
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 02 November 2023
Sec. Bone Research
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1231527
This article is part of the Research Topic Osteoporosis and Adipokines: the Potential for Future Treatment View all 6 articles
Background: The visceral adiposity index (VAI) is a marker of abdominal fat distribution and adipose tissue function. However, the association between VAI and femur bone mineral density (BMD) and osteoporosis is unclear among the U.S. older adults.
Methods: Cross-sectional data for adults aged 60 years and older from the 2007–2020 National Health and Nutrition Examination Survey (NHANES) were included. Multivariable linear and logistic regression were used to evaluate the association between VAI and femur BMD and osteoporosis. We used the smooth curve fitting to address nonlinearity. Moreover, a two-piecewise linear regression model was used to explain the nonlinearity further.
Results: The findings of the multivariable logistic regression models showed that as the VAI value increased by one unit, the prevalence of osteoporosis decreased by 1.2% after adjusting for covariates associated with osteoporosis. The multivariable linear regression models demonstrated that VAI was positively correlated with femur BMD. Further analysis revealed an inverted L-shaped and inverted U-shaped relationship between VAI and femur BMD at different sites.
Conclusions: Our findings indicated that an increased VAI is independently linked to a higher prevalence of osteoporosis among the U.S. older adults. Further analysis reveals that once VAI reaches a certain threshold, femur BMD no longer increases and may even decrease. This suggests that a moderate accumulation of visceral fat may be beneficial for bone health, while excessive visceral fat could potentially have detrimental effects.
Osteoporosis is a group of metabolic bone diseases characterized by reduced bone density, degradation of bone tissue microarchitecture, and an imbalance in bone homeostasis. This condition poses a risk of fragility fractures, which can increase mortality rates among patients. Bone mineral density (BMD) is a widely used metric for assessing bone health (1). Elderly individuals are at a higher risk of developing osteoporosis due to increased disruption of bone synthesis homeostasis with age (2). Femur BMD is a highly effective indicator for the detection and diagnosis of osteoporosis, with a strong correlation to all-cause mortality and mortality from other causes in patients with this condition (3). Especially for older people, hip fractures can be devastating (4). Therefore, it is of great significance to identify the risk factors related to low femur BMD and osteoporosis in elderly people.
Obesity has a complex impact on the development of osteoporosis, with both positive and negative effects on skeletal homeostasis. Several studies have identified a threshold effect of adiposity on BMD based on body mass index (BMI) (5, 6). The percentage of body fat has also been observed to influence BMD. This may be due to abnormal glycolipid metabolism triggered by obesity that affects bone strength (7). Additionally, a moderate amount of fat can contribute to bone protection, and body weight can stimulate bone growth via mechanical stress (8).
In recent years, the visceral adiposity index (VAI), calculated by combining anthropometric measurements and blood biomarkers, has emerged as a novel indicator for assessing obesity. It takes into account various factors such as BMI, waist circumference, triglycerides, and HDL cholesterol to assess the amount of visceral fat accumulation. This compensates for the limitations of BMI and waist circumference in differentiating between muscle and fat content. Some studies have suggested that VAI is superior to traditional BMI or waist circumference in predicting the risk of cardiometabolic disease (9, 10). Moreover, it has shown promising results in predicting and evaluating the risk of diabetes, metabolic syndrome, blood pressure, and endometrial cancer development (11–14). Previous research has indicated that individuals with osteopenia and osteoporosis typically have a smaller waist circumference compared to those with normal BMD values (15). Furthermore, excessive buildup of visceral and subcutaneous fat may negatively impact bone health in women, whether premenopausal or postmenopausal (16).
At present, there is insufficient evidence to establish a relationship between VAI and the prevalence of osteoporosis. The objective of our study was to examine the association of VAI with femur BMD and osteoporosis among the U.S. older adults to fill these knowledge gaps. The secondary goal was to assess the dose–response relationship between VAI and femur BMD at different sites.
The National Health and Nutrition Examination Survey (NHANES) is a comprehensive investigation designed to assess the health and nutritional status of the non-institutionalized population in the US. It makes use of a multistage, probability-based survey methodology with stratification to ensure accuracy and precision. The National Center for Health Statistics Research Ethics Review Board granted approval for this project, and all participants provided written informed consent prior to their participation (17). According to the related policies, the Institutional Review Board did not require a review for the secondary analysis (18). For this cross-sectional study, we merged the NHANES data from 2005–2010, 2013–2014, and 2017–2020. The participants in our study were all over 60 years of age and had completed interviews and assessments. Participants without complete data regarding BMI, waist circumference (WC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), femur BMD, and covariates were excluded from the study.
BMD measurements were conducted using dual-energy x-ray absorptiometry scans with Hologic QDR-4500A fan-beam densitometers (Hologic, Inc., Bedford, MA, United States). According to the classification criteria established by the World Health Organization, BMD values in any femur region can be defined as osteoporosis if they fall below −2.5 standard deviations from the reference group for young adults (19). The femoral regions that were evaluated in the study included the total femur, femoral neck, trochanter, and intertrochanter. The corresponding thresholds for osteoporosis were 0.68 g/cm2, 0.59 g/cm2, 0.49 g/cm2, and 0.78 g/cm2, respectively (20).
The VAI was used as an exposure variable and was calculated using gender-specific equations, as detailed below. Male: [WC (cm)/39.68 + (1.88 × BMI)] × (TG (mmol/L)/1.03) × (1.31/HDL (mmol/L)); Female: [WC (cm)/36.58 + 1.89 × (BMI)] × (TG (mmol/L)/0.81) × (1.52/HDL (mmol/L)) (21). TG was measured using the Wahlefeld method and HDL was measured using the magnesium sulfate/glucan method (22). The measurements of both TG and HDL were obtained from serum, and participants were required to fast for 9 h. BMI was calculated by dividing the weight in kilograms by the height in meters squared (kg/m2). WC was measured using electronic Sports Measurements (Seca Ltd, Medical Scales and Measurement Systems, Birmingham, UK) with an accuracy of millimeters (22). Please refer to the official NHANES website for more detailed information.
