
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 31 August 2023
Sec. Cellular Endocrinology
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1231520
Chronic inflammation and fibrosis are significant factors in the pathogenesis of metabolic-associated fatty liver disease (MAFLD). In this study, we conducted a bibliometric analysis of publications on inflammation and fibrogenesis in MAFLD, with a focus on reporting publication trends. Our findings indicate that the USA and China are the most productive countries in the field, with the University of California San Diego being the most productive institution. Over the past 23 years, Prof. Diehl AM has published 25 articles that significantly contributed to the research community. Notably, the research focus of the field has shifted from morbid obesity and adiponectin to metabolic syndrome, genetics, and microbiome. Our study provides a comprehensive and objective summary of the historical characteristics of research on inflammation and fibrogenesis in MAFLD, which will be of interest to scientific researchers in this field.
MAFLD, formerly known as non-alcoholic fatty liver disease (NAFLD), is distinguished by hepatic steatosis and the presence of one or more of the following factors: overweight/obesity, type 2 diabetes mellitus (T2DM), or indications of metabolic dysregulation (1). Strikingly, MAFLD affects approximately a quarter of the population worldwide with a rising trend (2) and involves a spectrum of liver diseases that range from simple steatosis to its progressive form, non-alcoholic steatohepatitis (NASH), characterized by inflammation and progressive tissue fibrosis, and may lead to the development of cirrhosis and even hepatocellular carcinoma (HCC) (2). Moreover, MAFLD aggravates the deteriorative progression of T2DM and its complications (3), and significantly increases the risk of chronic kidney disease (CKD) (4) along with cardiovascular disease (5). Nevertheless, the clinical management of MAFLD is currently restricted to lifestyle interventions with no approved drug therapy for the disease.
It has been widely accepted that chronic inflammation and fibrosis formation play pivotal roles in the pathogenesis of MAFLD (6). In a physiological state, the hepatic inflammatory response is a response to various stress conditions, which is beneficial to repairing tissue damage and promoting hepatic homeostasis (7). However, prolonged or intense inflammatory reactions may result in irreversible liver damage, such as liver fibrosis, which is triggered by the activation of hepatic stellate cells (HSCs) and their transdifferentiation into myofibroblasts (8). According to the “multiple hit” hypothesis, a comprehensive and detailed theory focusing on the pathomechanism of MAFLD, inflammation may precede steatosis in NASH, and inflammatory mechanisms are involved in the entire process of MAFLD (9). Moreover, synergistic effects of pathological events, such as endoplasmic reticulum (ER) stress (10), insulin resistance (11), aberrant lipid metabolism (12), oxidative stress (13), and mitochondrial dysfunction (14), make great contributions to the exacerbation of inflammation and the deterioration of fibrogenesis via various pathways, and have all been implicated in the progression of MAFLD. Therefore, it is meaningful to illustrate and summarize the research trend on inflammation and fibrogenesis in MAFLD with the hope of discovering drug targets and developing effective therapies.
Comparatively to literature reviews, bibliometrics conducts quantitative research on the field’s literature by analyzing its characteristics with the help of visualizing processing tools, like CiteSpace or VOSviewer, to identify the predominant institutions/countries, leading authors and journals, top-cited references, research trend, or hotspots (15). Considering that hepatic inflammation and fibrogenesis are of great importance in the progress of MAFLD, no bibliometric study has been reported on this topic. Therefore, our study aims to identify the publication trends and potentially significant hotspots on inflammation and fibrogenesis in MAFLD by analyzing the records published from 2000 to 2022.
In our study, we selected the Web of Science Core Collection Science Citation Index Expanded (WoSCC-SCIE) database for the literature search from 2000 to 2022 on 6 January 2023. All data extraction and downloads were completed on the same day to avoid bias in database updates. The search strategy is as follows: (TS = (inflammation and fibrosis)) AND (TS = (mafld or nafld or ‘‘nonalcoholic fatty liver disease’’ or ‘‘non-alcoholic fatty liver disease’’ or ‘‘metabolic associated fatty liver disease’’ or ‘‘metabolic-associated fatty liver disease’’)), then non-English literature and other types of literature were excluded, and only articles were enrolled in this study. Then, the raw data were downloaded from WoSCC-SCIE as text files involving full records and cited references. A total of 2,348 articles were ultimately analyzed in our study. The detailed flowchart is shown in Figure 1.
We mainly analyzed the data by VOSviewer (version 1.6.18) and CiteSpace software (version 6.2.R2 advance). The specific method was described before (16). VOSviewer is a software tool for constructing and visualizing bibliometric networks, and is often used to summarize the most prolific countries/regions, institutions, journals, and authors (see www.vosviewer.com). In our study, VOSviewer was used to show the top 10 most cited journals and achievements of different countries/regions and institutions. CiteSpace is another metrological analysis software developed by Prof. Chen C for bibliometric analysis and visualization (17). In this study, CiteSpace was used to evaluate multiple indicators, including the collaboration between countries/regions and authors, co-citation analysis, citation burst, clustered networks of co-cited references, and keywords with the strongest citation bursts.
A total of 2,348 documents were retrieved from the WoSCC-SCIE databases between 2000 and 2022 according to the flowchart shown in Figure 1. As shown in Figure 2, there was an overall upward trend in the amount of literature on inflammation and fibrogenesis in MAFLD, although in some years, the amount of literature could be declining. It is worth noting that 1,412 articles were published in the past 5 years, accounting for 60.14% of the total, implying that MAFLD has become a common chronic disease that has attracted the attention of researchers worldwide.
To determine which countries or regions have contributed the most to the development of this field during the past 23 years, we counted the number of articles published by different countries and regions using VOSviewer, and the top 10 most productive countries or regions are shown in Table 1. We found that the USA ranked first, followed by China, Japan, Italy, and Germany. Meanwhile, we concluded that the number of publications from the USA and China far exceeded those other countries/regions. In addition, the number of publications in China first surpassed that in the USA in 2021.
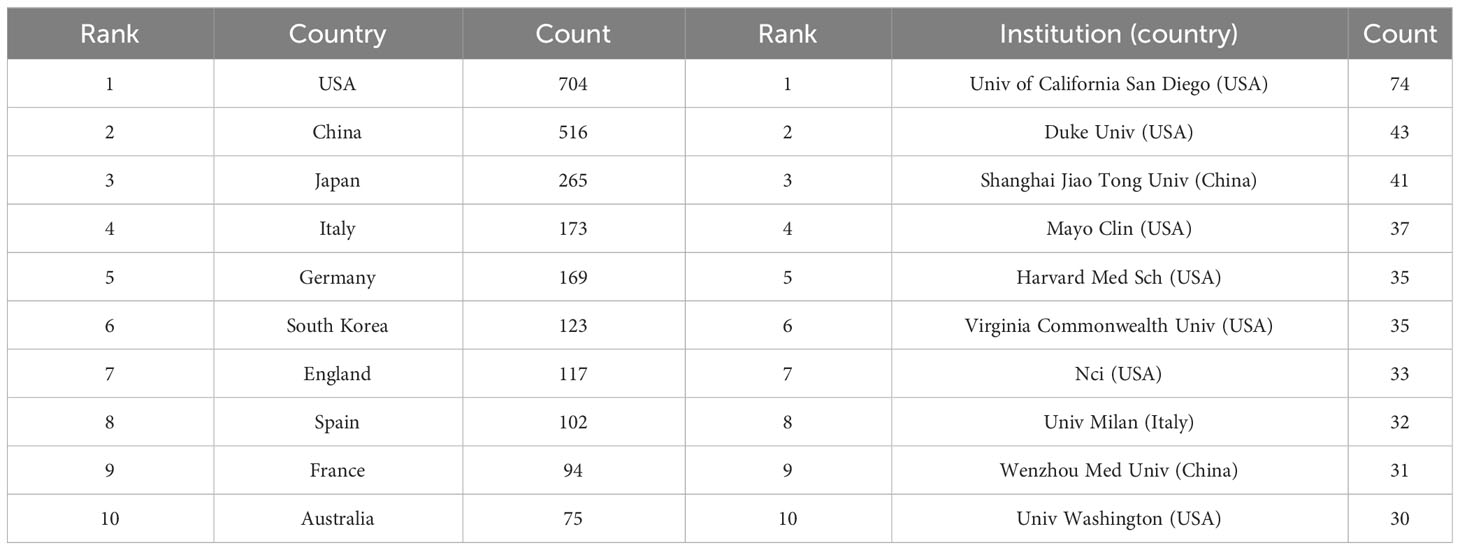
Table 1 The top 10 productive countries and institutions in research of inflammation and fibrosis in MAFLD from 2000 to 2022.
