
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 06 November 2023
Sec. Clinical Diabetes
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1238825
This article is part of the Research Topic The Underlying Mechanisms and Novel Approaches for Diabetes and its Related Complications View all 36 articles
A correction has been applied to this article in:
Corrigendum: Quantification of joint mobility limitation in adult type 1 diabetes
 Sanat Phatak1*†
Sanat Phatak1*† Pranav Mahadevkar2
Pranav Mahadevkar2 Kaustubh Suresh Chaudhari1
Kaustubh Suresh Chaudhari1 Shreya Chakladar3
Shreya Chakladar3 Swasti Jain1
Swasti Jain1 Smita Dhadge1
Smita Dhadge1 Sarita Jadhav1
Sarita Jadhav1 Rohan Shah1
Rohan Shah1 Aboli Bhalerao1
Aboli Bhalerao1 Anupama Patil2
Anupama Patil2 Jennifer L. Ingram4
Jennifer L. Ingram4 Pranay Goel3
Pranay Goel3 Chittaranjan S. Yajnik1
Chittaranjan S. Yajnik1Aims: Diabetic cheiroarthropathies limit hand mobility due to fibrosis and could be markers of a global profibrotic trajectory. Heterogeneity in definitions and lack of a method to measure it complicate studying associations with organ involvement and treatment outcomes. We measured metacarpophalangeal (MCP) joint extension as a metric and describe magnetic resonance (MR) imaging determinants of MCP restriction.
Methods: Adults with type 1 diabetes were screened for hand manifestations using a symptom questionnaire, clinical examination, and function [Duruoz hand index (DHI) and grip strength]. Patients were segregated by mean MCP extension (<20°, 20°–40°, 40°–60°, and >60°) for MR imaging (MRI) scanning. Patients in the four groups were compared using ANOVA for clinical features and MRI tissue measurements (tenosynovial, skin, and fascia thickness). We performed multiple linear regression for determinants of MCP extension.
Results: Of the 237 patients (90 men), 79 (33.8%) with cheiroarthropathy had MCP extension limitation (39° versus 61°, p < 0.01). Groups with limited MCP extension had higher DHI (1.9 vs. 0.2) but few (7%) had pain. Height, systolic blood pressure, and nephropathy were associated with mean MCP extension. Hand MRI (n = 61) showed flexor tenosynovitis in four patients and median neuritis in one patient. Groups with MCP mobility restriction had the thickest palmar skin; tendon thickness or median nerve area did not differ. Only mean palmar skin thickness was associated with MCP extension angle on multiple linear regression.
Conclusion: Joint mobility limitation was quantified by restricted mean MCP extension and had structural correlates on MRI. These can serve as quantitative measures for future associative and interventional studies.
What is already known:
- Diabetic cheiroarthropathies (limited joint mobility, carpal tunnel syndrome, flexor tenosynovitis, and carpal tunnel syndrome) limit hand function in type 1 diabetes.
- Associations with vascular complications are inconsistent, and a relationship with internal organ fibrosis is not known.
- A lack of a method to measure the amount of hand fibrosis contributes to difficulties in establishing associations.
What is the key question:
Is average metacarpophalangeal (MCP) joint extension a marker of joint stiffness in type 1 diabetes?
What are the new findings:
- Cheiroarthropathies limit extension at the MCP joint, despite being rarely symptomatic.
- Mean MCP extension was associated with other indicators of tissue stiffness such as blood pressure and proteinuria.
- MCP extension limitation has structural correlates on magnetic resonance imaging, chiefly skin thickening.
How this study might affect research:
- Mean MCP extension angle in the clinic, and standardized measurements of tissue thickness on hand MRI could both potentially be used as metrics of hand fibrosis in diabetes. With further validation, they could be used for associations with internal organ fibrosis as well as outcome measures for anti-fibrotic therapies.
Limitation of joint mobility in type 1 diabetes was described by Rosenbloom in 1974 (1). “Diabetic cheiroarthropathies,” hand conditions with a higher prevalence in diabetes, include limited joint mobility (LJM), carpal tunnel syndrome (CTS), flexor tenosynovitis (FTS), and Dupuytren’s contracture (DC) (2–4). Prevalence in type 1 diabetes ranges widely (8% to 66% LJM, 30% CTS, 28% FTS, and 9% DC) (2, 5–8). Their presence correlates with some but not all microvascular complications; these associations have often not been replicated (9). This variability owes to a heterogeneity in definitions and diagnostic methods in addition to population differences.
All cheiroarthropathies are fibrotic on histomorphology; biopsies demonstrate excessive collagen deposition in periarticular connective tissue (10). Irrespective of diabetes, hand tissue fibrosis in DC is associated internal organ fibrosis, especially the liver (11). Diabetes is associated with increased organ fibrosis, affecting the kidneys, heart, and liver, that leads to considerable morbidity and mortality (12). With a perceived common pathology, it is tempting to speculate that hand fibrosis externally reflects a more global profibrotic trajectory (13, 14). In such a situation, examining the hand could serve as a useful clinical biomarker to select out patients for internal organ fibrosis. However, establishing such associations is currently hampered by a lack of consensus in measuring the severity of hand involvement.
It is only in severe cases that flexion contractures ensue, affecting professional and self-care activities, as well as metabolic complications (15–17). However, an ideal metric should be able to detect subclinical involvement, encapsulate all the differing manifestations, and be easy and reproducible. Measurement of joint mobility has been previously used, and subjects with type 1 diabetes had limitations in wrist and interphalangeal (IP) flexion (18). All cheiroarthropathies result in a preferential inelasticity of structures on the palmar aspect of the hand, thus principally limiting finger extension. This typically involves all fingers in LJM, and one or two in FTS and DC. We therefore explored the utility of measuring mean metacarpophalangeal (MCP) extension as a measure of joint stiffness in diabetes in a cohort of adult patients with type 1 diabetes. In a subset of patients chosen across the spectrum of MCP extension limitation, we report magnetic resonance imaging (MRI) findings, including tissue thickening and contributors to joint stiffness.
The study was conducted at the Diabetes Unit, KEM Hospital Research Centre, a tertiary care specialized unit in Pune, India. We screened consecutive adult patients (>18 years of age) with type 1 diabetes, from March 2021 to December 2022. We excluded pregnant patients, those who needed hospital admission, and those with hand trauma or concurrent active inflammatory arthritis that precluded hand examination. We recorded age, gender, education, and occupation. Manual work was classified as agriculture work, manual labor, or operating heavy machinery for more than 6 h a day; keyboard work if usage was more than 6 h a day. Smoking (current, previous, and never) and alcohol habits were recorded.
