- 1Department of Radiation Oncology, Beijing Geriatric Hospital, Beijing, China
- 2Outpatient Department of Feng Tai District No.4 Retired Cadres Retreat Center, Army PLA, Beijing, China
- 3Department of Radiation Oncology, Air Force Medical Center PLA, Beijing, China
- 4Department of Radiation Oncology, Peking University Shou Gang Hospital, Beijing, China
- 5Center for Ion Medicine, The First Affiliated Hospital, University of Science and Technology of China, Hefei, China
Background: The continuous exploration of oligometastatic disease has led to the remarkable achievements of local consolidative therapy (LCT) and favorable outcomes for this disease. Thus, this study investigated the potential benefits of LCT in patients with single-organ metastatic pancreatic ductal adenocarcinoma (PDAC).
Methods: Patients with single-organ metastatic PDAC diagnosed between 2010 - 2019 were identified from the Surveillance, Epidemiology, and End Results (SEER) database. Propensity score matching (PSM) was performed to minimize selection bias. Factors affecting survival were assessed by Cox regression analysis and Kaplan-Meier estimates.
Results: A total of 12900 patients were identified from the database, including 635 patients who received chemotherapy combined with LCT with a 1:1 PSM with patients who received only chemotherapy. Patients with single-organ metastatic PDAC who received chemotherapy in combination with LCT demonstrated extended median overall survival (OS) by approximately 57%, more than those who underwent chemotherapy alone (11 vs. 7 months, p < 0.001). Furthermore, the multivariate Cox regression analysis revealed that patients that received LCT, younger age (< 65 years), smaller tumor size (< 50 mm), and lung metastasis (reference: liver) were favorable prognostic factors for patients with single-organ metastatic PDAC.
Conclusion: The OS of patients with single-organ metastatic pancreatic cancer who received LCT may be prolonged compared to those who received only chemotherapy. Nevertheless, additional prospective randomized clinical trials are required to support these findings.
1 Introduction
Pancreatic ductal adenocarcinoma (PDAC) is identified globally as a tumor with a poor prognosis (1). Patients often present with distant metastasis during diagnosis due to the insidious symptoms of pancreatic cancer, rendering surgery a non-viable treatment option (2, 3). Consequently, systemic chemotherapy remains the mainstay therapeutic modality for metastatic PDAC. Despite the tremendous advancements of these treatments, the prognosis remains extremely poor, with a 5-year overall survival (OS) rate of only 3% (4).
In 1995, Hellman and Weichselbaum proposed the concept of oligometastatic disease as a transitional state between localized and diffused metastatic burden characterized by limited metastatic tumors in particular sites (5). Clinical studies have shown that systemic treatment combined with local consolidative therapy (LCT) to all lesions significantly improved the prognosis of oligometastatic cancer patients (6–10). Traditionally, clinical practice guidelines deem surgery a contraindication for metastatic PDAC (11, 12). However, recent studies discovered that patients with metastatic pancreatic cancer who received systemic treatment and surgery exhibited significantly improved survival compared to chemotherapy alone (13, 14). Meanwhile, a retrospective cohort study of oligometastatic pancreatic cancer (OPanc) highlighted the benefits of stereotactic ablative radiation therapy (SABR) to all active metastatic sites with a median OS of 42 months, which was greater than chemotherapy alone (15).
Despite the proven benefits of LCT in metastatic PDAC, the previous studies were performed on a small sample size. Thus, recent studies have utilized data from the Surveillance, Epidemiology, and End Results database (SEER) and reported that LCT was associated with improved OS for metastatic PDAC (16–18). Currently, surgery and radiotherapy are viable LCT options for patients with cancer, but most studies focused primarily on the contribution of surgery in OPanc and neglected the role of radiotherapy. Most surgeries are performed on selected patients with OPanc and do not fully reflect the role of LCT in general cases. Earlier studies have not addressed the bias caused by data discrimination in the SEER database. Furthermore, these studies considered multi-organ metastasis of PDAC, which may have introduced confounders into the analysis. Therefore, the purpose of this study was to analyze results from the SEER database to assess the prognostic value of the LCT to patients with single-organ metastatic PDAC who received chemotherapy.
2 Patients and methods
2.1 Data source
The data used in this study were acquired from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) 17 registries Plus database (2000 - 2019) using SEER*Stat software version 8.4.0.1. The SEER data is publicly available and de-identified; thus, the data analysis does not require approval from the Institutional Ethics Committee. The researchers obtained authorization to access the database (Username: 10232-Nov2021).
2.2 Population study cohort
Patients with pancreatic cancer were first identified using the SEER primary site code based on the International Classification of Diseases for Oncology (ICD-0-3: C25.0 - C25.4, C25.7 - C25.9). In addition, patients with histology/behavior code 8140/3 (adenocarcinoma) or 8500/3 (invasive ductal adenocarcinoma) were selected for this study. As the database offers “SEER Combined Mets at DX liver/lung/bone/brain” information starting from 2010, this study only included patients diagnosed from 2010 to 2019. Furthermore, it should be noted that SEER has records of metastatic sites but not the number of metastatic sites.
The following patients were excluded from the study cohort: 1) patients who did not have pathological confirmation, 2) patients with pancreatic cancer that were not the first primary tumor or combined with other malignancies, 3) patients whose M stage was M0 or unclear, 4) patients with unknown T/N stage, 5) patients with multiple organ metastases (≥ 2) or metastases to organs other than lung, liver, brain, or bone, and 6) patients with single-organ metastatic pancreatic cancer who had not undergone chemotherapy. Despite the different TNM staging criteria used by the SEER database in 2010 - 2015, 2016 - 2017, and 2018-2019, the data collection for this study was not affected as the focus is on patients with metastatic pancreatic cancer. The original T/N staging criteria were used in this study to include more patients (T staging: T1/T2/T3/T4, N staging: N- and N+).
