
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 08 September 2023
Sec. Thyroid Endocrinology
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1227530
 Ning Ning1†
Ning Ning1† Juan Li2†
Juan Li2† Wenwu Sun1†
Wenwu Sun1† Chaoping Ma2†
Chaoping Ma2† Jiaoyan Li1
Jiaoyan Li1 Huiqiu Sheng1
Huiqiu Sheng1 Ying Chen1
Ying Chen1 Bing Zhao1
Bing Zhao1 Jiyuan Zhang2
Jiyuan Zhang2 Jiyue Zhu3
Jiyue Zhu3 Chengjin Gao2*
Chengjin Gao2* Enqiang Mao1*
Enqiang Mao1*Background: Nonthyroidal illness syndrome (NTIS) is a common endocrine dysfunction predicting unfavorable outcomes in critical illness. The objective of the study is to evaluate the association between different NTIS subtypes with outcomes in septic patients.
Methods: Septic patients in two Chinese academic centers from October 2012 and October 2022 are enrolled in analysis. Multivariable regressions are used to assess associations between NTIS and outcomes. Outcomes include in-hospital mortality, length of stay in hospital (LOS), non-invasive ventilation failure and weaning failure. Patients with NTIS are categorized into 4 types according to the different levels of FT4 and TSH. The association between different NTIS subtypes and mortality are further analyzed. Survival curve is plotted using the Kaplan–Meier method.
Results: After screening, a total of 1226 septic patients with complete thyroid hormones result are eventually enrolled. Among them, 520 (42.4%) patients are diagnosed as NTIS. In multivariable regression analysis, NTIS is independently associated with increased 30-days mortality (OR=1.759, CI 1.009-3.104, p=0.047), but has no association with 60-days mortality (OR=1.524, CI 0.893-2.618, p=0.123), 90-days mortality (OR=1.411, CI 0.831-2.408, p=0.203), LOS, non-invasive ventilation failure or weaning failure. In NTIS subtypes, NTIS patients with low FT3 and TSH levels, regardless of the FT4 values, have significantly higher mortality than euthyroid patients (30-days mortality, OR= 6.488, CI 1.546-27.808, p=0.01; 60-days mortality, OR=3.973, CI 1.006-15.579, p=0.046; 90-days mortality, OR=3.849, CI 0.977-15.088, p=0.051). This result is consistent in patients with low FT3 and FT4 levels, regardless of the TSH values (30-days mortality, OR=3.349, CI 1.402-7.957, p=0.006; 60-days mortality, OR= 2.594, CI 1.122-5.930, p=0.024; 90-days mortality, OR=2.55, CI 1.110-5.804, p=0.025). There is no survival difference between NTIS patients with low FT3 only and euthyroid patients. Survival plot shows the worst prognosis is in NTIS patients with low FT3, FT4 and TSH level.
Conclusions: NTIS is frequent in sepsis. A reduction of FT3 together with FT4 or TSH, but not FT3 only, is associated with an increased risk of mortality.
Sepsis is an infection induced life-threatening syndrome, which impacts millions of people around the world each year (1). Because of the aging populations, the reported incidence of sepsis is still increasing (2). For the better prognosis of septic patients, the treatment and management methods are still evolving.
Neuroendocrine systems dysfunction could contribute to sepsis-induced organ failure (3). Nonthyroidal illness syndrome (NTIS), also called “low T3 syndrome” or “euthyroid sick syndrome”, is the most common endocrine dysfunction in critical illness (4). The most obvious laboratory finding in NTIS is a dropped triiodothyronine (T3) with low or normal levels of thyroxine (T4) or thyroid stimulating hormone (TSH) in the absence of previous thyroidal diseases (5). The mechanism of NTIS in sepsis is not fully understood yet. One explanation is the theory of the malfunction of hypothalamic-pituitary-thyroid axis by inflammatory cytokine or some drugs (6, 7). This theory is supported by the evidence that TSH level is decreased in a part of critically ill patients. Another explanation is the theory of impaired T4 metabolism in peripheral tissues. This theory is supported by the evidence that the reverse T3 (rT3), an inactive peripheral metabolite of T4, is increased in critically ill patients (8).
