- Department of Endocrinology, Shidong Hospital, Yangpu District, Shidong Hospital Affiliated to University of Shanghai for Science and Technology, Shanghai, China
Background: There has existed controversy regarding the use of Ginkgo biloba (GKB) for blood metabolism among type 2 diabetes mellitus(T2DM) patients, and we tried to analyze the effects and safety of GKB on T2DM patients.
Methods: We conducted a literature search between January 2003 and December 2022 of seven online databases (PubMed, Scopus, Embase, Google Scholar, Web of Sciences, Cochrane Library, and China National Knowledge Infrastructure). A systematic literature review and meta-analysis were performed to compare the effects and safety of GKB among T2DM patients. Four groups of parameters were extracted and analyzed: hemorheology parameters, lipid profile, glycemic control markers, and adverse events.
Results: In the end, 13 eligible articles with 11 indicators among 1573 patients were included. In the hemorheology parameters section, GKB showed significantly lower plasma viscosity (PV) (SMD=-0.91, 95%CI [-1.45, -0.36], P<0.01) and hematocrit (Hct) (SMD=-0.60, 95%CI [-0.97, -0.24], P<0.01) than the control group. GKB shoed higher velocity of the dorsalis pedis artery (VDPA) (SMD=0.51, 95%CI [0.26, 0.76], P<0.01) and ankle brachial index (ABI) (SMD=0.71, 95%CI [0.32, 1.10], P<0.01) than the control. In both the lipid profile and glycemic control markers sections, we did not find any difference between GKB and control groups, including total cholesterol (TC), triglyceride (TG), low-density lipoprotein (LDL), high-density lipoprotein (HDL), hemoglobin A1c (HbA1c), and fasting serum glucose (FSG). In addition, we saw no difference in adverse events (AE). The sensitivity analysis and funnel plot showed that the results in this research were robust and had no publication bias.
Conclusion: In conclusion, GKB might safely reduce the risk of peripheral arterial or even systemic cardiovascular disease. However, GKB did not directly improve lipid and blood glucose levels in T2DM patients.
Systematic review registration: https://inplasy.com/, identifier INPLASY202350096.
Introduction
Diabetes has become a top global health problem since its incidence has substantially expanded over the past few decades in both industrialized and developing nations (1, 2). In 2021, an estimated 537 million persons (aged 20 to 79) had diabetes, and by 2045, that number was projected to rise to 783 million according to research by the World Health Organization (WHO) and Global Burden of Disease (GBD) (3).
A serious concern for healthcare was diabetes mellitus (DM), which has several features including chronic hyperglycemia and aberrant protein, fat, and carbohydrate metabolism (2, 4–6). Diabetes is one of the five priority noncommunicable diseases (NCDs) in WHO’s NCDs control strategy (7, 8). Ongoing complex, expensive efforts are required to prevent and treat chronic problems in patients with diabetes, especially type 2 diabetes mellitus (T2DM). Peripheral arterial disease (PAD) was one of the most prevalent long-term consequences of T2DM, particularly in elderly patients (5).
Ginkgo biloba (GKB), a deciduous tree of the Ginkgo family, is sometimes referred to as a “living fossil” since it is the oldest gymnosperm that remains after the quaternary glaciation. The pharmacological effects of GKB included antioxidant, anticancer, hepatoprotective, and cardiovascular protection (9). GKB is used to treat metabolism disorders and is frequently classified as a “safe dietary supplement” that can be sold without restriction in pharmacies and health food shops all over the world (4). Several medications for T2DM have had an unsatisfactory effect because of their high costs, unclear safety, side effects, low drug tolerance, and drug resistance. In this situation, phytotherapy has gained a lot of popularity as a viable alternative to pharmacological therapy (4).
There is still controversy about the clinical effects of GKB on blood metabolism in patients with T2DM. Some studies have suggested that GKB was beneficial in improving T2DM-related complications (2, 7, 10), but others found that the efficacy of GKB on diabetic macrovascular complications was not yet significant (11). Therefore, in this study, we analyzed the effects of ginkgo on blood metabolism in diabetic patients based on a large sample with comprehensive parameters.
Methods
Literature search strategy
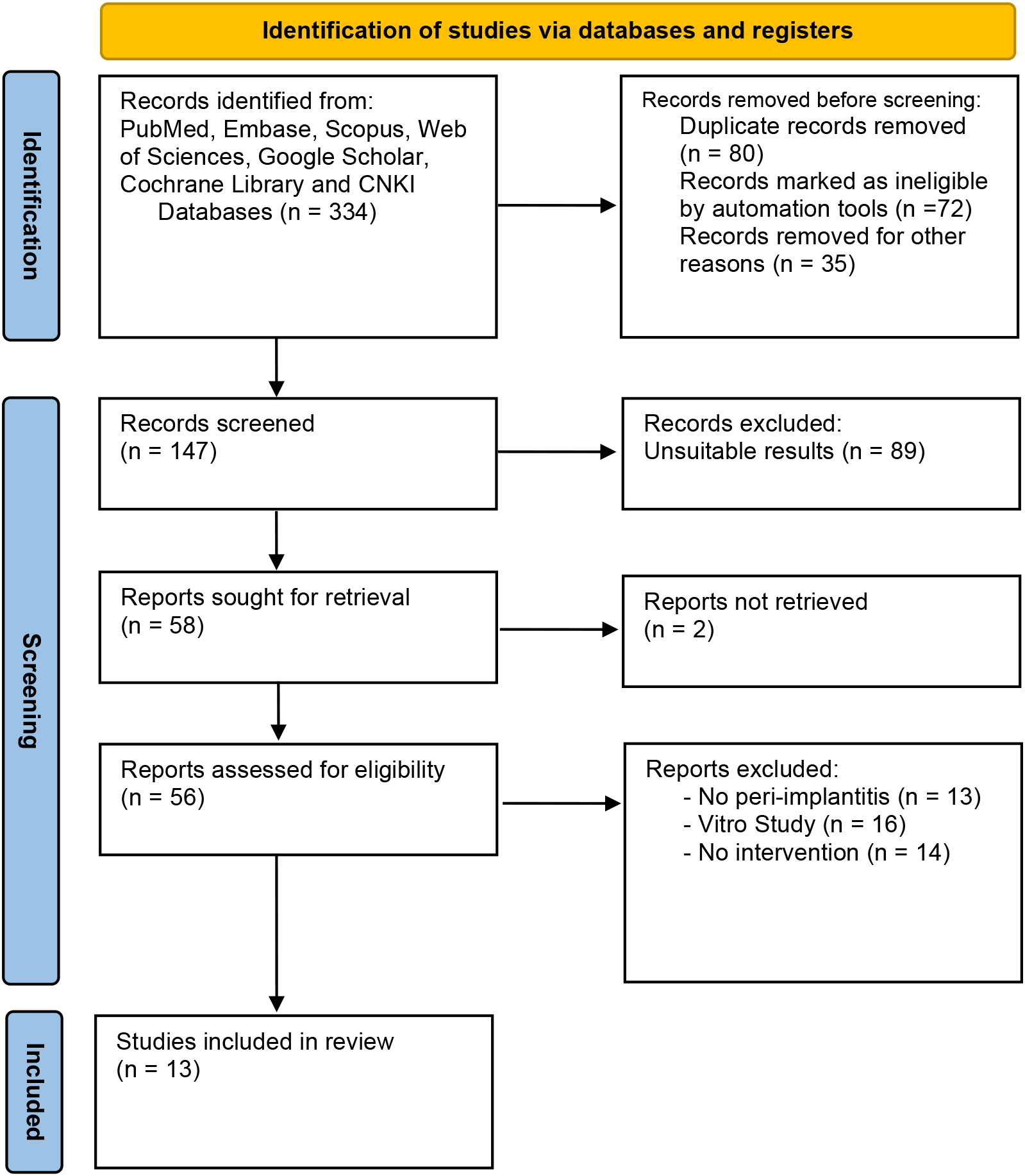
This research was conducted based on the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) flow diagram and checklist, which was registered at the International Platform of Registered Systematic Review and Meta-analysis Protocols (INPLASY202350096). Yu Han and Xue Hu searched seven online databases (PubMed, Embase, Scopus, Web of Sciences, Google Scholar, Cochrane Library, and China National Knowledge Infrastructure) for all randomized controlled trials reporting blood tests of GKB on T2DM patients. After screening literature published between January 2003 and December 2022, 13 articles were included without any language restrictions.
