
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 11 August 2023
Sec. Pediatric Endocrinology
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1227886
This article is part of the Research Topic Transgender Health: Exploring Diversity in the Endocrine Field View all 5 articles
 Eric Morris Bomberg1,2*
Eric Morris Bomberg1,2* Bradley Scott Miller1
Bradley Scott Miller1 Oppong Yaw Addo3
Oppong Yaw Addo3 Alan David Rogol4
Alan David Rogol4 Mutaz M. Jaber5
Mutaz M. Jaber5 Kyriakie Sarafoglou1,5
Kyriakie Sarafoglou1,5Introduction: The Centers for Disease Control and Prevention (CDC) and World Health Organization (WHO) created separate growth charts for girls and boys because growth patterns and rates differ between sexes. However, scenarios exist in which this dichotomizing “girls versus boys” approach may not be ideal, including the care of non-binary youth or transgender youth undergoing transitions consistent with their gender identity. There is therefore a need for growth charts that age smooth differences in pubertal timing between sexes to determine how youth are growing as “children” versus “girls or boys” (e.g., age- and sex-neutral, compared to age- and sex-specific, growth charts).
Methods: Employing similar statistical techniques and datasets used to create the CDC 2000 growth charts, we developed age-adjusted, sex non-specific growth charts for height, weight, and body mass index (BMI), and z-score calculators for these parameters. Specifically, these were created using anthropometric data from five US cross-sectional studies including National Health Examination Surveys II-III and National Health and Nutrition Examination Surveys I-III. To illustrate contemporary clinical practice, we overlaid our charts on CDC 2000 girls and boys growth charts.
Results: 39,119 youth 2-20 years old (49.5% female; 66.7% non-Hispanic White; 21.7% non-Hispanic Black) were included in the development of our growth charts, reference ranges, and z-score calculators. Respective curves were largely superimposable through around 10 years of age after which, coinciding with pubertal onset timing, differences became more apparent.
Discussion: We conclude that age-adjusted, sex non-specific growth charts may be used in clinical situations such as transgender youth in which standard “girls versus boys” growth charts are not ideal. Until longitudinal auxological data are available in these populations, our growth charts may help to assess a transgender youth’s growth trajectory and weight classification, and expectations surrounding these.
Because growth patterns and rates differ between sexes, the Centers for Disease Control and Prevention (CDC) and World Health Organization (WHO) created separate growth charts for girls and boys (1–3). The CDC 2000 growth charts for US children 2–19 years old, WHO 2006 growth charts for US children 0–2 years old and, in many countries the 1990 United Kingdom reference charts for children 0–5 years old, have been considered “reference” or “standard” for at least 15 years (3–5). However, scenarios exist in which this binary “girl versus boy” approach may not be ideal, including in care of non-binary youth or transgender youth undergoing transition consistent with their gender identity.
An increasing number of transgender youth are seeking medical care to establish treatment regimens geared towards developing physical characteristics congruent with experienced gender (6).
These interventions include gonadotropin releasing hormone agonists (GnRHa) to suppress puberty and reduce endogenous sex hormone production of one’s natal sex, and hormonal therapies (i.e., testosterone and estradiol) to induce secondary sex characteristics consistent with affirmed gender identity (7). Such therapies can impact anthropometrics, including linear growth, body mass index (BMI), and body composition (8, 9). For example, testosterone may increase muscle mass and BMI, while estradiol can lower waist-to-hip ratios due to hip fat deposition (10–12). Overall, the understanding of how hormonal interventions impact changes in pubertal growth spurt patterns is still in its infancy (13–15).
To monitor growth in non-binary youth or transgender youth and their response to medical interventions, currently clinicians have limited tools. It has been proposed that use of growth charts corresponding to both affirmed gender and genetic sex should/might be considered (10). For example, when evaluating growth parameters in a transgender male or female, the clinician must plot their height, height velocity, weight, and BMI on both the girls and boys CDC 2000 charts. Since all these growth parameters are sex dependent, assessments as to whether the growth response is appropriate become arbitrary as the determination is made by looking at the plot points of these parameters on both the male and female charts side-by-side. Because of the current challenges in monitoring growth in transgender youths, and the extended time it will take to develop gender-specific charts based on longitudinally collected data, an intermediate tool is needed.
Our group previously developed pubertal- and chronological-age adjusted growth charts and reference ranges using cross-sectional data from nationally representative US health surveys (e.g., National Health Examination Surveys (NHES), National Health and Nutrition Examination Surveys (NHANES) and Hispanic Health and Nutrition Examination Surveys (HHANES)) to assess how puberty affects anthropometrics including height, weight, and BMI (16–18). For example, we showed that considering race/ethnicity differences in pubertal timing affects shortness, tallness, and overweight/obesity prevalence quantification (16–18). Towards this end and utilizing similar techniques including use of pooled data from large US cross-sectional nationally-representative samples, we have now developed age-adjusted, sex non-specific growth charts and reference ranges for height, weight, and BMI, and z-score calculators for these parameters. In addition to sharing our growth charts and reference ranges, we exemplify how they can be used in clinical practice. Our goal in creating these tools is two-fold: to age-smooth growth across sexes in order to help clinicians monitor how one is growing as a “child” (sex non-specific) as opposed to specifically as a “girl or boy,” and as a research tool to monitor growth parameters until long-term auxological data can be systematically collected.
To develop our age-adjusted, sex non-specific growth charts, reference ranges, and z-score calculators, we pooled data from five complex US cross-sectional nationally representative surveys that included children and adolescents: NHES II (1963-1965), NHES III (1966-1970), NHANES I (1971-1974), NHANES II (1976-1980), and NHANES III (1988-1994) (19–24). CDC/National Center for Health Statistics institutional review board approval and documented consent was obtained from participants.
We chose to use data from these surveys for two main reasons. First, all included nationally represented US youth. Second, CDC 2000 growth charts, the most widely used US reference for 2-20 year olds, are based upon data from these surveys, making our growth charts analogous (25). If we used more contemporaneous NHANES surveys, we could not compare our results to those from CDC 2000. We note that these surveys took place largely prior to the US obesity epidemic and, therefore, CDC 2000 growth charts and this analysis both excluded weights from NHANES III for youth ≥6 years to avoid upward shifts in weight- and BMI-for-age curves due to rising obesity prevalence.
