- 1Department of Urology, Xiangya Hospital, Central South University, Changsha, China
- 2National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, China
- 3Department of Internal Medicine, Section Endocrinology, Yale University School of Medicine, New Haven, CT, United States
Background and objective: The early identification of modifiable risk factors is important for preventing kidney stones but determining causal associations can be difficult with epidemiological data. We aimed to genetically assess the causality between modifiable factors (lifestyle factors, serum parameters, and metabolic comorbidities) and the risk of kidney stones. Additionally, we aimed to explore the causal impact of education on kidney stones and its potential mediating pathways.
Methods: We conducted a two-sample Mendelian randomization (MR) study to explore the causal association between 44 modifiable risk factors and kidney stones. The FinnGen dataset initially explored the causal relationship of risk factors with kidney stones and the UK Biobank dataset was used as the validation set. Then, a meta-analysis was conducted by combining discovery and validation datasets. We used two-step MR to assess potential mediators and their mediation proportions between education and kidney stones.
Results: The combined results indicated that previous exposures may increase the risk of kidney stones, including sedentary behavior, urinary sodium, the urinary sodium/potassium ratio, the urinary sodium/creatinine ratio, serum calcium, 25-hydroxyvitamin D (25OHD), the estimated creatinine-based glomerular filtration rate (eGFRcrea), GFR estimated by serum cystatin C (eGFRcys), body mass index (BMI), waist circumference, type 2 diabetes mellitus (T2DM), fasting insulin, glycated hemoglobin, and hypertension. Coffee intake, plasma caffeine levels, educational attainment, and the urinary potassium/creatinine ratio may decrease the risk of kidney stones. Ranked by mediation proportion, the effect of education on the risk of kidney stones was mediated by five modifiable risk factors, including sedentary behavior (mediation proportion, 25.7%), smoking initiation (10.2%), BMI (8.2%), T2DM (5.8%), and waist circumference (3.2%).
Conclusion: This study provides MR evidence supporting causal associations of many modifiable risk factors with kidney stones. Sedentary lifestyles, obesity, smoking, and T2DM are mediating factors in the causal relationship between educational attainment and kidney stones. Our results suggest more attention should be paid to these modifiable factors to prevent kidney stones.
Introduction
Kidney stones is a common disease, with an overall prevalence of 1.7–14.8%, and appears to be increasing in nearly all countries (1). The annual incidence of new cases is estimated to be 15–20 per 10,000 people, 25% of whom need hospitalization (2, 3). The annual cost is predicted to double from 2 billion (2000) by 2030 in the USA (4). Unfortunately, the recurrence rate of kidney stones is up to 50% within 10 years (5). Kidney stones have been associated with a 10-year risk of a future atherosclerotic cardiovascular disease event (6). Recent population studies have found symptomatic kidney stone formers to be at increased risk for chronic and end-stage kidney disease (7, 8). Therefore, early identification and treatment of risk factors for kidney stones can help reduce the medical and financial burden.
Several lifestyle factors have been reported to be associated with kidney stones, such as smoking (9, 10), drinking (11), coffee consumption (12), sleep duration (13) and physical activity (14). Previous studies suggested that some serum and urine parameters are predictors of kidney stones, including the urinary sodium/potassium ratio (15), serum calcium (16), 25-hydroxyvitamin D (25OHD) (16), C-reactive protein (CRP) (17), urate (18), testosterone (19), estradiol (20), and lipids (21). In addition, metabolic comorbidities are highly associated with kidney stones, including obesity (22), hypertension (23), dyslipidemia (24), and diabetes (23). However, it remains difficult to measure the causal relationship between these factors and the formation of urinary stones because of limited studies, potential residual confounders, and reverse causalities. Therefore, it is of great important to disentangle whether these modifiable factors are the causations of the development of kidney stones.
Mendelian randomization (MR) is an emerging method that can be used to explore the causal relationship in the presence of potential confounders and reverse causations as it uses genetic variants as instrumental variables (IVs) and genetic variants are randomly assigned at conception (25). However, only a few risk factors of MR studies have been established to be associated with kidney stones. Here, we conducted a two-sample MR study to explore the causal relationship between kidney stones and 44 modifiable risk factors categorized as lifestyle factors, serum and urine parameters, and metabolic comorbidities. Furthermore, we conducted a meta-analysis based on the only extensive genome-wide association studies (GWASs) associated with kidney stones. Additionally, we aimed to explore the causal impact of education on kidney stones and its potential mediating pathways.
Methods
MR design
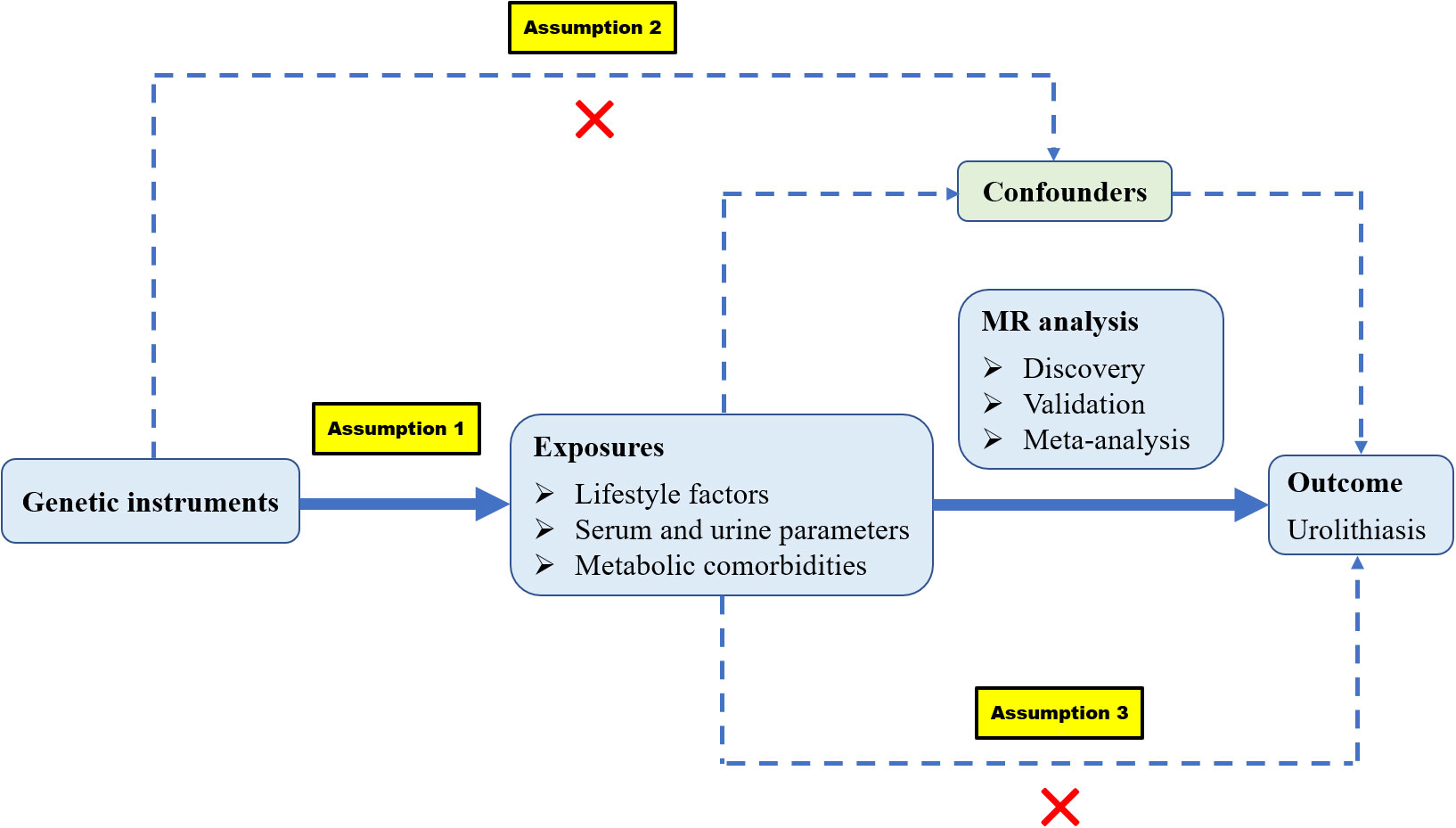
The following are the three assumptions of MR: (1) genetic variants are robustly associated with risk factors; (2) genetic variants are not associated with any confounders; and (3) genetic variants should affect the outcome merely through the risk factors (Figure 1). We identified 44 modifiable risk factors that may be associated with kidney stones. These risk factors can be categorized into three groups: (1) lifestyle factors, including diet, sleep habits, physical activity, and education levels; (2) serum and urine parameters, including serum micronutrients, biochemical indices, inflammatory indices, lipid traits, sex hormones, and urinary ion excretion; and (3) metabolic comorbidities, including obesity traits, type 2 diabetes mellitus (T2DM) and related traits, hypertension and related traits, and cardiovascular diseases. The FinnGen dataset was initially used to explore the causal relationship of risk factors with kidney stones and the UK Biobank (UKBB) dataset was used as the validation set. Then, a meta-analysis was conducted by combining discovery and validation datasets.