Generally, LCT is defined as receiving surgery, local radiotherapy, or surgery combined with local radiotherapy. External beam RT was the modality to receive radiation therapy; the patients whose “Radiation recode” code was “Beam radiation” were considered to receive local radiotherapy. Meanwhile, patients with Code 0 (surgery performed) were considered to have undergone surgery. Finally, the patients in this study were grouped as follows: 1) Chemotherapy group: patients who received chemotherapy; 2) Chemotherapy and LCT group: patients who received chemotherapy and LCT; 3) LCT group: patients who received LCT alone, and 4) Palliative group: patients who did not undergo chemotherapy and LCT.
2.3 Data collection
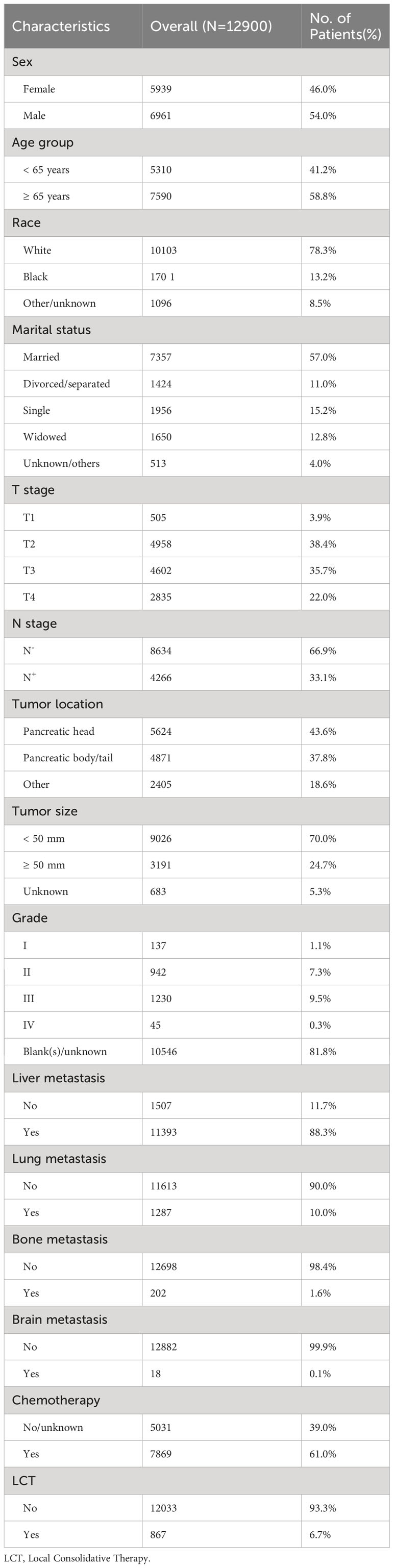
The following information was collected from the patient’s records: (i) Sex (Female/male), (ii) Age group (< 65 years/≥ 65 years), (iii) Race (White/black/other-unknown), (iv) Marital status (Married/divorced-separated/single/widowed/unknown-others), (v) T-stage (T1/T2/T3/T4), (vi) N-stage (N-/N+), (vii) Tumor location (Pancreatic head/pancreatic body - tail/other), (viii) Tumor size (< 50 mm/50 – 100 mm/unknown), (ix) Grade (I/II/III/IV/Blank(s)-unknown), (x) Metastasis location (Liver/lung/bone/brain), (xi) LCT (No/Yes).
2.4 Statistical analysis
Data analyses were performed using R v.4.2.2 statistical software (http://www.R-project.org, The R Foundation, Vienna, Austria), IBM Statistical Package for the Social Sciences (SPSS) software, version 26.0 (SPSS Inc., Chicago, IL, USA), and GraphPad Prism 9 version 9.40 (GraphPad Software, Inc., La Jolla, California USA). The categorical variables were expressed as proportions, and the differences were analyzed using Chi-square or Fisher’s exact test. The Cox regression, Kaplan-Meier plots, and the R survival package were also used for the survival analysis. A Cox proportional hazard model was applied to calculate each variable’s hazard ratio (HR) and 95% confidence interval (CI).
The multivariate Cox proportional hazard model utilized variables with p-values of < 0.2 in the univariate analysis. The multivariate analysis used backward stepwise Cox proportional hazards regression to determine the predictive factors. Survival curves were plotted via the Kaplan-Meier method and compared using the log-rank test. All the statistical tests used in this study were two-sided, and differences were considered significant at p < 0.05.
The PSM was used to adjust measured confounders, thus creating more comparable groups (19). A logistic regression analysis was undertaken using sex, age group, race, marital status, T stage, N stage, tumor location, tumor size, metastasis location, and LCT to calculate propensity scores for propensity score-matched analysis. Data relating to grade was mostly missing (blank(s)-unknown accounted for nearly 70% or more of each group), hence, unmatched in the PSM to ensure the accuracy of the study findings. The Chemotherapy group was matched to the Chemotherapy and LCT group using a 1:1 ratio with the caliper width set to 0.05 (20). The intergroup differences in categorical variables were compared using the Chi-squared test before and after matching.
3 Results
3.1 Baseline patient characteristics before PSM
A total of 81831 patients with pancreatic cancer were recorded in the SEER database from 2010 to 2019. In the present study, 12900 patients with single-organ metastatic pancreatic cancer fulfilled the inclusion and exclusion criteria (see Table 1; Figure 1). Patients with a single metastatic site often involved the liver (88.3%), followed by the lung (10.0%), followed by the bone (1.6%), and the brain (0.1%). Remarkably, N0 (n = 8634, 66.9%) was most common among patients with single-organ metastatic pancreatic cancer at the time of diagnosis. Furthermore, 7869 (61%) patients received systemic chemotherapy, and 867(6.7%) patients were treated with LCT.