Standing height to the nearest 0.1 cm and weight were measured by trained technicians following standardized protocols and using calibrated stadiometers and scales, respectively (26). In our analyses leading to creation of our growth charts, reference ranges, and z-score calculators, we included participants 2–20 years old to mirror CDC 2000 growth charts. We excluded children with missing data from any of the following: age, weight, height, and/or sex.
We used the Lambda, Mu, Sigma (LMS) semi-parametric approach in a Generalized Additive Models for Location, Scale, and Shape (GAMLSS) technique to model growth (27). This approach has been used in many growth reference analyses, including those from the CDC and WHO (5, 28–30). Box-Cox Power distribution families in GAMLSS with additive age splines were used to calculate estimates of our sex non-specific height, weight, and BMI reference data tables (31, 32). We accounted for sampling weights to generate nationally representative chronological age-based growth charts for height, weight, and BMI without stratification by sex. Detailed statistical and visual diagnostic tools were used to select the best fitting model for generating reference data (33). Statistical analyses were conducted in R 3.6.0 (The R Foundation for Statistical Computing and Graphics, Vienna, Austria) and data management was performed using SAS 9.4 (SAS Institute, Cary NC, USA).
Data from 39,119 participants (49.5% female; 66.7% non-Hispanic White (NHW); 21.7% non-Hispanic Black (NHB)) were included in the development of our growth charts, reference ranges, and z-score calculators (see Table 1). Analytic sample sizes used to estimate our growth charts were large and robust.
To illustrate the clinical utility of our age-adjusted sex non-specific height, weight, and BMI growth charts, we overlaid them on the CDC 2000 girls and boys growth charts. Of note, when modeled using GAMLSS methods, the combined data (girls and boys) have a broader distribution resulting in a new set of percentiles that differ from those for either sex-specific cohort. Because of this, at certain age points, especially before puberty, the height and weight of children plotted on the sex non-specific growth chart may appear slightly taller and/or heavier than on the CDC 2000 boy and girl charts. As shown in Figures 1–3, the respective curves are nearly superimposable up to around 10 years of age (e.g., 10-12 years old) in terms of height and around 8 years of age (e.g., 8-10 years old) in terms of weight and BMI, after which differences become more apparent. For example, in a 5 year old, the median (p50) height on our age-adjusted sex non-specific curve is 111 cm, compared to 108 cm on the girl and 109 cm on the boy CDC 2000 charts. However, in a 15 year old the p50 height on our curve is 167 cm, compared to 162 cm and 170 cm on the CDC girl and boy charts, respectively.
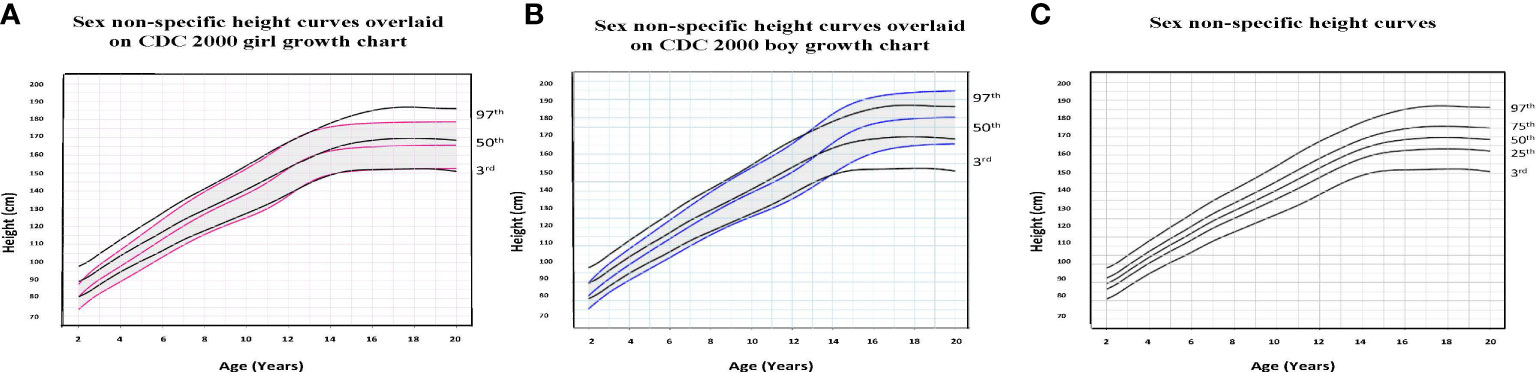
Figure 1 Comparison of age-adjusted sex non-specific height curves with CDC 2000 girl and boy age- and sex-adjusted height curves. (A) shows our age-adjusted sex non-specific height curves overlaid on the CDC 2000 girl age- and sex-adjusted height curves, highlighting the 3rd, 50th, and 97th percentiles. (B) shows our age-adjusted sex non-specific height curves overlaid on the CDC 2000 boy age- and sex-adjusted height curve, highlighting the 3rd, 50th, and 97th percentiles. (C) shows our age-adjusted sex non-specific height growth curves. Here, we highlight the 3rd, 25th, 50th, 75th, and 97th percentiles given their clinical utility.