The medical subject terms and keywords used for the search were “Ginkgo biloba”, “G. biloba”, “GKB”, “Type 2 diabetes mellitus”, and “T2DM” in the Boolean expression: [(Ginkgo biloba) OR (G. biloba) OR (GKB)] AND [(Type 2 diabetes mellitus) OR (T2DM)]. Related articles from the references of included research were also searched. Studies were eligible if they contained the following information: random control trials or observational studies reporting blood metabolism: hematological parameters, lipid profile, glycemic control markers, and adverse events.
Jian Meng and Fang Huang screened the titles, abstracts, and full text of identified articles during the search and evaluated the risk of bias, extracting the data using a standardized data form. When there was any discrepancy, it was resolved by discussion with a third author (Hui Xu).
Inclusion and exclusion criteria
After the primary selection of the studies, the texts of the studies that were potentially relevant were reviewed, and the studies were required to meet the following inclusion criteria:
(1) Research should be on the comparison between GKB and control in T2DM patients;
(2) The design of the research should be randomized control trials (RCTs);
(3) Studies should contain blood metabolism indicators evaluating the efficacy and safety of GKB;
(4) The full text of the studies should be available.
Studies were excluded based on the following pre-determined exclusion criteria:
(1) The study contains research on other drugs;
(2) The study contains other topics about T2DM;
(3) The study lacks available data;
(4) The study type is review or abstract.
Data extraction and quality assessment
From each published study, two writers (Minhui Zou and Jingxian Fang) retrieved the following data: the name of the initial author, publication year, author’s nationality, groups based on intervention (GKB and placebo), research design, sex, average age, and research cycle. The outcome items were blood metabolism parameters.
The quality of the included studies was evaluated by two independent reviewers (Qing Gu and Suijun Wang) using the ROB 2.0 scale (a revised Cochrane risk-of-bias tool for randomized trials).
Statistical analysis
Using the Cochrane Q statistic and Higgins and Thompsons’ I², we evaluated heterogeneity. I² was used to categorize heterogeneity as low, moderate, or high depending on its value: 25%, 50%, or 75%. The fixed-effect model was utilized for the meta-analyses when I² was less than 50%. Otherwise, the random-effect model was adopted if I² was greater than 50%. More specifically, data for standard mean difference (SMD) with a 95% confidence interval (CI) were retrieved or recalculated for effect sizes of continuous outcomes. For categorical results, odds ratios (ORs) were employed. R version 4.2.1 (R Core Team, Vienna, Austria) and the R package meta (version 6.2.0) were used for statistical analyses. A P value lower than 0.05 was considered statistically significant for all reported P values.
Using Egger’s regression test and a funnel plot, publication bias was evaluated for the primary outcome of blood metabolism. We performed a sensitivity analysis to evaluate the robustness of the final results by removing one included article in turns.
Results
Search process
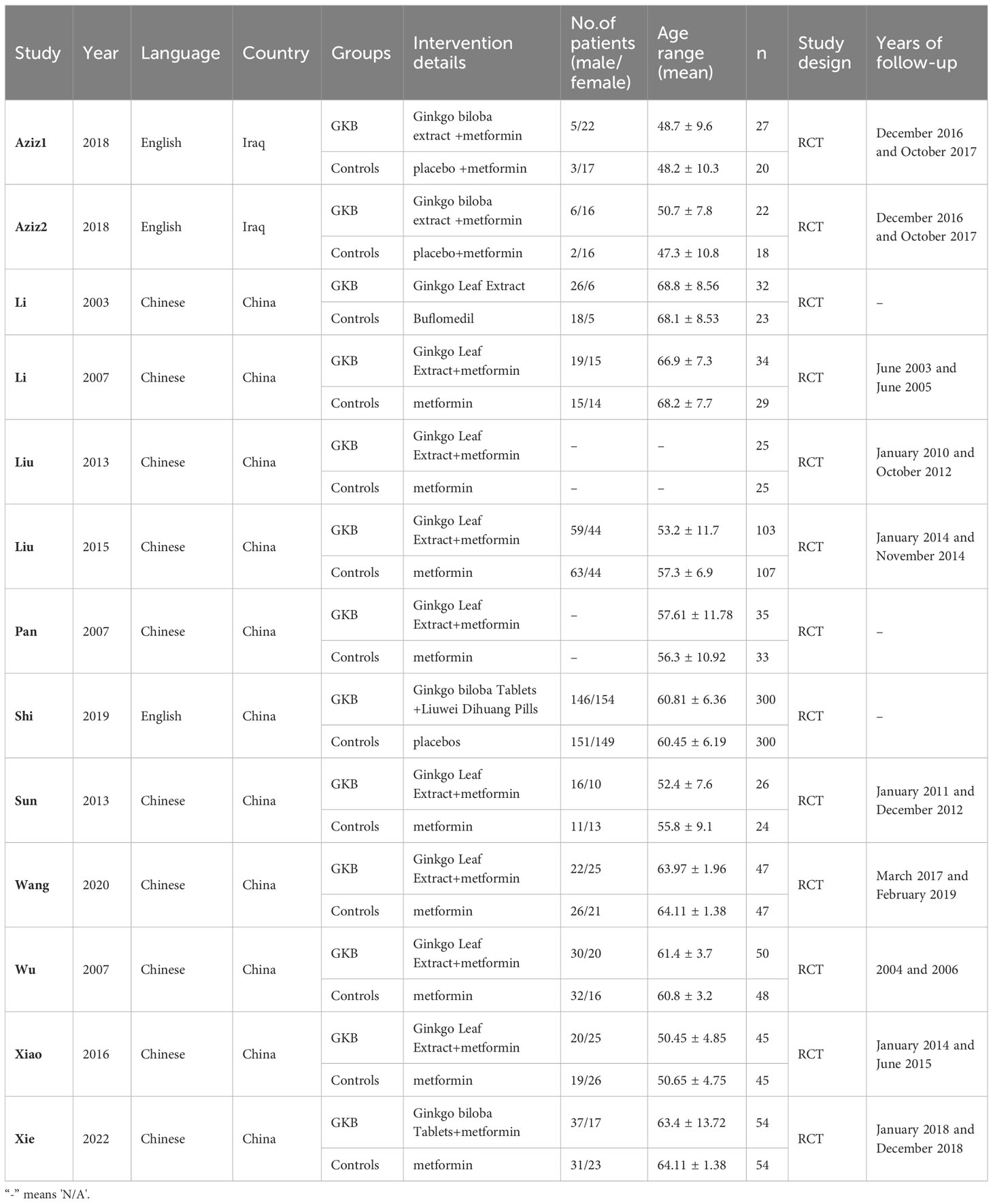
Based on the PRISMA flow diagram and inclusion/exclusion criteria, the systematic search of seven online databases identified 334 articles. After duplicate exclusion and screening for eligible research, 13 articles with 1573 patients were suitable for this meta-analysis (12–24). Figure 1 shows the flow chart of research selection, and the characteristics of the included studies are shown in Table 1. The risk of bias assessment for each included study is shown in Supplementary Files 1, 2.
