 1/2 (ERK1/2) proteins, caspase-3, -7, -8, Bad, Bax, and p53 proteins. The ability of sea buckthorn (50) and isorhamnetin (52) to affect ROS, the known promoters of apoptosis, indicates that this sea buckthorn flavonoid can impact ovarian cell viability and result in events impacting oxidative stress. Finally, sea buckhorn constituents isorhamnetin (52, 70) and quercetin (69, 81) can affect the production of estrogens and other ovarian hormones, which are considered as key regulators of ovarian functions and female reproduction and fecundity (81). This fact indicates that sea buckthorn might impact reproductive processes through hormonal mechanisms, too. The few relevant published findings suggest that sea buckthorn may be useful in the treatment of endometriosis by influencing extracellular regulators of inflammatory processes such as cytokines and VEGF. However, the intracellular mediators of this therapeutic activity still need to be identified and verified. It is uncertain whether this plant or its active ingredients have an effect on the healthy vagina and uterus. More is known about the mechanisms/mediators of sea buckthorn and its components’ impact on ovarian cells. Sea buckthorn flavonoids have been shown to suppress ovarian cancer cells by downregulating VEGF, anti-apoptotic proteins, upregulating pro-apoptotic proteins, suppressing the cell cycle at various checkpoints, p-AKT, and inducing oxidative and endoplasmic reticulum stress and autophagy. Hormones may also play a role in modulating the effects of plant constituents’ isorhamnetin and quercetin on female reproductive organs. However, it should be emphasized that the mediators and processes of sea buckthorn or its components are postulated primarily on the basis of indirect evidence - since these regulators have been altered following the administration of the plant or its constituents. More direct experimental data is needed to understand the functional interrelationships between plant compounds and reproductive process regulators. When compared to known mediators of its effects on non-reproductive processes, the number of recognized mediators of sea buckthorn on female reproductive organs is small. More research would very certainly add to the list of mediators of sea buckthorn’s physiological and therapeutic effects on female reproductive systems. Although hierarchical interrelationships between numerous mediators of sea buckthorn’s activities on the ovary, vagina, and uterus are plausible, they have yet to be thoroughly investigated.
Sea buckthorn is being studied as a functional food as well as a herbal medication for animal and human health, including the treatment of numerous female reproductive diseases. Although there are some publications on the effects of sea buckthorn chemicals quercetin and isorhamnetin on healthy ovarian cells, it is uncertain if sea buckthorn extract or its constituents could be effective in influencing healthy female reproductive processes. More data, however, is available on the use of sea buckthorn and its components to prevent and/or perhaps treat ovarian cancer. Flavonoids found in sea buckthorn can decrease cancer cell proliferation, cause apoptosis, prevent cell cycle arrest, and slow tumor development. This might point to the possible use of sea buckthorn flavonoids in the prevention and treatment of ovarian cancer.
Furthermore, ovarian cancer is linked to other gynecological diseases such as endometriosis (82). The potential use of sea buckthorn and its active ingredients in the treatment of gynecological problems such as uterine inflammation, endometriosis, and symptoms of vulvovaginal atrophy in postmenopausal women has been proven (82). No toxicity of sea buckthorn berries (50) or sea buckthorn berry oil (8) has been reported including no treatment-related maternal toxicity or embryotoxicity (8). According to the data, sea buckthorn products can be used as functional foods, nutritional supplements, and medicines. However, it cannot be ruled out that extracted and purified sea buckthorn compounds/molecules might be used in place of dietary sea buckthorn or its extract. Although such a substitute may be more expensive, the dose and ingredients may be easier to define, and the biological and/or therapeutic efficiency may be greater and more predictive than the raw plant product. Taken together, the available evidence on sea buckthorn’s beneficial effects suggests that it has potential therapeutic applications in phytotherapy of cancer, endometriosis, and/or other reproductive dysfunctions.