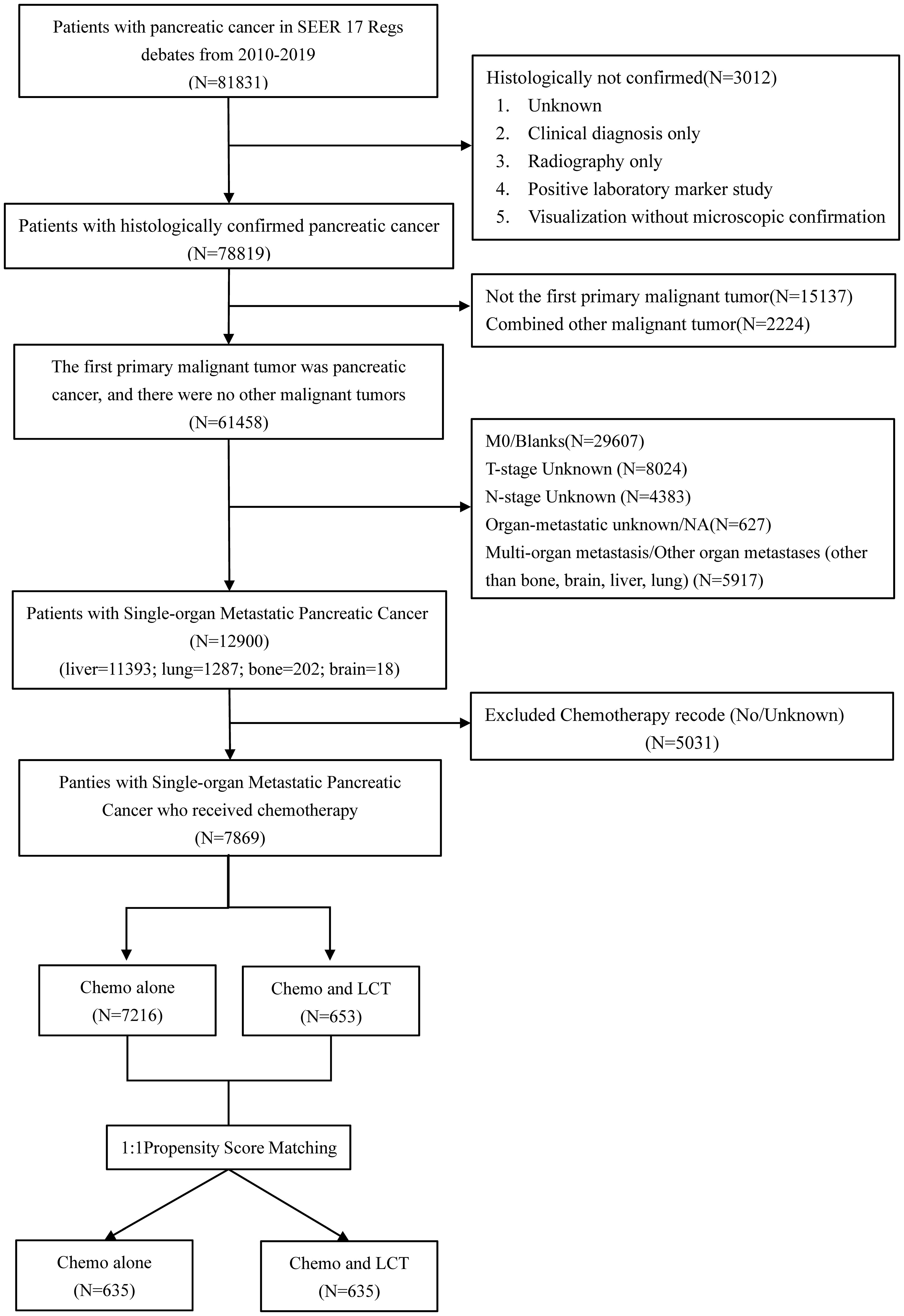
Figure 1 Flow chart depicting the patient selection process. SEER, Surveillance, Epidemiology, and End Results, LCT, local consolidative therapy, Chemo, chemotherapy.
In the chemotherapy group, 7216 patients underwent chemotherapy alone. Meanwhile, 653 patients received chemotherapy combined with LCT in the chemotherapy and LCT group, where 246 patients underwent surgery as their primary treatment, 376 patients received radiotherapy alone, and 31 were subjected to surgery and radiotherapy. There were significant differences between the two groups before matching in terms of age, race, T-stage, N-stage, tumor location, and metastatic sites (see Table 2).