The choice of variables was determined using previous research (23–25) and clinical observations. NHANES researchers created standardized questionnaires to collect demographic information, including gender, age, race, education level, poverty-to-income ratio (PIR), marital status, smoking status, and work activity. Race was classified as Mexican American, other Hispanic, non-Hispanic white, non-Hispanic black, or other. Education level was categorized as did not graduate from high school, graduated from high school, and college education or above. Marital status was reported as married/living with partner, widowed/divorced/separated, and never married. Smoking status was created from the question: “smoked at least 100 cigarettes in life”. Moderate activity was defined as activity that results in only small elevations in respiration and heart rate, such as brisk walking or carrying lightweight objects for a minimum of 10 min without interruption. The blood urea nitrogen, serum calcium, serum phosphorus, and serum uric acid were measured according to standardized protocols. More information can be obtained from the NHANES website (www.cdc.gov/nchs/nhanes/).
Continuous variables with a normal distribution were reported as mean (standard deviation, [SD]). Alternatively, variables with skewness were reported as median (interquartile range, [IQR]). Categorical variables were presented as frequencies (%). The clinical characteristics of VAI quartiles were compared using one-way ANOVA when the data were normally distributed, Kruskal–Wallis H when the distribution was skewed, and the chi-square test for categorical variables analysis. The association between VAI and femur BMD was evaluated using linear regression models (regression coefficients β and 95% confidence interval [CI]). We used logistic regression to investigate the associations between VAI with osteoporosis (odds ratio [OR] and 95% confidence interval [CI]). Both non-adjusted and multivariable adjusted models were applied. In this study, Model 1 was adjusted for gender, age, and race. Model 2 was adjusted for sociodemographic characteristics, including gender, age, race, education level, marital status, PIR, smoking status, and work activity. Model 3 was completely adjusted, including sociodemographic characteristics, blood urea nitrogen, serum calcium, serum phosphorus, and serum uric acid.
In addition, we transformed the VAI into a categorical variable based on the quartile and analyzed the trend p-value to validate the outcomes of the VAI as a continuous variable and assess the potential for nonlinearity. To address the nonlinear relationship between the VAI and femur BMD, we utilized a Generalized Additive Model along with smooth curve fitting techniques. Additionally, a two-piecewise linear regression model was employed to further explain the nonlinearity. We further used restricted cubic spline with three knots located at the 5th, 50th, and 95th percentiles of the exposure distribution to assess the dose–response relationship in Model 3. Specifically, when a ratio is represented in a smooth curve, the recursive method can detect inflection points and determine the maximum likelihood model for that point. To ensure the validity and reliability of the results obtained in this study, subgroup analyses were evaluated by multivariable logistic regression models. Potential modifications of the relationship between the VAI and osteoporosis were assessed, including gender, age, race, marital status, and smoking status. The interaction among subgroups was evaluated using the likelihood ratio test. Moreover, two sensitivity analyses were conducted as follows: (1) Participants with extreme VAI outside the mean ± 3 SD were excluded. (2) Participants who had ever taken prednisone or cortisone daily, treated for osteoporosis, had cancer or malignancy, had liver condition, had ever taken estrogen, or had celiac disease were excluded. All analyses were conducted using the statistical software packages R (“rms” R package for conducting restricted cubic spline analysis) and Free Statistics software version 1.7. A two-sided p-value <0.05 was regarded as having statistical significance.
This study included 56,769 prospective participants from NHANES (2005–2010, 2013–2014, and 2017–2020), of which 4,927 older adults (≥60 years) completed interviews and were subjected to mobile examination center (MEC) screening. Participants with missing data for femur BMD, BMI, WC, TG, and HDL-C (n = 1,147) were excluded. Our cross-sectional study included 3,342 participants after the exclusion of those with missing covariate data (n = 438) eventually. The study population selection flowchart is shown in Figure 1.
The mean participant age was 69.8 ± 6.9 years, and 1,759 (52.6%) were men. The median VAI was 1.5 (0.9, 2.4). There were 471 (14.1%) participants with osteoporosis. The detailed characteristics of the population by the VAI quartiles are available in Table 1. Participants with higher VAI often tended to be younger, female, had a lower educational level and family income, smoked more cigarettes, had less work activity, and had higher serum phosphorus and serum uric acid level.
The univariate analysis demonstrated that gender, age, education level, marital status, PIR, smoking status, work activity, blood urea nitrogen, serum phosphorus, and serum uric acid were associated with osteoporosis (Supplementary Table S1).
After adjustment in multivariable analyses, the VAI was significantly associated with femur BMD and osteoporosis. When considering the VAI as a continuous variable, the adjusted OR for osteoporosis was 0.88 (95% CI: 0.81–0.96, p = 0.002) in the fully adjusted model. Compared with participants with the lowest VAI in the 1st Quartile (≤0.92), the adjusted OR value for the VAI and osteoporosis in the 4th Quartile (≥2.39) was 0.61 (95% CI: 0.44–0.85, p = 0.003) in Model 3 (Table 2). When the VAI was assessed as a continuous variable, the regression coefficients β were 0.006 (95% CI: 0.004–0.009, p < 0.001), 0.004 (95% CI: 0.002–0.006, p = 0.001), 0.005 (95% CI: 0.003–0.007, p < 0.001), and 0.007 (95% CI: 0.004–0.010, p < 0.001) for total femur BMD, femur neck BMD, trochanter BMD, and intertrochanter BMD in the fully adjusted model (Model 3), respectively. After adjusting for all variables, analysis using quartiles revealed a significant positive correlation between VAI and femur BMD (Table 3). All of the models were statistically significant (Tables 2, 3, p for trend<0.001).
