- 1Chair for Biomarkers of Chronic Diseases, Department of Biochemistry, College of Science, King Saud University, Riyadh, Saudi Arabia
- 2Department of Physiology, College Medicine, King Saud University, Riyadh, Saudi Arabia
Objective: The present cross-sectional study examined the association between circulating levels of sex hormone-binding globulin (SHBG) and testosterone with bone mineral density (BMD) in middle-aged Arab men.
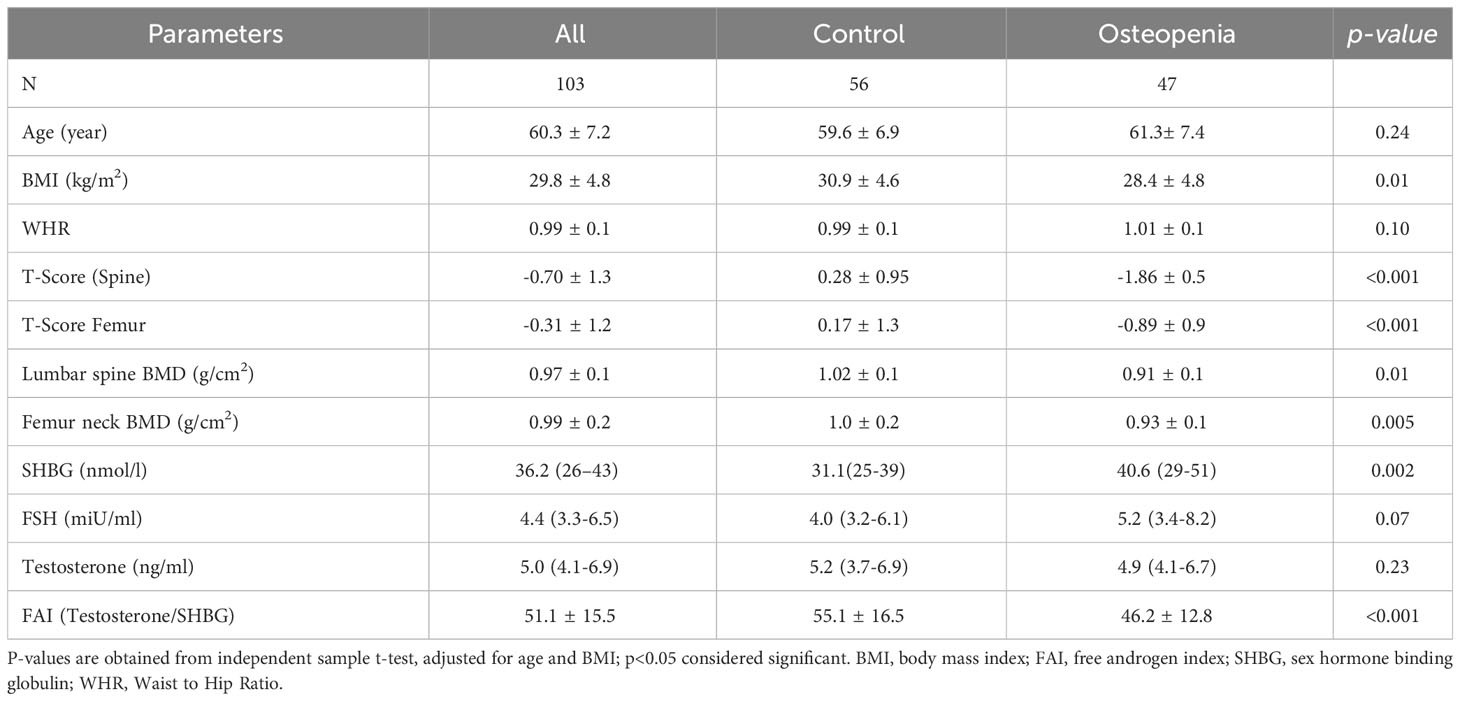
Methods: Clinical data of 103 middle-aged Saudi men (mean age 60.7±7.2) were extracted from the Osteoporosis Registry of the Chair for Biomarkers of Chronic Diseases, King Saud University in Riyadh, Saudi Arabia. Participants were categorized according to the presence of osteopenia (T-score -1.0 to -2.5) (N=47) and controls (N=56). Data collected included demographics and anthropometrics as well as levels of sex hormone-binding globulin (SHBG), testosterone and follicle-stimulating hormone (FSH) which were measured using commercially available assays. Free androgen index (FAI) was calculated.
Results: Those with osteopenia had significantly lower levels of FAI (p<0.05), and higher levels of SHBG (p<0.004) and FSH (p<0.005). In the osteopenia group, SHBG was positively correlated with age (r=0.33, p<0.05), while it was inversely correlated with BMD spine (r = -0.39, p<0.05) and T-score femur (r= -0.35, p<0.05) in the same group. Furthermore, testosterone was inversely correlated with BMI in the osteopenia group (r= -0.33, p<0.05) while FAI was positively correlated with T-score femur (r = 0.36, p<0.05) as well as in all participants (r= 0.24, p<0.05). Among controls, FAI had an inverse correlation with FSH (r= -0.28, p<0.05) and over-all (r= -0.22, p<0.05).
Conclusion: In summary, the associations elicited suggest that circulating levels of SHBG and FAI may be against age-related bone loss in middle-aged men.
1 Introduction
The aging population is growing quickly around the world, making age-related diseases such as osteoporosis a major public concern (1). Osteopenia, often regarded as a precursor to osteoporosis, can go unnoticed until it progresses, leading to an increased fracture risk in elderly patients, psychological and physical harm (2). This can also be a financial burden on families and society. Prevention is the best way to manage osteoporosis, so it is essential to pay close attention to those at risk, e.g., those with osteopenia and take early action for prevention and treatment.
