
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 29 September 2023
Sec. Reproduction
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1243402
This article is part of the Research Topic Recurrent Pregnancy Loss and Endocrine Dysfunction View all 10 articles
Objective: The objective of this study was to examine the influence of repeated embryo implantation failures on pregnancy outcomes among patients under 40 years of age undergoing in vitro fertilization/intracytoplasmic sperm injection embryo transfer (IVF/ICSI-ET).
Materials and methods: A retrospective analysis was conducted on the clinical data of 13,172 patients who underwent 16,975 IVF/ICSI-ET treatment cycles at Henan Reproductive Hospital between January 1, 2015, and December 31, 2018. Patients were categorized into four groups based on the number of previous embryo implantation failure cycles: Group A=no implantation failure, Group B= 1 implantation failure, Group C=2 implantation failures, Group D=≥3 implantation failures. Baseline characteristics and pregnancy outcomes were compared among the four groups. The impact of the number of previous embryo implantation failures on pregnancy outcomes among IVF/ICSI-ET patients was investigated using univariate and multiple regression analyses.
Results: Univariate logistic regression analysis demonstrated that factors such as the number of previous embryo implantation failures, female age, basal follicle count, endometrial thickness, total number of oocytes retrieved, type of cycle, number of high-quality embryos transferred, and stage of embryo development significantly affected implantation rate, clinical pregnancy rate, early spontaneous abortion rate, and live birth rate (all P < 0.05). The duration of infertility and anti-Mullerian hormone (AMH) levels were also found to influence implantation rate, clinical pregnancy rate, and live birth rate (all P < 0.05). Upon conducting multivariate logistic regression analysis and adjusting for confounding factors such as age, AMH levels, basal follicle count, endometrial thickness, total number of oocytes obtained, cycle type, number of high-quality embryos transferred, ovarian stimulation protocol, and stage of embryo development, it was revealed that, compared to Group A, Groups B, C, and D exhibited significantly lower implantation and live birth rates, as well as a significantly higher risk of early spontaneous abortion (all P < 0.05).
Conclusions: The number of previous embryo implantation failures is an independent factor affecting implantation rate, clinical pregnancy rate, spontaneous abortion rate and live birth rate of patients underwent IVF/ICSI-ET. With the increase of the number of previous embryo implantation failures, the implantation rate, clinical pregnancy rate and live birth rate of patients underwent IVF/ICSI-ET decreased significantly, and the rate of early spontaneous abortion gradually increased.
Infertility has increasingly become a significant global public health and sociological issue that profoundly impacts human development and health (1). The World Health Organization reports that infertility affects approximately 8–14% of women of reproductive age in western developed countries, with the prevalence rising to 25–30% in some developing regions, such as Africa and the Middle East (2). Currently, the infertile population in China exceeds 40 million, with an incidence of 12.5% among women of childbearing age (3). With the rapid advancement of assisted reproductive technology (ART), the implantation rate for a single high-quality blastocyst transfer has improved to as high as 65% (4). The in vitro fertilization/intracytoplasmic sperm injection-embryo transfer (IVF/ICSI-ET) technique has helped numerous infertile families achieve a healthy baby. However, approximately 10% of these individuals are unable to attain a clinical pregnancy even after three or more embryo transfers (5). Despite facing considerable emotional and financial strain, most of these patients still desire to continue their IVF/ICSI-ET treatment in hopes of increasing the “take-home-baby” rate.
Numerous factors influence the embryo implantation rate, including embryo chromosomal abnormalities, diminished endometrial receptivity, asynchrony between embryo and endometrium, maternal endocrine disorders, and thrombotic tendencies. The number of previous embryo implantation failures also plays an essential role in the embryo implantation rate (6). As the number of previous embryo implantation failures rises, the financial and psychological burden on patients grows as well. These individuals require clinicians to help predict the success probability of subsequent embryo transfer cycles based on the number of previous embryo implantation failures. However, the impact of the number of previous embryo implantation failures on the pregnancy outcome of the next IVF/ICSI-ET for infertile patients remains a contentious issue (7–11). Consequently, our study aimed to investigate the effect of the number of prior embryo implantation failures on the pregnancy outcome of the subsequent IVF/ICSI-ET treatment for patients aged <40 years.
