
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 23 November 2023
Sec. Clinical Diabetes
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1243992
This article is part of the Research Topic Deepening the Relationship between Diabetes, Oral Health and Periodontal Disease View all 8 articles
Aim: The objective was to investigate the association of periodontitis and tooth loss with extent of diabetic coronary atherosclerosis.
Materials and methods: 272 patients who were hospitalized at Shanghai East hospital and underwent a coronary artery calcium (CAC) CT scan were enrolled in this study. Individuals were grouped based on their CAC scores into a normal-to-mild coronary atherosclerosis (AS) group (0 ≤ score ≤ 100, n=184) and a moderate-to-severe group (score≥101, n=88). Periodontitis parameters and number of missing teeth were evaluated for every patient. The severity of periodontitis was categorized as mild, moderate, or severe. The taxonomic composition of the microbiota was determined using full-length 16S ribosomal RNA gene sequencing. Salivary inflammatory factors were tested by ELISA.
Results: Clinical attachment loss (CAL) (P =0.05) and the number of teeth lost (P = 0.016) were significantly higher in the moderate-to-severe coronary AS group, with these differences being more obvious in younger patients and patients with short-duration diabetes. Multivariate logistic regression analysis revealed that CAL (OR = 1.231, 95% CI = 1.066–1.214, P = 0.047) and having 10–19 missing teeth (OR = 1.604, 95% CI = 1.393–6.555, P = 0.05) were strongly associated with the presence of moderate-to-severe coronary AS. Salivary IL-6 and TNF-α levels, as well as levels of Porphyromonas gingivalis and Neisseria mucosa, were significantly elevated in the moderate-to-severe coronary AS group.
Conclusion: It was found that both tooth loss and CAL were related to the extent of diabetic coronary AS. Saliva inflammatory factors and oral bacteremia may be new biomarkers for moderate-to-severe coronary AS.
Diabetes mellitus is a risk factor for both atherosclerosis and periodontal disease (1). Cardiovascular diseases (CVDs) are the primary cause of mortality and morbidity in diabetes. Diabetes is unquestionably the most important risk factor for CVDs (2). Numerous epidemiological studies have identified a high degree of association between diabetes and periodontal disease, and periodontal diseases have even been proposed as a sixth complication of diabetes (3). The past decade has produced a growing number of investigations surrounding the idea that infection and inflammation occurring as a result of oral pathogenesis can lead to the progression of systemic disease (4–6). It is believed that infection-mediated upregulation of cytokines and other inflammatory mediators plays a central role in this pathologic process (7–9). The high prevalence of CVDs and periodontitis in individuals with diabetes may be attributed to an increased inflammatory response leading to atherosclerosis; this response is usually more extensive and develops at an earlier age in individuals with diabetes compared to those without diabetes (10).
Periodontal diseases are a group of common chronic infections that produce an exaggerated inflammatory response to the oral pathogenic microflora. Periodontitis is the main category of periodontal diseases. It affects the attachment of connective tissue and the supporting bone around the teeth, leading to tooth mobility and subsequent tooth loss (11, 12). Since an initial report in 1989, several further case–control studies, cross-sectional studies, and cohort studies have demonstrated a positive and independent correlation between periodontitis and CVDs (13–16). It should be noted that the specific criteria for periodontitis and CVDs used in these studies have varied greatly. This diversity may account for some of the variability seen among studies in terms of the strength of association between these two conditions. Although no clear explanation for the association between periodontitis and CVDs has been presented so far, there are several proposed mechanisms, both direct and indirect, accounting for this relationship (17–19). Periodontal bacteria and their products may enter systemic circulation (20, 21), inducing platelet aggregation, foam-cell formation (22), and the development of atheroma (a direct mechanism) (23, 24). As a result of the host-mediated immune response to this microbial challenge, serum concentrations of inflammatory mediators, including interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), and prostaglandin E2, which are elevated in periodontal disease (25). These mediators trigger atherosclerosis through recruitment of inflammatory cells to blood vessels, proliferation of vascular smooth muscle cells, and induction of vascular fat degeneration and intravascular coagulation (an indirect mechanism) (26).
There is strong evidence for a link between oral health and cardiometabolic health. Recent evidence suggests that exposure to the adverse oral microbial communities that underlie periodontitis might also contribute to the etiology of cardiometabolic diseases, including diabetes (27–29). However, few studies have investigated the relationship between oral microbial communities and the extent of diabetic coronary atherosclerosis. The identification of microbial signatures that emerge early in disease could help to elucidate the potential shared microbial etiology of periodontitis and cardiometabolic disease and provide biomarkers that can aid in recognition of severe coronary atherosclerosis (AS) in patients with type 2 diabetes mellitus (T2DM).
Although studies have reported separately on associations between periodontitis and diabetes (30, 31) and between periodontitis and coronary heart disease (32, 33), the impact of periodontitis on coronary AS in the presence of diabetes is not well delineated. It is remains unclear whether the relationship between periodontitis and coronary AS in patients with T2DM has its own characteristics, and how the duration of diabetes or the level of blood glucose control can affect this relationship. Previous studies have mainly examined the association between periodontitis and myocardial infarction risk, CVD mortality, fatal coronary events, or incidence of CVDs. It remains unclear whether a clinically significant association exists between the periodontitis and the extent of coronary atherosclerosis. Measurement of coronary artery calcium (CAC) score using non-contrast cardiac gated computed tomography provides a successful and simple scoring algorithm for quantification of CAC and estimation of the extent of coronary artery disease per atherosclerotic lesion (34). In the present study, we have chosen CAC score as an indicator of the extent of coronary AS. Moreover, few studies have focused on the relationship between changes in salivary inflammatory factors or oral microflora and coronary AS in patients with T2DM. However, it should be noted that epidemiological research cannot identify the cause of such a relationship. Therefore, there is a need for studies evaluating the relationship between periodontitis and extent of coronary AS in patients with T2DM and providing evidence to support the identification of moderate and severe coronary artery disease in dental clinics.
In this article, we present cross-sectional data on (1) the relationship of clinical measures of periodontitis and tooth loss with the extent of diabetic coronary AS, and (2) the changes in salivary inflammatory factors and oral microbial communities that occur in patients with varying degrees of diabetic coronary AS.
A total of 272 patients with T2DM, all of Chinese Han ethnicity, who were hospitalized at the Department of Endocrinology, Shanghai East Hospital Affiliated to Tongji University, from November 2020 to December 2021 were included in our study. Patients were diagnosed with T2DM according to the 1999 World Health Organization (WHO) criteria. We excluded patients with type 1 diabetes mellitus or specific types of diabetes mellitus, acute complications of diabetes, liver or renal dysfunction, serious cardiac arrhythmias or acute cardiac insufficiency, diabetic foot ulcers, or history of acute myocardium infarction, percutaneous coronary intervention, cerebral infarction, tumor, or psychosis. We obtained written informed consent from all of the participants. The study was approved by the Human Research and Ethics Committee of Shanghai East Hospital and adhered to the tenets of the Declaration of Helsinki.
Participants’ demographic details, frequency of dental visits, education level, and medical history were carefully recorded. Medical histories mainly covered age, sex, smoking habits, current medications, body mass index (BMI), CVD, cerebral apoplexy, peripheral arterial disease (PAD), hypertension, and duration of diabetes. Patients’ medical details, including presence of hypertension, hyperlipidemia, and diabetes mellitus, were obtained from patient records. BMI was calculated as weight (kg) divided by the square of height (m). In terms of education level, patients were categorized as unschooled or as having attended secondary school, high school, or university. Diabetes of less than 10 years’ duration was regarded as short-duration diabetes, and 10 years’ duration or more as long-duration diabetes in this study. Additionally, we divided patients into two age groups: an older group (more than 60 years old) and a younger group (60 years old or younger).
All the included patients underwent a CAC CT scan, which was specified in the informed consent materials. The CAC scan of the heart was rapidly acquired, prospectively electrocardiogram-triggered and without contrast. The CAC score was quantified using the Agatston method, in which the area of calcified atherosclerosis (defined as an area of at least 1 mm2 with a CT density >130 Hounsfield units [HU]) is multiplied by a density weighting factor and summed for the entire coronary artery tree using a CT dataset with 2.5 to 3.0 mm slice thickness. According to the recommendations of related guidelines, a CAC score of 0 is considered to represent no identifiable plaque burden; a CAC score of 1–10 to represent a minimal identifiable plaque burden; a CAC score of 11–100 to represent a mild atherosclerotic plaque burden; a CAC score of 101–400 to represent a moderate atherosclerotic plaque burden; and a CAC score of more than 400 to represent an extensive atherosclerotic plaque burden. Although a negative or extremely low calcium score (10 or lower) cannot be taken to fully exclude the presence of coronary atherosclerosis, it is consonant with the absence of a fixed (significant) coronary obstructive lesion (likelihood, 5 to 10% or lower) (35, 36). Since a CAC score of less than 100 indicates a low probability of coronary artery disease and coronary events (35–37), we chose 100 as cut-off point. Thus, all patients were assigned to one of two groups according to CAC score: a normal-to-mild group (0 ≤ score ≤ 100), or a moderate-to-severe group (≥101) (34).
