- 1The First Clinical Medical College, Shandong University of Traditional Chinese Medicine, Jinan, Shandong, China
- 2Department of Gynecology, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan, Shandong, China
- 3College of Acupuncture and Tuina, Shandong University of Traditional Chinese Medicine, Jinan, Shandong, China
Infertility and menstrual abnormalities in endometriosis patients are frequently caused by aberrant follicular growth or a reduced ovarian reserve. Endometriosis typically does not directly harm the oocyte, but rather inhibits the function of granulosa cells, resulting in a decrease in oocyte quality. Granulosa cells, as oocyte nanny cells, can regulate meiosis, provide the most basic resources required for oocyte development, and influence ovulation. Endometriosis affects oocyte development and quality by causing granulosa cells apoptosis, inflammation, oxidative stress, steroid synthesis obstacle, and aberrant mitochondrial energy metabolism. These aberrant states frequently interact with one another, however there is currently relatively little research in this field to understand the mechanism of linkage between abnormal states.
1 Introduction
Endometriosis (EMS) is a common condition in reproductive-age women. Its most common clinical signs are pelvic pain, dyspareunia, a prolonged menstrual period, and a rise in menstrual volume, which can lead to infertility, anxiety, and depression. Ovarian endometriosis (OEM) has the potential to progress to ovarian cancer (1). It is concerning that the etiology of EMS is unclear and has been debated for a long time. There are numerous contentious causes of EMS at the moment, including endometrial cell implantation hypothesis, body cavity metaplasia theory, induction theory, genetic variables, immune factors, and so on (2). The prevalence of this condition has risen in recent years as contemporary medical diagnosis and treatment technology has advanced. At the moment, the primary method of treatment is surgical intervention and hormone therapy, but it is easy to relapse after surgical treatment, which not only costs more but also causes repeated trauma to the patient, especially when patients with OEM and fertility needs are repeatedly stripped by laparoscopic surgery, which will further damage the patient’s reproductive ability (3, 4). Although hormone is effective in treating this disease, it does not improve the ovarian reserve of EMS patients. As a result, we must first understand how EMS lowers patient fertility, which will allow us to develop effective therapies and preventive measures to assist EMS patients in conceiving.
Reduced ovarian reserve and follicle quality, changes in normal pelvic physical environment, decreased endometrial receptivity, and immunological dysfunction are the main reasons for EMS impeding female fertility. The most concerning aspect is that certain EMS patients are unable to generate qualifying eggs, which reduces the success rate of natural conception and assisted reproduction (5). Despite the fact that the meta-analysis found a minor variation in the success rate of assisted reproduction between patients with EMS and those without EMS. However, there are numerous risk factors for long-term pregnancy in EMS patients (6, 7). Because of the delay in diagnosing EMS, some patients with EMS are clinically diagnosed when their ovarian reserve has been compromised, and this damage is difficult to reverse (8). Oocytes are discharged during the maturity of the follicle, the ovary’s most essential functional unit. Follicular granulosa cells(GCs) are the “guards” that accompany oocytes as they grow in size. During egg cell development, they rely mainly on GCs promotion and communication between oocyte and GCs (9). Recent research has demonstrated that the presence of EMS disrupts energy metabolism, apoptosis, and steroid hormone synthesis in GCs, lowering oocyte quality and limiting patients’ reproductive hopes. As a result, this paper explains how EMS injury lowers oocyte quality by causing follicular GCs damage.
2 Ovarian reserve and oocyte quality are reduced in EMS patients
The essence of EMS is that endometrial cells occur in places other than the uterine cavity, with the most common locations being the surface of tissues or organs such as the ovary, utero-rectal pouch, sacral ligament, bladder, and ureter. EMS pathophysiology is comparable to tumor biology in that it involves enhanced proliferation, adhesion, and invasion, increased neovascularization in ectopic endometrial lesions, and decreased apoptosis (10). Endometrial stromal cells from ectopic lesions had significantly increased proliferation, migration, and invasion capacities as compared to normal female endometrial stromal cells, according to research (11). Endometriotic lesions, like cancers, rely on angiogenesis to proliferate. Vascular endothelial growth factor may be produced by the endometrium in the uterine cavity of EMS patients, and the amount of Vascular endothelial growth factor in peritoneal fluid of EMS patients is much higher than that of normal individuals (12). Ectopic endometrial cells respond to estrogen and progesterone in the same way as eutopic endometrial cells do. Surprisingly, some ectopic endometrial cells can also manufacture estrogen on their own (13). Estrogen production is closely related to EMS-associated inflammation. Estrogen can stimulate Cytochrome c oxidase subunit 2 synthesis, and Cytochrome c oxidase subunit 2 can enhance Prostaglandin E2(PGE2) expression. PEG2 can increase the expression of aromatase, which in turn increases estrogen synthesis (14). Estrogen works on the highly expressed estrogen receptors in EMS lesions, promoting endometrial stromal cell survival and invasion, producing pro-inflammatory factors, and perpetuating inflammation (15, 16). The ultimate result of EMS is fibrosis, which is frequently histologically characterized as overly dense fibrous tissue around endometrial glands and stroma (17). Long-term inflammation of endometriotic lesions, which activates the Transforming growth factor beta-1 proprotein(TGF-β) signaling pathway, results in the creation of fibrotic lesions (18). Transforming growth factor beta-1 proprotein levels were observed to be higher in the serum and peritoneal fluid of EMS patients compared to healthy women (19).
Inflammatory, fibrotic, and oxidative responses caused by EMS can all harm a patient’s ovarian reserve. OEM, in particular, can have a direct impact on a patient’s ovarian reserve. In the ovaries afflicted by EMS cysts, the number of primordial follicles and AMH level fell, whereas the number of atretic follicles and primary follicles increased (20). This could be because EMS causes inflammation and oxidative stress, which leads to the recruitment of dormant primordial follicles into the growth and development track, while the local inflammatory response of the foci leads to ovarian fibrosis, affecting ovarian blood supply, and the growing follicles cannot get enough nutritional support and enter the atresia state (21, 22). A vicious cycle ensues, resulting in a patient’s decreasing ovarian reserve and oocyte quality. The most obvious impact is EMS patients have a lower oocyte retrieval rate, a lower oocyte fertilization rate, a lower number of final available embryos and high-quality embryos, and a worse cumulative live birth rate of IVF cycles (23, 24). The ovarian damage of EMS patients is more severe as the disease progresses. According to other research, compared to normal women, the fertilization rate of oocytes in stage I/II EMS patients is around 7% lower, and the fertilization rate in stage III/IV EMS patients is even lower (25). The ultrastructure of EMS patients’ oocytes revealed brown degeneration, deeper cytoplasm, larger refractive body, incomplete protrusion or separation of the first polar body, and a prolonged disintegration time of the zona pellucida (26, 27). Not only is the maturation ability of oocytes from EMS patients reduced, but also cortical granule loss, spindle fragmentation, and zona pellucis sclerosis may develop in immature oocytes following maturation and culture to meiosis II in vitro, which may impede with sperm penetration (28).