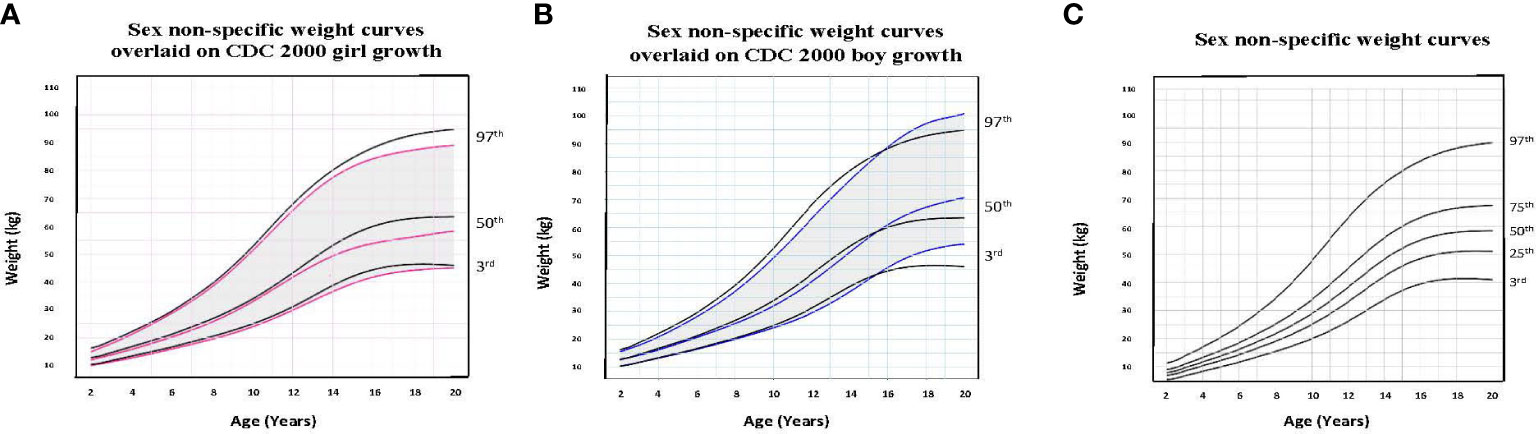
Figure 2 Comparison of age-adjusted sex non-specific weight curves with CDC 2000 girl and boy age- and sex-adjusted weight curves. (A) shows our age-adjusted sex non-specific weight curves overlaid on the CDC 2000 girl age- and sex-adjusted weight curves, highlighting the 3rd, 50th, and 97th percentiles. (B) shows our age-adjusted sex non-specific weight curves overlaid on the CDC 2000 boy age- and sex-adjusted weight curve, highlighting the 3rd, 50th, and 97th percentiles. (C) shows our age-adjusted sex non-specific weight growth curves. Here, we highlight the 3rd, 25th, 50th, 75th, and 97th percentiles given their clinical utility.
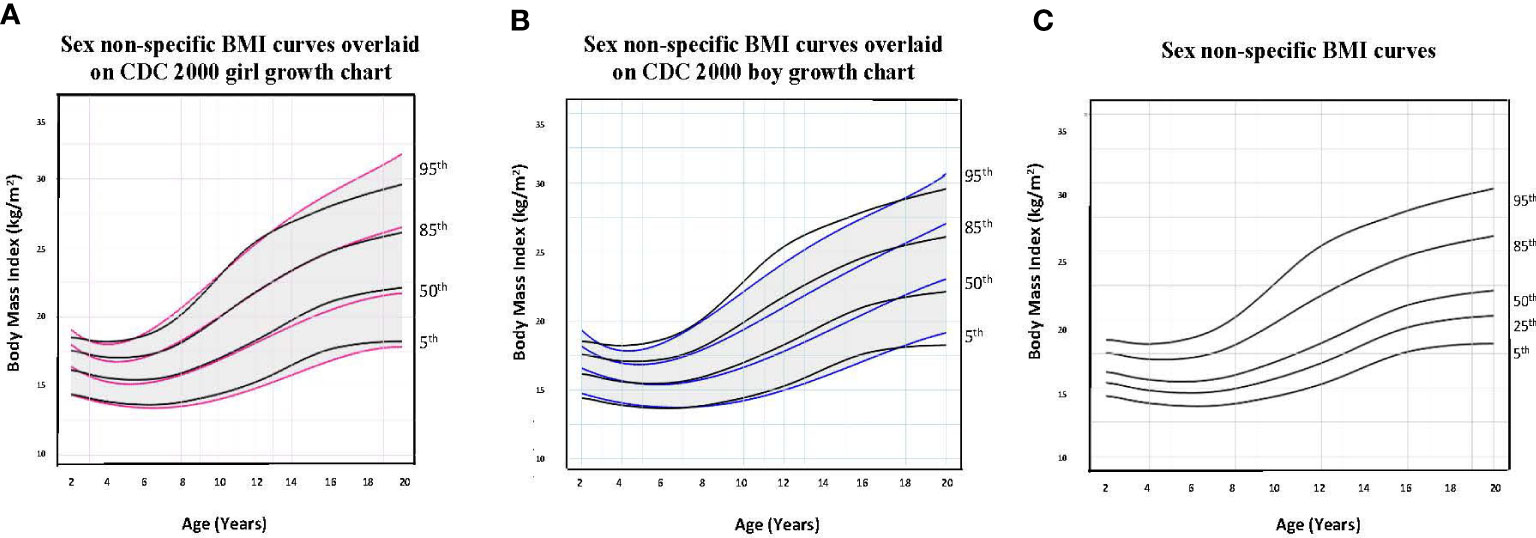
Figure 3 Comparison of age-adjusted sex non-specific body mass index (BMI) curves with CDC 2000 girl and boy age- and sex-adjusted BMI curves. (A) shows our age-adjusted sex non-specific BMI curves overlaid on the CDC 2000 girl age- and sex-adjusted BMI curves, highlighting the 5th, 50th, 85th, and 95th percentiles. (B) shows our age-adjusted sex non-specific BMI curves overlaid on the CDC 2000 boy age- and sex-adjusted weight curve, highlighting the 3rd, 50th, 85th, and 95th percentiles. (C) shows our age-adjusted sex non-specific BMI growth curves. Here, we highlight the 5rd (underweight), 25th, 50th, 85th (overweight), and 95th (obesity) percentiles given their clinical utility.
As for median near adult height, our age-adjusted sex non-specific height curve shows p50 of 169 cm (range p3: 151 cm, p97: 186 cm), compared to 163 cm (p3: 151 cm, p97: 176 cm) and 177 cm (p3: 163 cm, p97: 190 cm) on the girl and boy CDC 2000 curves, respectively. A comparison of clinically useful percentiles (height/weight: 3rd, 25th, 50th, 75th, 97th; BMI: 5th, 25th, 50th, 85th, 95th) from our age-adjusted sex non-specific curves with those from the CDC 2000 girl and boy curves can be seen in Supplemental Tables 1-3.