Two trained examiners performed all oral examinations. All patients were examined with a dental explorer, mouth mirror, and (Williams and WHO) periodontal probe. They all underwent a clinical periodontal test and a panoramic radiograph was taken. The gingival index (GI) was measured according to the Silness and Loe method. Additionally, bleeding on probing (BOP), clinical attachment loss (CAL), calculus index (CI), probing depth (PD), and periodontal index (PI) were measured according to O’Leary’s index around the six Rumford index teeth (38). Periodontitis severity staging (39) was calculated accordingly: for each tooth, the CAL of the most severe site was recorded; a CAL of 1–2 mm (with no tooth loss due to periodontitis) was defined as Stage I, a CAL of 3–4 mm (with no tooth loss due to periodontitis) as Stage II (moderate), a CAL of ≥ 5 mm (with loss of ≤ 4 teeth due to periodontitis) as Stage III, and a CAL of ≥ 5 mm (with loss of ≥ 5 teeth due to periodontitis) as Stage IV (severe). All patients with periodontitis were categorized into three groups (40): a mild periodontitis group (Stage I), a moderate periodontitis group (Stage II), and a severe periodontitis group (Stage III-IV). Tooth loss was assessed via panoramic radiography readings. Extractions of third molars and extractions performed for orthodontic treatment were not considered to represent tooth loss, whereas extractions for the purpose of dental implant treatment were considered to represent tooth loss. All radiography readings and assessments were carried out by one dentist. Tooth loss was categorized as 0 to 9, 10 to 19, or 20 to 31 missing teeth (41).
Saliva and blood samples were obtained on the same day at the time of the patient’s first visit. After overnight fasting, venous blood samples were collected from all patients. Unstimulated whole buccal saliva was collected from individuals as previously described (42). Briefly, saliva was collected during the course of 3 min after an overnight fast and without prior oral hygiene measures. Saliva samples were immediately frozen at -80°C and stored for less than 3 months before biochemical analysis.
Levels of total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides (TGs), fasting plasma glucose (FPG), glycosylated hemoglobin (HbA1c), and serum creatinine were measured using suitable laboratory techniques. ELISA assay kits supplied by Abcam (Cambridge, MA, USA) were used on saliva samples stored at −80°C to determine interleukin-6 (IL-6), matrix metalloproteinase-9 (MMP-9), and TNF-α levels.
Microbial DNA was extracted from saliva samples using the E.Z.N.A.® Soil DNA Kit (Omega Bio-tek, Norcross, GA, U.S.) according to the manufacturer’s protocols. The V1–V9 regions of the bacteria 16S ribosomal RNA gene were amplified by PCR (95°C for 2 min, followed by 27 cycles at 95°C for 30 s, 55°C for 30 s, and 72°C for 60 s and a final extension at 72°C for 5 min) using primers 27F 5′-AGRGTTYGATYMTGGCTCAG-3′ and 1492R 5′-RGYTACCTTGTTACGACTT-3′, where the barcode represents an eight-base sequence unique to each sample. PCR reactions were performed in triplicate using a 20 μL mixture containing 4 μL of 5 × FastPfu Buffer, 2 μL of 2.5 mM dNTPs, 0.8 μL of each primer (5 μM), 0.4 μL of FastPfu Polymerase, and 10 ng of template DNA. Amplicons were extracted from 2% agarose gels and purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, U.S.) according to the manufacturer’s instructions.
SMRTbell libraries were prepared from the amplified DNA by blunt-ligation according to the manufacturer’s instructions (Pacific Biosciences). Purified SMRTbell libraries from the Zymo and HMP mock communities were sequenced on dedicated PacBio Sequel II 8M cells using the Sequencing Kit 2.0 chemistry. Purified SMRTbell libraries from the pooled and barcoded samples were sequenced on a single PacBio Sequel II cell. All amplicon sequencing was performed by Shanghai Biozeron Biotechnology Co. Ltd (Shanghai, China).
PacBio raw reads were processed using the SMRT Link Analysis software package, version 9.0, to obtain demultiplexed circular consensus sequence (CCS) reads with the following settings: minimum number of passes = 3, minimum predicted accuracy = 0.99. Raw reads were processed through SMRT Portal to filter sequences for length (<800 or >2500 bp) and quality. Sequences were further filtered by removing the barcode, primer sequences, chimeras, and sequences containing 10 consecutive identical bases. OTUs were clustered with 98.65% similarity cut-off using UPARSE (version 7.1; http://drive5.com/uparse/), and chimeric sequences were identified and removed using UCHIME. The phylogenetic affiliation of each 16S rRNA gene sequence was analyzed using RDP Classifier (http://rdp.cme.msu.edu/) against the silva (SSU132)16S rRNA database, with a confidence threshold of 70% (43). A rarefaction analysis based on Mothur v.1.21.1 (44) was conducted to reveal the diversity indices, including the Richness and Shannon diversity indices. A Mann–Whitney U test was performed to assess the statistical significance of differences in diversity indices between samples. Differences were considered significant at p < 0.05. For identification of biomarkers for highly dimensional colonic bacteria, LEfSe (linear discriminant analysis effect size) analysis was conducted (45). The Kruskal–Wallis rank-sum test was performed to examine the changes and dissimilarities among classes, followed by LDA analysis to determine the effect size for each of the distinctively abundant taxa (46).
SPSS 21.0 (IBM Corp., Armonk, NY, USA) was used for statistical analysis. Data are expressed in the form mean ± standard deviation (SD) for continuous variables and percentages (%) for categorical variables. Between-group differences were analyzed using a two-tailed Student’s t-test or non-parametric t-test. Categorical variables were compared using the chi-squared test or Fisher’s exact test. The association between the severity of periodontal disease or missing teeth and the presence of moderate-to-severe coronary atherosclerosis was analyzed via multivariate logistic regression, adjusted for main effects of all covariates, including age, sex, education level, smoking, LDL, HbA1c, BMI, duration of diabetes, and history of statin treatment. All p-values were two-tailed, and p < 0.05 was considered statistically significant.
184 patients (92 male and 92 female; mean age, 63 ± 10 years) were allocated to the normal-to-mild group, and 88 (54 male and 34 female; mean age, 65 ± 7 years) to the moderate-to-severe group. Demographical, medical, and biochemical data, including medications, dental habits, and education status, are presented in Table 1. Patients in the moderate-to-severe group were older than those in the normal-to-mild group (P <0.001), and male subjects constituted the majority in the moderate-to-severe group (P <0.001). More subjects in the moderate-to-severe group were current smokers (P<0.01). The duration of diabetes was longer in the moderate-to-severe group than in the normal-to-mild group (P<0.001). However, there was no statistically significant difference in HbA1c levels between the two groups. Lower TC and LDL-C levels unexpectedly occurred in the moderate-to-severe group, with the differences compared to the normal-to-mild group being statistically significant (P<0.001). It is noteworthy that the rate of statin use at baseline was also higher in the moderate-to-severe group, which may be the reason for the lower TC and LDL-C levels. Regarding the salivary markers measured, IL-6 and TNF-α levels were significantly higher in the moderate-to-severe group than in the normal-to-mild group, whereas salivary MMP-9 levels did not differ significantly between the groups.
The periodontitis parameters of the subjects are shown in Table 2. CAL values were much higher among patients in the moderate-to-severe coronary atherosclerosis group (P=0.05). Additionally, the results revealed that individuals in the moderate-to-severe group had more tooth loss compared to patients in the normal-to-mild group (P=0.016). In analyzing patients’ tooth counts, we observed that more subjects in the normal-to-mild group had more than 25 teeth and 11–24 teeth (P =0.0001, P =0.001, respectively). Severe periodontitis occurred more frequently among patients in the moderate-to-severe coronary atherosclerosis group, and this increase was statistically stronger in male patients (P=0.005). Among female patients, there was an increase in the proportion of moderate periodontitis among patients with moderate-to-severe coronary atherosclerosis, with the difference being statistically significant (P=0.027).
The results of univariate logistic regression analyses, with ORs and CIs, are listed in Table 3 (model 1). CAL (P=0.021) and having 10–19 missing teeth (p=0.01) were found to be highly associated with the presence of moderate-to-severe coronary atherosclerosis. The ORs calculated for CAL and for 10–19 missing teeth were 1.536 (95% CI: 1.066–1.214) and 4.106 (95% CI: 1.404–12.009), respectively. The results of multivariate logistic regression analysis are also presented in Table 3 (model 2). Adjusting for parameters such as age, sex, education level, smoking, LDL, HbA1c, BMI, duration of diabetes, and history of statin treatment in the multivariate logistic regression, we again observed a correlation with moderate-to-severe coronary atherosclerosis among patients with T2DM for CAL (OR: 1.231; 95% CI: 1.004–1.516, P = 0.047) and for having 10-19 missing teeth (OR: 1.604; 95% CI: 1.393–6.555, P=0.05). Unexpectedly, severity staging of periodontitis was not significantly associated with the presence of moderate-to-severe diabetic coronary atherosclerosis in either the unadjusted or the adjusted model.

Table 3 Multivariate logistic regression models of the association of CAL and tooth loss with the presence of moderate or severe coronary atherosclerosis in patients with T2DM.
When we compared the parameters of periodontitis between patients with different durations of diabetes, we found that CAL values were higher in patients with long-duration diabetes. However, when patients with short-duration diabetes and moderate-to-severe coronary AS were compared to patients with long-duration diabetes and normal-to-mild coronary AS, the inter-group difference in CAL values disappeared (Table 4). Additionally, the results revealed that, among patients with short-duration diabetes, individuals in the moderate-to-severe group had more tooth loss compared to those in the normal-to-mild group. However, there was no relationship between tooth loss and extent of coronary AS among patients with long-duration diabetes (Table 5).