Previous studies have proved NTIS is associated with an unfavorable outcomes in septic patients (9). However, the pathophysiological significance of NTIS in sepsis could not be confirmed. The thyroid hormones change in critically ill patients has been regarded as similar as the condition of starvation, in which low T3 level is seen as the part of an adaptive metabolic response in an attempt to ameliorate the stress by lowering the metabolic activity (10). But this view is challenged by some suggesting that NTIS represents a maladaptive response to illness that requires correction. The question of whether or not septic patients will gain benefit from treating NTIS could not be answered yet. This question could only be answered in large randomized control trials (RCT). Some RCTs have been published not specifically for septic patients. However, the result is still inclusive (11). Surely, it should be pointed out that normalizing thyroid hormone concentrations is not necessarily equal to normalizing local thyroid hormone action, especially in critical illness (12). Astonishingly, no RCT result has been published to assess the treatment effects in septic patients with NTIS.
The objective of the study is therefore to identify subgroups of septic patients who might benefit from endocrine treatment in future RCTs.
This retrospective study was conducted in emergency intensive care unit (ICU), respiratory ICU and general ICU of Ruijin Hospital, Shanghai Jiaotong University School of Medicine, an academic 2100 beds hospital; and Xinhua Hospital, Shanghai Jiaotong University School of Medicine, an academic 2000 beds hospital. The protocol was approved by the institutional ethics board of each hospital (RJ-2022-312, XHEC-D-2023-074). The informed consent was waived because of the non-interventional, retrospective design of the study. Data analysis was performed in accordance with the 1964 Helsinki Declaration and its later amendments.
Consecutive adult patients with a diagnosis of sepsis between September 2015 and May 2022 (Ruijin Hospital, Shanghai Jiaotong University School of Medicine), and between October 2012 and October 2022 (Xinhua Hospital, Shanghai Jiaotong University School of Medicine) were screened. All patients were treated according to our local protocol, which included administering antibiotics as early as possible and initiating fluid resuscitation with crystalloids in addition to source control. Patient exclusion criteria included (1): without thyroid hormones test within 24h after admission (2) less than 48 hours from admission to discharge or death; (3) history of malignancy or autoimmune diseases; (4) history of thyroidal or pituitary diseases; (5) using drug that interfere with the hypothalamic-pituitary-thyroid axis before thyroid hormones test, such as contrast agents, amiodarone, thyroxine, thyreostatic agents, dopamine, lithium, antidepressants, and glucocorticoids.
The clinical variables are extracted from the paper-based or electronic medical records for each patient. Baseline demographic information includes age, sex, comorbidities (hypertension, diabetes and chronic kidney diseases). Infection sites are classified into respiratory system, gastrointestinal tract, urogenital tract, and others. Laboratory indicators were collected within 24 hours after admission included white blood cell count, alanine aminotransferase, total bilirubin, creatinine, lactate, C-reactive protein, procalcitonin and platelets count. Thyroid function (including TT3, FT3, TT4, FT4, TSH and rT3), Acute Physiology and Chronic Health Evaluation II scores (APACHE II) and Sequential Organ Failure Assessment (SOFA) scores were collected within 24 hours after admission. The use of non-invasive ventilation, invasive ventilation, and vasopressors were reported as well. Outcomes include in-hospital mortality, length of stay in hospital (LOS), non-invasive ventilation failure and weaning failure. Non-invasive ventilation failure is defined as oxygenation could not be maintained by non-invasive ventilation, and invasive ventilation with intubation or tracheotomy should be used. Weaning failure is defined as oxygenation could not be maintained after spontaneous breathing trial, and tracheostomy should be used; or patients suffered second intubation within 24h after extubation.
The diagnosis of sepsis is defined as SOFA score >= 2 points consequent to the infection (Sepsis-3) (1). NTIS in this study is defined as a low serum FT3 level (<2.63pmol/L) without an elevated TSH level (< 4.94μIU/mL). The normal range of serum thyroid hormones in this study: FT3, 2.63–5.7pmol/L; FT4, 9.01–19.04pmol/L; TT3, 0.89–2.44nmol/L; TT4, 62.67–150.84nmol/L; rT3, 31-95ng/dl; and TSH, 0.35–4.94μIU/mL. Patients with NTIS are categorized into 4 types: type 1, low FT3, normal FT4 and TSH; type 2, low FT3, normal FT4, and low TSH; type 3, low FT3, low FT4, and normal TSH; type 4, low FT3, low FT4 and TSH.
Categorical data will be described with frequency or ratio. Continuous variables will be described using median and interquartile range. Categorical variables will be compared using χ2 test or Fisher exact test. Continuous variables will be compared using the t-test for normally distributed variables or Wilcoxon rank-sum test for non-normally distributed variables. We implement multiple predictive mean matching imputations using chained equation and created 5 independent datasets to compute missing data. Variables with missing data more than 20% are deleted.