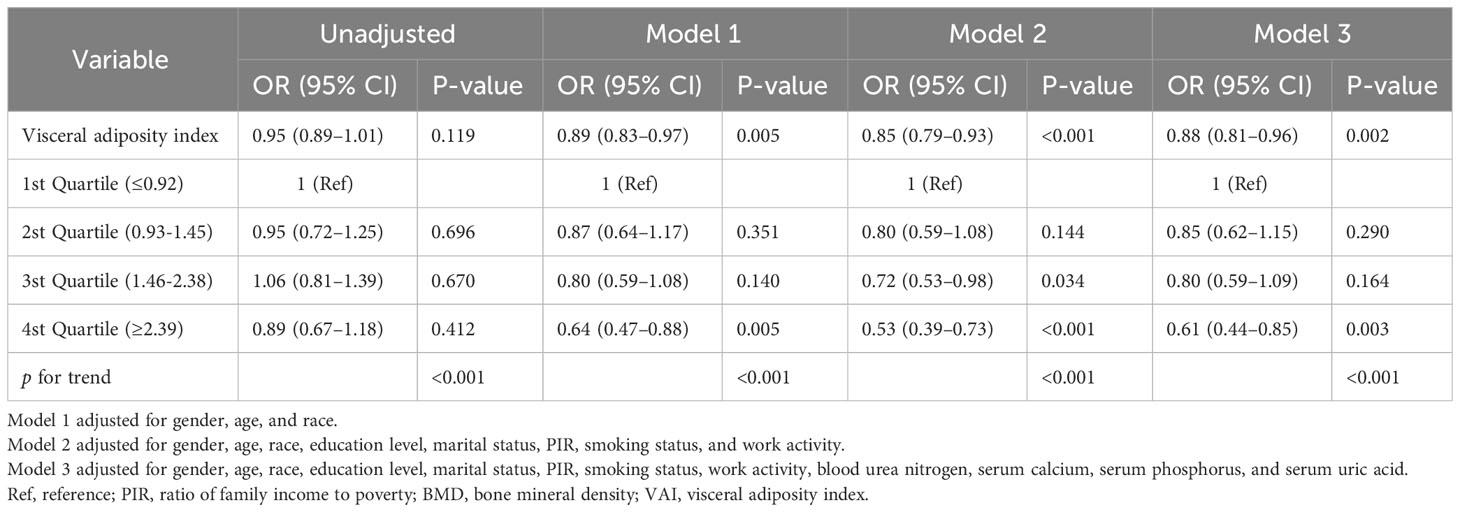
Table 2 Association between visceral adiposity index and osteoporosis in the multiple regression model.
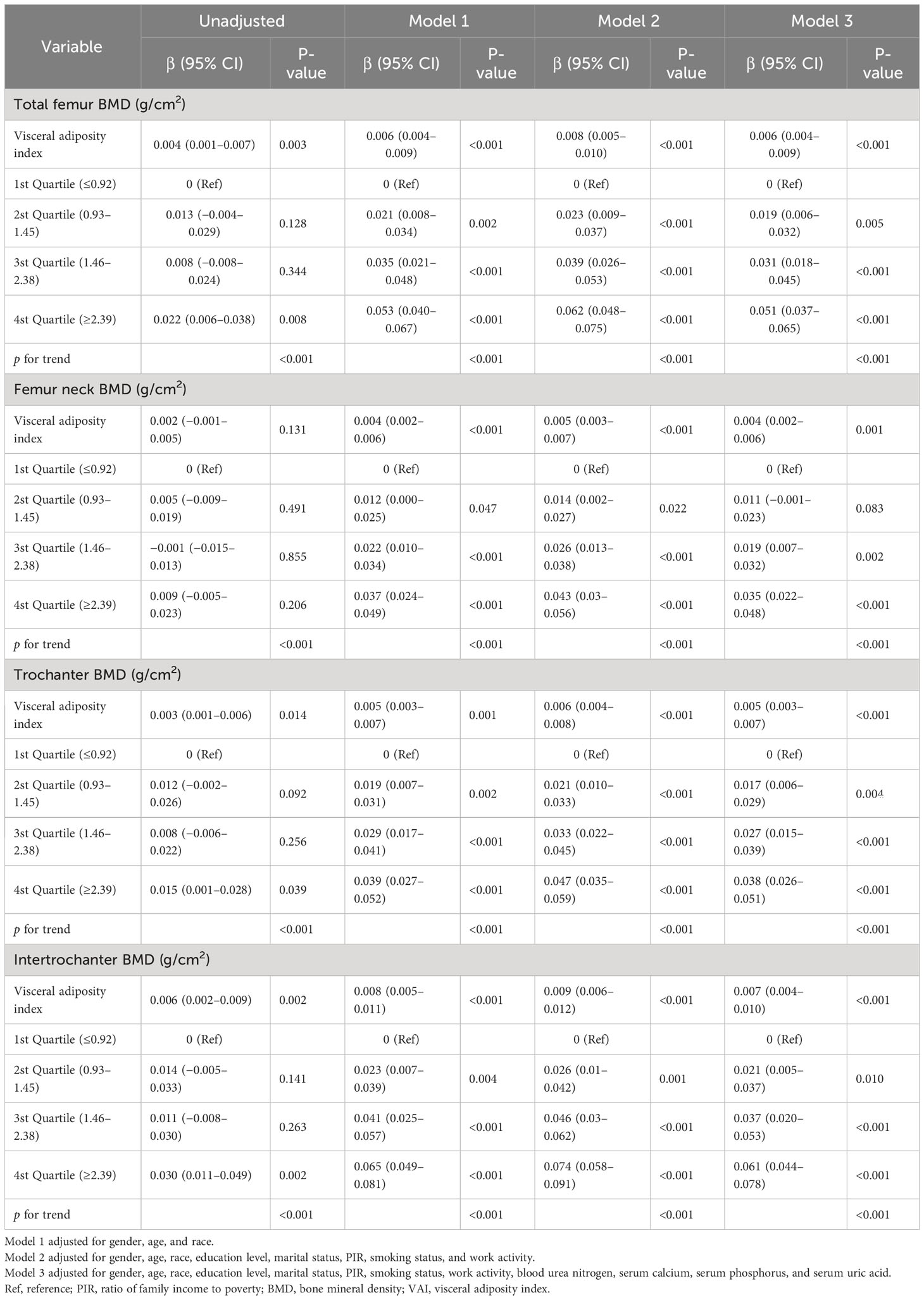
Table 3 Association between visceral adiposity index and femur BMD in the multiple regression model.