3 GSs’ effect on oocytes
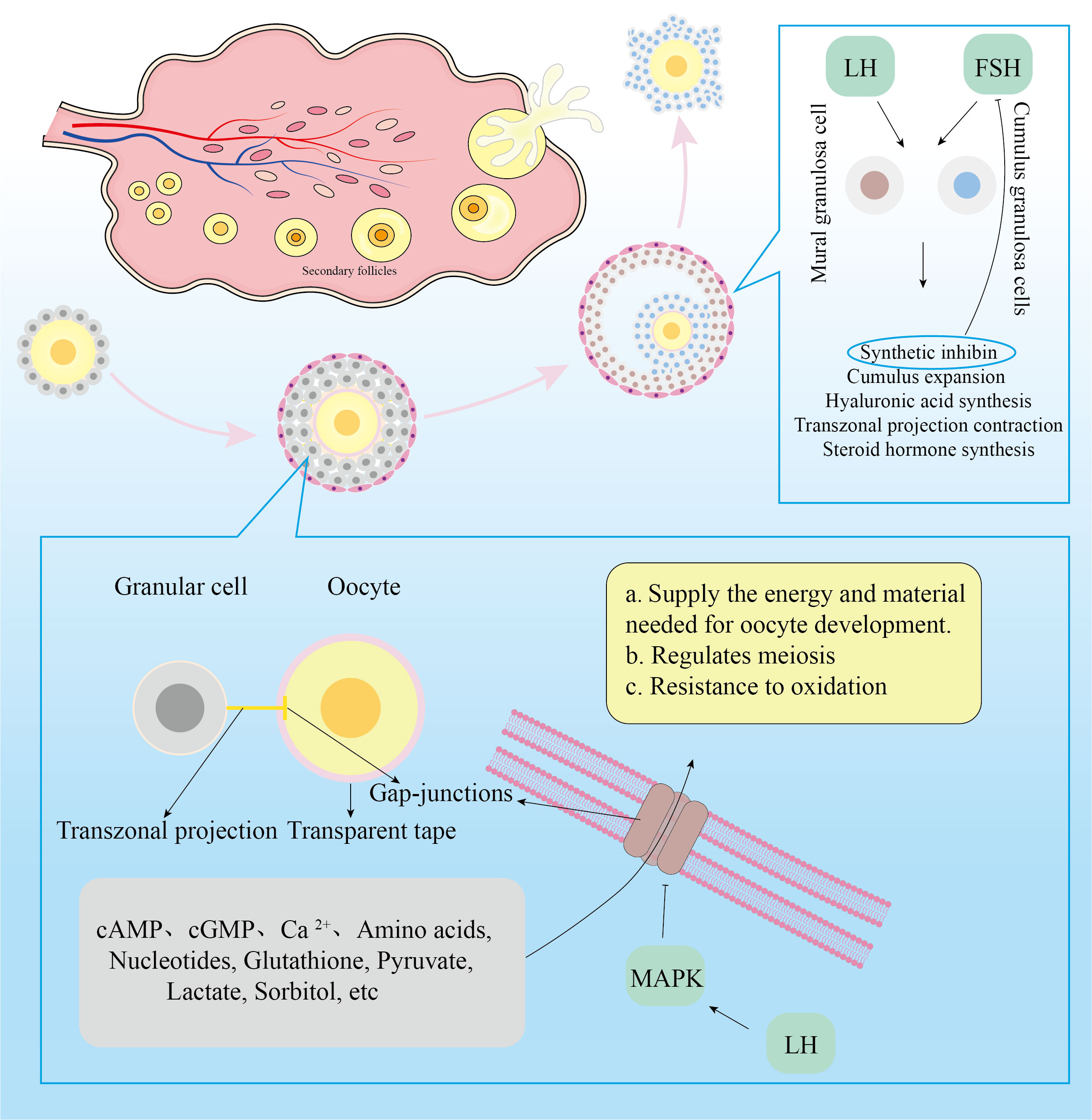
The female reproductive system’s fundamental functioning component is the follicle. Oocytes, GCs, theca cells, and follicular antrum make up the mature follicle. The oocyte is surrounded by flat undifferentiated GCs at the primordial follicle stage. A zona pellucida forms around the oocyte as the follicle develops, separating the GCs. Flat, undifferentiated GCs will become cuboidal and proliferate progressively. Transzonal projections(TZPs) connect the cumulus granulosa cells to the oocyte primarily through the zona pellucida (29). TZPS bind to the oocyte via Gap-junctions (GJs), which allow low-molecular-weight messages or nutrients to be transferred from cell to cell. Cumulus granulosa cells(CGCs) that are distant from the oocyte will form filopodia to interact with the oocyte as it develops (30). Undifferentiated GCs will gradually differentiate into mural granulosa cells(MGCs) and CGCs during the follicle development stage to preantral follicles. MGCs primarily execute endocrine tasks and are the first to accept Follicle stimulating hormone(FSH) and Luteinizing hormone(LH) action.
The function of follicular GCs is to help oocyte development and fertilization. These functions are mostly manifested in four areas (29): 1)Involved in maintaining of oocyte meiotic arrest; 2)Oocyte meiosis was induced to recover; 3)Provide the most fundamental substance needed for oocyte maturation; 4)Influences oocyte ejection from the follicle. Following female birth, the high level of Cathelicidin antimicrobial peptide(cAMP) secreted by undifferentiated GCs can keep cyclin Maturation promoting factor inactive by activating adenylate cyclase in oocytes, causing oocytes to enter the prophase stage of meiosis I (31). Following the appearance of the zona pellucida in oocytes, cyclic guanosine monophosphate(cGMP) secreted by GCs enters the oocyte via GJs, inactivating cGMP-inhibited 3’,5’-cyclic phosphodiesterase 3A and maintaining a high level of cAMP in oocytes that cannot be degraded, thereby inhibiting the meiotic process (32). When women enter the period of sexual development, gonadotropins can act on the follicles, causing them to develop further. When LH secretion was at its height, it might activate mitogen-activated protein kinase3/1(MAPK3/1) in CGCs and decrease the concentration of natriuretic peptide precursor type C in MGCs, resulting in cAMP hydrolysis (33). Activated MAPK also lowered GJs permeability, limiting cAMP and cGMP transport to oocytes and resulting in meiotic recovery of oocytes (34). Furthermore, after receiving the LH signal, the CGCs depolarized, increasing the intracellular calcium ion(Ca2+)concentration (35). Elevated Ca2+ will enter the oocyte via GJs, resulting in a temporary increase in Ca2+ concentration (36). The rise in Ca2+ concentration in oocytes will facilitate meiosis recovery (37).
Because oocytes have a limited ability to utilize nutrients, CGCs provide the majority of them. Through TZPs and GJs, GCs can deliver amino acids, nucleotides, glutathione, and carbohydrate metabolites to oocytes (38). Because of the limited activity of phosphofructokinase and lactate dehydrogenase in oocytes, as well as the sluggish glycolysis process, glucose is primarily glycolyzed by CGCs to create pyruvate and lactate, which are then transported to oocyte mitochondria (39). Oocytes, in turn, can ensure pyruvate availability by increasing the expression of glycolytic genes in CGCs. In addition, sorbitol dehydrogenase in granulosa cells reduces glucose to form sorbitol, which is then fed by oocytes’ indirect fructose synthesis (40). GCs not only provide necessary resources for oocyte formation, but they also control germinal vesicle rupture. FSH and LH control follicle growth, however the oocyte lacks Follicle stimulating hormone receptors (FSHR) and Luteinizing hormone receptors (LHR). As a result, FSH and LH influence follicular growth by acting on FSHR and LHR in CGCs and MGCs. FSH primarily works on MGCs, promoting estrogen synthesis in MGCs as well as GC proliferation. When GCs receive FSH signals, they produce inhibin, which inhibits FSH secretion and promotes the development of dominant and atresia non-dominant follicles (41). FSH and E2 synergistically encourage GCs to create LHR in preparation for the subsequent LH signal. FSH has the ability to stimulate CGC proliferation and hyaluronic acid synthesis. FSH stimulates CGC proliferation, hyaluronic acid synthesis, and then promotes the expansion of cumulus oocyte complex (42). Cumulus oocyte complex quality represents oocyte quality and is required for oocyte maturation and fertilization (43–45). When LH levels rise, LH and FSH work together to promote the production of progesterone and progesterone receptors in CGCs and to withdraw TZP from the oocyte membrane to the CGCs in preparation for the oocyte germinal vesicle to rupture (46, 47). Figure 1 depicts a more intuitive role for granule cells.
4 EMS causes GCs destruction
4.1 Apoptosis in GCs was triggered by EMS
When the granulosa cells die, the oocyte suffers from a quality decline or atresia due to a lack of various growth substances required for development. EMS can severely disrupt the cell cycle of follicular granulosa cells and increase the apoptotic rate of granulosa cells in patients (48, 49). Apoptotic bodies are extracellular vesicles containing nuclear and cytoplasmic debris that form as a result of apoptosis. The number of apoptotic bodies in undifferentiated GCs was considerably higher in patients with EMS compared to patients without EMS, and it was higher in patients with OEM compared to patients with EMS at other sites (50). Undifferentiated GC apoptosis can predict the result of in vitro fertilization (51). The more apoptotic bodies there are in EMS undifferentiated GCs, the lower the rate of recovered oocytes and the higher the rate of empty follicles.
The targets of differentially expressed CircRNAs were predominantly connected to apoptosis, Phosphatidylinositol3-kinase-RAC-beta serine/threonine-protein kinase(PI3K-AKT), and Cellular tumor antigen p53 signaling pathways by sequencing the CGCs of women with EMS and women without EMS (52). The PI3K-Akt signaling pathway is a critical regulator of cellular transcription, translation, proliferation, growth, and survival. After being phosphorylated, AKT participates in important biological processes such as apoptosis, protein synthesis, and cell cycle progressionAfter being phosphorylated, AKT participates in important biological processes such as apoptosis, protein synthesis, and cell cycle progression. Apoptosis of granulosa cells is higher in patients, which may be associated to decreased serum testosterone levels. In the CV434 granulosa cell line, testosterone can inhibit the PI3K-AKT signaling pathway and reduce cell death (49). Furthermore, the over-activation of primordial follicles in EMS patients’ ovaries is linked to the activation of the PI3K-AKT pathway (53). It was discovered by sequencing the follicular GCs of OEM patients and normal female follicular GCs that the differently expressed genes were primarily abundant in the MAPK, Protein WNT(WNT) signaling pathway, apoptosis, and steroid hormone response (54). Among these, the Wnt signaling pathway is linked to cell proliferation, death, and migration (55). WNT4 and WNT5a transcription levels were significantly increased in luteinizing granulosa cells from EMS patients, while WNT1 transcription levels were significantly decreased, β-catenin and its dephosphorylated active form expression was decreased, and the expression of apoptosis inhibitor genes was decreased, while apoptosis was enhanced. This shows that Wnt signaling dysregulation is linked to granulosa cell death and follicular cell atresia (48).