Sea buckthorn elements appear to alter healthy ovarian cell proliferation, death, and hormone release, as well as decrease ovarian cancer (by triggering ovarian cancer cell apoptosis and autophagy, decreasing cell growth, invasion, and halting the cell cycle). Furthermore, sea buckthorn and its bioactive ingredients may be effective in the treatment of gynecological problems such as uterine inflammation, endometriosis, and easing symptoms of vulvovaginal atrophy in postmenopausal women by targeting inflammatory cytokines and VEGF, as previously indicated. Nonetheless, many elements of sea buckhorn activity and application remain unknown to science. Inadequate research has been conducted on the effects of sea buckhorn extract on female reproductive processes and the roles of major individual elements. There is no information concerning the possible functional interrelationship among various plant constituents in the regulation of reproductive and non-reproductive processes, for example.
The mediators of sea buckthorn action have also been studied insufficiently, whilst the role and hierarchical interrelationships between signaling molecules mediating sea buckthorn actions remain rather speculative so far. They are based mainly on similar interrelationships between mediators of other substances. For example, it is possible that plant flavonoids with antioxidant properties could block ROS, prevent oxidative stress, and resulting inflammatory processes, mutagenesis, apoptosis, and arrest cell cycle (81, 83). It is possible that this is a case of sea buckhorn isoflavones, too. Nevertheless, such mechanisms might be proposed based on indirect indications only – the action of flavones on some indices of oxidative, inflammatory processes, apoptosis, or proliferation. Furthermore, plant flavonoids usually have phytoestrogenic properties – the ability to affect the receptors of steroid hormones, which in turn are the important regulators of cell proliferation, apoptosis, and cancerogenesis (81, 84). Sea buckthorn components/molecules can affect steroid hormones and steroid hormones-dependent processes, as discussed earlier. However, it is uncertain whether steroid hormone receptors, ROS, inflammatory regulators, and so on actually cause sea buckhorn function. Although research on this plant has concentrated on its medicinal potential and use, the function of sea buckhorn extract and some of its important components on a healthy female reproductive system is still mostly unknown. It is also necessary to identify and/or standardize optimal methods of delivering biologically active molecules of sea buckhorn. This plant’s and its compounds’ medicinal potential should be validated not just in vitro and in animal research, but also in clinical trials. The findings of the few reported studies listed above may just be the beginning steps toward understanding the biology and therapeutic potential of this interesting plant and its active ingredients, which will require further confirmatory research.
Conceptualization: MM, SR, AK. Writing – original draft preparation: MM. Writing – review and editing: SR, AK. Supervision: AK.
This study was supported by the Ministry of Education, Science, Research and Sport of the Slovak Republic projects APVV-18-0312, VEGA 1/0266/20, and KEGA 033SPU-4/2021.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sahoo DK, Chainy GBN. Chapter Eight - Hormone-linked redox status and its modulation by antioxidants. In: Litwack G, editor. Vitamins and hormones, vol. 121. Cambridge, Massachusetts, USA: Academic Press (2023). p. 197–246. Available at: https://www.sciencedirect.com/science/article/pii/S0083672922000826.
2. Chainy GBN, Sahoo DK. Hormones and oxidative stress: an overview. Free Radic Res (2020) 54(1):1–26. doi: 10.1080/10715762.2019.1702656
3. Hernández-Rabaza V, López-Pedrajas R, Almansa I. Progesterone, lipoic acid, and sulforaphane as promising antioxidants for retinal diseases: A review. Antioxidants (2019) 8(3):53. doi: 10.3390/antiox8030053
4. Pundir S, Garg P, Dviwedi A, Ali A, Kapoor VK, Kapoor D, et al. Ethnomedicinal uses, phytochemistry and dermatological effects of Hippophae rhamnoides L.: A review. J Ethnopharmacol (2021) 266:113434. doi: 10.1016/j.jep.2020.113434
5. Kalia RK, Singh R, Rai MK, Mishra GP, Singh SR, Dhawan AK. Biotechnological interventions in sea buckthorn (Hippophae L.): current status and future prospects. Trees (2011) 25(4):559–75. doi: 10.1007/s00468-011-0543-0
6. Grey C, Widén C, Adlercreutz P, Rumpunen K, Duan RD. Antiproliferative effects of sea buckthorn (Hippophae rhamnoides L.) extracts on human colon and liver cancer cell lines. Food Chem (2010) 120(4):1004–10. doi: 10.1016/j.foodchem.2009.11.039
7. Ozturk M, Hakeem KR, Ashraf M, Ahmad MSA. Global perspectives on underutilized crops. Cham: Springer International Publishing (2018). Available at: http://link.springer.com/10.1007/978-3-319-77776-4.
