
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Endocrinol., 09 August 2023
Sec. Obesity
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1230206
 Pejman Rohani1†
Pejman Rohani1† Nasser Malekpour Alamdari2†
Nasser Malekpour Alamdari2† Seyedeh Elaheh Bagheri3
Seyedeh Elaheh Bagheri3 Azita Hekmatdoost4
Azita Hekmatdoost4 Mohammad Hassan Sohouli1,5*
Mohammad Hassan Sohouli1,5*Background: Despite the fact that obesity and overweight are serious major health problems worldwide, fighting against them is also considered a challenging issue. Several interventional studies have evaluated the potential weight-reduction effect of Tirzepatide. In order to obtain a better viewpoint from them, this study aimed to comprehensively investigate the effects of subcutaneous Tirzepatide on obesity and overweight.
Methods: Scopus, PubMed/Medline, Web of Science, Cochrane, and Embase databases were searched using standard keywords to identify all controlled trials investigating the weight loss effects of Tirzepatide. Pooled weighted mean difference and 95% confidence intervals were achieved by random-effects model analysis for the best estimation of outcomes. The statistical heterogeneity and publication bias were determined using the Cochran’s Q test and I2 statistics and using the funnel plot and Egger’s test, respectively.
Results: Twenty three treatments arm with 7062 participants’ were included in this systematic review and meta‐regression analysis. The pooled findings showed that Tirzepatide vs placebo significantly reduced body weight (weighted mean difference (WMD): -11.34 kg, 95% confidence interval (CI): -12.79 to -9.88, P< 0.001), body mass index (BMI) (WMD: -3.11 kg/m2, 95% CI: -4.36 to -1.86, P< 0.001), and waist circumference (WC) (WMD: -7.24 cm, 95% CI -10.12 to -4.36, P< 0.001). These reductions were even greater, especially with higher doses and duration of Tirzepatide.
Conclusions: Tirzepatide medication had significant effects on weight management with the reduction of body weight, BMI, and WC. Administration of Tirzepatide can be considered a therapeutic strategy for overweight or obese people.
Obesity and overweight are two of the main health challenges worldwide that affect over a third of the population around the world (1). They are the leading causes of death, contributing to at least 2.8 million deaths and 35.8 million global disability-adjusted life years (DALYs), according to the most recent reports from the World Health Organization (WHO) (2). A wide range of metabolic alterations and clinical anomalies are present in people with persistent excessive weight gain or obesity. Additionally, untreated overweight or obese individuals are linked to a number of complications, such as chronic diseases like diabetes mellitus, hypertension, cardiovascular disease, and stroke (3–6). Moreover, recent evidence has shown that these population groups are not only at a higher risk of contracting non-communicable diseases but also at a higher risk of poorer outcomes from communicable diseases, such as increased rate of hospitalization and mortality from viral infections like severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) as compared with the general population (3, 4).
In general, the causes of obesity are multifaceted and difficult to pinpoint (1, 7), although they can include environmental and lifestyle variables including physical activity and food as well as hereditary factors (6, 8). So, it is expected that lifestyle change strategies should be done to prevent and combat obesity. However, the fight against obesity has been one of the greatest challenges. Indeed, it is found that even with lifestyle changes, the success rate in weight loss is not always satisfactory (6, 9). Therefore, given the high importance of obesity as the most common risk factor for developing the disease (1, 5, 6), clinical management primarily emphasizes weight loss, which can be achieved through medication.
According to several new clinical guidelines, the role of anti-obesity medications for obese or overweight people who have weight-related complications is highlighted as a recommended treatment for obesity (10). Glucagon-like peptide-1 (GLP-1) and gastric inhibitory polypeptide (GIP), as two of the main incretin peptide hormones, are responsible for glucose homeostasis and enhance glucose-stimulated insulin secretion after nutrient ingest (11). The distal ileum and colon’s L cells generate the 30 amino acid peptide GLP-1, whereas the duodenum and jejunum’s cells create the 42 amino acid peptide GIP (12). It is probable that the successful application of incretin peptide hormones has the potential to reduce weight and consider an obesity treatment (13, 14). Interestingly, more recent evidence has shown that the combination of both GIP and GLP-1 through multiple metabolic mechanisms synergistically affect each other. Therefore, it is conceivable that compared to administering each hormone separately, this may result in significantly increased insulin and glucagonostatic responses, which may have a greater impact on the effectiveness of weight loss (15, 16).
The synthetic peptide tirzepatide (LY3298176) as a dual agonist of GIP and GLP-1, which is used as a subcutaneous injection once a week, has recently attracted the attention of scientists as a peptide containing 39 amino acids (17, 18) that raises insulin secretion, lowers glucagon secretion, delays gastric emptying, lowers dietary intake, and ultimately lowers body weight (19–21). Reduced hepatic glucose synthesis and plasma glucose levels seem to be linked to GIP and GLP-1 agonist’s capacity to inhibit glucagon release. This medicine is an FDA-approved treatment for type 2 diabetic mellitus (T2DM) based on scientific data (15, 22, 23). Moreover, in a phase 3 clinical trial study on 2400 people living with obesity and overweight, it showed the effectiveness of Tirzepatide on anthropometry parameters (10). Despite some of the gastrointestinal adverse events of Tirzepatide including nausea, vomiting, and diarrhea (6, 24, 25), it has superiority to other similar agents and is considered a promising anti-obesity therapeutic drug due to its multiple pharmacological targets on nutrient-stimulated hormone receptor agonists (6, 26). However, the appropriate dose and duration of Tirzepatide therapy which is effective in weight loss is an issue that needs more comprehensive studies (27). The present systematic review and meta-regression analysis, based on clinical trials, aimed to investigate the effects of Tirzepatide on weight loss.
The Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) criteria were followed for conducting this study (28). Without regard to language or time restrictions, a thorough search was carried out in the PubMed/MEDLINE, Web of Science, SCOPUS, and Embase databases from the December 2018 to August 2022. Additionally, similar papers and gray literature were considered in the search. Medical subject headings (MeSH) and Emtree (Embase subject headings) were selected to search the online databases, as follow: (Tirzepatide OR ly3298176) AND (“weight” OR “Waist Circumference” OR “Body Mass Index”) AND (“Clinical Trials as Topic” OR “Cross-Over Studies” OR “Double-Blind Method” OR “Single-Blind Method” OR “Random Allocation” OR “Clinical Trial”) (The search strategy was added to the appendix as an example in the PubMed search database). In order to discover potentially overlooked eligible trials, the reference lists of the papers found and associated review studies were also manually examined.
Using titles, abstracts, or the complete texts of the research, two writers separately removed duplicate articles before finding and reviewing relevant publications. Discrepancy rate between reviewers was less than 4%, which was resolved by the third reviewer. In the end, the papers were separated based on the following standards: 1) Randomized clinical trials studies; 2) Tirzepatide was given as an intervention to adults; 3) The existence of a control group in the form of placebo or insulin, and 4) Baseline and post in both group (intervention and control) weight, WC, and BMI were recorded. If a study revealed outcomes at more than one follow-up period, the most recent or most extensive follow-up time was taken into account. Studies with duplicated data, studies with unclear information, studies in which Tirzepatide was an intervention in conjunction with other widely used medications, non-randomized trial designs, animal studies, studies without a control group, and reviews or meta-analysis studies were also omitted. Also, if Tirzepatide is compared with other common drugs or drugs with similar performance, the desired study will be removed due to lack of sufficient effect of Tirzepatide.