4.2 EMS promotes inflammation and oxidative stress in GCs
Inflammation and oxidative stress can impair oocyte quality and potentially cause follicular atresia. EMS, especially OEM, can lead to follicular inflammation and oxidative stress. According to previous research (56), the levels of C-C motif chemokine 2(CCL2) and Interleukin(IL)-8 in the follicular fluid produced by EMS-affected ovaries are higher than those produced by normal ovaries. CCL2 is a tiny cytokine that has the ability to cause inflammation. It can not only attract inflammatory cells like neutrophils, monocytes, and lymphocytes to the lesion site, but it can also stimulate the production of additional cytokines including IL-2, IL-6, and cell adhesion molecules (57). IL-8 is also a chemokine cytokine, and its involvement and regulation of human reproductive physiological and pathological processes has been established. IL-8’s primary biological activity is to recruit and activate neutrophils, hence promoting inflammation (58). The inflammatory reaction involving the follicles may become more severe as the lesions progress. IL-23 levels in follicular fluid and serum, for example, were considerably greater in individuals with III-IV EMS compared to those with I-II stages (59). In autoimmune inflammatory illnesses, IL-23 is a related factor that can mediate inflammatory and immunological responses by T cells, NK cells, and macrophages (60). When granulosa cells from EMS patients were cultivated in vitro, the levels of Tumor necrosis factor ligand superfamily member(TNF)-α, IL-8, and IL-1β in the cell supernatant were greater than in the control group (61). Furthermore, Nuclear Factor Kappa B(NFκB), Inhibitor of nuclear factor kappa-B kinase subunit beta, and NF-kappa-B inhibitor alpha expression was increased in granulosa cells from ovarian EMS patients, and the NFκB signaling pathway was significantly activated when granulosa cells were cultured with TNF-α (62). NFκB is an essential regulator of cellular inflammatory response and is involved in increasing the inflammatory cascade through cytokine activation. Telomerase activity is high in healthy follicles, whereas NF-kB expression in granulosa cells is inversely associated to oocyte mass and telomerase activity, implying that post-inflammatory alterations in granulosa cells are deleterious to oocyte development (63). However, Liang found no significant differences in chemokines and inflammatory cytokines between patients with surgically removed endometrial cysts and those with untreated EMS in follicular fluid (64).
Inflammation and oxidative stress are interrelated, and increases in oxidative stress can cause acute and chronic inflammation (65). Oxidative stress can cause inflammation via a number of mechanisms, including Nucleotide-binding oligomerization domain-like receptors, TOLL receptors, and NFκB pathways (66). Simultaneously, inflammation can result in oxidative stress (67, 68). The oxidative stress level in the ovarian cortex around endometriotic cysts was much higher than in dermoid cysts (69). The composition of follicular fluid revealed that patients with EMS had higher levels of oxidative substances such as 8-hydroxy-2 deoxyguanosine, reactive oxygen species, peroxynitrite ion, Nitric oxide, and malondialdehyde, and lower levels of antioxidant substances such as peroxide dismutase, catalase, vitamin A, vitamin C, vitamin E, and reduced glutathione (70–72). This could be connected to EMS-induced senescence, endoplasmic reticulum stress, and oxidative stress in CGCs (73, 74). When mouse cumulus oocyte complexes were cultured with endometrial cyst fluid, CGC mitochondrial performance was impaired, glutathione content was reduced, reactive oxygen species levels rose, and oxidative damage to oocytes was hastened (75). This shows that EMS can impact oocyte quality by generating oxidative stress in GCs. However, Donabela’s study found that the expression of superoxide dismutase 1, an antioxidant, was elevated in CGCs from individuals with moderate-to-severe EMS (76). As a result, we cannot say if the presence of EMS causes adaptive changes in CGCs, such as increased antioxidant capability. Oxidative stress may hinder ovulation in addition to lowering oocyte quality. Lin discovered that oxidative stress might decrease the expression of the histone-lysine N-methyltransferase EZH2 and the level of lysine 27 of the histone H3 protein in GCs while increasing the expression of Interleukin-1 receptor type 2 to suppress ovulation signals (77).
4.3 EMS influences GCs steroid hormone production
The synthesis and secretion of steroid hormones by GCs is critical for follicular growth and, as a result, can impact the quality of oocytes. The amount of FSHR and LHR on the surface of MGCs steadily increases as follicles expand, and estrogen released by MGCs can enhance CGCs proliferation. The aberrant follicular development in EMS patients is linked to a defective FSH signaling pathway operating on GCs, and EMS patients respond to FSH less effectively during ovulation induction (78). Although FSHR and cytochrome P450 family 19 subfamily A member 1(CYP19A1) expression levels are lower in GCs from EMS patients (77), it is unknown how EMS specifically changes FSHR signaling. According to other research (79, 80), the levels of estrogen and testosterone in the follicular fluid of patients with EMS are lower than those of patients without EMS, but the amount of progesterone is higher. When estrogen levels in the follicle are low, it frequently represents a deterioration in oocyte quality and the failure of in vitro fertilization (81). Follicular fluid progesterone levels rise as EMS severity rises, whereas testosterone levels fall as EMS severity rises (82, 83). However, another investigation found no change in progesterone synthesis by granulosa-lutein cells between patients with and without EMS (84). As a result, more information is required to establish the status of steroid synthesis by GCs in EMS.
The GCs produce and secrete the majority of the hormones in the follicle, and the hormone level in the follicular fluid represents the GCs’ steroid hormone secretion ability. The increased progesterone release by GCs may be related to increased autophagy of GCs in EMS patients, higher expression of Beclin-1(BECN1), and greater low-density lipoprotein degradation capacity. BECN1 inhibition decreases GCs autophagy and lowers low-density lipoprotein-induced progesterone synthesis (85). Furthermore, unusually increased PGE2 levels in EMS patients’ follicular fluid can enhance the expression of Steroidogenic acute regulatory protein(StAR) and the synthesis of progesterone in GCs (78). STAR may transport cholesterol from the outside mitochondrial membrane to the inner mitochondrial membrane and convert cholesterol to pregnenolone. Pregnenolone is a progestogen precursor that causes progesterone to be produced in response to 3β-HSD. However, Sreerangaraja’s (79) study found that the expression of STAR and 3β-HSD was reduced in CGCs from EMS patients. As a result, the mechanism by which EMS influences progesterone production by granulosa cells is unclear and requires additional investigation.
GCs from EMS patients exhibited lower expression of not just 3β-HSD, but also CYP19, as well as impaired ability to release inhibin B and estrogen (79, 86, 87). Among them, 3β-HSD was capable of converting dehydroepiandrosterone to androstenediol.CYP19 catalyzes the conversion of androstenedione and testosterone into estrone and estradiol, respectively, and is the rate-limiting enzyme in estrogen biosynthesis. Both proteins are essential regulators of the estrogen synthesis process and have a rate-limiting effect on the synthesis of steroid hormones, which may explain why GCs produce less estrogen. The mechanism through which EMS interferes with estrogen synthesis by GCs is currently unknown. A reliable theory is that the presence of EMS activates the extracellular regulated protein kinase(ERK)1/2 signaling pathway in GCs. When the ERK1/2 signaling pathway is activated, it inhibits estrogen production by CYP19 and promotes progesterone generation by STAR (88). Elevated IL-6 levels in EMS patients’ follicular fluid may activate the ERK1/2 signaling pathway in GCs. When Deura utilized IL-6 to culture granulosa tumor cell lines, it could enhance ERK1/2 phosphorylation, limit CYP19 expression, and diminish estrogen release (89). Li’s research also discovered that ERK1/2 signaling is enhanced in granulosa cells from EMS patients (90).