Although one generally desires to target an appropriate near adult height for one’s chosen gender, this is often an unreasonable expectation, particularly in a transgender male (assigned female at birth) youth. Figure 4 (transgender male, assigned female at birth, height chart) illustrates how using these sex non-specific charts can be useful in the clinical setting compared to the common practice of comparing male and female charts side-by side for clinical decision-making. Figure 5 (transgender female, assigned male at birth, BMI chart) illustrates differences in BMI percentiles between the sexes that may lead to misclassification of weight-related disorders in a transgender youth.
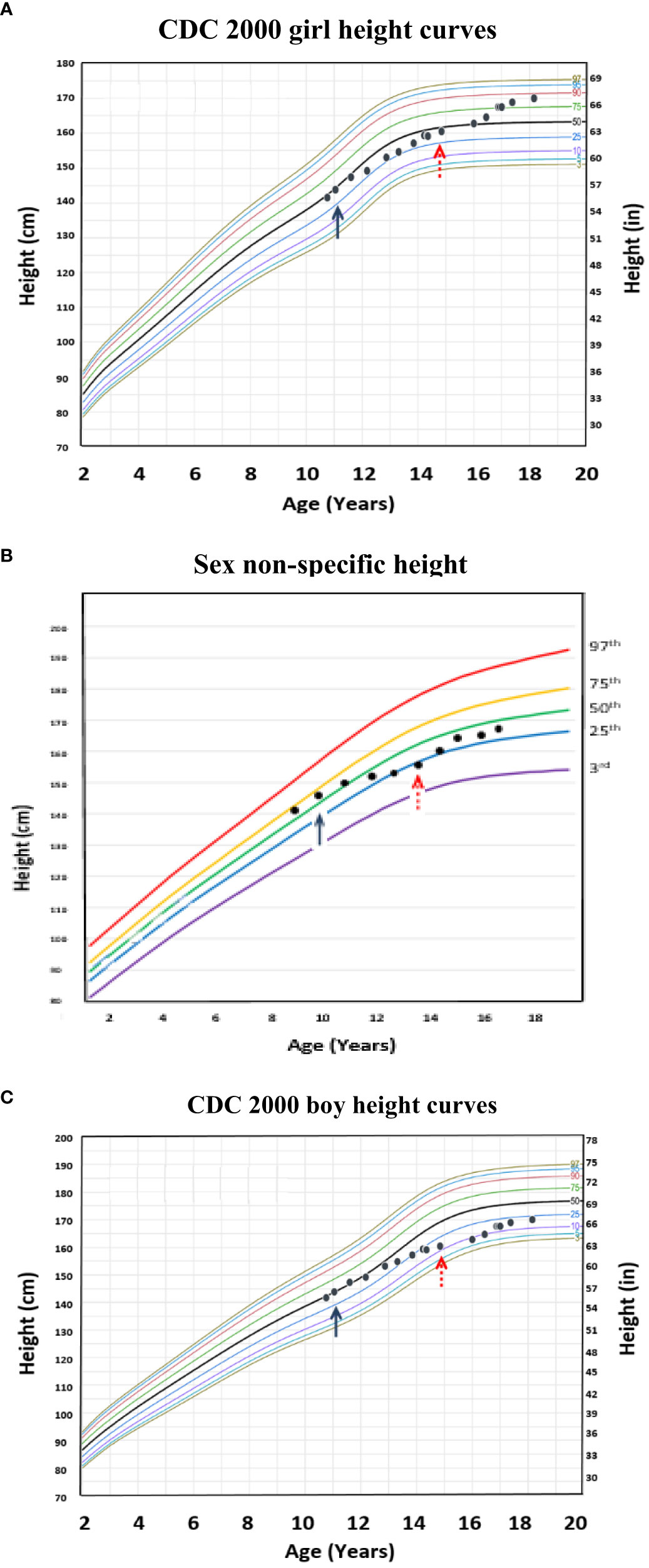
Figure 4 Example of a transgender male (assigned female at birth) individual with height plotted on the age-adjusted sex non-specific height curves. (B) shows height plotted on the age-adjusted sex non-specific height curves, while (A) and (C) show height plotted on the CDC 2000 girl and boy height curves, respectively. This individual received pubertal suppression with gonadotropin releasing hormone agonist therapy (pubertal blocker) beginning around age 12 years (↑) and subsequently began receiving testosterone for cross-sex hormonal therapy beginning around age 15 years (↑).
