- 1Iranian Research Center for HIV/AIDS, Iranian Institute for Reduction of High-Risk Behaviors, Tehran University of Medical Sciences, Tehran, Iran
- 2Trauma Research Center, Kashan University of Medical Sciences, Kashan, Iran
- 3Department of Radiology, Tabriz University of Medical Sciences, Tabriz, Iran
- 4School of Medicine, Islamic Azad University of Medical Sciences, Tehran, Iran
- 5School of Medicine, Bushehr University of Medical Sciences, Bushehr, Iran
- 6Department of Midwifery, School of Nursing and Midwifery, Kermanshah University of Medical Sciences, Kermanshah, Iran
- 7School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 8School of Medicine, Tehran University of Medical Sciences, Tehran, Iran
- 9Students’ Scientific Research Center (SSRC), Tehran University of Medical Sciences, Tehran, Iran
- 10Department of Health Information Technology, Khalkhal University of Medical Sciences, Khalkhal, Iran
- 11Bergen Addiction Research, Department of Addiction Medicine, Haukland University Hospital, Bergen, Norway
Introduction: Bone density regulation is considered one of the systems affected by thyroid hormones, leading to low bone density that can result in pathologic fractures, including hip fractures. This review aimed to update clinicians and researchers about the current data regarding the relationship between hip fractures and thyroid disorders.
Methods: English papers were thoroughly searched in four main online databases of Scopus, Web of Science, PubMed, and Embase. Data extraction was done following two steps of screening/selection using distinct inclusion/exclusion criteria. This study used the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) checklist and the Newcastle-Ottawa Scale (NOS) as bias assessment.
Results: In total, 19 articles were included in the research. The risk of hip fractures in women with differentiated thyroid cancer (DTC) is higher than hip fractures caused by osteoporosis. Men with hyperthyroidism and subclinical hyperthyroidism are at higher risk for hip fracture. Also, a decrease in serum thyroid stimulating hormone (TSH) may be associated with an increased risk of hip fracture.
Conclusion: Reaching a consensus conclusion regarding the association between subclinical thyroid dysfunction and hip fracture is not feasible due to the heterogenicity of evidence; however, there may be a higher risk of fracture in individuals with subclinical hyperthyroidism.
Introduction
Regulating metabolism and cell adjustment are just examples of what thyroid hormones do in the human body. Changes in these hormone levels occur in hypothyroidism, hyperthyroidism, subclinical hypothyroidism, and subclinical hyperthyroidism (1). Hypothyroidism is a common endocrine disorder caused by autoimmune thyroiditis (Hashimoto thyroiditis), iodine deficiency, or following surgery or radioiodine therapy (2). Hyperthyroidism is defined by elevated circulating free thyroid hormones, and overt hyperthyroidism is recognized as a low bone density or osteoporosis risk factor in older women. However, the relationship between biochemically defined subclinical hypothyroidism or hyperthyroidism and fracture risk is unknown. Still, in patients with subclinical hyperthyroidism, studies have shown that minor changes in thyroid hormone and/or thyroid stimulating hormone (TSH) levels can worsen bone mineral density (BMD) (3).
The bone remodeling cycle is what we call a continuous process of bone formation and bone resorption throughout the lifetime, and apart from local factors from osteoblasts and osteoclasts, the bone remodeling process is regulated by systemic factors such as calcitonin, parathyroid hormone (PTH), vitamin D3, estrogen, thyroid hormones, glucocorticoids, and growth hormones (4). T3 hormone increases bone formation through TRα receptors on osteoblasts and osteoclasts, but it can also increase osteoclast formation and the resorption process (5). Additionally, TSH action on the TSHR found in both osteoblasts and osteoclasts can also affect the bone remodeling cycle like T3 (6).
Changes in these hormone levels greatly affect bone metabolism and density and can lead to a decreased bone mineral density (BMD) that presents as osteoporosis. About 30–40% of osteoporosis patients are at great risk of osteoporotic bone fractures with a high mortality risk. The most frequent osteoporotic fractures are vertebral, distal radius, and hip fractures. Vertebral and hip fractures are considered life-threatening pathologies in the elderly (3). Hip fractures are a significant and incapacitating condition that disproportionately affects older women (7–15). While the epidemiology of hip fractures varies across countries, it is estimated that approximately 18% of women and 6% of men globally will be affected by this condition. Although the age-standardized incidence rate has decreased in many nations, the aging population generates a much greater impact (7–15). Therefore, the number of hip fractures globally is expected to swell from 1.26 million in 1990 to 4.5 million by the year 2050. The financial burden associated with this ailment is colossal since it requires long hospital stays and subsequent rehabilitation. Additionally, hip fracture is correlated with other adverse effects such as disability, depression, and cardiovascular diseases, which further exacerbates societal costs (7–15).
This review aimed to update clinicians and researchers about the current evidence regarding the relationship between hip fractures and thyroid disorders.
Methods
According to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA), this systematic review was carried out (16). The Newcastle-Ottawa Scale (NOS) quality assessment tool was used to evaluate methodological quality.
Data sources
Systematic searches were conducted in Embase, PubMed, Scopus, and Web of Science databases without time limitation. Manual checks were made for any additional studies bibliography of relevant studies.