Two authors independently reviewed the eligible studies. Name of first author, study site, year of publication, RCT design (crossover or parallel), sample size (intervention and control groups), participant characteristics (gender, BMI, age, and health status), type of outcomes, length of intervention, dosage of intervention, and means and standard deviations (S.D.s) of intended outcomes at baseline, post-intervention, and/or changes between baseline and post-intervention were all extracted.
The details of the study quality assessment are presented in Table 1. The quality of the included RCTs was methodologically assessed using the Cochrane risk-of-bias test for randomized trials (RoB 2), version 2 (37). Two authors independently rated each study as having a low, high, or unclear risk of bias based on the following potential sources of bias: blinding of outcome assessment, allocation concealment, participant and staff blinding, random sequence generation, incomplete outcome data, selective reporting, and other bias. Any disagreements were discussed with a third author to find a solution. In order to assess the quality of the current analysis study, the NutriGrade (Grading of Recommendations Assessment, Development, and Evaluation) grading system was also utilized (38). A reliable 10-point assessment system that assesses elements affecting study quality is the NutriGrade checklist. This scale has seven components: (1) risk of bias, (2) precision, (3) heterogeneity, (4) directness, (5) publishing bias, (6) funding bias, and (7) study design.
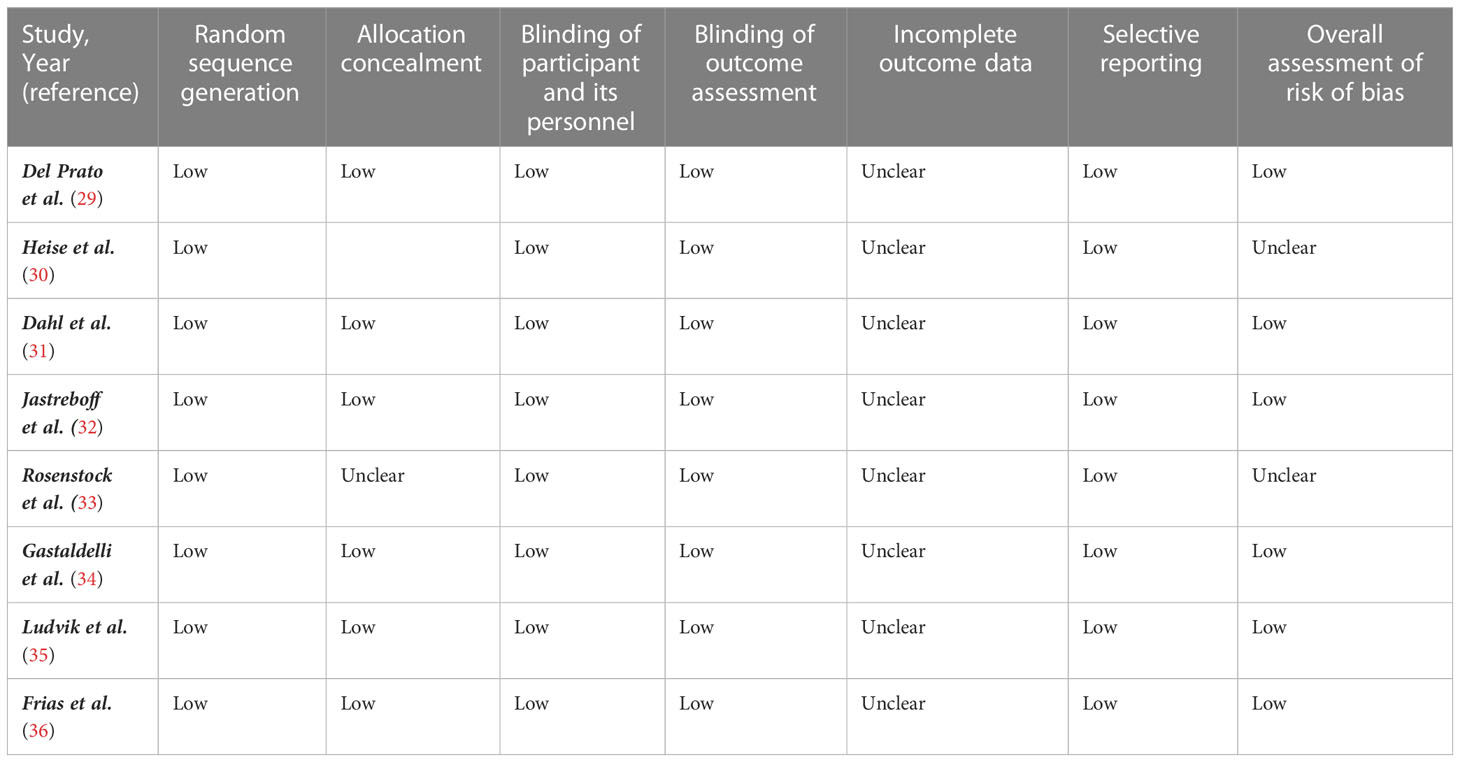
Table 1 Risk of bias assessment according to the Cochrane collaboration's risk of bias assessment tool.
STATA version 12.0 software was used to analyze the data. Different data formats were converted to the mean and standard deviations (S.D.s) using established formula (39, 40). For instance, we estimated the change using the formula below in the absence of standard deviations: Square root [(S.D. baseline 2 + SD final 2) - (2R S.D. baseline 2 S.D. final)] is the definition of S.D. changes. The following formula is used to convert the standard error of the mean (SEM) to standard deviation: S.D. is equal to SEM × √n, where n is the total number of participants in each group. The meta-analysis of study findings was conducted using the random-effects model. The general inverse variance approach was used to weight the research. Multiple evaluations within a single research group were handled by using the values from the longest time point for the analysis. The status of study heterogeneity was assessed using Q Statistics and I-squared (I2). With I2 values ranging from 0% to 25, 26% to 50%, 5% to 75%, and 76% to 100%, respectively, insignificant, low, moderate, and high heterogeneity were detected (41). Meta-regression investigates whether particular covariates (potential effect modifiers) explain any of the heterogeneity of treatment effects between studies. Thus, meta-regression between subcutaneous Tirzepatide and absolute mean differences in body weight based on dosage, baseline of mean age and BMI, and duration of intervention was performed using random effect model. A pre-defined subgroup analysis based on the dosage of the intervention was carried out to find potential sources of heterogeneity. To determine the contribution of each study to the total mean difference, a sensitivity analysis was used. We used the official Egger’s test to determine whether there was publication bias (42).