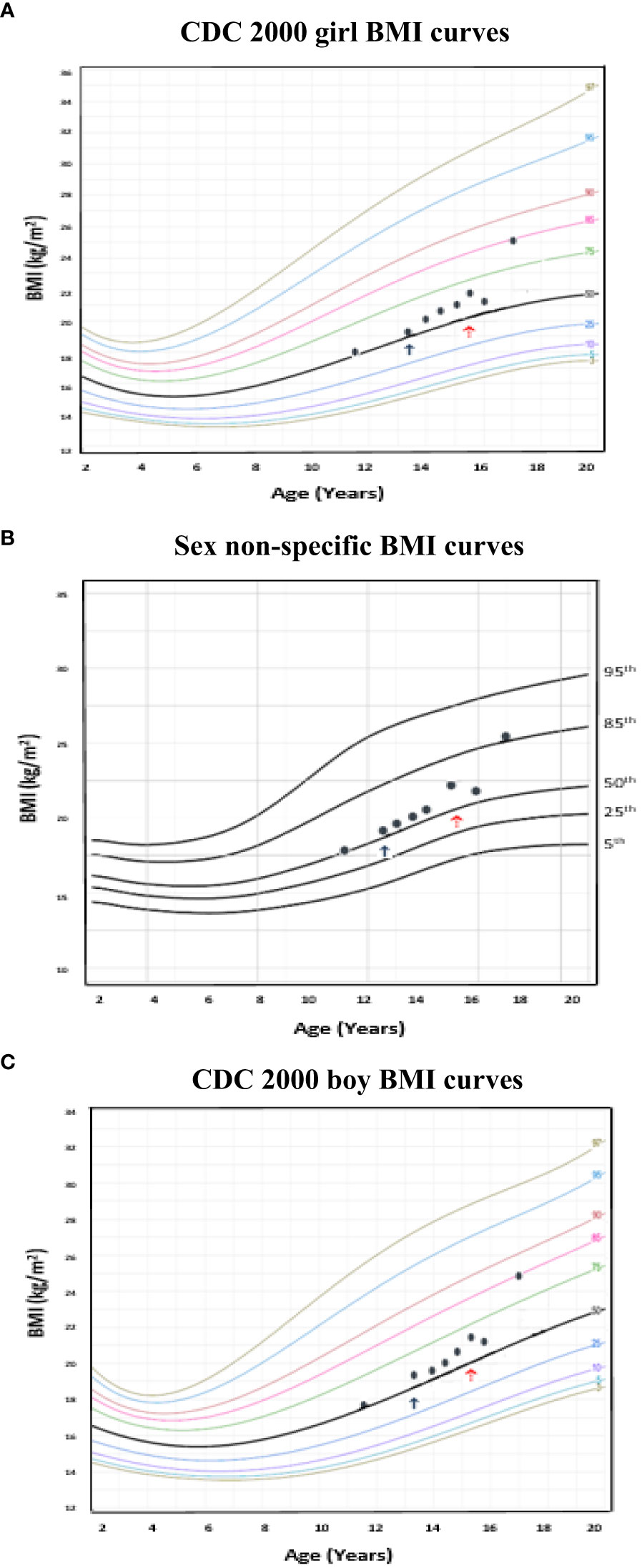
Figure 5 Example of a transgender female (assigned male at birth) individual with body mass index (BMI) plotted on the age-adjusted sex non-specific BMI curves. (B) shows BMI plotted on the age-adjusted sex non-specific BMI curves, while (A) and (C) show BMI plotted on the CDC 2000 girl and boy BMI curves, respectively. This individual received pubertal suppression with gonadotropin releasing hormone agonist therapy (pubertal blocker) beginning around age 13 years (↑) and subsequently began receiving estradiol for cross-sex hormonal therapy beginning around age 15 years (↑). At 17 years old, BMI was in the normal weight category (83rd percentile) on the CDC 2000 girl chart, however, overweight category (86th percentile) on the age-adjusted sex non-specific growth chart.
Finally, we created age-adjusted sex non-specific z-score calculators for height, weight, and BMI (http://tsaheight2020.shinyapps.io/gender0growthcharts). This website also includes comparative age-adjusted, sex non-specific growth curves, their CDC 2000 girl and boy counterpart curves, and data tables.
Using data from large multi-ethnic cross-sectional populations of US youth, we created age-adjusted, sex non-specific growth charts; reference ranges for height, weight, and BMI; and z-score calculators that age-smooth differences between sexes to assess how a youth is growing “as a child.” While challenging to specifically quantify, and with some differences among height, weight, and BMI, our growth charts are largely superimposable with the CDC 2000 girl and boy charts for about the first 10 years of life, after which divergence becomes more apparent, largely coinciding with general pubertal onset (34, 35). Therefore, clinical utility of these growth charts may have less impact during the pre-pubertal years, however become more important during pubertal onset and thereafter.
While the most commonly used US growth charts have been the WHO (for 0-2 year olds) and CDC 2000 (for 2-19 year olds) charts over the last at least 15 years, clinicians and researchers have realized the necessity of creating additional growth charts to address arisen needs among specific populations. For example, there are now specialized growth charts for Down, Turner, Noonan, and Williams syndromes (36–39). Additionally, pediatric severe obesity (defined as BMI ≥1.2 times the 95th BMI percentile (40)) charts have been generated from CDC 2000 data to better document varying degrees of obesity (41). Of note, creation of these additional charts has largely been made possible through the use of newer statistical techniques and/or larger numbers of youth within these subpopulations available for analysis, and more continue to be developed (31, 32).
The prevalence of youth identifying as transgender has been increasing (42). While difficult to determine, estimates suggest 1.2–4.1% of adolescents report a gender identity different from their genetic sex, with a similar number being variant in gender expression (43). Concurrently, the number of youth presenting for transgender care is growing with a steadily increasing demand for services in multidisciplinary clinics on several continents (6). Methods for tracking growth parameters in this population are lacking, and while it has been proposed that such youth be dually-tracked on girl and boy growth charts, this approach has limitations (i.e., may misclassify diagnoses of weight-related disorders; lead to difficulties predicting near adult height) (7, 10, 44). Therefore, there is a need to track how one is growing as a “transgender child” compared to specifically as a “girl” or “boy.” Our growth charts and z-score calculators can serve as an intermediate reference between the male-specific or female-specific data points until longitudinal growth data are available for the creation of transgender-specific growth charts. Further, as we do not yet know attitudes toward height growth in non-binary young people, sex non-specific charts can serve as a way to monitor overall growth and weight status, and to collect longitudinal data that can be linked to their attitudes/perceptions of growth.
There are a number of clinical applications whereby use of age-adjusted, sex non-specific growth charts may be helpful. For one, differences in BMI percentiles between sexes may misclassify diagnoses of weight-related disorders (i.e., overweight/obesity, underweight) in transgender youth (10). Kidd et al. presents examples illustrating this, including a 16 year old transgender male (assigned female at birth) adolescent on GnRHa and testosterone who would be classified as overweight on the girl but obese on the boy growth chart (10). Similarly, we present (Figure 5) a transgender male (assigned female at birth) adolescent who, at 17 years of age had a BMI in the normal weight category (83rd percentile) on the CDC 2000 girls growth chart, however, in the overweight category (86th percentile) on the sex non-specific growth chart. Longitudinal studies linking body composition measures of transgender youth to age-adjusted, sex non-specific BMI percentiles versus BMI percentiles for one’s sex and gender may help to more accurately assess a transgender youth’s weight classification. As the diagnosis of pediatric obesity accompanies medical and psychological sequelae and is associated with increased healthcare utilization, accurate diagnosis is imperative (18, 45–47). Further, if a child or adolescent is not diagnosed with overweight/obesity when they indeed have this, opportunities for earlier intervention and prevention of complications may be missed (18).