8. Wen P, Zhao P, Qin G, Tang S, Li B, Zhang J, et al. Genotoxicity and teratogenicity of seabuckthorn (Hippophae rhamnoides L.) berry oil. Drug Chem Toxicol (2020) 43(4):391–7. doi: 10.1080/01480545.2018.1497047
9. Ting HC, Hsu YW, Tsai CF, Lu FJ, Chou MC, Chen WK. The in vitro and in vivo antioxidant properties of seabuckthorn (Hippophae rhamnoides L.) seed oil. Food Chem (2011) 125(2):652–9. doi: 10.1016/j.foodchem.2010.09.057
10. Gunenc A, Khoury C, Legault C, Mirrashed H, Rijke J, Hosseinian F. Seabuckthorn as a novel prebiotic source improves probiotic viability in yogurt. LWT - Food Sci Technol (2016) 66:490–5. doi: 10.1016/j.lwt.2015.10.061
11. Ilango S, Sahoo DK, Paital B, Kathirvel K, Gabriel JI, Subramaniam K, et al. A review on annona muricata and its anticancer activity. Cancers (2022) 14(18):4539. doi: 10.3390/cancers14184539
12. Chhabria S, Mathur S, Vadakan S, Sahoo DK, Mishra P, Paital B. A review on phytochemical and pharmacological facets of tropical ethnomedicinal plants as reformed DPP-IV inhibitors to regulate incretin activity. Front Endocrinol (2022) 13. doi: 10.3389/fendo.2022.1027237
13. Wang K, Xu Z, Liao X. Bioactive compounds, health benefits and functional food products of sea buckthorn: a review. Crit Rev Food Sci Nutr (2022) 62(24):6761–82. doi: 10.1080/10408398.2021.1905605
14. Sajfrtová M, Ličková I, Wimmerová M, Sovová H, Wimmer Z. β-sitosterol: supercritical carbon dioxide extraction from sea buckthorn (Hippophae rhamnoides L.) seeds. Int J Mol Sci (2010) 11(4):1842–50. doi: 10.3390/ijms11041842
15. Enkhtaivan G, Maria John KM, Pandurangan M, Hur JH, Leutou AS, Kim DH. Extreme effects of Seabuckthorn extracts on influenza viruses and human cancer cells and correlation between flavonol glycosides and biological activities of extracts. Saudi J Biol Sci (2017) 24(7):1646–56. doi: 10.1016/j.sjbs.2016.01.004
16. Jurevičiūtė I, Keršienė M, Bašinskienė L, Leskauskaitė D, Jasutienė I. Characterization of berry pomace powders as dietary fiber-rich food ingredients with functional properties. Foods (2022) 11(5):716. doi: 10.3390/foods11050716
17. Yang B, Kallio HP. Fatty acid composition of lipids in sea buckthorn ( Hippophaë rhamnoides L.) berries of different origins. J Agric Food Chem (2001) 49(4):1939–47. doi: 10.1021/jf001059s
18. Ma X, Yang W, Kallio H, Yang B. Health promoting properties and sensory characteristics of phytochemicals in berries and leaves of sea buckthorn ( Hippophaë rhamnoides ). Crit Rev Food Sci Nutr (2022) 62(14):3798–816. doi: 10.1080/10408398.2020.1869921
19. Guo R, Guo X, Li T, Fu X, Liu RH. Comparative assessment of phytochemical profiles, antioxidant and antiproliferative activities of Sea buckthorn (Hippophaë rhamnoides L.) berries. Food Chem (2017) 221:997–1003. doi: 10.1016/j.foodchem.2016.11.063
20. Zhamanbayeva GT, Aralbayeva AN, Murzakhmetova MK, Tuleukhanov ST, Danilenko M. Cooperative antiproliferative and differentiation-enhancing activity of medicinal plant extracts in acute myeloid leukemia cells. BioMed Pharmacother (2016) 82:80–9. doi: 10.1016/j.biopha.2016.04.062