We extracted date of diagnosis, insulin compliance, and micro- and macro-vascular complications from patient files. Retinopathy was diagnosed on fundus photographs, nephropathy with proteinuria and/or end stage renal disease, neuropathy on biothesiometry, and clinical composite score. Common comorbidities (hypertension and hypothyroidism) and conditions or medications that could contribute to fibrosis (systemic sclerosis, skin disease involving the palms, malignancy, controlled inflammatory arthritis; methotrexate, amiodarone, aspirin, statins, and anti-epileptics) were recorded. Height, weight, and blood pressure were recorded using standard procedures. We looked for keloid scars, lipo-hypertrophy, or atrophy at insulin injection sites. We used skin autofluorescence as an indirect measurement of advanced glycation end-product (AGE) deposition, using the AGE reader on the non-dominant forearm in standard light conditions (19).
We screened for hand involvement using a structured history and measurements by trained research staff. The musculoskeletal history included presence of palmar pain, symptoms of compressive neuropathy (sensory and motor), grip difficulty, finger triggering, and perceived stiffness and tightness of palmar skin. We used the Duruoz Hand Index (DHI) that assesses activity limitation in 18 daily activities on a visual analogue scale, for hand function (20).
We recorded maximum possible passive extension at the MCP joint (second to fifth of both hands) until restriction or pain, with the palm approximated on a flat surface, using a protractor (Figure 1A). Mean passive MCP extension was calculated for each hand as the average of four finger extension angles. A prayer sign was defined as a visible gap between the two palms with an inability to approximate them fully. We measured the distance between the two fifth MCP and fifth proximal IP (PIP) joints, viewed from the ulnar aspect (Figure 1B). The presence of flexor tendon thickening, nodularity, triggering, and crepitus was noted. For CTS, we examined for the Tinel sign, the Phalen sign, and sensation in the median nerve distribution, recorded as normal, reduced, or absent. Hand grip strength was measured as an average of three readings using a Jamar hand dynamometer (Patterson Medical, Warrenville, IL). A physician (rheumatologist or diabetologist) examined all patients independently without access to the above measurements and assessed if one or more hand manifestation was present (LJM, FTS, CTS, and DD). Inter-rater reliability between two physicians (SD and SP), seen in 30 patients, was 0.88.
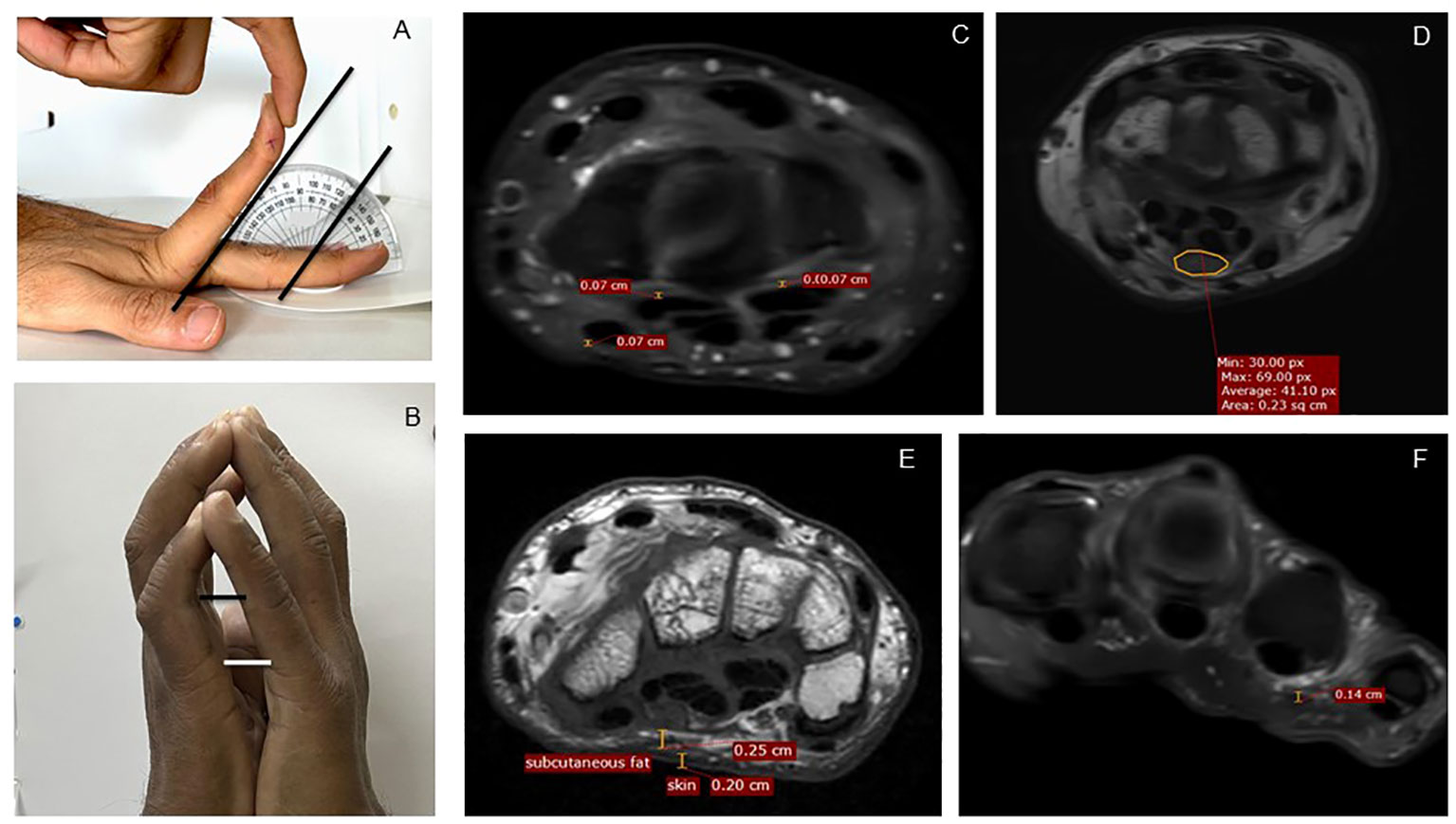
Figure 1 Measurement methodology, clinical and MRI. (A) Measurement of maximum passive extension at MCP joint of the left second finger, here showing 50° extension. (B) Measurements of distance between the fifth proximal interphalangeal joints and the fifth MCP joints as a quantification of the prayer sign. (C) Measurement of tenosynovial thickness at the level of carpal tunnel. (D) Measurement of median nerve area at the level of the carpal tunnel. (E) Measurement of skin and subcutaneous fat. (F) Measurement of palmar fascia overlying the fourth flexor tendon on PDFS images. MRI, magnetic resonance imaging; PDFS, proton density fat saturation.