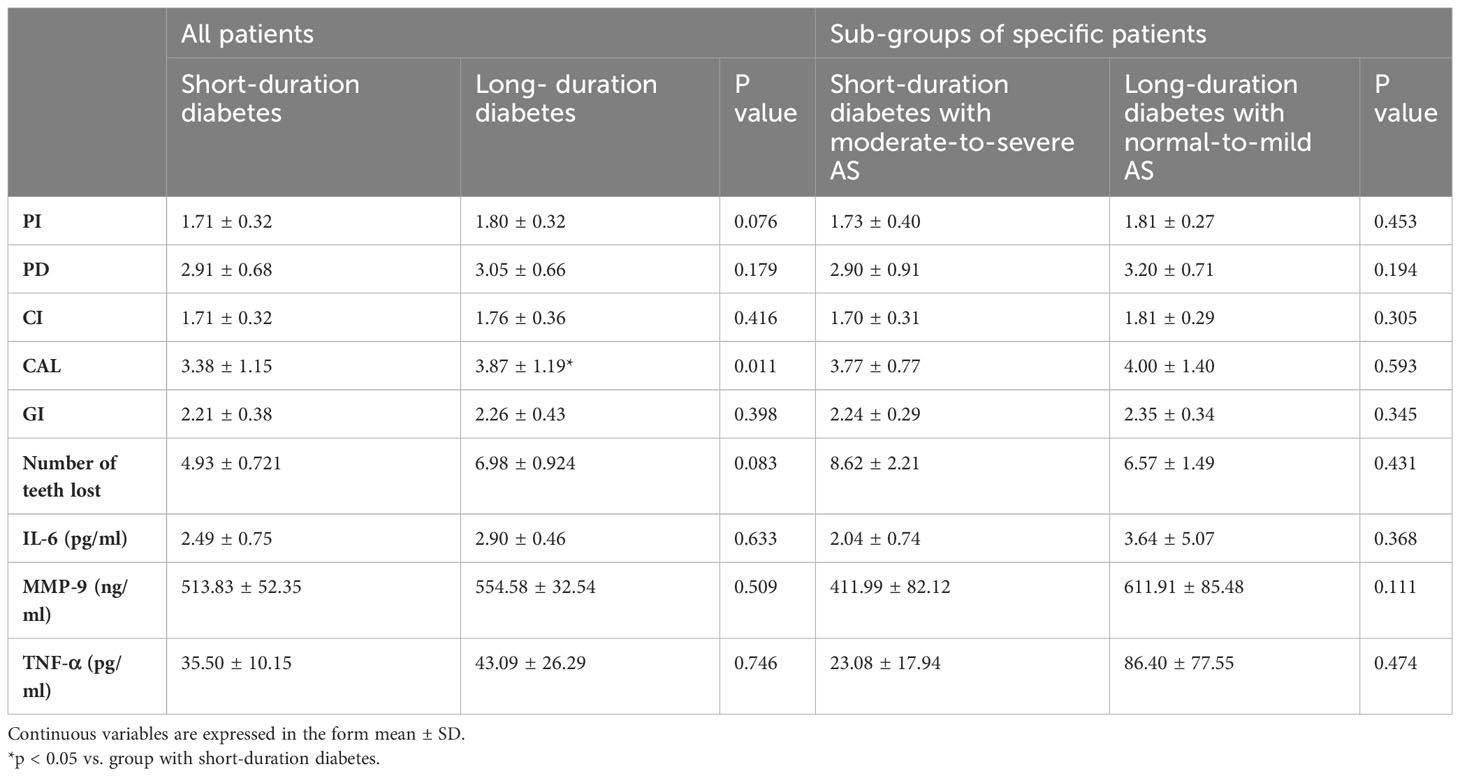
Table 4 Periodontitis parameters and salivary inflammatory markers in patients with short- vs. long-duration diabetes.
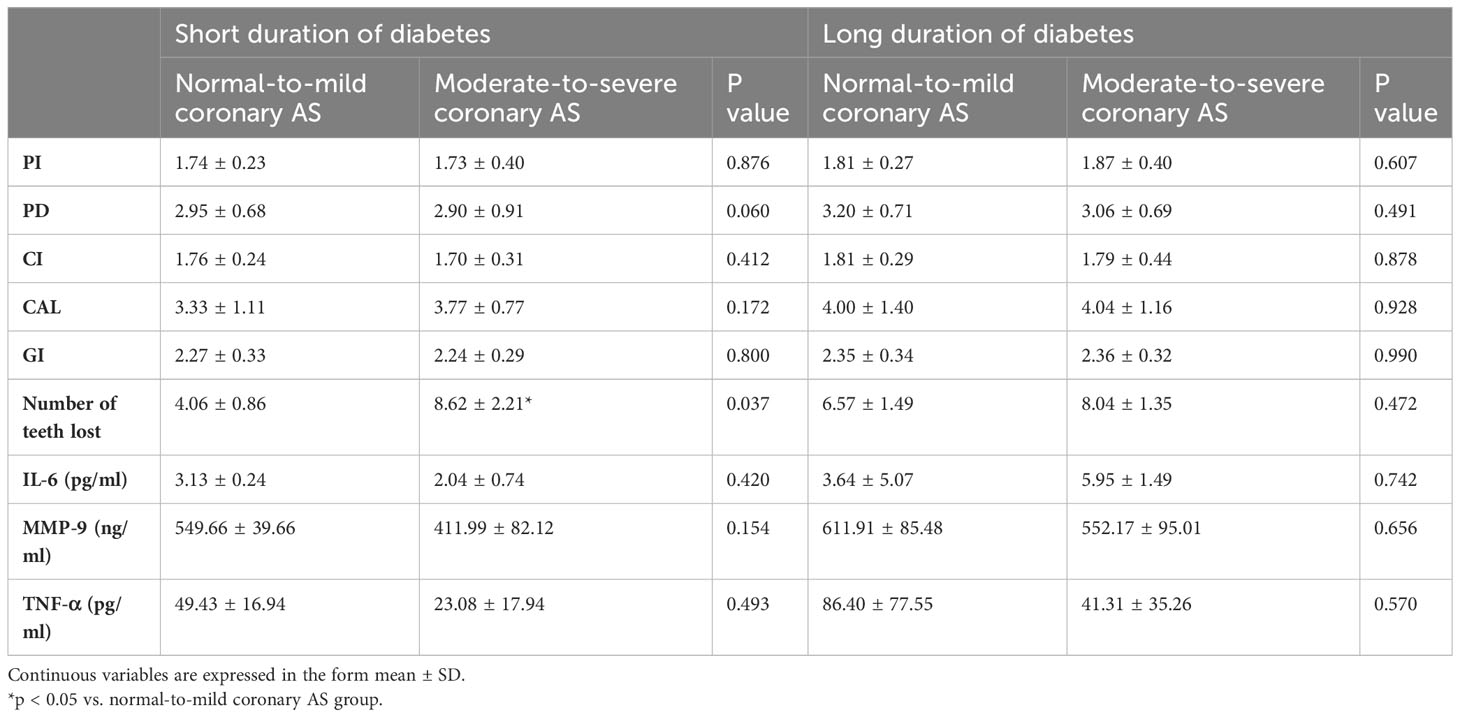
Table 5 Periodontitis parameters and salivary inflammatory markers in patients with short- vs. long-duration diabetes and different degrees of coronary atherosclerosis.
The results showed that older patients had higher CAL values than younger patients. However, among younger patients with moderate-to-severe coronary AS, all the parameters of periodontitis and tooth loss were higher than in older patients with normal-to-mild coronary AS, with no statistically significant differences (Table 6). Evaluating the results in younger patients with varying degrees of coronary AS, we observed that individuals with moderate-to-severe coronary AS had higher CAL and GI values, as well as more tooth loss, compared to individuals with normal-to-mild coronary AS. However, we did not observe this difference between the normal-to-mild and the moderate-to-severe group among older patients (Table 7).

Table 6 Periodontitis parameters and salivary inflammatory markers in patients in different age groups.