The present study analyzed the clinical data of 16,975 cycles of IVF/ICSI from 13,172 patients who were recruited from the Center of Henan Reproductive Hospital between January 2015 and December 2018. The inclusion criteria comprised the presence of indications for IVF/ICSI-ET and the absence of contraindications, in addition to an age < 40 years. The exclusion criteria included incomplete data or loss to follow-up, ultrasound-identified abnormal uterine structure, such as single or bicornuate uterine diaphragm, chromosomal abnormalities in either partner, previous pregnancy from IVF/ICSI-ET cycles, and the use of donor eggs or sperm. The IVF/ICSI-ET cycles were categorized into four groups according to the number of previous embryo implantation failures: Group A included 13172 cycles without any implantation failures; Group B included 2989 cycles with one embryo implantation failure; Group C included 658 cycles with two embryo implantation failures; and Group D included 156 cycles with three or more embryo implantation failures. The Ethics Committee of Henan Provincial People’s Hospital approved the study (No. (28) Len Audit (2022).
The appropriate protocol for controlled ovulation was selected based on the patient’s age, body mass index (BMI), basal follicle count, basal sex hormone levels, anti-müllerian hormone (AMH) levels, and previous IVF/ICSI-ET protocols. Patients underwent controlled ovulation stimulation (COS) and the dosage of gonadotropin was adjusted according to follicular size and hormonal levels. When the follicular diameter was ≥18 mm in three or more follicles, 4000–10000 IU of human chorionic gonadotropin (hCG) (Lizhu Pharmaceutical Company, China) was administered to induce ovulation. Oocyte retrieval guided by vaginal ultrasound was performed 36–37 h later. Routine luteal support was performed after oocyte retrieval, and one to two available embryos were transferred on either the third or fifth day after oocyte retrieval.
Patients with regular menstrual cycles and normal ovulation underwent natural cycle preparation of the endometrium. Transvaginal ultrasound was used to monitor follicular development and endometrial condition starting from day 11 of the menstrual cycle. Endometrial transformation occurred on the day of ovulation, and the endometrium was transformed 3 or 5 days before the transfer of cleavage-stage or blastocyst-stage embryos, respectively. For patients with irregular menstruation, hormone replacement therapy was employed using estradiol valerate (Progynova, 1 mg/tablet, Bayer, Germany). Oral administration of 4–8 mg/day was started on day 2–4 of the menstrual cycle. The dose of Progynova was maintained when the endometrial thickness was ≥8 mm, and the duration of administration was ≥11 days, while luteal support was given at the same time. After progesterone was administered, embryos were transferred on the third or fifth day. Endometrial transformation was performed on day 4 or 6 for the transfer of cleavage-stage or blastocyst-stage embryos, respectively.
Luteal support was provided through daily administration of vaginal progesterone gel (Crinone, 90 mg/unit, Merck & Serono) at a dosage of 90 mg, along with a once-daily oral dose of 20 mg dydrogesterone. The estrogen and progesterone doses remained unchanged after embryo transfer until blood β-hCG levels were measured on the 14th day post-embryo transfer. In case of pregnancy (β-hCG > 50 U/L), the original luteal support was maintained and the dosage was gradually reduced until the 10th week of pregnancy.
Clinical pregnancy was defined as the presence of an intrauterine gestational sac, confirmed by vaginal ultrasonography 4–6 weeks after embryo transfer. Spontaneous miscarriage was defined as pregnancy termination before 28 weeks of gestation with a fetal weight of less than 1000g. When spontaneous miscarriage occurs before 12 weeks of gestation, it is considered early pregnancy loss. Newborns delivered after 28 weeks of gestation and surviving are considered live births.
Other observed indicators included age, duration of infertility, BMI, type of infertility, cycle type, stage of embryo development, number(No.) of embryos transferred, endometrial thickness, No. of good-quality embryos, AMH levels, antral follicle count (AFC), infertility factors, and ovulation protocol.
Statistical analysis was conducted using Empower Stats software based on R language. Continuous variables were presented as mean ± standard deviation (SD), and categorical variables were presented as N (%). Intergroup comparisons were performed using one-way analysis of variance (ANOVA) and χ2 tests for categorical variables. Univariate regression analysis was used to identify potential factors affecting pregnancy outcomes. Furthermore, multivariate Logistic regression model with generalized estimating equation (GEE) was chosen to conduct univariate and multivariate analysis of the association of effect of the number of previous embryo implantation failures on pregnancy outcomes to account for the correlation between cycles from the same patient and to obtain ORs and 95% CIs for the risk of the number of implantation failures associated with pregnancy outcomes, while adjusting for confounding factors. A value of P < 0.05 was considered statistically significant.