A flowchart of the study selection procedure with exclusion criteria is shown in Figure 1. The aforementioned electronic databases produced 225 publications, according to this number. There were 165 papers overall after duplicate research were eliminated. Following a review of the titles and abstracts of the research, 128 publications were eliminated because they did not match the criteria for inclusion. During the secondary screening, 32 articles were located using full-text. 24 of the studies were eliminated for the aforementioned reasons. Finally, 8 papers (29–36) with 23 treatments arm were included in the quantitative meta-analysis since they matched the qualifying requirements.
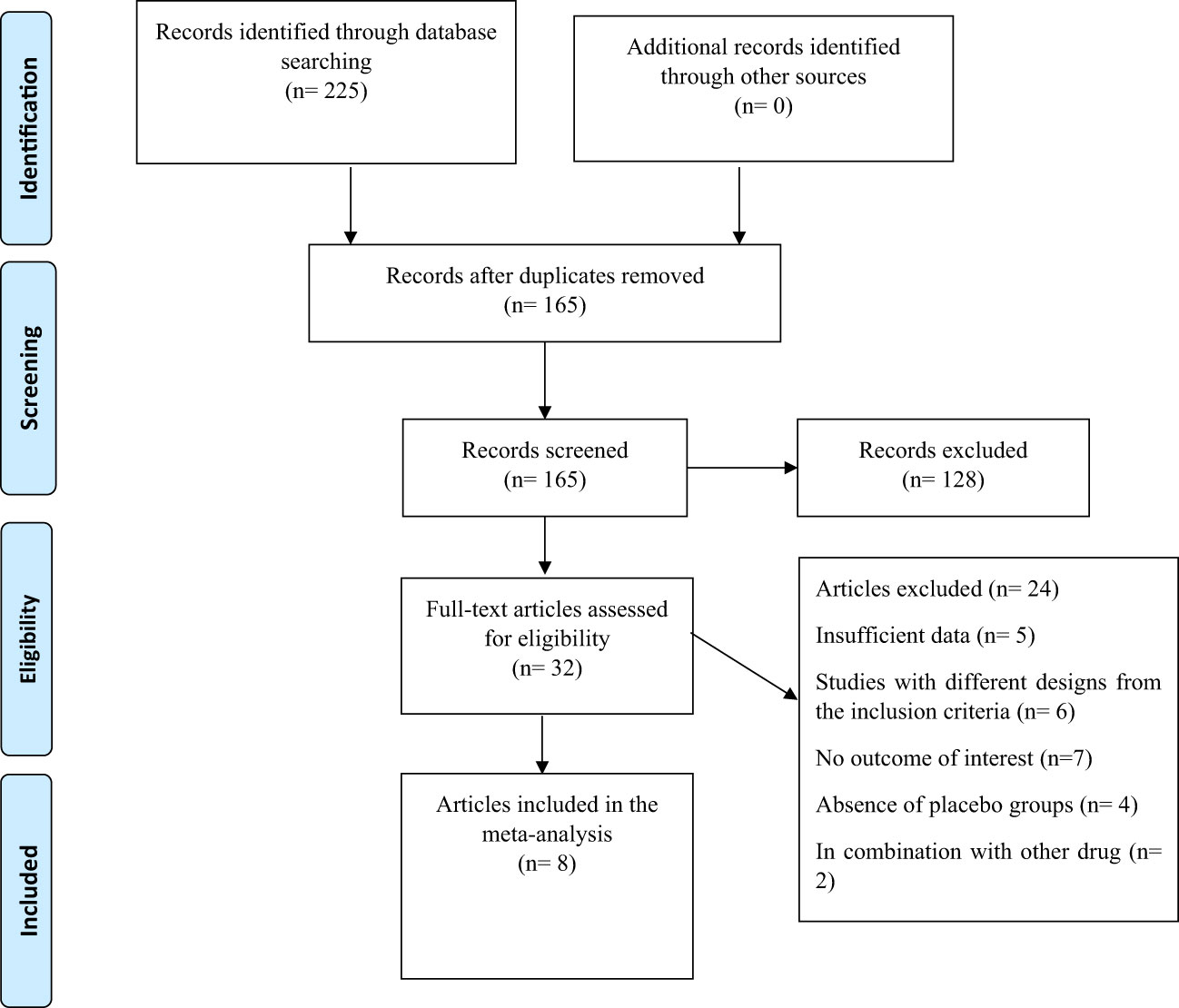
Figure 1 Flow chart of the study, including identification, screening, eligibility, and the final sample included.
The features of the pooled articles are shown in Table 2. Our surveys reveal that one study have been carried out in Germany and other studies were conducted in a multi-center or multi-country manner. Every study was released between 2018 and 2022. The all of the research utilized a parallel design and follow up intervention ranged from 26 to 72 weeks. The mean age and percentage of male participants ranged from 45 to 63.4 years and 31-62%, respectively, at the baseline. Six studies conducted on individuals with type 2 diabetes and two studies on participants living with obesity. Also, intervention doses ranged from 1 to 10 mg subcutaneously.
The findings of the evaluation of the eligible studies’ quality are shown in Table 1. Additionally, a score of 9.6 (very good quality) was determined after the NutriGrade score system was used to assess the quality of the current meta-analysis.
Pooled findings from the random-effects model indicated that body weight (weighted mean difference (WMD): -11.34 kg, 95% confidence interval (CI): -12.79 to -9.88, P< 0.001), body mass index (BMI) (WMD: -3.11 kg/m2, 95% CI: -4.36 to -1.86, P< 0.001), and waist circumference (WC) (WMD: -7.24 cm, 95% CI -10.12 to -4.36, P< 0.001) were significantly reduced after subcutaneous Tirzepatide compared to control group. Also, subgroup results showed that changes in weight loss following subcutaneous Tirzepatide at a dose of 15 mg (WMD: -13.02 kg, 95% CI: -15.36 to -10.69, I2 = 96.1%) were higher compared to other doses (10 mg (WMD: -11.66 kg, 95% CI: -14.16 to -9.16, I2 = 96.5%), 5 mg (WMD: -9.08 kg, 95% CI: -10.75 to -7.42, I2 = 92.2%)). Furthermore, significant heterogeneity was found among the studies for weight (Cochran Q test, P< 0.001, I2 = 96.8%), BMI (Cochran Q test, P< 0.001, I2 = 94.2%), and WC (Cochran Q test, P< 0.001, I2 = 89.2%; Figures 2–5).