The overall quality of the included studies based on ROB 2.0 was found to be adequate with regard to selection bias, comparability quality, and outcome quality.
Characteristics of included studies
Of all studies, 3 articles included >100 patients and 10 studies included <100 patients. The research period ranged from 1 to 3 years. The study design of all the included articles was RCTs. The mean age ranged from 47.3 to 68.8 among the included articles. The total number of males and females was close. The detailed characteristics of the included papers are shown in Table 1.
Results of quality assessment
The ROB 2.0 summary and details are shown in Supplementary Files 1, 2, respectively. In summary, less than 20% of articles showed a high risk of bias while over 50% showed a low risk of bias. The details showed that only two articles had an overall high risk of bias and seven had an overall low risk of bias.
Results of heterogeneity test
Firstly, we analyzed the hemorheology parameters. Concerning plasma viscosity (PV), pooled estimates of six studies showed that GKB had significantly lower PV than the control group (SMD=-0.91, 95%CI [-1.45, -0.36], P<0.01) (Figure 2A). Five articles reported hematocrit (Hct), and meta-analysis results showed that GKB also had lower Hct than control (SMD=-0.60, 95%CI [-0.97, -0.24], P<0.01) (Figure 2B). In terms of velocity of dorsalis pedis artery (VDPA) analysis, a meta-analysis of four articles indicated that GKB had higher VDPA than the control (SMD=0.51, 95%CI [0.26, 0.76], P<0.01) (Figure 2C). The pooled result of the ankle brachial index (ABI) in GKB was higher than the control (SMD=0.71, 95%CI [0.32, 1.10], P<0.01) (Figure 2D).
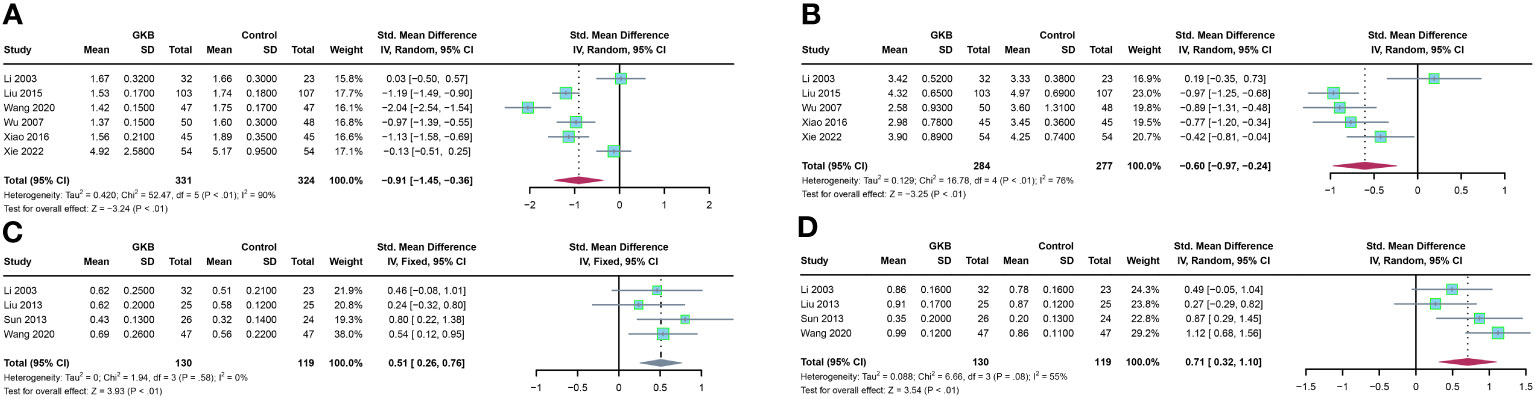
Figure 2 Effects of Ginkgo biloba on hematological parameters of T2DM patients maintained treated with GKB extract and placebo. (A) Plasma viscosity (mPa/s). (B) Hematocrit (%). (C) Velocity of dorsalis pedis artery (m/s). (D) Ankle brachial index. SD, standard deviation; 95% CI, 95% confidence interval; ChI², chi-squared test; Tau2, Tau-squared; I², I-squared; P, probability.
Furthermore, we analyzed the lipid profile after treatment of GKB. Upon total cholesterol (TC)(mmol/L) comparison, GKB showed no difference compared to the control (SMD=-0.37, 95%CI [-0.84, 0.09], P=0.11) (Figure 3A). Pooled analysis of triglyceride (TG) (mmol/L) showed no difference between GKB and control (SMD=-0.60, 95%CI [-1.25, 0.04], P=0.06) (Figure 3B). Both low-density lipoprotein (LDL) (mmol/L) (SMD=-0.34, 95%CI [-0.73, 0.04], P=0.08) (Figure 3C) and high-density lipoprotein (HDL) (mmol/L) (SMD=0.31, 95%CI [-0.60, 1.22], P=0.51) (Figure 3D) showed no difference compared to the GKB and control groups.
