- Digital Women's Health Initiative, Mary Elizabeth Conover Foundation, Inc., Tysons, VA, United States
Introduction
An “old wives’ tale” is a traditional truth not based on fact (1). The term is now an outmoded and disrespectful vernacular about women (2). Many spurious truths about menopause have become culturally embedded. To improve understanding and provide better care, a new perspective, and more accurate biologic terminology is needed for “the menopause.”
A recent report in The New York Times, “Women have been misled about menopause,” (3) demonstrates this subject remains a difficult conversation (4, 5). Importantly, women younger than 45 who experience signs and symptoms of menopause have a metabolic derangement associated with increased morbidity and mortality (6, 7). The typical midlife hormonal transition women experience, in many cases, adversely impacts their quality of life and creates economic burdens (8, 9).
The truth usually lives between arbitrary categories (10). The categorical and absolute terms “premenopausal” and “postmenopausal” are problematic, misleading, and sources of confusion. The midlife physiologic hormonal transition women experience is a complex process, gradually evolving and constantly in flux. This transition takes years to develop and is not two static states, one before and one after the last menses. Categorical thinking treats the categorical structure imposed as if the categories are static states (11). This way of thinking prevents clear communication and effective management. Another unhelpful way of categorical thinking is viewing the midlife physiologic hormonal transition as purely dichotomous, as either 1) a natural event needing no medical intervention or 2) a disease needing active intervention (11).
The social experience of the midlife transition in women related to the cessation of menses is culturally mediated (12). Menopause is a fascinating biological phenomenon that has puzzled scientists for decades. Women live many years after their reproductive capacity has ended (13). Researchers still debate the nature of the evolutionary pressure that caused menopause to develop in women. Essentially, this midlife transition has adaptive advantages by redirecting resources away from the dangers of reproduction at a later age and toward other essential and less risky needs of family and society, such as being a source of wisdom (13–15). Regardless of the exact reasons, post-reproductive longevity highlights the complexity of aging and the importance of understanding this phenomenon in greater depth. This midlife physiologic transition is an integral part of a woman’s life and has played a vital role in the survival and evolution of our species.
The essential biology of this physiologic midlife transition in women is a waning of ovarian endocrine function. The biological basis for the timing of this normal transition is the decline and eventual exhaustion of the number of potentially functional ovarian primordial follicles (16). The essential endocrinology involved is transitioning away from high average serum levels of 17-beta estradiol (E2) during reproductive years. These high levels support fertility and maintain health and resilience when menses are regular and ovulatory. The midlife physiologic transition is toward dramatically lower E2 levels in the later years, levels which are known to contribute to increased health risks. While menopause is the permanent cessation of menses and reproductive capacity, there is much more to it concerning public health (17).
Accumulating evidence begs an essential public health question. “Is there a safe and effective selection process and treatment regimen to spare those women at risk of the detrimental effects of low E2 in later life?” Evidence is clear, extremely low E2 levels increase the risk for some women. For example, there is a 2.5-fold increase in hip and vertebral fractures in older women with total E2 levels less than 5.0 pg/ml. Men have a similar association between E2 levels and fractures (18). Intriguingly, even minimal increases in E2 serum concentrations have a proven beneficial effect on bone mineral density in menopausal women, with little effect on endometrial proliferation (19, 20). These observations suggest a potentially safe therapeutic window of low physiologic E2 replacement may exist in other areas of a woman’s health. This same therapeutic window of low doses of E2, proven to improve bone mineral density, could theoretically also improve health for menopausal women regarding their cardiovascular health, central nervous system health, mood, and related cosmetic benefits to skin and hair (21, 22).
Accumulating evidence begs another essential public health question. “Is there a unifying biologic terminology to describe the physiology of ovarian hormone function across a woman’s lifespan?” The answer is clearly yes. More than 80 years ago, Fuller Albright, an endocrinologist at Harvard University, provided us with the scientific language for this purpose (23). He used the term “Primary Ovarian Insufficiency” to first define the scientific mechanism of failure of pubertal development in Turner Syndrome. His work showed that the pathologic defect was in the ovaries, not the hypothalamus or pituitary. Some women experience normal puberty and then develop amenorrhea due to Primary Ovarian Insufficiency before age 40 (7). In scientific terms, women in the midlife physiologic hormone transition are experiencing “Physiologic Primary Ovarian Insufficiency.” This midlife transition is a process, not an event.
Transitioning to a scientific perspective
P-POI (Physiologic POI) is a transition away from reproduction risks. Menopause is a categorical, absolute state. P-POI is a transition providing women the freedom to undertake other contributions to family and society based on accumulated wisdom. Speaking truth charts a path to being well. As noted, there is confusion and misinformation about the role of estrogen in women’s health and, specifically, the management of E2 deficiency in women across the lifespan (3, 5). Notably, the scientific community recognizes many biological effects of the most potent endogenous estrogenic sex steroid hormone, E2 (24). These hormonal effects go beyond reproduction and carry broad and profound public health implications.
A hallmark of science is the ability to self-correct. Deviation from seeking the truth as the priority seriously impairs the ability of science to self-correct (25). This self-correction may take decades in some cases and requires new evidence and new ways of thinking. The process does not happen by default. Emphasizing the political nature of progress, another example of categorical thinking impairs progress in social justice and health (26). Correcting misunderstandings regarding the effects of E2 deficiency on public health requires clear insight into the best evidence about E2 and creating more and better evidence in the future. Getting this evidence will require a clear and unifying global strategy for women’s health (27).
The physiologic midlife transition to menopause is a state of low serum E2, and early menopause is associated with significant morbidity and early mortality (28, 29). A few prospective population-based cohort studies provide convincing evidence that women with early onset menopause, and the associated E2 deficiency, have 1) a shorter life expectancy, 2) increased risk of type II diabetes, 3) adverse effects on cognitive function, 4) significant correlation between age at menopause and age at diagnosis of dementia, and 5) a significant correlation between age at menopause and age at death (6, 30, 31). This midlife transition to ultra-low serum E2 levels is associated with significant cardiovascular morbidity and mortality. Prospective epidemiologic studies correlate earlier menopause with earlier death, ischemic stroke, and a combined effect of earlier menopause and high-risk factors on death and cardiovascular disease, osteoporosis, and fragility fracture (32–34).
Physiologic Primary Ovarian Insufficiency:
A continuum of women’s health across the lifespan
As noted above, in 1942, Fuller Albright used the term “Primary Ovarian Insufficiency” when he first reported a condition involving cessation of normal ovarian function in young women (23). He concluded that the defect in function in these patients was primarily in the ovaries when his bioassay revealed high urinary FSH levels. Endocrinologists generally classify disorders of endocrine glands as “primary” when the defect within the gland renders the gland unresponsive to stimulation. On the other hand, the central component of the axis (hypothalamus and pituitary) functions normally.