There was a linear relationship between VAI and osteoporosis (Figure 2). Additionally, an inverted L-shaped curve was observed in the correlation between VAI and BMD in different areas of the femur, including total femur, trochanter, and intertrochanter regions (nonlinear, p = 0.002, p = 0.001, p = 0.001, respectively) after adjusting for all covariates. Interestingly, the relationship between the VAI and femur neck BMD exhibited an inverted U-shaped curve (Figure 3). Femur neck BMD was positively correlated with the VAI until it peaked at 3.9 (β = 0.014 [95% CI: 0.009–0.020]). However, when the VAI was higher than 3.9, the femur neck BMD decreased significantly (β = −0.044 [95% CI: −0.075 to −0.013]) (Table 4). The solid line represents the estimated risk, and the light-shaded area represents a point-wise 95% confidence interval adjusted for Model 3 (Figures 2, 3).
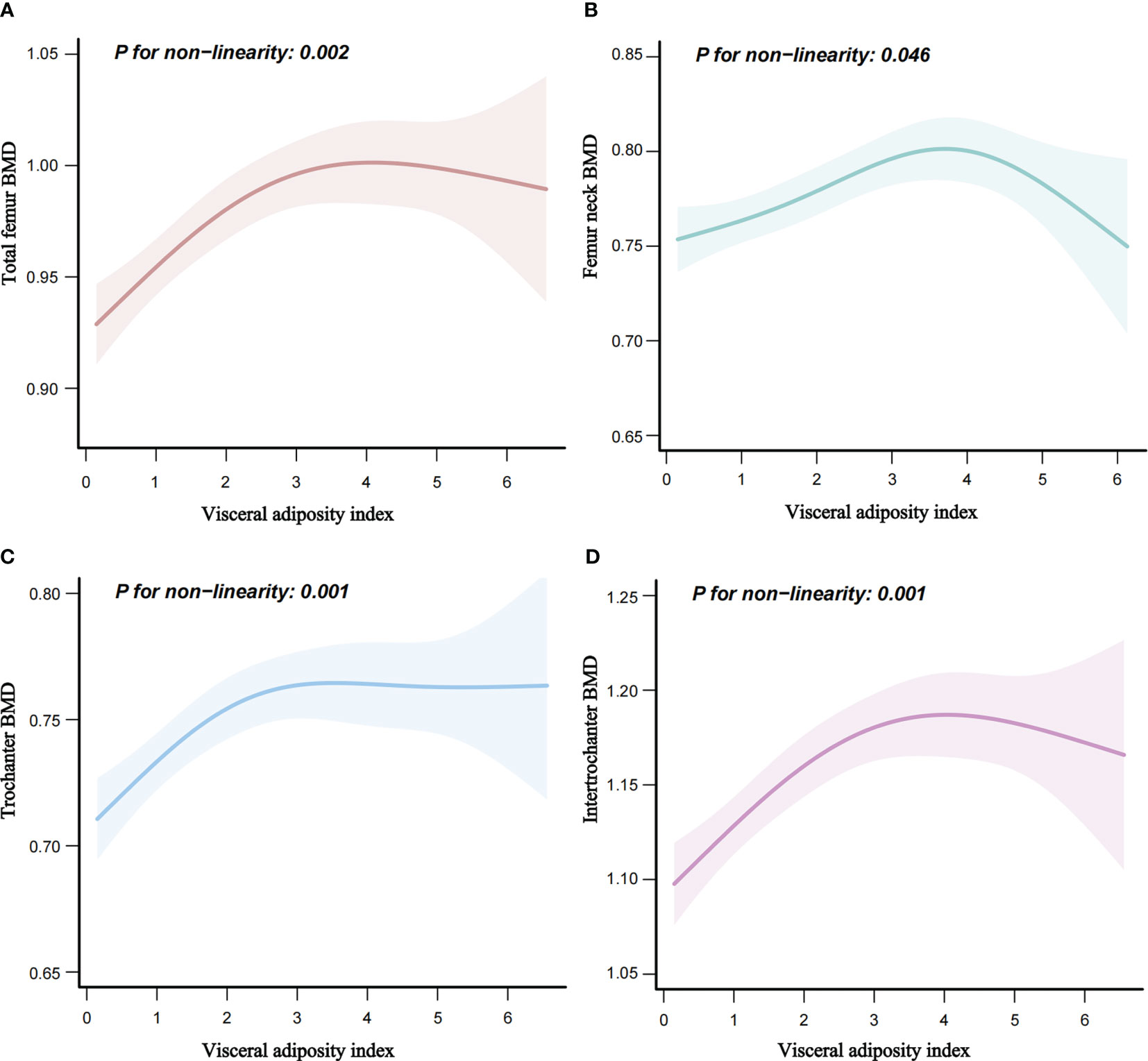
Figure 3 Nonlinear relationship of VAI and femur BMD. (A) Nonlinear relationship of VAI and total femur BMD. (B) Nonlinear relationship of VAI and femur neck BMD. (C) Nonlinear relationship of VAI and trochanter BMD. (D) Nonlinear relationship of VAI and intertrochanter BMD. BMD, bone mineral density (g/cm2).
Stratified analyses were performed in several subgroups to assess the potential effect modification of the relationship between VAI and osteoporosis. Significant interactions were not observed in any of the subgroups after stratifying by gender, age, race, marital status, and smoking status (Figure 4).