Selection of genetic variants
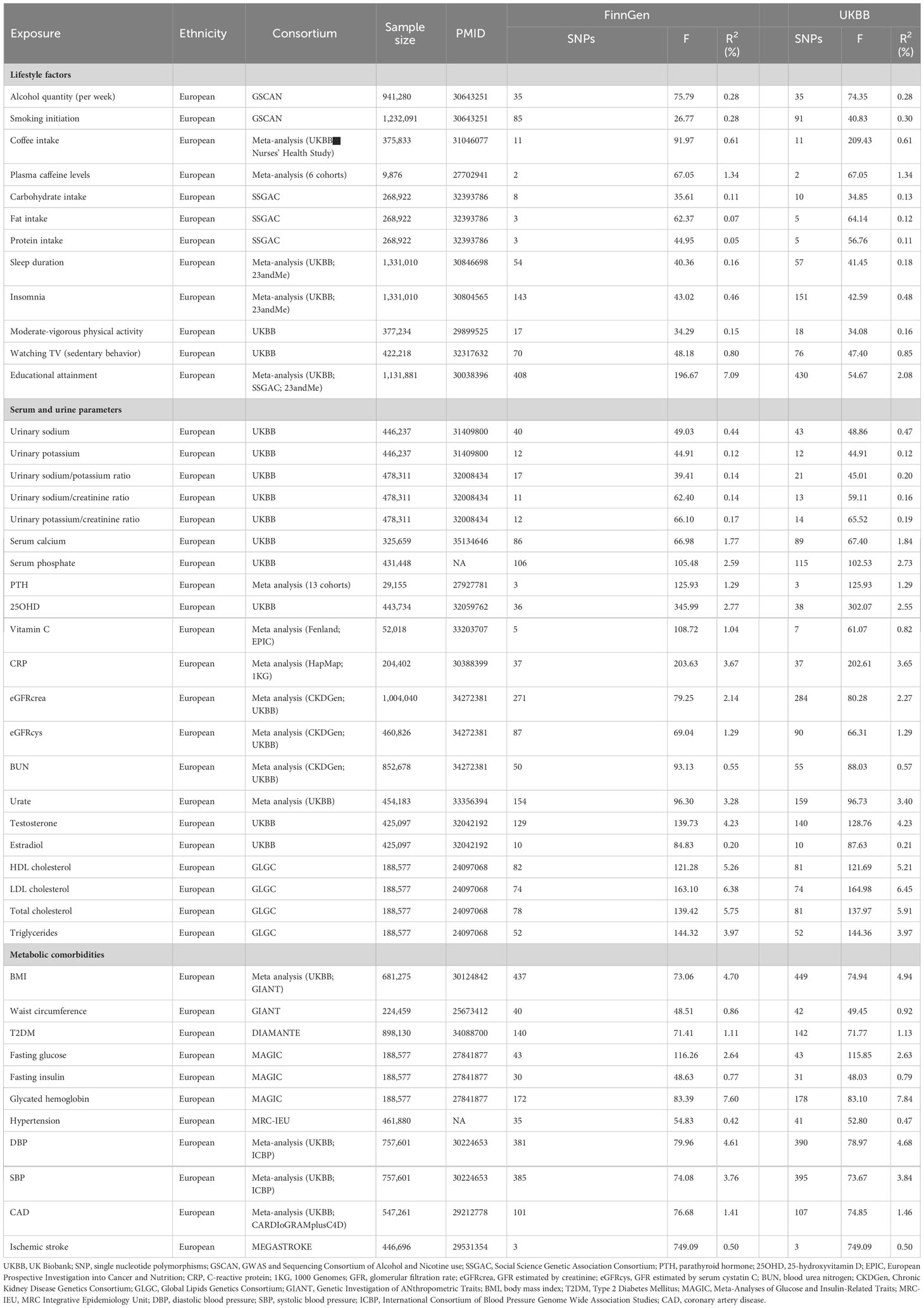
IVs were used to explore the association between modifiable risk factors and kidney stones. IVs of drinking and smoking were extracted from the GWAS and Sequencing Consortium of Alcohol and Nicotine use (GSCAN) (26). We identified the IVs of relative intakes of carbohydrate, fat, and protein from the Social Science Genetic Association Consortium (SSGAC) (27). We obtained IVs of moderate-vigorous physical activity (28), watching TV duration (sedentary behavior) (29), serum calcium (30), serum phosphate, urinary sodium (31), urinary potassium (31), 25OHD (32), the urinary sodium/potassium ratio (33), the urinary sodium/creatinine ratio (33), the urinary potassium/creatinine ratio (33), testosterone (34), and estradiol (34) based on a published GWAS from the UKBB. We extracted IVs of coffee intake (35), plasma caffeine levels (36), insomnia (37), sleep duration (37), educational attainment (38), vitamin C (39), parathyroid hormone (PTH) (40), CRP (41), the estimated creatinine-based glomerular filtration rate (eGFRcrea) (42), the GFR estimated by serum cystatin C (eGFRcys) (42), blood urea nitrogen (BUN) (42), urate (43), body mass index (BMI) (44), waist circumference (45), T2DM (46), diastolic blood pressure (DBP) (47), systolic blood pressure (SBP) (47), and coronary artery disease (CAD) (48) from some large GWAS meta-analyses. IVs of HDL cholesterol, LDL cholesterol, total cholesterol, and triglycerides were extracted from the Global Lipids Genetics Consortium (GLGC) with 188,577 European participants (49). IVs of fasting glucose and fasting insulin were obtained from Meta-Analyses of Glucose and Insulin-Related Traits (MAGIC). IVs of hypertension and ischemic stroke were extracted from the MRC Integrative Epidemiology Unit (MRC-IEU) and MEGASTROKE consortium, respectively. We extracted single nucleotide polymorphisms (SNPs) associated with each risk factor at a genome-wide significance level (P<5×10−8) and removed the existing linkage disequilibrium (r2 <0.01 and clump distance > 5,000 kb).
