- 1Department of Ultrasonography, The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, Wenzhou, China
- 2Department of Pulmonary and Critical Care Medicine, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China
- 3Department of Pulmonary and Critical Care Medicine, The Lu’an People’s Hospital of Anhui Province, The Lu’an Hospital Affiliated to Anhui Medical University, Lu’an, China
- 4Key Laboratory of Interventional Pulmonology of Zhejiang Province, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China
- 5The Quzhou Affiliated Hospital of Wenzhou Medical University, Quzhou People’s Hospital, Quzhou, China
Background: The oxidative balance score (OBS), an encompassing scoring mechanism for assessing oxidative stress, is formulated based on nutritional and lifestyle components. The emergence of metabolic syndrome (MetS) is intricately linked to oxidative stress. Nonetheless, the correlation between OBS and MetS displays variability within distinct cohorts.
Objective: We worked on the relationships between OBS and the risk of MetS, MetS severity, and all-cause mortality of MetS patients.
Methods: A total of 11,171 adult participants were collected from the U.S. National Health Examination Survey (NHANES) 2007-2018. Employing survey-weighted logistic models, we evaluated the relationship between OBS and MetS risk. Furthermore, survey-weighted linear models were utilized to investigate the connection between OBS and MetS severity. Among the participants, 3,621 individuals had their survival status recorded, allowing us to employ Cox proportional hazards regression models in order to ascertain the association between OBS and the all-cause mortality within the subset of individuals with MetS. The OBS (where a higher OBS signified an increased prevalence of anti- or pro-oxidant exposures) weighed the 20 factors, while the MetS severity score weighed the five factors.
Results: After multivariable adjustment, individuals with elevated OBS were found to exhibit a decreased susceptibility to MetS [odds ratio (OR) 0.95; 95% CI 0.94-0.96]. The adjusted OR was 0.42 (95% CI 0.33-0.53) for MetS risk in the fourth OBS quartile compared with those in the first OBS quartile (P for trend < 0.001). A one-unit increase in OBS was linked to a 3% reduction in MetS severity score by 3% (mean difference, -0.03; 95% CI, -0.04 to -0.03). Moreover, increased OBS correlated with decreased hazard of all-cause mortality risk among MetS subjects (adjusted hazard ratio, 0.95; 95% CI, 0.93-0.98). These associations retained their strength even subsequent to the introduction of sensitivity analyses. There existed a statistically significant negative correlation between diet/lifestyle OBS and both MetS risk as well as MetS severity.
Conclusions: An inverse correlation was observed between OBS and the susceptibility to MetS, MetS severity, and all-cause mortality of MetS patients. Health outcomes for MetS patients were positively related to antioxidant diets and lifestyles.
1 Introduction
Metabolic syndrome (MetS) and its associated disorders, a major risk factor for cardiometabolic complications (1), are responsible for the majority of non-communicable diseases’ deaths (2). MetS, a current silent epidemic disease, is a multi-faceted disorder with a highly complex and unclear pathogenesis (3). Growing evidence backs up that pro-oxidant/antioxidant imbalance plays a decisive role in a cluster of symptoms (4), including hypertension (5), obesity (6), insulin resistance (7), and NAFLD (8). Furthermore, antioxidant therapy has been proposed to have a beneficial effect on the prevention of MetS-related diseases (9–11), even though the benefits of antioxidant supplementation have not been confirmed by many epidemiological studies (12, 13).
Oxidative stress, which is to blame for in the development of many diseases (14), arises from an imbalance stemming from the excessive generation of harmful reactive oxygen and nitrogen species (15). This intricate equilibrium is governed by a complex network of antioxidants (16, 17). In a healthy state, this balance is upheld by the generation of highly reactive derivatives of oxygen metabolism (reactive oxygen species (ROS)) and their elimination by enzymatic/nonenzymatic antioxidants (18). An assortment of dietary (vitamin C, vitamin E, some carotenoids and flavonoids (17)) as well as non-dietary components (cigarette smoke and alcohol (19, 20)) can directly or indirectly contribute to the perturbation of the pro-oxidant/antioxidant equilibrium. Furthermore, different types of nutrient-mediated pro-oxidant/antioxidant imbalance possess the capacity to incite inflammatory responses (21). Notably, in postmenopausal Mexican women, markers of oxidative stress were significantly altered in those with MetS compared to those without, such as a drop in blood vitamin C (22).
An oxidative balance score (OBS) has been conceptualized through a series of observational investigations as a means to encompass the cumulative oxidative impacts arising from diverse exposures to pro-oxidants/antioxidants (23, 24). An OBS is constructed by aggregating scores based on quantiles or categories pertaining to dietary/lifestyle exposures. Moreover, the components included in this study were all discerned and validated within the framework of the National Health and Nutrition Examination Survey (NHANES) (23, 24), rendering them notably comprehensive within the ambit of this present study. Furthermore, the interaction between OBS and many diseases was evaluated, such as diabetes (25) and cardiovascular disease (26). However, the relationship between OBS and MetS risk has not been consistently demonstrated in all cohorts. For example, a small population-based study of Tehranian adults failed to confirm a remarkable correlation between OBS and the risk of MetS (27). Although the OBS approach is promising, the lack of consistent findings across different populations suggests that additional research is required to refine the methodology and establish its validity in diverse contexts.
Maximizing the comprehensiveness of OBS components and utilizing data from the U.S. National Health and Nutrition Examination Survey (NHANES) for analysis could enhance the precision of this correlation. The aim was to delve deeper into the potential connections between OBS (including dietary OBS and lifestyle OBS) and the susceptibility of MetS, MetS severity, and all-cause mortality of MetS patients. It was posited that oxidative stress was essential in the context of MetS, and there existed a conjecture regarding the potential variability of the OBS-MetS relationship across diverse populations. This investigation was carried out within the framework of the U.S. NHANES dataset.
2 Research design and methods
2.1 Study population
Participants in NHANES (2007-2018) were employed. Participants without even one valid diet recall, participants without information on OBS components, participants with tumors and cardiovascular disease, pregnant women, participants with implausible energy intakes (<500 kcal/day or >3,500 kcal/day for females and <800 kcal/day or >4,200 kcal/day for males) (23, 24, 28), participants without information on covariates (educational level and poverty income ratio (PIR)), and participants without information components of MetS were excluded. Eventually, 11,171 subjects were included (Figure 1).