The following keywords were used in combination:
A: “Hip fracture” OR “Trochanteric fracture” OR “Intertrochanteric fracture” OR “Sub trochanteric fracture” OR “Femoral fracture” [Title/Abstract]
B: “Thyroid disease” OR “Thyroid disorder” OR “Thyroid dysfunction” [Title/Abstract]
C: [A] AND [B]
Study selection
In two stages of screening and selection, publications of interest were included. First, titles and abstracts were evaluated, and relevant publications were chosen for the second stage. This step involved reading through the complete text of these papers. Studies were selected for analysis using the following inclusion and exclusion criteria:
1. Studies that addressed hip fractures and thyroid disorders.
2. Original articles.
3. English studies.
Exclusion criteria:
1. A systematic review, meta-analysis, qualitative studies, case report, and letter to the editor.
2. Articles that do not have full text, or in a language other than English.
Data extraction
For data extraction, the records were divided among four impartial assessors to retrieve the following details: study type, nation, first author, publication year, target population, comparison, and data on bone metabolism, including biochemical parameters, parameters of bone damage, and fracture data.
Quality assessment and risk evaluation
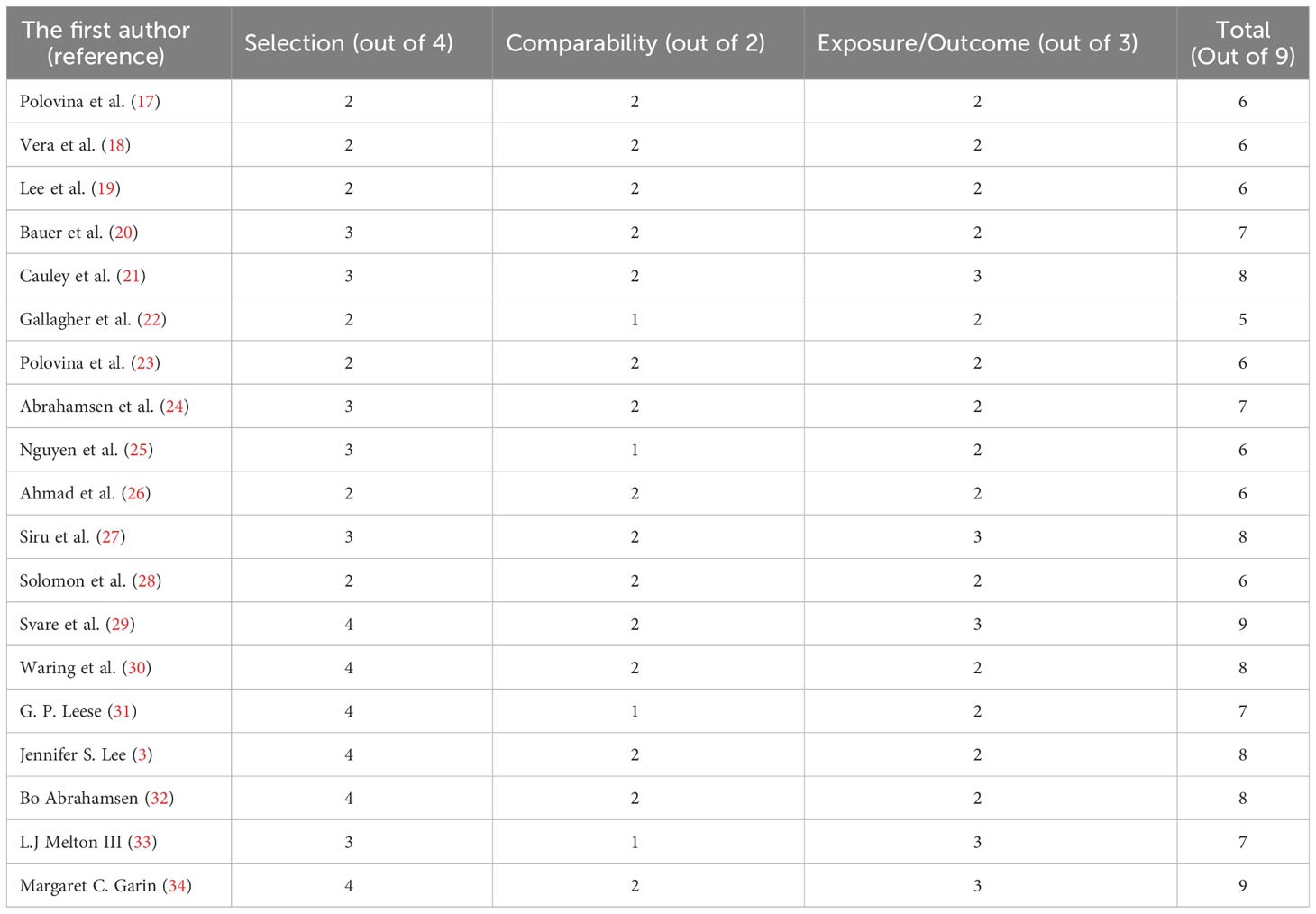
The study’s methodological quality was assessed using the NOS. It focused on three areas, including participant selection (0-4 points), comparability of study groups (0-2 points), and ascertainment of exposure (0-3 points), containing eight questions with a total score of nine. Finally, based on the total number of stars received, each study was assigned one of three grades: excellent, fair, or poor. When a study received 3 or 4 stars in the selection domain, 1 or 2 stars in the comparability domain, and 2 or 3 stars in the outcome/exposure domain, it was considered to have “excellent” quality. In the selection domain, “fair” was used for 2 stars, in the comparability domain for 1 or 2 stars, and in the outcome/exposure domain for 2 or 3 stars. “Poor” was used when the selection domain, comparability domain, or outcome/exposure domain received 0 stars, 1 star, or no stars, respectively (Table 1). Also, this review study complies with the PRISMA checklist to increase soundness and reliability (35).