Multivariable logistic regression is used to assess associations between 30-days mortality, 60-days mortality, 90-days mortality and NTIS. Multivariable linear regression analysis is used to assess association between LOS and NTIS. Effect sizes are reported as odds ratios (OR) and β estimates, respectively. Baseline information include age, sex, comorbidities, infection sites are adjusted. Moreover, total bilirubin represents liver function; creatinine represents renal function; lactate represents oxygen debt; C-reactive protein represents overall inflammation; procalcitonin represents infection severity; platelets count represents coagulation function; APACHE II score, use of non-invasive ventilation, invasive ventilation, and vasopressors are known associated with illness severity. These variables are also adjusted in the models. A subgroup of patients underwent non-invasive ventilation is selected to estimate the effect of NTIS on the incidence of non-invasive ventilation failure. A subgroup of patients underwent invasive ventilation is selected to estimate the effect of NTIS on the incidence of weaning failure. Multivariable logistic regression is used and OR is reported. Considering the limited number of patients in subgroups, only variables with significant difference in univariable logistic regression (p<0.05) are adjusted. Survival curve is plotted using the Kaplan–Meier method. All statistical analyses are performed using R software (version 4.2.1). A two-sided significance level less than 0.05 are defined as statistical significance.
A total of 1226 septic patients are eventually enrolled in our study. Among them, 605 (49.3%) patients are euthyroid with normal serum level of FT3, FT4 and TSH; 520 (42.4%) patients are diagnosed as NTIS with a low serum level of FT3 without elevated TSH. The other 101 (8.3%) patients are diagnosed with other thyroid hormones abnormality, but not included in the following analysis. The selection flowchart is shown in Figure 1.
A total of 1125 septic patients with euthyroid or NTIS are included in analysis. Patients with NTIS are older (median 72 vs 70, p =0.01), with fewer male (56.3% vs 65.3%, p=0.002), and have higher percentage of diabetes (42.1% vs 31.6%, p<0.001) and chronic kidney diseases (27.3% vs 17.5%, p<0.001). Most of the laboratory indicators on admission are worse in patients with NTIS, except for alanine aminotransferase (median 28 vs 28, p=0.238) and lactate (median 1.9 vs 1.7, p=0.404). As expected, the NTIS is associated with the severity of disease. APACHEII score (median 14 vs 10, p<0.001) and SOFA score (median 6 vs 3, p<0.001) are much higher in patients with NTIS. Patients with NTIS are more likely to receive non-invasive ventilation (24.6% vs 12.6%, p<0.001), invasive ventilation (21.5% vs 6.4%, p<0.001), and use vasopressors (34.6% vs 12.9%, p<0.001) than euthyroid patients (Table 1).
In multivariable logistic regression, after adjustment for confounders, NTIS is independently associated with increased 30-days mortality (OR=1.759, CI 1.009-3.104, p=0.047), but has no association with 60-days mortality (OR=1.524, CI 0.893-2.618, p=0.123) and 90-days mortality (OR=1.411, CI 0.831-2.408, p=0.203). In multivariable linear regression analysis, after adjustment for confounders, there is no association between NTIS and LOS (β estimate=-1.973, CI -5.485 to 1.537, p=0.270). A subgroup of patients underwent non-invasive ventilation (n=204). The incidence of non-invasive ventilation failure is 34.3% (n=70). Baseline characteristics between patients with or without non-invasive ventilation failure are shown in Supplemental Table 1. In multivariable logistic regression, after adjustment for age, sex, C-reactive protein, APACHEII score and use of vasopressors, there is no association between NTIS and non-invasive ventilation failure (OR=1.774, CI 0.840-3.828, p=0.136). A subgroup of patients underwent invasive ventilation (n=151). The incidence of weaning failure is 35.7% (n=54). Baseline characteristics between patients with or without weaning failure are shown in Supplemental Table 2. In multivariable logistic regression, after adjustment for age, sex, chronic kidney diseases and APACHEII score, there is no association between patients NTIS and weaning failure (OR=1.437, CI 0.624-3.453, p=0.402). The associations are shown in Table 2.