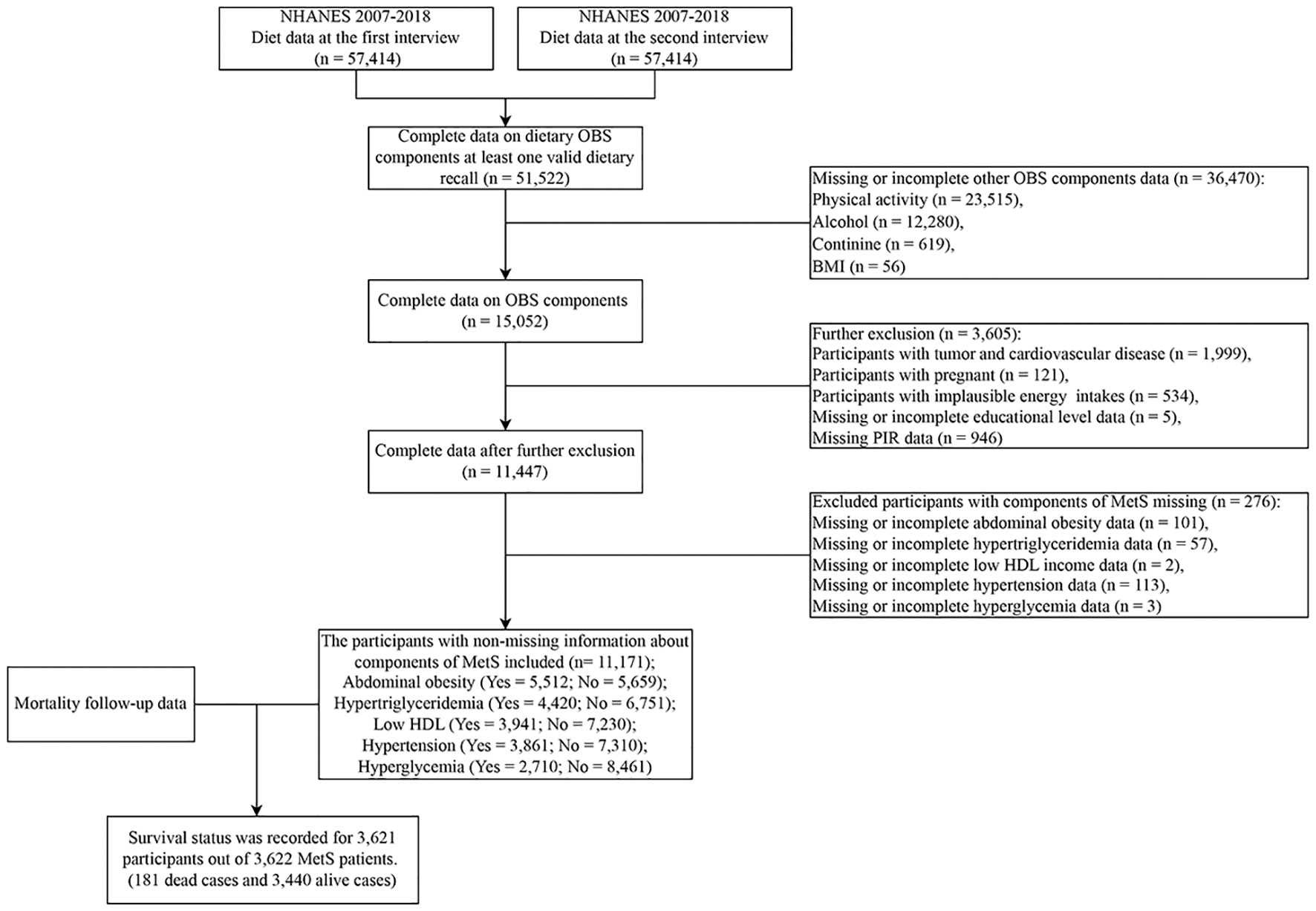
Figure 1 Flowchart of population included in our final analysis, U.S. National Health and Nutrition Examination Survey (NHANES), 2007-2018. OBS, oxidative balance score; MetS, Metabolic syndrome.
Utilizing a sophisticated sampling framework, the NHANES allows for the extrapolation of estimations to the broader spectrum of the U.S. populace. The NHANES includes interviews and examinations (29, 30). Ethical clearance for the execution of NHANES was obtained from the Ethics Review Board of the National Center for Health Statistics Moreover, comprehensive written informed consent was diligently obtained from each participant.
2.2 Main exposure
The main exposure was the OBS. Four lifestyle variables and sixteen nutrients were combined to create the OBS, and the information was described in Table S1. The 20 components in this study-five prooxidants and fifteen antioxidants (23, 24)-represented the pertinent variables identified in the NHANE. It is acknowledged that divergent datasets utilized in alternative studies may encompass a slightly disparate array of factors (27, 31) (Table S2). Nonetheless, given due consideration to the distinctive attributes of NHANES and a thorough review of antecedent investigations, our selection of factors is deemed comprehensive and reflective of the prevailing research landscape. Variables were categorically scored from 0 to 2 based on the sex-specific tertile of each component, and the point assignment for antioxidants and prooxidants was inverse. To enable generalization of the results, the calculation of the tertiles for each component was weighted. Then, the total score was calculated by the sum of the points for each of the 20 OBS components, and the possible OBS values were on a scale of 0 to 40. Moreover, the higher OBS indicated predominantly antioxidant exposures and lower OBS represented predominantly pro-oxidant exposures.
The sixteen nutrients were derived from the average nutrients’ intake during the 2 days. The two dietary interviews were conducted sequentially, in person and by telephone, respectively. We could obtain at least one 24-hour dietary recall of the participant and the participants’ dietary information from the U.S. Department of Agriculture Food and Nutrient Database for Dietary Studies.
The metabolic equivalent (MET) scores, which were calculated from data collected by the Physical Activity Questionnaire (PAQ), were assigned for physical activity. MET serves to quantify the relative energy expenditure associated with various activities. Within the PAQ survey, the MET values corresponding to distinct categories of activities, encompassing vigorous work-related takes, moderate work-related takes, walking or bicycling for transportation, vigorous leisure-time physical activities, and moderate leisure-time physical activities, were meticulously documented. Moreover, the quantification of physical activity was ascertained through the computation of the product derived by multiplying the MET value by the weekly frequency and duration of each specific physical activity (32).
Alcohol consumption was collected from the Alcohol Use Questionnaire, the average amount (drinks) of alcoholic beverages on those days when alcohol was consumed in the past 12 months was treated as sex-specific tertiles and subsequently assigned points. Body mass index (BMI) was from NHANES body measures collected by trained health technicians. Cotinine is generally regarded as the marker of active smoking and secondhand smoke exposure. This is owing to its distinct advantages, including a high concentration and a prolonged half-life. Moreover, the method of measurement of serum cotinine was described in detail in the NHANES website.
2.3 Main outcomes
The outcomes were the diagnosis of MetS and each of its components. The criteria of MetS are based on the National Cholesterol Education Program Adult Treatment Panel III (33, 34). Participants considered to be with MetS required the presence of at least three of five components (33). Table S3 showed the definition of MetS.
The data on blood pressure was collected from the acquisition of a minimum of three successive readings during blood pressure measurements. These readings were subsequently computed to derive the arithmetic mean. Details concerning the anti-hypertensive treatment and the diagnosis of hypertension were collected from the blood pressure/cholesterol section in the interview data. Similarly, information regarding anti-diabetic drugs, taking insulin, and the diagnosis of diabetes were meticulously collated from the interview data’s diabetes section.
However, although previous studies have reported several methods to quantify MetS, there is still no validated tool. We chose a continuous MetS severity score developed by Gurka and colleagues (35), because it has been applied in other NHANES studies (36). The MetS severity score weighs the five factors in a way that takes into account gender and race/ethnicity differences in risk status. Operationalized definitions in NHANES have been documented in other studies (36). Moreover, the Homeostatic Model Assessment for insulin resistance (HOMA-IR) was applied to reflect the status of patients.
Furthermore, mortality follow-up data spanning from the date of survey participation through December 31, 2019, were compiled by leveraging information from the National Death Index data (https://www.cdc.gov/nchs/data-linkage/mortality-public.htm).
2.4 Covariate definitions
Potential covariates included demographic data, such as age (years), gender (male, female), race/ethnicity (non-Hispanic White, non-Hispanic Black, Mexican American, and other), educational level (<high school, high school/general educational development, and >high school), PIR, and dietary energy intake.
2.5 Statistical analyses
Data on demographic characteristics, dietary intake, and outcomes (continuous variables) were shown as the median (P25, P75), while categorical variables were represented as numbers (percentages). The Kruskal-Wallis test was employed for the comparison of continuous baseline characteristics, as the continuous variables analyzed in this study were determined to exhibit non-normal distribution, and the Rao-Scott chi-squared test was adopted to test for categorical variables.
Survey weights from full sample 2-year interview weights (WTINT2YR) were used as recommended by NHANES (1/6 * WTINT2YR), and weighted models were conducted in the following regression analyses. Logistic models were conducted to estimate associations of OBS with the presence of MetS (dichotomous outcomes), the OBS was also categorized into quartiles and served as a categorical variable. Furthermore, linear models were performed to estimate the relationships of OBS with continuous outcomes (HOMA-IR and MetS severity score). Three models were conducted: Model one was the crude model; Model two adjusted for age, gender, race/ethnicity, educational level, and PIR; Model three further adjusted for dietary energy intake based on Model two. These covariates were chosen because they were regarded as clinically relevant confounders of the relationship between OBS and MetS. Moreover, weighted Cox proportional hazards regression models were employed to ascertain and quantify the potential associations of OBS with all-cause mortality among MetS participants.