A total of 3,296 participants were left after excluding the participants with extreme VAI (outside the mean ± 3 SD were excluded), and the association between VAI and osteoporosis remained stable. When considering the VAI as a continuous variable, the adjusted OR for osteoporosis was 0.88 (95% CI: 0.80–0.96, p = 0.007) in the fully adjusted model. Compared with participants with the lowest VAI in the 1st Quartile (≤0.92), the adjusted OR value for the VAI and osteoporosis in 4th Quartile (≥2.39) was 0.63 (95% CI: 0.46–0.88, p = 0.007) (Supplementary Table S2). After excluding participants who had ever taken prednisone or cortisone daily, been treated for osteoporosis, had cancer or malignancy, had liver conditions, ever taken estrogen, or had celiac disease, 2,246 participants remained, and the association between VAI and osteoporosis remained consistent. When considering the VAI as a continuous variable, the adjusted OR for osteoporosis was 0.86 (95% CI: 0.77–0.96, p = 0.007) in the fully adjusted model. Compared with participants with the lowest VAI in the 1st Quartile (≤0.92), the adjusted OR value for the VAI and osteoporosis in 4th Quartile (≥2.40) was 0.52 (95% CI: 0.33–0.81, p = 0.004) (Supplementary Table S3).
In this large population-based cross-sectional study of the older adults in the United States using 2005–2020 NHANES data, the results showed that VAI, whether as a continuous or categorical variable, was positively and linearly associated with osteoporosis when adjusted for potential confounding factors. We further revealed a nonlinear relationship between VAI and femur BMD. Specifically, an inverted L-shaped correlation was found between VAI and BMD across some regions of the femur such as the total femur, trochanter, and intertrochanter areas, with an inflection point of almost 3.0, 2.4, and 3.2, respectively. What is of interest is that the relationship between VAI and femur neck BMD displayed a curvilinear pattern resembling an inverted U-shape. The femur neck BMD was significantly reduced with the excessive VAI values. The most beneficial VAI value for femur neck BMD is 3.9. As far as we know, there are no other studies investigating the association of the VAI with femur BMD and osteoporosis among the U.S. older adults.
BMD typically showed higher values in individuals with greater weight or BMI (26, 27). Furthermore, a study has indicated that total body fat emerges as the most significant predictor of BMD throughout the entire skeletal structure (28), while a lower amount of body fat is identified as an independent risk factor contributing to greater lumbar spine bone loss in women (29). The research also indicated that obesity significantly reduces the risk of osteoporosis, osteopenia, and low bone mass in all participants (30). One reasonable mechanism explaining the increase in BMD in obese individuals is associated with the increased mechanical load and strain related to obesity (8). As body fat increases in obese individuals, it not only leads to passive loading but also increases muscle strain, which has a favorable impact on bone geometry and modeling (31). In fact, increased body fat from moderate obesity can have beneficial effects on bone health by promoting skeletal plasticity and modeling, which assists in maintaining bone mass and reducing bone loss, especially in the older adults (8). This has significant advantages in preventing fragility fractures. However, severe obesity, in particular, may be associated with an elevated risk of fractures (32, 33). Studies have indicated that women with abdominal obesity experience lower concentrations of serum dihydrotestosterone (DHT) (34), and that DHT has a significant inhibitory effect on estrogen synthesis in vivo (35). In low DHT environments, estrogen accumulation can play a role in maintaining optimal bone health. In contrast, moderate visceral fat accumulation in men is significantly positively correlated with endogenous adrenal steroid levels (36). Endogenous adrenal steroids have a noteworthy contribution to elevated estrogen levels, which could be why elevated VAI may be associated with reducing the risk of osteoporosis (37). In a cohort study involving postmenopausal women in Turkey, it was discovered that after controlling for potential confounding risks, higher VAI may contribute to a reduced risk of osteoporosis (38). This study supports our results.
In addition, we discovered an inverted L-shaped and inverted U-shaped relationship between VAI and femur BMD at different sites. The beneficial effect of increasing the level of VAI on femur BMD seemed to peak in those with considerably high VAI. Individuals with extremely high VAI levels tend to experience a decrease in femur BMD, which may indicate a potential negative impact on bone health. Several published research findings suggest a possible association between more visceral adipose tissue and reduced BMD, indicating that excessive VAI may elevate the risk of osteoporosis. When body fat percentage is below 33%, BMD is positively correlated with body fat content, which can decrease the risk of fragility fracture. However, when the body fat percentage exceeds 33%, body fat content in most skeletal sites is negatively related to BMD, which increases the risk of osteoporosis and fragility fracture (39–42). Activation of the Wnt/β-catenin signaling pathway has been shown to effectively inhibit the proliferation and differentiation of adipocytes, leading to reduced accumulation of visceral fat (43). The positive establishment and activation of the Wnt/β-catenin signaling pathway is crucial for the value-added of osteoblasts, and mutations in the LRP5 gene within this pathway have been identified as an important cause of osteoporosis (44). Therefore, it is reasonable to suspect that excessive obesity or a high VAI may impact the development of osteoporosis by disrupting the Wnt/β-catenin signaling pathway. High levels of VAI are an independent predictor of the development of type 2 diabetes (45), which is an important risk factor for osteoporosis (46, 47). Abnormally high levels of visceral and ectopic fat accumulation can lead to massive infiltration of local environmental macrophages and induce the release of inflammatory factors such as IL-6. These factors have been shown to cause reduced bone content and enhanced bone resorption, ultimately inducing the development of osteoporosis (48). The visceral adipose tissue derived from CT scans has demonstrated a statistically significant correlation with decreased bone and muscle density, even after adjusting for age, gender, and BMI (49). Another study has already demonstrated that visceral adipose tissue has an adverse effect on BMD in premenopausal obese women and has proposed that this association may be mediated by IGF-1 (50). Visceral adipose tissue has been shown to be negatively associated with BMD in a study based on the NHANES database; however, this study did not include older adults (45).