Non-dominant hand mean MCP extension was used as a metric of joint mobility limitation to segregate patients into 20° bins (0°–20°, 20°–40°, 40°–60°, and >60°). Consecutive patients within each group were approached for MRI with a plan of including 15 per group, regardless of physician opinion.
Unless manifestations were unilateral, an MRI of the non-dominant hand was performed at the Star Imaging Research Centre using a 3T MRI Superconducting system with eight-channel extremity coil (Ingenia Release 5, Philips Healthcare, Amsterdam, The Netherlands). Consenting patients were invited to the Diabetes Unit, where earlier clinical findings were confirmed; contraindications to MRI were excluded (metallic bone; cardiac, cochlear, or dental implants; metallic intrauterine contraceptive devices; pregnancy; and claustrophobia). Two patients were on insulin pumps, and both the pump and sensor-transmitters were removed for the scan. Random plasma glucose measurements were performed, and the diabetologist (SD) managed insulin dosages accordingly, to prevent hypoglycemia while inside the MRI scanner.
The following MR sequences were performed: axial and coronal T2 weighted images, and pre-contrast fat-saturated T1-weighted axial coronal with fat saturation. All MRI scans were read by the same musculoskeletal radiologist (PM) who provided a qualitative report (altered signals, thickening, and edema) on the status of bones, tendons, joints, median nerve, and other salient findings. Quantitative measurements were performed by one of two trained researchers (SC and SJ) on axial images, in addition to the radiologist (PM). The readers had good internal consistency (Cronbach alpha of 0.95) and agreement with each other (correlation coefficient of 0.78). Measurements included tenosynovial thickness for the four flexor digitorum longus tendons and the flexor hallucis longus tendon, at four levels: MCP joint, proximal phalanx midpoint, metacarpal bone midpoint, and carpal tunnel midpoint (Figure 1C). Mean tenosynovial thickness was calculated as average of these 20 data points. In addition, the thickness at a point of visually perceived maximum thickness for each tendon sheath was recorded. Skin and subcutaneous tissue thickness was measured at four points at the level of MCP joint and carpal tunnel, respectively, and an average was calculated (Figure 1E). Palmar fascia was visible only when thickened; it was measured at the site of thickening and was considered zero in others (Figure 1F). The median nerve cross-sectional area was measured at the level of the carpal tunnel outlet and median nerve signal abnormalities were noted (Figure 1D). An instinctive “total hand fibrosis” score was calculated by adding mean tenosynovial thickness, palmar fascia thickness, and palmar skin thickness. A similar score was also calculated using Z-scores of mean tenosynovial thickness, palmar fascia thickness, and palmar skin thickness (total hand fibrosis Z-score).
Data are presented as mean (standard deviation) and median (interquartile range) as appropriate. Patients with cheiroarthropathy were compared with those without, using T-tests. Patients with different levels of MCP extension (0°–20°, 20°–40°, 40°–60°, and >60°) were compared with each other with respect to demographics, diabetes characteristics, and MRI characteristics using ANOVA; we report p-value for the trend, with <0.05 considered significant. Univariate linear regression adjusted for age and sex was used for determinants of hand stiffness, using mean MCP extension of non-dominant hand as the dependent variable. Because this was explorative and MRI parameters were not known previously, the sample size for MRI scanning was not hypothesis based. MRI quantitative descriptors are described as mean (standard deviation) for each group. Univariate and then multiple linear regression were used for structural determinants of hand stiffness (mean MCP angle of the imaged hand as the dependent variable, average skin thickness, average subcutaneous thickness, tenosynovial thickness, palmar fascia thickness, and median nerve area as predictor variables). We first standardized the independent and the dependent variables of the dataset into their corresponding Z-scores. All statistical analysis were performed using SPSS (IBM Corporation, Armonk, NY) and R (R Foundation, Vienna, Austria).
This study received ethics permission from the KEM Hospital Research Centre Ethics Committee (KEMHRC/RVC/EC/1518), and patients signed separate informed consent forms for clinical examination and MRI scans. The study is registered with the Clinical Trials Registry of India (CTRI/2020/12/030057). Data sharing agreements were signed with Star Imaging and Research Centre and Indian Institute of Science Education and Research (IISER), Pune. The study received a waiver from the IISER Ethics Committee for Human Research (IEHCR/Admin/2021/007). All clinical and imaging data are stored at the Diabetes Unit, KEM Hospital Research Centre. Individual MRI results were made available to patients immediately; abnormalities found were offered treatment, such as perilesional steroids. Patient groups will be involved in disseminating the results of this study.
This study is funded through a DBT/Wellcome India Alliance Clinical and Public health fellowship (IA/CPHE/19/504607). The funding body had no role in study design or analysis.
We examined 237 adults with type 1 diabetes (90 male subjects, median age of 26.8 years). Fifteen (6%) patients were manual workers, and three patients were keyboard workers. The median duration of diabetes was 13.7 years; apart from insulin, 57 patients received metformin. One-fifth of the cohort (20 patients, 8.5%) had retinopathy, 28 (11.8%) patients had nephropathy, and 45 (19%) patients had neuropathy on clinical examination (Table 1).
Only nine (3.8%) patients complained of hand pain; 26 patients had a history of trigger finger, and 16 (6.8%) patients had a history of paresthesia. Prayer sign was seen in 73 (30%) patients. Fifty-three patients had CTS on clinical examination. Seventy-nine (33.8%) patients had a cheiroarthropathy manifestation on physician’s assessment; 30, 46, 24, and 3 patients had FTS, LJM, CTS, and DC, respectively; 24 patients had more than one condition. Patients with a physician-diagnosed cheiroarthropathy had a significantly different average MCP extension angle (39° vs. 61°, p < 0.01) and a significantly higher DHI (1.67 vs. 0.21, p < 0.01).