21. Leskinen HM, Suomela JP, Yang B, Kallio HP. Regioisomer compositions of vaccenic and oleic acid containing triacylglycerols in sea buckthorn (Hippophaë rhamnoides) pulp oils: influence of origin and weather conditions. J Agric Food Chem (2010) 58(1):537–45. doi: 10.1021/jf902679v
22. Zheng WH, Bai HY, Han S, Bao F, Zhang KX, Sun LL, et al. Analysis on the constituents of branches, berries, and leaves of hippophae rhamnoides L. by UHPLC-ESI-QTOF-MS and their anti-inflammatory activities. Nat Prod Commun (2019) 14(8):1–10. doi: 10.1177/1934578X19871404
23. Tkacz K, Gil-Izquierdo Á, Medina S, Turkiewicz IP, Domínguez-Perles R, Nowicka P, et al. Phytoprostanes, phytofurans, tocopherols, tocotrienols, carotenoids and free amino acids and biological potential of sea buckthorn juices. J Sci Food Agric (2022) 102(1):185–97. doi: 10.1002/jsfa.11345
24. Upadhyay NK, Yogendra Kumar MS, Gupta A. Antioxidant, cytoprotective and antibacterial effects of Sea buckthorn (Hippophae rhamnoides L.) leaves. Food Chem Toxicol (2010) 48(12):3443–8. doi: 10.1016/j.fct.2010.09.019
25. Wang Z, Zhao F, Wei P, Chai X, Hou G, Meng Q. Phytochemistry, health benefits, and food applications of sea buckthorn (Hippophae rhamnoides L.): A comprehensive review. Front Nutr (2022) 9:1036295. doi: 10.3389/fnut.2022.1036295
26. Kim SJ, Hwang E, Yi SS, Song KD, Lee HK, Heo TH, et al. Sea buckthorn leaf extract inhibits glioma cell growth by reducing reactive oxygen species and promoting apoptosis. Appl Biochem Biotechnol (2017) 182(4):1663–74. doi: 10.1007/s12010-017-2425-4
27. Zhu Y, Ji X, Yuen M, Yuen T, Yuen H, Wang M, et al. Effects of ball milling combined with cellulase treatment on physicochemical properties and in vitro hypoglycemic ability of sea buckthorn seed meal insoluble dietary fiber. Front Nutr (2022) 8:820672. doi: 10.3389/fnut.2021.820672
28. Dadhwal P, Dhingra HK, Dwivedi V, Alarifi S, Kalasariya H, Yadav VK, et al. (sea buckthorn) mediated green synthesis of copper nanoparticles and their application in anticancer activity. Front Mol Biosci (2023) 10:1246728. doi: 10.3389/fmolb.2023.1246728
29. Kashyap P, Riar CS, Sea Buckthorn JN. Antioxidants in fruits: properties and health benefits. Nayik GA, Gull A, editors. Singapore: Springer Singapore (2020) p. 201–25. doi: 10.1007/978-981-15-7285-2_11
30. Liu X, Lv M, Maimaitiyiming R, Chen K, Tuerhong N, Yang J, et al. Development of fermented sea buckthorn (Hippophae rhamnoides L.) juice and investigation of its antioxidant and antimicrobial activity. Front Nutr (2023) 10:1120748. doi: 10.3389/fnut.2023.1120748
31. Kwon DJ, Bae YS, Ju SM, Goh AR, Choi SY, Park J. Casuarinin suppresses TNF-α-induced ICAM-1 expression via blockade of NF-κB activation in HaCaT cells. Biochem Biophys Res Commun (2011) 409(4):780–5. doi: 10.1016/j.bbrc.2011.05.088
32. Jain M, Ganju L, Katiyal A, Padwad Y, Mishra KP, Chanda S, et al. Effect of Hippophae rhamnoides leaf extract against Dengue virus infection in human blood-derived macrophages. Phytomedicine (2008) 15(10):793–9. doi: 10.1016/j.phymed.2008.04.017
33. Chauhan AS, Negi PS, Ramteke RS. Antioxidant and antibacterial activities of aqueous extract of Seabuckthorn (Hippophae rhamnoides) seeds. Fitoterapia (2007) 78(7):590–2. doi: 10.1016/j.fitote.2007.06.004