At the start of the 21st century, reports showed that the prevalence of osteoporosis in Saudi Arabia was more severe than in other parts of the world (3). Early findings indicated that as many as 48% of people had osteopenia (4–6). Gouhar et al. observed that 29.7% of people over the age of 60 with osteoporosis were in this age group (7). Al Quaiz et al. also used DXA to check the bones of 362 healthy women and found that 58.6% of them had low bone density, even though most of them were between 40 and 50 years old (8).
Despite the extensive research conducted on osteoporosis in Saudi Arabia, there is a notable gap when it comes to studying patients specifically diagnosed with osteopenia. Consequently, there is a crucial need to identify and address the occurrence of osteopenia in Saudi men, with the ultimate aim of enhancing targeted interventions for both prevention and management of osteoporosis. Addressing this gap is essential to devise effective strategies for the prevention and management of osteoporosis in this demographic.
In men, the deficit of sex hormones is linked to diminished bone strength and heightened fracture risk, with the quality of the bone being a determining factor (9, 10). Bone mineral density (BMD) is affected by both genetic and environmental factors and is closely linked to osteoporosis and bone fractures. Numerous studies have investigated into the relationship between adult male sex hormone levels and BMD, and a prevalent finding has been an inverse correlation between Sex Hormone-Binding Globulin (SHBG) levels and BMD (11–13). SHBG, a protein binding firmly to sex steroids in the bloodstream, has its levels influenced by a plethora of factors (14). While certain studies propose that elevated SHBG levels in men could elevate osteoporosis and fracture risks, even after accounting for sex hormone levels (15–18), other research has found no significant impact of SHBG on BMD after considering other factors (19).
Despite advances in understanding, there remains an unsettling gap in patient care. Many men at risk are not routinely screened to ascertain fracture probability nor are they educated about fracture prevention or prevention and treatment of osteoporosis (20). Globally, questions arise regarding the frequency of BMD and DXA screenings in men. How often are men, especially those at risk, screened to ascertain their fracture probability? What guidelines dictate the criteria for these screenings? Particularly in Saudi Arabia, understanding SHBG’s role in bone health among aging males remains underexplored. This study aims to bridge this gap by investigating the correlations between SHBG levels, testosterone and other sex hormones with BMD in middle-aged Saudi men, thereby providing valuable insights to enhance osteoporosis prevention and management strategies.
2 Materials and methods
2.1 Study subjects
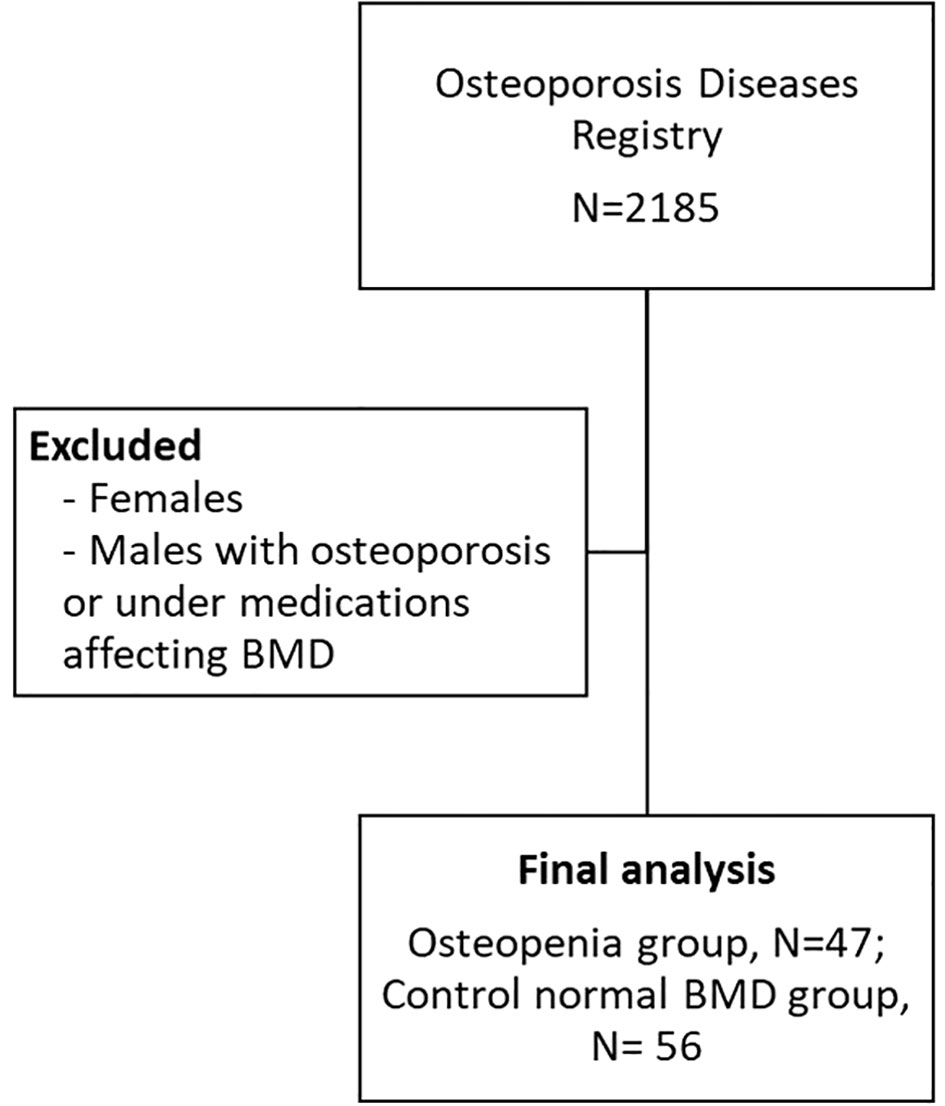
This study included a total of 103 Saudi men from the Osteoporosis Registry of the Chair for Biomarkers of Chronic Diseases (CBCD) at King Saud University in Riyadh, Saudi Arabia (21). In brief, the osteoporosis registry is a database of adult patients aged 55 years and above, from all over Riyadh tertiary hospitals who underwent DXA scan and provided blood samples and consent to be included in the registry for research purposes. Mean value of Bone mineral density (BMD) measurements were taken at the lumbar spine, left hip, and right hip using DXA (Hologic QDR 2000 Inc., Woltham, MA, USA). A patient with a T-score between -1 to -2.5 (mean value standard deviations, SD) is classified as having osteopenia (22). The exclusion criteria included men with pre-existing bone diseases other than low BMD or under medications that can affect BMD for at least 6 months. The participants were categorized into two groups: 47 individuals with osteopenia and 56 individuals with normal BMD. Osteopenia was defined as having a T-score between −2.5 and −1.0, while those with a T-score above −1.0 were considered to have normal BMD. Various anthropometric measurements were recorded, including height, weight, waist and hip circumferences, as well as systolic and diastolic blood pressure. Body mass index (BMI) was calculated by dividing weight (kg) by height (m2).