We do not believe that use of our age-adjusted, sex non-specific growth charts would impact decisions on when to start pubertal blockade, as early pubertal suppression leads to better psychological and physical outcomes (7). That said, our growth curves may help in guiding medical therapy to potentially augment height. For example, although one generally desires to target an appropriate near adult height for one’s chosen gender, this is often an unreasonable expectation, particularly in a transgender male (assigned female at birth) child during medical intervention with pubertal suppression and cross-hormone sex therapy.
Figure 4 shows how using these sex non-specific growth charts can be a more practical tool compared to the common practice of comparing the girl and boy charts side-by-side for clinical decision-making in terms of near adult height prediction. Prior to starting puberty blockers at 11 years old, the growth patterns of this transgender male (assigned female at birth) child were similar on the sex non-specific, CDC 2000 boy, and CDC 2000 girl growth charts at around the 50th percentile. However, after three years of pubertal suppression, his growth patterns began to diverge on the CDC 2000 boy and girl charts, a difference that became more pronounced after starting testosterone therapy. On the female chart, growth plots increased from the 25th toward the 50th percentile and, after starting testosterone, further increased to the 85th percentile. On the male chart, growth plots decreased from the 25th to the 10th percentile after starting testosterone, and increased to only the 20th percentile by age 18 years compared to the 85th on the female chart. This level of divergence makes it difficult to assess the impact of testosterone on growth solely by comparing the boy and girl CDC 2000 growth charts. In contrast, on the sex non-specific charts, after three years on pubertal suppression, growth decreased from the 50th to the 25th percentile and, after initiating testosterone, increased back toward the 50th percentile where he was growing prior to pubertal suppression. Therefore, the growth patterns seen on the sex non-specific chart provide an easily interpretable growth trajectory and target for medical interventions that can be used until longitudinal growth charts specific for transgender children are developed.
Our study has several strengths. Creation of our growth charts and z-score calculators was done using data from large nationally representative multi-ethnic cross-sectional cohorts (19–24). Specifically, we utilized the same datasets and similar statistical techniques used to create CDC 2000 growth charts, allowing for a direct comparison between our charts and those most commonly used in the US (25, 26). Further, our large sample size afforded us adequate statistical power to create these growth charts.
Our study also has limitations. Data used to create our weight and BMI charts were from 1963-1994, largely predating the obesity epidemic. Therefore, these growth charts may be different if more contemporaneous datasets were used. Pediatric severe obesity was rare from 1963-1994, whereas now it is the fastest growing pediatric obesity category (48). In terms of whether the use of more contemporaneous height data would also affect our growth charts (i.e., whether children are now comparatively taller), we suspect this not to be the case based upon current analyses of NHANES data from our group suggesting no secular trends (unpublished). Finally, we note that our z-scores have wider variability compared to those from CDC 2000 resulting from combining girls and boys in our analyses, with differences in pubertal growth spurt timing and long bone growth between sexes.
Although we believe that creation of our age-adjusted, sex non-specific growth charts is an important step towards addressing a critical need in the transgender population, we recognize the imperative need for future research and longitudinal data collection leading to separate growth charts and z-score calculators for transgender male and female youth. This will require significant time and effort. For example, NHANES data for the CDC 2000 growth charts were collected from 1960-1994. Further, it may be helpful to have sex non-specific height velocity charts and z-score calculators given their importance and practicality for tracking changes in height over shorter time durations (49). This is especially important given that height velocity may be altered by GnRHa and/or cross-sex hormonal therapy use (44, 50). Given the inherent limitations of creating height velocity charts using cross-sectional data, development of these charts would be best done using longitudinal cohorts and data registries tracking individuals’ growth over time (51).
In conclusion, we developed age-adjusted, sex non-specific growth charts that may eventually be used in scenarios in which standard “girls versus boys” growth charts may not be ideal. Presently, our sex non-specific growth charts should be considered a research tool that needs validation before they can be applied to clinical practice. Given the increasing prevalence of youth seeking transgender care and recognized limitations of current approaches, a critical need has arisen in terms of tracking growth parameters in these individuals. Until longitudinal data, including body composition measures, are available in this population, our growth charts may help to assess a transgender youth’s growth trajectory and weight classification, and expectations surrounding this.
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs.
The studies involving human participants were reviewed and approved by CDC/National Center for Health Statistics institutional review board. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
EB contributed to the research concept and design, statistical analyses, and data analysis and interpretation, wrote the first draft of the manuscript, and made critical revisions for intellectual content. BM contributed to the research concept and design, data interpretation, and manuscript write-up, and made critical revisions for intellectual content. OA and MJ contributed to the research concept and design, data interpretation, and manuscript write-up; performed statistical analyses, and made critical revisions for intellectual content. AR contributed to data interpretation, manuscript write-up, and critical revisions for intellectual content. KS developed the research concept and design, contributed to data analysis, interpretation, and manuscript write-up; and made critical revisions for intellectual content. All authors contributed to the article and approved the submitted version.
Research reported in this publication was supported by the NIDDK of the National Institutes of Health under Award Number K23DK125668. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
EB is a site principal investigator and site co-investigator for Novo Nordisk. BM is a consultant for Abbvie, Ascendis, BioMarin, Bluebird Bio, Endo Pharmaceuticals, Novo Nordisk, Pfizer, Sandoz, Sanofi Genzyme, Tolmar, and Vertice, and has received research support from Alexion, Abbvie, Amgen, Ascendis, BioMarin, Novo Nordisk, Opko, Protalix, Sandoz, Sangamo, Sanofi Genzyme and Takeda. ADR is a consultant for Antares Pharma, Ascendis Pharma A/S, Clarus Therapeutics, Lumos Pharma, the United States Anti-doping Agency USADA, and Ultragenyx Pharmaceutical. KS receives research support from the DHHS Federal Food and Drug Administration, NIH National Cancer Institute, National Science Foundation, Spruce Biosciences, Alexion and Neurocrine.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1227886/full#supplementary-material
1. Tanner JM, Davies PSW. Clinical longitudinal standards for height and height velocity for North American children. J Pediatr (1985) 107(3):317–28. doi: 10.1136/adc.51.3.170
2. de Onis M, Garza C, Onyango AW, Martorell R. WHO child growth standards. Acta Paediatr (2006) 95(450):1–106. doi: 10.1080/08035320500495373
3. Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat (2002) 11(246):1–190.