34. Yogendra Kumar MS, Tirpude RJ, Maheshwari DT, Bansal A, Misra K. Antioxidant and antimicrobial properties of phenolic rich fraction of Seabuckthorn (Hippophae rhamnoides L.) leaves in vitro. Food Chem (2013) 141(4):3443–50. doi: 10.1016/j.foodchem.2013.06.057
35. Widén C, Renvert S, Persson GR. Antibacterial activity of berry juices, an in vitro study. Acta Odontol Scand (2015) 73(7):539–43. doi: 10.3109/00016357.2014.887773
36. Wang X, Liu J, Zhang X, Zhao S, Zou K, Xie J, et al. Seabuckthorn berry polysaccharide extracts protect against acetaminophen induced hepatotoxicity in mice via activating the Nrf-2/HO-1-SOD-2 signaling pathway. Phytomedicine (2018) 38:90–7. doi: 10.1016/j.phymed.2017.11.007
37. Ma Z, Sun Q, Chang L, Peng J, Zhang M, Ding X, et al. A natural anti-obesity reagent derived from sea buckthorn polysaccharides: Structure characterization and anti-obesity evaluation in vivo. Food Chem (2022) 375:131884. doi: 10.1016/j.foodchem.2021.131884
38. Ali I, Zahra N ul A RR, Sharif H MM, Bhatti HA. A New Potent Anti-cancer Corosolic Ester Identified from the Super Miracle Plant Hippophae rhamnoides (Sea buckthorn). Biochem Mod Appl (2019) 2:24–9. doi: 10.33805/2638-7735.119
39. Ko JH, Sethi G, Um JY, Shanmugam MK, Arfuso F, Kumar AP, et al. The role of resveratrol in cancer therapy. Int J Mol Sci (2017) 18(12):2589. doi: 10.3390/ijms18122589
40. Yang S, Si L, Jia Y, Jian W, Yu Q, Wang M, et al. Kaempferol exerts anti-proliferative effects on human ovarian cancer cells by inducing apoptosis, G0/G1 cell cycle arrest and modulation of MEK/ERK and STAT3 pathways. J BUON (2019) 24(3):975–81.
41. Kristo A, Klimis-Zacas D, Sikalidis A. Protective role of dietary berries in cancer. Antioxidants (2016) 5(4):37. doi: 10.3390/antiox5040037
42. Han Y, Yuan C, Zhou X, Han Y, He Y, Ouyang J, et al. Anti-inflammatory activity of three triterpene from hippophae rhamnoides L. @ in lipopolysaccharide-stimulated RAW264.7 cells. Int J Mol Sci (2021) 22(21):12009. doi: 10.3390/ijms222112009
43. Sun WL, Li XY, Dou HY, Wang XD, Li JD, Shen L, et al. Myricetin supplementation decreases hepatic lipid synthesis and inflammation by modulating gut microbiota. Cell Rep (2021) 36(9):109641. doi: 10.1016/j.celrep.2021.109641
44. He N, Wang Q, Huang H, Chen J, Wu G, Zhu M, et al. A comprehensive review on extraction, structure, detection, bioactivity, and metabolism of flavonoids from sea buckthorn (Hippophae rhamnoides L.). J Food Biochem (2023) 2023:e4839124. doi: 10.1155/2023/4839124
45. Rédei D, Kúsz N, Rafai T, Bogdanov A, Burián K, Csorba A, et al. 14-Noreudesmanes and a phenylpropane heterodimer from sea buckthorn berry inhibit Herpes simplex type 2 virus replication. Tetrahedron (2019) 75(10):1364–70. doi: 10.1016/j.tet.2019.01.050
46. Huang H, Li Y, Gui F, Yang P, Zhang J, Li W, et al. Optimizing the purification process of polyphenols of sea buckthorn seed and its potential freshness effect. LWT (2023) 173:114380. doi: 10.1016/j.lwt.2022.114380