Certain exclusion criteria were applied in this study. Men with pre-existing bone diseases other than low BMD, those using or under medications that can affect BMD for at least 6 months, individuals with a history of treatment with pulsed electromagnetic fields (PEMFs) were excluded. The study was conducted in accordance with the Declaration of Helsinki and received approval from the Ethics Committee of the College of Science, King Saud University, Riyadh, Kingdom of Saudi Arabia (Approval# 8/25/454266, 30 September 2013). Figure 1 shows the flowchart of participants.
2.2 Biochemical analysis
Blood samples were collected from participants after an overnight fasting period. The measurement of SHBG was performed using an electrochemiluminescence immunoassay with the Roche Cobas-e411 kit (Roche Diagnostics, Mannheim, Germany). The assay had a detection limit of 0.35 nmol/L, and the intra-assay coefficient of variation (CV) ranged from 2.6% to 5.6%. FSH and testosterone levels were also measured using the COBAS e411 analyzer, following the standard protocol provided with the commercially available kit from Roche Diagnostics. The free androgen index (FAI) was calculated using the formula: FAI = (total testosterone/SHBG) x 100 (23).
2.3 Data analysis
Data analysis was performed using the Statistical Package for Social Sciences (SPSS) version 22.0 (SPSS, Inc., Chicago, IL, USA). Descriptive statistics were used to report normal variables as mean and standard deviation (SD), while non-Gaussian variables were presented as median (25th and 75th) percentiles. Categorical variables were expressed as frequencies and percentages. Independent Student T test and Mann-Whitney U test was performed for mean and median difference between control and osteopenia group. Bivariate associations between normal variables were assessed using Pearson’s correlation, while Spearman’s correlation was employed for non-normal variables. P-value less then <0.05 consider significant statistically.
3 Results
Table 1 presents the key characteristics of the study participants categorized by their respective groups. The study consisted of 103 men aged 60 years or older, with an average age of 60.3 ± 7.2 years. The majority of participants (82.4%) were over the age of 60. Approximately 46% of the men were diagnosed with osteopenia or low bone mass, and within this group, individuals had a significantly lower BMI compared to those in the normal group (p<0.001). Furthermore, the osteopenia group exhibited significantly lower BMD in the spine and femoral neck, as well as lower T-scores in the spine (p<0.001) in comparison to the normal group. In terms of hormone levels, the osteopenia group displayed significantly lower free androgen index (FAI) and higher levels of SHBG and FSH compared to the control group. However, there were no significant differences observed in age and testosterone levels between the two groups (Table 1). Figure 1 provides a graphical representation of the circulating levels of SHBG and FAI in both study groups, based on our collected data.
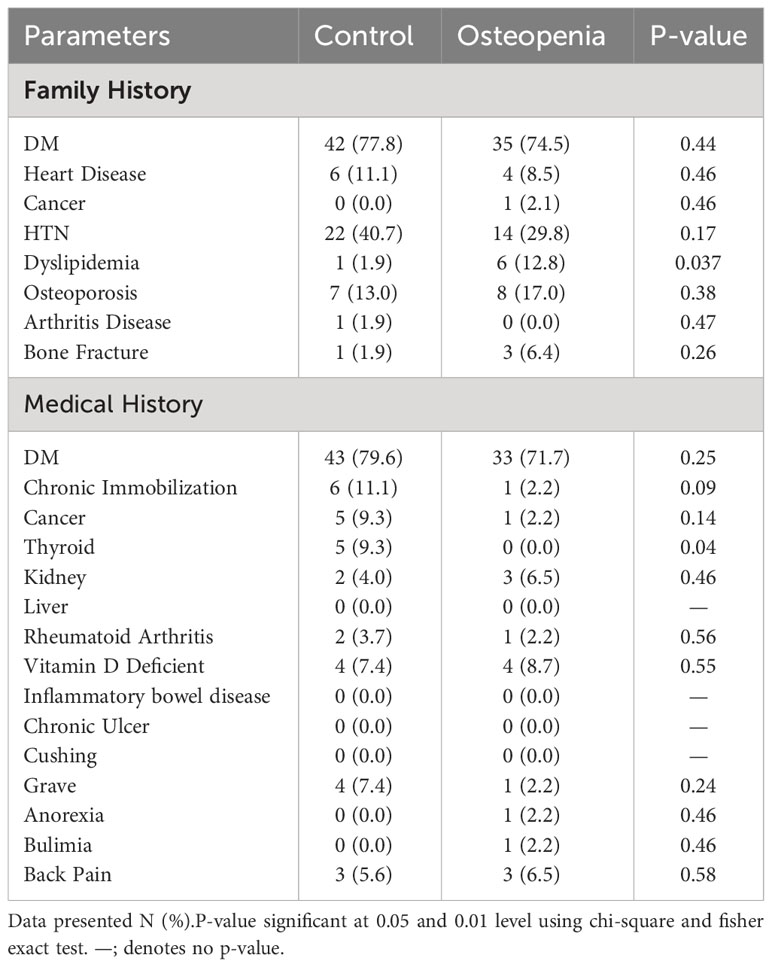
Table 2 showed patient’s characteristic regarding comorbidities and risk factors. In the family history assessment, a significant prevalence of diabetes mellitus (DM) was observed in both the control (77.8%) and osteopenia groups (74.5%). However, dyslipidemia was notably higher in the osteopenia group at 12.8% compared to 1.9% in the control group (p=0.037). For medical history, diabetes mellitus (DM) was again prominent in both groups, with 79.6% in controls and 71.7% in osteopenia. A marked difference was seen in thyroid diseases, prevalent in 9.3% of the control group but absent in the osteopenia group (p=0.04). Other conditions showed comparable frequencies between the groups.