A total of 13,172 patients who underwent 16,957 IVF/ICSI cycles were recruited and categorized into four groups based on the number of previous embryo implantation failures. The patient demographics and characteristics are presented in Table 1. The four groups exhibited significant differences in terms of age, duration of infertility, AMH levels, AFC, endometrial thickness, number of eggs retrieved, stage of embryo development, infertility factors, No. of high-quality embryos transferred, cycle type, and ovarian stimulation protocol (P < 0.05). However, no significant differences were observed in BMI, type of infertility, No. of embryos transferred, or No. of quality embryos transferred among the groups (P > 0.05).
The pregnancy outcomes of the four groups are presented in Table 2. The implantation rate, clinical pregnancy rate, and live birth rate exhibited significant decreases with an increase in the number of previous embryo implantatfailures (P<0.001). Conversely, the rate of early spontaneous abortion increased significantly (P<0.001).
The univariate logistic regression analysis revealed that the number of previous embryo implantation failures, female age, AFC, endometrial thickness, total number of eggs obtained, cycle type, No. of high-quality embryos transferred, and stage of embryo development had an impact on the implantation rate, clinical pregnancy rate, early spontaneous abortion rate, and live birth rate (P < 0.05). The factors affecting implantation, clinical pregnancy, and live birth rates were duration of infertility, AMH levels, and AFC (P < 0.05). (Table 3)
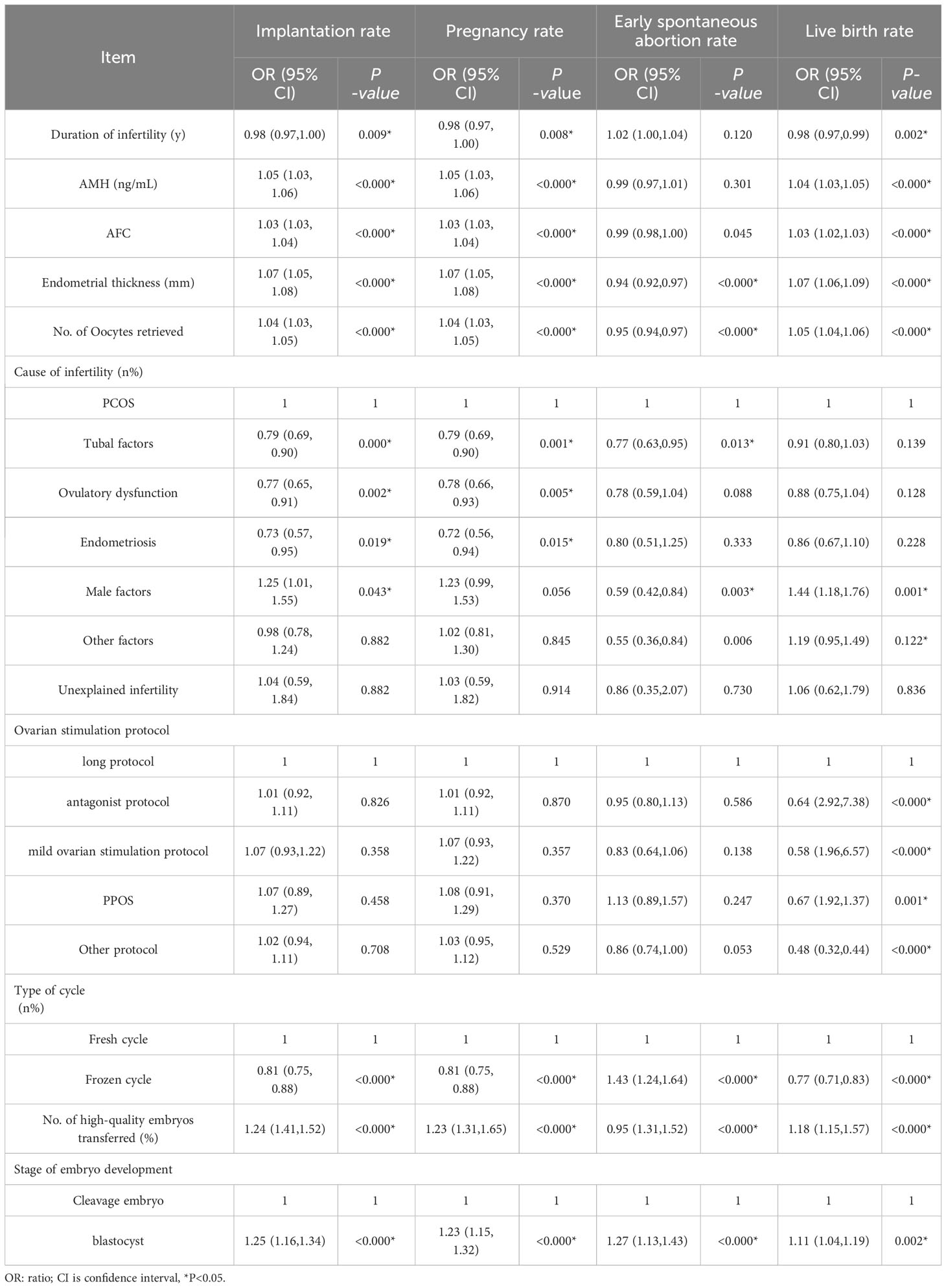
Table 3 Univariate analysis of clinical outcomes affecting the number of previous embryo implantation failures.