At the same time, we analyzed cooperative relationships between these countries and regions via CiteSpace. As shown in Figure 3, the size of the concentric circle is positively related to the number of articles published by each country and region; the fuchsia ring indicates a node with a centrality value greater than 0.1, signifying its close relationship with other nodes. We found that 6 of the top 10 fruitful countries worked closely with others. Among them, China ranked second in terms of the number of published articles, but its international cooperation with other countries needs to be strengthened.
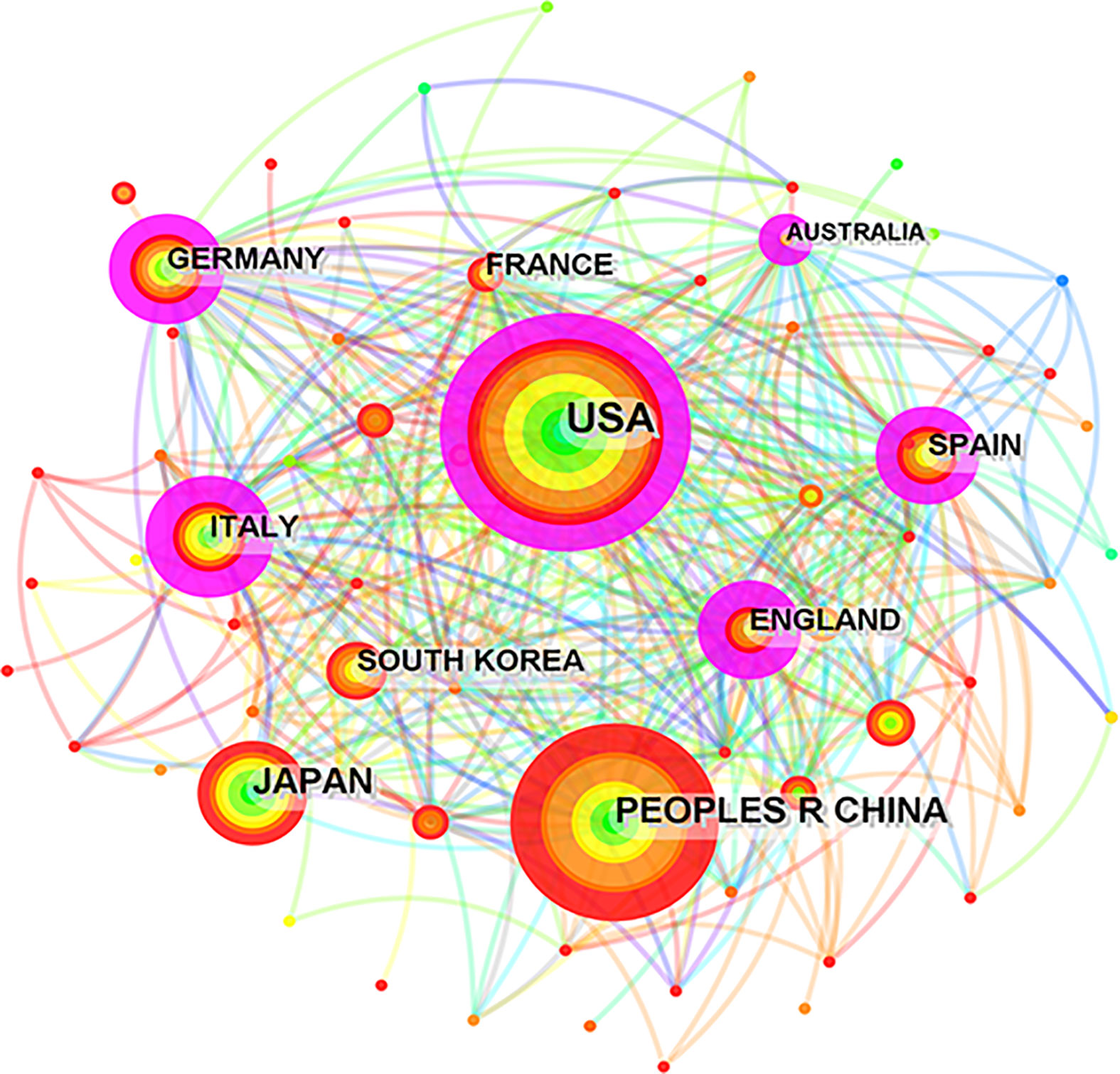
Figure 3 CiteSpace network map of the collaboration analysis of the studies on inflammation and fibrogenesis MAFLD among countries/ regions in 2000-2022, the top 10 countries/ regions are shown in the picture. The size of the concentric circle is positively related with the number of articles published by each country and area, the fuchsia ring indicates a node with a centrality value greater than 0.1.
Moreover, we analyzed the contribution of global institutions. The most yielding institutions are shown in Table 1. Interestingly, among the top 10 productive institutions, 7 were from the USA, 2 came from China, and 1 was from Italy. The University of California San Diego published the most articles (74 articles), followed by Duke University (n = 43), Shanghai Jiao Tong University (n = 41), Mayo University (n = 37), and Harvard Medical School (n = 35).
Over the past 23 years, 606 scholarly journals have published a total number of 2,348 original articles. We used VOSviewer to show journal influence. The top 10 most cited journals related to the topic of inflammation and fibrogenesis in MAFLD are presented in Table 2. According to the analysis, hepatology publications had the most publications (141 papers) and the most citations (22934) during the past 23 years, followed by those in Gastroenterology (9,737), Journal of Hepatology (6191), Plos One (3,941), and New England Journal of Medicine (3,421). In addition, eight journals were in the Q1 Journal Citation Reports (JCR) division, indicating their high academic standing. Notably, seven of these journals are from the USA, and the remaining three are from the Netherlands and the UK, with all of them being developed countries and therefore providing an important platform for the research development in this field.
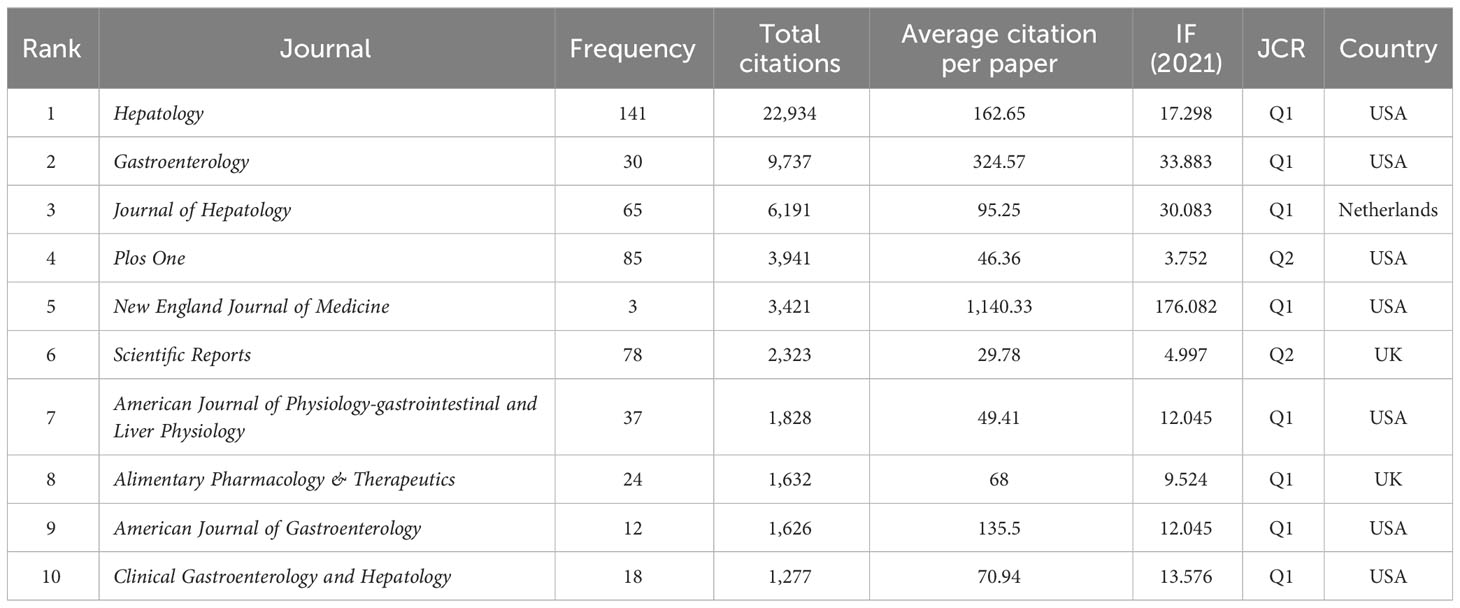
Table 2 The top 10 most active journals in research of inflammation and fibrosis in MAFLD (sorted by total citation) from 2000 to 2022.