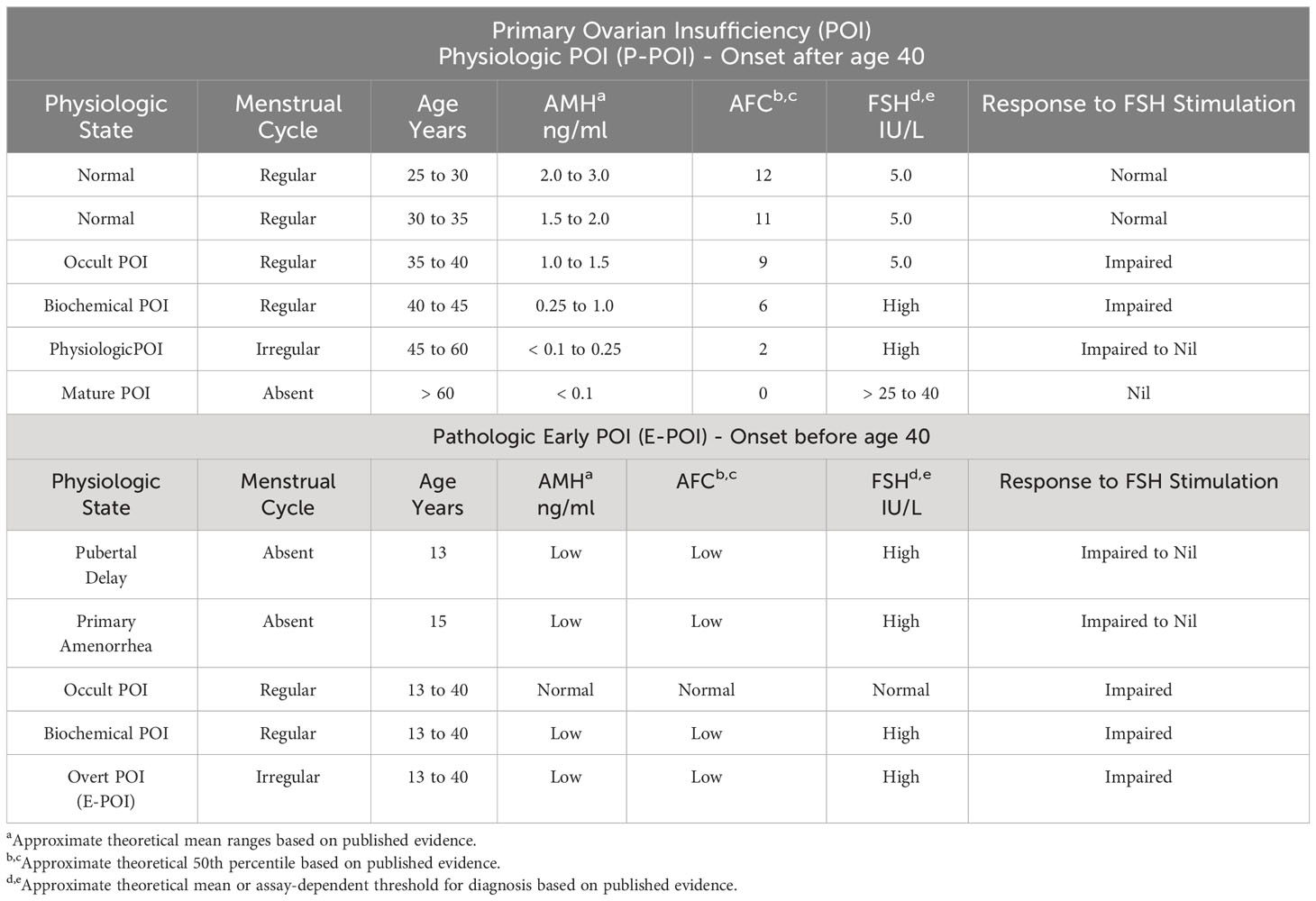
Hence, in Primary Ovarian Insufficiency (POI), serum gonadotropin levels are high due to the absence of feedback inhibition from the ovary. This same biology is the situation in women undergoing the age-related physiologic transition to waning ovarian function. From this perspective, the midlife transition to the cessation of menses in women is a natural and physiologic process of “Physiologic Primary Ovarian Insufficiency” (P-POI), programmed by the decline and exhaustion of ovarian follicle number (Table 1).
It appears serum AMH level identifies the endocrinologic leading edge in the age-related decline in ovarian function, which is best termed P-POI (35). When this process occurs before age 40, the unifying term would be Early POI (E-POI). In a longitudinal study of a cohort of women in midlife who had repeated hormone measures and experienced natural menopause, AMH decreased markedly before menopause and preceded the increase in FSH. Thus, AMH is an earlier and more sensitive marker of the POI, whether pathologic at an early age or physiologic at midlife (35).
Pathologic Primary Ovarian Insufficiency:
A continuum of women’s health across the lifespan
In 1938, Henry Turner published cases of what today is known as Turner Syndrome (36). The paradigm clinical presentation is a young girl who fails to enter pubertal development, i.e., no breast development and no first menses. At the time of Turner’s report, there was debate about whether the cause was dysfunction centrally (hypothalamus/pituitary) or peripherally (ovaries). Fuller Albright settled this debate by publishing evidence of high urinary FSH levels in the syndrome. Thus, Albright documented the first Early Primary Ovarian Insufficiency (E-POI) cases (23) (Table 1).
I saw my first case of pathologic E-POI while in private practice in Lynchburg, Virginia, USA. The woman also had autoimmune thyroiditis, which intrigued me as a possible case of polyglandular autoimmune syndrome. This experience changed the course of my career. Eventually, my interest in the enigma brought me to the Intramural Research Program (IRP) of the US National Institutes of Health (NIH). I worked as a Principal Investigator in the NIH Clinical Center. For many years I led a multidisciplinary team to take an integrated approach blending basic science with clinical trials. The New England Journal of Medicine requested I write a Clinical Practice review on the condition (7). Now, after I retired from the NIH in 2017, I have been advancing the cause of E-POI as President of the Mary Elizabeth Conover Foundation, Inc (37, 38). Our mission is to establish a global digital medical hub for E-POI clinical care and natural history research, as recommended more than 10 years ago by the proceedings of an NIH conference on the subject (39).