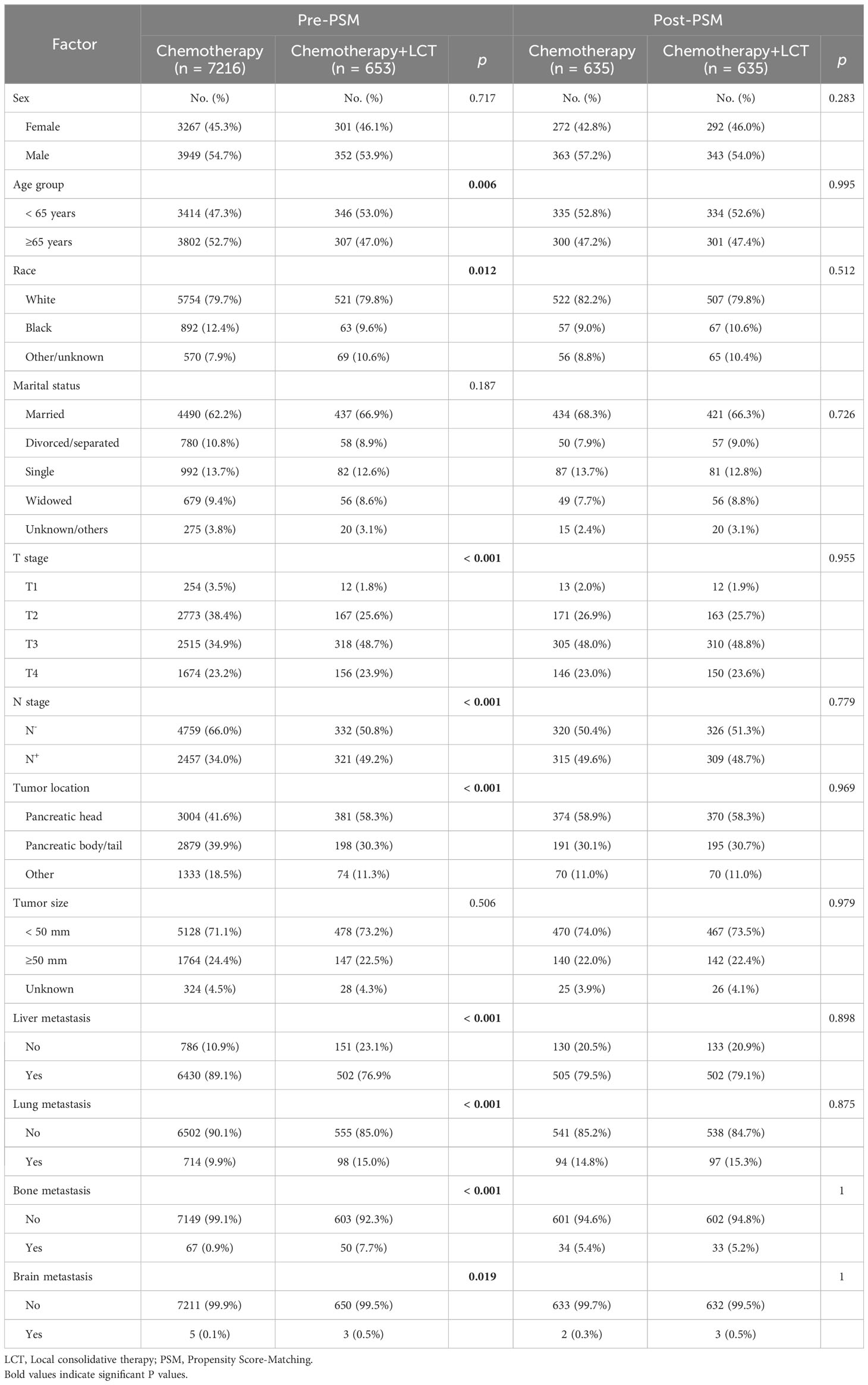
Table 2 Characteristics of patients in the chemotherapy alone and chemotherapy + LCT groups before and after the propensity score matching.
3.2 Baseline patient characteristics after PSM
After a 1:1 PSM analysis, 1270 patients were included in this study (635 patients/group). The statistically significant differences in baseline characteristics between groups decreased after PSM—nonetheless, the number of patients with brain metastases was negligible between the two groups. The baseline characteristics of patients after matching are shown in Table 2.
3.3 Survival outcomes before PSM
Patients with single-organ metastasis demonstrated better prognosis with a median OS of four months (95% CI: 3.87 - 4.13) than those with multiple-organ metastasis (OS, log-rank test, p < 0.001) (Figure 2).
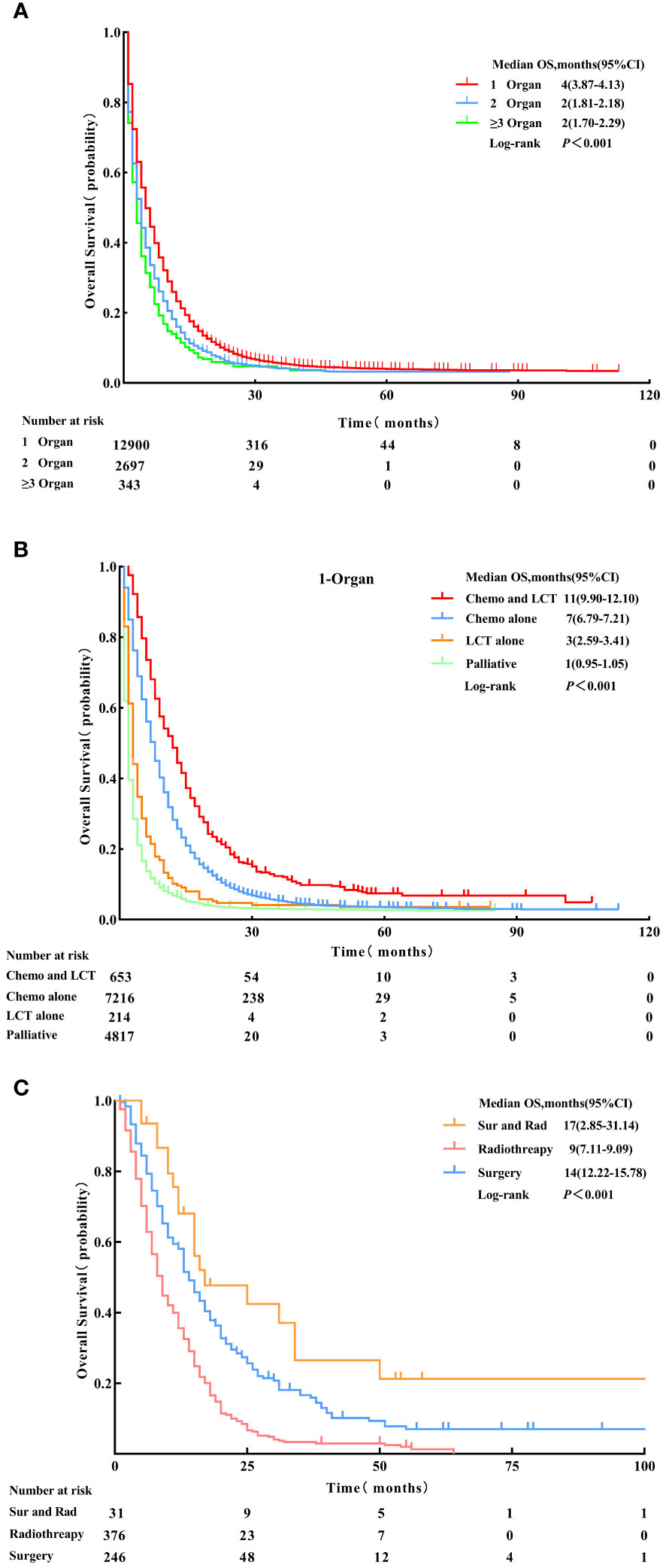
Figure 2 Overall survival for patients with 1-organ metastasis, 2-organs metastasis, and ≥ 3-organs metastasis. (A) Patients who received Chemo combined with LCT, Chemo alone, LCT alone, and palliative treatment (B) Patients who received Sur and Rad, Rad alone and Sur alone (C) before Propensity Score-Matching. LCT, local consolidative therapy, Chemo, chemotherapy, Sur, surgery, Rad, radiotherapy.