The top 10 most productive authors in this field are shown in Figure 4A and Table 3. Diehl AM from the Department of Gastroenterology of Duke University published 25 articles in this field and was ranked first, followed by Nobili V, Alisi A, Sanyal AJ, and Feldstein AE. She seemed interested in the connection between the Hedgehog (Hh) pathway and MAFLD (18–20) and carried out an in-depth study in this field. Furthermore, she took part in a clinical research discussing the efficacy of pioglitazone versus vitamin E versus placebo in non-diabetic patients with NASH, showing that vitamin E was a potential treatment well accepted with its high citation rate (21). Furthermore, she discussed the relationship between MAFLD and different reproductive life cycles, such as puberty and menopause (22, 23). The second one was Nobili V, from the Department of Pediatrics of Sapienza university of Rome, who has published 23 articles in this field and made great contributions to the field of children with MAFLD. His most cited article published in Hepatology studied lifestyle intervention and antioxidant therapy in children with MAFLD (24). Several articles suggested that various genetic mutations were associated with MAFLD, such as the PCSK7 gene variation (25) and the I148M patatin-like phospholipase domain-containing 3 gene mutation (26). Interestingly, Alisi A, from the same institution as Nobili V, published 23 articles and was ranked third. Most of her works were cooperated with Dr. Nobili V, showing their close cooperation and strong scientific research ability.
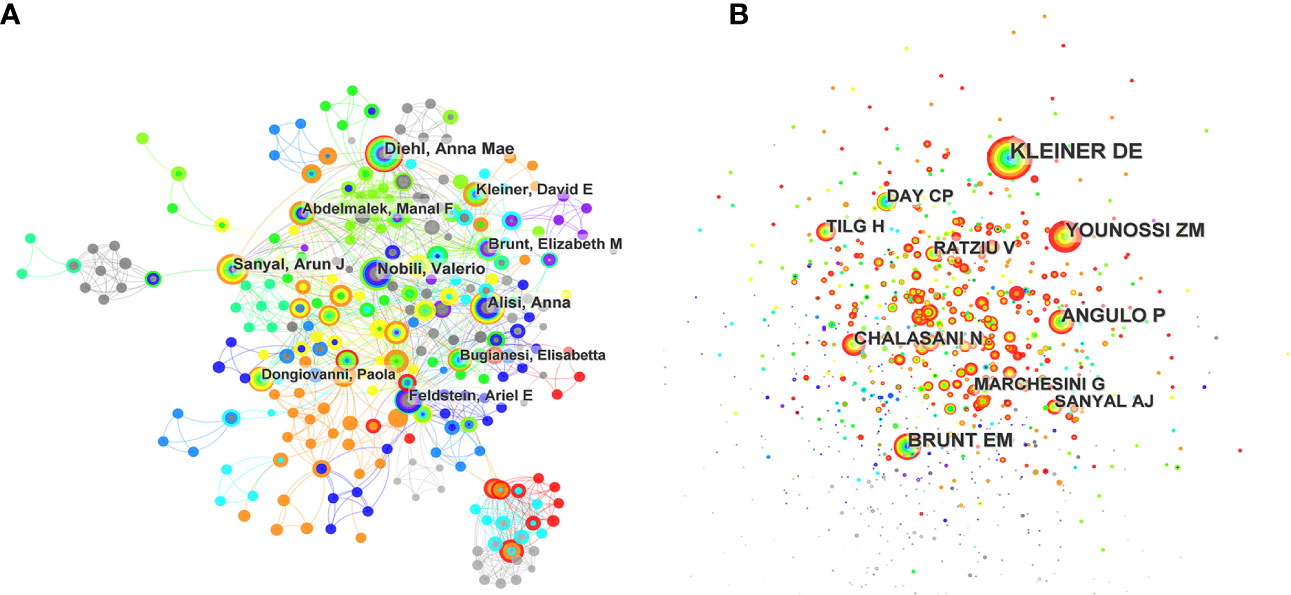
Figure 4 (A) CiteSpace visualization map of the top 10 authors. Each circle represents an author, and a link between two circles means a collaboration between each other. (B) CiteSpace visualization map of the top co-cited authors involved in inflammation and fibrogenesis in MAFLD research.

Table 3 The top 10 productive authors and co-cited authors in research of inflammation and fibrosis in MAFLD from 2000 to 2022.
Co-cited authors are those whose works were cited in more than one study at the same time. The network visualization map for the co-cited authors is shown in Figure 4B and Table 3. Kleiner DE ranked first with a total citation of 949 times, followed by Angulo P, Younossi ZM, Brunt EM, and Chalasani N. Among the top 10 most co-cited authors, 3 were the top 10 productive authors, and 4 were the first authors of the most cited articles of derived papers shown in Table 4. In general, the low centrality value indicated a lack of cooperation among them.
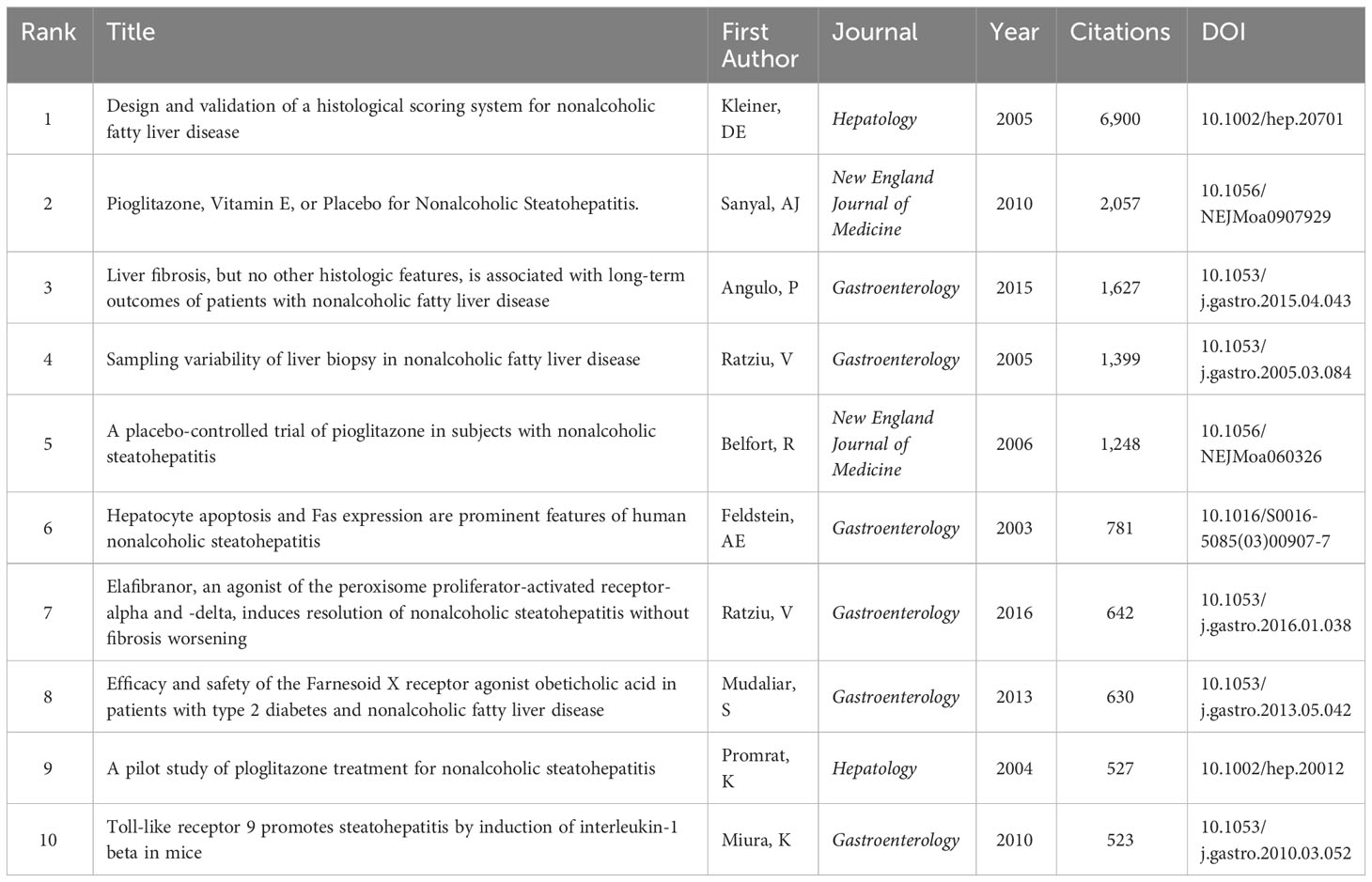
Table 4 The top 10 most cited articles in research of inflammation and fibrosis in MAFLD from 2000 to 2022.