47. Dadhwal P, Dhingra HK, Dwivedi V, Alarifi S, Kalasariya H, Yadav VK, et al. Frontiers | Hippophae rhamnoides L. (sea buckthorn) mediated green synthesis of copper nanoparticles and their application in anticancer activity. Front Mol Biosci (2023) 10:1246728 doi: 10.3389/fmolb.2023.1246728
48. Zhao H, Kong L, Shao M, Liu J, Sun C, Li C, et al. Protective effect of flavonoids extract of Hippophae rhamnoides L. @ on alcoholic fatty liver disease through regulating intestinal flora and inhibiting TAK1/p38MAPK/p65NF-κB pathway. J Ethnopharmacol (2022) 292:115225. doi: 10.1016/j.jep.2022.115225
49. Saeed GN, Ahsin S, Sarwar M. HEPATOPROTECTIVE EFFECT OF SEA BUCKTHORN BERRY SEED OIL IN CYCLOPHOSPHAMIDE-INDUCED HEPATIC TOXICITY IN BALB/c MICE. Pak J Physiol (2023) 19(2):20–4.
50. Olas B. Berry phenolic antioxidants – implications for human health? Front Pharmacol (2018) 9:78. doi: 10.3389/fphar.2018.00078
51. Li C, Yang X, Chen C, Cai S, Hu J. Isorhamnetin suppresses colon cancer cell growth through the PI3K-Akt-mTOR pathway. Mol Med Rep (2014) 9(3):935–40. doi: 10.3892/mmr.2014.1886
52. Li X, Chen H, Zhang Z, Xu D, Duan J, Li X, et al. Isorhamnetin promotes estrogen biosynthesis and proliferation in porcine granulosa cells via the PI3K/akt signaling pathway. J Agric Food Chem (2021) 69(23):6535–42. doi: 10.1021/acs.jafc.1c01543
53. Qin Y, Jiang W, Li A, Gao M, Liu H, Gao Y, et al. The combination of paraformaldehyde and glutaraldehyde is a potential fixative for mitochondria. Biomolecules (2021) 11(5):711. doi: 10.3390/biom11050711
54. Mulati A, Zhang X, Zhao T, Ren B, Wang L, Liu X, et al. Isorhamnetin attenuates high-fat and high-fructose diet induced cognitive impairments and neuroinflammation by mediating MAPK and NFκB signaling pathways. Food Funct (2021) 12(19):9261–72. doi: 10.1039/D0FO03165H
55. Farooqi AA, Jabeen S, Attar R, Yaylim I, Xu B. Quercetin-mediated regulation of signal transduction cascades and microRNAs: Natural weapon against cancer. J Cell Biochem (2018) 119(12):9664–74. doi: 10.1002/jcb.27488
56. Ferenczyova K, Kalocayova B, Bartekova M. Potential implications of quercetin and its derivatives in cardioprotection. Int J Mol Sci (2020) 21(5):1585. doi: 10.3390/ijms21051585
57. Carrasco-Pozo C, Cires MJ, Gotteland M. Quercetin and epigallocatechin gallate in the prevention and treatment of obesity: from molecular to clinical studies. J Med Food (2019) 22(8):753–70. doi: 10.1089/jmf.2018.0193
58. Xiao PT, Liu SY, Kuang YJ, Jiang ZM, Lin Y, Xie ZS, et al. Network pharmacology analysis and experimental validation to explore the mechanism of sea buckthorn flavonoids on hyperlipidemia. J Ethnopharmacol (2021) 264:113380. doi: 10.1016/j.jep.2020.113380
59. Lan Y, Sun Q, Ma Z, Peng J, Zhang M, Wang C, et al. Seabuckthorn polysaccharide ameliorates high-fat diet-induced obesity by gut microbiota-SCFAs-liver axis. Food Funct (2022) 13(5):2925–37. doi: 10.1039/D1FO03147C
60. Saha S, Buttari B, Panieri E, Profumo E, Saso L. An overview of nrf2 signaling pathway and its role in inflammation. Molecules (2020) 25(22):5474. doi: 10.3390/molecules25225474
61. Cavak N, Gumustas MK, Cekic SD. The protective role of hippophae rhamnoides L. @ on rat brain and liver tissues exposed to cold plus immobilization stress model. Turk Neurosurg (2022) 32(4):587–94. doi: 10.5137/1019-5149.JTN.23766-18.3