GWAS summary statistics of kidney stones
We used the kidney stones GWAS summary statistics from the UKBB and FinnGen consortium (https://r7.finngen.fi/). In the UKBB, the GWAS dataset contained 6,536 cases and 388,508 controls, with adjustments for age, sex, and genotyping platform (50). We used the seventh release data from the FinnGen consortium, with 7,433 cases and 301,094 controls, with adjustments for age, sex, 10 principal components, and a genotyping batch.
Mediation MR analysis
A two-stage MR analysis was undertaken to evaluate the mediating impact of intermediate risk factors on the causal relationships between education and the occurrence of kidney stones. First, we calculated the causal influence of genetically determined education on the mediators (β1). Second, we assessed the effect of each mediator on the risk of kidney stones (β2) in the UK Biobank. Third, the mediation proportion of each mediator within the comprehensive impact of education on kidney stones was determined by dividing the indirect effect, derived from the combined estimates of the two sequential steps (β1*β2), by the overall effect.
Statistical analysis
In the study, the following multiple methods were used to evaluate the causal relationship: random-effect inverse variance weighted (IVW), MR-Egger, weighted median, simple mode, and weighted mode. IVW is a meta-analysis method that obtains an overall estimate of the effect of each risk factor on the risk of kidney stones by combining the Wald ratios of each single nucleotide polymorphism (SNP). MR-Egger not only obtains a causal effect estimate but also assesses potential horizontal pleiotropic effects. Weighted median, simple mode, and weighted mode were regarded as supplements to IVW. The MR-PRESSO approach can detect pleiotropic outliers, which are then removed manually. The strength of IVs for each risk factor was evaluated by F statistics (F = beta2/se2). Cochrane’s Q statistic was used to assess the heterogeneity of IVs. Scatter plots and funnel plots allow the visualization of MR analysis results and potential outliers. Additionally, we performed “Leave-one-out” analysis to identify potentially heterogeneous SNPs. We conducted a meta-analysis using the fixed-effect model to combine the IVW analysis results from the UKBB and FinnGen consortium. All data analysis were completed using R software (version 4.1.3).
Results
The number of SNPs of risk factors varied from 2 to 449 and the explained phenotypic variances ranged from 0.07% to 7.84%. The general F statistics of each risk factor were greater than 10, suggesting there were no weak IVs biases (Table 1).
Discovery results of kidney stones in the FinnGen consortium
In the discovery phase, the IVW method showed that a total of 13 exposures were associated with the risk of kidney stones (P ≤ 0.05) (Tables S1, S2). Among them, urinary sodium, urinary potassium, the urinary sodium/creatinine ratio, serum calcium, eGFRcrea, eGFRcys, fasting insulin, and hypertension might increase the risk of kidney stones. Alcohol quantity (per week), coffee intake, plasma caffeine levels, educational attainment, and serum phosphate may decrease the risk of kidney stones. Heterogeneities were detected in the SNPs of the following exposures: alcohol quantity (per week), educational attainment, urinary sodium, the urinary sodium/creatinine ratio, serum calcium, serum phosphate, eGFRcrea, eGFRcys, and hypertension. However, no horizontal pleiotropy was found in the exposures associated with kidney stones.
Replication results of kidney stones in the UKBB consortium
In the replication phase, the IVW method showed that a total of 19 exposures were associated with the risk of kidney stones (P ≤ 0.05) (Tables S3, S4). The following risk factors may increase the risk of kidney stones: smoking initiation, watching TV (sedentary behavior), urinary sodium, the urinary sodium/potassium ratio, the urinary sodium/creatinine ratio, serum calcium, 25OHD, eGFRcrea, eGFRcys, BMI, waist circumference, T2DM, fasting insulin, glycated hemoglobin, and hypertension. Coffee intake, plasma caffeine levels, educational attainment, and the urinary potassium/creatinine ratio may decrease the risk of kidney stones. We observed heterogeneities in the following SNPs of the following exposures: smoking initiation, educational attainment, urinary sodium, the urinary sodium/potassium ratio, serum calcium, serum phosphate, eGFRcrea, eGFRcys, BMI, T2DM, and hypertension. However, we failed to find horizontal pleiotropy in the exposures.
Combined results of kidney stones from meta-analysis
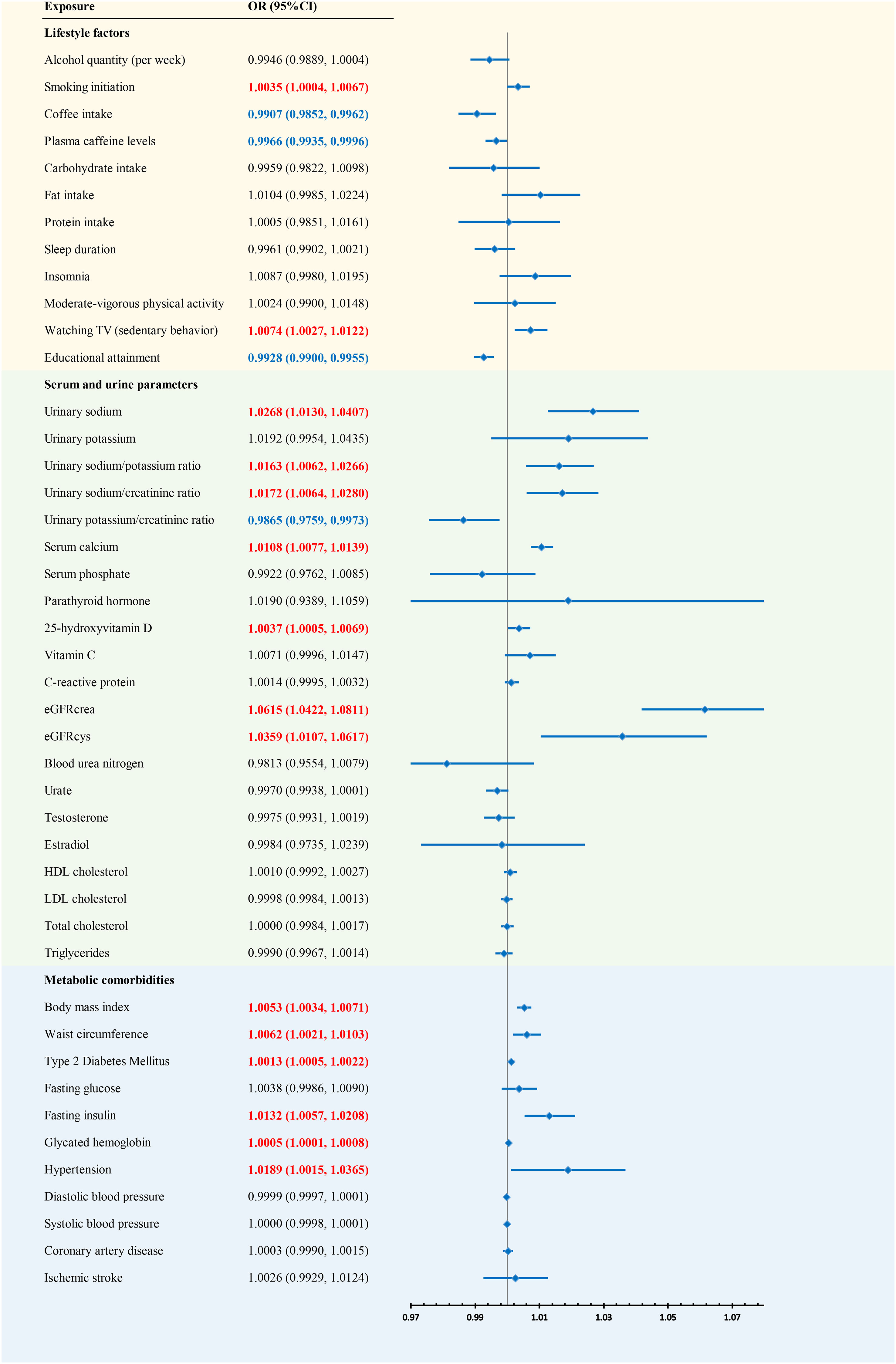
We conducted a meta-analysis combining the analysis results from the FinnGen and UKBB consortia (Figure 2). The combined results indicated that previous exposures may increase the risk of kidney stones, including smoking initiation, watching TV (sedentary behavior), urinary sodium, the urinary sodium/potassium ratio, the urinary sodium/creatinine ratio, serum calcium, 25OHD, eGFRcrea, eGFRcys, BMI, waist circumference, T2DM, fasting insulin, glycated hemoglobin, and hypertension. Coffee intake, plasma caffeine levels, educational attainment, and the urinary potassium/creatinine ratio may decrease the risk of kidney stones.