Results
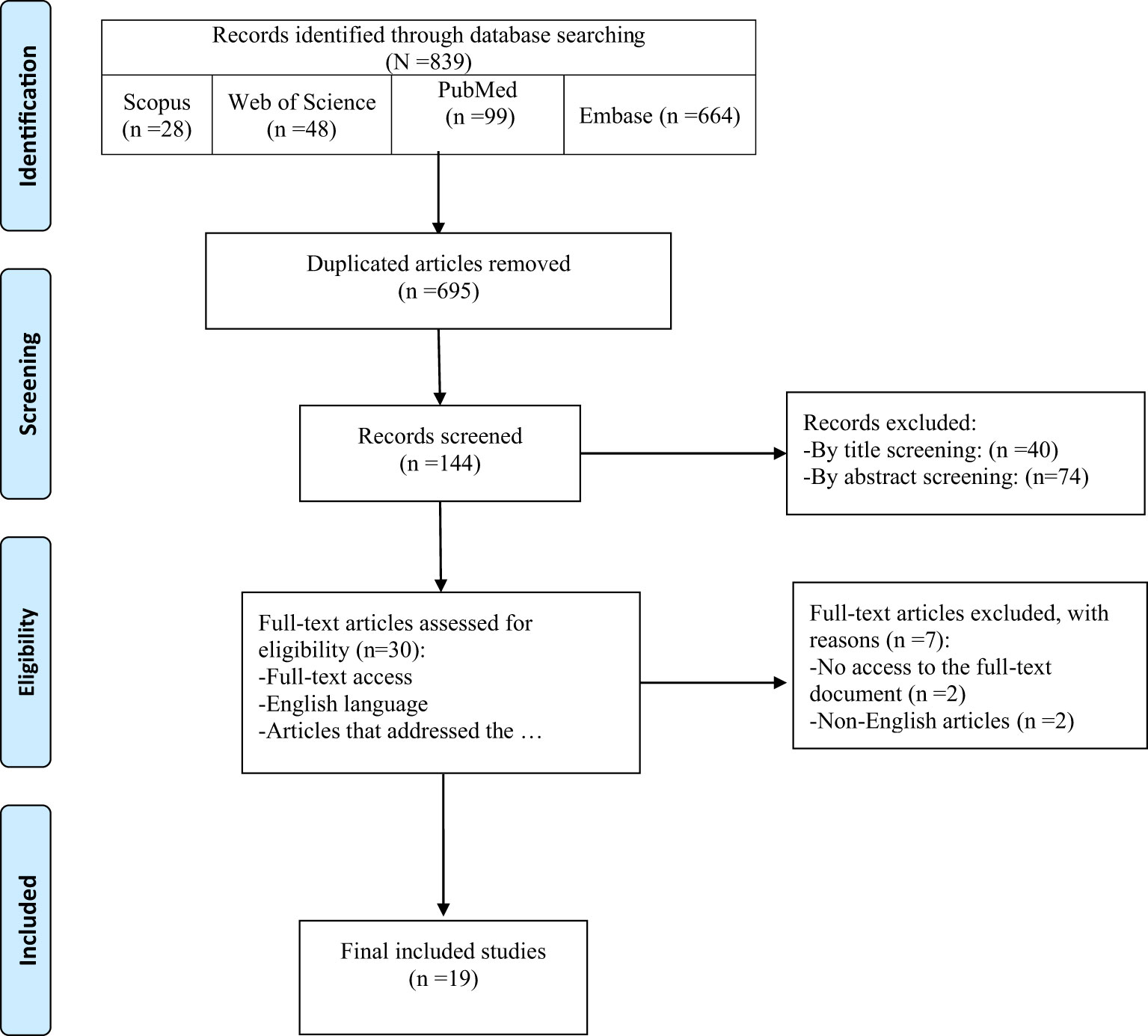
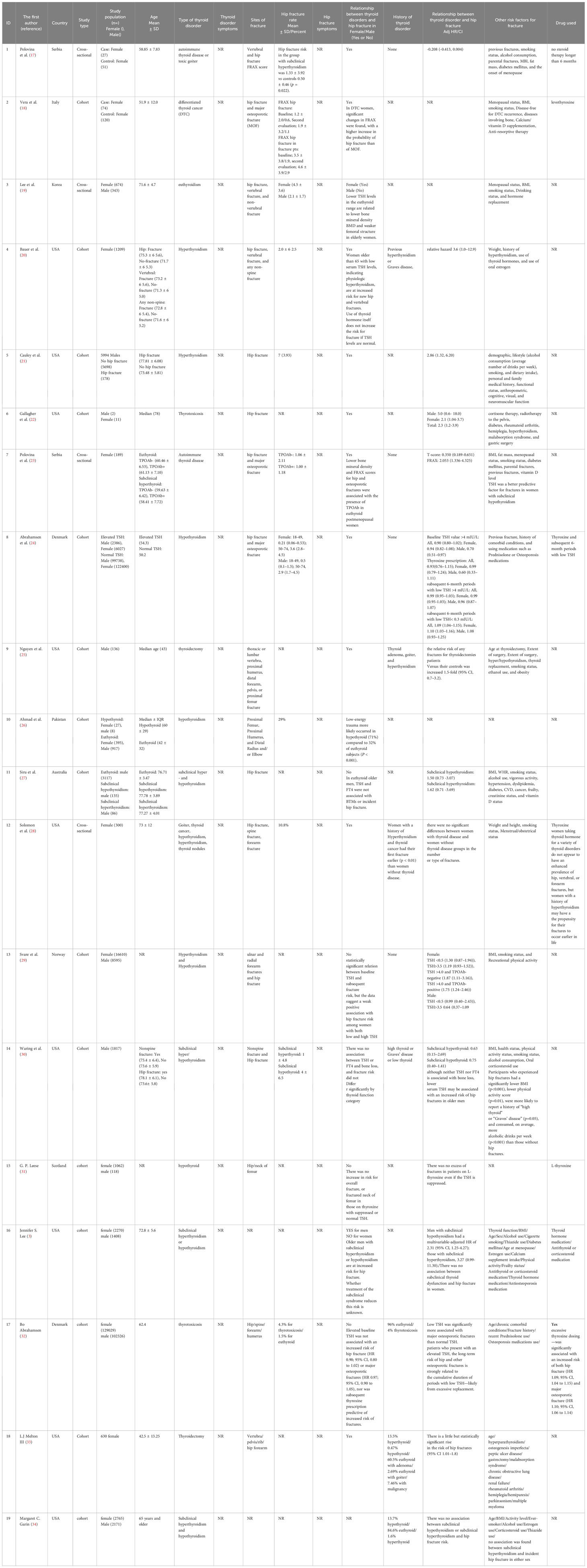
Among 839 records identified by the search, nineteen studies were included in this review (Figure 1). Table 2 provides an overview of the included studies and the extracted data. A total of 15 cohorts and 4 cross-sectional studies reported the data of 229,294 males and 2,838,789 females.