The basic science of 17-beta estradiol action
Actions of E2 in men
A recent genome-wide association study demonstrated a causal effect of endogenous serum E2 levels on increased bone mineral density in both men and women (40). Another recent genetic study showed evidence that higher serum E2 levels in men are associated with a reduced incidence of a thromboembolic phenomenon and a lower incidence of ischemic stroke (41). Surprisingly, men matched for age with menopausal women have higher serum E2 concentrations (42). In men, biological actions formerly attributed to testosterone may be actions of E2 resulting from the aromatization of testosterone (43). In this sense, testosterone is a “prehormone” in men. Furthermore, many male somatic and reproductive tissues express E2 receptors. In the rare cases of men lacking aromatase or a functional estrogen receptor alpha, evidence suggests vital actions of E2 in regulating the insulin-like growth factor-1 axis, bone growth, maintenance of skeletal health, body composition, glucose metabolism, vasomotor stability in addition to regulating male reproductive function and the hypothalamic-pituitary-testicular axis (43).
Actions of E2 in women
Actions of E2 in the brain
Evidence associates shorter cumulative life exposure to E2 in women with a higher risk of dementia (44). Notably, the scientific community now recognizes the neuromodulator effects of the potent sex steroid hormone E2 (24). E2 induces both spinogenesis and synaptogenesis (45, 46). These actions are mediated not only by classical slow-acting genomic effects by ER α and ER β receptors but also by rapid activity by membrane-bound ER α, ER β, and G protein-coupled estrogen receptor 1 (GPER1) mediating immediate nongenomic effects (47).
In female animal models, considerable evidence supports the crucial role of E2 in regulating learning and memory. A growing body of literature indicates a similar role in male animals. In animal models, E2 signaling affects spatial memory, object recognition memory, social memory, and fear memory (24). In female mice, E2 reduces anxiety by activating estrogen receptor-β (ERβ) (48). These laboratory science findings may have important clinical implications for managing E2 deficiency in women across the lifespan. In female rodents, E2 rapidly activates numerous cellular events in the brain, including cell signaling, histone modification, and local protein translation. This rapid E2 action in the brain consolidates spatial and object recognition memories (49). E2 also facilitates higher cognitive functions by exerting effects on the prefrontal cortex.
The evidence in animal models begs, “Is there a minimal neuroprotective level of E2 in humans?” The E2 effects related to higher cognitive function and synaptic health go well beyond the traditional role wrongly confined to reproduction. These findings support the critical role of later-life E2 deficiency in exacerbating the effects of aging on cognitive functions. Studies in nonhuman primate models are relevant for developing effective interventions for menopausal women (47).
Actions of E2 in the heart and cardiovascular system
The leading cause of death in women is cardiovascular disease, and there is a notable increase in the risk for this disease after menopause (50). Presenting myocardial infarction and stroke symptoms differ between men and women. Traditional symptoms defined in men are less commonly observed in women. For example, women are more likely than men to report nausea, vomiting, referred pain, cough, and fatigue when experiencing a myocardial infarction and less likely than men to report chest pain or sweating. These acute situations must be recognized and treated expeditiously. Delay in care leads to worse outcomes. Work remains to ensure early recognition of myocardial infarction and stroke in women (51, 52).
E2 plays a significant role in the modulation of cardiovascular physiology and pathophysiology (53). E2 regulates related gene expression, contractile function, microvascular function, metabolic processes, and calcium signaling (53). Notably, a prospective controlled study providing transdermal physiologic E2 replacement to menopausal women (in a manner mimicking premenopausal levels) significantly lowered blood pressure (54). The G protein-coupled estrogen receptor 1 (GPER1), expressed ubiquitously in the cardiovascular system and activated by E2, mediates vascular, renal, and cardiac mechanisms that influence blood pressure regulation and are active in vasodilation (55).
The evidence begs the question, “Is there a minimal cardiovascular protective level of E2 in humans?” Investigators in the SWAN Heart Study (Study of Women Across the Nation) detected changes in arterial stiffness within one year of the final menstrual period (56). The Multi-Ethnic Study of Atherosclerosis (MESA), a prospective cohort study, used Cox hazard models to evaluate associations of E2 with the outcome. After adjusting for demographics, risk factors, and use of hormone therapy, they found higher E2 levels associated with a lower risk of coronary artery disease (57). A longitudinal study of midlife women over up to 9 years showed that those with lower endogenous E2 levels had increased subclinical atherosclerosis progression (58). Vasomotor symptoms (hot flashes and night sweats) related to E2 deficiency are an independent indicator of increased risk of coronary artery disease after correcting for traditional cardiovascular risk factors (59).
Actions of E2 in bone
E2 is the crucial regulator of bone metabolism in both men and women. Bone, a metabolically active and complex tissue, plays five significant roles in health:
1. Mechanical, i.e., support and locomotion
2. Protection of vital organs (brain, heart, lungs)
3. Hematopoietic
4. Metabolic, as a reservoir for calcium, phosphorus, and mineral ions
5. Endocrine, i.e., the production of osteocalcin and FGF23 (60, 61)
Osteoclasts and osteoblasts are highly differentiated cells. Osteoclasts resorb bone; osteoblasts produce new bone. Osteoblasts transition to osteocytes, mechanosensory cells able to sense and respond to mechanical forces (62). Bone modeling, a life-long process, involves cycles of bone resorption and formation and determines bone health, development, and maintenance. Maintenance of bone mass throughout life requires a strict balance between bone resorption and formation linked in time and space. This strict correlation between bone resorption and formation is called coupling, with an adequate number of osteoblasts forming at resorption sites (63). Peak bone mass, an E2-dependent process, is an essential predictor for bone strength and osteoporotic fracture risk later in life. Peak bone mass is acquired at the end of the adolescent growth period (64).
In adolescent girls and women, an abrupt decline in serum E2 levels is associated closely with increased osteoclastic bone resorption. Low E2 levels stimulate circulating macrophages to produce osteoclastic cytokines that activate RANK and promote osteoclast activation. Additionally, the loss of direct pro-apoptotic effects of E2 on osteoclasts prolongs osteoclast lifespan, accelerating trabecular bone loss (65, 66).
The midlife physiologic transition to P-POI and the accompanying loss of E2 are associated with bone mineral density declines. E2-dependent bone loss has a predictable pattern. Initially, the loss is relatively rapid, affecting trabecular bone preferentially. After 10 to 15 years of E2 deficiency, the skeletal mass may be one-third to one-half of peak bone mass. The resulting skeletal fragility permits even minimal trauma to cause spine and wrist fractures. In later years hip fractures are alarmingly frequent (67). Timely restoration of E2 levels prevents E2-dependent bone loss and significantly reduces fracture risk. High calcium intake in the face of deficient E2 is ineffective in reducing the risk of bone fracture across the menopause transition (68).