3.1 Bivariate correlations of SHBG and FAI with other parameters
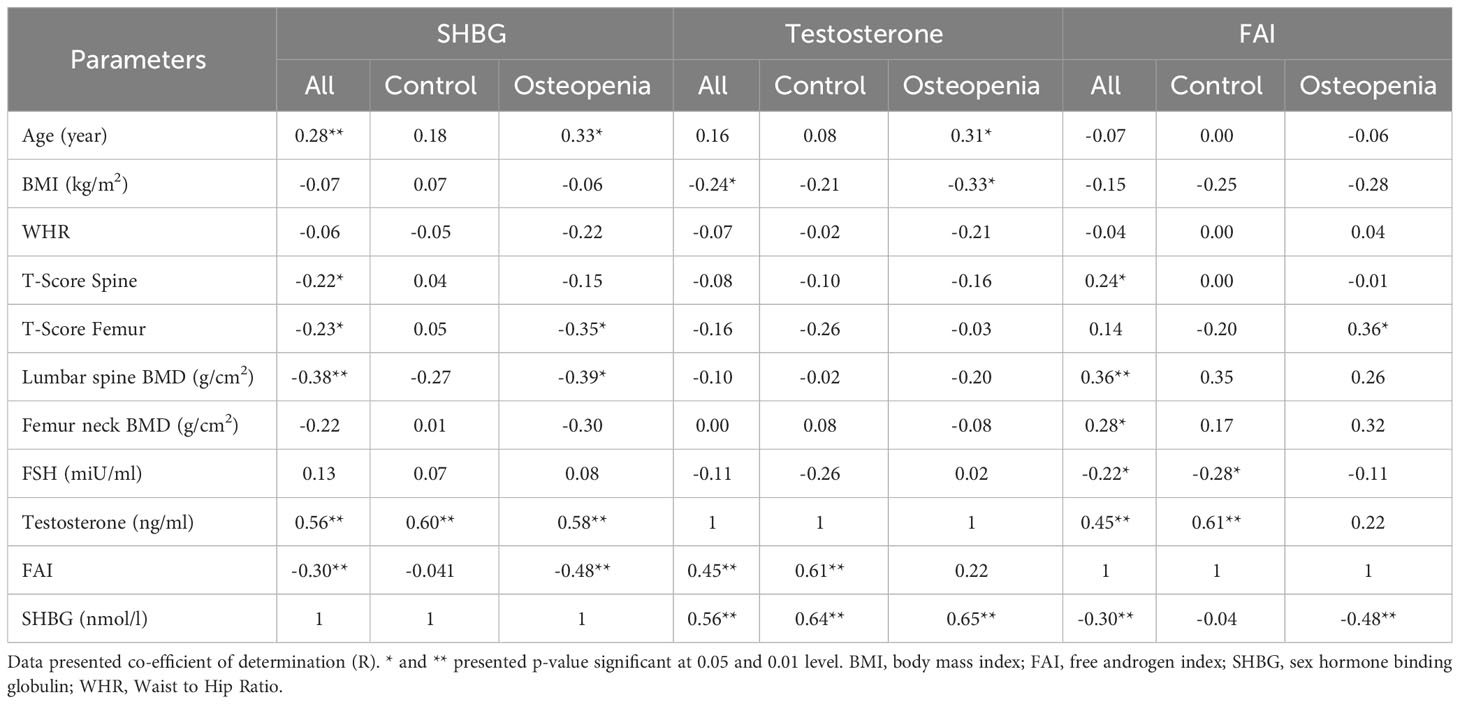
The relationships between SHBG, age, testosterone, T-score, BMD spine, FAI, and BMI in different study groups are presented in Table 3. The findings demonstrate that SHBG levels were positively and significantly associated with age in both the control and osteopenia groups. Furthermore, there was a positive and significant correlation between SHBG levels and testosterone across all groups. However, in the osteopenia group, SHBG levels exhibited a significant inverse correlation with T-score femur, BMD spine, and FAI. Testosterone showed a significant positive correlation with age in the osteopenia group, while displaying an inverse correlation with BMI within the same group. No significant correlations were observed between testosterone and T-score (spine) or BMD spine in any of the study groups. Additionally, there were no significant correlations found between testosterone and FSH. On the other hand, the FAI demonstrated a positive and significant correlation with T-score femur in the osteopenia group and all subjects, while exhibiting an inverse correlation with FSH in the control group and all subjects. Figure 2 provides a visual representation of the bivariate correlations between SHBG and T-score femur, as well as BMD (spine).

Figure 2 Scatterplots showing the associations of SHBG with spin BMD and T-score in the control and study groups.