4.4 EMS influences mitochondrial energy metabolism in GCs
Because GCs are the nanny cells that feed the oocyte, mitochondria play a vital role in energy metabolism. Therefore, mitochondrial morphology, the amount of mitochondrial DNA(mtDNA) expression, and the efficiency of adenosine-triphosphate(ATP) synthesis in GCs all influence oocyte developmental potential to some extent (91). Mitochondria from CGCs from mild EMS patients showed morphological edema, hazy mitochondria, and reduced mtDNA expression abundance (92). After surgery, the quantity of mtDNA expression in follicular GCs of females with severe EMS was enhanced compared to women without EMS (93). This could be related to a compensatory increase in mtDNA expression in GCs to compensate for the ovary’s lack of mitochondrial energy metabolism in order to adapt to the hypoxia in the follicular development microenvironment. According to Hsu’s research (94), CGCs in EMS patients produced less ATP, although mtDNA expression abundance remained unaffected. The respiratory chain’s structural proteins are encoded by mtDNA. The loss of its nucleic acid sequence will hamper oxidative phosphorylation and decrease ATP generation. As a result, when the energy metabolism of GCs mitochondria is insufficient, oocyte quality suffers. EMS also decreases mitochondrial membrane potential in GCs (79). The stability of mitochondrial membrane potential promotes the preservation of normal cellular physiological function. Normal mitochondrial membrane potential is required for oxidative phosphorylation and the generation of ATP. Increased glucose intake and lactate generation are also signs of abnormal mitochondrial energy metabolism in GCs from EMS patients. This could be due to elevated Prohibitin 1(PHB1) expression in GCs of EMS patients. When PHB1 expression was reduced, GCs expression of enzymes involved in glucose metabolism, glucose consumption, and lactate generation decreased (95). Furthermore, Sirtuin 2(SIRT2) inhibits phosphoenolpyruvate carboxykinase 1 degradation, and Phosphoenolpyruvate carboxykinase, cytosolic [GTP] is the rate-limiting enzyme in gluconeogenesis. SIRT2 expression is enhanced in granulosa cells of EMS patients, indicating that EMS impacts GCs metabolic pathways, which may be mediated by SIRT2 (96).
5 The relationship between EMS-induced GCs abnormalities and oocyte quality
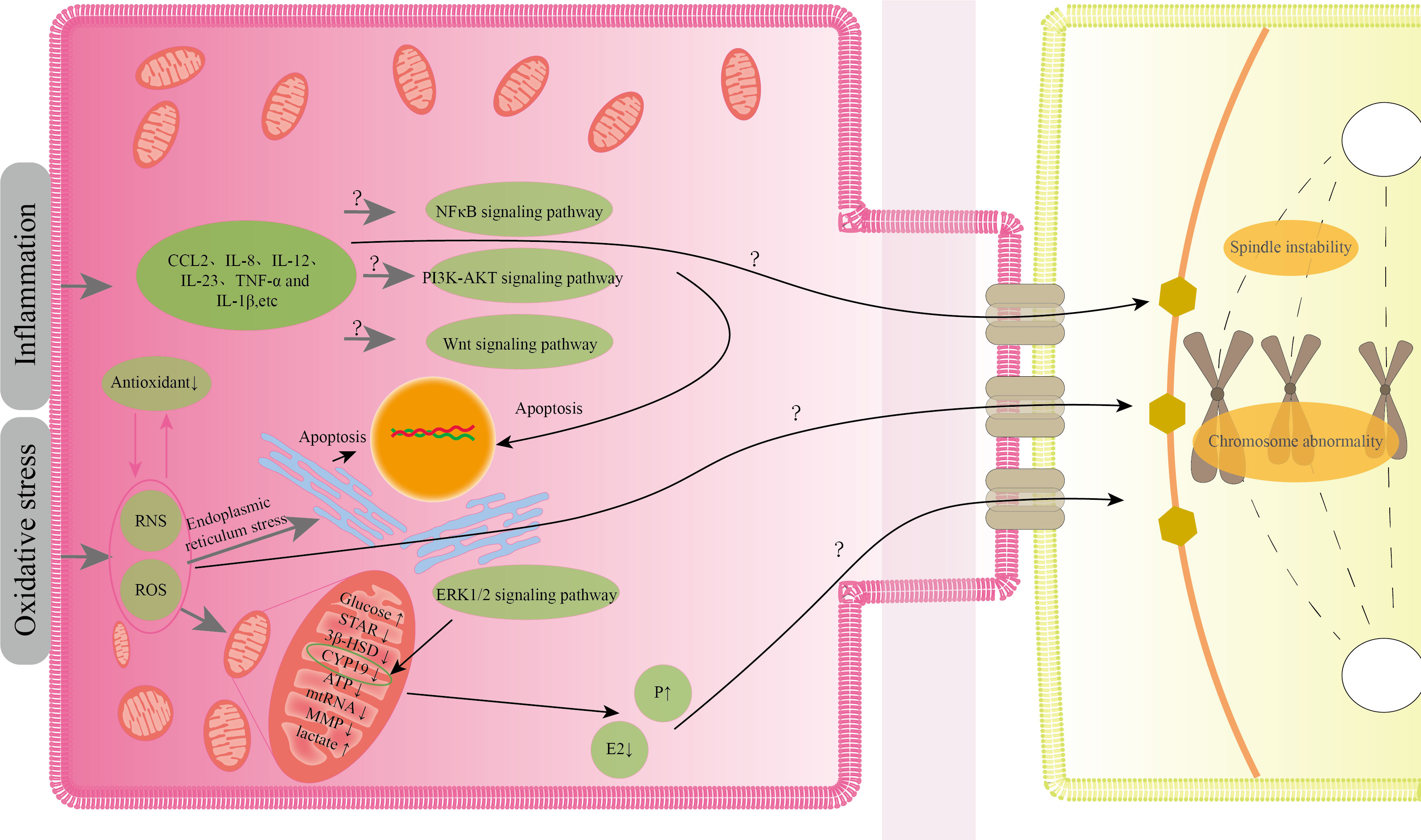
Current research indicates that EMS-induced oxidative stress, inflammation, aberrant mitochondrial energy metabolism, inappropriate steroid production, and apoptosis in GCs can all impair the quality of oocytes to variable degrees. Although there have been few studies on the abnormal pathological states of GCs, it is known from the existing studies that the abnormal condition of GCs generated by EMS does not exist alone, but interacts with one another. A transcriptome analysis of CGCs revealed that the genes that differed between mature and immature CGCs were mostly involved in steroid metabolism, inflammation, apoptosis, cell cycle regulation, and extracellular matrix remodeling (97). This also implies that the aberrant status of GCs generated by EMS will have an effect on oocyte maturation. According to the current research, oxidative stress appears to be the root cause of various aberrant conditions. EMS-induced oxidative stress generates reactive oxygen species, and an increase in reactive oxygen species in the follicle leads to spindle instability, aberrant chromosomal formation, and impaired oocyte developmental capability (98). Furthermore, protein nitration is enhanced in follicular GCs of EMS patients, and protein nitration is a marker of peroxynitrite ions, which are reactive nitrogen free radicals (70). Peroxynitrite ions can change oocyte spindle shape and chromosomal organization in a dose-dependent way (99). Oxidative stress can damage GCs mitochondria, affecting mitochondrial energy metabolism and steroid hormone production. Mitochondrial dysfunction generates free radicals, which exacerbates oxidative stress (100). Furthermore, the lower mitochondrial membrane potential of GCs may cause nuclear and cytoplasmic maturation to be out of sync, eventually leading to embryo development failure (101). The failure of mitochondrial steroidogenesis in GCs can result in aberrant oocyte development, especially when estrogen levels in follicular fluid are low and progesterone levels are high, affecting proper meiosis and late cleavage of oocytes (80, 102, 103). Nevertheless, it is unknown whether the level of progesterone in follicular fluid of patients with EMS differs from that of normal individuals, and we do not yet know the mechanism by which progesterone influences oocyte quality. Oxidative stress can also cause GCs inflammation and apoptosis. GCs inflammation frequently interferes with oocyte meiotic capacity. Lipopolysaccharide-induced inflammation enhances IL-6 and IL-8 secretion in bovine GCs, resulting in meiotic block and failure of germinal vesicle rupture in oocytes (104). The IL-6 described above may interfere with estrogen synthesis in GCs, impacting oocyte development (88). Several investigations in embryo culture have demonstrated that IL-8, IL-12, and TNF-α in EMS patients’ follicular fluid are inversely connected with oocyte maturity and embryo quality (105). Among these, TNF-α production by CGCs can increase senescence of mouse oocytes after ovulation, which may be the cause of oocyte quality decline (106). Although the mechanism of EMS-induced granulosa cell death is unknown, oxidative stress-induced apoptosis could be produced by endoplasmic reticulum stress. When EMS follicles were compared to normal women, GCs apoptosis was enhanced and endoplasmic reticulum stress was visible. GCs apoptosis was reduced after endoplasmic reticulum stress was relieved (73). MtDNA depletion causes apoptosis in granulosa cells (107). We noted previously that EMS patients’ granulosa cells had abnormal mtDNA expression, but we don’t know how EMS influences the abnormal expression of mtRNA. Figure 2 illustrates a potential process by which the granulosa cells' aberrant condition influences the quality of the oocyte.