Citation analysis is a reliable indicator for assessing the quality of articles, the results were derived from WoSCC-SCIE, and the top 10 most cited articles are shown in Table 4. The article published in Hepatology in 2005 ranked first. It presented a scoring system to assess a large range of histological features of NAFLD for pediatric and adult NAFLD (27). The next one was published in the New England Journal of Medicine in 2010, which was a clinical study discussing the efficacy of pioglitazone and vitamin E for the treatment of NASH in adults without diabetes. It was conducted in cooperation with Diehl AM, who ranked first in the number of published articles (21). The third one also focused on histological features and approved the crucial role of fibrosis stage in managing and monitoring in NAFLD patients (28). Among the top 10 most cited articles, 6 were from Gastroenterology, 2 were from Hepatology, and 2 were from the New England Journal of Medicine. It is worth noting that all of the above three journals were top-cited, enhancing the reliability of the results shown in Table 2.
Co-cited references are two or more references cited by another paper or more papers simultaneously. From 2000 to 2022, a total of 2,348 articles and their 53,816 references retrieved from WoSCC-SCIE were analyzed by CiteSpace, the first authors of the top 10 most co-cited references are presented in Figure 5A, and the summary of the top 10 most co-cited references is shown in Table 5. Interestingly, it seems to be well-accepted that the fibrosis stage of patients with MAFLD is an independent factor for the long-term outcomes, such as mortality, liver transplantation, and liver-related events (28, 29). In brief, the most cited references shown in Table 5 made great contributions to the development of inflammation and fibrogenesis in the MAFLD scientific community and were the most recognized papers in this field.
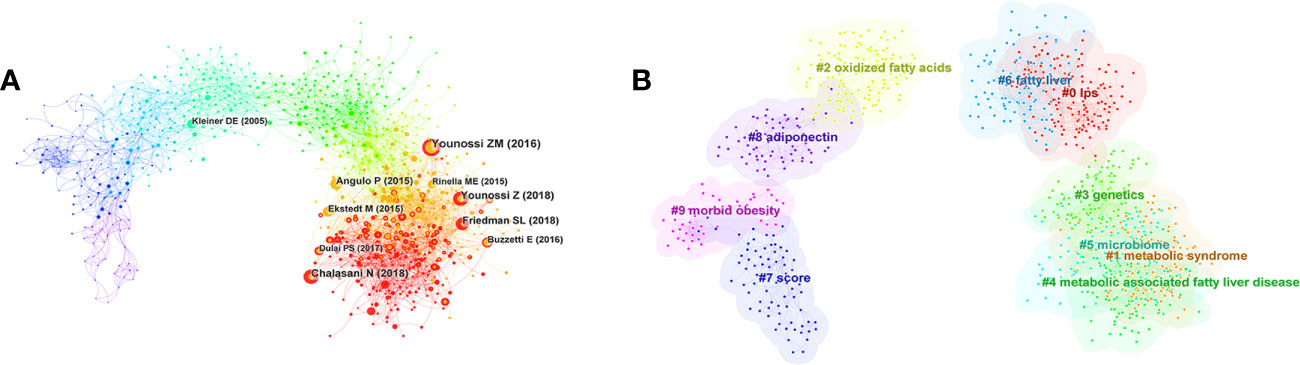
Figure 5 (A) CiteSpace co-citation map of references involved in inflammation and fibrogenesis in MAFLD. The first authors of the top 10 most co-cited references are presented. (B) Clustered networks of the co-citation analysis of the investigated reference and the 2348 citing articles via CiteSpace. The top 10 largest clusters are shown.
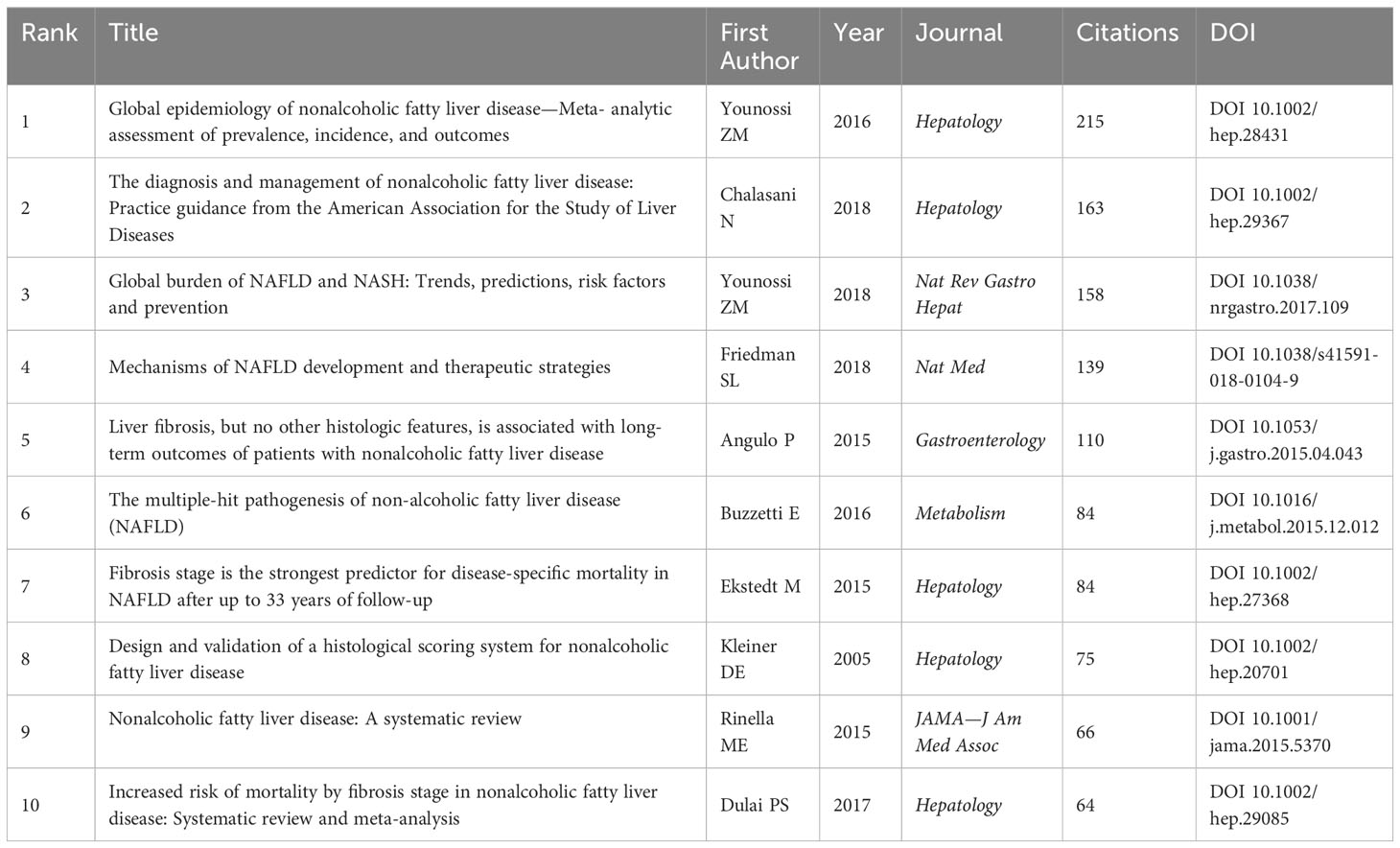
Table 5 The top 10 high-cited references in research of inflammation and fibrosis in MAFLD from 2000 to 2022.
In addition, we also analyzed the strong citation burst of references in this topic. The references of the top 20 references with the strongest citation bursts are shown in Figure 6. “Year” refers to the publication date, “Begin” refers to the first citation, and “End” refers to the last citation. We found that the two papers with 4 years’ duration were all focused on seeking effective treatment methods for MAFLD, indicating experts’ concern for this disease (21, 30). The strongest citation reference was a meta-analysis review published by Hepatology in 2016, which is still widely cited and was the top high-cited reference, discussing the epidemiology of NAFLD, including prevalence, incidence, and long-term outcomes (2). Five other pieces of literature still widely cited described the mechanism, diagnosis, treatment, and global burden of MAFLD (31–33).