While little recognized and little employed, published evidence demonstrated many years ago that there is a very low dose of E2 (only 14 micrograms per day) administered by a transdermal patch that effectively protects bone density in menopausal women (19, 20). Long-term studies confirm that women in midlife who regularly use menopausal hormone therapy have greater bone mass and fewer osteoporotic fractures (69). A recent meta-analysis clarified the effects of transdermal E2 replacement on BMD in menopausal women. According to pooled estimates, transdermal E2 significantly increased lumbar spine BMD one and two years after initiation of therapy (70).
Physiologic hormone replacement
Physiologic replacement of 17-Beta Estradiol is administered transdermally or transvaginally to avoid adverse “hepatic first-pass effects” of oral administration of estrogens (71). Oral estrogens are known to increase the risk of potentially fatal thromboembolic events (71). In 1986, Judd and colleagues published evidence indicating that transdermal estradiol can elicit many of the desirable actions of estrogen while avoiding the pharmacologic effects of oral estrogens on hepatic proteins (72). For this reason, when the NIH POI research team initiated clinical studies of pathologic POI in 1991, they chose the transdermal route of estradiol administration as the safer alternative. Prospective, randomized, double-blind, controlled studies provide the best evidence for clinical decisions (73). Over the past 20 years, the US National Institutes of Health (NIH) Intramural Research Program (NIH-IRP) conducted the only such study on hormone replacement in women with pathologic early POI (74). The study provided these women with the average daily production rate of estradiol (100 micrograms per day) by transdermal patch and cyclic, monthly oral progestogen. Over the three-year study, the NIH-IRP hormone replacement regimen restored bone mineral density to normal. Women tolerated the treatment well.
Physiologic E2 replacement is also available for women in the midlife transition, i.e., what we are terming, Physiologic Primary Ovarian insufficiency (P-POI). Physiologic E2 replacement is proven safe and effective for women experiencing the midlife transition symptoms of E2 deficiency, such as vasomotor instability and vaginal atrophy (75–77). Physiologic E2 in the low dose administered by the patch is also proven safe and effective in mitigating midlife transition bone loss as prevention of osteoporosis (19, 20).
A change in course: the US Women’s Health Initiative
Women’s health issues other than reproduction received little attention and little research funding before 1986. These omissions are regrettable regarding heart disease, long considered less risky for women than men (78). Coronary artery disease (CAD) is the most common reason for death in men and women in the US. Trends in the US suggest one-half of 40-year-old men will develop CAD in the future, and one in three healthy 40-year-old women (79).
In a 1990 report in the journal Science, this “malign neglect” of women’s health was attributed to the NIH (80). The awareness shifted focus toward women’s health issues and a greater interest in understanding how heart disease might affect women differently than men. The NIH effort seemed to some to be “too little too late” to address the issue of women’s participation in research. In 1991, Dr. Bernadine Healy, newly appointed as the first woman director of the NIH, announced her plan for the Women’s Health Initiative (WHI). She obtained funding directly from Congress as a discrete line item, with a projected budget of $625 million over the life of the 15-year study (81).
The initiative set out to bring greater attention to the significant causes of death and disability among women, particularly those related to heart disease, cancer, and osteoporosis. The funding by Congress provided the necessary resources for establishing several clinical trials involving thousands of women across the United States. The WHI has increased attention to the pressing need to address the unique health concerns of women. Indeed, the United States Government established the US Office of Women’s Health (OWH) as part of the Department of Health and Human Services. Its mission is to improve the health of women and girls through advocacy, education, and research (82).
In this role, the OWH has played a critical role in advancing women’s health issues and addressing health disparities that affect women. Over the years, the office has launched numerous initiatives to improve women’s health outcomes, including campaigns to increase awareness about breast cancer, heart disease, and other health issues that disproportionately affect women. The OWH has also been instrumental in advocating for policies and programs that promote women’s health, such as the Affordable Care Act, which expanded access to preventive services like mammograms and contraception. Today, the OWH remains a crucial player in the fight for women’s health equity and remains committed to improving the health of all women and girls across the United States.
The WHI also aimed to expand research on the significant causes of death, disability, and frailty among women. Thus, Congress also established the Office of Research on Women’s Health (ORWH) as part of the US National Institutes of Health (NIH) (83). Congress recognized that women’s health research needs more funding and more inclusion of women in clinical trials. These deficiencies created a gap in knowledge about women’s health. The ORWH promotes and supports research on women’s health and differences based on sex and gender. It works to ensure that all NIH-funded research considers sex as a biological variable and advocates for women’s inclusion in clinical studies. The ORWH also funds research on conditions that disproportionately affect women, such as breast cancer, osteoporosis, and autoimmune diseases. Through its efforts, the ORWH has made significant contributions to advancing women’s health research and improving the health outcomes of women.
Critical appraisal: the US NIH Women's Health Initiative
Critics give mixed reviews about the WHI. Some praise the efforts to improve women’s health. In contrast, others have criticized its origins as based more on political correctness and less on good scientific methods, and some have seriously questioned the validity of its results (84). This 1993 report in the prestigious journal Nature went so far as “Critics condemn NIH women’s study.” (84) The headline read, “NIH may be wasting $625 million or more in pursuit of an ambition to be seen to be politically correct.” This severe criticism of the NIH WHI came from a committee of the Institute of Medicine appointed by Congress to appraise one of NIH’s most expensive programs ever. The report was “a scathing indictment of its conception and design.” The committee criticized the WHI on several fronts based on (I) scientifically weak premises (2); flawed statistically by poor design (3); inadequate informed consent; and (4) a probable underestimate of the true cost. Surprisingly, the committee acknowledged politics rather than science and had the committee come short of an outright call to cancel the NIH WHI study.
A major concern with the design of the NIH WHI study was the age of the participants. In the WHI studies, women averaged approximately 12 years after menopause. Many of these women would have had significant asymptomatic atherosclerosis upon entry into the trial. Substantial data demonstrate athero-preventive effects of estrogen before vascular damage occurs, whereas adverse effects of oral estrogen on thrombosis and inflammation may predominate once complex atheromas are present. As a result, the study was not a primary prevention trial. From this perspective, the study may have caused great harm to participants (85).
The NIH WHI study has significantly impacted women’s health, but many unanswered questions and controversies remain. Not all the impact has been salutary to women’s health. Despite criticisms, the US WHI remains an essential milestone in women’s health history, some for good, some otherwise.