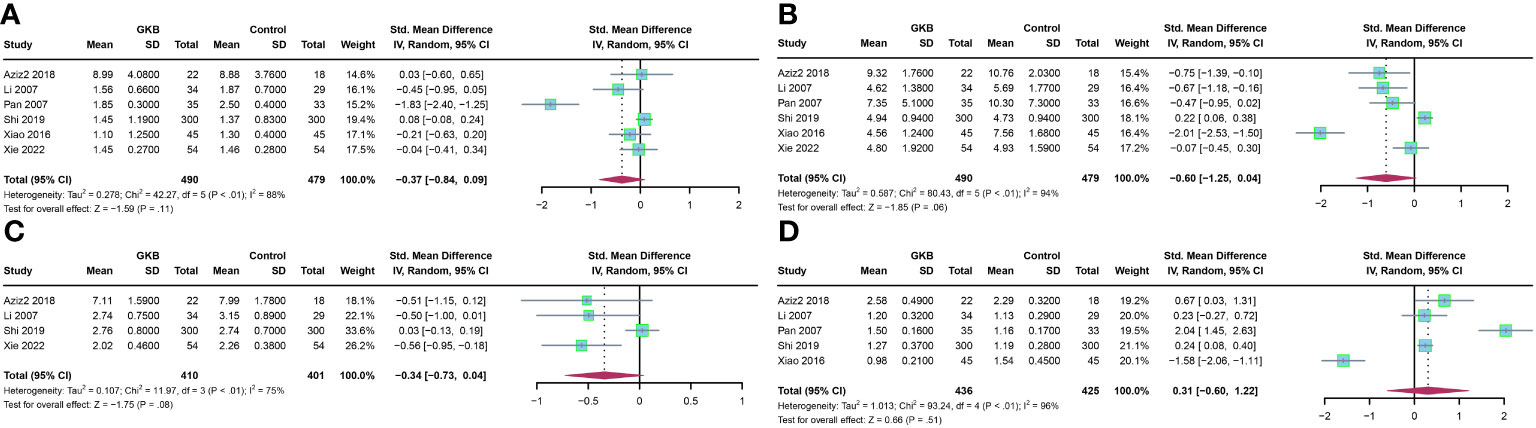
Figure 3 Effects of Ginkgo biloba on lipid profile of T2DM patients maintained treated with GKB extract and placebo. (A) Total cholesterol (mmol/L). (B) Triglyceride (mmol/L). (C) Low-density lipoprotein (mmol/L). (D) High-density lipoprotein(mmol/L).
Finally, we evaluated the glycemic control markers and adverse events in the GKB and control groups. Meta-analysis of both hemoglobin A1c (HbA1c)(%) (SMD=-0.15, 95%CI [-0.54, 0.24] P=0.44) (Figure 4A) and fasting serum glucose (FSG)(mmol/L) (SMD=0.07, 95%CI [-0.75, 0.88] P=0.87) (Figure 4B) showed no difference between the GKB and control. There was also no difference between the two groups in adverse events (AE) (OR=0.95, 95%CI [0.59, 1.53], P=0.84) (Figure 4C).
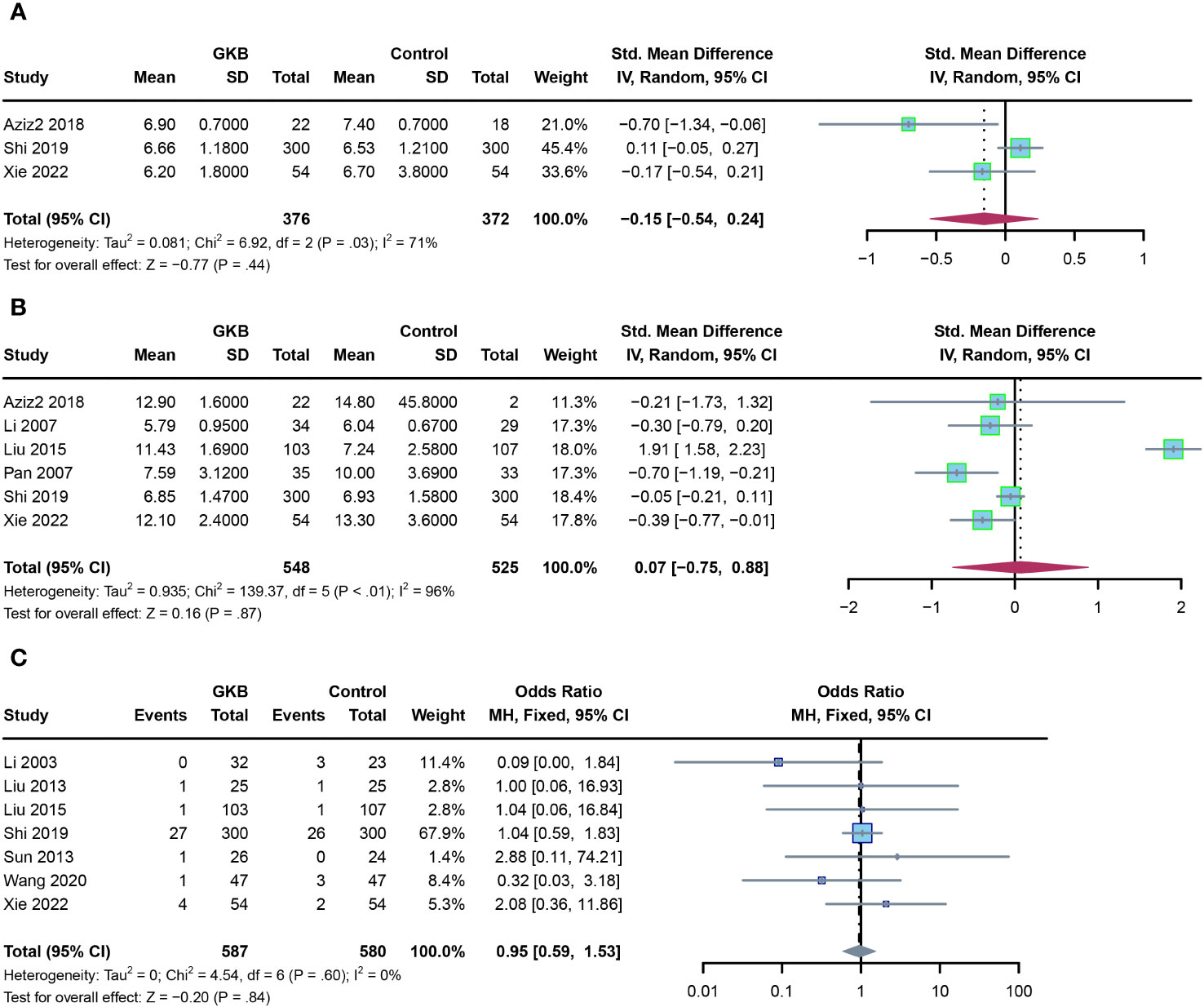
Figure 4 Effects of Ginkgo biloba on glycemic control markers of T2DM patients maintained treated with GKB extract and placebo, and adverse events. (A) Hemoglobin A1c (%). (B) Fasting serum glucose (mmol/L). (C) Adverse events.