Thyroid cancer
Women with differentiated thyroid cancer (DTC) showed significant changes in Fracture Risk Assessment Tool (FRAX), with a higher increase in the probability of hip fracture than of major osteoporotic fracture (TSH [n.v. 0.3~4.2 mIU/L]: 0.66 ± 1.22 (0.16)) (18). Also, women with a history of hyperthyroidism and thyroid cancer had their first fracture earlier (P<0.01) than women without thyroid disease (28), but there were no significant differences between women with thyroid disease and women without thyroid disease in the number or type of fractures (28).
Hyperthyroidism
Low serum TSH levels (0.1 mU/L) as an indicator of hyperthyroidism in women older than 65 were correlated with higher new hip fractures (20). Males with hyperthyroidism (TSH <0.10 mIU/L) (3, 21) and subclinical hyperthyroidism (17) are at increased risk for hip fracture. Interestingly, thyrotoxicosis, without the aid of other risk factors such as hypogonadism, particularly in men receiving gonadotropin-releasing hormone (GnRH) agonist therapy for prostate cancer, were responsible for the 5-fold increased hip fracture risk in males and 2.1-fold in females (22). Whether treatment of the subclinical syndrome reduces this risk remains unknown (3).
Euthyroid
In euthyroid older men, TSH and FT4 were not associated with Bone Turnover Markers (BTMs) or hip fracture incidence (27). Lower TSH levels in the euthyroid range were related to lower BMD and weaker femoral structure in elderly women but not men (19). Another study on older men reported that although neither TSH nor FT4 was associated with bone loss, lower serum TSH may be associated with an increased risk of hip fractures (relative hazard [RH] 1.31 per SD decrease in TSH, 95% CI 1.01 – 1.71) (30).
Thyroid hormone therapy
Women taking thyroid hormone for various thyroid disorders do not appear to have an enhanced prevalence of hip, vertebral, or forearm fractures (28). In another study, excessive thyroxine dosing was significantly but slightly associated with an increased risk of hip fracture (HR= 1.09; 95% CI: 1.04 to 1.15) (32).
Hypothyroidism
In hypothyroid people, low-energy trauma more likely occurred (71%) compared to 32% of euthyroid subjects (P<0.001) (26). Patients with hypothyroidism presenting with fractures are more likely females with low-energy trauma (26). TSH was a predictive factor for fractures in women with subclinical hypothyroidism (23, 24). No statistically significant relation was found between baseline TSH and subsequent fracture risk, but the data suggest a weak positive association with hip fracture risk among women with both low and high TSH (29–32).
Other outcomes
Lower BMD and FRAX scores for hip and osteoporotic fractures were associated with TPO-Ab in euthyroid postmenopausal women (23). The relative risk of any fractures for patients with thyroidectomy versus their controls was increased 1.5-fold (95% CI, 0.7–3.2) (25). There is a little but statistically significant rise in the risk of hip fractures among thyroidectomized patients (33).
Since some studies focused on women, results may be influenced by involutional osteoporosis (25). Osteoporosis was identified in 90% of hypothyroid subjects who underwent a DEXA scan (26).
Other risk factors for hip fracture
Risk factors for hip fracture reported to be age (3, 32), sex (3), previous fractures (21, 23, 24, 32), smoking status (3, 17–19, 21, 23, 28–31), alcohol consumption (3, 17, 19, 21, 25, 30), parental fractures (17, 23), body mass index (BMI) (3, 17–19, 21, 23, 28–30), fat mass and weight (17, 20, 23, 25), menopausal status (3, 17–19, 23), disease-free for DTC recurrence, diseases involving bone anti-resorptive therapy (18), vitamin D level (23), calcium/vitamin D supplementation (3, 18), hormone replacement and use of oral estrogen (3, 19, 20), history of hyperthyroidism (3, 20, 22, 25), use of thyroid hormones (3, 20, 25, 32) were among factors related to hip fracture.