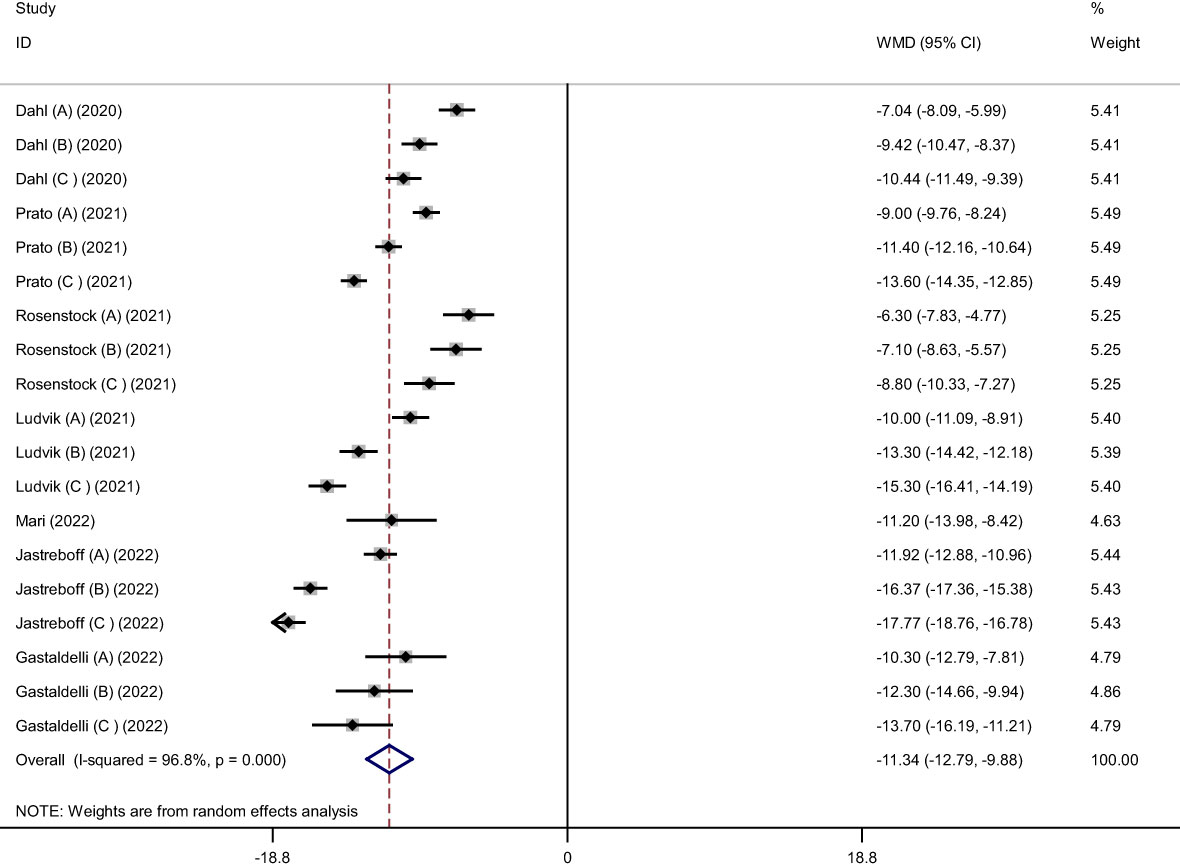
Figure 2 Forest plot of randomized controlled trials investigating the effects of Tirzepatide on weight (kg).
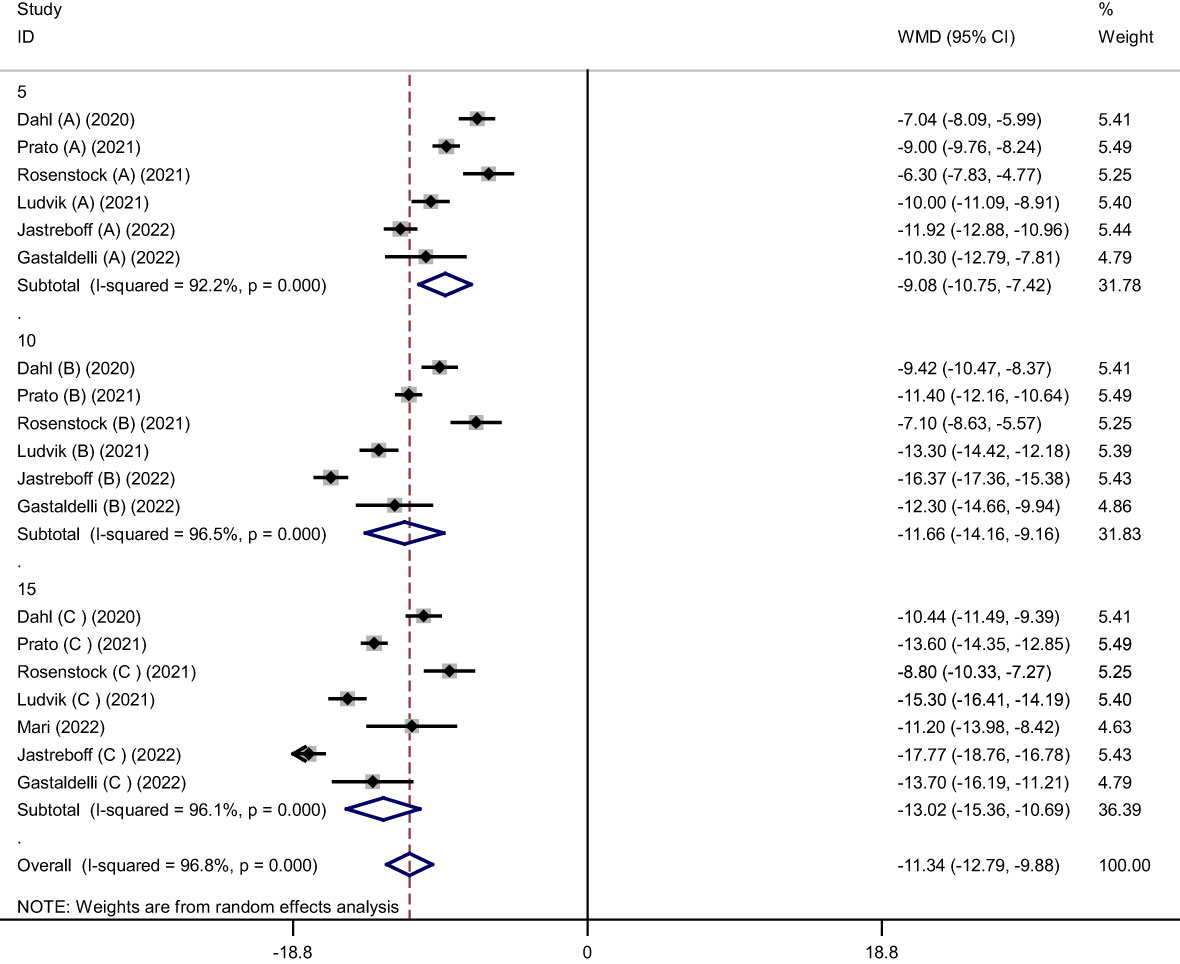
Figure 3 Forest plot of randomized controlled trials investigating the effects of Tirzepatide on weight based on dose of intervention (mg).

Figure 4 Forest plot of randomized controlled trials investigating the effects of Tirzepatide on body mass index (BMI) (kg/m2).