Mediation MR analysis
Tables S5 displays the effects of genetically predicted education and five modifiable risk factors through univariable MR analyses. Tables S6 displays the causal associations of each mediator with kidney stones with adjustment for education. Each 1-SD increase in education was linked to a reduced BMI (IVW β1: −0.252; 95% CI: −0.327, -0.177), waist circumference (IVW β1: −0.122; 95% CI: −0.167, −0.077), smoking initiation (IVW β1: −0.443; 95% CI: −0.501, −0.385), sedentary behavior (IVW β1: −0.603; 95% CI: −0.636, −0.570), and T2DM (IVW β1: −0.659; 95% CI: −0.771, −0.547). Each 1-SD higher BMI (IVW β2: 0.005; 95% CI: 0.003, 0.007), waist circumference (IVW β2: 0.004; 95% CI: 0.002, 0.006), smoking initiation (IVW β2: 0.004; 95% CI: 0.001, 0.007), sedentary behavior (IVW β2: 0.007; 95% CI: 0.003, 0.010), and T2DM (IVW β2: 0.001; 95% CI: 0.0002, 0.002) were associated with an increased risk of kidney stones after adjusting for education. Ranked by mediation proportion, the effect of education on the risk of kidney stones was mediated by five modifiable risk factors, including sedentary behavior (mediation proportion: 25.7%; 95% CI: 23.3%, 28.1%), smoking initiation (mediation proportion: 10.2%; 95% CI: 7.6%,12.9%), BMI (mediation proportion: 8.2%; 95% CI: 6.1%,10.4%), T2DM (mediation proportion: 5.8%; 95% CI: 0%,13.1%), and waist circumference (mediation proportion: 3.2%; 95% CI: 2.2%,4.1%).
Discussion
The present MR study analyzed the causal relationship between 44 modifiable risk factors and kidney stones. Using the latest and most extensive kidney stone GWAS, we not only confirmed some of the previous MR studies results, but also found new risk factors associated with kidney stones. Mediation MR analysis showed the causal mediators included sedentary behavior (25.7%), smoking initiation (10.2%), BMI (8.2%), T2DM (5.8%), and waist circumference (3.2%) in the association between education and kidney stones.
Lifestyle factors
Yuan et al. discovered that higher coffee and caffeine consumption may decrease the risk of kidney stones (51). Although we analyzed the data from the seventh release of the FinnGen consortium and the UKBB, which included more cases and controls, the results were consistent with this. The possible mechanisms are that caffeine has diuretic properties and may reduce the calcium oxalate crystal adhesion of renal tubular epithelial cells (52, 53). After including the most comprehensive GWAS dataset, we observed that lower educational attainment was associated with a higher risk of kidney stones, which is in keeping with the previous study (54). A possible reason is that education could affect kidney stones mediated by a variety of factors, such as smoking, dietary habits, obesity, and so on. We genetically found the associations between smoking initiation and an increased risk of kidney stones. Smoking may increase vasopressin levels, leading to low urine output, which increases the risk of kidney stones (55). Moreover, increased oxidative stress in the kidneys is also a potential mechanism (55). Consistent with a large meta-analysis (56), we failed to discover an association between moderate-vigorous physical activity and kidney stones, which runs counter to many previous studies. However, we observed that sedentary behavior can increase the risk of kidney stones, and whether this is mediated by obesity or not needs to be further investigated.
Serum and urine parameters
We first genetically discovered that levels of urinary sodium, the urinary sodium/potassium ratio, and the urinary sodium/creatinine ratio were positively associated with the risk of kidney stones. In the 1990s, Cirllo et al. revealed that the urinary sodium/potassium ratio and urinary sodium/creatinine ratio were significantly related to the prevalence of urinary stone diseases (57). High urinary calcium excretion can increase the relative risk of urinary stone formation (58). Many experiments demonstrated the level of urinary calcium excretion increased with urinary sodium excretion and dietary sodium intake in stone formers and healthy individuals (59–61). A low sodium diet may reduce urinary calcium excretion levels and thus it might be an effective approach for preventing urinary stones.
We also found lower levels of eGFRcrea and eGFRcys may decrease the risk of kidney stones, which can be largely explained by renal failure shortening the lifespan of patients, as the risk of kidney stones increases with the age (62), and lower urinary calcium excretion in people with chronic kidney disease than those with normal kidney function (63, 64). A cross-sectional study showed a progressive decrease of urinary calcium excretion with the progression of chronic kidney disease (64). However, our results only suggested a causal relationship between eGFR and kidney stones; it is clearly impossible to prevent urinary stones by reducing renal function. Moreover, we genetically confirmed that higher levels of serum calcium and 25(OH)D were significantly associated with kidney stones (65). Several studies indicated that a high level of vitamin D and vitamin D supplementation can increase hypercalcemia and hypercalciuria (66, 67).
Metabolic comorbidities
Obesity and T2DM were revealed to be positively associated with kidney stones based on cross-sectional and cohort studies (68, 69), which was consistent with our MR results and others (70). We further discovered that waist circumference, fasting insulin, glycated hemoglobin, and hypertension are also significantly associated with a higher risk of kidney stones. A meta-analysis showed that there were positive non-linear associations between BMI and waist circumference and kidney stones, and the relative risk of kidney stones increased by 21% per 5 kg/m (2) increase in BMI, 16% per 10 cm increase in waist circumference, and 16% among diabetes patients (56). Obesity can increase the risk of kidney stones in multiple ways. Excessive food intake may cause metabolic disorders of calcium, sodium, oxalate, and uric acid (71), which is the reason some obese people are prone to urinary stones. Additionally, fatty acid-binding protein 4 (FABP4), an essential member of the fatty acid-binding protein family (72), was downregulated in collecting duct epithelial cells in renal papillae with Randall plaques, and FABP4 knockout mice developed both interstitial calcium and renal tubular crystals (73). Moreover, the chronic inflammatory state in obesity plays an essential role in the development of kidney stones. Weinberg et al. investigated associations between diabetic severity and the risk of kidney stones and found that a history of T2DM, fasting plasma insulin, and glycosylated hemoglobin A1c was significantly associated with kidney stones even after adjusting for potential confounders (74). Insulin resistance may induce derangements of urine pH and the renal handling of calcium and ammonium, which can largely explain the increased risk of urinary stones for diabetics. Some studies have indicated the diabetics have increased urinary calcium and phosphorus excretion, and others have shown an increased urinary oxalate excretion in patients with kidney stones and T2DM (75, 76). Additionally, studies have demonstrated increased uric acid excretion in diabetics, which may be a mechanism in which uric acid is the main component of urinary stones in this instance (77). Furthermore, obesity is associated with insulin resistance, which can promote urinary stone formation. Hypercalciuria may be the pathogenetic factor of kidney stones in patients with hypertension (78).