Analysis was performed in R 4.1.1. P for trend was calculated by converting categorical variables to continuous variables. P for interaction was also calculated. Statistical significance was defined at a two-sided P value < 0.05.
2.6 Sensitivity analysis
To estimate the robustness of the results, some sensitivity analyses were undertaken. 1) Stratified analyses by stratifying factors including gender, age, education level, and PIR were performed. 2) The P for interaction was carried out. 3) Examination of the relationships based on dietary OBS and lifestyle OBS. 4) The analysis of removing one component from the total score at one time was conducted.
3 Results
3.1 Baseline characteristics
Among 11,171 participants, a total of 3,621 subjects were identified as having MetS. The distribution of OBS exhibited statistically significant variations across demographic parameters such as age, gender, race, and education level (P<0.05), as delineated in Table S4. Notably, individuals afflicted with MetS demonstrated comparatively lower OBS scores (19 [14, 25]) in contrast to their counterparts without MetS (21 [15, 26]). Furthermore, an elevated OBS was discerned among survivors in comparison to individuals who succumbed, underscoring a potential association between OBS and survival outcomes. According to the results, the variations in the studied variables across different quartiles of OBS were revealed in Table 1. The prevalence of MetS was observed to be comparatively lower among those in the 4th OBS quartile (OBS ≥26) in contrast to in the other three quartiles. Moreover, individuals exhibiting lower OBS values displayed elevated levels of both the HOMA-IR and MetS severity scores in comparison to those with higher OBS values. Notably, statistically significant differences were detected between the OBS quartile groups for each component of MetS (P < 0.05). Persons in a higher quartile were more prone to have higher age, PIR, energy intake, and educational level. No significant differences in gender across OBS quartiles were observed.
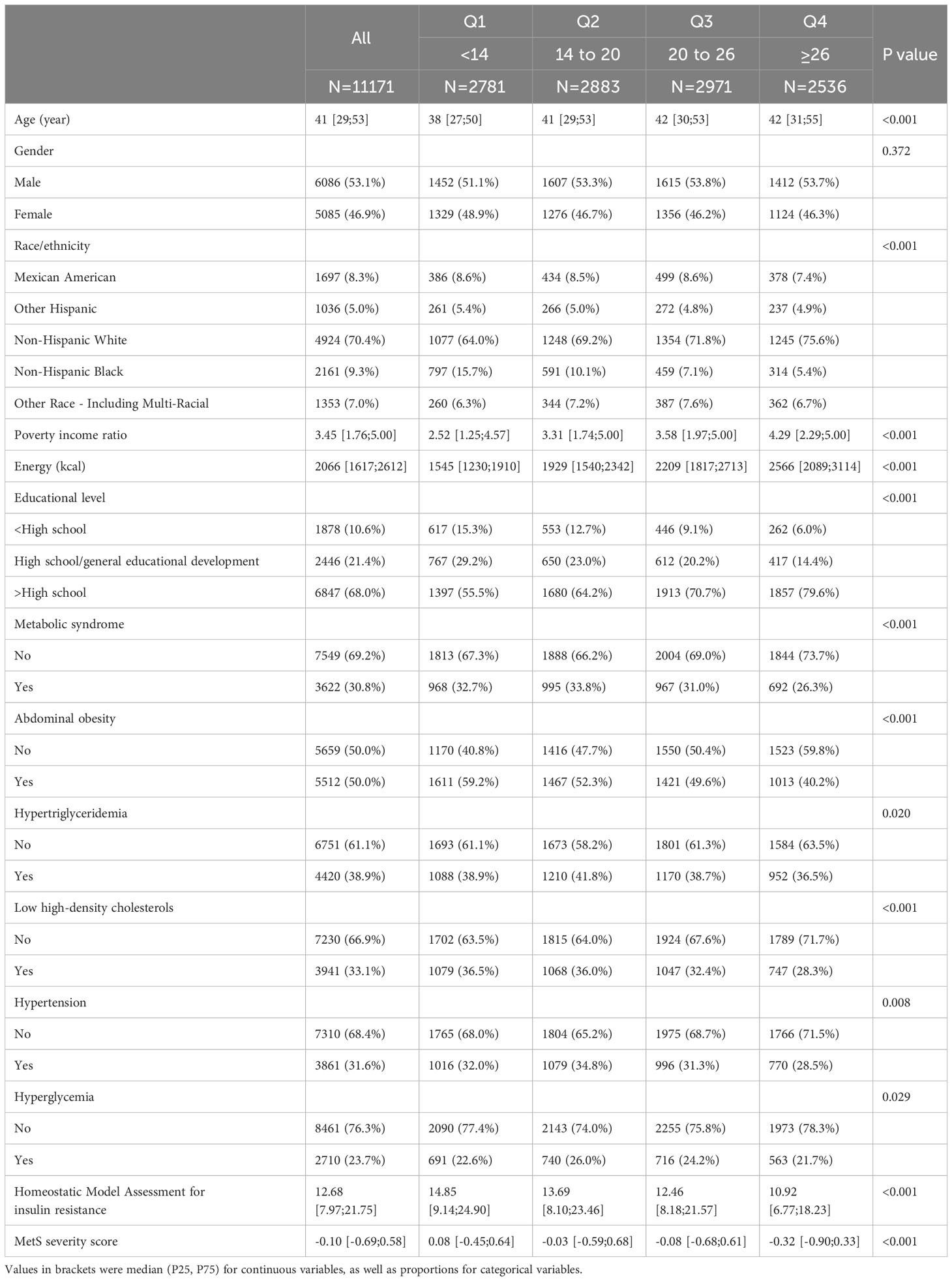
Table 1 Characteristics of participants based on quartiles of oxidative balance score, National Health and Nutrition Examination Survey (NHANES) 2007-2018.
3.2 Association of oxidative balance score with metabolic syndrome and its components
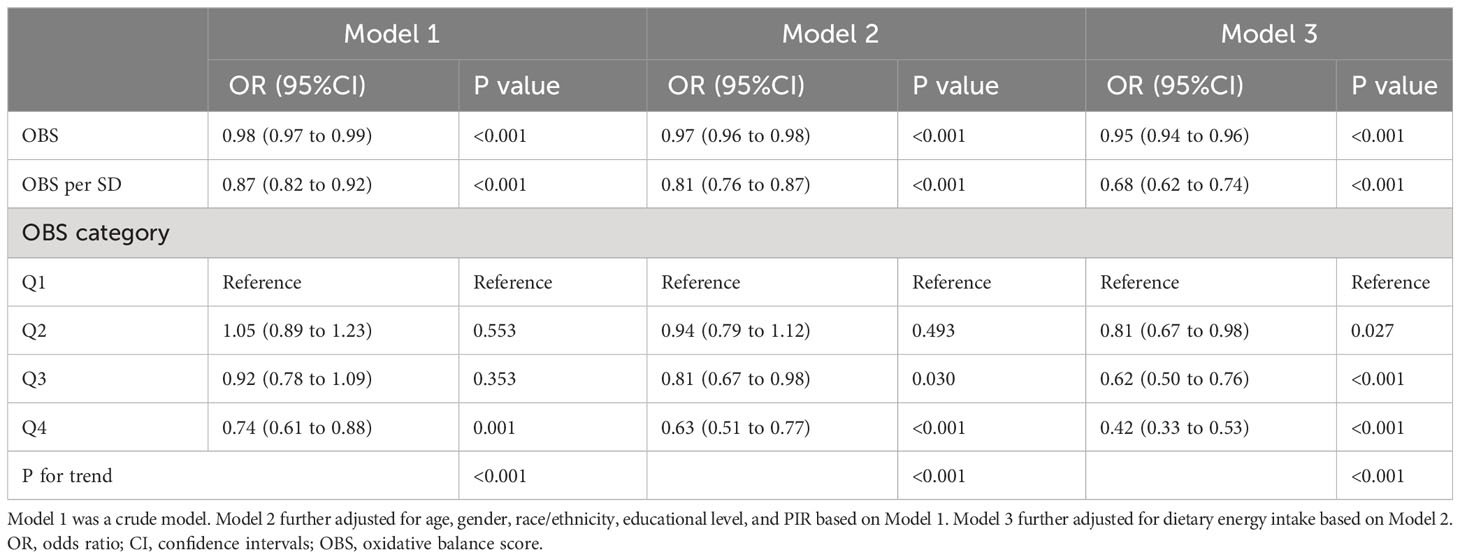
Table 2 and Table S5 summarized the results of correlations between OBS and MetS. The OBS was significantly negatively related to MetS (adjusted odds ratio (OR): 0.95; 95%CI: 0.94-0.96) after full adjustment. Every one-SD increase in OBS was linked with 32% decreased odds of MetS after full adjustment. When OBS was deemed as a categorical variable, there was also a negative correlation between the highest quartile OBS and MetS (adjusted OR: 0.42; 95%CI: 0.33-0.53), after full adjustment, compared with the lowest quartile OBS. The relationship was also observed in Model 1 and Model 2. Moreover, significant associations were also discovered in the 3rd quartile OBS (Model 2 & Model 3) and the 2nd quartile OBS (Model 3). Importantly, the P for trend was <0.001 in all models.