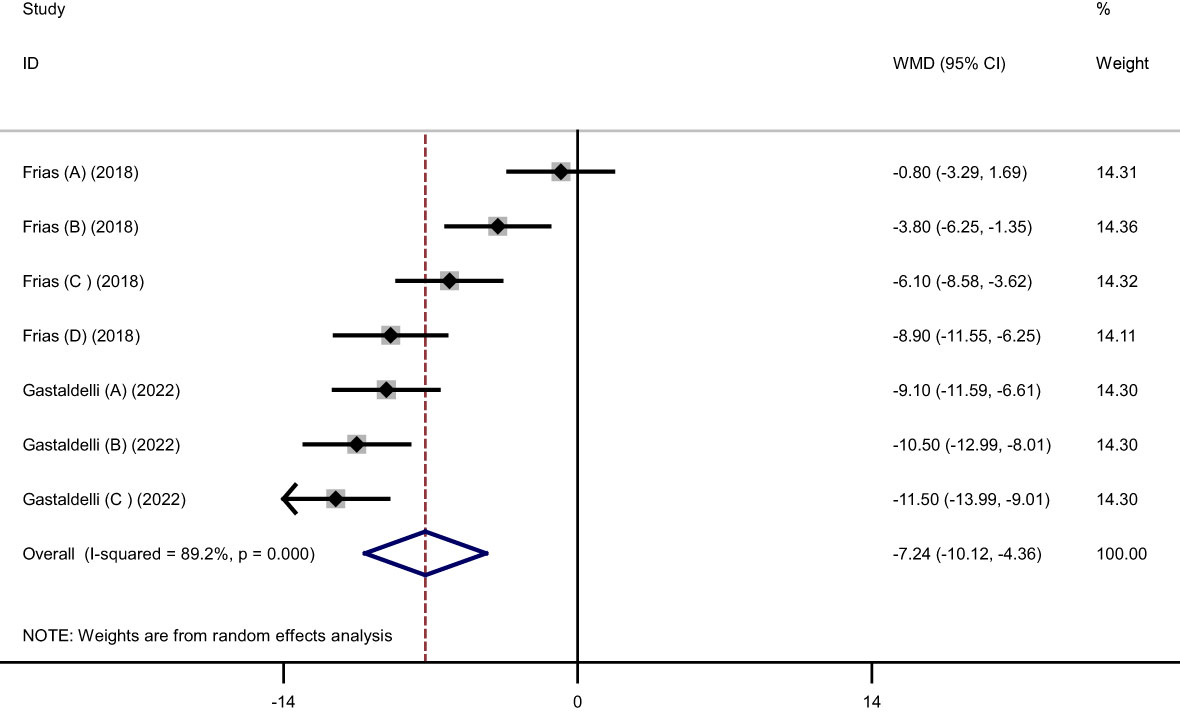
Figure 5 Forest plot of randomized controlled trials investigating the effects of Tirzepatide on waist circumference (WC) (cm).
Meta-regression between subcutaneous Tirzepatide and absolute mean differences in body weight based on dosage, baseline of mean age and BMI, and duration of intervention was performed. There was a significant relationship between duration of intervention ( ), baseline of mean BMI (
), baseline of mean BMI ( ) and dose of intervention (*) with changes in body weight (coefficient (Coef)= -0.1904459, P= 0.001 for
) and dose of intervention (*) with changes in body weight (coefficient (Coef)= -0.1904459, P= 0.001 for  ; Coef= -0.990465, P= 0.004 for
; Coef= -0.990465, P= 0.004 for  ; Coef= -0.3924448, P= 0.023 for *) (Supplementary Figure 1).
; Coef= -0.3924448, P= 0.023 for *) (Supplementary Figure 1).
We gradually removed each trial from the analysis to determine the impact of each article on the pooled effect size for the levels of weight, BMI, and WC. The robustness of the findings was demonstrated by the leave-one-out sensitivity analysis (Supplementary Figure 2).
Inspecting the funnel visually to determine publication bias, the Egger’s tests for weight (P= 0.326), BMI (P= 0.056), and WC (P= 0.293) revealed no indication of bias (Supplementary Figure 3).
As far as we are aware, this is the first research to thoroughly assess and analyze the results of intervention trials on the impacts of subcutaneous Tirzepatide on weight reduction. According to the study’s findings, subcutaneous Tirzepatide significantly reduced weight, BMI, and waist circumference in the intervention group compared to the control group. Additionally, analyses revealed that parameters were particularly impacted by the dosage and duration of the Tirzepatide drug.
Tirzepatide has been approved by FDA in May 2022. Since it has revealed significant improvement in glycemic control and weight loss compared to other alternatives, currently it can now be recommended off-label for the treatment of obesity and implemented as a second-line T2DM medication, maximizing similar advantages that are reported with established GLP-1 medications (43, 44). On this matter, The SURPASS clinical trial program’s goal in this area was to examine the effectiveness and safety of tirzepatide in T2DM patients. In comparison to other well-known GLP-1 agonists used for weight loss management, such as Semaglutide, Tirzepatide has a strong effect on reducing glucose and body weight, according to the SURPASS Pre-clinical studies, 4-week-long phase 1 and 26-week-long phase 2 clinical trials. Tirzepatide showed dose-dependent benefits on the decrease of HbA1c and weight greater than 2.4% and 11.3 kg, respectively, in phase 1 and phase 2 studies (45). Moreover, a study conducted by Heise et al. reported a significant reduction in weight after intervention with Tirzepatide versus placebo or Semaglutide after 28 weeks in T2DM adults aged 20 to 74 years. Also due to the potent glucose-lowering effects of Tirzepatide, a significant reduction in glucagon secretion, as well as improvements in insulin sensitivity and β-cell function were observed (30). Consistent with the previous studies, Frias et al. investigated the efficacy and safety of Tirzepatide after 26 weeks among T2DM patients aged 18–75 years. They found that Tirzepatide caused greater reductions in HbA1, BMI, and WC compared to placebo. Also, greater dose of Tirzepatide had a higher efficacy on HbA1 control and weight loss (36).
Del Prato et al. assessed the efficacy and safety of Tirzepatide after 52 weeks on T2DM adults with high cardiovascular risk aged equal to or more than 18 years. They showed that tirzepatide improved weight at the end of the intervention compared to baseline. Also, the amount of weight loss was higher in the higher dose of tirzepatide. Regarding the cardiovascular perspective, both the risk and numbers of cardiovascular events were significantly lower among treatment groups (29). Another study conducted by Wilson et al. compared the effects of subcutaneous Tirzepatide versus placebo added to titrated insulin Glargine after 40 weeks on T2DM patients with mean age of 60 years. There was a significant reduction in weight after the intervention, especially with a greater dose of Tirzepatide. Also, changes in weight were higher in Tirzepatide receivers compared to placebo receivers (46). Singh et al. compared the effects of Tirzepatide versus insulin glargine after 52 weeks on T2DM patients with high cardiovascular risk aged equal to or more than 18 years. The improvement in weight changes between treatment groups suggests that Tirzepatide can be considered a good option (47).