Although the analytical outcomes of the two GWAS databases largely converge, disparities are observed in the causal relationships between alcohol quantity, smoking initiation, sedentary behavior, educational attainment, urinary potassium, 25OHD, BMI, waist circumference, T2DM, and kidney stone disease. These distinctions may be attributed to factors such as population disparities, research methodologies, lifestyle patterns, and educational levels. In terms of population differences, the Finnish database primarily consists of individuals of Finnish descent, representing a homogenous Northern European population with distinct genetic characteristics. On the other hand, the UK Biobank database encompasses a much more diverse range of ethnic backgrounds due to the multicultural nature of the UK, encompassing various ethnicities such as Caucasian, Asian, and African. Regarding analytical methods, the Finnish database might have tailored statistical approaches to accommodate the genetic makeup of the Finnish population. By contrast, the UK Biobank would have likely employed diverse analytical strategies to consider the multitude of ethnic groups represented in the dataset. Disparities in lifestyle patterns and education levels are evident. Finland’s lifestyle habits, dietary preferences, and exercise routines may be influenced by local customs and Northern European cultural norms. Meanwhile, the UK Biobank database reflects the diverse lifestyle practices and dietary choices associated with the various ethnic groups residing in the UK.
Mediation MR analysis
Educational attainment is closely intertwined with human health. We used a two-step MR method to identify causal mediators that significantly elucidate the causal relationship between education and the occurrence of kidney stones. These findings align with the primary mechanisms underlying kidney stone development, in which sedentary habits, obesity, smoking, and T2DM emerge as pivotal risk factors for kidney stone formation (9, 22, 23). Low educational attainment increases susceptibility to kidney stone diseases, potentially by reducing access to economic, cultural, and social resources. These reductions may contribute to risk factors such as smoking, body weight, and some metabolic diseases (79, 80).
Strengths and limitations
Our study has several strengths. First, the MR design is suitable for exploring the causal relationships in the presence of potential confounders and reverse causation. Second, we used the latest and most extensive kidney stones GWAS and genetically found quite a few new risk factors associated with kidney stones, such as smoking initiation, sedentary behavior, urinary sodium, the urinary sodium/potassium ratio, the urinary sodium/creatinine ratio, waist circumference, fasting insulin, glycated hemoglobin, and hypertension. Third, our study included discovery, validation, and meta-analysis stages, enhancing the causal relationship between exposures and kidney stones. Finally, the study population was entirely of European ancestry, which reduced population stratification bias.
However, there are some limitations in our MR study. The biggest challenge is possible horizontal pleiotropy, meaning genetic variants influence the risk of kidney stones not via the exposure that we are studying. MR-Egger intercept was applied to detect the horizontal pleiotropy, and most of the results were stable. In addition, the heterogeneity of some IVs was not avoided due to the differences in study type, subgroup population, region, and so on. Finally, our study was restricted to European populations and the results may not be generalizable to other ethnic populations.
Conclusions
Our MR study shows causal associations of earlier smoking initiation, increased sedentary behavior, urinary sodium, the urinary sodium/potassium ratio, the urinary sodium/creatinine ratio, serum calcium, 25OHD, eGFRcrea, eGFRcys, BMI, waist circumference, fasting insulin, glycated hemoglobin, a history of T2DM, and hypertension with an increased risk of kidney stones. Increased coffee intake, plasma caffeine levels, educational attainment, and the urinary potassium/creatinine ratio are associated with a decreased risk of kidney stones. Mediation MR analysis expounded upon the causal impacts of educational attainment on kidney stones and quantified distinct causal mediators within relevant pathways, including sedentary lifestyles, obesity, smoking, and T2DM. Our results suggest more attention should be paid to these modifiable factors to prevent kidney stones.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The Mendelian randomization studies conducted using the UK Biobank data might not require ethical review. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
ML: project development, data analysis, and manuscript writing. JW and MG: data collection and analysis. WX: data collection. JC and ZC: manuscript editing. ZZ and HC: project development and manuscript editing. All authors contributed to the article and approved the submitted version.
Funding
The study was supported by Fundamental Research Funds for the Central Universities of Central South University (2022ZZTS0294 to ML; 2021zzts0348 to ZZ), the National Natural Science Foundation of China (82170781 to HC), and the Natural Science Foundation of Hunan Province (2021JJ31050 to HC).
Acknowledgments
We express our gratitude to the participants and investigators of the UKBB and FinnGen study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1240171/full#supplementary-material
Supplementary Figure 1 | Scatter plots for the causal association between 44 modifiable risk factors and kidney stones in the FinnGen consortium. (TIF)
Supplementary Figure 2 | Scatter plots for the causal association between 44 modifiable risk factors and kidney stones in the UK Biobank consortium. (TIF)
Supplementary Figure 3 | Funnel plots for the causal association between 44 modifiable risk factors and kidney stones in the FinnGen consortium. (TIF)
Supplementary Figure 4 | Funnel plots for the causal association between 44 modifiable risk factors and kidney stones in the UK Biobank consortium. (TIF)
Supplementary Figure 5 | Leave-one-out plots for the causal association between 44 modifiable risk factors and kidney stones in the FinnGen consortium. (TIF)
Supplementary Figure 6 | Leave-one-out plots for the causal association between 44 modifiable risk factors and kidney stones in the UK Biobank consortium. (TIF)
Abbreviations
25OHD, 25-hydroxyvitamin D; CRP, C-reactive protein; MR, Mendelian randomization; IVs, instrumental variables; GWAS, genome-wide association studies; T2DM, type 2 diabetes mellitus; UKBB, UK Biobank; PTH, parathyroid hormone; eGFRcrea, estimated creatinine-based glomerular filtration rate; eGFRcys, GFR estimated by serum cystatin C; BUN, blood urea nitrogen; BMI, body mass index; DBP, diastolic blood pressure; SBP, systolic blood pressure; CAD, coronary artery disease; SNPs, single nucleotide polymorphisms.
References
1. Romero V, Akpinar H, Assimos Dg. Kidney stones: a global picture of prevalence, incidence, and associated risk factors. Rev Urol. (2010) 12(2-3).