The map of the co-citation cluster according to keywords generated from the references of 2,348 citing articles by CiteSpace is shown in Figure 5B. The clustering modularity Q and the mean silhouette value were 0.6833 and 0.8729, respectively, demonstrating a credible structure and clustering results. Furthermore, the number of cluster labels is inversely proportional to the number of articles included in each cluster. Thus, the “#0 LPS” cluster contains the most papers, while the “#9 morbid obesity” cluster contains the fewest. The summary of clusters is listed in Table 6.
In order to analyze the change in research hotspots and trends, a timeline view is displayed in Figure 7. We found that early research concentrated on “#8 adiponectin” and “#9 morbid obesity”, interim studies concentrated on “#0 LPS”, “#2 oxidized fatty acids”, and “#6 fatty liver”, whereas current studies concentrated on “#1 metabolic syndrome (MS),” “#3 genetics”, and “#5 microbiome”, indicating changes in research hotspots.
Keyword burst detection is another effective way to explore research hotspots. Figure 8 shows the top 25 keywords with the strongest citation bursts on research from 2000 to 2022. Among the identified keyword bursts, chronic hepatitis C began in 2000 and ended in 2015 with the longest lasting time, which was associated with liver steatosis and was a risk factor for MAFLD. Moreover, three keyword bursts continue to last until the end of 2022, including management, stress, and fibrosis stage.
In this bibliometric analysis study, we found 2,348 articles regarding inflammation and fibrogenesis in MAFLD from 2000 to 2022 in the WoSCC-SCIE. With the help of VOSviewer and CiteSpace software, our study analyzed publication trends about inflammation and fibrogenesis in MAFLD from all aspects to shed light on researchers interested in this field.
Between 2000 and 2013, less than 100 articles concerning inflammation and fibrogenesis in MAFLD were published per year globally. However, the number of papers has increased rapidly since 2018, which was associated with the renaming of NAFLD and its high morbidity, indicating that the research on inflammation and fibrogenesis in MAFLD has caught the attention of researchers worldwide. Table 1 shows that the USA played a vital role in the research on inflammation and fibrogenesis in MAFLD. Notably, with the development of China’s medical and scientific research capability, the number of articles published in China has rapidly increased and firstly surpassed the USA in 2021. Moreover, with the development of globalization, international cooperation has become a new trend that benefits the output of high-quality research. The collaborations between countries/regions are shown in Figure 3, most (n = 6) of the top 10 productive countries/regions cooperated closely with other countries, but China greatly needs to strengthen its cooperation with other countries.
Remarkably, 7 of the top 10 productive institutions and 7 of the top 10 most cited journals are from the USA, reasonably explaining that the USA occupies the main contribution on total numbers of published papers. Therefore, these results demonstrated that the USA played a dominant role in the world’s academic activities. However, China may be a rising star in the next few years, considering the number of papers published in this field and its rising trends.
The timeline view in Figure 7 indicates the evolution of the research trend. We found that early research concentrated on adiponectin and morbid obesity. Adiponectin is an adipocyte-specific secretory protein that plays a pivotal role in glycolipid metabolism (34) and extracellular matrix (ECM) metabolism (35) and has made a great contribution to preventing the liver from steatosis, inflammation, and fibrosis (36, 37). It has been reported that MAFLD patients have significantly lower plasma adiponectin levels (38). Multivariate regression analysis identifies decreased adiponectin as an independent risk factor of hepatic steatosis (37). Furthermore, abundant clinical and basic studies have illustrated that adiponectin agonists are therapeutic targets for NAFLD therapy (39, 40). Morbid obesity was defined as a body mass index (BMI) of at least 40 kg/m2. Many researchers suggest that Roux-en-Y gastric bypass (RYGB) surgery is a decent curative method in morbid obesity patients with MAFLD to improve inflammation and fibrosis and then inhibit malignant progression to NASH (41–43). Subsequently, interim research mainly focused on identifying novel markers and cellular and molecular mechanisms in MAFLD (44, 45).
With the deepening study in this field, there is increasing evidence to show that MS is an important risk factor in MAFLD, vividly illustrated by the renaming of NAFLD to MAFLD. MS mainly refers to hyperglycemia, abdominal obesity, and dyslipidemia. A systematic review attempted to estimate the prevalence of MAFLD among patients with T2DM and found that the prevalence of MAFLD was 55.5% among 49,419 T2DM patients (46). Another research suggested that more than 90% of obese patients with T2DM also have MAFLD (47), indicating that MAFLD is strongly associated with hyperglycemia. Moreover, studies among T2DM participants have demonstrated that plasma glucose level is positively correlated with the risk of developing advanced liver disease (HCC) (48). Furthermore, glycemic variability, except for hyperinsulinemia and hyperglycemia, is an independent predictive factor for the progression of hepatic fibrosis in MAFLD (49). Considering that there is no approved drug for NASH to date, lifestyle interventions and combinations of drugs, which can effectively regulate glucose and lipid metabolism and reduce liver inflammation and fibrosis, might be a beneficial option to curb the deteriorated progression of MAFLD. Even new drug development focuses on the restitution of metabolic derangements and halting inflammatory and fibrogenic pathways, showing that MS plays an important role in the development of MAFLD (50).
Furthermore, it has been reported that genetic factors took part in the progression of MAFLD due to the upgrading and improvement of new genomic and proteomic technologies (51), and many risk variants of the NAFLD population were identified by a genome-wide association study (GWAS) (52). Currently, at least five variants in different genes are associated with the development and progression of MAFLD, namely, PNPLA3, TM6SF2, GCKR, MBOAT7, and HSD17B13 (53–55). Some of them are associated with an increased risk of T2DM. Others are associated with the risk of developing obesity (56, 57), indicating that MAFLD might have shared mechanisms that are involved in the pathogenesis of T2DM and obesity, emphasizing the importance of MS in MAFLD, and supporting the renaming of NAFLD to MAFLD (55).
Gut microbiota is another research hotspot now. Many studies showed that microbiota might improve or aggravate MAFLD through multiple mechanisms (58–60), including changing the permeability of the intestine (61), altering the expression of genes involved in the de novo lipogenesis (62), and regulating choline (63) and bile acid metabolism (64). The most extensively studied microbial molecule is lipopolysaccharide (LPS), which is produced by Gram-negative bacteria. It has been reported that systemic LPS concentration was significantly elevated in rats treated with HFD and high-sucrose diet (65) and in MAFLD patients (66). In related animal studies, the effect of LPS on the development of MAFLD has also been shown in mice injected with LPS and mice lacking toll-like receptor 4 (TLR4) (67, 68). A number of studies investigated that modulation of the gut microbiota may be a potential therapeutic target for MAFLD, including using antibiotics, prebiotics, and probiotics (69).
Generally speaking, Figure 7 demonstrates that the research hotspots have shifted their direction to MS, genetics, and the microbiome in the study of inflammation and fibrosis in MAFLD.
Another effective method of reflecting the transition of hotspots in an academic area is to use keyword bursts. The top 25 keywords with the strongest citation bursts are shown in Figure 8. Among them, three keywords continue to last by the end of 2022. The first one is management. The related research with the highest citation was published in Hepatology in 2019, elucidating that gut microbiota profile and systemic inflammatory response in NAFLD were closely related, further promoting the process of HCC (70). Some of the works concerned the relationship between MAFLD and common chronic diseases (71, 72), and most publications explored effective methods to curb the development of MAFLD and its complications (73–76). The second one was fibrosis stage, a valuable parameter to predict all-cause and liver disease-related mortality in MAFLD (77). The related articles mainly focused on seeking potential ways to restrain the adverse progression of fibrosis stage and thus improve the prognosis (78–80). For example, Khurana and Wang attempted to find an invasive method that is helpful for early diagnosis, distinguishing disease staging, and giving personalized treatments (81, 82). The third keyword burst, which started in 2019, was stress. Articles related to this keyword refer to a metabolic stress state relevant to the dysfunction of mitochondria and ER and describe its potential mechanisms that have a significant impact on the progress of MAFLD. Zhang et al. suggested that impaired mitophagy, which may lead to the accumulation of excessive ROS production and oxidative stress, triggered hepatic NLRP3 inflammasome activation during the progress of MAFLD (83). In addition, another research noted that down-regulating the NLRP3/NF-κB signaling pathway can attenuate inflammation in mouse liver (84). Meanwhile, the authors identified apoptosis signal-regulating kinase 1 (ASK1) as a suppressor of NASH and fibrosis formation via ASK1 knockout experiments. All of them reminded us that inflammation and fibrosis are key factors in the development of MAFLD and drugs that act on them may have potential as a clinical treatment to prevent MAFLD in humans.