4. Cole TJ. The development of growth references and growth charts. Ann Hum Biol (2012) 39(5):382.394. doi: 10.3109/03014460.2012.694475
5. Freeman JV, Cole TJ, Chinn S, Jones PR, White EM, Preece MA. Cross sectional stature and weight reference curves for the UK 1990. Arch Dis Child (1995) 73(1):17–24. doi: 10.1136/adc.73.1.17
6. Rosenthal SM. Transgender youth: current concepts. Ann Pediatr Endocrinol Metab (2016) 21:185–92. doi: 10.6065/apem.2016.21.4.185
7. Hembree WC, Cohen-Kettenis PT, Gooren L, Hannema SE, Meyer WJ, Murad MH, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab (2017) 102(11):3869–903. doi: 10.1210/jc.2017-01658
8. Leschek EW, Flor AC, Byant JC, Jones JV, Barnes KM, Cutler GB. Effect of antiandrogen, aromatase inhibitor, and gonadotropin-releasing hormone analog on adult height in familial male precocious puberty. J Pediatr (2017) 190:229–35. doi: 10.1016/j.jpeds.2017.07.047
9. Krishna KB, Fuqua JS, Rogol AD, Klein KO, Popovic J, Houk CP, et al. Use of gonadotropin-releasing hormone analogs in children: update by an international consortium. Horm Res Paediatr (2019) 91:357–72. doi: 10.1159/000501336
10. Kidd KM, Sequeira GM, Dhar CP, Montano GT, Witchel SF, Rofey D. Gendered body mass index percentile charts and transgender youth: making the case to change charts. Transgend Health (2019) 4(1):297–9. doi: 10.1089/trgh.2019.0016
11. Klaver M, de Blok CJM, Wiepjes CM, Nota NM, Dekker MJHJ, de Mutsert R, et al. Changes in regional body fat, lean body mass, and body shape in trans persons using cross-sex hormonal therapy: results from a multicenter prospective study. Eur J Endocrinol (2018) 178:163–71. doi: 10.1530/EJE-17-0496
12. Velho I, Fighera TM, Ziegelmann PK, Apritzer PM. Effects of testosterone therapy on BMI, blood pressure, and laboratory profile of transgender men: a systematic review. Andrology (2017) 5:881–8. doi: 10.1111/andr.12382
13. Boogers LS, Wiepjes CM, Klink DT, Hellinga I, van Trotsenburg ASP, den Heijer M, et al. Transgender girls grow tall: adult height is unaffected by GnRH analogue and estradiol treatment. J Clin Endocrinol Metab (2022) 107(9):e3805–15. doi: 10.1210/clinem/dgac349
14. Willemsen LA, Boogers LS, Wiepjes CM, Klink DT, van Trotsenburg ASP, den Heijer M, et al. Just as tall on testosterone: a neutral to positive effect on adult height of GnRHa and testosterone in trans boys. J Clin Endocrinol Metab (2023) 108(2):414–21. doi: 10.1210/clinem/dgac571
15. Stack A, Butler G, Cole T. Using SITAR analysis to explore the impact of gonadotropin-releasing hormone analogues on the pubertal growth spurt in adolescents with gender dysphoria. Endocrine Abstracts (2021) 78:P32. doi: 10.1530/endoabs.78.P32
16. Addo OY, Sarafoglou K, Miller BS. Effect of adjusting for tanner stage age on prevalence of short and tall stature of youths in the United States. J Pediatr (2018) 201:93–9. doi: 10.1016/j.jpeds.2018.05.051
17. Miller BS, Sarafoglou K, Addo OY. Development of tanner stage age adjusted CDC height curves for research and clinical applications. J Endocr Soc (2020) 4(9):bvaa098. doi: 10.1210/jendso/bvaa098
18. Bomberg EM, Addo OY, Sarafoglou K, Miller BS. Adjusting for pubertal status reduces overweight and obesity prevalence in the United States. J Pediatr (2021) 231:200–6. doi: 10.1016/j.jpeds.2020.12.038
19. Centers for Disease Control and Prevention, National Center for Health Statistics. NHES II (1963-1965). Available at: https://wwwn.cdc.gov/nchs/nhanes/nhes2/Default.aspx (Accessed 18 May 2023).
20. Centers for Disease Control and Prevention, National Center for Health Statistics. NHES III (1966-1970). Available at: https://wwwn.cdc.gov/nchs/nhanes/nhes3/Default.aspx (Accessed 18 May 2023).
21. Centers for Disease Control and Prevention, National Center for Health Statistics. NHANES I (1971-1974). Available at: https://wwwn.cdc.gov/nchs/nhanes/nhanes1/Default.aspx (Accessed 18 May 2023).
22. Centers for Disease Control and Prevention, National Center for Health Statistics. NHANES II (1976-1980). Available at: https://wwwn.cdc.gov/nchs/nhanes/nhanes2/Default.aspx (Accessed 18 May 2023).
23. Centers for Disease Control and Prevention, National Center for Health Statistics. NHANES III (1988-1994). Available at: https://wwwn.cdc.gov/nchs/nhanes/nhanes3/Default.aspx (Accessed 18 May 2023).
24. Centers for Disease Control and Prevention, National Center for Health Statistics. NHANES response rates and population totals. Available at: https://wwwn.cdc.gov/nchs/nhanes/ResponseRates.aspx#population-totals (Accessed 18 May 2023).