Mean extension at the MCP joint was 50.8° (SD) on the right hand and 54.2° on the left hand, with a range of 0° to 85° (Table 2). The largest group of patients (103, 43%) had mean extension in the range of 40° to 60°, whereas 64 (27%) patients had extension limited to less than 40°. The group with the most severe limitation (<20°) was predominantly male subjects (11/15, 73%), unlike all the other groups. Groups with limitation (0°–20° and 20°–40°) tended to be older and had diabetes for nearly a decade longer. One-fifth of the <20° group were manual workers; the group also had a much higher prevalence of smoking and/or alcohol use (nearly 30%) as compared with the other groups (approximately 10%). The group with the most severe restriction also had the highest prevalence of retinopathy (20%) and nephropathy (33%) but not neuropathy. Body composition did not vary across groups; subjects with MCP restriction had higher systolic blood pressure but not diastolic. They also had the highest prevalence of lipohypertrophy and keloid scar formation. Autofluorescence on AGE reader did not differ in the four groups.
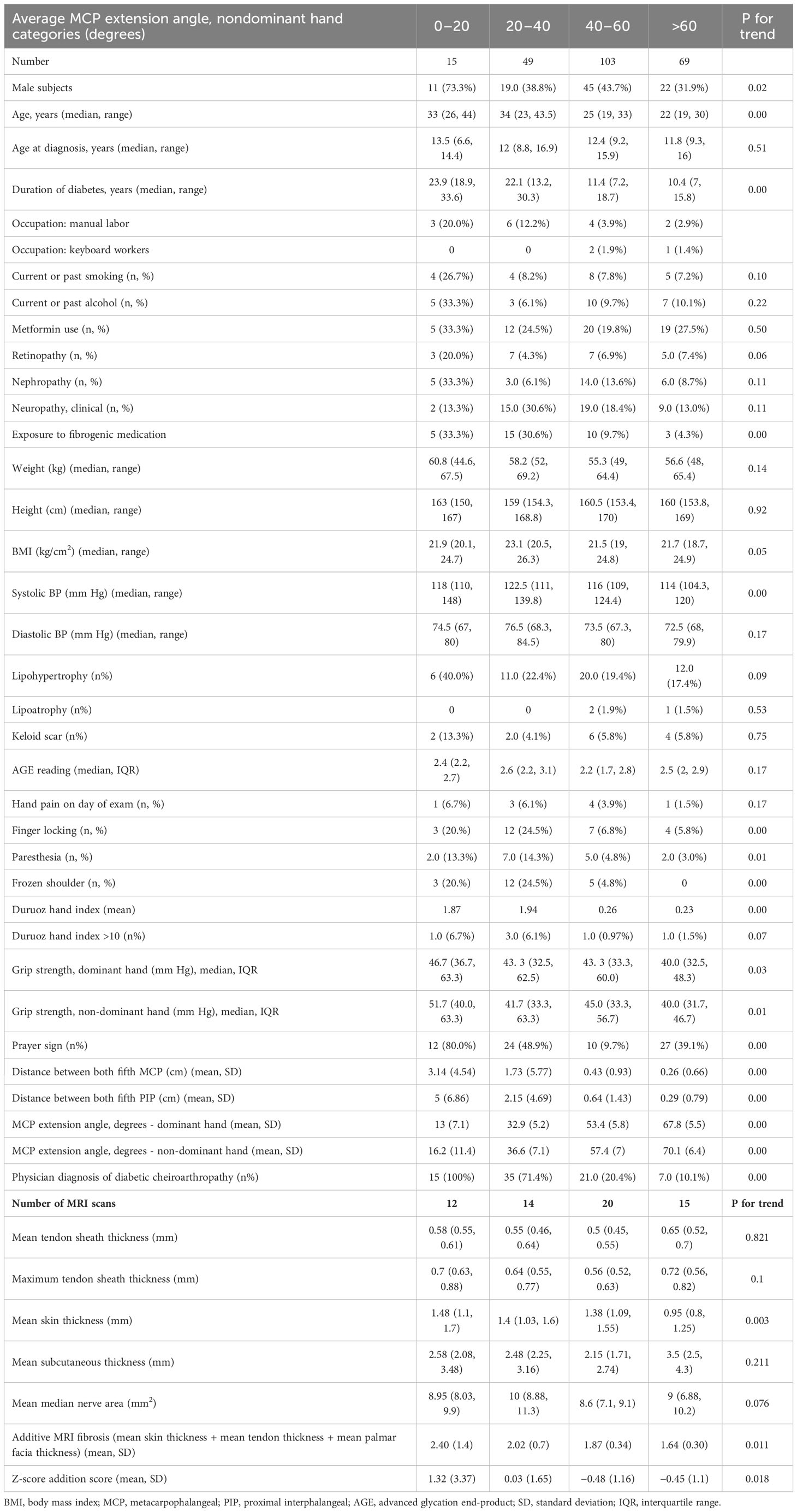
Table 2 Clinical and MRI findings in patients grouped by degree of metacarpophalangeal joint mobility restriction.
Hand joint restriction was rarely painful: Only 7% complained of hand pain even in the most limited group. Similarly, although more often seen in the restricted groups, only a small fraction had symptoms (14%) of neuropathy. Both observations translated into a small but statistically significant higher DHI in the restricted groups (mean of 1.9 compared with that of 0.2). Only six percent in the <20° group had a DHI of more than 10, which we considered clinically relevant hand function restriction. Most patients (80%) were observed to have a prayer sign in the <20° group. All patients in the <20° group and three-fourths of the 20°–40° group had at least one described cheiroarthropathy phenotype on physician opinion. In univariate linear regression analyses adjusted for gender and duration of diabetes, height, systolic blood pressure, urine–albumin creatinine ratio/clinically determined nephropathy, and the presence of frozen shoulder were significantly associated with average non-dominant hand MCP extension angle (Table 3).
MRI scans of the hand were performed in 61 patients (12 in the <20° group, 14 in the 20°–40° group, 20 in the 40°–60° group, and 15 in the >60° group). The radiologist’s findings included flexor tendon thickening or edema in four, whereas one has tenosynovial edema in the abductor pollicis longus tendon (De-Quervain tenosynovitis). One patient had median nerve neuritis, and one had bifid median nerve. Eight patients had ganglion cysts, five of which in dorsal scapholunate ligament. One patient had early MCP degenerative changes; other MRI scans were reported as normal.
When analyzed by group, there was no significant difference within the groups in mean tendon sheath thickness, maximum tendon sheath thickness or median nerve area (Table 2). The group with the most stiffness had the highest skin thickness and reduced progressively; but subcutaneous thickness did not differ across groups (Figure 2). Both the total hand fibrosis score and the total hand fibrosis Z-score were able to differentiate between the four groups (Table 2).