Multiple logistic regression was performed to analyze the impact of the number of previous embryo implantation failures on the implantation, clinical pregnancy, early spontaneous abortion, and live birth rates after IVF/ICSI-ET, while adjusting for confounding factors such as age, duration of infertility, AMH levels, AFC, endometrial thickness, total number of oocytes retrieved, infertility factors, embryonic developmental stage, and cycle type. The implantation rate, clinical pregnancy rate, and live birth rate of patients in groups B, C, and D exhibited a gradual decrease compared to group A, while early spontaneous abortion rates showed a significant increase. (Table 4)
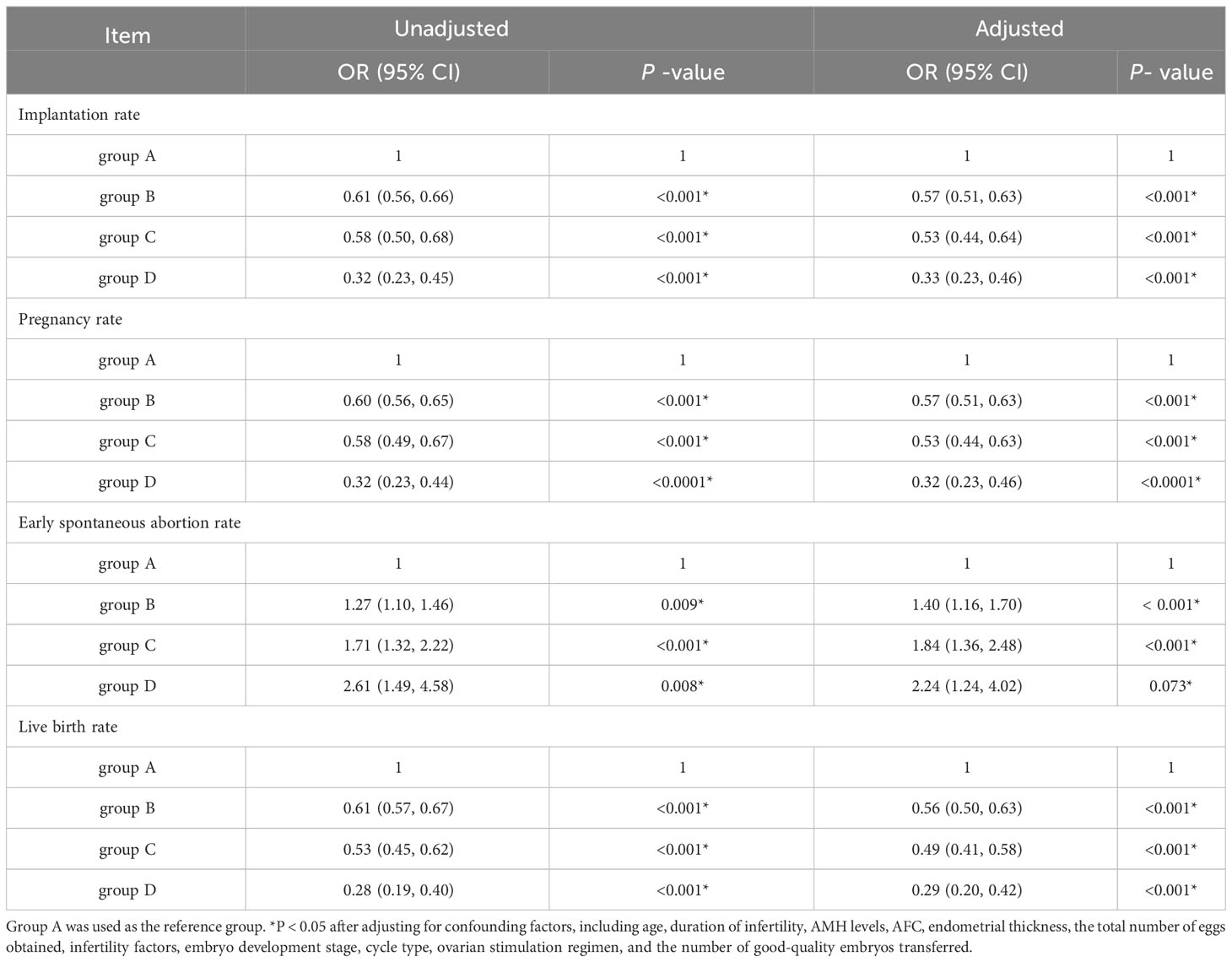
Table 4 Multifactorial logistic regression analysis of the number of previous embryo implantation failures on pregnancy outcome of IVF/ICSI-ET patients.