Regarding the MetS components, significant negative associations were observed between OBS with abdominal obesity (adjusted OR: 0.93; 95%CI: 0.92-0.93), hypertriglyceridemia (adjusted OR: 0.96; 95%CI: 0.95-0.97), low high-density cholesterols (adjusted OR: 0.96; 95%CI: 0.95-0.97), hypertension (adjusted OR: 0.96; 95%CI: 0.95-0.97), and hyperglycemia (adjusted OR: 0.98; 95%CI: 0.97-0.99). Similarly, the relationships between OBS quartiles and each MetS component were consistently apparent. Remarkably, these relationships were found to be statistically significant within the highest OBS quartile group (all P values for trend < 0.05), as illustrated in Table S5.
3.3 The relationship between oxidative balance score and metabolic syndrome severity
The continuous MetS severity score, quantified by the Mets Z-score, is exclusively accessible for adult individuals belonging to Hispanic, white, and black adults (35). The adjusted formula can also be applied to Asian populations (37). Importantly, the score was applied in other NHANES studies (36). However, no formula is currently available for the Mexican-American population. Therefore, we excluded Mexican Americans and some incomplete information, and eventually, 4,380 of 11,171 individuals were calculated for the MetS Z-score.
The adjusted linear regression model indicated that, even after accounting for potential confounders, for each one-unit increment in OBS, a person’s MetS Z-score decreased by 3%. The adjusted mean differences (MDs) for MetS were as follows: -0.17 (-0.29, -0.06) for the 2nd OBS quartile, -0.29 (-0.39, -0.20) for the 3rd OBS quartile, and -0.56 (-0.68, -0.44) for the 4th OBS quartile, in comparison to this reference group (participants in the lowest OBS quartile as the reference). These findings suggested a consistent trend of decreasing MetS severity scores with higher OBS quartiles, as supported by a statistically significant trend (P for trend < 0.05) (Table 3).
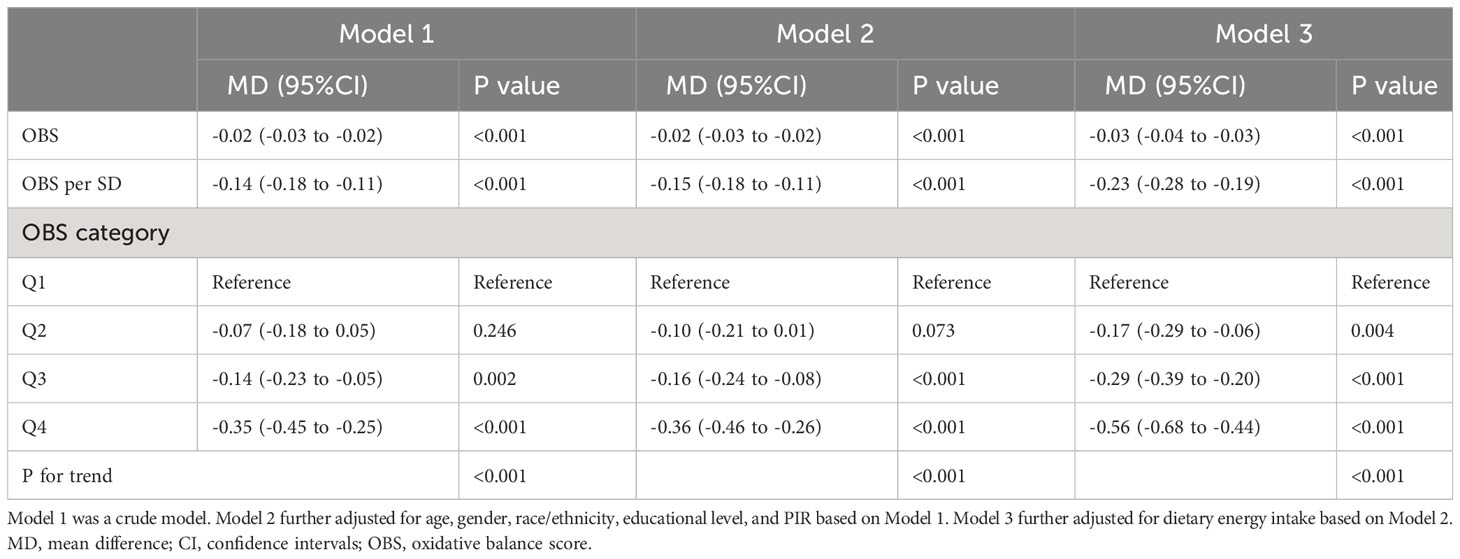
Table 3 The relationship between oxidative balance score and metabolic syndrome severity (MetS Z-score).
Furthermore, the degree of insulin resistance was also evaluated through the HOMA-IR, with calculations available for 5,378 out of the total 11,171 individuals possessing complete data. Increased OBS was related to lower HOMA-IR (adjusted MD, -0.53; 95% CI: -0.71, -0.35). The adjusted MD for the highest OBS quartile was -8.62 (-12.64, -4.60) (P for trend < 0.001) (Table S6).
3.4 The association between oxidative balance score and all-cause mortality among metabolic syndrome patients
Survival status was recorded for 3,622 participants out of 3,621 MetS patients, with 181 dead cases and 3,440 alive cases. Due to the low prevalence of deaths among MetS patients (45 cases of coronary heart disease-related dead cases and 49 cases of cancer-related dead cases), we will not explore the correlation of OBS with cause-specific mortality. The weighting algorithm enabled the sampling sample to reflect the overall sample.
For MetS patients, a one-unit OBS increase was related to a significant decrease in the risk of all-cause mortality (adjusted hazard ratio (HR) 0.96; 95% CI; 0.92-0.9995). When the subjects in the lowest quartile of the OBS were served as a reference, the participants in the high quartiles had a decreased risk of all-cause mortality [3rd quartile: 0.66 (0.18-0.82); 4th quartile: 0.38 (0.18-0.82)] after full adjustment (Table 4). Both dietary OBS and lifestyle OBS played an important role, and they were both negatively related to the risk of all-cause mortality in subjects with MetS. The strength of the association between OBS and the risk of all-cause mortality among males with MetS might be pronounced, but the association was statistically significant for male participants solely in Model 1 and Model 2, but not in Model 3 (Table 4).