Of note, magnetic resonance elastography (MRE), a keyword that lasted from 2017 to 2019, is a non-invasive evaluation to distinguish healthy people from those with NAFLD and assess the degree of fibrosis to discriminate simple steatosis from NASH (85), showing that colleagues are actively looking for non-invasive methods to determine the severity of NAFLD, and thus ensure timely and optimal treatment.
Notably, the number of publications focusing on inflammation and fibrosis in MAFLD increased rapidly. However, previous studies on inflammation and fibrogenesis in MAFLD still have certain limitations, and there is much to be improved in the future.
1. It is urgent to identify more valuable non-invasive biomarkers to meet the need to accurately stage the progression of MAFLD, make a definitive diagnosis as soon as possible, and provide the patients with timely and effective treatment.
2. More research on developing novel agents targeting hepatic inflammation and fibrosis are needed as there are no approved drugs for NASH.
3. There are numerous basic studies, but few can be applied to the clinic. The use of non-human primate models rather than rodents in mechanistic investigations would likely allow for a higher chance of translating basic discoveries into clinical practice.
Our study has some limitations. First, we only analyzed data exported from WoSCC-SCIE to undertake relevant analysis, which may result in selection bias. Second, this study excluded non-English literature, and some high-quality non-English literature was excluded. Third, VOSviewer and CiteSpace have certain defects that may output discredited results. Nevertheless, our study still provides significant information and insights for researchers interested in this field.
From 2000 to 2022, the number of articles focusing on inflammation and fibrosis in MAFLD increased rapidly, especially in the last 5 years. The USA played a vital role in the development of this topic regarding the number of publications, international cooperation with other countries, and achievements of authors. However, China may be a rising star in this research field with its increasing trend in the number of publications and its huge population. Current research mainly focuses on MS, genetics, and microbiome. Considering the urgent and emergent situation of inflammation and fibrosis in MAFLD, more studies are needed to focus on developing novel drugs and identifying non-invasive biomarkers. In short, our study provides a comprehensive overview of this discipline, which could more precisely direct scholars in future research and provide valuable guidance for clinical diagnosis, appropriate treatment, and individualized prevention.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
All the authors have contributed significantly. HL designed the study. KL and YC wrote the manuscript. KL, YC, SF, SW, and ZW collected and analyzed the data, participated in discussion. SF and HL supervised the study and corrected the manuscript. All authors contributed to the article and approved the submitted version.
The National Natural Science Foundation of China (grant numbers 81974111 to HL).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol (2020) 73(1):202–9. doi: 10.1016/j.jhep.2020.03.039
2. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence and outcomes. Hepatology (2016) 64(1):73–84. doi: 10.1002/hep.28431
3. Mantovani A, Scorletti E, Mosca A, Alisi A, Byrne CD, Targher G. Complications, morbidity and mortality of nonalcoholic fatty liver diseasme. Metab - Clin Exp (2020) 111S:154170. doi: 10.1016/j.metabol.2020.154170
4. Su W, Chen M, Xiao L, Du S, Xue L, Feng R, et al. Association of metabolic dysfunction-associated fatty liver disease, type 2 diabetes mellitus, and metabolic goal achievement with risk of chronic kidney disease. Front Public Health (2022) 10:1047794. doi: 10.3389/fpubh.2022.1047794
5. Anstee QM, Mantovani A, Tilg H, Targher G. Risk of cardiomyopathy and cardiac arrhythmias in patients with nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol (2018) 15(7):425–39. doi: 10.1038/s41575-018-0010-0
6. Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med (2018) 24(7):908–22. doi: 10.1038/s41591-018-0104-9
7. Kubes P, Mehal WZ. Sterile inflammation in the liver. Gastroenterology. (2012) 143(5):1158–72. doi: 10.1053/j.gastro.2012.09.008
8. Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol (2017) 14(7):397–411. doi: 10.1038/nrgastro.2017.38
9. Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: The multiple parallel hits hypothesis. Hepatology (2010) 52(5):1836–46. doi: 10.1002/hep.24001
10. Flessa C-M, Kyrou I, Nasiri-Ansari N, Kaltsas G, Kassi E, Randeva HS. Endoplasmic reticulum stress in nonalcoholic (metabolic associated) fatty liver disease (NAFLD/MAFLD). J Cell Biochem (2022) 123(10):1585–606. doi: 10.1002/jcb.30247
11. Sakurai Y, Kubota N, Yamauchi T, Kadowaki T. Role of insulin resistance in MAFLD. Int J Mol Sci (2021) 22(8):4156. doi: 10.3390/ijms22084156
12. Geng Y, Faber KN, de Meijer VE, Blokzijl H, Moshage H. How does hepatic lipid accumulation lead to lipotoxicity in non-alcoholic fatty liver disease? Hepatol Int (2021) 15(1):21–35. doi: 10.1007/s12072-020-10121-2
13. Clare K, Dillon JF, Brennan PN. Reactive oxygen species and oxidative stress in the pathogenesis of MAFLD. J Clin Transl Hepatol (2022) 10(5):939–46. doi: 10.14218/JCTH.2022.00067
14. Pirola CJ, Gianotti TF, Burgueño AL, Rey-Funes M, Loidl CF, Mallardi P, et al. Epigenetic modification of liver mitochondrial DNA is associated with histological severity of nonalcoholic fatty liver disease. Gut. (2013) 62(9):1356–63. doi: 10.1136/gutjnl-2012-302962
15. Chen C. Searching for intellectual turning points: progressive knowledge domain visualization. Proc Natl Acad Sci USA (2004) 101(Suppl 1):5303–10. doi: 10.1073/pnas.0307513100
16. Pei Z, Chen S, Ding L, Liu J, Cui X, Li F, et al. Current perspectives and trend of nanomedicine in cancer: A review and bibliometric analysis. J Control Release. (2022) 352:211–41. doi: 10.1016/j.jconrel.2022.10.023
17. Chen C, Song I-Y, Yuan X, Zhang J. The thematic and citation landscape of Data and Knowledge Engineering (1985–2007). Data Knowledge Engineering. (2008) 67(2):234–59. doi: 10.1016/j.datak.2008.05.004
18. Sundaram SS, Swiderska-Syn M, Sokol RJ, Halbower AC, Capocelli KE, Pan Z, et al. Nocturnal hypoxia activation of the hedgehog signaling pathway affects pediatric nonalcoholic fatty liver disease severity. Hepatol Commun (2019) 3(7):883–93. doi: 10.1002/hep4.1354
19. Swiderska-Syn M, Suzuki A, Guy CD, Schwimmer JB, Abdelmalek MF, Lavine JE, et al. Hedgehog pathway and pediatric nonalcoholic fatty liver disease. Hepatology (2013) 57(5):1814–25. doi: 10.1002/hep.26230
20. Guy CD, Suzuki A, Zdanowicz M, Abdelmalek MF, Burchette J, Unalp A, et al. Hedgehog pathway activation parallels histologic severity of injury and fibrosis in human nonalcoholic fatty liver disease. Hepatology (2012) 55(6):1711–21. doi: 10.1002/hep.25559
21. Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med (2010) 362(18):1675–85. doi: 10.1056/NEJMoa0907929
22. Suzuki A, Abdelmalek MF, Schwimmer JB, Lavine JE, Scheimann AO, Unalp-Arida A, et al. Association between puberty and features of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol (2012) 10(7):786–94. doi: 10.1016/j.cgh.2012.01.020