The impact of the number of previous implantation failures on pregnancy outcomes remains a controversial topic that requires further investigation. Cimadomo Danilo et al (12) reported in an observational study that the embryo implantation rate and early spontaneous abortion rate in the first four cycles of IVF/ICSI-ET treatment could not be predicted based on the number of previous embryo implantation failures. However, they found that the live birth rate decreased significantly in patients with a history of ≥3 implantation failures compared to those who underwent their first IVF/ICSI cycle. A retrospective cohort study in Israel reported that the live birth rate per IVF/ICSI-ET cycle decreased significantly with the increasing number of embryo transfer cycles. The study found that the live birth rate was almost zero in patients who underwent the fourth embryo transfer cycle, suggesting that infertile patients who did not achieve a clinical pregnancy with four consecutive embryo transfer cycles should discontinue treatment or consider alternative options such as donor eggs and sperm (7). Conversely, a large multicenter study by Andrew D. A. C. Smith et al (8) showed that the success rate of IVF/ICSI-ET tended to decrease with the number of embryo transfer cycles until the ninth cycle, after which live birth was almost impossible. They support the notion that, although the live birth rate decreased after 3–4 cycles of repeated treatment, there was still a possibility of live birth. Wang et al. (10) found no significant correlation between clinical pregnancy rate and the number of the first three transfer cycles, but observed significantly lower implantation and clinical pregnancy rates from the fourth embryo transfer cycle. The inconsistencies among these studies may be due to differences in study population characteristics, inclusion and exclusion criteria, quality of transferred embryos, level of assisted reproductive technology in each country, and sample size. Our findings demonstrate that the number of previous embryo implantation failures is an independent factor affecting implantation rate, clinical pregnancy rate, spontaneous abortion rate and live birth rate of patients underwent IVF/ICSI-ET. With the increase of the number of previous embryo implantation failures, the implantation rate, clinical pregnancy rate and live birth rate of patients underwent IVF/ICSI-ET decreased significantly, and the rate of early spontaneous abortion increased markedly.
Embryo implantation is a crucial step in the success of IVF/ICSI-ET treatment. However, recurrent embryo implantation failure (RIF) is a challenging condition that hinders the improvement of clinical pregnancy rates in these patients. Diagnostic and therapeutic challenges arise due to the diverse etiologies of RIF. Improving embryo implantation rates has become a major challenge in improving the clinical outcomes of IVF/ICSI-ET patients. Therefore, further research on RIF patients is necessary. The definition of RIF lacks a unified international standard. Currently, most experts accept the criteria based on the patient’s age, the number of failed IVF/ICSI cycles, and the number of good-quality embryos transferred (13). In China, the 2023 expert consensus defines RIF as the failure to achieve clinical pregnancy after transferring at least three good-quality embryos in three fresh or frozen cycles (14). The etiology of embryo implantation failure is complex, diverse, and partially unknown (15). Some studies have reported that the number of previous embryo implantation failures is an independent risk factor affecting implantation rates (5, 8, 9, 12). However, other studies have suggested that patient outcomes in IVF/ICSI cycles are not significantly related to the number of embryo transfer cycles (16). In our study, we found that patients without any implantation failures had significantly higher implantation rates, clinical pregnancy rates, and live birth rates, and a lower rate of early spontaneous abortion compared to those in the other three groups. The pregnancy outcomes were comparable between one and two previous embryo implantation failure with no significant differences in implantation rate, clinical pregnancy rate, and live birth rate (see attachment for details). However, the outcomes of patients with ≥3 previous implantation failures were worse than those of the other three groups of patients.
It is well known that age is the most critical factor affecting the development of oocytes and the quality of embryos. As female age increases, the decline in ovarian function and the increased probability of aneuploidy, she is prone to embryo developmental delay and stagnation, which can lead to embryo implantation failure, and the implantation rate decreases gradually (17, 18). We selected patients less than 40 years old and adjusted age by multiple logistic regression to avoid the impact of age on pregnancy outcomes. Impaired endometrial receptivity is also an important factor that causes embryo implantation failure (19). Therefore, patients underwent hysteroscopy after the first cycle of implantation failure to exclude interference from endometrial polyps, uterine adhesions, and chronic endometritis on implantation rates (20).