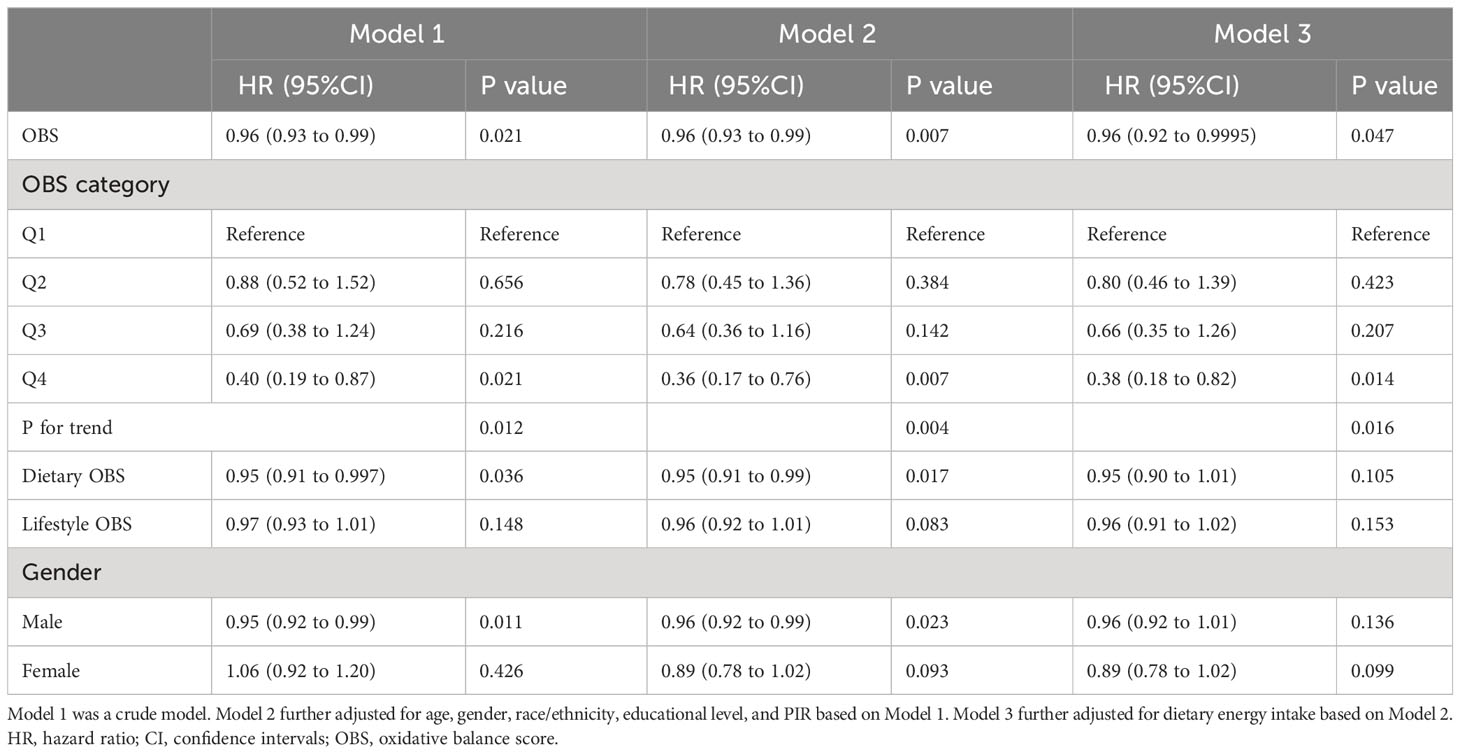
Table 4 Associations between oxidative balance score and the risk of all-cause mortality among metabolic syndrome patients.
3.5 Sensitivity analyses
The relationships of dietary/lifestyle OBS with the susceptibility of MetS were also estimated (Table 5). Notably, both dietary OBS and lifestyle OBS exhibited inverse associations with the risk of MetS, alongside reductions in MetS severity score (P for interaction <0.05). Additionally, the negative correlations between OBS and the prevalence of MetS, as well as MetS severity, persisted even after conducting stratified analyses based on gender, age, education, and PIR (Figure 2 and Table S7). Furthermore, the exclusion of any individual OBS component did not yield a substantial impact on the association between OBS and the risk of MetS, nor on the severity of MetS or all-cause mortality of MetS patients, as elucidated in Table S8.

Table 5 Associations between the dietary/lifestyle OBS and metabolic syndrome/metabolic syndrome severity score.
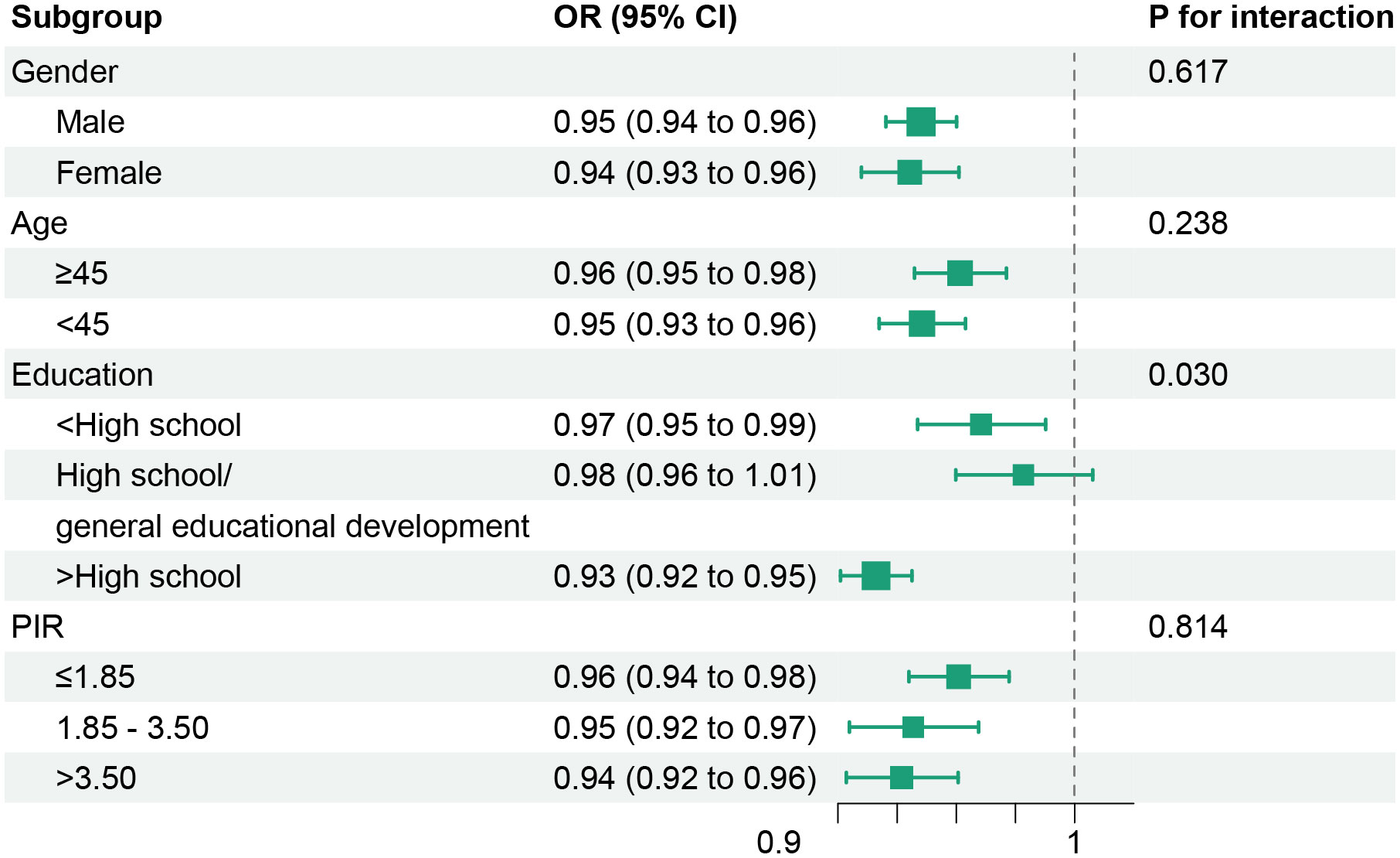
Figure 2 Stratified analyses of associations between oxidative balance score with metabolic syndrome risk. Models were adjusted for age, gender, race/ethnicity, educational level, PIR, and dietary energy intake (stratification factor itself was not included when stratifying). OR, odds ratio; CI, confidence intervals; PIR, poverty income ratio.