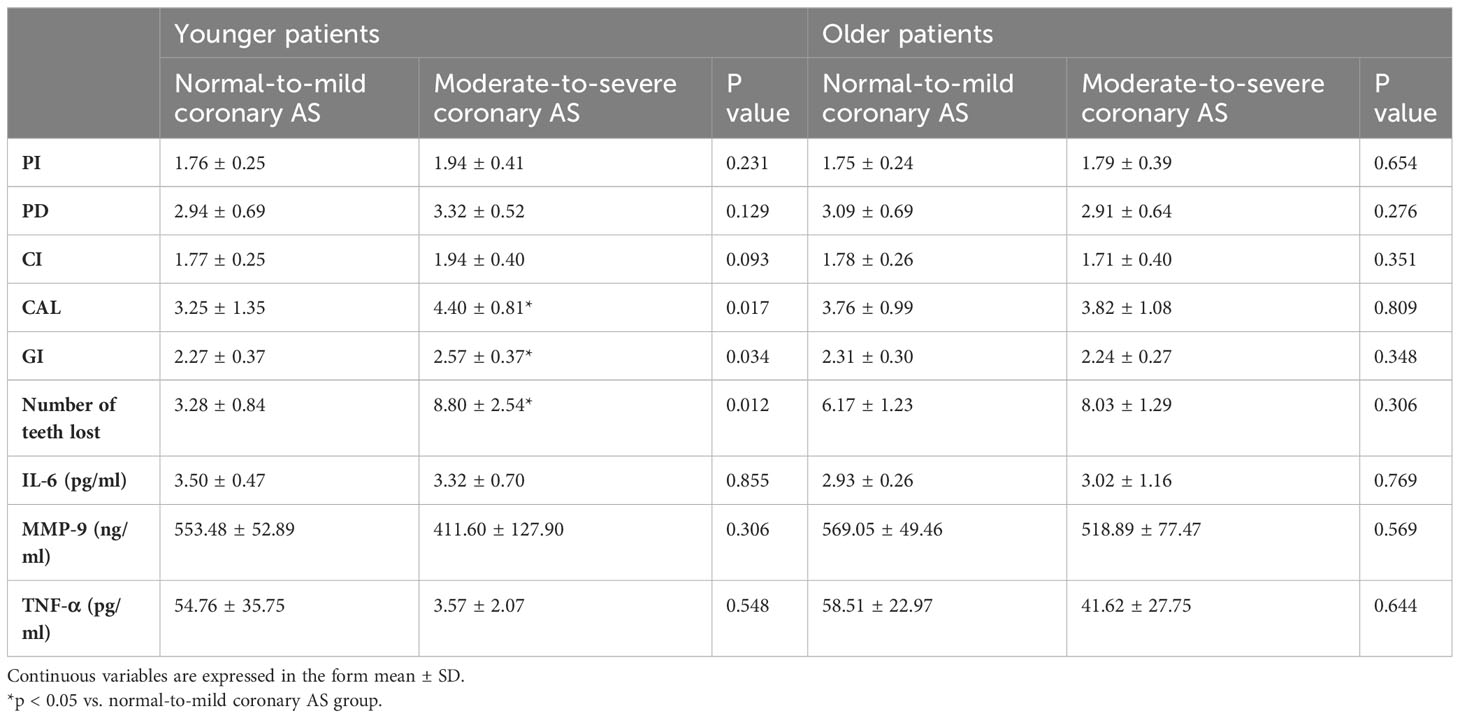
Table 7 Periodontitis parameters and salivary inflammatory markers in older and younger patients with different degrees of coronary atherosclerosis.
The CAC scores of patients in different severity staging groups for periodontitis are presented in Figure 1. CAC scores were higher in the severe group (436.17 ± 162.424) than in the mild group (122.3 ± 69.394), with the difference reaching statistical significance (P=0.043).

Figure 1 The CAC scores of patients grouped by severity of periodontitis. *p <0.05 vs. the mild group.
Alpha diversity indices indicated significant differences in Shannon index and Richness between the two coronary AS groups (p = 0.037 and p = 0.05, respectively), indicating higher abundance and greater diversity in the normal-to-mild group (Figures 2A, B).
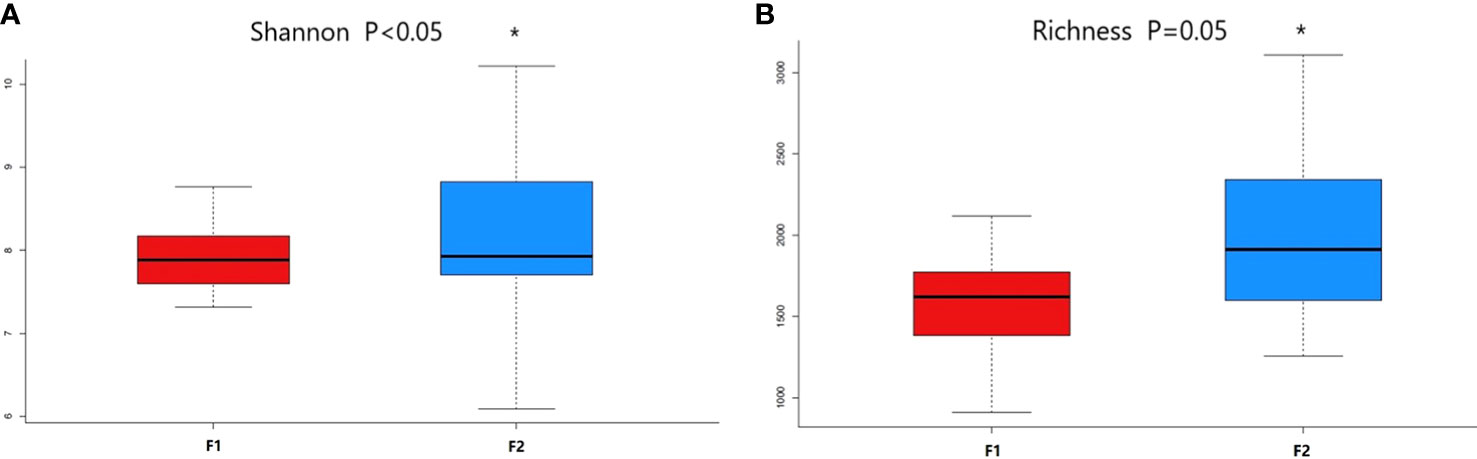
Figure 2 Comparison of alpha diversity metrics of plasma bacterial communities between the normal-to-mild and moderate-to-severe coronary AS groups. (A) Boxplots for comparison of species richness between the two study groups (Richness). (B) Boxplots for comparison of species diversity (Shannon index). (F1: moderate-to-severe coronary AS group; F2: normal-to-mild coronary AS group). * P≤0.05 vs F1 group.
The predominant taxa identified in the dental plaque samples of oral microbiota from the normal-to-mild and moderate-to-severe coronary AS groups at different taxonomic levels are shown in Figure 3. At the phylum level, Firmicutes, Proteobacteria, Bacteroidetes, and Actinobacteria accounted for the majority of the total sequences (Figure 3A). Levels of Actinobacteria were significantly lower in the moderate-to-severe coronary AS group (p =0.045, Mann–Whitney U test). At the class level, Bacteroidia, Betaproteobacteria, Bacilli, Clostridia, Alphaproteobacteria, Negativicutes, Chitinophagia, Gammaproteobacteria, Actinomycetia, and Fusobacteriia were the ten most common (Figure 3B), among which, levels of Actinomycetia were consistently more abundant in the normal-to-mild coronary AS group (p =0.035, Mann–Whitney U test). At the order level, Bacteroidales, Lactobacillales, and Neisseriales were predominant (Figure 3C). At the family level, the predominant families were Neisseriaceae, Streptococcaceae, Prevotellaceae, Chitinophagaceae, Veillonellaceae, and Porphyromonadaceae (Figure 3D), among which, levels of Porphyromonadaceae were more abundant in the moderate-to-severe coronary AS group (p =0.016, Mann–Whitney U test). At the genus level, Neisseria, Streptococcus, Prevotella, Veillonella, and Porphyromonas were predominant (Figure 3E). Porphyromonas levels were significantly higher in the moderate-to-severe coronary AS group (p =0.017, Mann–Whitney U test). Finally, at the species level, the predominant species were Neisseria subflava, Sediminibacterium magnilacihabitans, Streptococcus mitis_AV, Pseudoclostridium thermosuccinogenes, Veillonella dispar, and Porphyromonas gingivalis (Figure 3F). Among these, the abundance of Porphyromonas gingivalis differed significantly between the normal-to-mild and the moderate-to-severe coronary AS groups (p =0.042, Mann–Whitney U test). These results indicated distinctive differences in the bacterial communities of the groups with different degrees of coronary AS.