3.2 Stepwise regression analysis
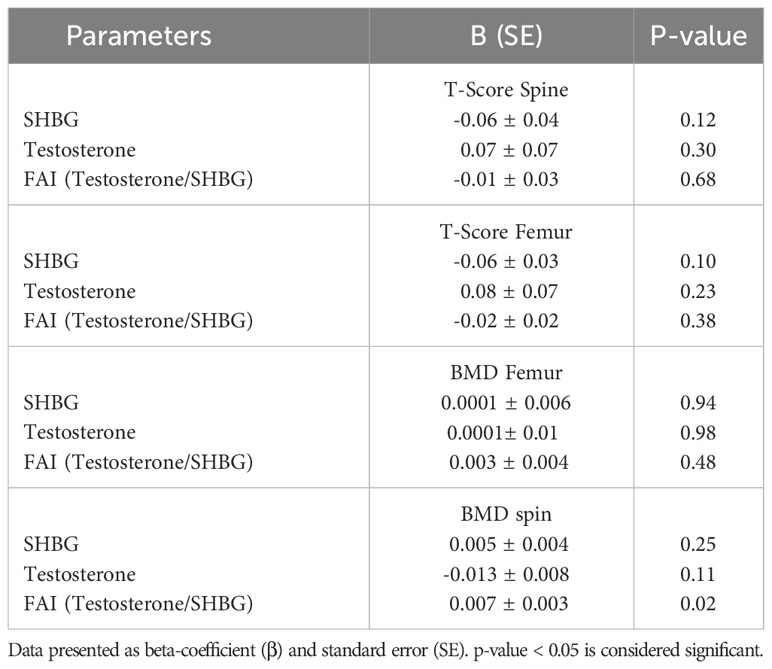
To investigate the influence of SHBG, testosterone, and FAI on T-scores and BMDs, a stepwise linear regression analysis was performed. T-score and BMD were considered as the dependent variables, while SHBG, testosterone, and FAI served as the independent variables. The results of this analysis are presented in Table 4. The study findings indicated that among men with osteopenia, FAI exerted a significant impact on spine BMD (p = 0.024). However, SHBG did not demonstrate a significant effect on either T-scores or BMDs across any of the three groups.
4 Discussion
SHBG and testosterone are essential components of bone metabolism, but their link with BMD in older Saudi men hasn’t been studied much. This study examined the relationship between BMD and serum levels of SHBG, testosterone, and FAI in 103 middle-aged Saudi men. The study found that osteopenia patients had significantly increased SHBG and FSH levels, while FAI levels were reduced, which may explain bone loss. The study also found interesting associations between these markers and anthropometric indices. A notable observation in the osteopenia group was the significant increase in SHBG and testosterone levels with advancing age. These findings align with previous studies conducted in diverse populations, further supporting their consistency (24–26). Previous research has consistently demonstrated a significant decline in BMD after the age of 50, and this decline has been linked to variations in sex hormones (27, 28). There is a well-established reduction in sex hormone levels and functional hormone receptors, which are known to play a crucial role in the development of osteoporosis (13). Correlations of these markers with men age also propose a modulatory influence of age on elderly men sex hormones that then effect on bone metabolism. SHBG is a primary carrier for testosterone and higher levels of SHBG bind more free testosterone. So both SHBG and testosterone increases with age. These results indicate that SHBG and testosterone levels may be useful aging-related biomarkers in a group at increased risk of age-related diseases such physical function impairment and osteopenia.
Our research emphasizes the significance of recognizing obesity as a risk factor for osteopenia in men due to its impact on reducing serum testosterone levels. Testosterone is inversely associated with BMI in all and osteopenia groups. The relationship between obesity and low serum testosterone levels in men is still being debated (29), possibly due to the conversion of testosterone to estradiol by adipose tissue (30). This evidence of a cause-and-effect relationship between BMI and serum testosterone is backed up by a meta-analysis study of the effects of body weight loss on serum testosterone, which found that both a low-calorie diet and bariatric surgery are linked to a significant rise in serum testosterone after body weight loss (31). Osuna et al. (32) showed that as the BMI score goes up, the concentration of testosterone and SHBG goes down in the same way. Shamim et al. (33) did a study that was similar to ours. They found that as the BMI index went up, the amount of testosterone in healthy men ages 30–50 went down. Our study shows that this relationship is also true for men between the ages of 50 and 70.
This study revealed a notable inverse association between sex hormone-binding globulin (SHBG) and bone mineral density (BMD) at the spine. Conversely, testosterone levels exhibited a significant positive correlation with SHBG. As a result, it was anticipated that higher levels of serum testosterone would be linked to improved bone health. However, some studies reported a significant relationship between testosterone levels and BMD (26, 34). Additionally, low testosterone levels were associated with bone loss in other studies (13, 35, 36). In our study, we did not find any significant associations between testosterone levels and BMD or T-Score. These findings are consistent with previous research (37–39).
Recent studies have presented conflicting findings regarding the association between SHBG and testosterone with bone metabolism (39–41). For instance, the European Male Ageing Study (EMAS) revealed that testosterone was not linked to bone metabolism parameters in middle-aged and elderly men (39, 41). Interestingly, one study indicated that SHBG, rather than total testosterone, exhibited a significant relationship with biochemical bone turnover markers (BTMs) (39). In our own study, we observed a significant negative correlation between SHBG levels and both Femur T-Score and spine BMD. However, only FAI levels showed a significant positive correlation with T-Score Femur. These findings suggest that FAI levels, rather than testosterone levels, may be more closely associated with bone metabolism. A commonly acknowledged hypothesis regarding the role of SHBG in bone metabolism revolves around its anti-estrogenic effect. It is believed that elevated levels of SHBG bind to estrogen, thereby decreasing its biologically active form. This, in turn, can lead to a reduction in bone mineral density (BMD) and an increased susceptibility to fractures.