Medical history (21, 24, 30, 32), cognitive, visual, and neuromuscular function (21), diabetes mellitus (3, 17, 22, 23), rheumatoid arthritis, hemiplegia, malabsorption syndrome, and gastric surgery, radiotherapy to the pelvis (22), and using medication such as Prednisolone or Osteoporosis medications (3, 22, 24, 30, 32) were among factors correlated with hip fracture. Also, thiazide use, frailty status (3), age at thyroidectomy, extent of surgery (3, 25), menstrual/obstetrical status (28), and physical activity status (3, 29, 30) were related to hip fracture.
Discussion
We have conducted a systematic literature review to investigate the potential association between thyroid dysfunction and hip fracture outcome. Results indicate that the association of subclinical hypo- and hyperthyroidism with increased risk of hip fracture is still unclear since there is inevitable heterogenicity in the methodology of the studies. Studies were different regarding sample size, follow-up duration, comorbidities, history of previous fracture, history of medication (background therapies), thyroid pathogenesis (thyroid cancer, Goiter, thyroid nodule, autoimmune thyroid disease, etc.), severity of disease, number of events or traumas that occurred, and menopause status in women.
The systematic review and meta-analysis of seven population-based cohorts reported that participants with subclinical hypo- and hyperthyroidism, particularly among those with TSH levels of less than 0.10 mIU/L, compared with euthyroid participants had higher hazard ratios for hip and non-spine fracture but without statistical differences (P>0.05) (36). In like manner, all articles mentioned TSH levels of lower than 0.10 mIU/L as a cut off value, however, various articles have reached diffrenet results regarding the association between subclinical thyroid disorders and fractures. A similar meta-analysis study by Zhu et al. investigated 17 prospective cohorts, including 313,557 individuals, and found that subclinical hyperthyroidism contributes to a significantly increased risk of hip, spine, and non-spine fractures by calculating relative risks; however, subclinical hypothyroidism was not associated with risk of any fracture (37). Additionally, in line with our findings, they concluded that age, cutoff value, and follow-up duration might play an important role in BMD, leading to higher fracture risk. Fang et al. evaluated sex-related differences between subclinical thyroid dysfunction and fractures. They demonstrated no significant sex-related differences. Unlike previous studies, they have argued that there is a greater risk of any fracture in men than in women with follow-ups of fewer than ten years; however, the risk of hip fracture was higher in women than men without a significant difference (38).
Mortenson et al., while focusing on the association of different medications with the risk of hip fracture, investigated the impact of thyroid hormone as one of the medications on hip fragility. They reported that patients who were overtreated or undertreated with exogenous thyroid hormone had a significantly higher risk of hip fracture (39). On the contrary, some studies hold up the view that endogenous subclinical hyperthyroidism has more effect on BMD than exogenous (40, 41). Also, Wirth et al. found that excluding all exogenous thyroid hormone recipients and limiting the analysis to individuals with endogenous subclinical hyperthyroidism showed an increased risk from 1.38 to 2.16 for hip fracture (36). A similar work by Ku et al. has demonstrated that TSH suppression therapy after thyroidectomy in postmenopausal women significantly decreased hip, lumbar spine, and femoral neck BMD; conversely, in premenopausal women, significantly increased lumbar spine and femoral neck BMD. Additionally, the case and control groups had no significant difference in men.
Different hypothetical mechanisms have been proposed to illustrate the relationship between thyroid hormone and BMD. First, osteoclasts have receptors for thyroid hormones which can directly influence its function, and since high thyroid hormone results in lower TSH hormone; therefore, besides the direct effect of thyroid hormone, it has an indirect impact on bone turnover and bone loss by regulating TSH (42, 43). Secondly, individuals with subclinical hyperthyroidism seem to have lower thigh muscle strength, possibly leading to increased fall-related fractures (44, 45). Thirdly, unlike osteoclasts, osteoblasts have receptors for both thyroid and estrogen hormones, indicating that these hormones play a crucial role in bone formation. As a result, subclinical hyperthyroidism and low estrogen levels, especially in postmenopausal women, are associated with osteoporosis and an increased risk of fractures (46, 47). Likewise, hypothyrodism has negative impacts on bone health, including reducing bone remodeling, provoking falls, reducing the osteoblast activity and decelerating secondary bone mineralization (5, 48). Notably, there is a possibility that hypothyroid patients who are already on treatment with thyroxine supplements were in fact iatrogenic hyperthyroid (26). Consequently, thyroid hormones profoundly impact BMD (39); however, individuals’ age might have a more important role due to the severity of osteoporosis, the number of traumas or fallings, and the previous history of fractures considerably increasing in elderlies (44). Moreover, many studies do not distinguish between underlying pathogenesis, such as thyroid cancers, thyroid tumors, goiter, thyroid nodules, autoimmune thyroid disease, etc. These conditions affect bone turnover in various ways, possibly responsible for confounding results of included studies and previous reviews.
Limitation
Different approaches and methodologies were applied in the included studies, resulting in significant heterogenicity. For instance, different follow-up duration, a wide variety of statistical analysis reports (hazard ratio, relative risk ratio, odds ratio, etc.), and the absence of clear control cases limited our interpretation. Additionally, there is an increase in the upper physiological TSH reference range with age (e.g. 97.5 percentile from 4.32 mUI/l at the age of 20-30 to 5.23 mUI/l around the age 80 and 5.71 mUI/l around age of 90). Thus, some older individuals (i.e. with an increased risk of fracture) may be misclassified as having subclinical hypothyroidism, while their TSH may be indeed within their age-specific reference range. Plus, considering the conditions in which the thyroid hormones are evaluated is very important. For instance, assessing hormone levels right after the fracture is not recommended since fractures can be one of the triggers of acute stress and a contributing factor to the change in TSH levels. Furthermore, selection bias may be present despite our efforts not to set a strict and narrow inclusion criterion. Nevertheless, it is essential to study the available literature to reach a consistent conclusion and recognize the gaps that still need to be addressed.