A total of 520 patients with NTIS are categorized into 4 types according to the different levels of FT4 and TSH: 349 (67.1%) patients are classified into type 1; 98(18.8%) patients are classified into type 2; 57 (10.9%) patients are classified into type 3; and 16 (3.2%) patients are classified into type 4. The patient number and thyroid hormones characteristics of different types NTIS are shown in Table 3; Figure 2. Figure 3 compares the cumulative survival rates between the euthyroid and different NTIS types. As shown in the survival curve, patients with NTIS type 4 (low FT3, FT4 and TSH) have the highest mortality rate compared with euthyroid or other types NTIS. Considering the low percentage of NTIS type 3and NTIS type 4 in the total patients, different NTIS types are grouped for further analysis. NTIS group 1: low FT3 and TSH levels, regardless of the FT4 values (NTIS type 2+NTIS type 4). NTIS group 2: low FT3 and FT4 levels, regardless of the TSH values (NTIS type 3+NTIS type 4). As shown in Table 4, patients with low FT3 and TSH levels, regardless of the FT4 values, have significantly higher incidence of mortality than euthyroid patients (30-days mortality, OR= 6.488, CI 1.546-27.808, p=0.01; 60-days mortality, OR=3.973, CI 1.006-15.579, p=0.046; 90-days mortality, OR=3.849, CI 0.977-15.088, p=0.051). But there is no significant difference between patients with low FT3 only and enthyroid (30-days mortality, OR=1.489, CI 0.788-2.827, p=0.219; 60-days mortality, OR=1.399, CI 0.762-2.570, p=0.276; 90-days mortality, OR=1.335, CI 0.730-2.241, p=0.345). Similarly, patients with low FT3 and FT4 levels, regardless of the TSH values, have significantly higher incidence of mortality than euthyroid patients (30-days mortality, OR=3.349, CI 1.402-7.957, p=0.006; 60-days mortality, OR= 2.594, CI 1.122-5.930, p=0.024; 90-days mortality, OR=2.55, CI 1.110-5.804, p=0.025). But there is no significant difference between patients with low FT3 only and enthyroid (30-days mortality, OR=1.564, CI 0.842-2.925, p=0.157; 60-days mortality, OR=1.376, CI 0.760-2.494, p=0.29; 90-days mortality, OR=1.282, CI 0.710-2.314, p=0.407).
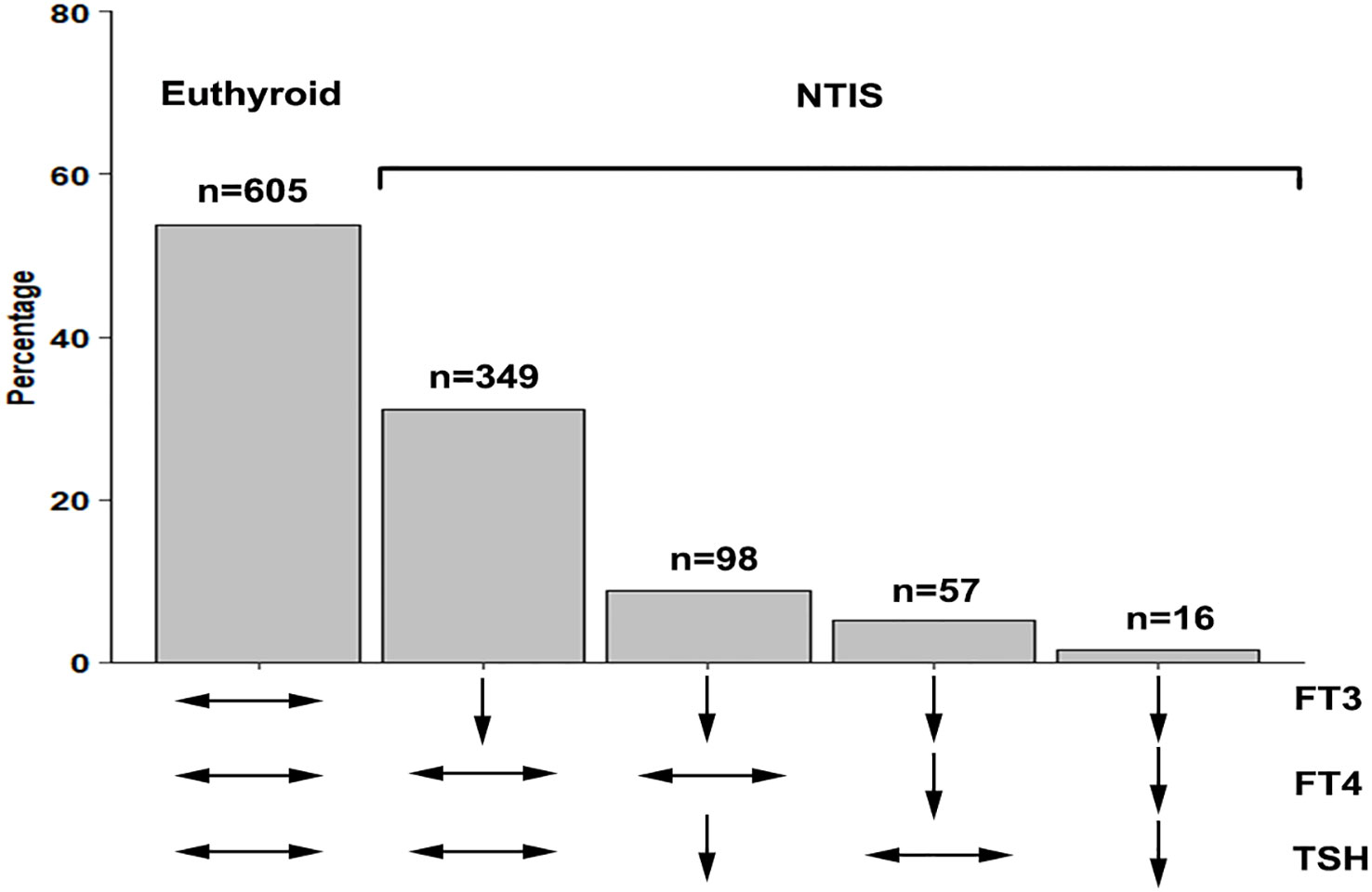
Figure 2 The patient number of different types NTIS. NTIS, nonthyroidal illness syndrome; FT3, free triiodothyronine; FT4, free thyroxine; TSH, thyroid stimulating hormone.