4 Discussion
In the present study, a range of models was employed to analyze data containing 11,171 participants of NHANES. We examined the relationships of OBS with the risk of MetS, MetS severity, and all-cause mortality of MetS patients. Elevated OBS was linked to a reduced risk of MetS, lower MetS severity, and diminished all-cause mortality of MetS patients. Adherence to an antioxidant diet and lifestyle would be beneficial to MetS patients. The implications of these findings underscore the potential benefits of adopting antioxidant-rich diets and lifestyles for individuals afflicted with MetS. The study underscores the significance of advocating for healthy antioxidant practices and dietary habits as integral strategies for the prevention and management of MetS.
One previous cross-sectional study explored the correlation between OBS and MetS risk in Iranian adults (27). This population-based cross-sectional study by Noruzi et al. (847 participants) exhibited no significant association between OBS and MetS (27). This OBS was derived by summing tertile scores of only thirteen pro-/antioxidant components. However, the components were different from those of our OBS. In addition to alcohol consumption, our composition includes a new selection of six components (riboflavin (38), niacin (39), vitamin B6 (40), vitamin B12 (41), magnesium (42), and copper (43)) in this study. Notably, these six components were chosen based on their relationship to both redox and MetS. Previous nhanes-based studies have revealed that increased intake of niacin and vitamin B6 were linked with reduced risk of MetS (44). Vitamin B2 deficiency consequence of insulin resistance and MetS (45). The results based on the CARDIA study indicated that both dietary consumption of vitamins B6 and B12 were negatively related to the incidence of MetS, and serum concentrations of them exhibited a similar pattern (46). In a prospective Chinese cohort study, it was shown that dietary magnesium consumption was inversely linked with MetS (47). One meta-analysis indicated an inverse correlation between dietary copper and MetS (48). Our study built on these previous findings by utilizing a different set of components to derive OBS and investigating its relationship with MetS, MetS severity, and all-cause mortality in a large NHANES-based sample.
In support of our findings, an observational study of 6,400 Koreans aged >40 years revealed a tight correlation between OBS and MetS risk (49). The study also demonstrated that subjects with the highest OBS quartile had a decreased risk for MetS than those with the lowest quartile (49). However, the OBS components included in this Korean cohort were different from ours, which included only 7 components (iron, β-carotene, vitamins C, retinol, smoking, alcohol consumption and physical activity). Moreover, we conducted a comprehensive assessment of outcomes, encompassing both the severity of MetS and all-cause mortality among MetS individuals. Furthermore, through evaluating the antioxidant status of Thai subjects with MetS, we could find that an alteration in antioxidant status was associated with MetS (50). Our results, together with these previous findings, underscore the importance of maintaining a balance between pro-oxidants and antioxidants in preventing and managing MetS patients.
Our results also exhibited that higher OBS was related to lower MetS severity. The degree of oxidative stress depends on the severity of MetS (51). Previous studies have shown that participants with higher MetS severity scores tended to have less physical activity, a pro-inflammatory dietary pattern, and lower adherence to the Mediterranean diet (52). However, a study that included 63 patients with MetS indicated no notable correlations between MetS severity and spot oxidative stress or exercise-induced oxidative stress biomarkers (53). This may be because the isolated contribution of a single component is difficult to ascertain. The imbalance between antioxidants and pro-oxidants is regarded as playing an essential role in both MetS and its constituent disorders (18).
Interestingly, for MetS participants, OBS was negatively associated with all-cause mortality, but the association was tighter in male subjects than female subjects. Previous investigations have revealed that males with MetS have a higher mortality risk than females (54, 55). Male subjects tended to have a higher prevalence of smoking, and smoking played an essential role in interpreting the gender difference in mortality (56). The correlation between MetS and unfavorable prognosis was likely influenced by a variety of controllable and uncontrollable factors (57). Our study suggests that adherence to an antioxidant diet and lifestyle might be beneficial to reducing all-cause mortality in individuals with MetS, particularly in males.
Our study had several strengths. Firstly, we employed the use of OBS, which integrated several dietary and lifestyle components into a comprehensive score, to better capture the intricate relationships among various factors associated with MetS. Moreover, a wide range of commonly used models were utilized to investigate not only the association of OBS with the risk of MetS but also the associations of OBS with MetS severity and all-cause mortality of MetS patients. Furthermore, the sensitivity analyses warranted the robustness of the associations.
Our study also had several limitations. Firstly, due to the utilization of only sixteen nutrients and four lifestyle factors being employed, our ability to acquire more accurate OBS compositions is probably limited. Considering that composite measures may provide a more precise representation of health outcomes in comparison to individual pro-oxidant/antioxidant exposure (58, 59), the OBS compositions are supposed to use a richer set of features, such as flavonoids, medication type, and additional information on diet and lifestyle. Secondly, OBS is unable to assess threshold effects and does not respond to dynamic alterations in antioxidant and pro-oxidant states. Thirdly, although our study included 11,171 participants, our analysis of mortality was limited by the relatively small number of deaths (n=181) among MetS patients. The effectiveness of OBS should be validated in larger data sets. Furthermore, the lack of biomarkers of oxidative stress in this study prevented us from verifying the effectiveness of OBS for oxidative balance assessment. Finally, in the examination concerning the relationship between OBS and MetS risk, as well as MetS severity, the outcomes attained solely indicated correlations between these factors due to the utilization of cross-sectional data.
Despite this limitation, we still found that the relationship between MetS and OBS was robust. In addition, although we were careful to perform sensitivity analyses to validate each model. This feature of NHANES’ complex sampling design enables our results to be extended to all noninstitutionalized resident adults in the US. Furthermore, it was necessary to confirm these correlations in additional retrospective and prospective populations, because we could not determine whether it was the same in other populations of different ethnicity or other cohorts.
5 Conclusion
This study illustrates inverse associations between OBS and the risk of MetS, as well as MetS severity and all-cause mortality of MetS patients. Higher OBS indicates greater exposure to antioxidants. This study advocates that an antioxidant diet and lifestyle contribute to the well-being of individuals with MetS. Our study underscores the pivotal role of antioxidant exposure in effectively managing MetS patients, thereby proposing a potential strategy to enhance clinical outcomes in this population. Future randomized controlled trials are necessary to firmly establish these findings and investigate the clinical effectiveness of antioxidant interventions among MetS management.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: NHANES website (https://www.cdc.gov/nchs/nhanes/index.htm). The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by National Center for Health Statistics Ethics Review Board (Protocol Number: Protocol #2005-06; Protocol #2011-17). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. This study involved secondary data analysis of the National Health and Nutrition Examination Survey, and this study we conducted was exempt from institutional review for this reason.