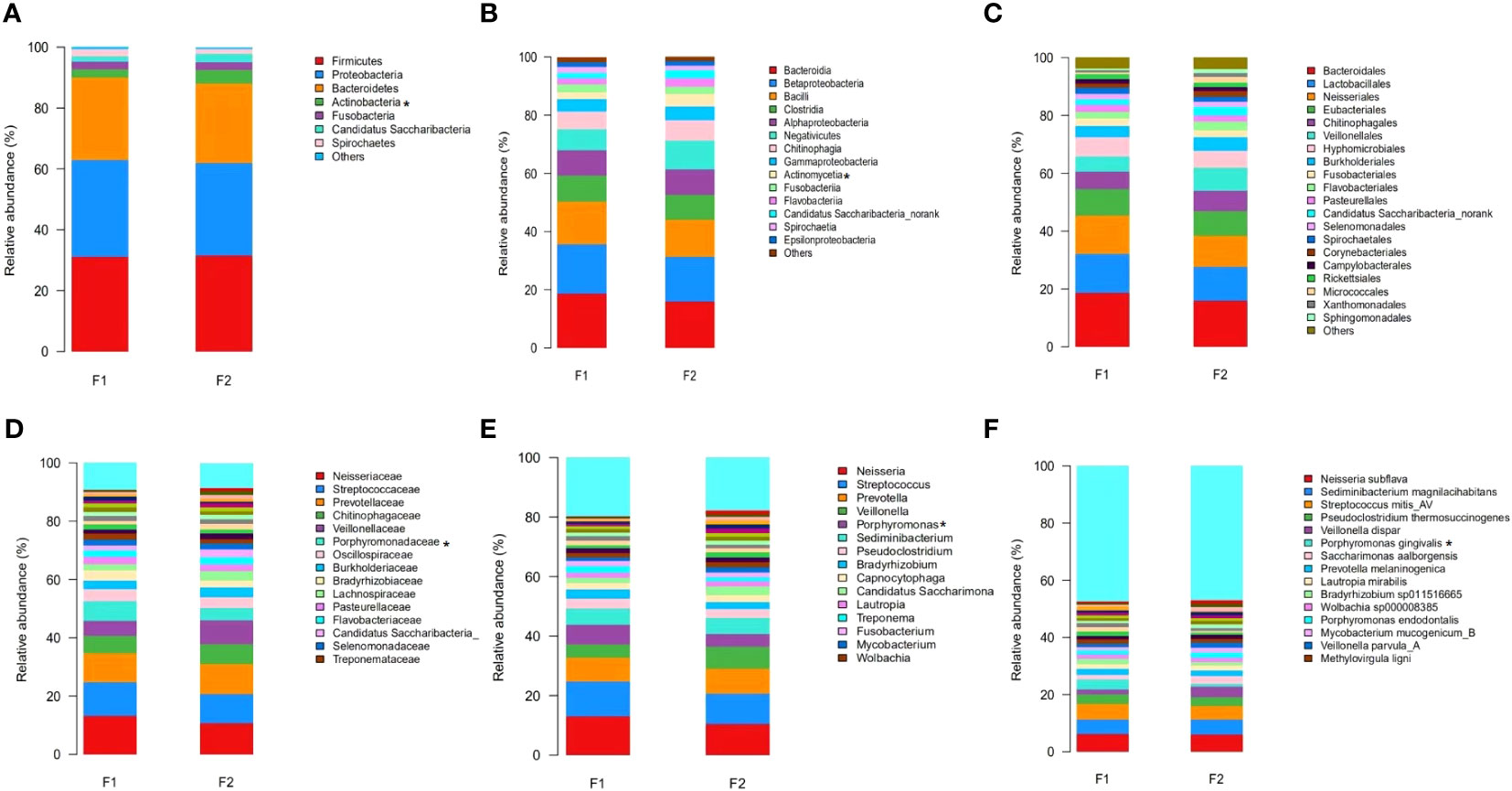
Figure 3 Relative abundance distribution at the level of phylum (A), class (B), order (C), family (D), genus (E), and species (F) in the normal-to-mild and moderate-to-severe coronary AS groups. (F1: moderate-to-severe coronary AS group; F2: normal-to-mild coronary AS group.) *p<0.05, F1 vs. F2.
To identify the specific bacterial taxa of oral microbiota that were associated with the moderate-to-severe group, we compared the microbial composition of the two groups using the LEfSe method. A cladogram representative of the structure of the oral microbiota and the predominant bacteria is shown in Figure 4A, with indications of the largest differences in the taxa represented in the two communities. The levels of members of bacterial taxa belonging to Porphyromonadaceae, Porphyromonas, Porphyromonas gingivalis, and Neisseria_mucosa were increased in the moderate-to-severe coronary AS group, and those of Actinomycetia, Rothia_dentocariosa, and Blautia coccoides were enriched in the normal-to-mild group; thus, these could be used as biomarkers for discrimination between the groups. The LDA bar graph (log 10) for discrimination is presented in Figure 4B.
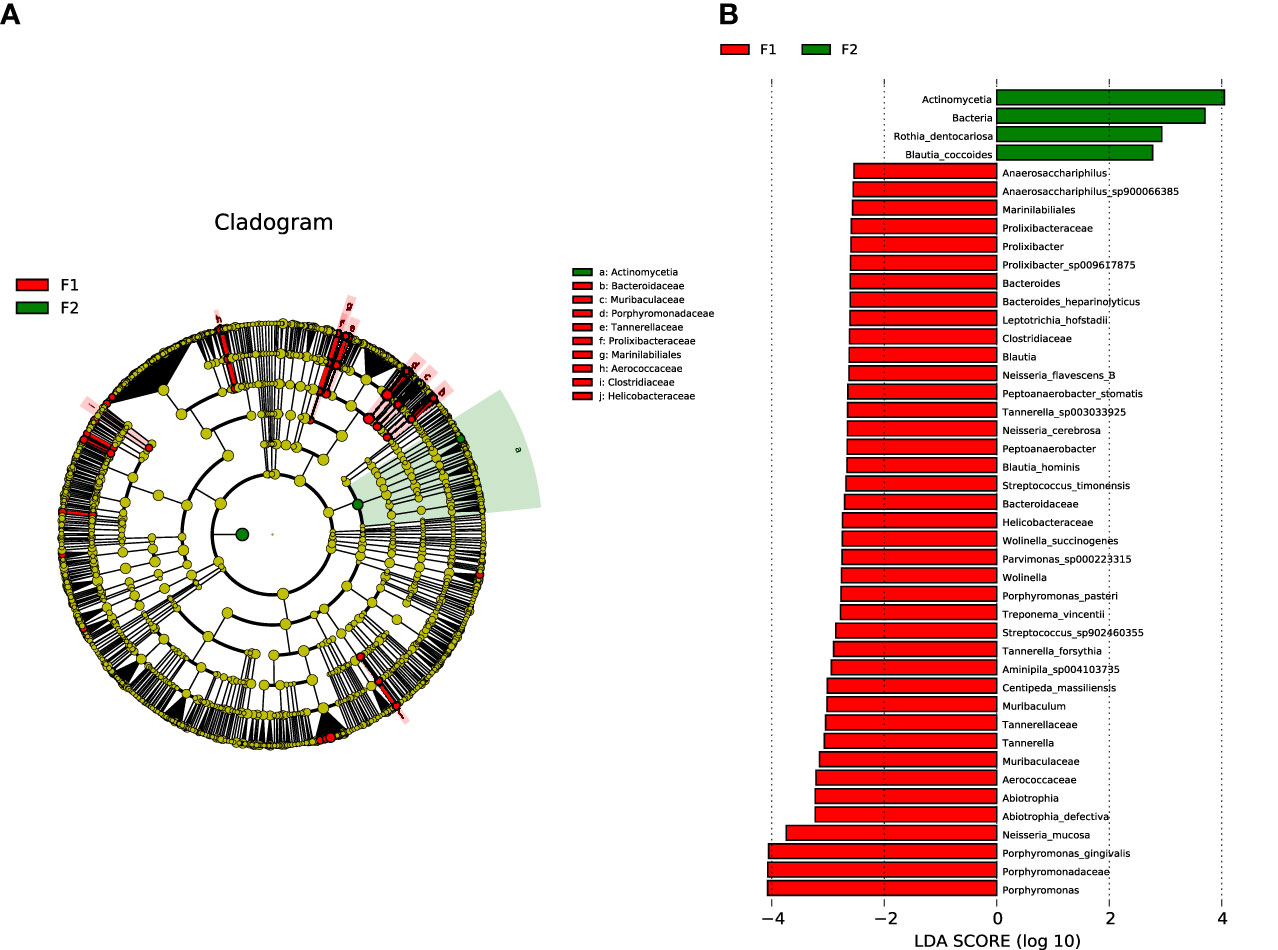
Figure 4 Analysis of species diversity in the normal-to-mild and moderate-to-severe coronary AS groups. (A) Cladistic map of microbial evolution. (B) Histogram of LDA value distribution. In the evolutionary branching diagram, circles radiating from the inside out indicate taxonomic levels from phylum to genus (or species). Each small circle for a different taxon class represents the taxa of that class, and the diameter of the small circle is proportional to the relative abundance. Species with no significant differences are yellow; red nodes represent the microbiota playing an important role in the moderate-to-severe group; and green nodes represent the microbiota playing an important role in the normal-to-mild group. Bar length in the histogram indicates the size of the impact of different species (LDA score). (F1: moderate-to-severe coronary AS group; F2: normal-to-mild coronary AS group).
The outcomes of the present study indicated that there exists a significant association of periodontitis and tooth loss with the presence of moderate-to-severe coronary atherosclerosis in patients with T2DM. Notably, we found that the nature of this association varies with sex, age, and duration of diabetes.
A number of other analyses have reported previously on the relationship between periodontitis and CVDs (47, 48). These studies have focused primarily on the risk of myocardial infarction or stroke and the prevalence of coronary heart disease in relation to periodontitis, whereas we have used the extent of atherosclerosis as the primary outcome. To the best of our knowledge, this is also the first study to identify a relationship between periodontitis and the extent of coronary atherosclerosis evaluated by CAC scores in patients with T2DM. Our findings will be beneficial in reducing cardiac deaths in patients with T2DM through the identification of individuals with a higher risk of incident cardiovascular events for early intervention.
Previous reports have similarly supported a relationship between severity staging of periodontitis and cardiovascular disease (13–15, 49) or sub-atherosclerosis (50, 51). In contrast, here we did not observe an association between severity staging of periodontitis and extent of coronary AS. This conflicting result may be due to variation in the criteria used for periodontitis severity staging and cardiovascular disease. Our results also reported sex differences. Our findings suggest that women with moderate periodontitis and men with severe periodontitis should be screened for moderate or severe coronary atherosclerosis in order to prevent cardiovascular events. The sex differences reported here might be attributable to the fact that women may have comparably less severe periodontitis because of their lower smoking rate. Another reason may be that postmenopausal women are prone to coronary AS. A recent publication has reported on sex differences in the relationship of periodontal disease with clinical end-points (52). Therefore, our data support this report with respect to coronary AS in patients with T2DM.