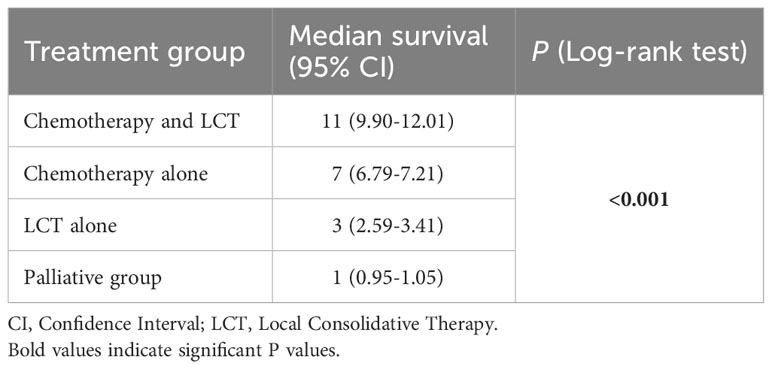
The median OS for patients with single-organ metastatic pancreatic cancer in all groups were as follows: chemotherapy and LCT group = 11 months (95% CI: 9.90-12.01), chemotherapy group = 7 months (95% CI: 6.79 - 7.21), LCT group = 3 months (95% CI:2.59-3.41), and palliative group = 1 month (95% CI: 0.95 - 1.05) (see Table 3). The most significant improvement in survival was observed in patients who underwent chemotherapy and LCT.
The survival analysis using Kaplan-Meier survival curves revealed distinct differences in clinical outcomes among the four groups (OS, log-rank test, p<0.001) (see Figure 2). In the analysis of the chemotherapy and LCT group by local treatment modality subgroups, the median OS was 17 months (95% CI: 2.85 - 31.14) in the surgery and radiotherapy group, 14 months (95% CI: 12.22 - 15.78) in the surgery group, and 9 months (95% CI: 7.11 - 9.09) in the radiotherapy group (OS, log-rank test, p < 0.001) (see Figure 2).
3.4 Survival outcomes after PSM
The principal findings post-PSM were generally consistent with those before matching. Patients with single-organ metastatic pancreatic cancer who received chemotherapy combined with LCT had significantly improved survival than those who received chemotherapy alone (11 vs.7 months, log-rank test, p < 0.001). Meanwhile, patients who were treated with LCT alone demonstrated better survival outcomes than those who did not receive either LCT or chemotherapy (3 vs.1 month, log-rank test, p < 0.001) (see Figure 3).
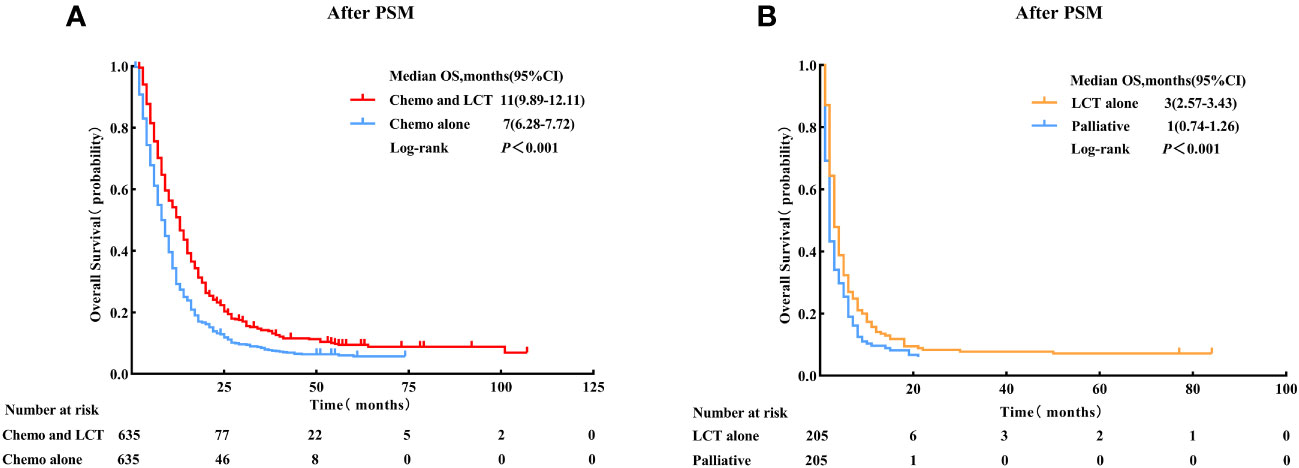
Figure 3 Overall survival for patients with single-organ metastatic PDAC who received Chemo combined with LCT and Chemo alone. (A) Patients who received LCT alone and palliative treatment, (B) after Propensity Score-Matching. LCT, local consolidative therapy, Chemo, chemotherapy.