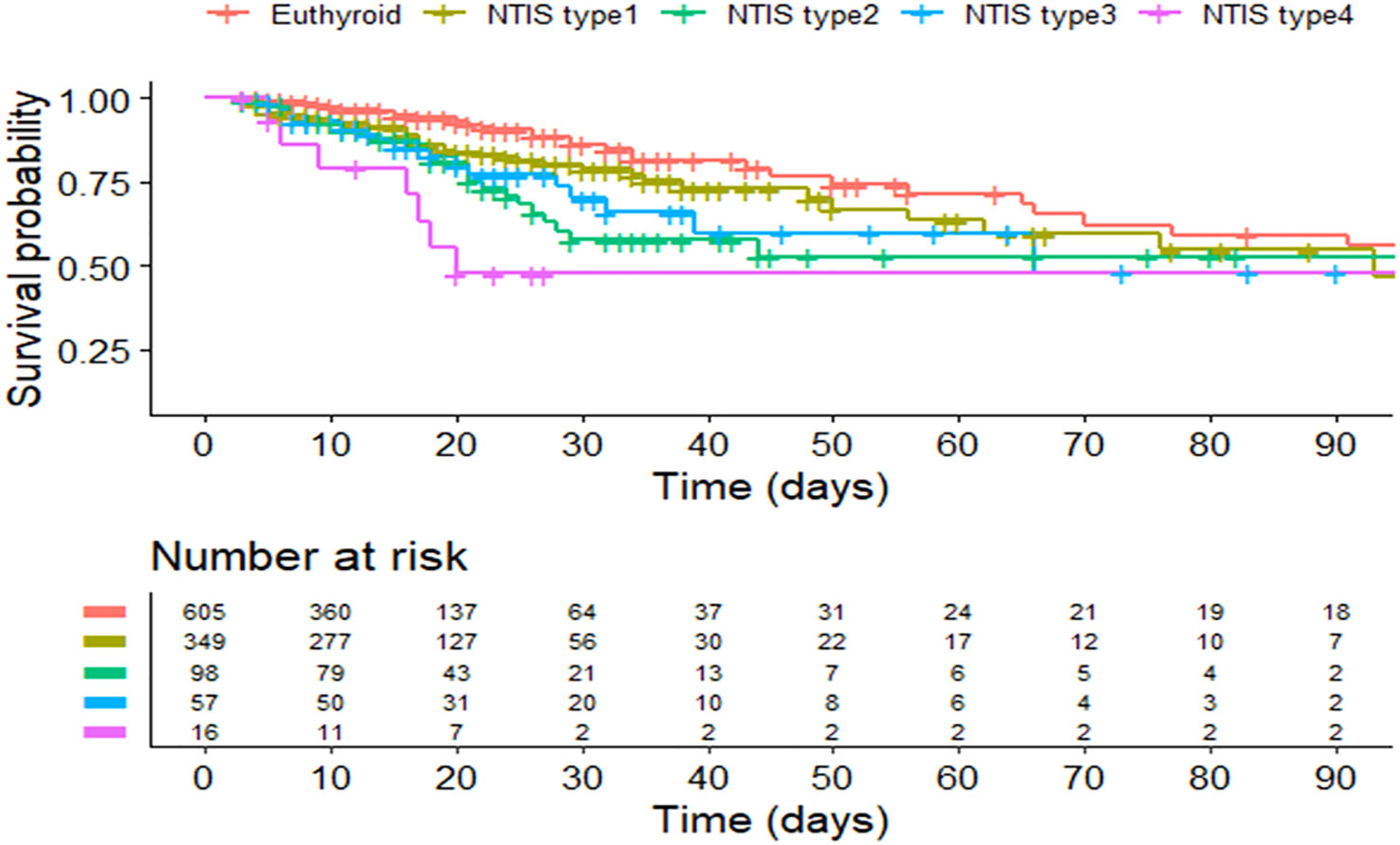
Figure 3 Kaplan–Meier curve of the euthyroid and different NTIS types. NITS, nonthyroidal illness syndrome.