The strengths of our study included adjusting for multiple biochemical indicators that were closely associated with osteoporosis (24, 25, 51) and the relatively large sample size. This study also has some limitations. Firstly, femur BMD data were not collected in NHANES 2011–2012 and 2015–2016. Therefore, we were unable to further validate the results with more cycles of data. Secondly, owing to the inherent limitations of cross-sectional studies, the causal association of the VAI with femur BMD and osteoporosis cannot be determined, and current relationships may be bidirectional. Thirdly, lumbar spine BMD was not included in this study mainly because degenerative changes in the lumbar spine are more pronounced in older adults, which can lead to falsely elevated measurements (52, 53). There are more missing values for lumbar spine bone density. However, there are guidelines that consider the lumbar spine to be the preferred site for efficacy assessment (54). This may have an impact on the results. Fourthly, an elevated BMD does not necessarily reflect superior bone quality. A more accurate assessment of bone quality could be obtained through HR-pQCT. However, this was not within the scope of our study. Lastly, despite adjusting for potential confounding factors and conducting sensitivity analyses, because of the limitations of the NHANES database, we were unable to access information regarding patients’ dietary patterns, specific dietary characteristics, and other potential confounders such as whether participants had diseases associated with absorption disorders, underwent abdominal surgeries leading to absorption issues, or had active infectious foci, which could still potentially impact the results. More research is needed to supplement our understanding of the bidirectional modulating effects of obesity on BMD and skeletal remodeling, as well as the impact threshold. Further high-quality prospective studies are required to clarify the potential mechanisms linking VAI and osteoporosis.
In this cross-sectional study based on the NHANES from 2005 to 2020, we found that an increased VAI is independently linked to a higher prevalence of osteoporosis among the U.S. older adults. Interestingly, our results also revealed a nonlinear relationship between VAI and femur BMD at various sites, characterized by an inverted L-shape and an inverted U-shape, suggesting that once VAI reaches a certain threshold, femur BMD no longer increases and may even decrease. This implies that a moderate buildup of visceral fat might have a positive impact on bone health, whereas an excessive amount of visceral fat could potentially lead to adverse effects. VAI could serve as a valuable indicator for clinically assessing the skeletal health of the older adults.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by National Center for Health Statistics research ethics review board. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from gifted from another research group. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Conceptualization, ZL, AS, and JH. Data curation, AS and SW. Formal analysis, AS. Funding acquisition, ZL. Methodology, AS. Writing—original draft, AS, JH, SW, and FY. Writing—review and editing, ZL, AS, and JH. All authors have read and agreed to the published version of the manuscript.
This research was funded by the “National key research and development program, grant number 2020YFC2006103” and “CACMS Innovation Fund, grant number CI2021A01412”.
We gratefully thank Qiushi Shi (Huawei Technologies Co., Ltd., Shanghai, China), Dr. Jie Liu (People’s Liberation Army of China General Hospital, Beijing, China) for their contribution to the statistical support and comments regarding the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1231527/full#supplementary-material
1. Xue S, Kemal O, Lu M, Lix LM, Leslie WD, Yang S. Age at attainment of peak bone mineral density and its associated factors: The National Health and Nutrition Examination Survey 2005-2014. Bone (2020) 131:115163. doi: 10.1016/j.bone.2019.115163
2. Kanis JA, Cooper C, Rizzoli R, Reginster JY, Scientific Advisory Board of the European Society for C, Economic Aspects of O, et al. Correction to: European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int (2020) 31(4):801. doi: 10.1007/s00198-020-05303-5
3. Cai S, Fan J, Zhu L, Ye J, Rao X, Fan C, et al. Bone mineral density and osteoporosis in relation to all-cause and cause-specific mortality in NHANES: A population-based cohort study. Bone (2020) 141:115597. doi: 10.1016/j.bone.2020.115597
4. Black DM, Rosen CJ. Clinical practice. Postmenopausal osteoporosis. N Engl J Med (2016) 374(3):254–62. doi: 10.1056/NEJMcp1513724
5. Sun RJ, Ma J, Duan LZ, Zhu JY, Yu SC, Huang H, et al. Threshold effects of body mass index on the bone mineral density of Chinese rural women in fluorosis area. Zhonghua Yu Fang Yi Xue Za Zhi (2020) 54(11):1295–9. doi: 10.3760/cma.j.cn112150-20200825-01150
6. Zheng R, Byberg L, Larsson SC, Hoijer J, Baron JA, Michaelsson K. Prior loss of body mass index, low body mass index, and central obesity independently contribute to higher rates of fractures in elderly women and men. J Bone Miner Res (2021) 36(7):1288–99. doi: 10.1002/jbmr.4298
7. Zhao C, Kan J, Xu Z, Zhao D, Lu A, Liu Y, et al. Higher BMI and lower femoral neck strength in males with type 2 diabetes mellitus and normal bone mineral density. Am J Med Sci (2022) 364(5):631–7. doi: 10.1016/j.amjms.2022.06.007
8. Gkastaris K, Goulis DG, Potoupnis M, Anastasilakis AD, Kapetanos G. Obesity, osteoporosis and bone metabolism. J Musculoskelet Neuronal Interact (2020) 20(3):372–81.