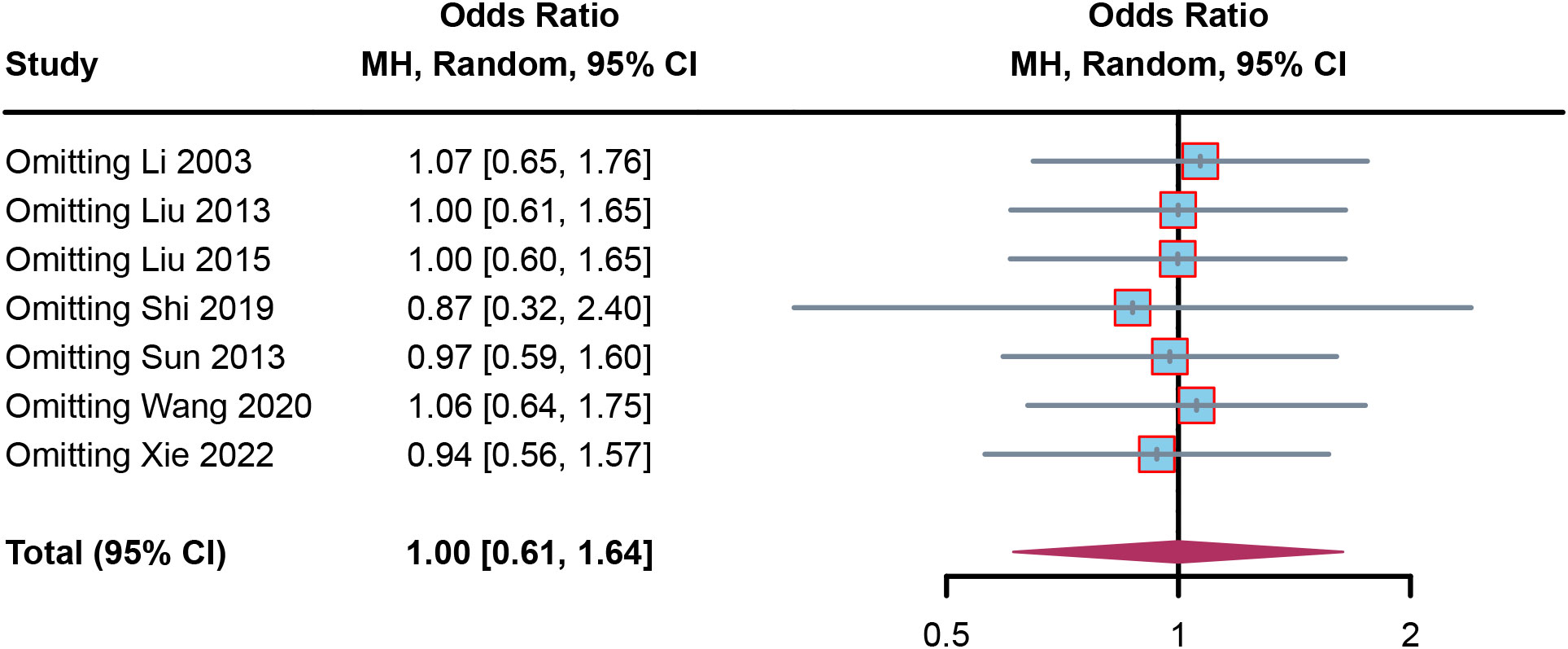
Results of sensitivity analysis and publication bias
To confirm the robustness of the results, sensitivity analysis was performed. In the sensitivity forest plot (Figure 5), we omitted each article in turn. In the adverse events meta-analysis, each included article had a similar OR for robustness; Li (2003) had the highest OR, which was a value of 1.07 [0.65, 1.76], while Shi (2019) had the lowest OR, which was a value of 0.87 [0.32, 2.40]. These results indicated the results were robust.
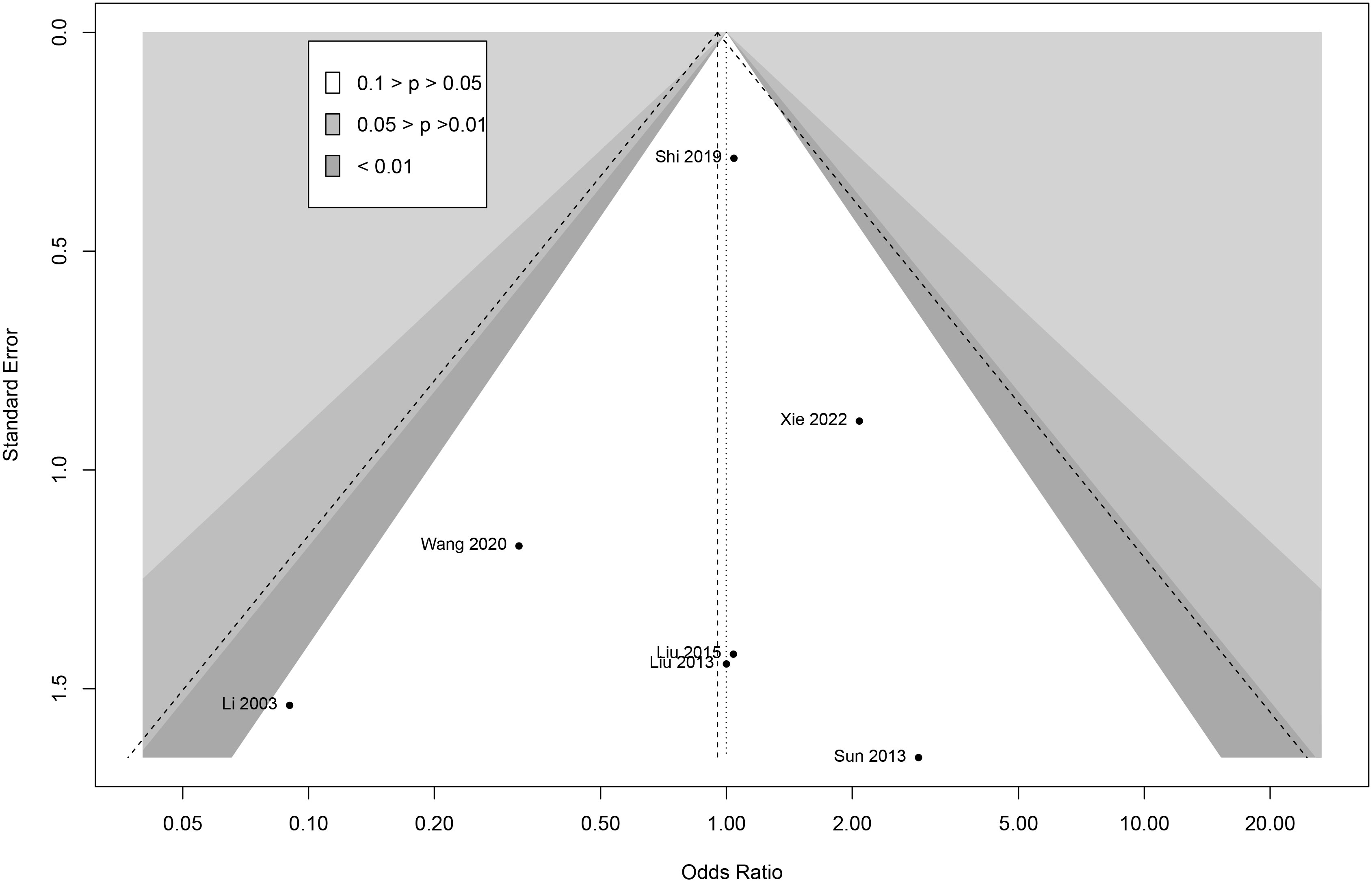
To analyze the publication bias, Begg funnel plots were performed for adverse events meta-analysis. The funnel plots showed visual symmetry and indicated that limited publication bias existed in this research (Figure 6). Egger regression was used to test for adverse events (z = 0.24; P = 0.21), which further demonstrated the absence of publication bias.