When it comes to conducting clinical research, ethics play a crucial role. Flawed research can have serious consequences, from misleading patients to wasting valuable resources. That is why ensuring that all research is conducted ethically is so important. One key ethical concern is the validity of the research itself. The results may be inaccurate or unreliable if a study is flawed. This can have serious implications for patients and the wider medical community. Researchers must take great care to design studies that are scientifically sound and free from bias. Another important ethical issue is patient safety. Clinical research often involves testing new treatments or procedures on human subjects. It is essential that patients are fully informed of the risks and benefits of participating in the research and that their safety is always a top priority.
Flawed clinical research with inadequate informed consent is unethical research. The quality of the informed consent obtained from the WHI participants is of particular concern. Were these women informed regarding the availability of safer transdermal E2 alternatives to the oral estrogen used in the study? There has been a call for an independent investigation of the NIH WHI to scrutinize every major WHI paper to determine whether the data justified the conclusions drawn (86). It may be time to call on Congress to initiate the process.
FDA black box warnings on estrogen
A problematic legacy of the US NIH Women’s Health Initiative
These FDA black box warnings on E2 may cause significant problems for patients and healthcare providers. Some see these warnings as a blatant interference with the mutual respect and trust between the individual patient and her clinician. Two glaring examples of what may be termed “heavy-handed regulation” are situations of administration of very low doses of E2 administered physiologically and with proven clinical benefit outweighing the risk. The first example is using 0.014 micrograms of transdermal E2 to prevent bone loss, an FDA-approved treatment (19, 20). What is the necessity for a boxed warning on such a low physiologic dose? The FDA black box warning on this minimal dose of E2 may have put millions of women at unnecessary risk of osteoporosis. A second glaring example is using the vaginal E2 ring for urogenital atrophy, which has proven low systemic absorption (87). What is the need for a boxed warning in this case? These warnings may likely cause many women to suffer needlessly in fear of proven treatment.
There are many avenues of concern regarding these box warnings on E2. First, the alarm may discourage providers from prescribing the medication, even if it is the most appropriate treatment for their patient. Second, the warning may cause patients to fear taking the therapy, leading them never to start the treatment or to stop the treatment altogether without consulting their clinician. Such action can be dangerous for treatments critical to their health. Third, the black box warning may discourage pharmaceutical companies from producing or marketing treatments of proven benefit.
FDA warnings may even have had broader public health spillover effects. For example, the National Institutes of Health Women’s Health Initiative Study and the subsequent FDA box warnings on estrogen caused menopausal hormone use to drop sharply. One analysis showed associated spillover effects of the WHI study on preventive care visits by women aged 60–69, who had statistically significant declines in their mammography, cholesterol, and blood stool tests (88).
Overall, issuing a black box warning is a serious matter that requires careful consideration of the potential benefits and risks of the treatment. Of concern, no uniform FDA guidelines exist on removing boxed warnings. To promote the safe use of approved therapies, the FDA should adopt a uniform and transparent process governing decisions to impose or, importantly, to remove boxed warnings when they cause more harm than good (89).
Paradoxically, the FDA black box warnings divert women away from FDA-approved hormone replacement. Instead, fear makes women vulnerable to unregulated therapies with unproven benefits and unknown risks as provided by purveyors of “Compounded Bioidentical Hormone Therapy.” (90)
Conclusion
“Physiologic Primary Ovarian Insufficiency” is a unifying scientific term describing the physiology and trajectory of ovarian hormone function across a woman’s lifespan into her later years. To improve understanding and provide better care, a new perspective and this more accurate biologic terminology is needed for “menopause.” The essential biology of this physiologic midlife transition in women is a waning of ovarian endocrine function. The physiologic midlife transition to so-called menopause is a state of low serum E2, and early E2 deficiency is associated with significant morbidity and early mortality. A change in terminology away from the culturally defined menopause and to the scientifically defined Physiologic Primary Ovarian Insufficiency (P-POI) is needed to increase awareness of the underlying biology and endocrinology, improve communication, and provide more effective evaluation and management of the associated role of physiologic E2 replacement in maintaining a woman’s health.
Author contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Merriam Webster. . Available at: https://www.merriam-webster.com/dictionary/old%20wives%27%20tale (Accessed 24 May 2023).
2. McCabe J. Old wives’ tales: feminist re-visions of film and other fictions. Fem Rev (2003) 74:116–9. doi: 10.1057/palgrave.fr.9400106
3. Dominus S. Women have been misled about menopause (2023). The New York Times. Available at: https://www.nytimes.com/2023/02/01/magazine/menopause-hot-flashes-hormone-therapy.html (Accessed February 12, 202).
4. Prober CG, Grousbeck HI, Meehan WF. Managing difficult conversations: an essential communication skill for all professionals and leaders. Acad Med J Assoc Am Med Coll (2022) 97(7):973–6. doi: 10.1097/ACM.0000000000004692
5. Spencer H, Simon JA, Nelson LM. Difficult conversations: Management of estradiol deficiency. Maturitas (2023) 171:24. doi: 10.1016/j.maturitas.2023.03.001
6. Asllanaj E, Bano A, Glisic M, Jaspers L, Ikram MA, Laven JSE, et al. Age at natural menopause and life expectancy with and without type 2 diabetes. Menopause (2019) 26(4):387–94. doi: 10.1097/GME.0000000000001246
7. Nelson LM. Clinical practice. Primary ovarian insufficiency. N Engl J Med (2009) 360(6):606–14. doi: 10.1056/NEJMcp0808697
8. Whiteley J, DiBonaventura Md, Wagner JS, Alvir J, Shah S. The impact of menopausal symptoms on quality of life, productivity, and economic outcomes. J Womens Health (Larchmt) (2013) 22(11):983–90. doi: 10.1089/jwh.2012.3719
9. D’Angelo S, Bevilacqua G, Hammond J, Zaballa E, Dennison EM, Walker-Bone K. Impact of menopausal symptoms on work: findings from women in the health and employment after fifty (HEAF) study. Int J Environ Res Public Health (2022) 20(1):295. doi: 10.3390/ijerph20010295
10. Meyer VF. The medicalization of menopause: critique and consequences. Int J Health Serv (2001) 31(4):769–92. doi: 10.2190/M77D-YV2Y-D5NU-FXNW
11. de Langhe B, Fernbach P. The dangers of categorical thinking. Harvard Business Review: Brighton, Massachusetts (2019). ProQuest. Web. 14 May 2023.