6 Summary
The decrease in oocyte quality in EMS patients will make pregnancy hard for women. Even with assisted reproductive technology, the rate of pregnancy failure remains high. In conclusion, EMS primarily reduces oocyte quality by causing GCs apoptosis, inflammation, oxidative stress, steroidogenesis disorders, and abnormal mitochondrial energy metabolism, which also provides a therapeutic direction for improving assisted reproductive technology success rates in EMS patients. It is troubling that these aberrant states frequently affect each other, but the mechanism underlying the link between the multiple abnormal states of GCs generated by EMS is yet unknown. In addition, it is unclear how the aberrant status of GCs influences the oocyte by which mechanism or pathway. Although patients with EMS can achieve pregnancy using assisted reproductive technology, it is unknown whether the offspring’s health would suffer as a result of poor oocyte quality. In terms of research technology, current study on the extraction of GCs from follicles leaves key questions unanswered. Because MGCs and CGCs serve different functions in follicles, most studies do not distinguish between the two when removing GCs from follicles. As a result of our summary, future research should concentrate on how to improve the abnormal condition of GCs induced by EMS and better understand the routes by which the abnormal state of GCs impacts oocyte quality. We can intervene earlier to achieve better GCs, enhance reproductive endocrinology, and so increase pregnancy rates and offspring health if we understand how EMS affects oocyte quality by altering GCs.
Author contributions
Writing—Original draft preparation: WF and ZY. Writing—review and editing, and supervision: ML and YZ. Draw diagram: WF. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by Scientific planning project of youth education in Shandong province Undergraduate Academic Project (23BSH251); The Natural Science Foundation of Shandong Province (ZR2021MH404); Ji ‘nan Science and Technology Innovation Development Plan (202225006).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Horne AW, Missmer SA. Pathophysiology, diagnosis, and management of endometriosis. BMJ (2022) 379:e070750. doi: 10.1136/bmj-2022-070750
2. Saunders PTK, Horne AW. Endometriosis: Etiology, pathobiology, and therapeutic prospects. Cell (2021) 184(11):2807–24. doi: 10.1016/j.cell.2021.04.041
4. Muzii L, Galati G, Mattei G, Chinè A, Perniola G, Di Donato V, et al. Expectant, medical, and surgical management of ovarian endometriomas. J Clin Med (2023) 12(5):1858. doi: 10.3390/jcm12051858
5. Bonavina G, Taylor HS. Endometriosis-associated infertility: From pathophysiology to tailored treatment. Front Endocrinol (Lausanne) (2022) 13:1020827. doi: 10.3389/fendo.2022.1020827
6. Qu H, Du Y, Yu Y, Wang M, Han T, Yan L. The effect of endometriosis on IVF/ICSI and perinatal outcome: A systematic review and meta-analysis. J Gynecol Obstet Hum Reprod (2022) 51(9):102446. doi: 10.1016/j.jogoh.2022.102446
7. Alshehre SM, Narice BF, Fenwick MA, Metwally M. The impact of endometrioma on in vitro fertilisation/intra-cytoplasmic injection IVF/ICSI reproductive outcomes: a systematic review and meta-analysis. Arch Gynecol Obstet (2021) 303(1):3–16. doi: 10.1007/s00404-020-05796-9
8. Kimber-Trojnar Ż, Pilszyk A, Niebrzydowska M, Pilszyk Z, Ruszała M, Leszczyńska-Gorzelak B. The potential of non-invasive biomarkers for early diagnosis of asymptomatic patients with endometriosis. J Clin Med (2021) 10(13):2762. doi: 10.3390/jcm10132762
9. Eppig JJ. Reproduction: Oocytes call, granulosa cells connect. Curr Biol (2018) 28(8):R354–6. doi: 10.1016/j.cub.2018.03.005
10. Wang X, Zhang M, Jiang L, Fang X, Zhang T. Exosomal AFAP1-AS1 binds to microRNA-15a-5p to promote the proliferation, migration, and invasion of ectopic endometrial stromal cells in endometriosis. Reprod Biol Endocrinol (2022) 20(1):77. doi: 10.1186/s12958-022-00942-1
11. Bulun SE, Yildiz S, Adli M, Chakravarti D, Parker JB, Milad M, et al. Endometriosis and adenomyosis: shared pathophysiology. Fertil Steril (2023) 119(5):746–50. doi: 10.1016/j.fertnstert.2023.03.006
12. Khodarahmian M, Amidi F, Moini A, Kashani L, Salahi E, Danaii-Mehrabad S, et al. A randomized exploratory trial to assess the effects of resveratrol on VEGF and TNF-α 2 expression in endometriosis women. J Reprod Immunol (2021) 143:103248. doi: 10.1016/j.jri.2020.103248
13. Poirier D, Nyachieo A, ROmano A, Roy J, Maltais R, Chai D, et al. An irreversible inhibitor of 17β-hydroxysteroid dehydrogenase type 1 inhibits estradiol synthesis in human endometriosis lesions and induces regression of the non-human primate endometriosis. J Steroid Biochem Mol Biol (2022) 222:106136. doi: 10.1016/j.jsbmb.2022.106136
14. Minami T, Tsuzuki Y, Tanaka Y, Kitawaki J, Mori T. The Tpl2-MEK pathway plays a critical role in spheroid-cultured endometriotic stromal cells. Am J Reprod Immunol (2023) 89(5):e13689. doi: 10.1111/aji.13689
15. Han SJ, Jung SY, Wu SP, Hawkins SM, Park MJ, Kyo S, et al. Estrogen receptor β Modulates apoptosis complexes and the inflammasome to drive the pathogenesis of endometriosis. Cell. (2015) 163(4):960–74. doi: 10.1016/j.cell.2015.10.034
16. Juhasz-Böss I, Fischer C, Lattrich C, Skrzypczak M, Malik E, Ortmann O, et al. Endometrial expression of estrogen receptor β and its splice variants in patients with and without endometriosis. Arch Gynecol Obstet (2011) 284(4):885–91. doi: 10.1007/s00404-010-1768-7
17. Matsuzaki S, Pouly JL, Canis M. Dose-dependent pro- or anti-fibrotic responses of endometriotic stromal cells to interleukin-1β and tumor necrosis factor α. Sci Rep (2020) 10(1):9467. doi: 10.1038/s41598-020-66298-x
18. Zubrzycka A, Migdalska-Sęk M, Jędrzejczyk S, Brzeziańska-Lasota E. The expression of TGF-β1, SMAD3, ILK and miRNA-21 in the ectopic and eutopic endometrium of women with endometriosis. Int J Mol Sci (2023) 24(3):2453. doi: 10.3390/ijms24032453
19. Khan KN, Yamamoto K, Fujishita A, Muto H, Koshiba A, Kuroboshi H, et al. Differential levels of regulatory T cells and T-helper-17 cells in women with early and advanced endometriosis. J Clin Endocrinol Metab (2019) 104(10):4715–29. doi: 10.1210/jc.2019-00350
20. Check JH. What role does decreased ovarian reserve play in the aetiology of infertility related to endometriosis? Hum Reprod (2003) 18(3):653–4; author reply 654-5. doi: 10.1093/humrep/deg086
21. Dolmans MM, Martinez-Madrid B, Gadisseux E, Guiot Y, WY Y, Torre A, et al. Short-term transplantation of isolated human ovarian follicles and cortical tissue into nude mice. Reproduction (2007) 134(2):253–62. doi: 10.1530/REP-07-0131
22. David A, Van Langendonckt A, Gilliaux S, MM D, Donnez J, Amorim CA. Effect of cryopreservation and transplantation on the expression of kit ligand and anti-Mullerian hormone in human ovarian tissue. Hum Reprod (2012) 27(4):1088–95. doi: 10.1093/humrep/des013