Discussion
To our knowledge, this research is the first comprehensive report of GKB on blood metabolism in T2DM with multiple parameters. GKB significantly reduced PV and Hct but increased VDPA and ABI in T2DM patients. In addition, GKB had no effects on TC, TG, LDL, HDL, HbA1c, and FSG. Furthermore, GKB was safe for T2DM patients.
All four significant indicators were associated with PAD in T2DM, and the viscosity of blood was an indicator for understanding and treating the disease (25). In a cross-sectional study in Edinburgh, Lowe et al. found that blood viscosity and its major determinants (Hct, PV, and fibrinogen) as well as leukocyte activation were significantly associated with an increase in the severity of PAD (26). Tzoulaki et al., also in the Edinburgh Arterial Prospective Study, found that the effect of blood viscosity on PAD was modest. Elevated levels of Hct maintained a significant correlation with PAD and showed a dose–response relationship with disease severity (27). Indeed, GKB could lower PV and Hct, which further decreased the risk of PAD. The mechanism by which higher Hct affects coronary blood flow velocity may be that as the hematocrit rises, blood viscosity increases, enhancing the shear force on the vessel wall. This results in damage to the vascular endothelium, which stimulates the release of multiple vasoactive factors from endothelial cells, resulting in an imbalance of vasodilator/constrictor factors (e.g., an imbalance in the secretion of nitric oxide/endothelin), which contributes to the development of PAD (28).
There is a higher prevalence of lesions in the dorsalis pedis artery than in popliteal or anterior/posterior tibial arteries; the dorsalis pedis artery was the first artery to develop diabetic macrovascular disease and was crucial for early screening (29). It could be viewed as a representation of the systemic vasculature in T2DM. The ABI consists of serial measurements of Doppler-recorded systolic blood pressure in the brachial, dorsalis pedis, and posterior tibial arteries. There is concordance between the VDPA and the ABI (30). The sensitivity and specificity of the ABI for the identification of PAD are greater than 90%. There is a well-known relationship between PAD and the number of diseased coronary vessels. The ABI may be a surrogate marker for systemic atherosclerosis. Low ABI is associated with a high prevalence of cardiovascular risk factors (31).
In conclusion, all four significant indices could assess PAD in T2DM, and the reduction of PV and Hct with an increase of VDPA and ABI might reduce the risk of PAD or even systemic vascular disease. However, there was no discernible difference between the GKB group and the control group in the study’s assessment of lipid and glucose indices, suggesting that GKB might not directly lower lipids and glucose in T2DM patients.
Previous articles reported that the pathogenesis of diabetes mellitus was similar to that of “thirst” in Chinese medicine (mainly showed ‘Yin’ deficiency and blood stasis), and Ginkgo biloba could nourish ‘Yin’ and activate blood (10, 11). It was suggested that GKB could improve insulin resistance and inflammation, reduce the serine phosphorylation of IRS-1 receptor, and decrease NFκB/JNK activation, thereby reducing the release of inflammatory adipokines (32). Besides, diabetes was associated with multiple inflammatory responses, including accumulation of AGEs, leukocyte infiltration, ECM deposition, and cytokine and adhesion molecule expression (32). sRAGE was described as the “sponge” of AGE, which counteracted the deleterious effects of cellular RAGE by binding to serum AGE and AGE-P. Studies showed that lower sRAGE levels were associated with a higher risk of T2DM, coronary heart disease, and all-cause mortality (33). Ginkgo was reported to reduce the relative total superoxide dismutase activity in patients with T2DM, leading to higher sRAGE levels and reducing the risk of T2DM (34).
In our results, GKB had no effects on HbA1c. Among the included articles, Aziz (13) and Xie (24) said GKB could decrease HbA1c with a limited sample size. However, Shi (19) reported that GKB did not influence HbA1c with a large sample size. In addition, the research period of Shi was 24 months, whereas Aziz’s was 3 months and Xie’s was 12 months. This situation seems controversial and has led to a hypothesis: if Aziz and Xie enlarge their sample size and study period, will the effects of GKB in T2DM be stable? This topic needs further analysis in the form of future large-scale, multicenter, randomized, double-blind, placebo-controlled trials.
GKB was thought to have the following properties: being cardioprotective and anti-apoptotic, having an anti-platelet activating factor, being an antioxidant, exhibiting free radical scavenging, being neuroprotective, and showing membrane stabilization. By decreasing the transcription of pro-apoptotic caspase-9 and JNK and increasing the activity of anti-apoptotic Bcl-2 protein, Ginkgo biloba extract EGb761 attenuated pro-inflammatory and pro-apoptotic processes, thus preventing the initiation of apoptotic signaling cascade and thus protecting the integrity of the mitochondrial membrane (35). Research has demonstrated that the administration of Ginkgo biloba extract can impede adipogenesis and modulate lipid metabolism, resulting in a decrease in body weight and food consumption (8).
Diabetic peripheral vasculopathy had a prevalence of about 20% in diabetic patients and was a common vascular complication of T2DM, with the lower limb arteries being the main site of onset (36, 37). Therefore, for patients with T2DM, in addition to glucose and lipid control, vascular protection was also important. About the vascular protection mechanism, in a study by Tsai et al, they found that GKB increased Krüppel-Like Factor 2 expression, which then increased endothelial NO synthase (eNOS) expression and NO production (38). GKB increased eNOS in a dose-dependent manner at both the RNA and protein levels. NO is an important mediator of endothelial cell function, inhibiting leukocyte adhesion and migration and preventing platelet aggregation. NO exerts its vascular protective effects by using diastolic smooth muscle cells to reduce vascular tone (39, 40).
Concerning the heterogeneity in part of our results, we have found several possible reasons. Firstly, the different publishing years of the included articles contributed to the heterogeneity. Secondly, the different dosages of GKB might influenced the heterogeneity. Thirdly, the different research measurements and staff might lead to heterogeneity. Fourthly, even though most of the included research was conducted in China, the different sampling cities might be related to heterogeneity since China had a large country scope with different lifestyle and eating habits. Finally, the age and sex distribution in different research varied a lot; the lowest average age was 47.3 ± 10.8 while the highest was 68.8 ± 8.56, while the lowest sex ratio (male/female) was 0.13 but the highest was 4.33. In this situation, we still want to conduct a comprehensive meta-analysis for GKB among T2DM and hope it can lay the foundation for better research in the future.