12. Leidy LE. Biological aspects of menopause: across the lifespan. Annu Rev Anthropol (1994) 23:231–53. doi: 10.1146/annurev.an.23.100194.001311
13. Johnstone RA, Cant MA. Evolution of menopause. Curr Biol (2019) 29(4):R112–5. doi: 10.1016/j.cub.2018.12.048
14. Peccei JS. A critique of the grandmother hypotheses: old and new. Am J Hum Biol (2001) 13(4):434–52. doi: 10.1002/ajhb.1076
16. Richardson SJ, Senikas V, Nelson JF. Follicular depletion during the menopausal transition: evidence for accelerated loss and ultimate exhaustion. J Clin Endocrinol Metab (1987) 65(6):1231–7. doi: 10.1210/jcem-65-6-1231
17. Burger HG, Hale GE, Robertson DM, Dennerstein L. A review of hormonal changes during the menopausal transition: focus on findings from the Melbourne Women’s Midlife Health Project. Hum Reprod Update (2007) 13(6):559–65. doi: 10.1093/humupd/dmm020
18. Cauley JA. Estrogen and bone health in men and women. Steroids (2015) 99(Pt A):11–5. doi: 10.1016/j.steroids.2014.12.010
19. Ettinger B, Ensrud KE, Wallace R, Johnson KC, Cummings SR, Yankov V, et al. Effects of ultralow-dose transdermal estradiol on bone mineral density: a randomized clinical trial. Obstet Gynecol (2004) 104(3):443–51. doi: 10.1097/01.AOG.0000137833.43248.79
20. Schaefers M, Muysers C, Alexandersen P, Christiansen C. Effect of microdose transdermal 17beta-estradiol compared with raloxifene in the prevention of bone loss in healthy postmenopausal women: a 2-year, randomized, double-blind trial. Menopause (2009) 16(3):559–65. doi: 10.1097/gme.0b013e31818ebfba
21. Lephart ED. A review of the role of estrogen in dermal aging and facial attractiveness in women. J Cosmet Dermatol (2018) 17(3):282–8. doi: 10.1111/jocd.12508
22. Zouboulis CC, Blume-Peytavi U, Kosmadaki M, Roó E, Vexiau-Robert D, Kerob D, et al. Skin, hair and beyond: the impact of menopause. Climacteric (2022) 25(5):434–42. doi: 10.1080/13697137.2022.2050206
23. Albright F, Smith PH, Fraser R. A syndrome characterized by primary ovarian insufficiency and decreased stature: report of 11 cases with a digression on hormonal control of axillary and pubic hair. Am J Med Sci (1942) 204:625–48.
24. Taxier LR, Gross KS, Frick KM. Oestradiol as a neuromodulator of learning and memory. Nat Rev Neurosci (2020) 21(10):535–50. doi: 10.1038/s41583-020-0362-7
25. Ioannidis JP. Why science is not necessarily self-correcting. Perspect Psychol Sci (2012) 7(6):645–54. doi: 10.1177/1745691612464056
26. Sochas L. Challenging categorical thinking: A mixed methods approach to explaining health inequalities. Soc Sci Med (2021) 283:114192. doi: 10.1016/j.socscimed.2021.114192
27. Hamoda H, Moger S. Board of Trustees and Medical Advisory Council of the British Menopause Society. Developing the Women’s health strategy: The British Menopause Society’s recommendations to the department of health and social care’s call for evidence. Post Reprod Health (2022) 28(1):13–8. doi: 10.1177/20533691211064037
28. Shuster LT, Rhodes DJ, Gostout BS, Grossardt BR, Rocca WA. Premature menopause or early menopause: long-term health consequences. Maturitas (2010) 65(2):161–6. doi: 10.1016/j.maturitas.2009.08.003
29. Malek AM, Vladutiu CJ, Meyer ML, Cushman M, Newman R, Lisabeth LD, et al. The association of age at menopause and all-cause and cause-specific mortality by race, postmenopausal hormone use, and smoking status. Prev Med Rep (2019) 15:100955. doi: 10.1016/j.pmedr.2019.100955
30. Ryan J, Scali J, Carrière I, Amieva H, Rouaud O, Berr C, et al. Impact of a premature menopause on cognitive function in later life. BJOG (2014) 121(13):1729–39. doi: 10.1111/1471-0528.12828
31. Coppus AM, Evenhuis HM, Verberne GJ, Visser FE, Eikelenboom P, van Gool WA, et al. Early age at menopause is associated with increased risk of dementia and mortality in women with Down syndrome. J Alzheimers Dis (2010) 19(2):545–50. doi: 10.3233/JAD-2010-1247
32. Li Y, Zhao D, Wang M, Sun JY, Liu J, Qi Y, et al. Combined effect of menopause and cardiovascular risk factors on death and cardiovascular disease: a cohort study. BMC Cardiovasc Disord (2021) 21(1):109. doi: 10.1186/s12872-021-01919-5
33. Yoshida Y, Chen Z, Baudier RL, Krousel-Wood M, Anderson AH, Fonseca VA, et al. Early menopause and cardiovascular disease risk in women with or without type 2 diabetes: A pooled analysis of 9,374 postmenopausal women. Diabetes Care (2021) 44(11):2564–72. doi: 10.2337/dc21-1107
34. Svejme O, Ahlborg HG, Nilsson JÅ, Karlsson MK. Early menopause and risk of osteoporosis, fracture and mortality: a 34-year prospective observational study in 390 women. BJOG (2012) 119(7):810–6. doi: 10.1111/j.1471-0528.2012.03324.x
35. Soares AG, Kilpi F, Fraser A, Nelson SM, Sattar N, Welsh PI, et al. Longitudinal changes in reproductive hormones through the menopause transition in the Avon Longitudinal Study of Parents and Children (ALSPAC). Sci Rep (2020) 10(1):21258. doi: 10.1038/s41598-020-77871-9
36. Classic pages in obstetrics and gynecology by Henry H. Turner. A syndrome of infantilism, congenital webbed neck, and cubitus valgus. Endocrinology, vol. 23, pp. 566-574, 1938. Am J Obstet Gynecol (1972) 113(2):279.