23. Horton J, Sterrenburg M, Lane S, Maheshwari A, TC Li, Cheong Y. Reproductive, obstetric, and perinatal outcomes of women with adenomyosis and endometriosis: a systematic review and meta-analysis. Hum Reprod Update (2019) 25(5):592–632. doi: 10.1093/humupd/dmz012
24. Zhou L, Wang L, Geng Q, Zhang H, Xu S, Diao L, et al. Endometriosis is associated with a lowered cumulative live birth rate: A retrospective matched cohort study including 3071 in vitro fertilization cycles. J Reprod Immunol (2022) 151:103631. doi: 10.1016/j.jri.2022.103631
25. Shebl O, Sifferlinger I, Habelsberger A, Oppelt P, RB M, Petek E, et al. Oocyte competence in in vitro fertilization and intracytoplasmic sperm injection patients suffering from endometriosis and its possible association with subsequent treatment outcome: a matched case-control study. Acta Obstet Gynecol Scand (2017) 96(6):736–44. doi: 10.1111/aogs.12941
26. Robin C, Uk A, Decanter C, Behal H, Collinet P, Rubod C, et al. Impact of endometriosis on oocyte morphology in IVF-ICSI: retrospective study of a cohort of more than 6000 mature oocytes. Reprod Biol Endocrinol (2021) 19(1):160. doi: 10.1186/s12958-021-00798-x
27. Kasapoglu I, Kuspinar G, Saribal S, Turk P, Avcı B, Uncu G. Detrimental effects of endometriosis on oocyte morphology in intracytoplasmic sperm injection cycles: a retrospective cohort study. Gynecol Endocrinol (2018) 34(3):206–11. doi: 10.1080/09513590.2017.1391203
28. Nada AM, El-Noury A, Al-Inany H, Bibars M, Taha T, Salama S, et al. Effect of laser-assisted zona thinning, during assisted reproduction, on pregnancy outcome in women with endometriosis: randomized controlled trial. Arch Gynecol Obstet (2018) 297(2):521–8. doi: 10.1007/s00404-017-4604-5
29. El-Hayek S, Yang Q, Abbassi L, FitzHarris G, Clarke HJ. MamMalian oocytes locally remodel follicular architecture to provide the foundation for germline-soma communication. Curr Biol (2018) 28(7):1124–1131.e3. doi: 10.1016/j.cub.2018.02.039
30. Anderson E, Wilkinson RF, Lee G, Meller S. A correlative microscopical analysis of differentiating ovarian follicles of mammals. J Morphol (1978) 156(3):339–66. doi: 10.1002/jmor.1051560303
31. Grieco D, Porcellini A, Avvedimento EV, Gottesman ME. Requirement for cAMP-PKA pathway activation by M phase-promoting factor in the transition from mitosis to interphase. Science. (1996) 271(5256):1718–23. doi: 10.1126/science.271.5256.1718
32. Clarke H. Control of mamMalian oocyte development by interactions with the maternal follicular environment. Results Probl Cell Differ (2017) 63:17–41. doi: 10.1007/978-3-319-60855-6_2
33. Kawamura K, Cheng Y, Kawamura N, Takae S, Okada A, Kawagoe Y, et al. Pre-ovulatory LH/hCG surge decreases C-type natriuretic peptide secretion by ovarian granulosa cells to promote meiotic resumption of pre-ovulatory oocytes. Hum Reprod (2011) 26(11):3094–101. doi: 10.1093/humrep/der282
34. Okudaira Y, Wakai T, Funahashi H. Levels of cyclic-AMP and cyclic-GMP in porcine oocyte-cumulus complexes and cumulus-free oocytes derived from small and middle follicles during the first 24-hour period of in vitro maturation. J Reprod Dev (2017) 63(2):191–7. doi: 10.1262/jrd.2016-156
35. Albertini DF, Combelles CM, Benecchi E, Carabatsos MJ. Cellular basis for paracrine regulation of ovarian follicle development. Reproduction (2001) 121(5):647–53. doi: 10.1530/rep.0.1210647
36. Mattioli M, Barboni B. Signal transduction mechanism for LH in the cumulus-oocyte complex. Mol Cell Endocrinol (2000) 161(1-2):19–23. doi: 10.1016/s0303-7207(99)00218-x
37. Wang Y, Kong N, Li N, Hao X, Wei K, Xiang X, et al. Epidermal growth factor receptor signaling-dependent calcium elevation in cumulus cells is required for NPR2 inhibition and meiotic resumption in mouse oocytes. Endocrinology (2013) 154(9):3401–9. doi: 10.1210/en.2013-1133
38. Tanghe S, Van Soom A, Nauwynck H, Coryn M, de Kruif A. Minireview: Functions of the cumulus oophorus during oocyte maturation, ovulation, and fertilization. Mol Reprod Dev (2002) 61(3):414–24. doi: 10.1002/mrd.10102
39. Harris SE, Leese HJ, Gosden RG, Picton HM. Pyruvate and oxygen consumption throughout the growth and development of murine oocytes. Mol Reprod Dev (2009) 76(3):231–8. doi: 10.1002/mrd.20945
40. Kaneko T, Iuchi Y, Takahashi M, Fujii J. Colocalization of polyol-metabolizing enzymes and immunological detection of fructated proteins in the female reproductive system of the rat. Histochem Cell Biol (2003) 119(4):309–15. doi: 10.1007/s00418-003-0516-5
41. Richani D, Constance K, Lien S, Agapiou D, Stocker WA, Hedger MP, et al. Cumulin and FSH cooperate to regulate inhibin B and activin B production by human granulosa-lutein cells in vitro. Endocrinology (2019) 160(4):853–62. doi: 10.1210/en.2018-01026
42. Liu H, Zhou D, Liu C, Zhuan Q, Luo Y, Mo X, et al. The Calcium-Sensing Receptor Is Involved in Follicle-Stimulating Hormone-Induced Cumulus Expansion in in vitro Cultured Porcine Cumulus-Oocyte Complexes. Front Cell Dev Biol (2021) 9:625036. doi: 10.3389/fcell.2021.625036
43. Mohan Jeena L, Kumar D, Rahangdale S, Pratap Singh A, Chandra Sarkhel B. Transcriptional profile of cumulus associated GJA1, PTX3, PRSS35, and SERPINE2 genes with oocytes and embryonic development in water buffalo. Mol Biol Rep (2022) 49(7):6285–93. doi: 10.1007/s11033-022-07435-9
44. Santiquet NW, Greene AF, Becker J, Barfield JP, Schoolcraft WB, Krisher RL. A pre-in vitro maturation medium containing cumulus oocyte complex ligand-receptor signaling molecules maintains meiotic arrest, supports the cumulus oocyte complex and improves oocyte developmental competence. Mol Hum Reprod (2017) 23(9):594–606. doi: 10.1093/molehr/gax032
45. Ploutarchou P, Melo P, Day AJ, Milner CM, Williams SA. Molecular analysis of the cumulus matrix: insights from mice with O-glycan-deficient oocytes. Reproduction (2015) 149(5):533–43. doi: 10.1530/REP-14-0503
46. Shimada M, Terada T. FSH and LH induce progesterone production and progesterone receptor synthesis in cumulus cells: a requirement for meiotic resumption in porcine oocytes. Mol Hum Reprod (2002) 8(7):612–8. doi: 10.1093/molehr/8.7.612
47. Abbassi L, El-Hayek S, Carvalho KF, Wang W, Yang Q, Granados-Aparici S, et al. Epidermal growth factor receptor signaling uncouples germ cells from the somatic follicular compartment at ovulation. Nat Commun (2021) 12(1):1438. doi: 10.1038/s41467-021-21644-z
48. Demirel LC, Cengiz B, Unlü C. Severe endometriosis and apoptotic granulosa cells. Fertil Steril (2001) 75(3):642. doi: 10.1016/s0015-0282(00)01771-4
49. Toya M, Saito H, Ohta N, Saito T, Kaneko T, Hiroi M. Moderate and severe endometriosis is associated with alterations in the cell cycle of granulosa cells in patients undergoing in vitro fertilization and embryo transfer. Fertil Steril (2000) 73(2):344–50. doi: 10.1016/s0015-0282(99)00507-5
50. Sanchez AM, Somigliana E, Vercellini P, Pagliardini L, Candiani M, Vigano P. Endometriosis as a detrimental condition for granulosa cell steroidogenesis and development: From molecular alterations to clinical impact. J Steroid Biochem Mol Biol (2016) 155(Pt A):35–46. doi: 10.1016/j.jsbmb.2015.07.023