Since first described in 1970s, NTIS has been viewed as a predictor of unfavorable outcomes in critical illness (4). However, there is a lack of RCT evaluating treatment effect on the NTIS in sepsis. One important reason may be that there are disappointing results in the trials of other critical illness. In previous studies, cardiac diseases and cardiac surgery was the interesting field of investigation. But the results showed that, even though the short duration thyroid hormones treatment could increase the cardiac index, there is no improvement on the post-operative mortality (13). In another meta-analysis of congenital heart surgery in children, thyroid hormone replacement could ameliorate inotrope score, but not effective on mechanical ventilation duration, LOS and post-operative mortality (14). However, it must be pointed out that, the conclusion could not be extrapolated to sepsis directly due to the small sample size and different etiologies of these trials.
It is very crucial to select valid endpoints to assess the potential benefit of treatment. Selection of non-appropriate endpoints could result in failure of the trial. Mortality is the most concerned endpoint in clinical trials on sepsis. Todd et al. reported that decreased T3 level at baseline is associated with mortality in 231 surgical septic patients (15). In Wang’s study, it was also reported that decreased serum T4 level can be a predictive marker for in-hospital mortality (16). In our study, the short term 30 days in-hospital mortality is independently associated with NTIS. This conclusion is consistent with previous reports. However, multiple organs and systems could be involved in sepsis. There is lack of evidence regarding the final targets of the thyroid hormones in sepsis. A large part of septic patients suffered respiratory failure, which is a main cause of mortality. Diaphragm and muscle weakness could be induced by sepsis and is correlated with poor outcomes (17, 18). Thyroid hormone is important to normal skeletal muscle function. So it was speculated that the weakness of muscular strength secondary to NTIS increases the risk of mortality in septic patients. We found that patients with NTIS have an increased need for non-invasive ventilation and invasive ventilation, which in addition to signifying a worse clinical condition, may also be an additional indication of skeletal muscle weakness, including the diaphragm. However, NTIS has no association with non-invasive ventilation failure and weaning failure in this study. In Flavia’s research, impaired mitochondrial function of diaphragm muscle could be observed and thyroid hormone treatment could improve mitochondrial parameters on the septic mouse model (19). But the result could not be repeated in human study, that thyroid hormone treatment did not yield any benefit on respiratory muscle function in critically ill patients with NTIS (20).
One important finding of our study is that different NTIS subtypes could significantly impact the mortality in sepsis, which means thyroid hormones pattern heterogeneity should be considered in the patients diagnosed with NTIS. In our study, after excluding hypothalamic-pituitary-thyroid axis diseases, we found NTIS patients with low FT4 independent from the TSH levels, or low TSH independent from the FT4 levels had significantly higher mortality than euthyroid patients. But there is no survival difference between NTIS patients with low FT3 only and euthyroid patients. The worst prognosis is in the group of patients with low FT3, FT4 and TSH levels. Similar conclusion is suggested in another study on 247 critical illness patients (21). But in this study, it was concluded that only low FT3 and FT4 levels is an independent risk factor for mortality; low TSH level has no impact on the prognosis. This conclusion should be interpreted carefully because of the limited number of low TSH level patients in this study. The reduced FT4 or TSH indicates the suppressed hypothalamic-pituitary-thyroid axis response. In previous RCTs, intravenous or oral thyroid hormones substitutions were the choice of intervention (11). However, thyroid hormones substitutions may further suppress the hypothalamic-pituitary-thyroid axis. In animal sepsis models, NTIS can be overcomed by thyrotropin releasing hormone (TRH) administration, confirming that stimulation of the pituitary may restore the decreased serum thyroid hormone levels in sepsis (22). In protracted critical ill patients, administration of TRH and growth hormone-releasing peptide can reactivate blunted TSH secretion and restore plasma thyroid hormone levels (23). These studies provide convincing evidence that the hypothalamic-pituitary-thyroid axis is a possible treatment target in NTIS.