Author contributions
Conceptualities, funding acquisition, and writing-review and editing, CC and RY; methodology, software, and writing-original draft preparation, ZX; validation, data curation and writing-review and editing, XL and WC; writing-review and editing, LW. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was financially supported by the Key Laboratory of Interventional Pulmonology of Zhejiang Province (2019E10014), the Zhejiang Provincial Key Research and Development Program (2020C03067), and the National Nature Science Foundation of China (82170017).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1233145/full#supplementary-material
References
1. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation (2009) 120(16):1640–5. doi: 10.1161/circulationaha.109.192644
2. Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep (2018) 20(2):12. doi: 10.1007/s11906-018-0812-z
3. Wang HH, Lee DK, Liu M, Portincasa P, Wang DQ. Novel insights into the pathogenesis and management of the metabolic syndrome. Pediatr Gastroenterol Hepatol Nutr (2020) 23(3):2234–8646. doi: 10.5223/pghn.2020.23.3.189
4. Spahis S, Borys JM, Levy E. Metabolic syndrome as a multifaceted risk factor for oxidative stress. Antioxid Redox Signal (2017) 26(9):445–61. doi: 10.1089/ars.2016.6756
5. Sinha N, Dabla PK. Oxidative stress and antioxidants in hypertension-a current review. Curr Hypertens Rev (2015) 11(2):132–42. doi: 10.2174/1573402111666150529130922
6. Bondia-Pons I, Ryan L, Martinez JA. Oxidative stress and inflammation interactions in human obesity. J Physiol Biochem (2012) 68(4):701–11. doi: 10.1007/s13105-012-0154-2
7. Henriksen EJ, Diamond-Stanic MK, Marchionne EM. Oxidative stress and the etiology of insulin resistance and type 2 diabetes. Free Radic Biol Med (2011) 51(5):993–9. doi: 10.1016/j.freeradbiomed.2010.12.005
8. Chen Z, Tian R, She Z, Cai J, Li H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic Biol Med (2020) 152:116–41. doi: 10.1016/j.freeradbiomed.2020.02.025
9. Abdali D, Samson SE, Grover AK. How effective are antioxidant supplements in obesity and diabetes? Med Princ Pract (2015) 24(3):201–15. doi: 10.1159/000375305
10. Gregório BM, De Souza DB, de Morais Nascimento FA, Pereira LM, Fernandes-Santos C. The potential role of antioxidants in metabolic syndrome. Curr Pharm Des (2016) 22(7):859–69. doi: 10.2174/1381612822666151209152352
11. Ahmad KA, Yuan Yuan D, Nawaz W, Ze H, Zhuo CX, Talal B, et al. Antioxidant therapy for management of oxidative stress induced hypertension. Free Radic Res (2017) 51(4):428–38. doi: 10.1080/10715762.2017.1322205
12. Katsiki N, Manes C. Is there a role for supplemented antioxidants in the prevention of atherosclerosis? Clin Nutr (2009) 28(1):3–9. doi: 10.1016/j.clnu.2008.10.011
13. Goszcz K, Deakin SJ, Duthie GG, Stewart D, Leslie SJ, Megson IL. Antioxidants in cardiovascular therapy: panacea or false hope? Front Cardiovasc Med (2015) 2:29. doi: 10.3389/fcvm.2015.00029
14. Rajendran P, Nandakumar N, Rengarajan T, Palaniswami R, Gnanadhas EN, Lakshminarasaiah U, et al. Antioxidants and human diseases. Clin Chim Acta (2014) 436:332–47. doi: 10.1016/j.cca.2014.06.004
15. Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biol (2015) 4:180–3. doi: 10.1016/j.redox.2015.01.002
16. Fang YZ, Yang S, Wu G. Free radicals, antioxidants, and nutrition. Nutrition (2002) 18(10):872–9. doi: 10.1016/s0899-9007(02)00916-4
17. Halliwell B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol (2006) 141(2):312–22. doi: 10.1104/pp.106.077073
18. Hutcheson R, Rocic P. The metabolic syndrome, oxidative stress, environment, and cardiovascular disease: the great exploration. Exp Diabetes Res (2012) 2012:271028. doi: 10.1155/2012/271028
19. Albano E. Alcohol, oxidative stress and free radical damage. Proc Nutr Soc (2006) 65(3):278–90. doi: 10.1079/pns2006496
20. Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact (2006) 160(1):1–40. doi: 10.1016/j.cbi.2005.12.009
21. Tan BL, Norhaizan ME, Liew WP. Nutrients and oxidative stress: friend or foe? Oxid Med Cell Longev (2018) 2018:9719584. doi: 10.1155/2018/9719584
22. Montoya-Estrada A, Veruete-Bedolla DB, Romo-Yañez J, Ortiz-Luna GF, Arellano-Eguiluz A, Najéra N, et al. Markers of oxidative stress in postmenopausal women with metabolic syndrome. J Obstet Gynaecol (2022) 42(6):2387–92. doi: 10.1080/01443615.2022.2062223
23. Xu Z, Xue Y, Wen H, Chen C. Association of oxidative balance score and lung health from the National Health and Nutrition Examination Survey 2007-2012. Front Nutr (2022) 9:961950. doi: 10.3389/fnut.2022.961950
24. Zhang W, Peng SF, Chen L, Chen HM, Cheng XE, Tang YH. Association between the oxidative balance score and telomere length from the national health and nutrition examination survey 1999-2002. Oxid Med Cell Longev (2022) 2022:1345071. doi: 10.1155/2022/1345071
25. Golmohammadi M, Ayremlou P, Zarrin R. Higher oxidative balance score is associated with better glycemic control among Iranian adults with type-2 diabetes. Int J Vitam Nutr Res (2021) 91(1-2):31–9. doi: 10.1024/0300-9831/a000596
26. Ilori TO, Wang X, Huang M, Gutierrez OM, Narayan KM, Goodman M, et al. Oxidative balance score and the risk of end-stage renal disease and cardiovascular disease. Am J Nephrol (2017) 45(4):338–45. doi: 10.1159/000464257
27. Noruzi Z, Jayedi A, Farazi M, Asgari E, Dehghani Firouzabadi F, Akbarzadeh Z, et al. Association of oxidative balance score with the metabolic syndrome in a sample of Iranian adults. Oxid Med Cell Longev (2021) 2021:5593919. doi: 10.1155/2021/5593919
28. Hou W, Han T, Sun X, Chen Y, Xu J, Wang Y, et al. Relationship between carbohydrate intake (Quantity, quality, and time eaten) and mortality (Total, cardiovascular, and diabetes): assessment of 2003-2014 national health and nutrition examination survey participants. Diabetes Care (2022) 45(12):3024–31. doi: 10.2337/dc22-0462
29. Curtin LR, Mohadjer LK, Dohrmann SM, Kruszon-Moran D, Mirel LB, Carroll MD, et al. National Health and Nutrition Examination Survey: sample design 2007-2010. Vital Health Stat 2 (2013) 160:1–23.
30. Johnson CL, Paulose-Ram R, Ogden CL, Carroll MD, Kruszon-Moran D, Dohrmann SM, et al. National health and nutrition examination survey: analytic guidelines 1999-2010. Vital Health Stat 2 (2013) 161:1–24.