Among all the parameters of periodontitis that we examined in this study, CAL levels merit the most attention. CAL mainly represents the severity of periodontitis, while other parameters (such as PD, PI, and GI, among others) indicate the complexity of management (53). Our results indicated that the absolute value of CAL was more meaningful in indicating the extent of coronary AS in patients with T2DM than other parameters of periodontitis or the severity staging of periodontitis.
Beck et al. emphasized that the cumulative effect of periodontal infection can be evaluated by measuring the number of teeth lost (54). Based on our results, we suggest that tooth loss could be used as a marker of the extent of coronary AS in T2DM by almost any healthcare worker. Our results are consistent with studies showing an association between tooth loss and CVDs (55–58). It was a little unexpected that our study indicated an association between 10–19 missing teeth and moderate-to-severe coronary AS, rather than 20–31 missing teeth. Although this is not currently very clear, it may be attributable to the fact that patients with 20–31 missing teeth may have more confounding factors, such as older age, longer duration of diabetes, or a higher prevalence of smoking or statin use (data not shown), which could affect both periodontitis and CVDs. Our results suggested that particular attention should be paid to CVDs in patients suffering from diabetes with ≥10 missing teeth.
The present study also demonstrated the influence of age and duration of diabetes on the association between periodontitis and coronary AS. Our results indicated that clinicians should pay more attention to CAL levels and missing teeth in younger patients or patients with short duration of diabetes. Age is a risk factor for both periodontitis and coronary AS. When we examined the specific group of younger patients with moderate-to-severe coronary AS, we found periodontal parameters indicating greater severity in comparison to those of older patients with normal and mild coronary AS. Although this difference was not statistically significant, it indicated at least that the younger patients with severe atherosclerosis were not better off in terms of periodontal status than older patients with mild atherosclerosis, despite having the advantage of age. Similar results were observed in patients with short duration of diabetes. Higher levels of CAL or more missing teeth in younger patients or patients with short-duration diabetes may be clues to the presence of moderate or severe coronary AS. We did not observe differences in CAL or number of missing teeth between the groups with different degrees of coronary AS among older patients or patients with long-duration diabetes. This is in accordance with previous reports showing that the association between periodontitis and atherosclerotic changes is stronger in younger people (16, 59, 60) and is sometimes not seen in older subjects (16, 61). One reasonable explanation for this may be the cumulative nature of periodontal disease and tooth loss, such that as subjects age there are fewer teeth available for periodontitis to act on (62).
Recently, there has been growing appreciation that saliva can reflect virtually the entire spectrum of health, from normality to disease states (63). Some saliva markers may be relevant for the prognosis, diagnosis, and management of periodontitis (64) and CVDs (65). The value of salivary biomarkers, such as inflammatory cytokines, ILs and TNFs, enzymes, and growth factors, in the diagnosis and monitoring of gingivitis and periodontitis has been verified (66). Our study also found that salivary IL-6 and TNF-α were higher in patients with T2DM and more severe coronary AS. However, we did not observe a statistically significant difference between groups when we divided patients into groups according to age, duration of diabetes, and extent of coronary AS. The reason for this may be that this study included a small number of participants. The findings of our study also suggested that salivary levels of IL-6 and TNF-α may be biomarkers of moderate-to-severe coronary AS in patients with T2DM. Although these results remain to be validated for clinical application, they suggest that saliva could be used to measure the levels of cardiometabolic risk markers in the near future; they also demonstrate its potential in the identification and management of diseases.
Bacteremia is frequent in periodontitis and provides a pathway for periodontal pathogens to access the arterial wall. Our study indicated that Porphyromonas gingivalis and Neisseria mucosa were increased in patients with diabetes and moderate-to-severe coronary AS. There is extensive evidence that P. gingivalis is particularly important as a potential microbial link between human periodontitis and atherosclerosis, as indicated by the correlation between circulating specific antibodies and risk of stroke (67) and cardiovascular disease (68–70). Our results are in agreement with these reports and demonstrate the important role of P. gingivalis in linking periodontitis and severe coronary atherosclerosis in patients with T2DM. The mechanisms underlying the role of P. gingivalis in connecting the triple axis of periodontitis, CVD, and T2DM are not very clear at present. One possible mechanism can be described as follows. P. gingivalis can increase the accumulation of reactive oxygen species (ROS), leading to oxidative stress (71). Oxidative stress contributes to damage to major cellular components, including DNA, proteins, and lipids. Autoantibodies may cross-react with oxidative epitopes, such as MDA, MAA, and AGE epitopes on sugars, OxLDL, oxPL, and P. gingivalis. These PAMPs and DAMPs may serve as bridges or links between periodontitis and atherosclerosis with T2DM (71, 72). Further research should be conducted to verify this hypothesis. Neisseria mucosa is one of the core bacterial groups in saliva and in supragingival and subgingival plaque (73). However, there has been no report of its role in atherosclerosis. It is not clear whether Neisseria mucosa contributes to the development of AS in patients with diabetes. Further studies should be conducted to determine its role in AS.
This study has several limitations that are inherent to cross-sectional analyses. The relationships reported here should not be interpreted as causal. Our cross-sectional study design lacked information on the time sequence of events and therefore did not permit the identification of causal relationships. Additionally, the sample size of this study was not large enough. In future, cohort studies will be required to determine the correlation between coronary atherosclerosis and periodontal disease based upon well-determined diagnostic criteria and to evaluate the effects of complicating variables for either atherosclerosis or periodontitis. Despite its advantages of being cost-effective and non-contrast, the use of CAC score as the primary outcome measure in this study may impose some limitations. Following important single-center and clinical registry studies, as well as large long-term population-based observational studies, clinical practice guidelines consider CAC scoring to be potentially useful as a way of improving cardiovascular risk assessment in asymptomatic individuals and as a guide for initiation or deferral of preventive therapies (74); however, the National Heart, Lung, and Blood Institute (NHLBI) only endorses its use in the study of subclinical cardiovascular disease. The prevalence of CAC has been found to differ by ethnicity, gender, and age. Taking this into consideration, the use of a CAC score cut-off of ≥75th percentile for age, sex, and race as a validated measure of atherosclerosis may to some extent be more precise than a cut-off value of CAC score ≥ 100 (75).
In conclusion, our data indicate that CAL and number of missing teeth are associated with extent of coronary atherosclerosis, especially in younger patients and patients with short-duration diabetes. Our results also suggest that saliva inflammatory factors may be biomarkers of moderate-to-severe coronary AS in patients with T2DM. Considering that CVDs are the primary cause of mortality and morbidity in patients with T2DM, the association between periodontitis and moderate-to-severe coronary AS may be of major importance and merits assessment as early as possible in dental clinics. Dentists may become more involved in the management of diabetic cardiovascular complications.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/sra/?term=SRP453053. The code can be found here: https://github.com/5798132/xulei.
The studies involving humans were approved by Human Research and Ethics Committee of Shanghai East Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
BF and LX contributed to the conception and design of the study. HL, XY, and ZL organized the database. MS and ZL performed the oral examinations. ZL performed the ELISA experiments. LX performed the statistical analysis. MS and LX wrote the first draft of the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (81870529).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AS, atherosclerosis; BMI, body mass index; BOP, bleeding on probing; CAC, coronary artery calcium; CAL, clinical attachment loss; CVD, cardiovascular disease; CI, calculus index; FPG, fasting plasma glucose; GI, gingival index; HDL-C, high-density lipoprotein cholesterol; HbA1c, glycosylated hemoglobin; IL, interleukin; LDL-C, low-density lipoprotein cholesterol; MMP-9, matrix metalloproteinase-9; PAD, peripheral arterial disease; PD, probing depth; PI, periodontal index; TNF-α, tumor necrosis factor-α; T2DM, type 2 diabetes mellitus; TC, total cholesterol; TGs, triglycerides.
1. Mattila KJ, Nieminen MS, Valtonen VV, Rasi VP, Kesäniemi YA, Syrjälä SL, et al. Association between dental health and acute myocardial infarction. BMJ (1989) 298:779–81. doi: 10.1136/bmj.298.6676.779
2. Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, et al. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation (1999) 100:1134–46. doi: 10.1161/01.cir.100.10.1134
3. Bascones-Martínez A, González-Febles J, Sanz-Esporrín J. Diabetes and periodontal disease. Review of the literature. Am J Dent (2014) 27:63–7.