The main strength of this study is that, in contrast to recent studies to find a positive trend for the impacts of subclinical thyroid dysfunction on hip fracture, our study tried to avoid biases and report reliable evidence in this matter. In this regard, we did not exclude studies due to heterogeneity or contradicted results. For future studies, we recommend that studies share their data in valid and authorized data banks to help big data scientists perform more detailed stratified analysis.
Conclusion
Reaching a consensus conclusion is not feasible regarding the association between subclinical thyroid dysfunction and hip fracture due to the heterogenicity of evidence, but we believe that confirming thyroid dsyfunction as a validated risk factor for hip fracture is yet to come. More studies with clear control selection are required to shed light on this matter which adjusts all possible potential confounders such as sex, age, endogenous or exogenous thyroid hormone, follow-up duration, age-adjusted cutoff values, body weight, cigarette smoking, previous fracture, and the epidemic of falls.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
(1) The conception and design of the study: EM, SS (2) Acquisition of data: SY, MD, AG (3) Analysis and interpretation of data: HS, AM (4) Drafting the article: EM, SM, KQ, GA, SP, MA, PM (5) Revising it critically for important intellectual content: SS, SY, OD (6) Final approval of the version to be submitted: SS, EM, OD. All authors contributed to the article and approved the submitted version.
Acknowledgments
The present study was conducted in collaboration with Khalkhal University of Medical Sciences, Tehran University of Medical Sciences, and Kashan University of Medical Sciences.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Apostu D, Lucaciu O, Oltean-Dan D, Mureșan A-D, Moisescu-Pop C, Maxim A, et al. The influence of thyroid pathology on osteoporosis and fracture risk: A review. Diagnostics (2020) 10(3):149. doi: 10.3390/diagnostics10030149
2. de Gouveia Dal Pino EM, Kowal G. Particle acceleration by magnetic reconnection. Magnetic Fields Diffuse Media (2015), 373–98. doi: 10.1007/978-3-662-44625-6_13
3. Lee JS, Bůžková P, Fink HA, Vu J, Carbone L, Chen Z, et al. Subclinical thyroid dysfunction and incident hip fracture in older adults. Arch Internal Med (2010) 170(21):1876–83. doi: 10.1001/archinternmed.2010.424
4. Siddiqui JA, Partridge NC. Physiological bone remodeling: systemic regulation and growth factor involvement. Physiology (2016) 31(3):233–45. doi: 10.1152/physiol.00061.2014
5. Bassett JD, Williams GR. Role of thyroid hormones in skeletal development and bone maintenance. Endocrine Rev (2016) 37(2):135–87. doi: 10.1210/er.2015-1106
6. Baliram R, Latif R, Zaidi M, Davies T. Expanding the role of thyroid-stimulating hormone in skeletal physiology. Front Endocrinol (2017) 8:252. doi: 10.3389/fendo.2017.00252
7. Veronese N, Maggi S. Epidemiology and social costs of hip fracture. Injury (2018) 49(8):1458–60. doi: 10.1016/j.injury.2018.04.015
8. Soleimani M, Barkhordari S, Mardani F, Shaarbafchizadeh N, Naghavi-Al-Hosseini F. Rationing access to total hip and total knee replacement in the Islamic Republic of Iran to reduce unnecessary costs: policy brief. East Mediterr Health J (2020) 26(11):1396–402. doi: 10.26719/emhj.20.109
9. Soleimani M, Babagoli M, Baghdadi S, Mirghaderi P, Fallah Y, Sheikhvatan M, et al. Return to work following primary total hip arthroplasty: a systematic review and meta-analysis. J Orthop Surg Res (2023) 18(1):95. doi: 10.1186/s13018-023-03578-y
10. Moharrami A, Mirghaderi SP, Marzban S, Moazen-Jamshidi SMM, Shakoor D, Mortazavi SMJ. Total Hip Arthroplasty via direct anterior approach for osteonecrosis; comparison with primary hip osteoarthritis in a mid term follow up. J Clin Orthop Trauma (2022) 34:102042. doi: 10.1016/j.jcot.2022.102042
11. Hoveidaei AH, Nakhostin-Ansari A, Hosseini-Asl SH, Khonji MS, Razavi SE, Darijani SR, et al. Increasing burden of hip osteoarthritis in the Middle East and North Africa (MENA): an epidemiological analysis from 1990 to 2019. Arch Orthop Trauma Surg (2023) 143(6):3563–73. doi: 10.1007/s00402-022-04582-3
12. Fard SB, Jamshidi SMMM, Hoveidaei AH, Razzaghof M, Mortazavi SMJ. Nonunion following valgus subtrochanteric osteotomy for neglected femoral neck fracture: A case report. Int J Surg Case Rep (2023) 103:107905. doi: 10.1016/j.ijscr.2023.107905