There are certain limitations in this study. Firstly, although the data in this study are prospectively collected, this remains a retrospective study, and missing data is inevitable. Even though we do our best to fill the missing value with multiple imputations, the bias still exists. Secondly, the thyroid hormone levels are changed dynamically during the sepsis process, longitudinal dynamic measurement of thyroid hormone levels is not available. Thirdly, although our study has a large sample size, it was performed on the Chinese population, and only two centers are included. Finally, the cause of the sepsis is not elucidated in this study. However, to our knowledge, this is the first study that comprehensively analyzes the association between different thyroid hormone patterns of NTIS and prognosis in sepsis. This study may provide information about stratification of septic patients and choice of treatment methods in future clinical trials.
In summary, almost half of septic patients complicated with NTIS. Septic patients with NTIS have significantly higher 30 days in-hospital mortality. NTIS patients with low serum FT4 or TSH level have lower survival rate than euthyroid patients. There is no survival difference between NTIS patients with low FT3 only and euthyroid. The worst prognosis is in NTIS patients with low FT3, FT4 and TSH level. Different patterns of NTIS should be considered when enrolling participants or choosing treatment methods in future randomized trials about sepsis.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the institutional ethics board of Ruijin Hospital, Shanghai Jiaotong University School of Medicine and Xinhua Hospital, Shanghai Jiaotong University School of Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because The informed consent was waived because of the non-interventional, retrospective design of the study.bnh
NN, Juan Li, WS and CM: original draft. WS: analysis. Jiaoyan Li, BZ, JZhang and JZhu: data collection. HS and YC: supervision. CG and EM: conceptualization. All authors read and approved the final manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1227530/full#supplementary-material
1. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. (2016) 315:801–10. doi: 10.1001/jama.2016.0287
2. Fleischmann-Struzek C, Mellhammar L, Rose N, Cassini A, Rudd KE, Schlattmann P, et al. Incidence and mortality of hospital- and ICU-treated sepsis: results from an updated and expanded systematic review and meta-analysis. Intensive Care Med (2020) 46:1552–62. doi: 10.1007/s00134-020-06151-x
3. Gheorghita V, Barbu AE, Gheorghiu ML, Caruntu FA. Endocrine dysfunction in sepsis: a beneficial or deleterious host response? Germs (2015) 5:17–25. doi: 10.11599/germs.2015.1067
4. Bermudez F, Surks MI, Oppenheimer JH. High incidence of decreased serum triiodothyronine concentration in patients with nonthyroidal disease. J Clin Endocrinol Metab (1975) 41:27–40. doi: 10.1210/jcem-41-1-27
5. Fliers E, Boelen A. An update on non-thyroidal illness syndrome. J endocrinological Invest (2021) 44:1597–607. doi: 10.1007/s40618-020-01482-4
6. Van den Berghe G, de Zegher F, Lauwers P. Dopamine and the sick euthyroid syndrome in critical illness. Clin Endocrinol. (1994) 41:731–7. doi: 10.1111/j.1365-2265.1994.tb02787.x
7. Haugen BR. Drugs that suppress TSH or cause central hypothyroidism. Best Pract Res Clin Endocrinol Metab (2009) 23:793–800. doi: 10.1016/j.beem.2009.08.003
8. Fliers E, Bianco AC, Langouche L, Boelen A. Thyroid function in critically ill patients. Lancet Diabetes Endocrinol. (2015) 3:816–25. doi: 10.1016/S2213-8587(15)00225-9
9. Angelousi AG, Karageorgopoulos DE, Kapaskelis AM, Falagas ME. Association between thyroid function tests at baseline and the outcome of patients with sepsis or septic shock: a systematic review. Eur J Endocrinol. (2011) 164:147–55. doi: 10.1530/EJE-10-0695
10. Boelen A, Wiersinga WM, Fliers E. Fasting-induced changes in the hypothalamus-pituitary-thyroid axis. Thyroid Off J Am Thyroid Assoc (2008) 18:123–9. doi: 10.1089/thy.2007.0253