2. Türk C, Petřík A, Sarica K, Seitz C, Skolarikos A, Straub M, et al. EAU guidelines on interventional treatment for urolithiasis. Eur Urol. (2016) 69(3):475–82. doi: 10.1016/j.eururo.2015.07.041
3. Khan SR, Pearle MS, Robertson WG, Gambaro G, Canales BK, Doizi S, et al. Kidney stones. Nat Rev Dis Primer. (2016) 2. doi: 10.1038/nrdp.2016.8
4. Antonelli JA, Maalouf NM, Pearle MS, Lotan Y. Use of the National Health and Nutrition Examination Survey to calculate the impact of obesity and diabetes on cost and prevalence of urolithiasis in 2030. Eur Urol. (2014) 66(4):724–9. doi: 10.1016/j.eururo.2014.06.036
5. Pearle MS, Goldfarb DS, Assimos DG, Curhan G, Denu-Ciocca CJ, Matlaga BR, et al. Medical management of kidney stones: AUA guideline. J Urol. (2014) 192(2):316–24. doi: 10.1016/j.juro.2014.05.006
6. Glover LM, Bass MA, Carithers T, Loprinzi PD. Association of kidney stones with atherosclerotic cardiovascular disease among adults in the United States: Considerations by race-ethnicity. Physiol Behav (2016) 157:63–6. doi: 10.1016/j.physbeh.2016.01.026
7. Rule AD, Krambeck AE, Lieske JC. Chronic kidney disease in kidney stone formers. Clin J Am Soc Nephrol CJASN. (2011) 6(8):2069–75. doi: 10.2215/CJN.10651110
8. El-Zoghby ZM, Lieske JC, Foley RN, Bergstralh EJ, Li X, Melton LJ, et al. Urolithiasis and the risk of ESRD. Clin J Am Soc Nephrol CJASN. (2012) 7(9):1409–15. doi: 10.2215/CJN.03210312
9. Hamano S, Nakatsu H, Suzuki N, Tomioka S, Tanaka M, Murakami S. Kidney stone disease and risk factors for coronary heart disease. Int J Urol Off J Jpn Urol Assoc (2005) 12(10):859–63. doi: 10.1111/j.1442-2042.2005.01160.x
10. Tamadon MR, Nassaji M, Ghorbani R. Cigarette smoking and nephrolitiasis in adult individuals. Nephro-Urol Mon. (2013) 5(1):702–5. doi: 10.5812/numonthly.5251
11. Goldfarb DS, Fischer ME, Keich Y, Goldberg J. A twin study of genetic and dietary influences on nephrolithiasis: a report from the Vietnam Era Twin (VET) Registry. Kidney Int (2005) 67(3):1053–61. doi: 10.1111/j.1523-1755.2005.00170.x
12. Geng J, Qiu Y, Kang Z, Li Y, Li J, Liao R, et al. The association between caffeine intake and risk of kidney stones: A population-based study. Front Nutr (2022) 9:935820. doi: 10.3389/fnut.2022.935820
13. Yin S, Wang J, Bai Y, Yang Z, Cui J, Wang J. Association between sleep duration and kidney stones in 34 190 American adults: A cross-sectional analysis of NHANES 2007-2018. Sleep Health (2022) 8(6):671–7. doi: 10.1016/j.sleh.2022.08.003
14. Sorensen MD, Chi T, Shara NM, Wang H, Hsi RS, Orchard T, et al. Activity, energy intake, obesity, and the risk of incident kidney stones in postmenopausal women: a report from the Women’s Health Initiative. J Am Soc Nephrol JASN. (2014) 25(2):362–9. doi: 10.1681/ASN.2013050548
15. Cirillo M, Laurenzi M, Panarelli W, Stamler J. Urinary sodium to potassium ratio and urinary stone disease. The Gubbio Population Study Research Group. Kidney Int (1994) 46(4):1133–9. doi: 10.1038/ki.1994.376
16. Kahwati LC, Weber RP, Pan H, Gourlay M, LeBlanc E, Coker-Schwimmer M, et al. Vitamin D. Calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: Evidence report and systematic review for the US preventive services task force. JAMA. (2018) 319(15):1600–12. doi: 10.1001/jama.2017.21640
17. Shoag J, Eisner Bh. Relationship between C-reactive protein and kidney stone prevalence. J Urol. (2014) 191(2). doi: 10.1016/j.juro.2013.09.033
18. Pak CY, Barilla DE, Holt K, Brinkley L, Tolentino R, Zerwekh JE. Effect of oral purine load and allopurinol on the crystallization of calcium salts in urine of patients with hyperuricosuric calcium urolithiasis. Am J Med (1978) 65(4):593–9. doi: 10.1016/0002-9343(78)90846-x
19. Li J-Y, Zhou T, Gao X, Xu C, Sun Y, Peng Y, et al. Testosterone and androgen receptor in human nephrolithiasis. J Urol. (2010) 184(6):2360–3. doi: 10.1016/j.juro.2010.08.009
20. Zhao Z, Mai Z, Ou L, Duan X, Zeng G. Serum estradiol and testosterone levels in kidney stones disease with and without calcium oxalate components in naturally postmenopausal women. PLoS One (2013) 8(9):e75513. doi: 10.1371/journal.pone.0075513
21. Torricelli FCM, De SK, Gebreselassie S, Li I, Sarkissian C, Monga M. Dyslipidemia and kidney stone risk. J Urol. (2014) 191(3):667–72. doi: 10.1016/j.juro.2013.09.022
22. Taylor EN, Stampfer MJ, Curhan GC. Obesity, weight gain, and the risk of kidney stones. JAMA. (2005) 293(4):455–62. doi: 10.1001/jama.293.4.455
23. Lieske JC, de la Vega LSP, Gettman MT, Slezak JM, Bergstralh EJ, Melton LJ, et al. Diabetes mellitus and the risk of urinary tract stones: a population-based case-control study. Am J Kidney Dis Off J Natl Kidney Found. (2006) 48(6):897–904. doi: 10.1053/j.ajkd.2006.09.002
24. Hung J-A, Li C-H, Geng J-H, Wu D-W, Chen S-C. Dyslipidemia increases the risk of incident kidney stone disease in a large Taiwanese population follow-up study. Nutrients. (2022) 14(7). doi: 10.3390/nu14071339
25. Smith GD, Hemani. G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet (2014) 23(R1). doi: 10.1093/hmg/ddu328
26. Liu M, Jiang Y, Wedow R, Li Y, Brazel DM, Chen F, et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet (2019) 51(2):237–44. doi: 10.1038/s41588-018-0307-5
27. Meddens SFW, de Vlaming R, Bowers P, Burik CAP, Linnér RK, Lee C, et al. Genomic analysis of diet composition finds novel loci and associations with health and lifestyle. Mol Psychiatry (2021) 26(6). doi: 10.1038/s41380-020-0697-5
28. Klimentidis YC, Raichlen DA, Bea J, Garcia DO, Wineinger NE, Mandarino LJ, et al. Genome-wide association study of habitual physical activity in over 377,000 UK Biobank participants identifies multiple variants including CADM2 and APOE. Int J Obes 2005. (2018) 42(6):1161–76. doi: 10.1038/s41366-018-0120-3
29. van de Vegte YJ, Said MA, Rienstra M, van der Harst P, Verweij N. Genome-wide association studies and Mendelian randomization analyses for leisure sedentary behaviours. Nat Commun (2020) 11(1):1770. doi: 10.1038/s41467-020-15553-w