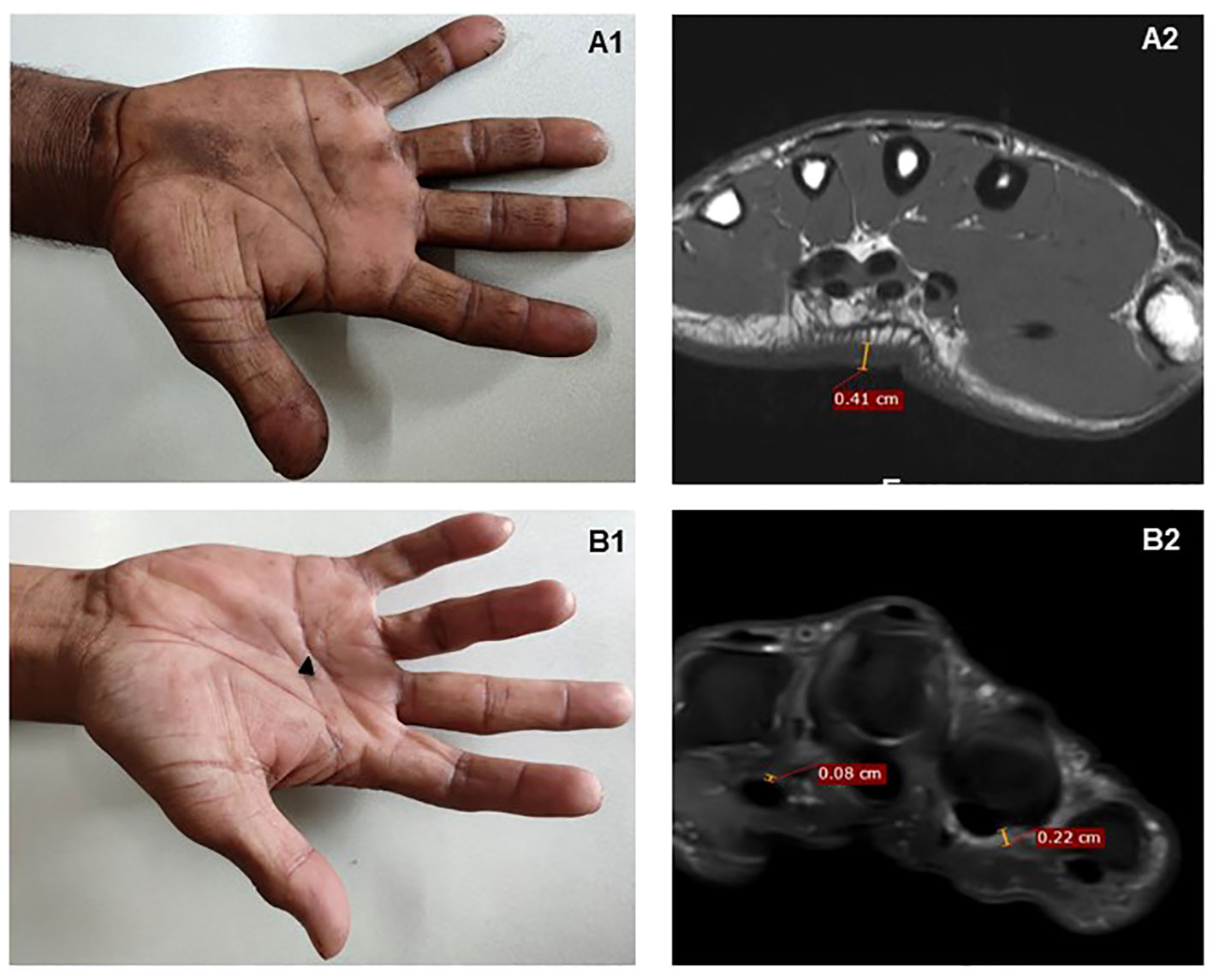
Figure 2 MRI findings in two patients with MCP extension restriction. (A1) A patient with severely restricted MCP extension – mean 10°. (A2) MRI demonstrates considerable palmar skin thickening alone on T1-weighted axial images, seen at mid-metacarpal level; there is no tenosynovial or palmar fascia thickening. (B1) A patient with bilateral Dupuytren’s contracture on the fourth finger (black arrow); (B2) PDFS scan shows ill-defined PDFS hyperintense soft tissue thickening in relation to flexor thickening also involving A1 pulley and the palmar fascia.
Mean palmar skin thickness correlated significantly with palmar fascia thickness (correlation coefficient of 0.298, p = 0.02) but not with mean tenosynovial thickness (p = 0.25). Similarly, mean tenosynovial thickness also correlated with palmar fascia thickness (correlation coefficient of 0.28, p = 0.03). Univariate linear regression showed that only palmar skin thickness and palmar fascia thickness correlated with MCP angle restriction (Table 4). Only mean palmar skin thickness remained significantly associated with MCP angle in multiple linear regression (Table 4).
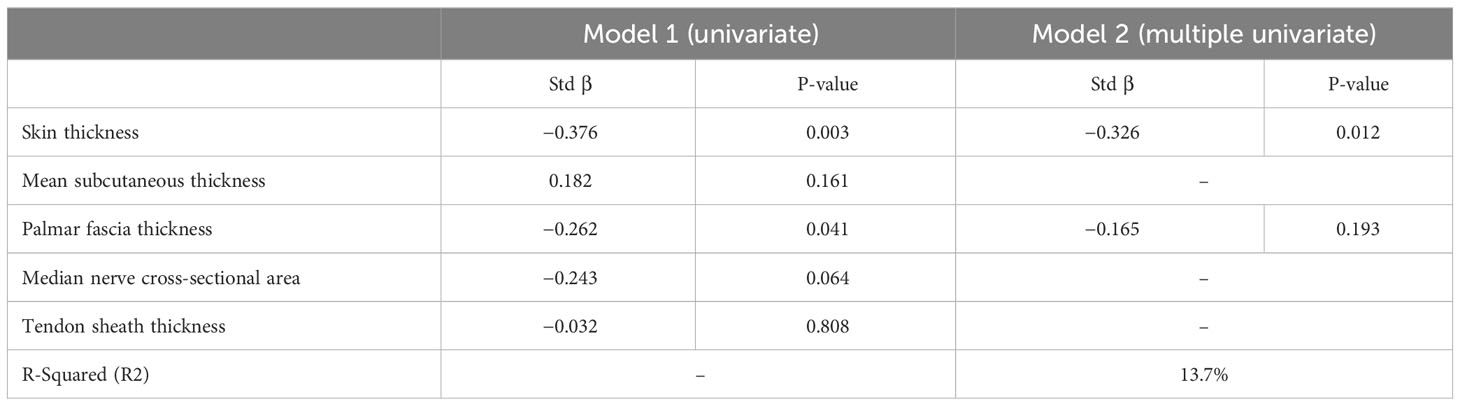
Table 4 Linear regression model for structural contributors to mean metacarpophalangeal extension on MRI.