11. Sciacchitano S, Capalbo C, Napoli C, Anibaldi P, Salvati V, De Vitis C, et al. Nonthyroidal illness syndrome: To treat or not to treat? Have we answered the question? A review of metanalyses. Front Endocrinol. (2022) 13:850328. doi: 10.3389/fendo.2022.850328
12. Peeters RP, van der Geyten S, Wouters PJ, Darras VM, van Toor H, Kaptein E, et al. Tissue thyroid hormone levels in critical illness. J Clin Endocrinol Metab (2005) 90:6498–507. doi: 10.1210/jc.2005-1013
13. Kaptein EM, Sanchez A, Beale E, Chan LS. Clinical review: Thyroid hormone therapy for postoperative nonthyroidal illnesses: a systematic review and synthesis. J Clin Endocrinol Metab (2010) 95:4526–34. doi: 10.1210/jc.2010-1052
14. Flores S, Loomba RS, Checchia PA, Graham EM, Bronicki RA. Thyroid hormone (Triiodothyronine) therapy in children after congenital heart surgery: A meta-analysis. Semin Thorac Cardiovasc Surg. (2020) 32:87–95. doi: 10.1053/j.semtcvs.2019.05.020
15. Todd SR, Sim V, Moore LJ, Turner KL, Sucher JF, Moore FA. The identification of thyroid dysfunction in surgical sepsis. J Trauma acute Care Surg. (2012) 73:1457–60. doi: 10.1097/TA.0b013e318270db2c
16. Wang Y, Sun F, Hong G, Lu Z. Thyroid hormone levels as a predictor marker predict the prognosis of patients with sepsis. Am J Emergency Med (2021) 45:42–7. doi: 10.1016/j.ajem.2021.02.014
17. Supinski GS, Callahan LA. Diaphragm weakness in mechanically ventilated critically ill patients. Crit Care (2013) 17:R120. doi: 10.1186/cc12792
18. Shabana TS, Anis SG, Ibrahim DM. Association between thyroid dysfunction and intensive care unit-acquired weakness: A case-control study. Crit Care Res Pract. (2021) 2021:8889036. doi: 10.1155/2021/8889036
19. Bloise FF, Santos AT, de Brito J, de Andrade CBV, Oliveira TS, de Souza AFP, et al. Sepsis impairs thyroid hormone signaling and mitochondrial function in the mouse diaphragm. Thyroid Off J Am Thyroid Assoc (2020) 30:1079–90. doi: 10.1089/thy.2019.0124
20. Bello G, Spinazzola G, Giammatteo V, Montini L, De Pascale G, Bisanti A, et al. Effects of thyroid hormone treatment on diaphragmatic efficiency in mechanically ventilated subjects with nonthyroidal illness syndrome. Respir Care (2019) 64:1199–207. doi: 10.4187/respcare.06770
21. Plikat K, Langgartner J, Buettner R, Bollheimer LC, Woenckhaus U, Scholmerich J, et al. Frequency and outcome of patients with nonthyroidal illness syndrome in a medical intensive care unit. Metabolism: Clin Exp. (2007) 56:239–44. doi: 10.1016/j.metabol.2006.09.020
22. Rocchi R, Kimura H, Tzou SC, Suzuki K, Rose NR, Pinchera A, et al. Toll-like receptor-MyD88 and Fc receptor pathways of mast cells mediate the thyroid dysfunctions observed during nonthyroidal illness. Proc Natl Acad Sci USA. (2007) 104:6019–24. doi: 10.1073/pnas.0701319104
23. Van den Berghe G, Wouters P, Weekers F, Mohan S, Baxter RC, Veldhuis JD, et al. Reactivation of pituitary hormone release and metabolic improvement by infusion of growth hormone-releasing peptide and thyrotropin-releasing hormone in patients with protracted critical illness. J Clin Endocrinol Metab (1999) 84:1311–23. doi: 10.1210/jcem.84.4.5636
Keywords: sepsis, nonthyroidal illness syndrome, low T3 syndrome, respiratory function, mortality
Citation: Ning N, Li J, Sun W, Ma C, Li J, Sheng H, Chen Y, Zhao B, Zhang J, Zhu J, Gao C and Mao E (2023) Different subtypes of nonthyroidal illness syndrome on the prognosis of septic patients: a two-centered retrospective cohort study. Front. Endocrinol. 14:1227530. doi: 10.3389/fendo.2023.1227530
Received: 23 May 2023; Accepted: 23 August 2023;
Published: 08 September 2023.
Edited by:
Terry Francis Davies, Icahn School of Medicine at Mount Sinai, United StatesReviewed by:
Pietro Locantore, Catholic University of the Sacred Heart, Rome, ItalyCopyright © 2023 Ning, Li, Sun, Ma, Li, Sheng, Chen, Zhao, Zhang, Zhu, Gao and Mao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Enqiang Mao, bWFvZXFAeWVhaC5uZXQ=; Chengjin Gao, Z2FvY2hlbmdqaW5AeGluaHVhbWVkLmNvbS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.