Furthermore, a study conducted by Jastreboff et al. evaluated the efficacy and safety of Tirzepatide after 72 weeks on participants living with obese or overweight without diabetes aged equal to or more than 18 years. The results showed that changes in weight and WC following Tirzepatide at a greater dose of 15 mg were higher compared to its lower doses and placebo (32). On the other hand, Rosenstock et al. found that the dose-dependent effects of intervention with Tirzepatide on weight loss were higher after 40 weeks compared to baseline and placebo in T2DM patients aged 54·1 years. Also, due to the robust improvements in controlling glycemia and body weight, without increased risk of hypoglycemia, authors proposed the use of Tirzepatide as a potential monotherapy for T2DM treatment (33).
Each drug may cause unwanted side effects in addition to its required effects. The studies list stomach pain as one of the side effects that are most frequently reported, along with other less frequent side effects like gaseous stomach pain, heartburn, recurrent fever, skin itching, rash, redness, fullness in the stomach, swelling of the face, throat, and tongue, vomiting, and yellow eyes or skin.
In a meta-analysis study on adults living with obesity and no diabetes, Liraglutide as a GLP-1 receptor agonist reduced body weight (WMD −3.35 kg; 95%CI −4.65 to −2.05), and BMI (WMD −1.45 kg/m2; 95%CI −1.98 to −0.91) in comparison to placebo (48). In another meta-analysis in 2021, Liraglutide caused a weight loss of -4.19 kg (95%CI, -4.84 to -3.55) compared to the control group (49). In the case of another GLP-1 agonist, a recent meta-analysis (including 4 clinical trial studies) was conducted to investigate the effectiveness of subcutaneous Semaglutide compared to placebo for weight loss in adults with obesity and without diabetes, and it was shown that this drug causes weight loss by − 11.62 kg (95% CI: −13.03 to −10.21) (50). These findings were also reported in another meta-analysis for weight and body mass index (decrease by -4.48 kg/m2) (51). However, in another meta-analysis study in diabetic subjects (52), Semaglutide reduced weight by WMD: -2.73 kg and -4.09 kg, for 0.5 mg and 1 mg, respectively. Overall, the efficacy of Tirzepatide appears to be much greater than Liraglutide, but comparing it with Semaglutide requires more study to confirm the findings. Anti-diabetic medications were divided into three categories depending on how well they helped people lose weight. We defined a weak impact as a weight loss of less than 3.2% of one’s starting weight, a moderate effect as a weight loss between 3.2% and 5%, and a strong effect as a weight loss of more than 5%. The majority of writers discovered that metformin caused a small decrease in body weight (less than 3.2% of initial weight in all trials) (53). Acarbose reduces intestinal glucose absorption, which results in a decrease in daily calorie intake, however the effect on body weight is minimal (54). SGLT-2 I causes a statistically significant decrease in body weight and, in a dose-dependent manner, increases urine glucose excretion (55). The majority of weight loss caused by SGLT-2i is fat loss rather than lean mass loss, with visceral fat loss being somewhat larger than subcutaneous fat loss in T2D patients (55). Except for empagliflozin, which causes a mild weight reduction, SGLT-2 I has a moderate effect on weight loss (56). The anti-diabetic drug class that has demonstrated the highest efficacy in terms of weight loss is GLP1-RA (9). Exenatide’s and dulaglutide’s weight-related effects were low and minor, respectively, but liraglutide—the only GLP1-RA authorized for the treatment of obesity—tirzepatide, and semaglutide had a significant weight-related effect (57). A potential treatment option for weight loss is the dual GIP and GLP-1 receptor agonist, LY3298176, which outperforms dulaglutide in terms of effectiveness. Intriguing results with regard to lowering waist circumference have also been seen with this medication (36).
The present study has several major strengths. First, this is the first systematic review and meta-regression analysis investigating the effects of subcutaneous Tirzepatide on weight loss. Second, the causal inference of our results is strong due to the design of meta-regression analysis based on eligible clinical trials. Third, we considered the Cochrane Bias Methods to minimize systematic errors and achieve reliable estimates of effects. Forth, the caliber of the included papers was fairly high and the major findings held up well after sensitivity analyses and Egger’s test. Finally, the results of our study may contribute to determining a medication that specialists should take in mind and consider at least for patients who are highly at risk for continued abnormal weight gain or obesity progression.
However, our study had some limitations that jeopardized the extraction of robust conclusions. Clinically and statistically significant heterogeneities were found. These may be explained by the differences in the intervention-specific factors (e.g., type, dose, administration route, and duration of drugs) and weight-specific factors (e.g., age, sex, physiology, genetics, familial history, race/ethnicity, physical activity, socioeconomic status, dietary intakes, and drug, tobacco, or alcohol consumption) (58). Nonetheless, we attempted to identify some possible sources of heterogeneity in data by performing a subgroup analysis.
Taken together, Tirzepatide is a novel medication approved for treating T2DM with the extra benefit of weight loss.
In general, the present systematic review and meta‐regression analysis demonstrated that subcutaneous Tirzepatide may be able to significantly improve body weight, BMI, and WC in the intervention group compared to the control. The beneficial effect seemed greatest in those trials with higher doses and duration of Tirzepatide. Multidimensional weight loss management, such as combination with other related medications and lifestyle interventions might optimize the therapeutic effect of Tirzepatide for overweight or obese people. Further homogeneous and well-powered clinical trials on the appropriate dose and duration of Tirzepatide medication for different ranges of overweight persons are required to confirm our findings and increase our understanding of the effects of subcutaneous Tirzepatide on weight loss.
The datasets presented in this article are not readily available due to privacy/ethical restrictions. Requests to access the datasets should be directed to bW9oYW1tYWRoYXNzYW5zb2hvdWxpQGdtYWlsLmNvbQ==.
PR, AH, and MS contributed in conception, design, and statistical analysis. MS, AH, NM, SB, and PR contributed in data collection and manuscript drafting. MS and AH supervised the study. All authors approved the final version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1230206/full#supplementary-material
WHO: World health organization, DALYs: Disability-adjusted life years, SARS-CoV-2: Severe acute respiratory syndrome coronavirus-2, GLP-1: Glucagon-like peptide-1, GIP: Gastric inhibitory polypeptide, FDA: Food and Drug Administration, T2DM: Type 2 diabetes mellitus, WMD: Weighted mean difference, CI: Confidence interval, BMI: Body mass index, WC: Waist circumference, Coef: Coefficient, HbA1c: Hemoglobin A1C, ADA: American Diabetes Association.