This research found no statistically significant relationship between SHBG and either BMD or t-scores when using multiple regression models. However, FAI was the only gonadal hormone that was a significant predictor of bone mass, and this relationship was only found to be significant at the spine. These results were the same as ones that had already been released (42). This could mean that androgens affect bone density in other ways, such as by affecting the function of the kidney’s 1a-hydroxylase, as suggested by Francis et al. (42) We used FAI as a measure of androgen state because it looks at both total testosterone and how much of it is bound to SHBG. Taxel et al. (43) found that when an aromatase inhibitor is given to older men, the levels of estradiol go down and bone resorption markers go up. Androgen receptors are present on osteoblasts (44), while both osteoblasts and osteoclasts have estrogen receptors (44, 45). Since osteoblasts make aromatase (46) it is likely that androgens are changed into estrogens at the tissue level.
One notable strength of this study is its novelty, as it is the first known investigation to examine the relationship between sex hormone-binding globulin (SHBG) and testosterone levels with BMD specifically in Arab middle-aged men. However, it is important to acknowledge the limitations of the study. The sample size was relatively small, which restricts the generalizability of the findings. Additionally, being a cross-sectional study, it only provides a snapshot of the proposed relationship, limiting our ability to establish cause and effect. Conducting a longitudinal study within this population would be valuable and informative in further exploring and understanding this relationship. Furthermore, it should be noted that we did not perform exclusions based on factors such as spondylarthrosis, which could potentially impact the accuracy of lumbar spine BMD measurements. This limitation is important to consider when interpreting our results and their relevance to the broader population of Arab middle-aged men.
In conclusion, the prevalence of osteopenia is related to greater SHBG concentrations and decreased FAI. In Saudi older men with osteopenia, SHBG was significantly inversely associated with BMD, notably at the spin. In addition, in the osteopenia group, FAI was positively and significantly correlated with femoral T-score. Age and menopause likely trigger a protective response of increased blood SHBG and testosterone levels in response to increased bone resorption. In comparison to BMD and DXA, that are direct measures of bone density, SHBG levels offer insights into the hormonal dynamics influencing bone health. As a diagnostic tool for osteoporosis or osteopenia, SHBG would act more as an adjunct or a supplementary test, rather than a replacement for BMD or DXA. More research is needed to understand the processes driving these correlations.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of the College of Science, King Saud University, Riyadh, Kingdom of Saudi Arabia (Approval# 8/25/454266, 30 September 2013). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SY drafted the first version of the manuscript, MK made the statistical analysis. supervised critically revised the manuscript. ME contributed to the data collection. NA and AAcritically revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia (grant no. IFKSURC-1-1608) for funding this research.
Acknowledgments
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
SHBG, Sex hormone-binding globulin; BMD, Bone mineral density; FAI, Free androgen index; FSH, Follicle-stimulating hormone; BMI, Body mass index; WHR, Waist to Hip Ratio.
References
1. Salari N, Darvishi N, Bartina Y, Larti M, Kiaei A, Hemmati M, et al. Global prevalence of osteoporosis among the world older adults: a comprehensive systematic review and meta-analysis. J Orthop Surg Res (2021) 16(1):669. doi: 10.1186/s13018-021-02821-8
2. Wang Y, Zhang Y, Zhao L, Wang G, Yang C, Zhao C, et al. Prevalence and distribution of osteopenia in chinese population: A system review and meta-analysis. Iran J Public Health (2022) 51(11):2435–48. doi: 10.18502/ijph.v51i11.11161
3. Sadat-Ali M, AlZamami JF, AlNaimi SN, Al-Noaimi DA, AlDakheel DA, AlSayed HN, et al. Osteoporosis: Is the prevalence increasing in Saudi Arabia. Ann Afr Med (2022) 21(1):54–7. doi: 10.4103/aam.aam_79_20
4. Sadat-Ali M, Al-Habdan I, Al-Mulhim AA, El-Hassan AY. Effect of parity on bone mineral density among postmenopausal Saudi Arabian women. Saudi Med J (2005) 26(10):1588–90.
5. Ardawi MS, Maimany AA, Bahksh TM, Nasrat HA, Milaat WA, Al-Raddadi RM. Bone mineral density of the spine and femur in healthy Saudis. Osteoporos Int (2005) 16(1):43–55. doi: 10.1007/s00198-004-1639-9
6. Sadat-Ali M, Al-Habdan IM, Al-Mulhim FA, El-Hassan AY. Bone mineral density among postmenopausal Saudi women. Saudi Med J (2004) 25(11):1623–5.