9. Derezinski T, Zozulinska-Ziolkiewicz D, Uruska A, Dabrowski M. Visceral adiposity index as a useful tool for the assessment of cardiometabolic disease risk in women aged 65 to 74. Diabetes Metab Res Rev (2018) 34(8):e3052. doi: 10.1002/dmrr.3052
10. Al-Daghri NM, Al-Attas OS, Wani K, Alnaami AM, Sabico S, Al-Ajlan A, et al. Sensitivity of various adiposity indices in identifying cardiometabolic diseases in Arab adults. Cardiovasc Diabetol (2015) 14:101. doi: 10.1186/s12933-015-0265-5
11. Nusrianto R, Tahapary DL, Soewondo P. Visceral adiposity index as a predictor for type 2 diabetes mellitus in Asian population: A systematic review. Diabetes Metab Syndr (2019) 13(2):1231–5. doi: 10.1016/j.dsx.2019.01.056
12. Bijari M, Jangjoo S, Emami N, Raji S, Mottaghi M, Moallem R, et al. The accuracy of visceral adiposity index for the screening of metabolic syndrome: A systematic review and meta-analysis. Int J Endocrinol (2021) 2021:6684627. doi: 10.1155/2021/6684627
13. Leite NN, Cota BC, Gotine A, Rocha D, Pereira PF, Hermsdorff HHM. Visceral adiposity index is positively associated with blood pressure: A systematic review. Obes Res Clin Pract (2021) 15(6):546–56. doi: 10.1016/j.orcp.2021.10.001
14. Bo LL, Wang YQ, Liu YY, Li XD, Zhou R, Wang JL. Analyze of obesity indicators and effect of fertility preservation treatment in patients with endometrial atypical hyperplasia and early endometrial cancer. Zhonghua Fu Chan Ke Za Zhi (2022) 57(10):767–74. doi: 10.3760/cma.j.cn112141-20220727-00487
15. Cohen A, Dempster DW, Recker RR, Lappe JM, Zhou H, Zwahlen A, et al. Abdominal fat is associated with lower bone formation and inferior bone quality in healthy premenopausal women: a transiliac bone biopsy study. J Clin Endocrinol Metab (2013) 98(6):2562–72. doi: 10.1210/jc.2013-1047
17. Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, Dostal J. National health and nutrition examination survey: plan and operations, 1999-2010. Vital Health Stat 1 (2013) 56):1–37.
18. Liu H, Wang L, Chen C, Dong Z, Yu S. Association between dietary niacin intake and migraine among american adults: national health and nutrition examination survey. Nutrients (2022) 14(15). doi: 10.3390/nu14153052
19. Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group Osteoporos Int (1994) 4(6):368–81. doi: 10.1007/BF01622200
20. Looker AC, Orwoll ES, Johnston CC Jr., Lindsay RL, Wahner HW, Dunn WL, et al. Prevalence of low femoral bone density in older U.S. adults from NHANES III. J Bone Miner Res (1997) 12(11):1761–8. doi: 10.1359/jbmr.1997.12.11.1761
21. Amato MC, Giordano C. Visceral adiposity index: an indicator of adipose tissue dysfunction. Int J Endocrinol (2014) 2014:730827. doi: 10.1155/2014/730827
22. Jiang K, Luan H, Pu X, Wang M, Yin J, Gong R. Association between visceral adiposity index and insulin resistance: A cross-sectional study based on US adults. Front Endocrinol (Lausanne) (2022) 13:921067. doi: 10.3389/fendo.2022.921067
23. Wang J, Xing F, Sheng N, Xiang Z. Associations of the geriatric nutritional risk index with femur bone mineral density and osteoporosis in american postmenopausal women: data from the national health and nutrition examination survey. Front Nutr (2022) 9:860693. doi: 10.3389/fnut.2022.860693
24. Wang G, Fang ZB, Liu DL, Chu SF, Li HL, Zhao HX. Association between caffeine intake and lumbar spine bone mineral density in adults aged 20-49: A cross-sectional study. Front Endocrinol (Lausanne) (2022) 13:1008275. doi: 10.3389/fendo.2022.1008275
25. Zhao S, Gao W, Li J, Sun M, Fang J, Tong L, et al. Dietary inflammatory index and osteoporosis: the National Health and Nutrition Examination Survey, 2017-2018. Endocrine (2022) 78(3):587–96. doi: 10.1007/s12020-022-03178-6
26. Bauer DC, Browner WS, Cauley JA, Orwoll ES, Scott JC, Black DM, et al. Factors associated with appendicular bone mass in older women. The Study of Osteoporotic Fractures Research Group. Ann Intern Med (1993) 118(9):657–65. doi: 10.7326/0003-4819-118-9-199305010-00001
27. Glauber HS, Vollmer WM, Nevitt MC, Ensrud KE, Orwoll ES. Body weight versus body fat distribution, adiposity, and frame size as predictors of bone density. J Clin Endocrinol Metab (1995) 80(4):1118–23. doi: 10.1210/jcem.80.4.7714079
28. Reid IR, Ames R, Evans MC, Sharpe S, Gamble G, France JT, et al. Determinants of total body and regional bone mineral density in normal postmenopausal women–a key role for fat mass. J Clin Endocrinol Metab (1992) 75(1):45–51. doi: 10.1210/jcem.75.1.1619030
29. Yang S, Center JR, Eisman JA, Nguyen TV. Association between fat mass, lean mass, and bone loss: the Dubbo Osteoporosis Epidemiology Study. Osteoporos Int (2015) 26(4):1381–6. doi: 10.1007/s00198-014-3009-6
30. Salamat MR, Salamat AH, Janghorbani M. Association between obesity and bone mineral density by gender and menopausal status. Endocrinol Metab (Seoul) (2016) 31(4):547–58. doi: 10.3803/EnM.2016.31.4.547
31. Addison O, Marcus RL, Lastayo PC, Ryan AS. Intermuscular fat: a review of the consequences and causes. Int J Endocrinol (2014) 2014:309570. doi: 10.1155/2014/309570
32. Rinonapoli G, Pace V, Ruggiero C, Ceccarini P, Bisaccia M, Meccariello L, et al. Obesity and bone: A complex relationship. Int J Mol Sci (2021) 22(24). doi: 10.3390/ijms222413662