31. Goodman M, Bostick RM, Dash C, Terry P, Flanders WD, Mandel J. A summary measure of pro- and anti-oxidant exposures and risk of incident, sporadic, colorectal adenomas. Cancer Causes Control (2008) 19(10):1051–64. doi: 10.1007/s10552-008-9169-y
32. Tian X, Xue B, Wang B, Lei R, Shan X, Niu J, et al. Physical activity reduces the role of blood cadmium on depression: A cross-sectional analysis with NHANES data. Environ pollut (2022) 304:119211. doi: 10.1016/j.envpol.2022.119211
33. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation (2002) 106(25):3143–421. doi: 10.1161/circ.106.25.3143
34. Godos J, Zappalà G, Bernardini S, Giambini I, Bes-Rastrollo M, Martinez-Gonzalez M. Adherence to the Mediterranean diet is inversely associated with metabolic syndrome occurrence: a meta-analysis of observational studies. Int J Food Sci Nutr (2017) 68(2):138–48. doi: 10.1080/09637486.2016.1221900
35. Gurka MJ, Lilly CL, Oliver MN, DeBoer MD. An examination of sex and racial/ethnic differences in the metabolic syndrome among adults: a confirmatory factor analysis and a resulting continuous severity score. Metabolism (2014) 63(2):218–25. doi: 10.1016/j.metabol.2013.10.006
36. Kariuki JK, Yang K, Scott PW, Chasens ER, Godzik C, Luyster FS, et al. Obstructive sleep apnea risk is associated with severity of metabolic syndrome: A secondary analysis of the 2015-2018 national health and nutrition examination survey. J Cardiovasc Nurs (2022) 37(5):482–9. doi: 10.1097/jcn.0000000000000868
37. Huh JH, Lee JH, Moon JS, Sung KC, Kim JY, Kang DR. Metabolic syndrome severity score in Korean adults: analysis of the 2010-2015 korea national health and nutrition examination survey. J Korean Med Sci (2019) 34(6):e48. doi: 10.3346/jkms.2019.34.e48
38. Ashoori M, Saedisomeolia A. Riboflavin (vitamin B2) and oxidative stress: a review. Br J Nutr (2014) 111(11):1985–91. doi: 10.1017/s0007114514000178
39. Kaplon RE, Gano LB, Seals DR. Vascular endothelial function and oxidative stress are related to dietary niacin intake among healthy middle-aged and older adults. J Appl Physiol (1985) (2014) 116(2):156–63. doi: 10.1152/japplphysiol.00969.2013
40. Hellmann H, Mooney S. Vitamin B6: a molecule for human health? Molecules (2010) 15(1):442–59. doi: 10.3390/molecules15010442
41. van de Lagemaat EE, de Groot L, van den Heuvel E. Vitamin B(12) in relation to oxidative stress: A systematic review. Nutrients (2019) 11(2):482. doi: 10.3390/nu11020482
42. Zheltova AA, Kharitonova MV, Iezhitsa IN, Spasov AA. Magnesium deficiency and oxidative stress: an update. Biomed (Taipei) (2016) 6(4):20. doi: 10.7603/s40681-016-0020-6
43. Gaetke LM, Chow CK. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology (2003) 189(1-2):147–63. doi: 10.1016/s0300-483x(03)00159-8
44. Wu Y, Li S, Wang W, Zhang D. Associations of dietary vitamin B1, vitamin B2, niacin, vitamin B6, vitamin B12 and folate equivalent intakes with metabolic syndrome. Int J Food Sci Nutr (2020) 71(6):738–49. doi: 10.1080/09637486.2020.1719390
45. Mazur-Bialy AI, Pocheć E. Vitamin B2 deficiency enhances the pro-inflammatory activity of adipocyte, consequences for insulin resistance and metabolic syndrome development. Life Sci (2017) 178:9–16. doi: 10.1016/j.lfs.2017.04.010
46. Zhu J, Chen C, Lu L, Shikany JM, D'Alton ME, Kahe K. Folate, vitamin B6, and vitamin B12 status in association with metabolic syndrome incidence. JAMA Netw Open (2023) 6(1):e2250621. doi: 10.1001/jamanetworkopen.2022.50621
47. Jiao Y, Li W, Wang L, Jiang H, Wang S, Jia X, et al. Relationship between dietary magnesium intake and metabolic syndrome. Nutrients (2022) 14(10):2013. doi: 10.3390/nu14102013
48. Ding J, Liu Q, Liu Z, Guo H, Liang J, Zhang Y. Associations of the dietary iron, copper, and selenium level with metabolic syndrome: A meta-analysis of observational studies. Front Nutr (2021) 8:810494. doi: 10.3389/fnut.2021.810494
49. Lee HS, Park T. Pathway-driven approaches of interaction between oxidative balance and genetic polymorphism on metabolic syndrome. Oxid Med Cell Longev (2017) 2017:6873197. doi: 10.1155/2017/6873197
50. Suriyaprom K, Kaewprasert S, Putpadungwipon P, Namjuntra P, Klongthalay S. Association of antioxidant status and inflammatory markers with metabolic syndrome in Thais. J Health Popul Nutr (2019) 38(1):1. doi: 10.1186/s41043-018-0158-9
51. Thomas MS, Huang L, Garcia C, Sakaki JR, Blesso CN, Chun OK, et al. The effects of eggs in a plant-based diet on oxidative stress and inflammation in metabolic syndrome. Nutrients (2022) 14(12):2548. doi: 10.3390/nu14122548
52. Gallardo-Alfaro L, Bibiloni MDM, Mascaró CM, Montemayor S, Ruiz-Canela M, Salas-Salvadó J, et al. Leisure-time physical activity, sedentary behaviour and diet quality are associated with metabolic syndrome severity: the PREDIMED-plus study. Nutrients (2020) 12(4):1013. doi: 10.3390/nu12041013
53. Mallard AR, Ramos JS, Roberts LA, Centner CM, Fassett RG, Coombes JS. The association between metabolic syndrome severity and oxidative stress induced by maximal exercise testing - a cross-sectional study. Biomarkers (2019) 24(4):394–400. doi: 10.1080/1354750x.2019.1600022
54. Franco OH, Massaro JM, Civil J, Cobain MR, O'Malley B, D'Agostino RB Sr. Trajectories of entering the metabolic syndrome: the framingham heart study. Circulation (2009) 120(20):1943–50. doi: 10.1161/circulationaha.109.855817
55. Moebus S, Balijepalli C, Lösch C, Göres L, von Stritzky B, Bramlage P, et al. Age- and sex-specific prevalence and ten-year risk for cardiovascular disease of all 16 risk factor combinations of the metabolic syndrome - A cross-sectional study. Cardiovasc Diabetol (2010) 9:34. doi: 10.1186/1475-2840-9-34
56. Jousilahti P, Vartiainen E, Tuomilehto J, Puska P. Sex, age, cardiovascular risk factors, and coronary heart disease: a prospective follow-up study of 14 786 middle-aged men and women in Finland. Circulation (1999) 99(9):1165–72. doi: 10.1161/01.cir.99.9.1165
57. Khang YH, Cho SI, Kim HR. Risks for cardiovascular disease, stroke, ischaemic heart disease, and diabetes mellitus associated with the metabolic syndrome using the new harmonised definition: findings from nationally representative longitudinal data from an Asian population. Atherosclerosis (2010) 213(2):579–85. doi: 10.1016/j.atherosclerosis.2010.09.009
58. Eastwood MA. Interaction of dietary antioxidants in vivo: how fruit and vegetables prevent disease? Qjm (1999) 92(9):527–30. doi: 10.1093/qjmed/92.9.527
Keywords: oxidative stress, metabolic syndrome, lifestyles, diet, mortality
Citation: Xu Z, Lei X, Chu W, Weng L, Chen C and Ye R (2024) Oxidative balance score was negatively associated with the risk of metabolic syndrome, metabolic syndrome severity, and all-cause mortality of patients with metabolic syndrome. Front. Endocrinol. 14:1233145. doi: 10.3389/fendo.2023.1233145
Received: 01 June 2023; Accepted: 18 December 2023;
Published: 12 January 2024.
Edited by:
Zhen-Yu Zhang, KU Leuven, BelgiumReviewed by:
Liangkai Chen, Huazhong University of Science and Technology, ChinaMika Enomoto, Kurume University, Japan
Copyright © 2024 Xu, Lei, Chu, Weng, Chen and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chengshui Chen, Y2hlbmNoZW5nc2h1aUB3bXUuZWR1LmNu; Ran Ye, cmFueWV3ZW56QDE2My5jb20=
†These authors have contributed equally to this work
 Zhixiao Xu
Zhixiao Xu Xiong Lei2
Xiong Lei2 Weiwei Chu
Weiwei Chu Chengshui Chen
Chengshui Chen Ran Ye
Ran Ye