7. Gouhar G, Tamimm R, Alhemaidi W, NB. A, Alharbi K, Qattan A, et al. Association of specific risk factors for osteoporosis in Saudi female patients referred from DEXA scan in Riyadh city. Int J Med Developing Countries. (2019) 3(4):353–7. doi: 10.24911/IJMDC.51-1545568941
8. AlQuaiz AM, Kazi A, Tayel S, Shaikh SA, Al-Sharif A, Othman S, et al. Prevalence and factors associated with low bone mineral density in Saudi women: a community based survey. BMC Musculoskelet Disord (2014) 15:5. doi: 10.1186/1471-2474-15-5
9. Zhang R, Mao J, Wang X, Nie M, Ma W, Ji W, et al. Increased bone mineral density in male patients with idiopathic hypogonadotropic hypogonadism who undergo sex hormone therapy: findings from cross-sectional and longitudinal studies. Endocr Pract (2021) 27(9):934–40. doi: 10.1016/j.eprac.2021.05.004
10. Qu Z, Jiang J, Yang F, Huang J, Zhao J, Yan S. Genetically predicted sex hormone-binding globulin and bone mineral density: A mendelian randomization study. Calcif Tissue Int (2021) 108(3):281–7. doi: 10.1007/s00223-020-00770-8
11. Veldhuis-Vlug AG, Woods GN, Sigurdsson S, Ewing SK, Le PT, Hue TF, et al. Serum FSH is associated with BMD, bone marrow adiposity, and body composition in the AGES-reykjavik study of older adults. J Clin Endocrinol Metab (2021) 106(3):e1156–e69. doi: 10.1210/clinem/dgaa922
12. Champakanath A, Keshawarz A, Pyle L, Snell-Bergeon JK, Shah VN. Fracture risk assessment (FRAX) without BMD and risk of major osteoporotic fractures in adults with type 1 diabetes. Bone (2021) 143:115614. doi: 10.1016/j.bone.2020.115614
13. Paller CJ, Shiels MS, Rohrmann S, Basaria S, Rifai N, Nelson W, et al. Relationship of sex steroid hormones with bone mineral density (BMD) in a nationally representative sample of men. Clin Endocrinol (Oxf). (2009) 70(1):26–34. doi: 10.1111/j.1365-2265.2008.03300.x
14. Hoppe E, Bouvard B, Royer M, Audran M, Legrand E. Sex hormone-binding globulin in osteoporosis. Joint Bone Spine. (2010) 77(4):306–12. doi: 10.1016/j.jbspin.2010.03.011
15. Legrand E, Hedde C, Gallois Y, Degasne I, Boux de Casson F, Mathieu E, et al. Osteoporosis in men: a potential role for the sex hormone binding globulin. Bone (2001) 29(1):90–5. doi: 10.1016/S8756-3282(01)00478-1
16. Mellstrom D, Vandenput L, Mallmin H, Holmberg AH, Lorentzon M, Oden A, et al. Older men with low serum estradiol and high serum SHBG have an increased risk of fractures. J Bone Miner Res (2008) 23(10):1552–60. doi: 10.1359/jbmr.080518
17. LeBlanc ES, Nielson CM, Marshall LM, Lapidus JA, Barrett-Connor E, Ensrud KE, et al. The effects of serum testosterone, estradiol, and sex hormone binding globulin levels on fracture risk in older men. J Clin Endocrinol Metab (2009) 94(9):3337–46. doi: 10.1210/jc.2009-0206
18. Tuck SP, Scane AC, Fraser WD, Diver MJ, Eastell R, Francis RM. Sex steroids and bone turnover markers in men with symptomatic vertebral fractures. Bone (2008) 43(6):999–1005. doi: 10.1016/j.bone.2008.08.123
19. Araujo AB, Travison TG, Leder BZ, McKinlay JB. Correlations between serum testosterone, estradiol, and sex hormone-binding globulin and bone mineral density in a diverse sample of men. J Clin Endocrinol Metab (2008) 93(6):2135–41. doi: 10.1210/jc.2007-1469
20. LeBoff MS, Greenspan SL, Insogna KL, Lewiecki EM, Saag KG, Singer AJ, et al. The clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int (2022) 33(10):2049–102. doi: 10.1007/s00198-021-05900-y
21. Wani K, Yakout SM, Ansari MGA, Sabico S, Hussain SD, Alokail MS, et al. Metabolic syndrome in arab adults with low bone mineral density. Nutrients (2019) 11(6):1405. doi: 10.3390/nu11061405
22. Al-Saleh Y, Sulimani R, Sabico S, Alshahrani FM, Fouda MA, Almohaya M, et al. Diagnosis and management of osteoporosis in Saudi Arabia: 2023 key updates from the Saudi Osteoporosis Society. Arch Osteoporos. (2023) 18(1):75. doi: 10.1007/s11657-023-01242-w
23. Al-Daghri NM, Yakout SM, Ansari MGA, Hussain SD, Wani KA, Sabico S. Vitamin D metabolites and sex steroid indices in postmenopausal women with and without low bone mass. Metabolites (2021) 11(2):86. doi: 10.3390/metabo11020086
24. Varsavsky M, Reyes-Garcia R, Garcia-Martin A, Gonzalez-Ramirez AR, Aviles-Perez MD, Munoz-Torres M. SHBG levels are associated with bone loss and vertebral fractures in patients with prostate cancer. Osteoporos Int (2013) 24(2):713–9. doi: 10.1007/s00198-012-2012-z
25. Lecomte P, Lecureuil N, Lecureuil M, Osorio Salazar C, Valat C. Age modulates effects of thyroid dysfunction on sex hormone binding globulin (SHBG) levels. Exp Clin Endocrinol Diabetes. (1995) 103(5):339–42. doi: 10.1055/s-0029-1211375
26. Cawthon PM, Schousboe JT, Harrison SL, Ensrud KE, Black D, Cauley JA, et al. Sex hormones, sex hormone binding globulin, and vertebral fractures in older men. Bone (2016) 84:271–8. doi: 10.1016/j.bone.2016.01.009
27. Zhang X, Hua T, Zhu J, Peng K, Yang J, Kang S, et al. Body compositions differently contribute to BMD in different age and gender: a pilot study by QCT. Arch Osteoporos. (2019) 14(1):31. doi: 10.1007/s11657-019-0574-5
28. Papaioannou A, Kennedy CC, Cranney A, Hawker G, Brown JP, Kaiser SM, et al. Risk factors for low BMD in healthy men age 50 years or older: a systematic review. Osteoporos Int (2009) 20(4):507–18. doi: 10.1007/s00198-008-0720-1
29. Eriksson J, Haring R, Grarup N, Vandenput L, Wallaschofski H, Lorentzen E, et al. Causal relationship between obesity and serum testosterone status in men: A bi-directional mendelian randomization analysis. PloS One (2017) 12(4):e0176277. doi: 10.1371/journal.pone.0176277
30. Fui MN, Dupuis P, Grossmann M. Lowered testosterone in male obesity: mechanisms, morbidity and management. Asian J Androl. (2014) 16(2):223–31.