One-third of an Indian cohort of adults with type 1 diabetes had diabetic cheiroarthropathy that was rarely symptomatic or functionally limiting but did substantially limit average MCP joint extension. The utility of the intuitively selected MCP extension limitation as a measure of hand stiffness is given plausibility by associations with structural correlates of fibrotic skin thickening on MRI. Expected features such as tenosynovial inflammatory edema or median nerve enlargement were rare. An additive score of tissue thickening was able to differentiate between levels of joint stiffness. Apart from novel insights into the patho-anatomy of hand stiffness, our data suggest a framework for quantification of hand fibrosis regardless of individual cheiroarthropathy manifestation, both on clinical examination and imaging. After further validation, they could be used as metrics for associative studies with other profibrotic manifestations and interventions.
Although cheiroarthropathy is often reported in both type 1 and type 2 diabetes, its implications in clinical practice are yet inconclusive. Age and duration of diabetes are pervasively associated, regardless of definitions and population (2, 7, 9). An older age and longer duration of the cohort explains the near double prevalence found in the Epidemiology of Diabetes Interventions and Complications/Diabetes Control and Complications Trial cohort as well as a Danish patient registry (2, 21) LJM prevalence reduced from 43% to 23% in adult patients with type 1 diabetes over two decades (7). Reduced prevalence has been attributed to better glycemic care; however, the association of hand manifestations with hemoglobin A1C is inconsistent (22). The pathobiology in the ones who do have hand manifestations is intriguing. Associations with microvascular disease including retinopathy are reported (2, 23) but not always replicated (9). Could hand stiffness demonstrate a profibrotic tendency not entirely explained by vascular disease? (14) We found some associative signals with systolic blood pressure, proteinuria, and adhesive capsulitis; each a proxy for arterial, renal, and musculoskeletal fibrosis, respectively, but not with other microvascular disease. Similar associations with nephropathy have been found with MCP and wrist flexion angles (18). Future studies of associations with markers of fibrotic liver and cardiac disease would be informative if the hand could be used as a clinical biomarker of internal fibrosis.
Although hand stiffness was common, it was rarely symptomatic or functionally limiting, thus making the case for active screening by a physician. Painful hand conditions are often encapsulated by functional indices such as the Health Assessment Questionnaire (HAQ); Disabilities of the Arm, Shoulder, and Hand (DASH); and the DHI (24–26). The higher DHI seen in our study in the stiffer group would not be considered clinically significant (20). Other studies echo the insubstantial functional impact of these conditions using different scores such as HAQ and the DASH score (2, 27). Only 10% of patients with diabetes volunteered symptoms in the hand, showing wide discrepancy in patient-volunteered symptoms and physician-examined manifestations (8). Symptomatic patients likely represent a subset with advanced stiffness; a quantitative metric would be useful if it can detect early manifestations, especially in irreversible but potentially preventable process like fibrosis. Most descriptions of joint stiffness use the table top test or prayer sign (28). Goniometry offers a more reproducible, quantitative metric. Using goniometry, authors showed that, although most joints assessed were less flexible in diabetics, the MCP and distal IP joints were most severely affected (29). We selected average resistance to MCP stretch as a metric that would intuitively encapsulate stiffness regardless of specific manifestation: Whereas LJM affects all fingers, DC and FTS may affect one or multiple digits. All of these would reduce mean MCP extension, but DC and FTS are not expected to reduce wrist extension. Although we did not check for MCP flexion, it is likely to be far less affected as attested by normal grip strengths; average MCP flexion was well preserved in a type 1 longitudinal cohort, with only a 10° loss over 15 years (18). Although ours is not a longitudinal study, the range of LJM extension was wide.
Previous MRI descriptions of diabetic cheiroarthropathy are limited to a single case report that showed flexor tenosynovial sheath thickening on T2-weighted images and tenosynovial proliferation on axial T1 fat saturation gadolinium enhancement (30). Similarly, flexor tendon sheath and subcutaneous tissue thickening were found on ultrasound in diabetic cheiroarthropathy (31). We found that skin thickening contributed most to LJM; these may be explained by differences in patient selection, population differences, and diagnostic modality. A notable feature in our cohort was the relatively normal size of the median nerve, suggesting that patients with type 1 diabetes may not have the expected increase in median nerve cross-sectional area routinely used to diagnose CTS (32). Median nerve cross-sectional area was smaller in diabetics with CTS (mean of 8.8 mm2) than with patients with CTS without diabetes (mean of 10.4 mm2) (33). Future work in conjunction with nerve conduction velocities is useful in determining whether patients with diabetes need separate cutoff values for MRI diagnosis of CTS.
Regardless of the degree of hand stiffness, inflammation on MRI was conspicuous by its absence, contrasting with MRI findings in FTS in rheumatoid arthritis (34) and systemic sclerosis (35). A small number of our patients, like the one in the previous case report, had tenosynovial edema (30). It is possible that the time of assessment along the temporal evolution of the manifestation makes a difference, with an inflammatory initiation continuing to fibrosis. Most inflammatory tenosynovitis cases are painful, although pain was rare in our group (34). It could be postulated that non-inflammatory profibrotic pathways such as AGE deposition and hypoxia might be more important disease mechanisms.
Our study has many strengths: To our knowledge, it is the first systematic evaluation of joint stiffness in type 1 diabetes, and the structural differences on MRI provide credence to MCP extension angle as a quantifiable clinical measure of hand fibrosis. Both clinical measurements and MRI measurements were performed by more than one assessor, suggesting a reproducibility of methods.
This study has limitations: Even the “controls” with no hand stiffness were diabetic, and future work would include age-matched non-diabetic controls. We restricted this study to type 1 diabetes, despite the hand conditions being seen in both types. We reckoned that patients with type 1 diabetes would have fewer clinical confounders, including age, medications with antifibrotic properties (such as metformin and pioglitazone), and accrual of damage with manual work. Future work would encompass both types of diabetes as well as prediabetes. We did not compare the performance of MCP extension to other joints. The groupings of 20° were arbitrarily decided on the basis of perceived convenience for a clinician, and each group had unequal numbers on MRI. The sample for imaging was not calculated statistically, because a goniometer was not used to measure MCP extension angles; however, the lack of specialized equipment would, in our opinion, make the screening measurement easier to perform in the community.