Patients were then subdivided based on the type of metastatic site to determine which group benefited most from LCT. The results of the subgroup analysis suggested that the OS rate was significantly improved with the use of LCT for patients with liver metastasis (11 vs. 7 months, log-rank test, p < 0.001) and lung metastasis (16 vs. 10 months, log-rank test, p < 0.001) (see Figure 4).
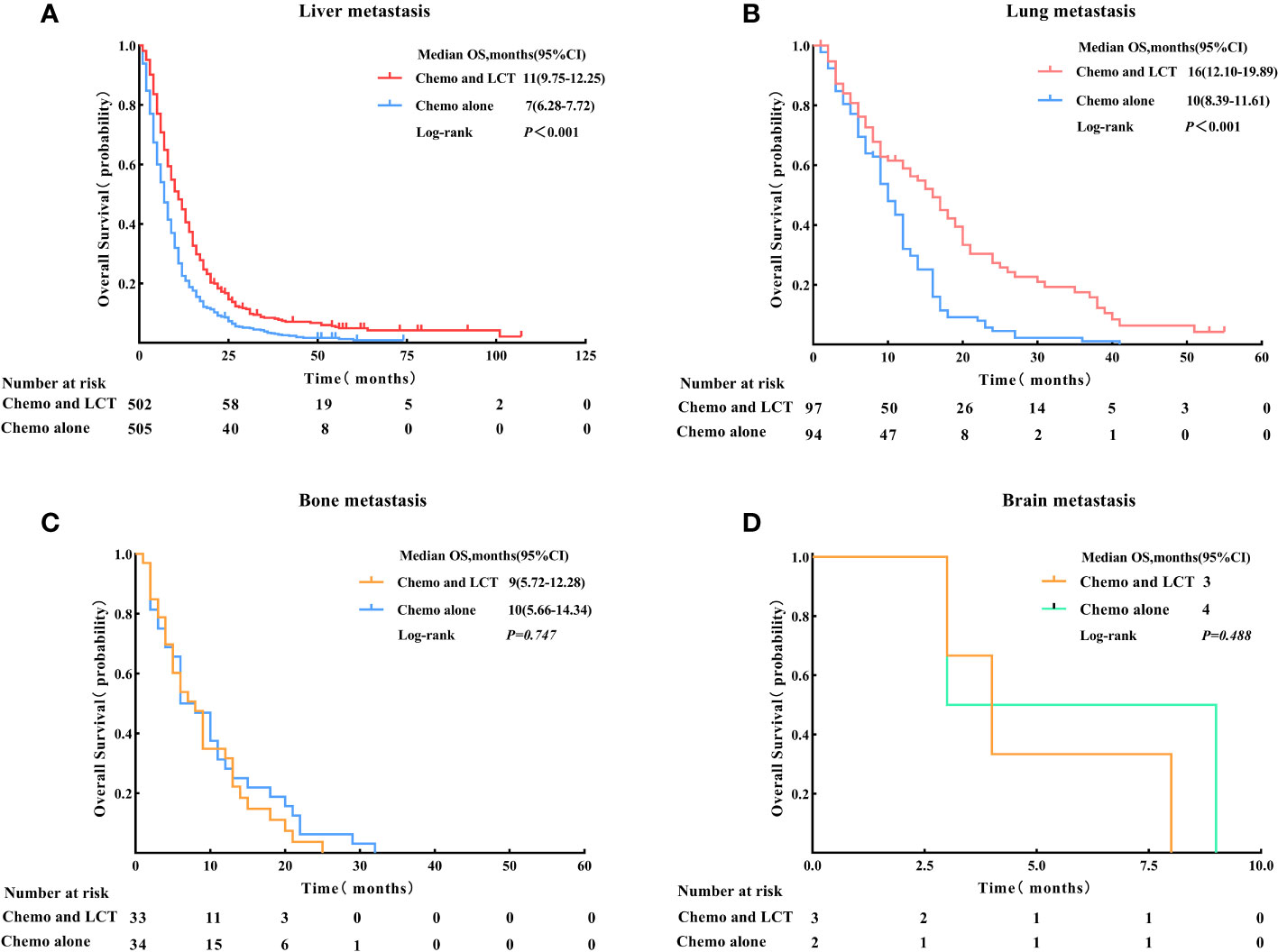
Figure 4 Overall survival for the type of metastatic site in patients with single-organ metastatic PDAC after Propensity Score-Matching. (A) Liver metastasis, (B) Lung metastasis, (C) Bone metastasis, and (D) Brain metastasis. LCT, local consolidative therapy, Chemo, chemotherapy.
3.5 Univariate and multivariate analyses of factors associated with OS
The univariate Cox analysis identified the factors associated with enhanced OS, including < 65 years (HR = 0.83, 95% CI: 0.74 - 0.94, p = 0.002), smaller tumor size (< 50 mm) (HR < 50 mm vs. ≥ 50 mm = 0.80, 95% CI:0.69 - 0.92, p = 0.002), metastasis site (lung) (HR lung vs. liver metastasis = 0.81, 95% CI: 0.69 - 0.96, p = 0.015) and LCT (HR = 0.63, 95% CI: 0.55 - 0.71, p < 0.001). Meanwhile, tumor site was not a significant prognostic factor for survival (p = 0.181) (see Table 4; Figure 5).