25. Grummer-Strawn LM, Garza C, Johnson CL. Childhood growth charts. Pediatrics (2002) 109(1):141–2. doi: 10.1542/peds.109.1.141
26. Lohman TG, Roche AF, Martorell R. Anthropometric standardization reference manual. Hum Kinet (1988) 6(1):1–177.
27. Rigby RRA, Stasinopoulos DM. Generalized additive models for location, scale and shape. Appl Statist (2005) 54(3):507–54. doi: 10.1111/j.1467-9876.2005.00510.x
28. Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ (2000) 320(7244):1240–3. doi: 10.1136/bmj.320.7244.1240
29. Cole TJ, Green PJ. Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med (1992) 11(10):1305–19. doi: 10.1002/sim.4780111005
30. de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ (2007) 85(9):660–7. doi: 10.2471/blt.07.043497
31. WHO. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Suppl (2006) 450:76–85. doi: 10.1111/j.1651-2227.2006.tb02378.x
32. Rigby RA, Stasinopoulos DM. Smooth centile curves for skew and kurtotic data modelled using the Box-Cox power exponential distribution. Stat Med (2004) 23(19):3053–76. doi: 10.1002/sim.1861
33. Stasinopolus M, Rigby B, Akantziliotou C. Instructions on how to use the gamlss package in R (2013). Available at: http://www.gamlss.com/wp-content/uploads/2013/01/gamlss-manual.pdf (Accessed 18 May 2023).
34. Biro FM, Greenspan LC, Galvez MP, Pinney SM, Teitelbaum S, Windham GC, et al. Onset of breast development in a longitudinal cohort. Pediatrics (2013) 132(6):1019–27. doi: 10.1542/peds.2012-3773
35. Herman-Giddens ME, Steffes J, Harris D, Slora E, Hussey M, Dowshen SA, et al. Secondary sexual characteristics in boys: data from the Pediatric Research in Office Settings Network. Pediatrics (2012) 130(5):e1058–68. doi: 10.1542/peds.2011-3291
36. Zemel BS, Pipan M, Stallings VA, Hall W, Schadt K, Freedman DS, et al. Growth charts for children with Down Syndrome in the United States. Pediatrics (2015) 136(5):e1204–1211. doi: 10.1542/peds.2015-1652
37. Lyon AJ, Preece MA, Grant DB. Growth curve for girls with Turner syndrome. Arch Dis Child (1985) 60(10):932–5. doi: 10.1136/adc.60.10.932
38. Isojima T, Sakazume Y. Development of disease-specific growth charts in Turner syndrome and Noonan syndrome. Ann Pediatr Endocrinol Metabl (2017) 22(4):240–6. doi: 10.6065/apem.2017.22.4.240
39. Martin NDT, Smith WR, Cole TJ, Preece MA. New height, weight and head circumference charts for British children with Williams syndrome. Arch Dis Child (2007) 92(7):598–601. doi: 10.1136/adc.2006.107946
40. Kelly AS, Barlow SE, Rao G, Inge TH, Hayman LL, Steinberger J, et al. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement from the American Heart Association. Circulation (2013) 128(15):1689–712. doi: 10.1161/CIR.0b013e3182a5cfb3
41. Gulati AK, Kaplan DW, Daniels SR. Clinical tracking of severely obese children: a new growth chart. Pediatrics (2012) 130(6):1136–40. doi: 10.1542/peds.2012-0596
42. Meerwijk EL, Sevelius JM. Transgender population size in the United States: a meta-regression of population-based probability samples. Am J Public Health (2017) 107:e1–8. doi: 10.2105/AJPH.2016.303578
43. Bonifacio JH, Maser C, Stadelman K, Palmert M. Management of gender dysphoria in adolescents in primary care. Can Med Assoc J (2019) 191:e69–75. doi: 10.1503/cmaj.180672
44. Roberts SA, Carswell JM. Growth, growth potential, and influences on adult height in the transgender and gender-diverse population. Andrology (2021) 9:1679–88. doi: 10.1111/andr.13034
45. Tremmel M, Gerdtham U, Nilsson PM, Saha S. Economic burden of obesity: a systematic literature review. Int J Environ Res Public Health (2017) 14:e435. doi: 10.3390/ijerph14040435
46. Pont SJ, Puhl R, Cook SR, Slusser W. Stigma experienced by children and adolescents with obesity. Pediatrics (2017) 140:e20173034. doi: 10.1542/peds.2017-3034
47. Grimmett C, Croker H, Carnell S, Wardle J. Telling parents their child's weight status: psychological impact of a weight-screening program. Pediatrics (2008) 122:e682–8. doi: 10.1542/peds.2007-3526
48. Spinelli A, Buoncristiano M, Kovacs VA, Yngve A, Spiroski I, Obreja G, et al. Prevalence of severe obesity among primary school children in 21 European countries. Obes Facts (2019) 12(2):244–58. doi: 10.1159/000500436
49. Kelly A, Winer KK, Kalkwarf H, Oberfield SE, Lappe J, Gilsanz V, et al. Age-based reference ranges for annual height velocity in US children. J Clin Endocrinol Metab (2014) 99:2104–12. doi: 10.1210/jc.2013-4455
50. de Waal DV, Cohen-Kettenis PT. Clinical management of gender identity disorders in adolescents: a protocol on psychological and pediatric endocrinology aspects. Eur J Endocrinol (2006) 155(Suppl1):s131–S137. doi: 10.1530/eje.1.02231
Keywords: growth charts, growth, transgender, body mass index, pediatric obesity, nutrition surveys
Citation: Bomberg EM, Miller BS, Addo OY, Rogol AD, Jaber MM and Sarafoglou K (2023) Sex non-specific growth charts and potential clinical implications in the care of transgender youth. Front. Endocrinol. 14:1227886. doi: 10.3389/fendo.2023.1227886
Received: 23 May 2023; Accepted: 21 July 2023;
Published: 11 August 2023.
Edited by:
Gianluca Tornese, Institute for Maternal and Child Health Burlo Garofolo (IRCCS), ItalyReviewed by:
Gary Butler, University College London Hospitals NHS Foundation Trust, United KingdomCopyright © 2023 Bomberg, Miller, Addo, Rogol, Jaber and Sarafoglou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eric Morris Bomberg, Ym9tYmUwMDJAdW1uLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.