33. Gonnelli S, Caffarelli C, Nuti R. Obesity and fracture risk. Clin cases Miner Bone Metab (2014) 11(1):9–14. doi: 10.11138/ccmbm/2014.11.1.009
34. Cote JA, Lessard J, Mailloux J, Laberge P, Rheaume C, Tchernof A. Circulating 5alpha-dihydrotestosterone, abdominal obesity and adipocyte characteristics in women. Horm Mol Biol Clin Investig (2012) 12(2):391–400. doi: 10.1515/hmbci-2012-0026
35. Conway BA, Mills TM. In vitro effects of dihydrotestosterone on granulosa cell production of estrogen and progesterone. Steroids (1991) 56(5):258–62. doi: 10.1016/0039-128x(91)90044-v
36. Tchernof A, Labrie F, Belanger A, Prud'homme D, Bouchard C, Tremblay A, et al. Relationships between endogenous steroid hormone, sex hormone-binding globulin and lipoprotein levels in men: contribution of visceral obesity, insulin levels and other metabolic variables. Atherosclerosis (1997) 133(2):235–44. doi: 10.1016/s0021-9150(97)00125-1
37. Klinge CM. Estrogenic control of mitochondrial function. Redox Biol (2020) 31:101435. doi: 10.1016/j.redox.2020.101435
38. Elmas H, Duran C, Can M, Tolu I, Guney I. The relationship between bone mineral densitometry and visceral adiposity index in postmenopausal women. Rev Bras Ginecol Obstet (2023) 45(2):82–8. doi: 10.1055/s-0043-1764497
39. Crivelli M, Chain A, da Silva ITF, Waked AM, Bezerra FF. Association of visceral and subcutaneous fat mass with bone density and vertebral fractures in women with severe obesity. J Clin Densitom (2021) 24(3):397–405. doi: 10.1016/j.jocd.2020.10.005
40. Jia L, Cheng M. Correlation analysis between risk factors, BMD and serum osteocalcin, CatheK, PINP, beta-crosslaps, TRAP, lipid metabolism and BMI in 128 patients with postmenopausal osteoporotic fractures. Eur Rev Med Pharmacol Sci (2022) 26(21):7955–9. doi: 10.26355/eurrev_202211_30147
41. Du D, Jing Z, Zhang G, Dang X, Liu R, Song J. The relationship between central obesity and bone mineral density: a Mendelian randomization study. Diabetol Metab Syndr (2022) 14(1):63. doi: 10.1186/s13098-022-00840-x
42. Zhang Y, Tan C, Tan W. BMI, socioeconomic status, and bone mineral density in U.S. adults: Mediation analysis in the NHANES. Front Nutr (2023) 10:1132234. doi: 10.3389/fnut.2023.1132234
43. Chen N, Wang J. Wnt/beta-catenin signaling and obesity. Front Physiol (2018) 9:792. doi: 10.3389/fphys.2018.00792
44. Cui Y, Hu X, Zhang C, Wang K. The genetic polymorphisms of key genes in WNT pathway (LRP5 and AXIN1) was associated with osteoporosis susceptibility in Chinese Han population. Endocrine (2022) 75(2):560–74. doi: 10.1007/s12020-021-02866-z
45. Jain RK, Vokes T. Visceral adipose tissue is negatively associated with bone mineral density in NHANES 2011-2018. J Endocr Soc (2023) 7(4):bvad008. doi: 10.1210/jendso/bvad008
46. Koromani F, Ghatan S, van Hoek M, Zillikens MC, Oei EHG, Rivadeneira F, et al. Type 2 diabetes mellitus and vertebral fracture risk. Curr Osteoporos Rep (2021) 19(1):50–7. doi: 10.1007/s11914-020-00646-8
47. Li H, Zhang X, Zhang Q, Zhang Q, Zhu X, Xie T, et al. The relationship between the monocyte-to-lymphocyte ratio and osteoporosis in postmenopausal females with T2DM: A retrospective study in Chinese population. Front Endocrinol (Lausanne) (2023) 14:1112534. doi: 10.3389/fendo.2023.1112534
48. Fischer V, Haffner-Luntzer M. Interaction between bone and immune cells: Implications for postmenopausal osteoporosis. Semin Cell Dev Biol (2022) 123:14–21. doi: 10.1016/j.semcdb.2021.05.014
49. Zhang P, Peterson M, Su GL, Wang SC. Visceral adiposity is negatively associated with bone density and muscle attenuation. Am J Clin Nutr (2015) 101(2):337–43. doi: 10.3945/ajcn.113.081778
50. Bredella MA, Torriani M, Ghomi RH, Thomas BJ, Brick DJ, Gerweck AV, et al. Determinants of bone mineral density in obese premenopausal women. Bone (2011) 48(4):748–54. doi: 10.1016/j.bone.2010.12.011
51. Pan K, Tu R, Yao X, Zhu Z. Associations between serum calcium, 25(OH)D level and bone mineral density in adolescents. Adv Rheumatol (2021) 61(1):16. doi: 10.1186/s42358-021-00174-8
52. Liu G, Peacock M, Eilam O, Dorulla G, Braunstein E, Johnston CC. Effect of osteoarthritis in the lumbar spine and hip on bone mineral density and diagnosis of osteoporosis in elderly men and women. Osteoporos Int (1997) 7(6):564–9. doi: 10.1007/BF02652563
53. Dalle Carbonare L, Giannini S, Sartori L, Nobile M, Ciuffreda M, Silva-Netto F, et al. Lumbar osteoarthritis, bone mineral density, and quantitative ultrasound. Aging (Milano) (2000) 12(5):360–5. doi: 10.1007/BF03339861
Keywords: visceral adiposity index, femur bone mineral density, osteoporosis, U-shaped, cross-sectional study, National Health and Nutrition Examination Survey
Citation: Sun A, Hu J, Wang S, Yin F and Liu Z (2023) Association of the visceral adiposity index with femur bone mineral density and osteoporosis among the U.S. older adults from NHANES 2005–2020: a cross-sectional study. Front. Endocrinol. 14:1231527. doi: 10.3389/fendo.2023.1231527
Received: 30 May 2023; Accepted: 18 October 2023;
Published: 02 November 2023.
Edited by:
Saba Tariq, University of Birmingham, United KingdomReviewed by:
Yusuke Osawa, Keio University, JapanCopyright © 2023 Sun, Hu, Wang, Yin and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhengtang Liu, ZG9jdG9yemh0QDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.