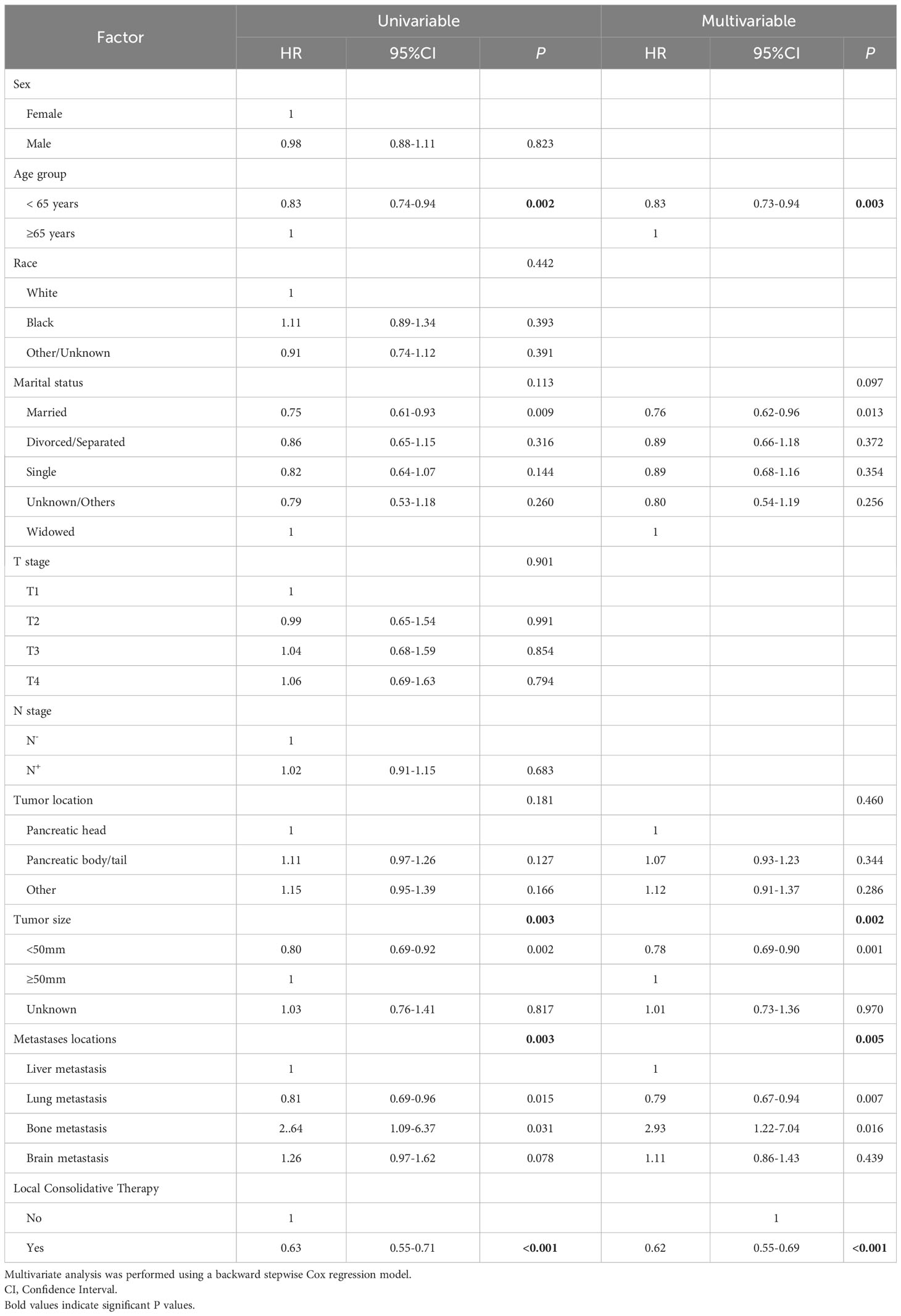
Table 4 Factors associated with overall survival in univariate and multivariate analyses in panties with single-organ metastatic pancreatic cancer who received chemotherapy.
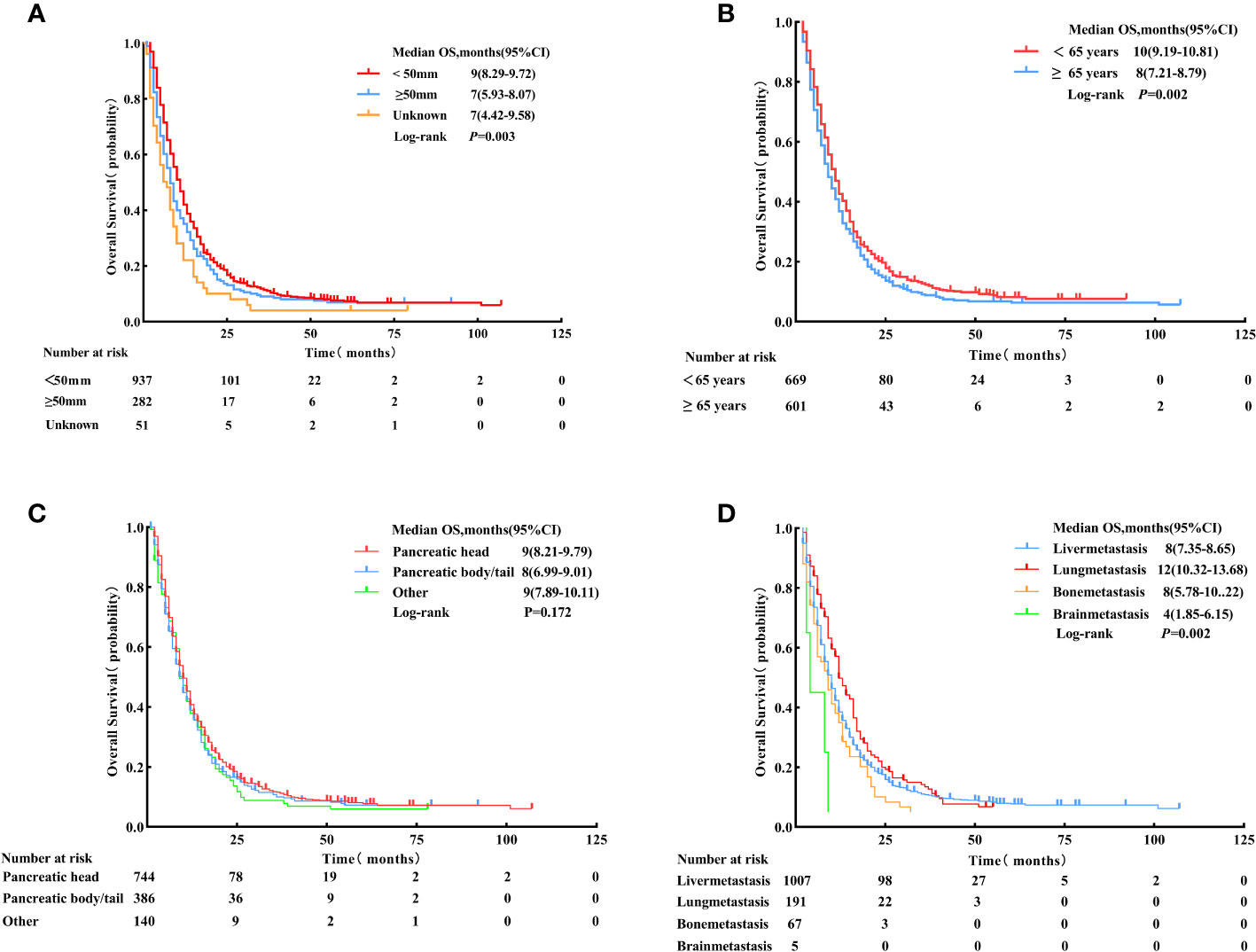
Figure 5 Overall survival for patients with different tumor sizes (< 50 mm, ≥ 50 mm, and unknown). (A) Age: < 65 years vs. ≥ 65 years, (B) Primary tumor location: pancreatic head, pancreatic body/tail, and other sites, (C) Metastasis site: liver, lung, bone, and brain, and (D) after Propensity Score-Matching.