13. Ebrahimpour A, Sadighi M, Hoveidaei AH, Chehrassan M, Minaei R, Vahedi H, et al. Surgical treatment for bisphosphonate-related atypical femoral fracture: A systematic review. Arch Bone Jt Surg (2021) 9(3):283–96. doi: 10.22038/abjs.2020.52698.2608
14. Ebrahimpour A, Chehrassan M, Hoveidaei AH, Jafari Kafiabadi M, Sadighi M, Manafi Rasi A, et al. Surgical management of extremity fractures in COVID-19 patients. J Orthop Spine Trauma (2022) 7(4):127–33. doi: 10.18502/jost.v7i4.8858
15. Beheshti Fard S, Moharrami A, Mirghaderi SP, Mortazavi SMJ. Broken pin removal from hip joint using arthroscopic grasper – A technical note and review of literature. Injury (2022) 53(11):3853–7. doi: 10.1016/j.injury.2022.08.054
16. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev (2015) 4(1):1–9. doi: 10.1186/2046-4053-4-1
17. Polovina S, Micić D, Miljić D, Milić N, Micić D, Popović V. The fracture risk assessment tool (FRAX® score) in subclinical hyperthyroidism. Vojnosanit Pregl (2015) 72(6):510–6. doi: 10.2298/VSP1506510P
18. Vera L, Gay S, Campomenosi C, Paolino S, Pera G, Monti E, et al. Ten-year estimated risk of bone fracture in women with differentiated thyroid cancer under TSH-suppressive levothyroxine therapy. Endokrynol Pol (2016) 67(4):350–8. doi: 10.5603/EP.a2016.0046
19. Lee SJ, Kim KM, Lee EY, Song MK, Kang DR, Kim HC, et al. Low normal TSH levels are associated with impaired BMD and hip geometry in the elderly. Aging Dis (2016) 7(6):734. doi: 10.14336/AD.2016.0325
20. Bauer DC, Ettinger B, Nevitt MC, Stone KL, Group SoOFR. Risk for fracture in women with low serum levels of thyroid-stimulating hormone. Ann Internal Med (2001) 134(7):561–8. doi: 10.7326/0003-4819-134-7-200104030-00009
21. Cauley JA, Cawthon PM, Peters KE, Cummings SR, Ensrud KE, Bauer DC, et al. Risk factors for hip fracture in older men: the osteoporotic fractures in men study (MrOS). J Bone Mineral Res (2016) 31(10):1810–9. doi: 10.1002/jbmr.2836
22. Gallagher JC, Melton L, Riggs BL. Examination of prevalence rates of possible risk factors in a population with a fracture of the proximal femur. Clin Orthop Relat Res (1980) 153:158–65. doi: 10.1097/00003086-198011000-00021
23. Polovina SP, Miljic D, Zivojinovic S, Milic N, Micic D, Brkic VP. The impact of thyroid autoimmunity (TPOAb) on bone density and fracture risk in postmenopausal women. Hormones (Athens) (2017) 16(1):54–61. doi: 10.14310/horm.2002.1719
24. Abrahamsen B, Jørgensen HL, Laulund AS, Nybo M, Bauer DC, Brix TH, et al. The excess risk of major osteoporotic fractures in hypothyroidism is driven by cumulative hyperthyroid as opposed to hypothyroid time: An observational register-based time-resolved cohort analysis. J Bone Mineral Res (2015) 30(5):898–905. doi: 10.1002/jbmr.2416
25. Nguyen TT, Heath IIIH, Bryant SC, O’Fallon WM, Melton LJ III. Fractures after thyroidectomy in men: A population-based cohort study. J Bone Mineral Res (1997) 12(7):1092–9. doi: 10.1359/jbmr.1997.12.7.1092
26. Ahmad T, Muhammad ZA, Nadeem S. Is hypothyroidism associated with outcomes in fracture patients? Data from a trauma registry. J Surg Res (2021) 268:527–31. doi: 10.1016/j.jss.2021.07.036
27. Siru R, Alfonso H, Chubb SP, Golledge J, Flicker L, Yeap BB. Subclinical thyroid dysfunction and circulating thyroid hormones are not associated with bone turnover markers or incident hip fracture in older men. Clin Endocrinol (2018) 89(1):93–9. doi: 10.1111/cen.13615
28. Solomon Bl, Wartofsky L, Burman K. Prevalence of fractures in postmenopausal women with thyroid disease. Thyroid (1993) 3(1):17–23. doi: 10.1089/thy.1993.3.17
29. Svare A, Nilsen TIL, Åsvold BO, Forsmo S, Schei B, Bjøro T, et al. Does thyroid function influence fracture risk? Prospective data from the HUNT2 study, Norway. Eur J Endocrinol (2013) 169(6):845–52. doi: 10.1530/EJE-13-0546
30. Waring AC, Harrison S, Fink HA, Samuels MH, Cawthon PM, Zmuda JM, et al. A prospective study of thyroid function, bone loss, and fractures in older men: the MrOS study. J Bone Mineral Res (2013) 28(3):472–9. doi: 10.1002/jbmr.1774
31. Leese G, Jung R, Guthrie C, Waugh N, Browning M. Morbidity in patients on L-thyroxine: a comparison of those with a normal TSH to those with a suppressed TSH. Clin Endocrinol (1992) 37(6):500–3. doi: 10.1111/j.1365-2265.1992.tb01480.x
32. Abrahamsen B, Jørgensen HL, Laulund AS, Nybo M, Brix TH, Hegedüs L. Low serum thyrotropin level and duration of suppression as a predictor of major osteoporotic fractures—the OPENTHYRO register cohort. J Bone Mineral Res (2014) 29(9):2040–50. doi: 10.1002/jbmr.2244