The success of ART largely depends on the quality of the embryo and the receptivity of the endometrium. In this study, the implantation rate, the clinical pregnancy rate and the live birth rate of patients underwent first embryo transfer cycle were 49.97%, 64.46% and 54.78% respectively, which was a higher rate of successful pregnancy compared to those who had previous failed cycles, which is similar to Shapiro B S study (21). Patients with one or two previous embryo implantation failures exhibited comparable pregnancy outcomes, while patients with three or more previous failures showed significantly lower rates of embryo implantation, clinical pregnancy, and live birth. Patients with three or more previous failures was RIF patients, whose infertility is often caused by multiple factors, including maternal endocrine and immune disorders and thrombophilia (22). Our cohort did not undergo etiology-related investigations and treatments for RIF patient. However, we have since put in place etiologic screening and appropriate treatment for patients with four or more embryo transfer cycles. Our findings highlight the need for further research into RIF patients, as the etiology of this condition is complex and not well understood. Therefore, patients with ≥3 previous implantation failures are recommended to continue subsequent cycles only after examination and treatment to improve pregnancy outcomes.
Spontaneous abortion is a common complication of pregnancy in obstetrics and gynecology. It is known that spontaneous abortion after IVF/ICSI-ET-assisted clinical pregnancy reduces the live birth rate. However, it is uncertain how the number of previous embryo implantation failures affects the rate of spontaneous abortion after assisted clinical pregnancy (10, 23). Previous studies have yielded conflicting results regarding the impact of the number of embryo transfer cycles on the rate of spontaneous abortion after clinical pregnancy in IVF/ICSI-assisted conception. Some studies have shown no significant change in the spontaneous abortion rate as the number of cycles increased, while others have demonstrated an increased risk of spontaneous abortion in patients with multiple embryo transfer cycles, which partially supports the results of our study (24, 25). In our study, we found that the early spontaneous abortion rate was lowest in the first embryo transfer group. Patients with ≥3 embryo implantation failures had a significantly higher early spontaneous abortion rate of 28.07%, which may be due to the fact that these patients are RIF population, and the etiology of RIF and recurrent spontaneous abortion (RSA) is similar (26). The etiology of RSA involves chromosomal or genetic abnormalities, anatomical abnormalities, autoimmune diseases, prethrombotic state (PTS), endocrine factors, infectious factors, male factors, and psychological factors (27). However, the etiological examination and correction were not carried out in our study for patients with ≥3 embryo implantation failures, which may explain the high risk of spontaneous abortion in these patients.
However, there are some limitations in this study. First, our study was a single-center, retrospective study and there might be some confounders that we did not control for. In addition, we prioritized some pregnancy outcomes and did not investigate neonatal outcomes. In the future, our findings need to be validated by expanding the sample size or by high-quality randomized clinical trial studies.
In conclusion, the number of previous embryo implantation failures is an independent factor affecting implantation rate, clinical pregnancy rate, spontaneous abortion rate and live birth rate of patients underwent IVF/ICSI-ET. Based on our study, patients with ≥3 previous implantation failures are recommended to undergo etiology-related investigations and treatments to continue subsequent cycles to improve pregnancy outcomes. Investigations for RIF includes: 1. General risk factors such as old age, poor lifestyle, smoking, etc. 2. Immune factors include autoimmunity and alloimmunity; 3. Prethrombotic states include hereditary and acquired thrombophilia; 4 Endometrial receptivity test 5. Factors of infection; 6 Reproductive anatomy; 7. Endocrine factors; 8. Male factor; 9. Chromosomes. Treatments of RIF: 1. General treatment such as weight control, healthy diet and appropriate exercise; 2.IVF-ET program optimization; 3. Regulation of immune disorders (glucocorticoids, low molecular weight heparin, immunoglobulin, etc.); 4. Low molecular weight heparin for treatment of pre-thrombotic state; 5. Doxycycline and metronidazole for chronic endometritis; 6. Treatment of submucosal myoma, polyps and hydrosalpinx and other normal anatomical structure abnormalities; 7. The man controls his weight, reduces smoking, etc. 8. Encourage both couples or those with chromosome abnormalities to perform PGT treatment.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Henan Medical Ethics Committee of Provincial People’s Hospital. The patients/participants provided their written informed consent to participate in this study.
YF and HQ conception and design, review and final approval of the version to be published. FJ, CT, XH analyses the data. YF and HQ draft and revise the article. YF and FJ collect and analyze the data. All authors contributed to the article and approved the submitted version.