Overall, the results of this study, based on a large sample and multiple indicators, confirmed that GKB might safely reduce the risk of PAD or even systemic cardiovascular disease in T2DM patients but did not directly improve lipid and blood glucose levels in T2DM patients.
The present study had some limitations. First, because studies of GKB affecting patients with T2DM were not retrieved in the United States or Europe, comprehensive results for multiple populations were lacking. Second, due to the variability of indicators reported in the included studies, there was no way to have a larger sample for each indicator. Finally, the related mechanism of included indicators was not complete, which shows the need for future mechanical research.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
Conception and design: QG and SW; Administrative support: LZ, XD, YY, and YG; Provision of study materials or patients: HX, CL, and YW; Collection and assembly of data: XH, JM, and FH; Data analysis and interpretation: HZ, JF, and YH; Manuscript writing: All authors. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1231053/full#supplementary-material
Supplementary File 1 | Risk of bias details assessed by ROB 2.0.
Supplementary File 2 | Risk of bias summary assessed by ROB 2.0.
Glossary
References
1. Magliano DJ, Islam RM, Barr ELM, Gregg EW, Pavkov ME, Harding JL, et al. Trends in incidence of total or type 2 diabetes: systematic review. BMJ (2019) 366:l5003. doi: 10.1136/bmj.l5003
2. Koren R, Lerner A, Tirosh A, Zaidenstein R, Ziv-Baran T, Golik A, et al. The use of complementary and alternative medicine in hospitalized patients with type 2 diabetes mellitus in Israel. J Altern Complementary Med (2015) 21(7):395–400. doi: 10.1089/acm.2015.0019
3. GBD 2019 Diabetes Mortality Collaborators. Diabetes mortality and trends before 25 years of age: an analysis of the Global Burden of Disease Study 2019. Lancet Diabetes Endocrinol (2022) 10(3):177–92. doi: 10.1016/S2213-8587(21)00349-1
4. Achete de Souza G, de Marqui SV, Matias JN, Guiguer EL, Barbalho SM. Effects of ginkgo biloba on diseases related to oxidative stress. Planta Med (2020) 86(6):376–86. doi: 10.1055/a-1109-3405
5. Derosa G, D'Angelo A, Preti PS, Maffioli P. Evaluation of the Effect on Sexual Performance of a Nutraceutical Combination Containing Alpha Lipoic Acid, Vitis vinifera L. and Ginkgo biloba, Compared to Placebo, Avanafil or a Combination of Nutraceutical Plus Avanafil in Males With Type 2 Diabetes Mellitus With Erectile Dysfunction. Front Endocrinol (Lausanne) (2022) 13:847240. doi: 10.3389/fendo.2022.847240
6. Han J, Pang X, Shi X, Zhang Y, Peng Z, Xing Y. Ginkgo biloba extract EGB761 ameliorates the extracellular matrix accumulation and mesenchymal transformation of renal tubules in diabetic kidney disease by inhibiting endoplasmic reticulum stress. BioMed Res Int (2021) 2021:1–11. doi: 10.1155/2021/6657206
7. Wang H, Yuan M, Zou X. Efficacy and safety of Ginkgo biloba for patients with early diabetic nephropathy. Medicine (2020) 99(35):e21959. doi: 10.1097/MD.0000000000021959
8. Xu S, Tang L, Qian X, Wang Y, Gong J, Yang H, et al. Molecular mechanism of Ginkgo biloba in treating type 2 diabetes mellitus combined with non-alcoholic fatty liver disease based on network pharmacology, molecular docking, and experimental evaluations. J Food Biochem (2022) 46(12):e14419. doi: 10.1111/jfbc.14419
9. Forman V, Luo D, Geu-Flores F, Lemcke R, Nelson DR, Kampranis SC, et al. A gene cluster in Ginkgo biloba encodes unique multifunctional cytochrome P450s that initiate ginkgolide biosynthesis. Nat Commun (2022) 13(1):5143. doi: 10.1038/s41467-022-32879-9
10. Palta P, Carlson MC, Crum RM, Colantuoni E, Sharrett AR, Yasar S, et al. Diabetes and cognitive decline in older adults: the ginkgo evaluation of memory study. Journals Gerontology: Ser A (2018) 73(1):123–30. doi: 10.1093/gerona/glx076
11. Zhao Y, Yu J, Liu J, An X. The role of liuwei dihuang pills and ginkgo leaf tablets in treating diabetic complications. Evidence-Based Complementary Altern Med (2016) 2016:1–8. doi: 10.1155/2016/1213090
12. Aziz TA, Hussain SA, Mahwi TO, Ahmed ZA. Efficacy and safety of Ginkgo biloba extract as an "add-on" treatment to metformin for patients with metabolic syndrome: a pilot clinical study. Ther Clin Risk Manag (2018) 14:1219–26. doi: 10.2147/TCRM.S169503
13. Aziz TA, Hussain SA, Mahwi TO, Ahmed ZA, Rahman HS, Rasedee A. The efficacy and safety of Ginkgo biloba extract as an adjuvant in type 2 diabetes mellitus patients ineffectively managed with metformin: a double-blind, randomized, placebo-controlled trial. Drug Des Devel Ther (2018) 12:735–42. doi: 10.2147/DDDT.S157113
14. Li X, Fu X, Lang X. Effects of Ginkgo biloba extract on the levels of intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in patients with early diabetes nephropathy. Chin J Integrated Traditional Western Med (2007) 05):412–4.
15. Liu C. Observation on the therapeutic effect of ginkgo biloba and dipyridamole injection on vascular disease of both lower limbs in diabetes. Chin Med Guide (2013) 11(30):181–2.
16. Liu R. Effects of Ginkgo biloba extract on hemorheology and microvascular circulation in patients with diabetes. Pharmacol clinic traditional Chin Med (2015) 31(04):239–41.
17. Minglong L, Shue Z, Guangye L. Clinical study of buflomedil and ginkgo dipyridamole in the treatment of lower limb vascular disease in elderly patients with diabetes. Chin J Gerontology (2003) 2003(11):744–5.
18. Pan Z. Effect of Ginkgo biloba extract on the improvement of microvascular disease in type 2 diabetes. Baotou Med (2007) 04):195–6.
19. Shi R, Wang Y, An X, Ma J, Wu T, Yu X, et al. Efficacy of co-administration of liuwei dihuang pills and ginkgo biloba tablets on albuminuria in type 2 diabetes: A 24-month, multicenter, double-blind, placebo-controlled, randomized clinical trial. Front Endocrinol (2019) 10:100. doi: 10.3389/fendo.2019.00100
20. Sun T, Lei W, Gao L. Clinical observation of ginkgo biloba dipyridamole combined with low-molecular-weight heparin calcium in the treatment of diabetes peripheral vascular disease. Seek Med advice (2013) 11(09):308–9.