23. Yang JD, Abdelmalek MF, Guy CD, Gill RM, Lavine JE, Yates K, et al. Patient sex, reproductive status, and synthetic hormone use associate with histologic severity of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol (2017) 15(1):127–31.e2. doi: 10.1016/j.cgh.2016.07.034
24. Nobili V, Manco M, Devito R, Di Ciommo V, Comparcola D, Sartorelli MR, et al. Lifestyle intervention and antioxidant therapy in children with nonalcoholic fatty liver disease: A randomized, controlled trial. Hepatology (2008) 48(1):119–28. doi: 10.1002/hep.22336
25. Dongiovanni P, Meroni M, Baselli G, Mancina RM, Ruscica M, Longo M, et al. PCSK7 gene variation bridges atherogenic dyslipidemia with hepatic inflammation in NAFLD patients. J Lipid Res (2019) 60(6):1144–53. doi: 10.1194/jlr.P090449
26. Valenti L, Alisi A, Galmozzi E, Bartuli A, Del Menico B, Alterio A, et al. I148M patatin-like phospholipase domain-containing 3 gene variant and severity of pediatric nonalcoholic fatty liver disease. Hepatology (2010) 52(4):1274–80. doi: 10.1002/hep.23823
27. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology (2005) 41(6):1313–21. doi: 10.1002/hep.20701
28. Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Hepatology (2015) 149(2):389–97.e10. doi: 10.1053/j.gastro.2015.04.043
29. Ekstedt M, Hagström H, Nasr P, Fredrikson M, Stål P, Kechagias S, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatol (Baltimore Md). (2015) 61(5):1547–54. doi: 10.1002/hep.27368
30. Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet (2015) 385(9972):956–65. doi: 10.1016/S0140-6736(14)61933-4
31. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology (2018) 67(1):328–57. doi: 10.1002/hep.29367
32. Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metab - Clin Experimental. (2016) 65(8):1038–48. doi: 10.1016/j.metabol.2015.12.012
33. Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatology. (2018) 15(1):11–20. doi: 10.1038/nrgastro.2017.109
34. Tao C, Sifuentes A, Holland WL. Regulation of glucose and lipid homeostasis by adiponectin: effects on hepatocytes, pancreatic β cells and adipocytes. Best Pract Res Clin Endocrinol Metab (2014) 28(1):43–58. doi: 10.1016/j.beem.2013.11.003
35. Zhao C, Wu M, Zeng N, Xiong M, Hu W, Lv W, et al. Cancer-associated adipocytes: emerging supporters in breast cancer. J Exp Clin Cancer Res (2020) 39(1):156. doi: 10.1186/s13046-020-01666-z
36. Heydari M, Cornide-Petronio ME, Jiménez-Castro MB, Peralta C. Data on adiponectin from 2010 to 2020: therapeutic target and prognostic factor for liver diseases? Int J Mol Sci (2020) 21(15):5242. doi: 10.3390/ijms21155242
37. Targher G, Bertolini L, Scala L, Poli F, Zenari L, Falezza G. Decreased plasma adiponectin concentrations are closely associated with nonalcoholic hepatic steatosis in obese individuals. Clin Endocrinol (Oxf). (2004) 61(6):700–3. doi: 10.1111/j.1365-2265.2004.02151.x
38. Pagano C, Soardo G, Esposito W, Fallo F, Basan L, Donnini D, et al. Plasma adiponectin is decreased in nonalcoholic fatty liver disease. Eur J Endocrinology. (2005) 152(1):113–8. doi: 10.1530/eje.1.01821
39. Gastaldelli A, Harrison S, Belfort-Aguiar R, Hardies J, Balas B, Schenker S, et al. Pioglitazone in the treatment of NASH: the role of adiponectin. Aliment Pharmacol Ther (2010) 32(6):769–75. doi: 10.1111/j.1365-2036.2010.04405.x
40. Alzahrani B, Iseli T, Ramezani-Moghadam M, Ho V, Wankell M, Sun EJ, et al. The role of AdipoR1 and AdipoR2 in liver fibrosis. Biochim Biophys Acta Mol basis disease. (2018) 1864(3):700–8. doi: 10.1016/j.bbadis.2017.12.012
41. de Almeida SR, Savassi Rocha PR, Dias Sanches M, Rios Leite VH, da Silva RAP, Costa Diniz MT, et al. Roux-en-Y gastric bypass improves the nonalcoholic steatohepatitis (NASH) of morbid obesity. Obes Surgery. (2006) 16(3):270–8. doi: 10.1381/096089206776116462
42. Furuya CK Jr., De Oliveira CPMS, De Mello ES, Faintuch J, Raskovski A, Matsuda M, et al. Effects of bariatric surgery on nonalcoholic fatty liver disease: Preliminary findings after 2 years. J Gastroenterol Hepatol (2007) 22(4):510–4. doi: 10.1111/j.1440-1746.2007.04833.x
43. Klein S, Mittendorfer B, Eagon JC, Patterson B, Grant L, Feirt N, et al. Gastric bypass surgery improves metabolic and hepatic abnorMalities associated with nonalcoholic fatty liver disease. Gastroenterology. (2006) 130(6):1564–72. doi: 10.1053/j.gastro.2006.01.042
44. Feldstein AE, Lopez R, Tamimi TA-R, Yerian L, Chung Y-M, Berk M, et al. Mass spectrometric profiling of oxidized lipid products in human nonalcoholic fatty liver disease and nonalcoholic steatohepatitis [S]. J Lipid Res (2010) 51(10):3046–54. doi: 10.1194/jlr.M007096
45. Subramanian S, Goodspeed L, Wang S, Kim J, Zeng L, Ioannou GN, et al. Dietary cholesterol exacerbates hepatic steatosis and inflammation in obese LDL receptor-deficient mice. J Lipid Res (2011) 52(9):1626–35. doi: 10.1194/jlr.M016246
46. Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, Fukui N, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J Hepatol (2019) 71(4):793–801. doi: 10.1016/j.jhep.2019.06.021
47. Tolman KG, Fonseca V, Dalpiaz A, Tan MH. Spectrum of liver disease in type 2 diabetes and management of patients with diabetes and liver disease. Diabetes Care (2007) 30(3):734–43. doi: 10.2337/dc06-1539
48. Pang Y, Kartsonaki C, Turnbull I, Guo Y, Clarke R, Chen Y, et al. Diabetes, plasma glucose, and incidence of fatty liver, cirrhosis, and liver cancer: A prospective study of 0.5 million people. Hepatol (Baltimore Md) (2018) 68(4):1308–18. doi: 10.1002/hep.30083
49. Hashiba M, Ono M, Hyogo H, Ikeda Y, Masuda K, Yoshioka R, et al. Glycemic variability is an independent predictive factor for development of hepatic fibrosis in nonalcoholic fatty liver disease. PloS One (2013) 8(11):e76161. doi: 10.1371/journal.pone.0076161
50. Rojas Á, Lara-Romero C, Muñoz-Hernández R, Gato S, Ampuero J, Romero-Gómez M. Emerging pharmacological treatment options for MAFLD. Ther Adv Endocrinol Metab (2022) 13:20420188221142452. doi: 10.1177/20420188221142452
51. Suppli MP, Rigbolt KTG, Veidal SS, Heebøll S, Eriksen PL, Demant M, et al. Hepatic transcriptome signatures in patients with varying degrees of nonalcoholic fatty liver disease compared with healthy normal-weight individuals. Am J Physiol Gastrointest Liver Physiol (2019) 316(4):G462–G72. doi: 10.1152/ajpgi.00358.2018
52. Eslam M, Valenti L, Romeo S. Genetics and epigenetics of NAFLD and NASH: Clinical impact. J Hepatol (2018) 68(2):268–79. doi: 10.1016/j.jhep.2017.09.003
53. Abul-Husn NS, Cheng X, Li AH, Xin Y, Schurmann C, Stevis P, et al. A protein-truncating HSD17B13 variant and protection from chronic liver disease. N Engl J Med (2018) 378(12):1096–106. doi: 10.1056/NEJMoa1712191
54. Kozlitina J, Smagris E, Stender S, Nordestgaard BG, Zhou HH, Tybjærg-Hansen A, et al. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat Genet (2014) 46(4):352–6. doi: 10.1038/ng.2901
55. Carlsson B, Lindén D, Brolén G, Liljeblad M, Bjursell M, Romeo S, et al. Review article: the emerging role of genetics in precision medicine for patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther (2020) 51(12):1305–20. doi: 10.1111/apt.15738
56. Mahajan A, Wessel J, Willems SM, Zhao W, Robertson NR, Chu AY, et al. Refining the accuracy of validated target identification through coding variant fine-mapping in type 2 diabetes. Nat Genet (2018) 50(4):559–71. doi: 10.1038/s41588-018-0084-1