4. Mattila KJ, Asikainen S, Wolf J, Jousimies-Somer H, Valtonen V, Nieminen M. Age, dental infections, and coronary heart disease. J Dent Res (2000) 79:756–60. doi: 10.1177/00220345000790020901
5. Schmidt MI, Duncan BB, Sharrett AR, Lindberg G, Savage PJ, Offenbacher S, et al. Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): a cohort study. Lancet (1999) 353:1649–52. doi: 10.1016/s0140-6736(99)01046-6
6. Rittie L, Berton A, Monboisse JC, Hornebeck W, Gillery P. Decreased contraction of glycated collagen lattices coincides with impaired matrix metalloproteinase production. Biochem Biophys Res Commun (1999) 264:488–92. doi: 10.1006/bbrc.1999.1519
7. Taylor GW, Borgnakke WS. Periodontal disease: associations with diabetes, glycemic control and complications. Oral Dis (2008) 14:204–5. doi: 10.1111/j.1601-0825.2008.01442.x
8. Taylor GW. Bidirectional interrelationships between diabetes and periodontal diseases: an epidemiologic perspective. Ann Periodontol (2001) 6:99–112. doi: 10.1902/annals.2001.6.1.99
9. Tsai C, Hayes C, Taylor GW. Glycemic control of type 2 diabetes and severe periodontal disease in the US adult population. Community Dent Oral Epidemiol (2002) 30:182–92. doi: 10.1034/j.1600-0528.2002.300304.x
10. Southerland JH, Moss K, Taylor GW, Beck JD, Pankow J, Gangula PR, et al. Periodontitis and diabetes associations with measures of atherosclerosis and CHD. Atherosclerosis (2012) 222:196–201. doi: 10.1016/j.atherosclerosis.2012.01.026
11. Savage A, Eaton KA, Moles DR, Needleman I. A systematic review of definitions of periodontitis and methods that have been used to identify this disease. J Clin Periodontol (2009) 36:458–67. doi: 10.1111/j.1600-051X.2009.01408.x
12. Houshmand M, Holtfreter B, Berg MH, Schwahn C, Meisel P, Biffar R, et al. Refining definitions of periodontal disease and caries for prediction models of incident tooth loss. J Clin Periodontol (2012) 39:635–44. doi: 10.1111/j.1600-051X.2012.01892.x
13. Berent R, Auer J, Schmid P, Krennmair G, Crouse SF, Green JS, et al. Periodontal and coronary heart disease in patients undergoing coronary angiography. Metabolism (2011) 60:127–33. doi: 10.1016/j.metabol.2009.12.016
14. Gotsman I, Lotan C, Soskolne WA, Rassovsky S, Pugatsch T, Lapidus L, et al. Periodontal destruction is associated with coronary artery disease and periodontal infection with acute coronary syndrome. J Periodontol (2007) 78:849–58. doi: 10.1902/jop.2007.060301
15. Dorn JM, Genco RJ, Grossi SG, Falkner KL, Hovey KM, Iacoviello L, et al. Periodontal disease and recurrent cardiovascular events in survivors of myocardial infarction (MI): the Western New York Acute MI Study. J Periodontol (2010) 81:502–11. doi: 10.1902/jop.2009.090499
16. Dietrich T, Jimenez M, Krall Kaye EA, Vokonas PS, Garcia RI. Age-dependent associations between chronic periodontitis/edentulism and risk of coronary heart disease. Circulation (2008) 117:1668–74. doi: 10.1161/CIRCULATIONAHA.107.711507
17. Akman PT, Fentoglu O, Yilmaz G, Arpak N. Serum plasminogen activator inhibitor-1 and tumor necrosis factor-alpha levels in obesity and periodontal disease. J Periodontol (2012) 83:1057–62. doi: 10.1902/jop.2011.110548
18. Spahr A, Klein E, Khuseyinova N, Boeckh C, Muche R, Kunze M, et al. Periodontal infections and coronary heart disease – Role of periodontal bacteria and importance of total pathogen burden in the coronary event and periodontal disease (CORODONT) study. Arch Intern Med (2006) 166:554–9. doi: 10.1001/archinte.166.5.554
19. Nguyen CM, Kim JW, Quan VH, Nguyen BH, Tran SD. Periodontal associations in cardiovascular diseases: The latest evidence and understanding. J Oral Biol Craniofac Res (2015) 5:203–6. doi: 10.1016/j.jobcr.2015.06.008
20. Pietiainen M, Kopra KAE, Vuorenkoski J, Salminen A, Paju S, Mäntylä P, et al. Aggregatibacter actinomycetemcomitans serotypes associate with periodontal and coronary artery disease status. J Clin Periodontol (2018) 45:413–21. doi: 10.1111/jcpe.12873
21. Liljestrand JM, Paju S, Buhlin K, Persson GR, Sarna S, Nieminen MS, et al. Lipopolysaccharide, a possible molecular mediator between periodontitis and coronary artery disease. J Clin Periodontol (2017) 44:784–92. doi: 10.1111/jcpe.12751
22. Qi M, Miyakawa H, Kuramitsu HK. Porphyromonas gingivalis induces murine macrophage foam cell formation. Microb Pathog (2003) 35:259–67. doi: 10.1016/j.micpath.2003.07.002
23. Lalla E, Lamster IB, Hofmann MA, Bucciarelli L, Jerud AP, Tucker S, et al. Oral infection with a periodontal pathogen accelerates early atherosclerosis in apolipoprotein E-null mice. Arterioscler Thromb Vasc Biol (2003) 23:1405–11. doi: 10.1161/01.ATV.0000082462.26258.FE
24. Li C, Lv Z, Shi Z, Zhu Y, Wu Y, Li L, et al. Periodontal therapy for the management of cardiovascular disease in patients with chronic periodontitis. Cochrane Database Syst Rev (2017) 11:CD009197. doi: 10.1002/14651858.CD009197.pub3
25. Montenegro MM, Ribeiro IWJ, Kampits C, Saffi MAL, Furtado MV, Polanczyk CA, et al. Randomized controlled trial of the effect of periodontal treatment on cardiovascular risk biomarkers in patients with stable coronary artery disease: Preliminary findings of 3 months. J Clin Periodontol (2019) 46:321–31. doi: 10.1111/jcpe.13085
26. Hamilton JA, Hasturk H, Kantarci A, Serhan CN, Van Dyke T. Atherosclerosis, periodontal disease, and treatment with resolvins. Curr Atheroscler Rep (2017) 19:57. doi: 10.1007/s11883-017-0696-4
27. Preshaw PM, Alba AL, Herrera D, Jepsen S, Konstantinidis A, Makrilakis K, et al. Periodontitis and diabetes: a two-way relationship. Diabetologia (2012) 55:21–31. doi: 10.1007/s00125-011-2342-y
28. Lalla E, Papapanou PN. Diabetes mellitus and periodontitis: a tale of two common interrelated diseases. Nat Rev Endocrinol (2011) 7:738–48. doi: 10.1038/nrendo.2011.106
29. Lockhart PB, Bolger AF, Papapanou PN, Osinbowale O, Trevisan M, Levison ME, et al. Periodontal disease and atherosclerotic vascular disease: does the evidence support an independent association?: a scientific statement from the American Heart Association. Circulation (2012) 125:2520–44. doi: 10.1161/CIR.0b013e31825719f3
30. Almas K, Al-Qahtani M, Al-Yami M, Khan N. The relationship between periodontal disease and blood glucose level among type II diabetic patients. J Contemp Dent Pract (2001) 2(4):18–25. doi: 10.5005/jcdp-2-4-1
31. Nishimura F, Murayama Y. Periodontal inflammation and insulin resistance—lessons from obesity. J Dent Res (2001) 80(8):1690–4. doi: 10.1177/00220345010800080201
32. Beck JD, Slade G, Offenbacher S. Oral disease, cardiovascular disease and systemic inflammation. Periodontol (2000) 23:110–20. doi: 10.1034/j.1600-0757.2000.2230111.x
33. Katz J, Marc H, Porter S, Ruskin J. Inflammation, periodontitis, and coronary heart disease. Lancet (2001) 358:1998. doi: 10.1016/S0140-6736(01)06989-6
34. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol (1990) 15:827–32. doi: 10.1016/0735-1097(90)90282-t
35. Rumberger JA, Brundage BH, Rader DJ, Kondos G. Electron beam computed tomographic coronary calcium scanning: a review and guidelines for use in asymptomatic persons. Mayo Clin Proc (1999) 74:243–52. doi: 10.4065/74.3.243
36. Patel MR, Calhoon JH, Dehmer GJ, Grantham JA, Maddox TM, Maron DJ, et al. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 appropriate use criteria for coronary revascularization in patients with stable ischemic heart disease: A report of the american college of cardiology appropriate use criteria task force, american association for thoracic surgery, american heart association, american society of echocardiography, american society of nuclear cardiology, society for cardiovascular angiography and interventions, society of cardiovascular computed tomography, and society of thoracic surgeons. J Am Coll Cardiol (2017) 69:2212–41. doi: 10.1016/j.jacc.2017.02.001
37. Tota-Maharaj R, Joshi PH, Budoff MJ, Whelton S, Zeb I, Rumberger J, et al. Usefulness of regional distribution of coronary artery calcium to improve the prediction of all-cause mortality. Am J Cardiol (2015) 115:1229–34. doi: 10.1016/j.amjcard.2015.01.555
38. Newman M, Takei H, Klokkevold P, Carranza F. Newman and Carranza’s Clinical Periodontology. 13th ed. USA: Elsevier (2018) p. 208–14.