62. Olas B, Skalski B, Ulanowska K. The anticancer activity of sea buckthorn [Elaeagnus rhamnoides (L.) A. Nelson]. Front Pharmacol (2018) 9:232. doi: 10.3389/fphar.2018.00232
63. Masoodi KZ, Wani W, Dar ZA, Mansoor S, Anam-ul-Haq S, Farooq I, et al. Sea buckthorn (Hippophae rhamnoides L.) inhibits cellular proliferation, wound healing and decreases expression of prostate specific antigen in prostate cancer cells in vitro. J Funct Foods (2020) 73:104102. doi: 10.1016/j.jff.2020.104102
64. Liu H, Zhou M. Antitumor effect of Quercetin on Y79 retinoblastoma cells via activation of JNK and p38 MAPK pathways. BMC Complement Altern Med (2017) 17(1):531. doi: 10.1186/s12906-017-2023-6
65. Wang Y, Nie F, Ouyang J, Wang X, Ma X. Inhibitory effects of sea buckthorn procyanidins on fatty acid synthase and MDA-MB-231 cells. Tumor Biol (2014) 35(10):9563–9. doi: 10.1007/s13277-014-2233-1
66. Goel HC, Samanta N, Kannan K, Kumar IP, Bala M. Protection of spermatogenesis in mice against gamma ray induced damage by Hippophae rhamnoides. Andrologia (2006) 38(6):199–207. doi: 10.1111/j.1439-0272.2006.00740.x
67. Sharma A, Kashyap D, Sak K, Tuli HS, Sharma AK. Therapeutic charm of quercetin and its derivatives: a review of research and patents. Pharm Pat Anal (2018) 7(1):15–32. doi: 10.4155/ppa-2017-0030
68. Plante-Dubé M, Picard C, Gilbert I, Robert C, Fievez V, Vlaeminck B, et al. Effects of a dietary supplement enriched in palmitoleic acid on fatty acid composition of follicular fluid, granulosa cell metabolism, and oocyte developmental capacity in early lactation dairy cows. J Dairy Sci (2021) 104(3):3693–706. doi: 10.3168/jds.2020-19191
69. Sirotkin AV, Štochmaľová A, Alexa R, Kádasi A, Bauer M, Grossmann R, et al. Quercetin directly inhibits basal ovarian cell functions and their response to the stimulatory action of FSH. Eur J Pharmacol (2019) 860:172560. doi: 10.1016/j.ejphar.2019.172560
70. Lu Df, Yang Lj, Wang F, Zhang Gl. Inhibitory effect of luteolin on estrogen biosynthesis in human ovarian granulosa cells by suppression of aromatase (CYP19). J Agric Food Chem (2012) 60(34):8411–8. doi: 10.1021/jf3022817
71. Bhat FA, Sharmila G, Balakrishnan S, Singh PR, Srinivasan N, Arunakaran J. Epidermal growth factor-induced prostate cancer (PC3) cell survival and proliferation is inhibited by quercetin, a plant flavonoid through apoptotic machinery. BioMed Prev Nutr (2014) 4(4):459–68. doi: 10.1016/j.bionut.2014.07.003
72. Teekaraman D, Elayapillai SP, Viswanathan MP, Jagadeesan A. Quercetin inhibits human metastatic ovarian cancer cell growth and modulates components of the intrinsic apoptotic pathway in PA-1 cell line. Chem Biol Interact (2019) 300:91–100. doi: 10.1016/j.cbi.2019.01.008
73. Li M, Zhang W, Yang L, Wang H, Wang Y, Huang K, et al. The mechanism of xiaoyao san in the treatment of ovarian cancer by network pharmacology and the effect of stigmasterol on the PI3K/akt pathway. Dis Markers (2021) 2021:1–10. doi: 10.1155/2021/4304507
74. Tavsan Z, Kayali HA. Flavonoids showed anticancer effects on the ovarian cancer cells: Involvement of reactive oxygen species, apoptosis, cell cycle and invasion. BioMed Pharmacother (2019) 116:109004. doi: 10.1016/j.biopha.2019.109004