31. Corona G, Rastrelli G, Monami M, Saad F, Luconi M, Lucchese M, et al. Body weight loss reverts obesity-associated hypogonadotropic hypogonadism: a systematic review and meta-analysis. Eur J Endocrinol (2013) 168(6):829–43. doi: 10.1530/EJE-12-0955
32. Osuna JA, Gomez-Perez R, Arata-Bellabarba G, Villaroel V. Relationship between BMI, total testosterone, sex hormone-binding-globulin, leptin, insulin and insulin resistance in obese men. Arch Androl. (2006) 52(5):355–61. doi: 10.1080/01485010600692017
33. Shamim MO, Ali Khan FM, Arshad R. Association between serum total testosterone and Body Mass Index in middle aged healthy men. Pak J Med Sci (2015) 31(2):355–9. doi: 10.12669/pjms.312.6130
34. Mellstrom D, Johnell O, Ljunggren O, Eriksson AL, Lorentzon M, Mallmin H, et al. Free testosterone is an independent predictor of BMD and prevalent fractures in elderly men: MrOS Sweden. J Bone Miner Res (2006) 21(4):529–35. doi: 10.1359/jbmr.060110
35. Cauley JA, Ewing SK, Taylor BC, Fink HA, Ensrud KE, Bauer DC, et al. Sex steroid hormones in older men: longitudinal associations with 4.5-year change in hip bone mineral density–the osteoporotic fractures in men study. J Clin Endocrinol Metab (2010) 95(9):4314–23.
36. Kuchuk NO, van Schoor NM, Pluijm SM, Smit JH, de Ronde W, Lips P. The association of sex hormone levels with quantitative ultrasound, bone mineral density, bone turnover and osteoporotic fractures in older men and women. Clin Endocrinol (Oxf). (2007) 67(2):295–303. doi: 10.1111/j.1365-2265.2007.02882.x
37. Rucker D, Ezzat S, Diamandi A, Khosravi J, Hanley DA. IGF-I and testosterone levels as predictors of bone mineral density in healthy, community-dwelling men. Clin Endocrinol (Oxf). (2004) 60(4):491–9. doi: 10.1111/j.1365-2265.2004.02006.x
38. Zha XY, Hu Y, Pang XN, Zhu JH, Chang GL, Li L. Sex hormone-binding globulin (SHBG) as an independent determinant of bone mineral density (BMD) among Chinese middle-aged and elderly men. Endocrine (2014) 47(2):590–7. doi: 10.1007/s12020-013-0155-0
39. Boonen S, Pye SR, O'Neill TW, Szulc P, Gielen E, Borghs H, et al. Influence of bone remodelling rate on quantitative ultrasound parameters at the calcaneus and DXA BMDa of the hip and spine in middle-aged and elderly European men: the European Male Ageing Study (EMAS). Eur J Endocrinol (2011) 165(6):977–86. doi: 10.1530/EJE-11-0353
40. Hsu B, Cumming RG, Seibel MJ, Naganathan V, Blyth FM, Bleicher K, et al. Reproductive hormones and longitudinal change in bone mineral density and incident fracture risk in older men: the concord health and aging in men project. J Bone Miner Res (2015) 30(9):1701–8. doi: 10.1002/jbmr.2493
41. Vanderschueren D, Pye SR, Venken K, Borghs H, Gaytant J, Huhtaniemi IT, et al. Gonadal sex steroid status and bone health in middle-aged and elderly European men. Osteoporos Int (2010) 21(8):1331–9. doi: 10.1007/s00198-009-1144-2
42. Francis RM, Peacock M, Aaron JE, Selby PL, Taylor GA, Thompson J, et al. Osteoporosis in hypogonadal men: role of decreased plasma 1,25-dihydroxyvitamin D, calcium malabsorption, and low bone formation. Bone (1986) 7(4):261–8. doi: 10.1016/8756-3282(86)90205-X
43. Taxel P, Kennedy DG, Fall PM, Willard AK, Clive JM, Raisz LG. The effect of aromatase inhibition on sex steroids, gonadotropins, and markers of bone turnover in older men. J Clin Endocrinol Metab (2001) 86(6):2869–74. doi: 10.1210/jc.86.6.2869
44. Colvard DS, Eriksen EF, Keeting PE, Wilson EM, Lubahn DB, French FS, et al. Identification of androgen receptors in normal human osteoblast-like cells. Proc Natl Acad Sci U S A. (1989) 86(3):854–7. doi: 10.1073/pnas.86.3.854
45. Eriksen EF, Colvard DS, Berg NJ, Graham ML, Mann KG, Spelsberg TC, et al. Evidence of estrogen receptors in normal human osteoblast-like cells. Science (1988) 241(4861):84–6. doi: 10.1126/science.3388021
Keywords: SHBG, testosterone, fAI, BMD, osteopenia
Citation: Yakout SM, Khattak MNK, Al-Daghri NM, Al-Masri AA and Elsaid MA (2023) Associations of bone mineral density with sex hormone-binding globulin (SHBG) and testosterone in middle-aged Saudi men: a cross-sectional study. Front. Endocrinol. 14:1230279. doi: 10.3389/fendo.2023.1230279
Received: 28 May 2023; Accepted: 10 November 2023;
Published: 24 November 2023.
Edited by:
Wanli W. Smith, Johns Hopkins University, United StatesReviewed by:
Nazareth Novaes Rocha, Fluminense Federal University, BrazilJoanna K. Filipowska, City of Hope National Medical Center, United States
Copyright © 2023 Yakout, Khattak, Al-Daghri, Al-Masri and Elsaid. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nasser M. Al-Daghri, bmRhZ2hyaUBrc3UuZWR1LnNh
 Sobhy M. Yakout1
Sobhy M. Yakout1 Nasser M. Al-Daghri
Nasser M. Al-Daghri