39. Papapanou PN, Sanz M, Buduneli N, Dietrich T, Feres M, Fine DH, et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri- Implant Diseases and Conditions. J Clin Periodontol (2018) 45:S162–70. doi: 10.1002/JPER.17-0721
40. Germen M, Baser U, Lacin CC, Fıratlı E, İşsever H, Yalcin F. Periodontitis prevalence, severity, and risk factors: A comparison of the AAP/CDC case definition and the EFP/AAP classification. Int J Environ Res Public Health (2021) 18:3459. doi: 10.3390/ijerph18073459
41. Desvarieux M, Demmer RT, Rundek T, Boden-Albala B, Jacobs DR Jr, Papapanou PN, et al. Oral infections and vascular disease epidemiology study (INVEST). Relationship between periodontal disease, tooth loss, and carotid artery plaque: the Oral Infections and Vascular Disease Epidemiology Study (INVEST). Stroke (2003) 34:2120–5. doi: 10.1161/01.STR.0000085086.50957.22
42. Bäck M, Hlawaty H, Labat C, Michel JB, Brink C. The oral cavity and age: a site of chronic inflammation? PloS One (2007) 2:e1351. doi: 10.1371/journal.pone.0001351
43. Amato KR, Yeoman CJ, Kent A, Righini N, Carbonero F, Estrada A, et al. Habitat degradation impacts black howler monkey (Alouatta pigra) gastrointestinal microbiomes. ISME J (2013) 7:1344–53. doi: 10.1038/ismej.2013.16
44. Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol (2009) 75:7537–41. doi: 10.1128/AEM.01541-09
45. Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, et al. Metagenomic biomarker discovery and explanation. Genome Biol (2011) 12:R60. doi: 10.1186/gb-2011-12-6-r60
46. Ijaz MU, Ahmed MI, Zou X, Hussain M, Zhang M, Zhao F, et al. Beef, casein, and soy proteins differentially affect lipid metabolism, triglycerides accumulation and gut microbiota of high-fat diet-fed C57BL/6J mice. Front Microbiol (2018) 9:2200. doi: 10.3389/fmicb.2018.02200
47. DeStefano F, Anda RF, Kahn HS, Williamson DF, Russell CM. Dental disease and risk of coronary heart disease and mortality. BMJ (1993) 306:688–91. doi: 10.1136/bmj.306.6879.688
48. Paunio K, Impivaara O, Tiekso J, Mäki J. Missing teeth and ischaemic heart disease in men aged 45–64 years. Eur Heart J (1993) 14:54–6.
49. Holmlund A, Holm G, Lind L. Severity of periodontal disease and number of remaining teeth are related to the prevalence of myocardial infarction and hypertension in a study based on 4,254 subjects. J Periodontol (2006) 77:1173–8. doi: 10.1902/jop.2006.050233
50. Cairo F, Castellani S, Gori AM, Nieri M, Baldelli G, Abbate R, et al. Severe periodontitis in young adults is associated with sub-clinical atherosclerosis. J Clin Periodontol (2008) 35:465–72. doi: 10.1111/j.1600-051X.2008.01228.x
51. Beck JD, Elter JR, Heiss G, Couper D, Mauriello SM, Offenbacher S. Relationship of periodontal disease to carotid artery intima-media wall thickness: the atherosclerosis risk in communities (ARIC) study. Arterioscler Thromb Vasc Biol (2001) 21:1816–22. doi: 10.1161/hq1101.097803
52. Grau AJ, Becher H, Ziegler CM, Lichy C, Buggle F, Kaiser C, et al. Periodontal disease as a risk factor for ischemic stroke. Stroke (2004) 35:496–501. doi: 10.1161/01.STR.0000110789.20526.9D
53. Caton JG, Armitage G, Berglundh T, Chapple ILC, Jepsen S, Kornman KS, et al. A new classification scheme for periodontal and peri-implant diseases and conditions - Introduction and key changes from the 1999 classification. J Clin Periodontol (2018) 45 Suppl 20:S1–8. doi: 10.1111/jcpe.12935
54. Beck JD, Eke P, Heiss G, Madianos P, Couper D, Lin D, et al. Periodontal disease and coronary heart disease: a reappraisal of the exposure. Circulation (2005) 112:19–24. doi: 10.1161/CIRCULATIONAHA.104.511998
55. Zanella SM, Pereira SS, Barbisan JN, Vieira L, Saba-Chujfi E, Haas AN, et al. Periodontal disease, tooth loss and coronary heart disease assessed by coronary angiography: a cross-sectional observational study. J Periodontal Res (2016) 51:221–7. doi: 10.1111/jre.12301
56. Kodovazenitis G, Pitsavos C, Papadimitriou L, Vrotsos IA, Stefanadis C, Madianos PN. Association between periodontitis and acute myocardial infarction: a case-control study of a nondiabetic population. J Periodontal Res (2014) 49:246–52. doi: 10.1111/jre.12101
57. De Angelis F, Basili S, Giovanni F, Dan Trifan P, Di Carlo S, Manzon L. Influence of the oral status on cardiovascular diseases in an older Italian population. Int J Immunopath Pharm (2018) 32:394632017751786. doi: 10.1177/0394632017751786
58. Lee HO, Kim H-L, Jin KN, Oh S, Han YS, Jung DU, et al. Association between dental health and obstructive coronary artery disease: an observational study. BMC Cardiovasc Disord (2019) 19:98. doi: 10.1186/s12872-019-1080-9
59. Sim SJ, Kim HD, Moon JY, Zavras AI, Zdanowicz J, Jang SJ, et al. Periodontitis and the risk for non-fatal stroke in Korean adults. J Periodontol (2008) 79:1652–8. doi: 10.1902/jop.2008.080015
60. Jimenez M, Krall EA, Garcia RI, Vokonas PS, Dietrich T. Periodontitis and incidence of cerebrovascular disease in men. Ann Neurol (2009) 66:505–12. doi: 10.1002/ana.21742
61. Geismar K, Stoltze K, Sigurd B, Gyntelberg F, Holmstrup P. Periodontal disease and coronary heart disease. J Periodontol (2006) 77:1547–54. doi: 10.1902/jop.2006.050405
62. Elter JR, Champagne CM, Offenbacher S, Beck JD. Relationship of periodontal disease and tooth loss to prevalence of coronary heart disease. J Periodontol (2004) 75:782–90. doi: 10.1902/jop.2004.75.6.782
63. Segal A, Wong DT. Salivary diagnostics: enhancing disease detection and making medicine better. Eur J Dent Educ (2008) 12:22–9. doi: 10.1111/j.1600-0579.2007.00477.x
64. Teles RP, Likhari V, Socransky SS, Haffajee AD. Salivary cytokine levels in subjects with chronic periodontitis and in periodontally healthy individuals: a cross-sectional study. J Periodontal Res (2009) 4:411–7. doi: 10.1111/j.1600-0765.2008.01119.x
65. Floriano PN, Christodoulides N, Miller CS, Ebersole JL, Spertus J, Rose BG. Use of saliva-based nano-biochip tests for acute myocardial infarction at the point of care: a feasibility study. Clin Chem (2009) 55:1530–8. doi: 10.1373/clinchem.2008.117713
66. Ebersole JL, Nagarajan R, Akers D, Miller CS. Targeted salivary biomarkers for discrimination of periodontal health and disease(s). Front Cell Infect Microbiol (2013) 19:62. doi: 10.3389/fcimb.2015.00062
67. Pussinen PJ, Alfthan G, Jousilahti P, Paju S, Tuomilehto J. Systemic exposure to Porphyromonas gingivalis predicts incident stroke. Atherosclerosis (2007) 193:222–8. doi: 10.1016/j.atherosclerosis.2006.06.027
68. Pussinen PJ, Jousilahti P, Alfthan G, Palosuo T, Asikainen S, Salomaa V. Antibodies to periodontal pathogens are associated with coronary heart disease. Arterioscler Thromb Vasc Biol (2003) 23:1250–4. doi: 10.1161/01.ATV.0000072969.71452.87
69. Pussinen PJ, Alfthan G, Tuomilehto J, Asikainen S, Jousilahti P. High serum antibody levels to Porphyromonas gingivalis predict myocardial infarction. Eur J Cardiovasc Prev Rehabil (2004) 11:408–11. doi: 10.1097/01.hjr.0000129745.38217.39
70. Bohnstedt S, Cullinan MP, Ford PJ, Palmer JE, Leishman SJ, Westerman B, et al. High antibody levels to P. gingivalis in cardiovascular disease. J Dent Res (2010) 89:938–42. doi: 10.1177/0022034510370817
71. Turunen SP, Kummu O, Harila K, Veneskoski M, Soliymani R, Baumann M, et al. Recognition of Porphyromonas gingivalis gingipain epitopes by natural IgM binding to malondialdehyde modified low-density lipoprotein. PloS One (2012) 7:e34910. doi: 10.1371/journal.pone.0034910
72. Ambrogini E, Que X, Wang S, Yamaguchi F, Weinstein RS, Tsimikas S, et al. Oxidation-specific epitopes restrain bone formation. Nat Commun (2018) 9:2193. doi: 10.1038/s41467-018-04047-5
73. Yamashita Y, Takeshita T. The oral microbiome and human health. J Oral Sci (2017) 59:201–6. doi: 10.2334/josnusd.16-0856
74. Goff DC Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation (2014) 129:S49–73. doi: 10.2174/1570161118666200403143518
Keywords: periodontitis, tooth loss, diabetes, coronary atherosclerosis, CAC scores
Citation: Shen M, Li Z, Li H, Yan X, Feng B and Xu L (2023) Association of periodontitis and tooth loss with extent of coronary atherosclerosis in patients with type 2 diabetes mellitus. Front. Endocrinol. 14:1243992. doi: 10.3389/fendo.2023.1243992
Received: 21 June 2023; Accepted: 06 November 2023;
Published: 23 November 2023.
Edited by:
Razia Abdool Gafaar Khammissa, University of Pretoria, South AfricaReviewed by:
Robert Kiss, McGill University, CanadaCopyright © 2023 Shen, Li, Li, Yan, Feng and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo Feng, ZmVuZ2JvZGZ5eUB0b25namkuZWR1LmNu; Lei Xu, c2hpcmxleV9vazFAMTI2LmNvbQ==
†These authors have contributed equally to this work and share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.