51. Nakahara K, Saito H, Saito T, Ito M, Ohta N, Takahashi T, et al. Ovarian fecundity in patients with endometriosis can be estimated by the incidence of apoptotic bodies. Fertil Steril (1998) 69(5):931–5. doi: 10.1016/s0015-0282(98)00038-7
52. Huang X, Yu Q. Bioinformatic analysis confirms differences in circular RNA expression profiles of cumulus cells between patients with ovarian and peritoneal endometriosis-associated infertility. Front Endocrinol (Lausanne) (2023) 14:1137235. doi: 10.3389/fendo.2023.1137235
53. Takeuchi A, Koga K, Satake E, Makabe T, Taguchi A, Miyashita M, et al. Endometriosis triggers excessive activation of primordial follicles via PI3K-PTEN-akt-foxo3 pathway. J Clin Endocrinol Metab (2019) 104(11):5547–54. doi: 10.1210/jc.2019-00281
54. Shi L, Wei X, Wu B, Yuan C, Li C, Dai Y, et al. Molecular Signatures Correlated With Poor IVF Outcomes: Insights From the mRNA and lncRNA Expression of Endometriotic Granulosa Cells. Front Endocrinol (Lausanne) (2022) 13:825934. doi: 10.3389/fendo.2022.825934
55. Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell (2003) 5(3):367–77. doi: 10.1016/s1534-5807(03)00266-1
56. Yland J, Carvalho LFP, Beste M, Bailey A, Thomas C, Abrão MS, et al. Endometrioma, the follicular fluid inflammatory network and its association with oocyte and embryo characteristics. Reprod BioMed Online (2020) 40(3):399–408. doi: 10.1016/j.rbmo.2019.12.005
57. Zhang H, Yang K, Chen F, Liu Q, Ni J, Cao W, et al. Role of the CCL2-CCR2 axis in cardiovascular disease: Pathogenesis and clinical implications. Front Immunol (2022) 13:975367. doi: 10.3389/fimmu.2022.975367
58. Vilotić A, Nacka-Aleksić M, Pirković A, Bojić-Trbojević Ž, Dekanski D, Jovanović Krivokuća M. IL-6 and IL-8: An overview of their roles in healthy and pathological pregnancies. Int J Mol Sci (2022) 23(23):14574. doi: 10.3390/ijms232314574
59. Zhang QF, Chen GY, Liu Y, Huang HJ, Song YF. Relationship between resistin and IL-23 levels in follicular fluid in infertile patients with endometriosis undergoing IVF-ET. Adv Clin Exp Med (2017) 26(9):1431–5. doi: 10.17219/acem/41149
60. Parham C, Chirica M, Timans J, Vaisberg E, Travis M, Cheung J, et al. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J Immunol (2002) 168(11):5699–708. doi: 10.4049/jimmunol.168.11.5699
61. Carlberg M, Nejaty J, Fröysa B, Guan Y, Söder O, Bergqvist A. Elevated expression of tumour necrosis factor alpha in cultured granulosa cells from women with endometriosis. Hum Reprod (2000) 15(6):1250–5. doi: 10.1093/humrep/15.6.1250
62. Li Y, Li R, Ouyang N, Dai K, Yuan P, Zheng L, et al. Investigating the impact of local inflammation on granulosa cells and follicular development in women with ovarian endometriosis. Fertil Steril (2019) 112(5):882–891.e1. doi: 10.1016/j.fertnstert.2019.07.007
63. Yamagata Y, Nakamura Y, Umayahara K, Harada A, Takayama H, Sugino N, et al. Changes in telomerase activity in experimentally induced atretic follicles of immature rats. Endocr J (2002) 49(6):589–95. doi: 10.1507/endocrj.49.589
64. Liang Y, Yang X, Lan Y, Lei L, Li Y, Wang S. Effect of Endometrioma cystectomy on cytokines of follicular fluid and IVF outcomes. J Ovarian Res (2019) 12(1):98. doi: 10.1186/s13048-019-0572-7
65. Rochette L, Lorin J, Zeller M, Guilland JC, Lorgis L, Cottin Y, et al. Nitric oxide synthase inhibition and oxidative stress in cardiovascular diseases: possible therapeutic targets? Pharmacol Ther (2013) 140(3):239–57. doi: 10.1016/j.pharmthera.2013.07.004
66. Lugrin J, Rosenblatt-Velin N, Parapanov R, Liaudet L. The role of oxidative stress during inflammatory processes. Biol Chem (2014) 395(2):203–30. doi: 10.1515/hsz-2013-0241
67. Al-Harbi NO, Nadeem A, Al-Harbi MM, Imam F, Al-Shabanah OA, Ahmad SF, et al. Oxidative airway inflammation leads to systemic and vascular oxidative stress in a murine model of allergic asthma. Int Immunopharmacol (2015) 26(1):237–45. doi: 10.1016/j.intimp.2015.03.032
68. Sun J, Jiao Z, Zhu W, Li X, Wang P, Wang J, et al. Astilbin attenuates cadmium-induced adipose tissue damage by inhibiting NF-κB pathways and regulating the expression of HSPs in chicken. Biol Trace Elem Res (2023) 201(5):2512–23. doi: 10.1007/s12011-022-03327-y
69. Matsuzaki S, Schubert B. Oxidative stress status in normal ovarian cortex surrounding ovarian endometriosis. Fertil Steril (2010) 93(7):2431–2. doi: 10.1016/j.fertnstert.2009.08.068
70. Da Broi MG, de Albuquerque FO, de Andrade AZ, Cardoso RL, Jordão Junior AA, Navarro PA. Increased concentration of 8-hydroxy-2'-deoxyguanosine in follicular fluid of infertile women with endometriosis. Cell Tissue Res (2016) 366(1):231–42. doi: 10.1007/s00441-016-2428-4
71. Goud PT, Goud AP, Joshi N, Puscheck E, Diamond MP, Abu-Soud HM. Dynamics of nitric oxide, altered follicular microenvironment, and oocyte quality in women with endometriosis. Fertil Steril (2014) 102(1):151–159.e5. doi: 10.1016/j.fertnstert.2014.03.053
72. Scutiero G, Iannone P, Bernardi G, Bonaccorsi G, Spadaro S, Volta CA, et al. Oxidative stress and endometriosis: A systematic review of the literature. Oxid Med Cell Longev (2017) 2017:7265238. doi: 10.1155/2017/7265238
73. Lin X, Dai Y, Tong X, Xu W, Huang Q, Jin X, et al. Excessive oxidative stress in cumulus granulosa cells induced cell senescence contributes to endometriosis-associated infertility. Redox Biol (2020) 30:101431. doi: 10.1016/j.redox.2020.101431
74. Kunitomi C, Harada M, Takahashi N, Azhary JMK, Kusamoto A, Nose E, et al. Activation of endoplasmic reticulum stress mediates oxidative stress-induced apoptosis of granulosa cells in ovaries affected by endometrioma. Mol Hum Reprod (2020) 26(1):40–52. doi: 10.1093/molehr/gaz066
75. Ren Z, Huang J, Zhou C, Jia L, Li M, Liang X, et al. Transferrin and antioxidants partly prevented mouse oocyte oxidative damage induced by exposure of cumulus-oocyte complexes to endometrioma fluid. J Ovarian Res (2020) 13(1):139. doi: 10.1186/s13048-020-00738-0
76. Donabela FC, Meola J, Padovan CC, de Paz CC, Navarro PA. Higher SOD1 gene expression in cumulus cells from infertile women with moderate and severe endometriosis. Reprod Sci (2015) 22(11):1452–60. doi: 10.1177/1933719115585146
77. Lin X, Tong X, Zhang Y, Gu W, Huang Q, Zhang Y, et al. Decreased expression of EZH2 in granulosa cells contributes to endometriosis-associated infertility by targeting IL-1R2. Endocrinology (2022) 164(2):bqac210. doi: 10.1210/endocr/bqac210
78. González-Fernández R, Peña Ó, Hernández J, Martín-Vasallo P, Palumbo A, Ávila J. Patients with endometriosis and patients with poor ovarian reserve have abnormal follicle-stimulating hormone receptor signaling pathways. Fertil Steril (2011) 95(7):2373–8. doi: 10.1016/j.fertnstert.2011.03.030
79. Wang J, Shen XX, Huang XH, Zhao ZM. Follicular fluid levels of prostaglandin E2 and the effect of prostaglandin E2 on steroidogenesis in granulosa-lutein cells in women with moderate and severe endometriosis undergoing in vitro fertilization and embryo transfer. Chin Med J (Engl) (2012) 125(22):3985–90.