This study was supported by the Science and Technology Project of Henan Province (182102310134).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Inhorn MC, Patrizio P. Infertility around the globe: new thinking on gender, reproductive technologies, and global movements in the 21st century0]. Hum ReprodUpdate (2015) 21(4):411–26. doi: 10.1093/humupd/dmv016
2. Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA, et al. National, regional, and global trends in infertility prevalence since 1990:a systematic analysis of 277 health surveys. PloS Med (2012) 9(12):e1001356. doi: 10.1371/journal.pmed.1001356
3. Sun H, Gong TT, Jiang YT, et al. Global, regional, and national prevalence and disability-adjusted life-years for infertility in 195 countries and territories, 1990-2017: results from a global burden of disease study, 2017. Aging (Albany NY) (2019) 11(23):10952–91. doi: 10.18632/aging.102497
4. Mao X, Zhang J, Chen Q, Kuang Y, Zhang S, et al. Short-term copper intrauterine device placement improves the implantation and pregnancy rates in women with repeated implantation failure. Fertil Steril (2017) 108(1):55–61 e1. doi: 10.1016/j.fertnstert.2017.05.014
5. Cimadomo D, Craciunas L, Vermeulen N, Vomstein K, Toth B. Definition, diagnostic and therapeutic options in recurrent implantation failure: an international survey of clinicians and embryologists. Hum Reprod (2021) 36(2):305–17. doi: 10.1093/humrep/deaa317
6. Wang Y, Tian Y, Liu L, Li TC, Tong X, Zhu H, et al. The number of previous failed embryo transfer cycles is an independent factor affecting implantation rate in women undergoing IVF/ICSI treatment: A retrospective cohort study. Med(Baltim) (2021) 100(9):e25034. doi: 10.1097/MD.0000000000025034
7. Simonstein F, Mashiach-Eizenberg M, Revel A, Younis JS, et al. Assisted reproduction policies in Israel: a retrospective analysis of in vitro fertilization-embryo transfer. Fertil Steril (2014) 102(5):1301–6. doi: 10.1016/j.fertnstert.2014.07.740
8. Smith ADAC, Tilling K, Nelson SM, Lawlor DA, et al. Live-birth rate associated with repeat in vitro fertilization treatment cycles. J Am Med Assoc (2015) 314(24):2654–62. doi: 10.1001/jama.2015.17296
9. Xu XL, Xu YY, Chen CH, Li J, Lu Y, Zh LH, et al. Impact of miscarriage in the first complete cycle of human assisted reproductive technology pregnancy on the outcome of subsequent cycles of assisted conception. J Zhengzhou Univ (Medical Edition) (2022) 057–001).:78–82. doi: 10.13705/j.issn.1671–6825.2021.09.053
10. Wang F, Sun H-X, Wang J-X, Zhang NY, Hu YL, et al. Pregnancy outcomes in repeated cycles of in vitro fertilization-embryo transfer. Chin J Male Sci (2010) 16(11):1007–11. doi: 10.13263/j.cnki.nja.2010.11.02
11. Homburg R, Meltcer S, Rabinson J, Scharf S, Anteby EY, Orvieto R, et al. Is there a limit for the number of in vitro fertilization cycles for an individual patient? Fertil Steril (2009) 91(4 Suppl):1329–31. doi: 10.1016/j.fertnstert.2008.03.010
12. Danilo C, Antonio C, Lisa D, Tacconi L, Soscia D, Giancani A, et al. Leave the past behind: women’s reproductive history shows no association with blastocysts’ euploidy and limited association with live birth rates after euploid embryo transfers. Hum Reprod 4(4):929–40. doi: 10.1093/humrep/deab014
13. Coughlan C, Ledger W, Wang Q, Liu F, Demirol A, Gurgan T, et al. Recurrent implantation failure: definition and management. Reprod BioMed Online (2014) 28(1):14–38. doi: 10.1016/j.rbmo.2013.08.011
14. Chinese Physicians Association of Reproductive Medicine, Chinese Women Physicians Association of Reproductive Medicine. Chinese expert consensus on the clinical management of recurrent implantation failure. Chin Med J (2023) 103(2):89–100. doi: 10.3760/cma.j.cn112137
15. Wang XP. New perspectives on the etiology and treatment of recurrent miscarriage and recurrent implantation failure. Chin J Obstetrics Gynecology (2019) 54(12):4. doi: 10.3760/cma.j.issn.0529–567x.2019.12.001