37. Fink DA, Nelson LM, Pyeritz R, Johnson J, Sherman SL, Cohen Y, et al. Fragile X associated primary ovarian insufficiency (FXPOI): case report and literature review. Front Genet (2018) 9:529. doi: 10.3389/fgene.2018.00529
38. Piedade KC, Spencer H, Persani L, Nelson LM. Optimizing fertility in primary ovarian insufficiency: case report and literature review. Front Genet (2021) 12:676262. doi: 10.3389/fgene.2021.676262
39. Cooper AR, Baker VL, Sterling EW, Ryan ME, Woodruff TK, Nelson LM. The time is now for a new approach to primary ovarian insufficiency. Fertil Steril (2011) 95(6):1890–7. doi: 10.1016/j.fertnstert.2010.01.016
40. Schmitz D, Ek WE, Berggren E, Höglund J, Karlsson T, Johansson Å. Genome-wide association study of estradiol levels and the causal effect of estradiol on bone mineral density. J Clin Endocrinol Metab (2021) 106(11):e4471–86. doi: 10.1210/clinem/dgab507
41. Nethander M, Quester J, Vandenput L, Ohlsson C. Association of genetically predicted serum estradiol with risk of thromboembolism in men: A mendelian randomization study. J Clin Endocrinol Metab (2021) 106(8):e3078–86. doi: 10.1210/clinem/dgab164
42. Carlson LE, Sherwin BB. Higher levels of plasma estradiol and testosterone in healthy elderly men compared with age-matched women may protect aspects of explicit memory. Menopause (2000) 7(3):168–77. doi: 10.1097/00042192-200007030-00007
43. Russell N, Grossmann M. MECHANISMS IN ENDOCRINOLOGY: Estradiol as a male hormone. Eur J Endocrinol (2019) 181(1):R23–43. doi: 10.1530/EJE-18-1000
44. Gong J, Harris K, Peters SAE, Woodward M. Reproductive factors and the risk of incident dementia: A cohort study of UK Biobank participants. PloS Med (2022) 19(4):e1003955. doi: 10.1371/journal.pmed.1003955
45. Prange-Kiel J, Fester L, Zhou L, Jarry H, Rune GM. Estrus cyclicity of spinogenesis: underlying mechanisms. J Neural Transm (Vienna) (2009) 116(11):1417–25. doi: 10.1007/s00702-009-0294-x
46. Kilinc D. The emerging role of mechanics in synapse formation and plasticity. Front Cell Neurosci (2018) 12:483. doi: 10.3389/fncel.2018.00483
47. Hara Y, Waters EM, McEwen BS, Morrison JH. Estrogen effects on cognitive and synaptic health over the lifecourse. Physiol Rev (2015) 95(3):785–807. doi: 10.1152/physrev.00036.2014
48. Oyola MG, Portillo W, Reyna A, Foradori CD, Kudwa A, Hinds L, et al. Anxiolytic effects and neuroanatomical targets of estrogen receptor-β (ERβ) activation by a selective ERβ agonist in female mice. Endocrinology (2012) 153(2):837–46. doi: 10.1210/en.2011-1674
49. Frick KM, Kim J. Mechanisms underlying the rapid effects of estradiol and progesterone on hippocampal memory consolidation in female rodents. Horm Behav (2018) 104:100–10. doi: 10.1016/j.yhbeh.2018.04.013
50. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. American heart association council on epidemiology and prevention statistics committee and stroke statistics subcommittee. Heart disease and stroke statistics-2019 update: A report from the american heart association. Circulation (2019) 139(10):e56–e528. doi: 10.1161/CIR.0000000000000659
51. Chen W, Woods SL, Puntillo KA. Gender differences in symptoms associated with acute myocardial infarction: a review of the research. Heart Lung (2005) 34(4):240–7. doi: 10.1016/j.hrtlng.2004.12.004
52. Hosman FL, Engels S, den Ruijter HM, Exalto LG. Call to action for enhanced equity: racial/ethnic diversity and sex differences in stroke symptoms. Front Cardiovasc Med (2022) 9:874239. doi: 10.3389/fcvm.2022.874239
53. den Ruijter HM, Kararigas G. Estrogen and cardiovascular health. Front Cardiovasc Med (2022) 9:886592. doi: 10.3389/fcvm.2022.886592
54. Seely EW, Walsh BW, Gerhard MD, Williams GH. Estradiol with or without progesterone and ambulatory blood pressure in postmenopausal women. Hypertension (1999) 33(5):1190–4. doi: 10.1161/01.hyp.33.5.1190
55. Gohar EY. G protein-coupled estrogen receptor 1 as a novel regulator of blood pressure. Am J Physiol Renal Physiol (2020) 319(4):F612–7. doi: 10.1152/ajprenal.00045.2020
56. Samargandy S, Matthews KA, Brooks MM, Barinas-Mitchell E, Magnani JW, Janssen I, et al. Arterial stiffness accelerates within 1 year of the final menstrual period: the SWAN heart study. Arterioscler Thromb Vasc Biol (2020) 40(4):1001–8. doi: 10.1161/ATVBAHA.119.313622
57. Zhao D, Guallar E, Ouyang P, Subramanya V, Vaidya D, Ndumele CE, et al. Endogenous sex hormones and incident cardiovascular disease in post-menopausal women. J Am Coll Cardiol (2018) 71(22):2555–66. doi: 10.1016/j.jacc.2018.01.083
58. El Khoudary SR, Wildman RP, Matthews K, Thurston RC, Bromberger JT, Sutton-Tyrrell K. Endogenous sex hormones impact the progression of subclinical atherosclerosis in women during the menopausal transition. Atherosclerosis (2012) 225(1):180–6. doi: 10.1016/j.atherosclerosis.2012.07.025
59. Muka T, Oliver-Williams C, Colpani V, Kunutsor S, Chowdhury S, Chowdhury R, et al. Association of vasomotor and other menopausal symptoms with risk of cardiovascular disease: A systematic review and meta-analysis. PloS One (2016) 11(6):e0157417. doi: 10.1371/journal.pone.0157417
60. Emmanuelle NE, Marie-Cécile V, Florence T, Jean-François A, Françoise L, Coralie F, et al. Critical role of estrogens on bone homeostasis in both male and female: from physiology to medical implications. Int J Mol Sci (2021) 22(4):1568. doi: 10.3390/ijms22041568
61. Oldknow KJ, MacRae VE, Farquharson C. Endocrine role of bone: recent and emerging perspectives beyond osteocalcin. J Endocrinol (2015) 225(1):R1–19. doi: 10.1530/JOE-14-0584
62. Tresguerres FGF, Torres J, López-Quiles J, Hernández G, Vega JA, Tresguerres IF. The osteocyte: A multifunctional cell within the bone. Ann Anat (2020) 227:151422. doi: 10.1016/j.aanat.2019.151422
63. Sims NA, Martin TJ. Osteoclasts provide coupling signals to osteoblast lineage cells through multiple mechanisms. Annu Rev Physiol (2020) 82:507–29. doi: 10.1146/annurev-physiol-021119-034425
64. Weaver CM, Gordon CM, Janz KF, Kalkwarf HJ, Lappe JM, Lewis R, et al. The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: a systematic review and implementation recommendations. Osteoporos Int (2016) 27(4):1281–386. doi: 10.1007/s00198-015-3440-3
65. Golden NH. Bones and birth control in adolescent girls. J Pediatr Adolesc Gynecol (2020) 33(3):249–54. doi: 10.1016/j.jpag.2020.01.003
66. Martin-Millan M, Almeida M, Ambrogini E, Han L, Zhao H, Weinstein RS, et al. The estrogen receptor-alpha in osteoclasts mediates the protective effects of estrogens on cancellous but not cortical bone. Mol Endocrinol (2010) 24(2):323–34. doi: 10.1210/me.2009-0354
67. Sambrook P, Kelly P, Eisman J. Bone mass and ageing. Baillieres Clin Rheumatol (1993) 7(3):445–57. doi: 10.1016/s0950-3579(05)80072-x
68. Bailey RL, Zou P, Wallace TC, McCabe GP, Craig BA, Jun S, et al. Calcium supplement use is associated with less bone mineral density loss, but does not lessen the risk of bone fracture across the menopause transition: data from the study of women’s health across the nation. JBMR Plus (2019) 4(1):e10246. doi: 10.1002/jbm4.10246