80. Sreerangaraja Urs DB, Wu WH, Komrskova K, Postlerova P, Lin YF, Tzeng CR, et al. Mitochondrial function in modulating human granulosa cell steroidogenesis and female fertility. Int J Mol Sci (2020) 21(10):3592. doi: 10.3390/ijms21103592
81. Wunder DM, Mueller MD, Birkhäuser MH, Bersinger NA. Steroids and protein markers in the follicular fluid as indicators of oocyte quality in patients with and without endometriosis. J Assist Reprod Genet (2005) 22(6):257–64. doi: 10.1007/s10815-005-5149-2
82. Garrido N, Navarro J, Remohí J, Simón C, Pellicer A. Follicular hormonal environment and embryo quality in women with endometriosis. Hum Reprod Update (2000) 6(1):67–74. doi: 10.1093/humupd/6.1.67
83. Pellicer A, Valbuena D, Bauset C, Albert C, Bonilla-Musoles F, Remohí J, et al. The follicular endocrine environment in stimulated cycles of women with endometriosis: steroid levels and embryo quality. Fertil Steril (1998) 69(6):1135–41. doi: 10.1016/s0015-0282(98)00085-5
84. Garrido N, Krüssel JS, Remohí J, Simón C, Pellicer A. Expression and function of 3beta hydroxisteroid dehydrogenase (3beta HSD) type II and corticosteroid binding globulin (CBG) in granulosa cells from ovaries of women with and without endometriosis. J Assist Reprod Genet (2002) 19(1):24–30. doi: 10.1023/a:1014058622697
85. Ding Y, Zhu Q, He Y, Lu Y, Wang Y, Qi J, et al. Induction of autophagy by Beclin-1 in granulosa cells contributes to follicular progesterone elevation in ovarian endometriosis. Transl Res (2021) 227:15–29. doi: 10.1016/j.trsl.2020.06.013
86. Lu X, Wu Y, Gao XH, Wang YW, Wang L, Sun XX. Effect of letrozole on estradiol production and P450 aromatase messenger RNA expression of cultured luteinized granulosa cells from women with and without endometriosis. Fertil Steril (2012) 98(1):131–5. doi: 10.1016/j.fertnstert.2012.03.055
87. Dokras A, Habana A, Giraldo J, Jones E. Secretion of inhibin B during ovarian stimulation is decreased in infertile women with endometriosis. Fertil Steril (2000) 74(1):35–40. doi: 10.1016/s0015-0282(00)00568-9
88. Miyoshi T, Otsuka F, Inagaki K, Otani H, Takeda M, Suzuki J, et al. Differential regulation of steroidogenesis by bone morphogenetic proteins in granulosa cells: involvement of extracellularly regulated kinase signaling and oocyte actions in follicle-stimulating hormone-induced estrogen production. Endocrinology (2007) 148(1):337–45. doi: 10.1210/en.2006-0966
89. Deura I, Harada T, Taniguchi F, Iwabe T, Izawa M, Terakawa N. Reduction of estrogen production by interleukin-6 in a human granulosa tumor cell line may have implications for endometriosis-associated infertility. Fertil Steril (2005) 83 Suppl 1:1086–92. doi: 10.1016/j.fertnstert.2004.12.014
90. Li Y, Liu YD, Chen SL, Chen X, Ye DS, Zhou XY, et al. Down-regulation of long non-coding RNA MALAT1 inhibits granulosa cell proliferation in endometriosis by up-regulating P21 via activation of the ERK/MAPK pathway. Mol Hum Reprod (2019) 25(1):17–29. doi: 10.1093/molehr/gay045
91. Cecchino GN, Seli E, Alves da Motta EL, García-Velasco JA. The role of mitochondrial activity in female fertility and assisted reproductive technologies: overview and current insights. Reprod BioMed Online (2018) 36(6):686–97. doi: 10.1016/j.rbmo.2018.02.007
92. Xu B, Guo N, Zhang XM, Shi W, Tong XH, Iqbal F, et al. Oocyte quality is decreased in women with minimal or mild endometriosis. Sci Rep (2015) 5:10779. doi: 10.1038/srep10779
93. Yamashita Y, Asano M, Morishima S, Fujino K, Terai Y, Ohmichi M. Mitochondrial gene expression in granulosa cells of severe endometriosis with in vitro fertilization and embryo transfer. Fertil Steril (2007) 88(6):1703–5. doi: 10.1016/j.fertnstert.2007.01.111
94. Liu Y, Han M, Li X, Wang H, Ma M, Zhang S, et al. Age-related changes in the mitochondria of human mural granulosa cells. Hum Reprod (2017) 32(12):2465–73. doi: 10.1093/humrep/dex309
95. Mao J, Zhang J, Cai L, Cui Y, Liu J, Mao Y. Elevated prohibitin 1 expression mitigates glucose metabolism defects in granulosa cells of infertile patients with endometriosis. Mol Hum Reprod (2022) 28(6):gaac018. doi: 10.1093/molehr/gaac018
96. González-Fernández R, Martín-Ramírez R, Rotoli D, Hernández J, Naftolin F, Martín-Vasallo P, et al. Granulosa-lutein cell sirtuin gene expression profiles differ between normal donors and infertile women. Int J Mol Sci (2019) 21(1):295. doi: 10.3390/ijms21010295
97. Wyse BA, Fuchs Weizman N, Kadish S, Balakier H, Sangaralingam M, Librach CL. Transcriptomics of cumulus cells - a window into oocyte maturation in humans. J Ovarian Res (2020) 13(1):93. doi: 10.1186/s13048-020-00696-7
98. Sasaki H, Hamatani T, Kamijo S, Iwai M, Kobanawa M, Ogawa S, et al. Impact of oxidative stress on age-associated decline in oocyte developmental competence. Front Endocrinol (Lausanne) (2019) 10:811. doi: 10.3389/fendo.2019.00811
99. Banerjee J, Shaeib F, Maitra D, Saed GM, Dai J, Diamond MP, et al. Peroxynitrite affects the cumulus cell defense of metaphase II mouse oocytes leading to disruption of the spindle structure in vitro. Fertil Steril (2013) 100(2):578–84.e1. doi: 10.1016/j.fertnstert.2013.04.030
100. Yang Q, Cong L, Wang Y, Luo X, Li H, Wang H, et al. Increasing ovarian NAD+ levels improve mitochondrial functions and reverse ovarian aging. Free Radic Biol Med (2020) 156:1–10. doi: 10.1016/j.freeradbiomed.2020.05.003
101. Song ZQ, Li X, Wang YK, Du ZQ, Yang CX. DMBA acts on cumulus cells to desynchronize nuclear and cytoplasmic maturation of pig oocytes. Sci Rep (2017) 7(1):1687. doi: 10.1038/s41598-017-01870-6
102. Smith DM, Tenney DY. Effects of steroids on mouse oocyte maturation in vitro. J Reprod Fertil (1980) 60(2):331–8. doi: 10.1530/jrf.0.0600331
103. Sakaguchi K, Huang W, Yang Y, Yanagawa Y, Nagano M. Relationship between in vitro growth of bovine oocytes and steroidogenesis of granulosa cells cultured in medium supplemented with bone morphogenetic protein-4 and follicle stimulating hormone. Theriogenology (2017) 97:113–23. doi: 10.1016/j.theriogenology.2017.04.030
104. Bromfield JJ, Sheldon IM. Lipopolysaccharide initiates inflammation in bovine granulosa cells via the TLR4 pathway and perturbs oocyte meiotic progression in vitro. Endocrinology (2011) 152(12):5029–40. doi: 10.1210/en.2011-1124
105. Corachán A, Pellicer N, Pellicer A, Ferrero H. Novel therapeutic targets to improve IVF outcomes in endometriosis patients: a review and future prospects. Hum Reprod Update (2021) 27(5):923–72. doi: 10.1093/humupd/dmab014
106. Kong QQ, Wang J, Xiao B, FH L, Zhu J, GY S, et al. Cumulus cell-released tumor necrosis factor (TNF)-α promotes post-ovulatory aging of mouse oocytes. Aging (Albany NY) (2018) 10(7):1745–57. doi: 10.18632/aging.101507
Keywords: endometriosis, granulosa cells, infertility, mechanism, oocytes
Citation: Fan W, Yuan Z, Li M, Zhang Y and Nan F (2023) Decreased oocyte quality in patients with endometriosis is closely related to abnormal granulosa cells. Front. Endocrinol. 14:1226687. doi: 10.3389/fendo.2023.1226687
Received: 22 May 2023; Accepted: 01 August 2023;
Published: 16 August 2023.
Edited by:
Iveta Yotova, Medical University of Vienna, AustriaReviewed by:
Michele Da Broi, University of Sao Paulo, BrazilAbdulsamed Kükürt, Kafkas University, Türkiye
Copyright © 2023 Fan, Yuan, Li, Zhang and Nan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zheng Yuan, Z3l5MTMzMTEyOEAxNjMuY29t
 Weisen Fan
Weisen Fan Zheng Yuan2*
Zheng Yuan2* Yingjie Zhang
Yingjie Zhang