In conclusion, we found a substantial subset of patients with adult type 1 diabetes had diabetic cheiroarthropathy and resulting limitations in MCP extension. Joint mobility limitation was rarely symptomatic or functionally limiting, warranting an active search in the clinic. Joint stiffness was driven by skin thickening, and inflammatory tenosynovial involvement was rare on MRI. Our data provide a framework for a quantitative assessment of hand fibrosis even in subclinical disease, using mean MCP extension in the clinic, and using additive tissue thickness scores on MRI. Pending more extensive validation, these measurements could potentially be used to elucidate associations with internal organ fibrosis, as well as for sample size calculations and outcome measures for trials for musculoskeletal fibrosis.
A version of this article has been posted on the medRxiv preprint server.
SP is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by KEM Hospital Research Centre Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
SP, JI, PG, and CS were involved in the conception and design of the study. SC, SaJ, RS, and SP were involved in the conduct of the study. SP, KC, SC, SwJ, and AB were involved in analysis and interpretation of the results. SP and KC wrote the first draft of the manuscript. SP is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors contributed to the article and approved the submitted version.
This study is funded through a DBT/Wellcome India Alliance Clinical and Public health fellowship (IA/CPHE/19/504607) awarded to SP. The funding body had no role in study design or analysis. Many participants with type 1 diabetes in this study are beneficiaries of financial and social support from a ‘type 1 diabetes initiative’ of the following philanthropic donors: 1) Hinduja Foundation, 2) Mukul Madhav Foundation, 3) Nityasha. The authors are grateful to these organisations for their continued support in the management of people with type 1 diabetes.
Authors thank Prof. Satyajit Rath for methodological guidance; Dr. Kalpana Jog, Vidya Gokhale, Swati Alekar, and Dr. Neelima Nagarkar from the type 1 diabetes clinic; and Rasika Ladkat for study related administration.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Rosenbloom AL, Frias JL. Diabetes mellitus, short stature and joint stiffness - a new syndrome. Clin Res (1974) 22:92A. doi: 10.1203/00006450-197404000-00608
2. Larkin ME, Barnie A, Braffett BH, Cleary PA, Diminick L, Harth J, et al. Musculoskeletal complications in type 1 diabetes. Diabetes Care (2014) 37(7):1863–9. doi: 10.2337/dc13-2361
3. Gutefeldt K, Lundstedt S, Thyberg ISM, Bachrach-Lindström M, Arnqvist HJ, Spångeus A. Clinical examination and self-reported upper extremity impairments in patients with long-standing type 1 diabetes mellitus. J Diabetes Res (2020) 2020:4172635. doi: 10.1155/2020/4172635
4. Rydberg M, Zimmerman M, Gottsäter A, Svensson AM, Eeg-Olofsson K, Dahlin LB. Diabetic hand: prevalence and incidence of diabetic hand problems using data from 1.1 million inhabitants in southern Sweden. BMJ Open Diabetes Res Care (2022) 10(1):e002614. doi: 10.1136/bmjdrc-2021-002614
5. Cagliero E. Rheumatic manifestations of diabetes mellitus. Curr Rheumatol Rep (2003) 5(3):189–94. doi: 10.1007/s11926-003-0065-x
6. Pandey A, Usman K, Reddy H, Gutch M, Jain N, Qidwai S. Prevalence of hand disorders in type 2 diabetes mellitus and its correlation with microvascular complications. Ann Med Health Sci Res (2013) 3(3):349–54. doi: 10.4103/2141-9248.117942
7. Lindsay JR, Kennedy L, Atkinson AB, Bell PM, Carson DJ, McCance DR, et al. Reduced prevalence of limited joint mobility in type 1 diabetes in a U.K. clinic population over a 20-year period. Diabetes Care (2005) 28(3):658–61. doi: 10.2337/diacare.28.3.658
8. Chen LH, Li CY, Kuo LC, Wang LY, Kuo KN, Jou IM, et al. Risk of hand syndromes in patients with diabetes mellitus: A population-based cohort study in Taiwan. Med (Baltimore). (2015) 94(41):e1575. doi: 10.1097/MD.0000000000001575
9. Frost D, Beischer W. Limited joint mobility in type 1 diabetic patients: associations with microangiopathy and subclinical macroangiopathy are different in men and women. Diabetes Care (2001) 24(1):95–9. doi: 10.2337/diacare.24.1.95
10. Kameyama M, Chen KR, Mukai K, Shimada A, Atsumi Y, Yanagimoto S. Histopathological characteristics of stenosing flexor tenosynovitis in diabetic patients and possible associations with diabetes-related variables. J Handb Surg Am (2013) 38(7):1331–9. doi: 10.1016/j.jhsa.2013.03.049
11. Broekstra DC, Groen H, Molenkamp S, Werker PMN, van den Heuvel ER. A systematic review and meta-analysis on the strength and consistency of the associations between dupuytren disease and diabetes mellitus, liver disease, and epilepsy. Plast Reconstr Surg (2018) 141(3):367e–79e. doi: 10.1097/PRS.0000000000004120
12. Tuleta I, Frangogiannis NG. Diabetic fibrosis. Biochim Biophys Acta Mol Basis Dis (2021) 1867(4):166044. doi: 10.1016/j.bbadis.2020.166044
13. Ban CR, Twigg SM. Fibrosis in diabetes complications: pathogenic mechanisms and circulating and urinary markers. Vasc Health Risk Manage (2008) 4(3):575–96. doi: 10.2147/vhrm.s1991
14. Phatak S, Ingram JL, Goel P, Rath S, Yajnik C. Does hand stiffness reflect internal organ fibrosis in diabetes mellitus? Front Clin Diabetes Healthc. (2023) 4:1198782. doi: 10.3389/fcdhc.2023.1198782