69. Ettinger B. Prevention of osteoporosis: treatment of estradiol deficiency. Obstet Gynecol (1988) 72(5 Suppl):12S–7S.
70. Abdi F, Mobedi H, Bayat F, Mosaffa N, Dolatian M, Ramezani Tehrani F. The effects of transdermal estrogen delivery on bone mineral density in postmenopausal women: A meta-analysis. Iran J Pharm Res (2017) 16(1):380–9.
71. Lievertz RW. Pharmacology and pharmacokinetics of estrogens. Am J Obstet Gynecol (1987) 156(5):1289–93. doi: 10.1016/0002-9378(87)90166-9
72. Chetkowski RJ, Meldrum DR, Steingold KA, Randle D, Lu JK, Eggena P, et al. Biologic effects of transdermal estradiol. N Engl J Med (1986) 314(25):1615–20. doi: 10.1056/NEJM198606193142505
73. Centre for evidence-based medicine (CEBM) . University of Oxford. Available at: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/oxford-centre-for-evidence-based-medicine-levels-of-evidence-march-2009 (Accessed February 12, 2023).
74. Popat VB, Calis KA, Kalantaridou SN, Vanderhoof VH, Koziol D, Troendle JF, et al. Bone mineral density in young women with primary ovarian insufficiency: results of a three-year randomized controlled trial of physiological transdermal estradiol and testosterone replacement. J Clin Endocrinol Metab (2014) 99(9):3418–26. doi: 10.1210/jc.2013-4145
75. Hedrick RE, Ackerman RT, Koltun WD, Halvorsen MB. Estradiol gel 0.1% relieves vasomotor symptoms independent of age, ovarian status, or uterine status. Menopause (2010) 17(6):1167–73. doi: 10.1097/gme.0b013e3181e04b75
76. Kovács G, Zelei T, Vokó Z. Comparison of efficacy and local tolerability of estradiol metered-dose transdermal spray to estradiol patch in a network meta-analysis. Climacteric (2016) 19(5):488–95. doi: 10.1080/13697137.2016.1221919
77. Tanmahasamut P, Jirasawas T, Laiwejpithaya S, Areeswate C, Dangrat C, Silprasit K. Effect of estradiol vaginal gel on vaginal atrophy in postmenopausal women: A randomized double-blind controlled trial. J Obstet Gynaecol Res (2020) 46(8):1425–35. doi: 10.1111/jog.14336
78. Thomas JL, Braus PA. Coronary artery disease in women. A historical perspective. Arch Intern Med (1998) 158(4):333–7. doi: 10.1001/archinte.158.4.333
79. Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, et al. Heart disease and stroke statistics–2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation (2007) 115(5):e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918
81. National Heart Lung and Blood Institute, Women’s Health Initiative. Available at: https://www.nhlbi.nih.gov/science/womens-health-initiative-whi (Accessed May 26, 2023).
82. Office on Women’s Health. US department of health and human services . Available at: https://www.womenshealth.gov/ (Accessed May 26, 2023).
83. Office of Research on Women’s Health, National Institutes of Health. Available at: https://orwh.od.nih.gov/ (Accessed May 26, 2023).
84. Culliton BJ. Critics condemn NIH women’s study. Nature (1993) 366(6450):11. doi: 10.1038/366011a0
85. Harman SM, Naftolin F, Brinton EA, Judelson DR. Is the estrogen controversy over? Deconstructing the Women’s Health Initiative study: a critical evaluation of the evidence. Ann N Y Acad Sci (2005) 1052:43–56. doi: 10.1196/annals.1347.004
86. Utian WH. A decade post WHI, menopausal hormone therapy comes full circle–need for independent commission. Climacteric (2012) 15(4):320–5. doi: 10.3109/13697137.2012.678916
87. Streff A, Chu-Pilli M, Stopeck A, Chalasani P. Changes in serum estradiol levels with Estring in postmenopausal women with breast cancer treated with aromatase inhibitors. Support Care Cancer (2021) 29(1):187–91. doi: 10.1007/s00520-020-05466-1
88. Meltem Daysal N, Orsini C. Spillover effects of drug safety warnings on preventive health care use. B.E J Economic Anal Policy (2015) 15:(1). doi: 10.1515/bejeap-2013-0038
89. Yeh JS, Sarpatwari A, Kesselheim AS. Ethical and practical considerations in removing black box warnings from drug labels. Drug Saf (2016) 39(8):709–14. doi: 10.1007/s40264-016-0419-8
Keywords: 17 beta-estradiol (E2), women’s health, public health, policy and administrative challenges, physiologic primary ovarian insufficiency, menopausal hormonal therapy, morbidity & mortality, FDA boxed warning
Citation: Nelson LM (2023) The truth about 17-beta estradiol: menopause beyond “old wives’ tales”. Front. Endocrinol. 14:1229804. doi: 10.3389/fendo.2023.1229804
Received: 02 June 2023; Accepted: 31 August 2023;
Published: 11 September 2023.
Edited by:
Liang Ma, Washington University in St. Louis, United StatesReviewed by:
Xuezhi (Daniel) Jiang, Reading Hospital, United StatesCopyright © 2023 Nelson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lawrence M. Nelson, RG9jQENvbm92ZXJGb3VuZGF0aW9uLm9yZw==
 Lawrence M. Nelson
Lawrence M. Nelson