33. Melton IIIL, Ardila E, Crowson C, O’Fallon W, Khosla S. Fractures following thyroidectomy in women: a population-based cohort study. Bone (2000) 27(5):695–700. doi: 10.1016/S8756-3282(00)00379-3
34. Garin MC, Arnold AM, Lee JS, Robbins J, Cappola AR. Subclinical thyroid dysfunction and hip fracture and bone mineral density in older adults: the cardiovascular health study. J Clin Endocrinol Metab (2014) 99(8):2657–64. doi: 10.1210/jc.2014-1051
35. da Maia TF, de Camargo BG, Pereira ME, de Oliveira CS, Guiloski IC. Increased risk of fractures and use of proton pump inhibitors in menopausal women: A systematic review and meta-analysis. Int J Environ Res Public Health (2022) 19(20):13501. doi: 10.3390/ijerph192013501
36. Wirth CD, Blum MR, da Costa BR, Baumgartner C, Collet T-H, Medici M, et al. Subclinical thyroid dysfunction and the risk for fractures: a systematic review and meta-analysis. Ann Internal Med (2014) 161(3):189–99. doi: 10.7326/M14-0125
37. Zhu H, Zhang J, Wang J, Zhao X, Gu M. Association of subclinical thyroid dysfunction with bone mineral density and fracture: a meta-analysis of prospective cohort studies. Endocrine (2020) 67:685–98. doi: 10.1007/s12020-019-02110-9
38. Fang H, Zhao R, Cui S, Wan W. Sex differences in major cardiovascular outcomes and fractures in patients with subclinical thyroid dysfunction: a systematic review and meta-analysis. Aging (Albany NY). (2022) 14(20):8448. doi: 10.18632/aging.204352
39. Mortensen SJ, Mohamadi A, Wright CL, Chan JJ, Weaver MJ, von Keudell A, et al. Medications as a risk factor for fragility hip fractures: a systematic review and meta-analysis. Calcified Tissue Int (2020) 107:1–9. doi: 10.1007/s00223-020-00688-1
40. Yan Z, Huang H, Li J, Wang J. Relationship between subclinical thyroid dysfunction and the risk of fracture: a meta-analysis of prospective cohort studies. Osteoporosis Int (2016) 27:115–25. doi: 10.1007/s00198-015-3221-z
41. Belaya ZE, Melnichenko GA, Rozhinskaya LY, Fadeev VV, Alekseeva TM, Dorofeeva OK, et al. Subclinical hyperthyroidism of variable etiology and its influence on bone in postmenopausal women. Hormones (Athens) (2007) 6(1):62–70.
42. Cooper DS, Biondi B. Subclinical thyroid disease. Lancet (2012) 379(9821):1142–54. doi: 10.1016/S0140-6736(11)60276-6
43. Baliram R, Sun L, Cao J, Li J, Latif R, Huber AK, et al. Hyperthyroid-associated osteoporosis is exacerbated by the loss of TSH signaling. J Clin Invest (2012) 122(10):3737–41. doi: 10.1172/JCI63948
44. Morrison A, Fan T, Sen SS, Weisenfluh L. Epidemiology of falls and osteoporotic fractures: a systematic review. Clinicoecon outcomes Res (2012) 5:9–18.
45. Brennan MD, Powell C, Kaufman KR, Sun PC, Bahn RS, Nair KS. The impact of overt and subclinical hyperthyroidism on skeletal muscle. Thyroid (2006) 16(4):375–80. doi: 10.1089/thy.2006.16.375
46. Van der Eerden B, Gevers E, Löwik C, Karperien M, Wit J. Expression of estrogen receptor α and β in the epiphyseal plate of the rat. Bone (2002) 30(3):478–85. doi: 10.1016/S8756-3282(01)00703-7
47. Robson H, Siebler T, Stevens DA, Shalet SM, Williams GR. Thyroid hormone acts directly on growth plate chondrocytes to promote hypertrophic differentiation and inhibit clonal expansion and cell proliferation. Endocrinology (2000) 141(10):3887–97. doi: 10.1210/endo.141.10.7733
Keywords: hip fracture, thyroid disease, thyroid disorder, thyroid dysfunction, thyroid
Citation: SeyedAlinaghi S, Yarmohammadi S, Dashti M, Ghasemzadeh A, Siami H, Molla A, Mahrokhi S, Qaderi K, Arjmand G, Parikhani SN, Amrollah MF, Mirghaderi P, Mehraeen E and Dadras O (2023) The relationship of hip fracture and thyroid disorders: a systematic review. Front. Endocrinol. 14:1230932. doi: 10.3389/fendo.2023.1230932
Received: 08 June 2023; Accepted: 25 September 2023;
Published: 10 October 2023.
Edited by:
Laurent M Sachs, Muséum National d’Histoire Naturelle, FranceReviewed by:
Krzysztof Cezary Lewandowski, Medical University of Lodz, PolandParaskevi Xekouki, University of Crete, Greece
Copyright © 2023 SeyedAlinaghi, Yarmohammadi, Dashti, Ghasemzadeh, Siami, Molla, Mahrokhi, Qaderi, Arjmand, Parikhani, Amrollah, Mirghaderi, Mehraeen and Dadras. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Esmaeil Mehraeen, ZXMubWVocmFlZW5AZ21haWwuY29t; Soudabeh Yarmohammadi, eWFybW9oYW1tYWRpc291ZGFiZWhAZ21haWwuY29t
 SeyedAhmad SeyedAlinaghi
SeyedAhmad SeyedAlinaghi Soudabeh Yarmohammadi2*
Soudabeh Yarmohammadi2* Sona Mahrokhi
Sona Mahrokhi Kowsar Qaderi
Kowsar Qaderi Ghazal Arjmand
Ghazal Arjmand Peyman Mirghaderi
Peyman Mirghaderi Esmaeil Mehraeen
Esmaeil Mehraeen Omid Dadras
Omid Dadras