1. Jaca A, Iwu C, Durão S, Onyango AW, Wiysonge CS. Understanding the underlying drivers of obesity in Africa: a scoping review protocol. BMJ Open (2020) 10(11):. doi: 10.1136/bmjopen-2020-040940
2. Pugliese G, Liccardi A, Graziadio C, Barrea L, Muscogiuri G, Colao A. Obesity and infectious diseases: pathophysiology and epidemiology of a double pandemic condition. Int J Obes (2005) 46(3):449–65. doi: 10.1038/s41366-021-01035-6
3. Loos RJ, Yeo GS. The genetics of obesity: from discovery to biology. Nat Rev (2022) 23(2):120–33. doi: 10.1038/s41576-021-00414-z
4. Oladeji O, Zhang C, Moradi T, Tarapore D, Stokes AC, Marivate V, et al. Monitoring information-seeking patterns and obesity prevalence in Africa with internet search data: observational study. JMIR Public Health Surveillance (2021) 7(4):. doi: 10.2196/24348
5. Kassie AM, Abate BB, Kassaw MW. Prevalence of overweight/obesity among the adult population in Ethiopia: a systematic review and meta-analysis. BMJ Open (2020) 10(8):e039200. doi: 10.1136/bmjopen-2020-039200
6. Chavda VP, Ajabiya J, Teli D, Bojarska J, Apostolopoulos VJM. Tirzepatide, a new era of dual-targeted treatment for diabetes and obesity: a mini-review. Molecules (2022) 27(13):4315. doi: 10.3390/molecules27134315
7. Baxter J, Armijo PR, Flores L, Krause C, Samreen S, Tanner T. Updates on monogenic obesity in a multifactorial disease. Obesity Surg (2019) 29(12):4077–83. doi: 10.1007/s11695-019-04200-z
8. Grundy SM. Multifactorial causation of obesity: implications for prevention. Am J Clin Nutr (1998) 67(3 Suppl):563s–72s. doi: 10.1093/ajcn/67.3.563S
9. Cignarella A, Busetto L, Vettor R. Pharmacotherapy of obesity: An update. Pharmacol Res (2021) 169:105649. doi: 10.1016/j.phrs.2021.105649
10. Ryan DH, Syndrome M. Next generation antiobesity medications: setmelanotide, semaglutide, tirzepatide and bimagrumab: what do they mean for clinical practice? J Obesity Metabol Syndrome (2021) 30(3):196. doi: 10.7570/jomes21033
11. Gasbjerg LS, Bergmann NC, Stensen S, Christensen MB, Rosenkilde MM, Holst JJ, et al. Evaluation of the incretin effect in humans using GIP and GLP-1 receptor antagonists. Peptides (2020) 125:170183. doi: 10.1016/j.peptides.2019.170183
12. Fisman EZ, Tenenbaum A. The dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist tirzepatide: a novel cardiometabolic therapeutic prospect. Cardiovascular Diabetol (2021) 20(1):1–5. doi: 10.1186/s12933-021-01412-5
13. Michałowska J, Miller-Kasprzak E, Bogdański P. Incretin hormones in obesity and related cardiometabolic disorders: The clinical perspective. Nutrients (2021) 13(2):351. doi: 10.3390/nu13020351
14. Del Prato S, Gallwitz B, Holst JJ, Meier JJ. The incretin/glucagon system as a target for pharmacotherapy of obesity. Obesity Rev (2022) 23(2):. doi: 10.1111/obr.13372
15. Karagiannis T, Avgerinos I, Liakos A, Del Prato S, Matthews DR, Tsapas A, et al. Management of type 2 diabetes with the dual GIP/GLP-1 receptor agonist tirzepatide: a systematic review and meta-analysis. Diabetologia (2022) 65:1–11. doi: 10.1007/s00125-022-05715-4
16. Samms RJ, Coghlan MP, Sloop KW. How may GIP enhance the therapeutic efficacy of GLP-1? Trends Endocrinol Metab (2020) 31(6):410–21. doi: 10.1016/j.tem.2020.02.006
17. Thomas MK, Nikooienejad A, Bray R, Cui X, Wilson J, Duffin K, et al. Dual GIP and GLP-1 receptor agonist tirzepatide improves beta-cell function and insulin sensitivity in type 2 diabetes. J Clin Endocrinol Metabol (2021) 106(2):388–96. doi: 10.1210/clinem/dgaa863
18. Frederick MO, Boyse RA, Braden TM, Calvin JR, Campbell BM, Changi SM, et al. Kilogram-scale GMP manufacture of tirzepatide using a hybrid SPPS/LPPS approach with continuous manufacturing. Organic Process Res Dev (2021) 25(7):1628–36. doi: 10.1021/acs.oprd.1c00108
19. Tirzepatide. LiverTox: clinical and research information on drug-induced liver injury. Bethesda (MD: National Institute of Diabetes and Digestive and Kidney Diseases (2012).
20. Wang L. Designing a dual GLP-1R/GIPR agonist from tirzepatide: comparing residues between tirzepatide, GLP-1, and GIP. Drug Design Dev Ther (2022) 16:1547–59. doi: 10.2147/DDDT.S358989
21. Kaneko S. Tirzepatide: A novel, once-weekly dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes. TouchREVIEWS Endocrinol (2022) 18(1):10–9. doi: 10.17925/EE.2022.18.1.10
22. Tall Bull S, Nuffer W, Trujillo JM. Tirzepatide: A novel, first-in-class, dual GIP/GLP-1 receptor agonist. J Diabetes its Complications (2022) 36(12):108332. doi: 10.1016/j.jdiacomp.2022.108332
23. Al-Horani RA, Chedid M. Tirzepatide: A new generation therapeutic for diabetes type 2. Endocrine Metab Immune Disord Drug Targets (2022) 23:36200219. doi: 10.2174/1871530322666221004151212
24. Willard FS, Douros JD, Gabe MB, Showalter AD, Wainscott DB, Suter TM, et al. Tirzepatide is an imbalanced and biased dual GIP and GLP-1 receptor agonist. JCI Insight (2020) 5(17):. doi: 10.1172/jci.insight.140532
25. Frías JP, Davies MJ, Rosenstock J, Pérez Manghi FC, Fernández Landó L, Bergman BK, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Eng J Med (2021) 385(6):503–15. doi: 10.1056/NEJMoa2107519
26. Lu VB, Gribble FM, Reimann F. Nutrient-induced cellular mechanisms of gut hormone secretion. Nutrients (2021) 13(3):883. doi: 10.3390/nu13030883
27. Bhagavathula AS, Vidyasagar K, Tesfaye W. Efficacy and safety of tirzepatide in patients with type 2 diabetes mellitus: a systematic review and meta-analysis of randomized phase II/III trials. Pharmaceuticals (2021) 14(10):991. doi: 10.3390/ph14100991
28. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic Rev (2015) 4(1):1. doi: 10.1186/2046-4053-4-1
29. Del Prato S, Kahn SE, Pavo I, Weerakkody GJ, Yang Z, Doupis J, et al. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): a randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet (London England) (2021) 398(10313):1811–24. doi: 10.1016/S0140-6736(21)02188-7
30. Heise T, Mari A, DeVries JH, Urva S, Li J, Pratt EJ, et al. Effects of subcutaneous tirzepatide versus placebo or semaglutide on pancreatic islet function and insulin sensitivity in adults with type 2 diabetes: a multicentre, randomised, double-blind, parallel-arm, phase 1 clinical trial. Lancet Diabetes Endocrinol (2022) 10(6):418–29. doi: 10.1016/S2213-8587(22)00085-7
31. Dahl D, Onishi Y, Norwood P, Huh R, Bray R, Patel H, et al. Effect of subcutaneous tirzepatide vs placebo added to titrated insulin glargine on glycemic control in patients with type 2 diabetes: the SURPASS-5 randomized clinical trial. Jama (2022) 327(6):534–45. doi: 10.1001/jama.2022.0078
32. Jastreboff AM, Aronne LJ, Ahmad NN, Wharton S, Connery L, Alves B, et al. Tirzepatide once weekly for the treatment of obesity. New Engl J Med (2022) 387:205–16. doi: 10.1056/NEJMoa2206038
33. Rosenstock J, Wysham C, Frías JP, Kaneko S, Lee CJ, Fernández Landó L, et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet (London England) (2021) 398(10295):143–55. doi: 10.1016/S0140-6736(21)01324-6
34. Gastaldelli A, Cusi K, Fernández Landó L, Bray R, Brouwers B, Rodríguez Á. Effect of tirzepatide versus insulin degludec on liver fat content and abdominal adipose tissue in people with type 2 diabetes (SURPASS-3 MRI): a substudy of the randomised, open-label, parallel-group, phase 3 SURPASS-3 trial. Lancet Diabetes Endocrinol (2022) 10(6):393–406. doi: 10.1016/S2213-8587(22)00070-5
35. Ludvik B, Giorgino F, Jódar E, Frias JP, Landó LF, Brown K, et al. Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): a randomised, open-label, parallel-group, phase 3 trial. Lancet (2021) 398(10300):583–98. doi: 10.1016/S0140-6736(21)01443-4
36. Frias JP, Nauck MA, Van J, Kutner ME, Cui X, Benson C, et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet (2018) 392(10160):2180–93. doi: 10.1016/S0140-6736(18)32260-8
37. Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Assessing risk of bias in a randomized trial. In: Cochrane handbook for systematic reviews of interventions (2019). p. 205–28.