21. Wang J, Yu Y, Zhang H. Effects of Ginkgo Biloba Extract Injected at Zusanli Point on Arterial Blood Flow, Hemorheology and Angiogenesis of Lower Extremity in Patients with diabetes Peripheral Vascular Disease. China Med Herald (2020) 17(18):81–6.
22. Wu T, You Y. Clinical study on intervention of ginkgo biloba and dipyridamole injection in the treatment of senile diabetes with hyperviscosity and peripheral vascular diseases. Chin Pract Med (2007) 2(29):4–6.
23. Xiao C, Zhan D, Meiliang X. Effects of Ginkgo biloba extract on VEGF and D-dimer in type 2 diabetes patients with microangiopathy. J Changchun Univ Traditional Chin Med (2016) 32(06):1203–5.
24. Xie Z, Hou D, Li J. Effects of ginkgo ketoester dropping pills combined with rosuvastatin on vascular endothelial function and hemorheology in patients with stable coronary heart disease and diabetes. World J Integrated Traditional Chin Western Med (2022) 17(03):551–555+560.
25. Chen T, Tu M, Huang L, Zheng Y. Association of serum adiponectin with intima media thickness of dorsalis pedis artery and macroangiopathy in type 2 diabetes. J Diabetes Res (2020) 2020:1–10. doi: 10.1155/2020/8358102
26. Lowe GD, Fowkes FG, Dawes J, Donnan PT, Lennie SE, Housley E. Blood viscosity, fibrinogen, and activation of coagulation and leukocytes in peripheral arterial disease and the normal population in the Edinburgh Artery Study. Circulation. (1993) 87(6):1915–20. doi: 10.1161/01.CIR.87.6.1915
27. Tzoulaki I, Murray GD, Lee AJ, Rumley A, Lowe GD, Fowkes FG. Inflammatory, haemostatic, and rheological markers for incident peripheral arterial disease: Edinburgh Artery Study. Eur Heart J (2007) 28(3):354–62. doi: 10.1093/eurheartj/ehl441
28. Liu W, Östberg N, Yalcinkaya M, Dou H, Endo-Umeda K, Tang Y, et al. Erythroid lineage Jak2V617F expression promotes atherosclerosis through erythrophagocytosis and macrophage ferroptosis. J Clin Invest. (2022) 132(13):e155724. doi: 10.1172/JCI155724
29. Sun H, Liu L, Jing Y, Wang J, Zhou Y, Chen K, et al. Post-exercise ankle–brachial index decline and risk of all-cause mortality: A meta-analysis. Eur J Prev Cardiol (2020) 27(11):1225–7. doi: 10.1177/2047487319849507
30. Lee JY, Lee SW, Lee WS, Han S, Park YK, Kwon CH, et al. Prevalence and clinical implications of newly revealed, asymptomatic abnormal ankle-brachial index in patients with significant coronary artery disease. JACC Cardiovasc Interv. (2013) 6(12):1303–13. doi: 10.1016/j.jcin.2013.08.008
31. Polonsky TS, McDermott MM. Lower extremity peripheral artery disease without chronic limb-threatening ischemia: A review. JAMA. (2021) 325(21):2188–98. doi: 10.1001/jama.2021.2126
32. Jeong HR, Shim YS, Lee HS, Hwang JS. Hemoglobin and hematocrit levels are positively associated with blood pressure in children and adolescents 10 to 18 years old. Sci Rep (2021) 11(1):19052. doi: 10.1038/s41598-021-98472-0
33. Smith EE, Abdullah AR, Petkovska I, Rosenthal E, Koroshetz WJ, Schwamm LH. Poor outcomes in patients who do not receive intravenous tissue plasminogen activator because of mild or improving ischemic stroke. Stroke (2005) 36(11):2497–9. doi: 10.1161/01.STR.0000185798.78817.f3
34. Sun J, Han K, Xu M, Li L, Qian J, Li L, et al. Blood viscosity in subjects with type 2 diabetes mellitus: roles of hyperglycemia and elevated plasma fibrinogen. Front Physiol (2022) 13:827428. doi: 10.3389/fphys.2022.827428
35. Tao Y, Zhu F, Pan M, Liu Q, Wang P. Pharmacokinetic, metabolism, and metabolomic strategies provide deep insight into the underlying mechanism of ginkgo biloba flavonoids in the treatment of cardiovascular disease. Front Nutr (2022) 9:857370. doi: 10.3389/fnut.2022.857370
36. Shareena G, Kumar D. Traversing through half a century research timeline on Ginkgo biloba, in transforming a botanical rarity into an active functional food ingredient. Biomedicine Pharmacotherapy (2022) 153:113299. doi: 10.1016/j.biopha.2022.113299
37. Tian Y, Han X, Qu Y, Zhang Y, Rong H, Wu K, et al. Genome-wide identification of the ginkgo (Ginkgo biloba L.) LBD transcription factor gene and characterization of its expression. Int J Mol Sci (2022) 23(10):5474. doi: 10.3390/ijms23105474
38. Tsai TN, Lin WS, Wu CH, Lin WY, Chu KM, Cheng CC, et al. Activation of krüppel-like factor 2 with ginkgo biloba extract induces eNOS expression and increases NO production in cultured human umbilical endothelial cells. Acta Cardiol Sin (2014) 30(3):215–22.
39. Tsai HY, Huang PH, Lin FY, Chen JS, Lin SJ, Chen JW. Ginkgo biloba extract reduces high-glucose-induced endothelial reactive oxygen species generation and cell adhesion molecule expression by enhancing HO-1 expression via Akt/eNOS and p38 MAP kinase pathways. Eur J Pharm Sci (2013) 48(4-5):803–11. doi: 10.1016/j.ejps.2013.01.002
Keywords: Ginkgo biloba, GKB, type 2 diabetes mellitus, T2DM, meta-analysis
Citation: Zou H, Fang J, Han Y, Hu X, Meng J, Huang F, Xu H, Lu C, Wang Y, Zhang L, Dong X, Yu Y, Guo Y, Gu Q and Wang S (2024) Effects and safety of Ginkgo biloba on blood metabolism in type 2 diabetes mellitus: a systematic review and meta-analysis. Front. Endocrinol. 14:1231053. doi: 10.3389/fendo.2023.1231053
Received: 01 June 2023; Accepted: 27 November 2023;
Published: 08 January 2024.
Edited by:
Jared Rutter, The University of Utah, United StatesReviewed by:
Sudhanshu Kumar Bharti, Patna University, IndiaCarmen Rubio, Manuel Velasco Suárez National Institute of Neurology and Neurosurgery, Mexico
Copyright © 2024 Zou, Fang, Han, Hu, Meng, Huang, Xu, Lu, Wang, Zhang, Dong, Yu, Guo, Gu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Suijun Wang, d3NqODAxQDE2My5jb20=; Qing Gu, Z3E5ODAxMjQ4QHNpbmEuY29t
†These authors share first authorship
 Huimin Zou†
Huimin Zou† Suijun Wang
Suijun Wang