57. Perez-Diaz-Del-Campo N, Abete I, Cantero I, Marin-Alejandre BA, Monreal JI, Elorz M, et al. Association of the SH2B1 rs7359397 gene polymorphism with steatosis severity in subjects with obesity and non-alcoholic fatty liver disease. Nutrients. (2020) 12(5):1260. doi: 10.3390/nu12051260
58. Boursier J, Mueller O, Barret M, MaChado M, Fizanne L, Araujo-Perez F, et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatol (Baltimore Md). (2016) 63(3):764–75. doi: 10.1002/hep.28356
59. Michail S, Lin M, Frey MR, Fanter R, Paliy O, Hilbush B, et al. Altered gut microbial energy and metabolism in children with non-alcoholic fatty liver disease. FEMS Microbiol Ecol (2015) 91(2):1–9. doi: 10.1093/femsec/fiu002
60. Im ST, Mun H, Park S, Kang H, Kim WC, Heo S-J, et al. Ishige okamurae Celluclast extract ameliorates non-alcoholic fatty liver in high-fructose diet-fed mice by modulation of lipid metabolism and gut microbiota composition. Food Chem Toxicol (2023) 177:113864. doi: 10.1016/j.fct.2023.113864
61. Luther J, Garber JJ, Khalili H, Dave M, Bale SS, Jindal R, et al. Hepatic injury in nonalcoholic steatohepatitis contributes to altered intestinal permeability. Cell Mol Gastroenterol Hepatol (2015) 1(2):222–32. doi: 10.1016/j.jcmgh.2015.01.001
62. Kang H, You HJ, Lee G, Lee SH, Yoo T, Choi M, et al. Interaction effect between NAFLD severity and high carbohydrate diet on gut microbiome alteration and hepatic de novo lipogenesis. Gut Microbes (2022) 14(1):2078612. doi: 10.1080/19490976.2022.2078612
63. Chen YM, Liu Y, Zhou RF, Chen XL, Wang C, Tan XY, et al. Associations of gut-flora-dependent metabolite trimethylamine-N-oxide, betaine and choline with non-alcoholic fatty liver disease in adults. Sci Rep (2016) 6:19076. doi: 10.1038/srep19076
64. Arab JP, Karpen SJ, Dawson PA, Arrese M, Trauner M. Bile acids and nonalcoholic fatty liver disease: Molecular insights and therapeutic perspectives. Hepatology (2017) 65(1):350–62. doi: 10.1002/hep.28709
65. Zhou X, Han D, Xu R, Li S, Wu H, Qu C, et al. A model of metabolic syndrome and related diseases with intestinal endotoxemia in rats fed a high fat and high sucrose diet. PloS One (2014) 9(12):e115148. doi: 10.1371/journal.pone.0115148
66. Sharifnia T, Antoun J, Verriere TGC, Suarez G, Wattacheril J, Wilson KT, et al. Hepatic TLR4 signaling in obese NAFLD. Am J Physiol Gastrointest Liver Physiol (2015) 309(4):G270–G8. doi: 10.1152/ajpgi.00304.2014
67. Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. (2007) 56(7):1761–72. doi: 10.2337/db06-1491
68. Spruss A, Kanuri G, Wagnerberger S, Haub S, Bischoff SC, Bergheim I. Toll-like receptor 4 is involved in the development of fructose-induced hepatic steatosis in mice. Hepatol (Baltimore Md). (2009) 50(4):1094–104. doi: 10.1002/hep.23122
69. Ma J, Zhou Q, Li H. Gut microbiota and nonalcoholic fatty liver disease: insights on mechanisms and therapy. Nutrients. (2017) 9(10):1124. doi: 10.3390/nu9101124
70. Ponziani FR, Bhoori S, Castelli C, Putignani L, Rivoltini L, Del Chierico F, et al. Hepatocellular carcinoma is associated with gut microbiota profile and inflammation in nonalcoholic fatty liver disease. Hepatology (2019) 69(1):107–20. doi: 10.1002/hep.30036
71. Viglino D, Jullian-Desayes I, Minoves M, Aron-Wisnewsky J, Leroy V, Zarski J-P, et al. Nonalcoholic fatty liver disease in chronic obstructive pulmonary disease. Eur Respir J (2017) 49(6):1601923. doi: 10.1183/13993003.01923-2016
72. Monteillet L, Gjorgjieva M, Silva M, Verzieux V, Imikirene L, Duchampt A, et al. Intracellular lipids are an independent cause of liver injury and chronic kidney disease in non alcoholic fatty liver disease-like context. Mol Metab (2018) 16:100–15. doi: 10.1016/j.molmet.2018.07.006
73. Chalasani N, Abdelmalek MF, Garcia-Tsao G, Vuppalanchi R, Alkhouri N, Rinella M, et al. Effects of belapectin, an inhibitor of galectin-3, in patients with nonalcoholic steatohepatitis with cirrhosis and portal hypertension. Gastroenterology. (2020) 158(5):1334–45.e5. doi: 10.1053/j.gastro.2019.11.296
74. Lee SM, Koh DH, Jun DW, Roh YJ, Kang HT, Oh JH, et al. Auranofin attenuates hepatic steatosis and fibrosis in nonalcoholic fatty liver disease via NRF2 and NF- κB signaling pathways. Clin Mol Hepatol (2022) 28(4):827–40. doi: 10.3350/cmh.2022.0068
75. Wang C, Duan X, Sun X, Liu Z, Sun P, Yang X, et al. Protective effects of glycyrrhizic acid from edible botanical glycyrrhiza glabra against non-alcoholic steatohepatitis in mice. Food Funct (2016) 7(9):3716–23. doi: 10.1039/C6FO00773B
76. Nakashima A, Sugimoto R, Suzuki K, Shirakata Y, Hashiguchi T, Yoshida C, et al. Anti-fibrotic activity of Euglena gracilis and paramylon in a mouse model of non-alcoholic steatohepatitis. Food Sci Nutr (2019) 7(1):139–47. doi: 10.1002/fsn3.828
77. Hagström H, Nasr P, Ekstedt M, Hammar U, Stål P, Hultcrantz R, et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol (2017) 67(6):1265–73. doi: 10.1016/j.jhep.2017.07.027
78. Akuta N, Kawamura Y, Fujiyama S, Sezaki H, Hosaka T, Kobayashi M, et al. SGLT2 inhibitor treatment outcome in nonalcoholic fatty liver disease complicated with diabetes mellitus: the long-term effects on clinical features and liver histopathology. Internal Med (2020) 59(16):1931–7. doi: 10.2169/internalmedicine.4398-19
79. Loomba R, Lawitz E, Mantry PS, Jayakumar S, Caldwell SH, Arnold H, et al. The ASK1 inhibitor selonsertib in patients with nonalcoholic steatohepatitis: A randomized, phase 2 trial. Hepatology (2018) 67(2):549–59. doi: 10.1002/hep.29514
80. Huber Y, Boyle M, Hallsworth K, Tiniakos D, Straub BK, Labenz C, et al. Health-related quality of life in nonalcoholic fatty liver disease associates with hepatic inflammation. Clin Gastroenterol Hepatol (2019) 17(10):2085–92.e1. doi: 10.1016/j.cgh.2018.12.016
81. Khurana S, Butt W, Khara HS, Johal AS, West SF, Chen Z-ME, et al. Bi-lobar liver biopsy via EUS enhances the assessment of disease severity in patients with non-alcoholic steatohepatitis. Hepatol Int (2019) 13(3):323–9. doi: 10.1007/s12072-019-09945-4
82. Wang Z-H, Zheng KI, Wang X-D, Qiao J, Li Y-Y, Zhang L, et al. LC-MS-based lipidomic analysis in distinguishing patients with nonalcoholic steatohepatitis from nonalcoholic fatty liver. Hepatobiliary Pancreatic Dis Int (2021) 20(5):452–9. doi: 10.1016/j.hbpd.2021.05.008
83. Zhang N-P, Liu X-J, Xie L, Shen X-Z, Wu J. Impaired mitophagy triggers NLRP3 inflammasome activation during the progression from nonalcoholic fatty liver to nonalcoholic steatohepatitis. Lab Invest (2019) 99(6):749–63. doi: 10.1038/s41374-018-0177-6
84. Wang Q, Ou Y, Hu G, Wen C, Yue S, Chen C, et al. Naringenin attenuates nonalcoholic fatty liver disease by down-regulating the NLRP3/NF-κB pathway in mice. Br J Pharmacol (2020) 177(8):1806–21. doi: 10.1111/bph.14938
Keywords: inflammation, fibrosis, MAFLD, bibliometric analysis, NAFLD
Citation: Luo K, Chen Y, Fang S, Wang S, Wu Z and Li H (2023) Study on inflammation and fibrogenesis in MAFLD from 2000 to 2022: a bibliometric analysis. Front. Endocrinol. 14:1231520. doi: 10.3389/fendo.2023.1231520
Received: 30 May 2023; Accepted: 08 August 2023;
Published: 31 August 2023.
Edited by:
Chaodong Wu, Texas A and M University, United StatesCopyright © 2023 Luo, Chen, Fang, Wang, Wu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huiqing Li, bGhxaW5nNUAxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.