15. Gokcen N, Cetinkaya Altuntas S, Coskun Benlidayi I, Sert M, Nazlican E, Sarpel T. An overlooked rheumatologic manifestation of diabetes: diabetic cheiroarthropathy. Clin Rheumatol (2019) 38(3):927–32. doi: 10.1007/s10067-019-04454-z
16. Merashli M, Chowdhury TA, Jawad AS. Musculoskeletal manifestations of diabetes mellitus. QJM. (2015) 108(11):853–7. doi: 10.1093/qjmed/hcv106
17. Casanova JE, Casanova JS, Young MJ. Hand function in patients with diabetes mellitus. South Med J (1991) 84(9):1111–3. doi: 10.1097/00007611-199109000-00013
18. Labad J, Rozadilla A, Garcia-Sancho P, Nolla JM, Montanya E. Limited joint mobility progression in type 1 diabetes: A 15-year follow-up study. Int J Endocrinol (2018) 2018:1897058. doi: 10.1155/2018/1897058
19. Maran A, Morieri ML, Falaguasta D, Avogaro A, Fadini GP. The fast-glycator phenotype, skin advanced glycation end products, and complication burden among people with type 1 diabetes. Diabetes Care (2022) 45(10):2439–44. doi: 10.2337/dc22-0980
20. Turan Y, Duruöz MT, Aksakalli E, Gürgan A. Validation of Duruöz Hand Index for diabetic hand dysfunction. J Investig Med (2009) 57(8):887–91. doi: 10.2310/JIM.0b013e3181b91c82
21. Wagner S, Nørgaard K, Willaing I, Olesen K, Andersen HU. Upper-extremity impairments in type 1 diabetes: results from a controlled nationwide study. Diabetes Care (2023) 46(6):1204–8. doi: 10.2337/dc23-0063
22. McCance DR, Crowe G, Quinn MJ, Smye M, Kennedy L. Incidence of microvascular complications in type 1 diabetic subjects with limited joint mobility: a 10-year prospective study. Diabetes Med (1993) 10(9):807–10. doi: 10.1111/j.1464-5491.1993.tb00170.x
23. Garg SK, Chase HP, Marshall G, Jackson WE, Holmes D, Hoops S, et al. Limited joint mobility in subjects with insulin dependent diabetes mellitus: relationship with eye and kidney complications. Arch Dis Child. (1992) 67(1):96–9. doi: 10.1136/adc.67.1.96
24. Ramchurn N, Mashamba C, Leitch E, Arutchelvam V, Narayanan K, Weaver J, et al. Upper limb musculoskeletal abnormalities and poor metabolic control in diabetes. Eur J Intern Med (2009) 20(7):718–21. doi: 10.1016/j.ejim.2009.08.001
25. Shah KM, Clark BR, McGill JB, Lang CE, Maynard J, Mueller MJ. Relationship between skin intrinsic fluorescence-an indicator of advanced glycation end products-and upper extremity impairments in individuals with diabetes mellitus. Phys Ther (2015) 95(8):1111–9. doi: 10.2522/ptj.20140340
26. Savaş S, Köroğlu BK, Koyuncuoğlu HR, Uzar E, Celik H, Tamer NM. The effects of the diabetes related soft tissue hand lesions and the reduced hand strength on functional disability of hand in type 2 diabetic patients. Diabetes Res Clin Pract (2007) 77(1):77–83. doi: 10.1016/j.diabres.2006.10.020
27. Franchignoni F, Vercelli S, Giordano A, Sartorio F, Bravini E, Ferriero G. Minimal clinically important difference of the disabilities of the arm, shoulder and hand outcome measure (DASH) and its shortened version (QuickDASH). J Orthop Sports Phys Ther (2014) 44(1):30–9. doi: 10.2519/jospt.2014.4893
28. Goyal A, Tiwari V, Gupta Y. Diabetic hand: A neglected complication of diabetes mellitus. Cureus. (2018) 10(6):e2772. doi: 10.7759/cureus.2772
29. Schulte L, Roberts MS, Zimmerman C, Ketler J, Simon LS. A quantitative assessment of limited joint mobility in patients with diabetes. Goniometric analysis of upper extremity passive range of motion. Arthritis Rheumatol (1993) 36(10):1429–43. doi: 10.1002/art.1780361016
30. Khanna G, Ferguson P. MRI of diabetic cheiroarthropathy. AJR Am J Roentgenol. (2007) 188(1):W94–5. doi: 10.2214/AJR.06.0672
31. Ismail AA, Dasgupta B, Tanqueray AB, Hamblin JJ. Ultrasonographic features of diabetic cheiroarthropathy. Br J Rheumatol (1996) 35(7):676–9. doi: 10.1093/rheumatology/35.7.676
32. Moran L, Perez M, Esteban A, Bellon J, Arranz B, del Cerro M. Sonographic measurement of cross-sectional area of the median nerve in the diagnosis of carpal tunnel syndrome: correlation with nerve conduction studies. J Clin Ultrasound. (2009) 37(3):125–31. doi: 10.1002/jcu.20551
33. Guillen-Astete CA, Luque-Alarcon M, Garcia-Montes N. Ultrasound assessment of the median nerve does not adequately discriminate the carpal tunnel syndrome among patients diagnosed with diabetes. Diabetology. (2021) 2(4):226–31. doi: 10.3390/diabetology2040020
34. Eshed I, Feist E, Althoff CE, Hamm B, Konen E, Burmester GR, et al. Tenosynovitis of the flexor tendons of the hand detected by MRI: an early indicator of rheumatoid arthritis. Rheumatol (Oxford). (2009) 48(8):887–91. doi: 10.1093/rheumatology/kep136
Keywords: magnetic resonance imaging (MRI), outcome measure (healthcare), tenosynovitis, metacarpophalangeal (MCP) joint, limited joint mobility (LJM), stiffness
Citation: Phatak S, Mahadevkar P, Chaudhari KS, Chakladar S, Jain S, Dhadge S, Jadhav S, Shah R, Bhalerao A, Patil A, Ingram JL, Goel P and Yajnik CS (2023) Quantification of joint mobility limitation in adult type 1 diabetes. Front. Endocrinol. 14:1238825. doi: 10.3389/fendo.2023.1238825
Received: 12 June 2023; Accepted: 17 October 2023;
Published: 06 November 2023.
Edited by:
Åke Sjöholm, Gävle Hospital, SwedenReviewed by:
Jue Ling, Nantong University, ChinaCopyright © 2023 Phatak, Mahadevkar, Chaudhari, Chakladar, Jain, Dhadge, Jadhav, Shah, Bhalerao, Patil, Ingram, Goel and Yajnik. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sanat Phatak, c2FuYXRwaGF0YWtAZ21haWwuY29t
†ORCID: Sanat Phatak, orcid.org/0000-0002-3548-9956
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.