30. Yuan S, Yu L, Gou W, Wang L, Sun J, Li D, et al. Health effects of high serum calcium levels: Updated phenome-wide Mendelian randomisation investigation and review of Mendelian randomisation studies. EBioMedicine. (2022) 76:103865. doi: 10.1016/j.ebiom.2022.103865
31. Pazoki R, Evangelou E, Mosen-Ansorena D, Pinto RC, Karaman I, Blakeley P, et al. GWAS for urinary sodium and potassium excretion highlights pathways shared with cardiovascular traits. Nat Commun (2019) 10(1):3653. doi: 10.1038/s41467-019-11451-y
32. Manousaki D, Mitchell R, Dudding T, Haworth S, Harroud A, Forgetta V, et al. Genome-wide association study for vitamin D levels reveals 69 independent loci. Am J Hum Genet (2020) 106(3):327–37. doi: 10.1016/j.ajhg.2020.01.017
33. Zanetti D, Bergman H, Burgess S, Assimes TL, Bhalla V, Ingelsson E. Urinary albumin, sodium, and potassium and cardiovascular outcomes in the UK biobank: Observational and mendelian randomization analyses. Hypertens Dallas Tex 1979. (2020) 75(3):714–22. doi: 10.1161/HYPERTENSIONAHA.119.14028
34. Ruth KS, Day FR, Tyrrell J, Thompson DJ, Wood AR, Mahajan A, et al. Using human genetics to understand the disease impacts of testosterone in men and women. Nat Med (2020) 26(2):252–8. doi: 10.1038/s41591-020-0751-5
35. Zhong VW, Kuang A, Danning RD, Kraft P, van Dam RM, Chasman DI, et al. A genome-wide association study of bitter and sweet beverage consumption. Hum Mol Genet (2019) 28(14):2449–57. doi: 10.1093/hmg/ddz061
36. Cornelis MC, Kacprowski T, Menni C, Gustafsson S, Pivin E, Adamski J, et al. Genome-wide association study of caffeine metabolites provides new insights to caffeine metabolism and dietary caffeine-consumption behavior. Hum Mol Genet (2016) 25(24). doi: 10.1093/hmg/ddw334
37. Jansen PR, Watanabe K, Stringer S, Skene N, Bryois J, Hammerschlag AR, et al. Genome-wide analysis of insomnia in 1,331,010 individuals identifies new risk loci and functional pathways. Nat Genet (2019) 51(3):394–403. doi: 10.1038/s41588-018-0333-3
38. Lee JJ, Wedow R, Okbay A, Kong E, Maghzian O, Zacher M, et al. Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat Genet (2018) 50(8):1112–21. doi: 10.1038/s41588-018-0147-3
39. Zheng J-S, Luan J, Sofianopoulou E, Imamura F, Stewart ID, Day FR, et al. Plasma vitamin C and type 2 diabetes: Genome-wide association study and mendelian randomization analysis in european populations. Diabetes Care (2021) 44(1):98–106. doi: 10.2337/dc20-1328
40. Robinson-Cohen C, Lutsey PL, Kleber ME, Nielson CM, Mitchell BD, Bis JC, et al. Genetic variants associated with circulating parathyroid hormone. J Am Soc Nephrol JASN. (2017) 28(5):1553–65. doi: 10.1681/ASN.2016010069
41. Ligthart S, Vaez A, Võsa U, Stathopoulou MG, de Vries PS, Prins BP, et al. Genome analyses of >200,000 individuals identify 58 loci for chronic inflammation and highlight pathways that link inflammation and complex disorders. Am J Hum Genet (2018) 103(5):691–706. doi: 10.1016/j.ajhg.2018.09.009
42. Stanzick KJ, Li Y, Schlosser P, Gorski M, Wuttke M, Thomas LF, et al. Discovery and prioritization of variants and genes for kidney function in >1.2 million individuals. Nat Commun (2021) 12(1):4350. doi: 10.1038/s41467-021-24491-0
43. Gill D, Cameron AC, Burgess S, Li X, Doherty DJ, Karhunen V, et al. Urate, blood pressure, and cardiovascular disease: Evidence from mendelian randomization and meta-analysis of clinical trials. Hypertens Dallas Tex 1979. (2021) 77(2):383–92. doi: 10.1161/HYPERTENSIONAHA.120.16547
44. Yengo L, Sidorenko J, Kemper KE, Zheng Z, Wood AR, Weedon MN, et al. Meta-analysis of genome-wide association studies for height and body mass index in ∼700000 individuals of European ancestry. Hum Mol Genet (2018) 27(20):3641–9. doi: 10.1093/hmg/ddy271
45. Shungin D, Winkler TW, Croteau-Chonka DC, Ferreira T, Locke AE, Mägi R, et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature. (2015) 518(7538):187–96. doi: 10.1038/nature14132
46. Mordi IR, Lumbers RT, Palmer CNA, Pearson ER, Sattar N, Holmes MV, et al. Type 2 diabetes, metabolic traits, and risk of heart failure: A mendelian randomization study. Diabetes Care (2021) 44(7):1699–705. doi: 10.2337/dc20-2518
47. Evangelou E, Warren HR, Mosen-Ansorena D, Mifsud B, Pazoki R, Gao H, et al. Genetic analysis of over 1 million people identifies 535 new loci associated with blood pressure traits. Nat Genet (2018) 50(10):1412–25. doi: 10.1038/s41588-018-0205-x
48. van der Harst P, Verweij N. Identification of 64 novel genetic loci provides an expanded view on the genetic architecture of coronary artery disease. Circ Res (2018) 122(3):433–43. doi: 10.1161/CIRCRESAHA.117.312086
49. Willer CJ, Schmidt EM, Sengupta S, Peloso GM, Gustafsson S, Kanoni S, et al. Discovery and refinement of loci associated with lipid levels. Nat Genet (2013) 45(11):1274–83. doi: 10.1038/ng.2797
50. Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med (2015) 12(3):e1001779. doi: 10.1371/journal.pmed.1001779
51. Yuan S, Larsson SC. Coffee and caffeine consumption and risk of kidney stones: A mendelian randomization study. Am J Kidney Dis Off J Natl Kidney Found. (2022) 79(1):9–14.e1. doi: 10.1053/j.ajkd.2021.04.018
52. Rieg T, Steigele H, Schnermann J, Richter K, Osswald H, Vallon V. Requirement of intact adenosine A1 receptors for the diuretic and natriuretic action of the methylxanthines theophylline and caffeine. J Pharmacol Exp Ther (2005) 313(1):403–9. doi: 10.1124/jpet.104.080432
53. Peerapen P, Thongboonkerd V. Caffeine prevents kidney stone formation by translocation of apical surface annexin A1 crystal-binding protein into cytoplasm: In vitro evidence. Sci Rep (2016) 6:38536. doi: 10.1038/srep38536
54. Wang M, Jian Z, Gao X, Yuan C, Jin X, Li H, et al. Causal associations between educational attainment and 14 urological and reproductive health outcomes: A mendelian randomization study. Front Public Health (2021) 9:742952. doi: 10.3389/fpubh.2021.742952
55. Sulaiman SK, Enakshee J, Traxer O, Somani BK. Which type of water is recommended for patients with stone disease (Hard or soft water, tap or bottled water): Evidence from a systematic review over the last 3 decades. Curr Urol Rep (2020) 21(1):6. doi: 10.1007/s11934-020-0968-3