38. Schwingshackl L, Knüppel S, Schwedhelm C, Hoffmann G, Missbach B, Stelmach-Mardas M, et al. Perspective: NutriGrade: A scoring system to assess and judge the meta-evidence of randomized controlled trials and cohort studies in nutrition research. Adv Nutr (2016) 7(6):994–1004. doi: 10.3945/an.116.013052
39. Higgins J. Cochrane handbook for systematic reviews of interventions. The Cochrane Collaboration (2011).
40. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Method (2005) 5(1):13. doi: 10.1186/1471-2288-5-13
41. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
42. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629
44. Ali R, Virendra SA, Chawla PA. Bumps and humps in the success of Tirzepatide as the first GLP1 and GIP receptor agonist. Health Sci Rev (2022) 4:100032. doi: 10.1016/j.hsr.2022.100032
45. Min T, Bain SC. The role of tirzepatide, dual GIP and GLP-1 receptor agonist, in the management of type 2 diabetes: the SURPASS clinical trials. Diabetes Therapy (2021) 12(1):143–57. doi: 10.1007/s13300-020-00981-0
46. Wilson JM, Lin Y, Luo MJ, Considine G, Cox AL, Bowsman LM, et al. The dual glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 receptor agonist tirzepatide improves cardiovascular risk biomarkers in patients with type 2 diabetes: A p ost hoc analysis. Diab Obesity Metabol (2022) 24(1):148–53. doi: 10.1111/dom.14553
47. Singh M. In type 2 diabetes with increased CV risk, tirzepatide reduced HbA1c vs. glargine at 52 wk. Ann Int Med (2022) 175(3):JC34. doi: 10.7326/J22-0009
48. Barboza JJ, Huamán MR, Melgar B, Diaz-Arocutipa C, Valenzuela-Rodriguez G, Hernandez AV. Efficacy of liraglutide in non-diabetic obese adults: a systematic review and meta-analysis of randomized controlled trials. J Clin Med (2022) 11(11):2998. doi: 10.3390/jcm11112998
49. Moon S, Lee J, Chung HS, Kim YJ, Yu JM, Yu SH, et al. Efficacy and safety of the new appetite suppressant, liraglutide: a meta-analysis of randomized controlled trials. Endocrinol Metab (2021) 36(3):647–60. doi: 10.3803/EnM.2020.934
50. Arastu N, Cummins O, Uribe W, Nemec EC. Efficacy of subcutaneous semaglutide compared to placebo for weight loss in obese, non-diabetic adults: a systematic review & meta-analysis. Int J Clin Pharm (2022) 44(4):852–9. doi: 10.1007/s11096-022-01428-1
51. Zhong P, Zeng H, Huang M, Fu W, Chen Z. Efficacy and safety of once-weekly semaglutide in adults with overweight or obesity: A meta-analysis. Endocrine (2022) 75(3):718–24. doi: 10.1007/s12020-021-02945-1
52. Zhong P, Zeng H, Huang M, He G, Chen Z. Efficacy and safety of subcutaneous and oral semaglutide administration in patients with type 2 diabetes: A meta-analysis. Front Pharmacol (2021) 12:695182. doi: 10.3389/fphar.2021.695182
53. Stumvoll M, Nurjhan N, Perriello G, Dailey G, Gerich JE. Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. New Engl J Med (1995) 333(9):550–4. doi: 10.1056/NEJM199508313330903
54. Wolever T, Chiasson J, Josse R, Hunt J, Palmason C, Rodger N, et al. Small weight loss on long-term acarbose therapy with no change in dietary pattern or nutrient intake of individuals with non-insulin-dependent diabetes. Int J Obes (1997) 21(9):756–63. doi: 10.1038/sj.ijo.0800468
55. Ferrannini E, Muscelli E, Frascerra S, Baldi S, Mari A, Heise T, et al. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest (2014) 124(2):499–508. doi: 10.1172/JCI72227
56. Bolinder J, Ljunggren Ö, Johansson L, Wilding J, Langkilde A, Sjöström C, et al. Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. Diabetes Obes Metab (2014) 16(2):159–69. doi: 10.1111/dom.12189
57. Nuffer WA, Trujillo JM. Liraglutide: a new option for the treatment of obesity. Pharmacotherapy: J Hum Pharmacol Drug Ther (2015) 35(10):926–34. doi: 10.1002/phar.1639
Keywords: tirzepatide, obesity, overweight, glycemic control, weight loss
Citation: Rohani P, Malekpour Alamdari N, Bagheri SE, Hekmatdoost A and Sohouli MH (2023) The effects of subcutaneous Tirzepatide on obesity and overweight: a systematic review and meta‐regression analysis of randomized controlled trials. Front. Endocrinol. 14:1230206. doi: 10.3389/fendo.2023.1230206
Received: 28 May 2023; Accepted: 19 July 2023;
Published: 09 August 2023.
Edited by:
Claire Joanne Stocker, Aston University, United KingdomReviewed by:
Raffaele Carraro, La Princesa University Hospital, SpainCopyright © 2023 Rohani, Malekpour Alamdari, Bagheri, Hekmatdoost and Sohouli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammad Hassan Sohouli, bW9oYW1tYWRoYXNzYW5zb2hvdWxpQGdtYWlsLmNvbQ==
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.