75. Kim T, Choi E. Equol induced apoptosis via cell cycle arrest in human breast cancer MDA-MB-453 but not MCF-7 cells. Mol Med Rep (2008) 1(2):239–44. doi: 10.3892/mmr.1.2.239
76. Imran M, Salehi B, Sharifi-Rad J, Aslam Gondal T, Saeed F, Imran A, et al. Kaempferol: a key emphasis to its anticancer potential. Molecules (2019) 24(12):2277. doi: 10.3390/molecules24122277
77. El-Kott AF, Shati AA, Al-Kahtani MA, Alharbi SA. Kaempferol induces cell death in A2780 ovarian cancer cells and increases their sensitivity to cisplatin by activation of cytotoxic endoplasmic reticulum-mediated autophagy and inhibition of protein kinase B. 66. Folia Biol (Praha) (2020) 66(1):36–46. doi: 10.14712/fb2020066010036
78. Larmo PS, Yang B, Hyssälä J, Kallio HP, Erkkola R. Effects of sea buckthorn oil intake on vaginal atrophy in postmenopausal women: a randomized, double-blind, placebo-controlled study. Maturitas (2014) 79(3):316–21. doi: 10.1016/j.maturitas.2014.07.010
79. De Seta F, Caruso S, Di Lorenzo G, Romano F, Mirandola M, Nappi RE. Efficacy and safety of a new vaginal gel for the treatment of symptoms associated with vulvovaginal atrophy in postmenopausal women: A double-blind randomized placebo-controlled study. Maturitas (2021) 147:34–40. doi: 10.1016/j.maturitas.2021.03.002
80. Nasir SA. Improved endometrial thickness and vascularity following vitamins E and C administration in infertile women undergoing controlled ovarian stimulation. Pharmaceuticals (Basel) (2021) 14(4):373. doi: 10.3390/ph14040373
81. Sirotkin AV, Alwasel SH, Harrath AH. The influence of plant isoflavones daidzein and equol on female reproductive processes. Pharmaceuticals (2021) 14(4):373. doi: 10.3390/ph14040373
82. Kvaskoff M, Mahamat-Saleh Y, Farland LV, Shigesi N, Terry KL, Harris HR, et al. Endometriosis and cancer: a systematic review and meta-analysis. Hum Reprod Update (2021) 27(2):393–420. doi: 10.1093/humupd/dmaa045
83. Scuto M, Ontario ML, Salinaro AT, Caligiuri I, Rampulla F, Zimbone V, et al. Redox modulation by plant polyphenols targeting vitagenes for chemoprevention and therapy: Relevance to novel anti-cancer interventions and mini-brain organoid technology. Free Radic Biol Med (2022) 179:59–75. doi: 10.1016/j.freeradbiomed.2021.12.267
Keywords: Hippophae rhamnoides, isorhamnetin, quercetin, reproduction, female, proliferation, apoptosis, cancer
Citation: Mihal M, Roychoudhury S, Sirotkin AV and Kolesarova A (2023) Sea buckthorn, its bioactive constituents, and mechanism of action: potential application in female reproduction. Front. Endocrinol. 14:1244300. doi: 10.3389/fendo.2023.1244300
Received: 22 June 2023; Accepted: 20 October 2023;
Published: 07 November 2023.
Edited by:
Dipak Kumar Sahoo, Iowa State University, United StatesReviewed by:
Suvranil Ghosh, University of Texas Southwestern Medical Center, United StatesCopyright © 2023 Mihal, Roychoudhury, Sirotkin and Kolesarova. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adriana Kolesarova, YWRyaWFuYS5rb2xlc2Fyb3ZhQHVuaWFnLnNr
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.