56. Aune D, Mahamat-Saleh Y, Norat T, Riboli E. Body fatness, diabetes, physical activity and risk of kidney stones: a systematic review and meta-analysis of cohort studies. Eur J Epidemiol. (2018) 33(11):1033–47. doi: 10.1007/s10654-018-0426-4
57. Cirillo M, Laurenzi M, Panarelli W, Stamler J. Urinary sodium to potassium ratio and urinary stone disease. The Gubbio Population Study Research Group. Kidney Int (1994) 46(4). doi: 10.1038/ki.1994.376
58. Curhan Gc, Taylor En. 24-h uric acid excretion and the risk of kidney stones. Kidney Int (2008) 73(4). doi: 10.1038/sj.ki.5002708
59. Phillips MJ, Cooke JN. Relation between urinary calcium and sodium in patients with idiopathic hypercalciuria. Lancet Lond Engl (1967) 1(7504):1354–7. doi: 10.1016/s0140-6736(67)91763-1
60. Nascimento L, Oliveros FH, Cunningham E. Renal handling of sodium and calcium in hypercalciuria. Clin Pharmacol Ther (1984) 35(3):342–7. doi: 10.1038/clpt.1984.41
61. Silver J, Rubinger D, Friedlaender MM, Popovtzer MM. Sodium-dependent idiopathic hypercalciuria in renal-stone formers. Lancet Lond Engl (1983) 2(8348):484–6. doi: 10.1016/s0140-6736(83)90513-5
62. Sorokin I, Mamoulakis C, Miyazawa K, Rodgers A, Talati J, Lotan Y. Epidemiology of stone disease across the world. World J Urol. (2017) 35(9):1301–20. doi: 10.1007/s00345-017-2008-6
63. Coyne D, Acharya M, Qiu P, Abboud H, Batlle D, Rosansky S, et al. Paricalcitol capsule for the treatment of secondary hyperparathyroidism in stages 3 and 4 CKD. Am J Kidney Dis Off J Natl Kidney Found. (2006) 47(2):263–76. doi: 10.1053/j.ajkd.2005.10.007
64. Liu J, Tio MC, Verma A, Schmidt IM, Ilori TO, Knauf F, et al. Determinants and outcomes associated with urinary calcium excretion in chronic kidney disease. J Clin Endocrinol Metab (2022) 107(1):e281–92. doi: 10.1210/clinem/dgab574
65. Jian Z, Huang Y, He Y, Jin X, Li H, Li S, et al. Genetically predicted lifelong circulating 25(OH)D levels are associated with serum calcium levels and kidney stone risk. J Clin Endocrinol Metab (2022) 107(3):e1159–66. doi: 10.1210/clinem/dgab758
66. Hu H, Zhang J, Lu Y, Zhang Z, Qin B, Gao H, et al. Association between circulating vitamin D level and urolithiasis: A systematic review and meta-analysis. Nutrients. (2017) 9(3):301. doi: 10.3390/nu9030301
67. Malihi Z, Wu Z, Stewart AW, Lawes CM, Scragg R. Hypercalcemia, hypercalciuria, and kidney stones in long-term studies of vitamin D supplementation: a systematic review and meta-analysis. Am J Clin Nutr (2016) 104(4):1039–51. doi: 10.3945/ajcn.116.134981
68. Abufaraj M, Xu T, Cao C, Waldhoer T, Seitz C, D’andrea D, et al. Prevalence and trends in kidney stone among adults in the USA: Analyses of national health and nutrition examination survey 2007-2018 data. Eur Urol Focus. (2021) 7(6):1468–75. doi: 10.1016/j.euf.2020.08.011
69. Ping H, Lu N, Wang M, Lu J, Liu Y, Qiao L, et al. New-onset metabolic risk factors and the incidence of kidney stones: a prospective cohort study. BJU Int (2019) 124(6):1028–33. doi: 10.1111/bju.14805
70. Yuan S, Larsson SC. Assessing causal associations of obesity and diabetes with kidney stones using Mendelian randomization analysis. Mol Genet Metab (2021) 134(1-2):212–5. doi: 10.1016/j.ymgme.2021.08.010
71. Asplin JR. Obesity and urolithiasis. Adv Chronic Kidney Dis (2009) 16(1):11–20. doi: 10.1053/j.ackd.2008.10.003
72. Hotamisligil GS, Bernlohr DA. Metabolic functions of FABPs–mechanisms and therapeutic implications. Nat Rev Endocrinol (2015) 11(10):592–605. doi: 10.1038/nrendo.2015.122
73. Taguchi K, Chen L, Usawachintachit M, Hamamoto S, Kang M, Sugino T, et al. Fatty acid-binding protein 4 downregulation drives calcification in the development of kidney stone disease. Kidney Int (2020) 97(5):1042–56. doi: 10.1016/j.kint.2020.01.042
74. Weinberg AE, Patel CJ, Chertow GM, Leppert JT. Diabetic severity and risk of kidney stone disease. Eur Urol. (2014) 65(1):242–7. doi: 10.1016/j.eururo.2013.03.026
75. Thalassinos NC, Hadjiyanni P, Tzanela M, Alevizaki C, Philokiprou D. Calcium metabolism in diabetes mellitus: effect of improved blood glucose control. Diabetes Med J Br Diabetes Assoc (1993) 10(4):341–4. doi: 10.1111/j.1464-5491.1993.tb00076.x
76. Eisner BH, Porten SP, Bechis SK, Stoller ML. Diabetic kidney stone formers excrete more oxalate and have lower urine pH than nondiabetic stone formers. J Urol. (2010) 183(6):2244–8. doi: 10.1016/j.juro.2010.02.007
77. Cook DG, Shaper AG, Thelle DS, Whitehead TP. Serum uric acid, serum glucose and diabetes: relationships in a population study. Postgrad Med J (1986) 62(733):1001–6. doi: 10.1136/pgmj.62.733.1001
78. Cappuccio FP, Strazzullo P, Mancini M. Kidney stones and hypertension: population based study of an independent clinical association. BMJ. (1990) 300(6734):1234–6. doi: 10.1136/bmj.300.6734.1234
79. Marmot M, Friel S, Bell R, Houweling TAJ, Taylor S, Commission on Social Determinants of Health. Closing the gap in a generation: health equity through action on the social determinants of health. Lancet Lond Engl (2008) 372(9650):1661–9. doi: 10.1016/S0140-6736(08)61690-6
Keywords: kidney stones, Mendelian randomization, lifestyle factors, metabolic comorbidities, mediation Mendelian randomization
Citation: Liu M, Wu J, Gao M, Li Y, Xia W, Zhang Y, Chen J, Chen Z, Zhu Z and Chen H (2023) Lifestyle factors, serum parameters, metabolic comorbidities, and the risk of kidney stones: a Mendelian randomization study. Front. Endocrinol. 14:1240171. doi: 10.3389/fendo.2023.1240171
Received: 14 June 2023; Accepted: 04 September 2023;
Published: 22 September 2023.
Edited by:
Mohiuddin Md. Taimur Khan, Washington State University Tri-Cities, United StatesReviewed by:
Jens Djurhuus, Aarhus University, DenmarkRicardo Adrian Nugraha, Airlangga University, Indonesia
Copyright © 2023 Liu, Wu, Gao, Li, Xia, Zhang, Chen, Chen, Zhu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zewu Zhu, emh1emV2QDE2My5jb20=; Hequn Chen, Y2hlbmhlcXVueHlAMTI2LmNvbQ==
†ORCID: Zewu Zhu, orcid.org/0000-0001-5320-9391
Hequn Chen, orcid.org/0000-0002-4286-9308
 Minghui Liu
Minghui Liu Jian Wu1,2
Jian Wu1,2 Weiping Xia
Weiping Xia Zewu Zhu
Zewu Zhu Hequn Chen
Hequn Chen