16. Silberstein T, Trimarchi JR, Gonzalez L, Keefe DL, Blazar AS, et al. Pregnancy outcome in in vitro fertilization decreases to a plateau with repeated cycles. Fertil Sterility (2005) 84(4):1043–5. doi: 10.1016/j.fertnstert.2005.04.026
17. Hatirnaz S, Ozer A, Hatirnaz E, Atasever M, Başaranoglu S, Kanat-Pektas M, et al. Preimplantation genetic screening among women experiencing recurrent failure of in vitro fertilization. Int J GYNECOL OBSTET (2017) 137(3):314–8. doi: 10.1002/ijgo.12135
18. May-Panloup P, Boucret L, Chao de la Barca JM, Desquiret-Dumas V, Ferré-L'Hotellier V, Morinière C, et al. Ovarian ageing: the role of mitochondria in oocytes and follicles. Hum Reprod Update (2016) 22(6):725–43. doi: 10.1093/humupd/dmw028
19. Bashiri A, Halper KI, Orvieto R. Recurrent Implantation Failure-update overview on etiology, diagnosis,treatment and future directions. Reprod Biol Endocrinol (2018) 16(1):121. doi: 10.1186/s12958–018–0414–2
20. Kuroda K, Horikawa T, Moriyama A, Nakao K, Juen H, Takamizawa S, et al. Impact of chronic endometritis on endometrial receptivity analysis results and pregnancy outcomes. Immunity Inflammation Dis (2020) 8(4):650–8. doi: 10.1002/iid3.354
21. Shapiro BS, Richter KS, Harris DC, Daneshmand ST, et al. Dramatic declines in implantation and pregnancy rates in patients who undergo repeated cycles of in vitro fertilization with blastocyst transfer after one or more failed attempts. Fertil Steril (2001) 76(3):538–42. doi: 10.1016/s0015–0282(01)01979–3
22. Franasiak JM, Alecsandru D, Forman EJ, Gemmell LC, Goldberg JM, Llarena N, et al. A review of the pathophysiology of recurrent implantation failure. Fertil Steril (2021) 116(6):1436–48. doi: 10.1016/j.fertnstert.2021.09.014
23. Zhang HZ, Xiong F, Li GG, Sun Q, Chen PL, Wan CY, et al. Clinical outcomes after repeated vitrification of frozen-thawed embryos for transfer. J Reprod Med (2018) 27(7):5. doi: 10.3969/.jissn.1004-3845
24. Pirtea P, De Ziegler D, Tao X, Zhan Y, Ayoubi JM, Seli E, et al. Rate of true recurrent implantation failure is low: results of three successive frozen euploid single embryo transfers. Fertil Steril (2021) 115(1):45–53. doi: 10.1016/j.fertnstert.2020.07.002
25. Yang R, Yang S, Li R, Chen X, Wang H, Ma C, et al. Biochemical pregnancy and spontaneous abortion in first IVF cycles are negative predictors for subsequent cycles: an over 10,000 cases cohort study. Arch Gynecol Obstet (2015) 292(2):453–8. doi: 10.1007/s00404–015–3639–8
26. Polanski LT, Baumgarten MN, Quenby S, Brosens J, Campbell BK, Raine-Fenning NJ, et al. What exactly do we mean by ‘recurrent implantation failure’? A systematic review and opinion. Reprod BioMed Online (2014) 28(4):409–23. doi: 10.1016/j.rbmo.2013.12.006
27. Obstetrics and Gynecology Section of the Chinese Medical Association, Expert Consensus Group on the Diagnosis and Treatment of Recurrent Miscarriage. Expert consensus on the diagnosis and treatment of recurrent miscarriage (2022). Chin J Obstetrics Gynecology (2022) 57(9):653–67. doi: 10.3760/cma.j.cn112141-20220421-00259
Keywords: IVF/ICSI, implantation rate (IR), Live birth rate (LBR), pregnancy outcome, recurrent implantation failure (RIF)
Citation: Fang Y, Jingjing F, Tiantain C, Huanhuan X and Qiaohua H (2023) Impact of the number of previous embryo implantation failures on IVF/ICSI-ET pregnancy outcomes in patients younger than 40 years: a retrospective cohort study. Front. Endocrinol. 14:1243402. doi: 10.3389/fendo.2023.1243402
Received: 20 June 2023; Accepted: 31 August 2023;
Published: 29 September 2023.
Edited by:
Federico Jensen, University of Buenos Aires, ArgentinaReviewed by:
Alicia Motta, University of Buenos Aires, ArgentinaCopyright © 2023 Fang, Jingjing, Tiantain, Huanhuan and Qiaohua. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: He Qiaohua, aHFodWFhYWFAMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.