The multivariate Cox regression analysis results were consistent with the univariate analysis after matching, indicating that the parameters were independent prognostic factors for OS. The results of the multivariate analysis are presented in Table 4; Figure 6.
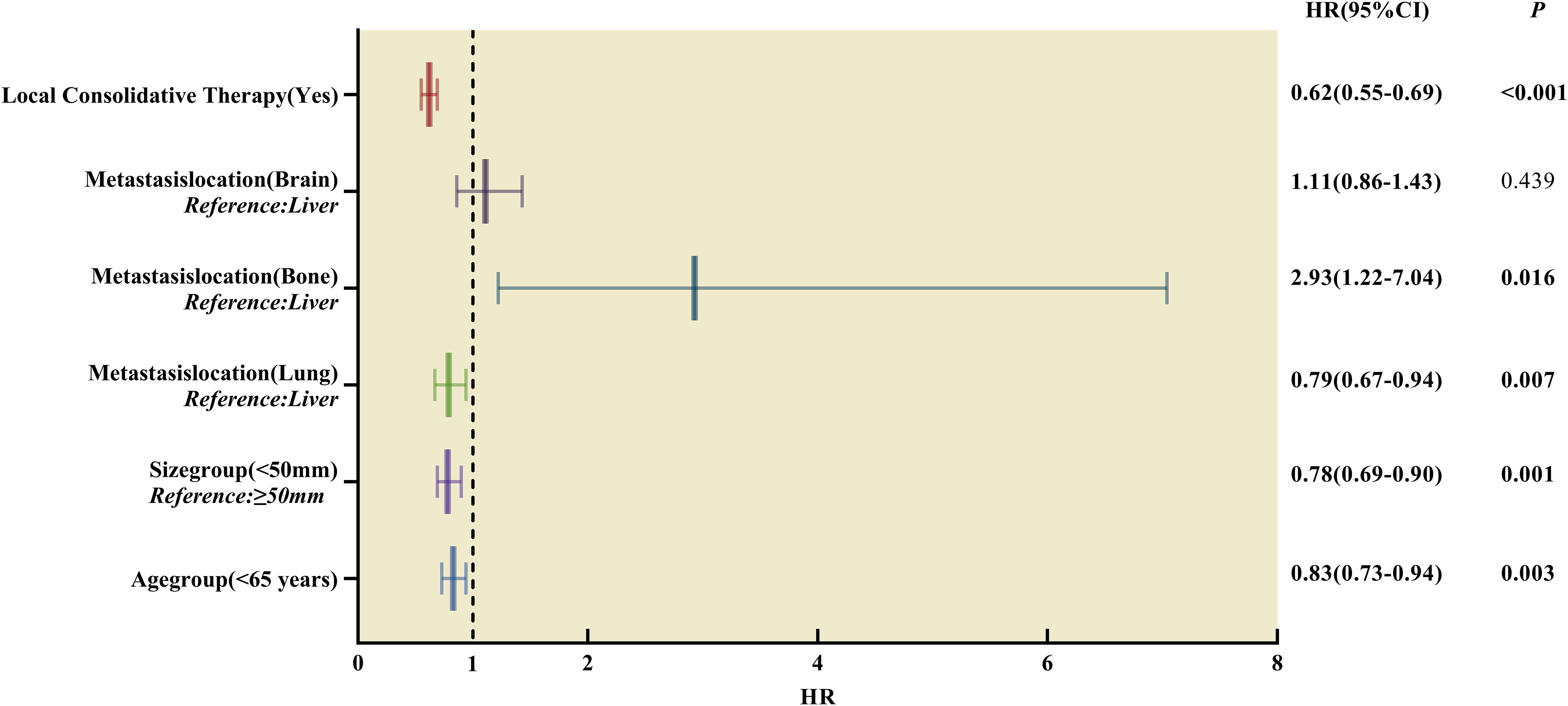
Figure 6 Forest plot of hazard ratios derived from multivariate Cox regression analysis of prognosis in patients with single-organ metastatic PDAC after Propensity Score-Matching. HR, hazard ratios, CI, confidence interval.
4 Discussion
Clinical and biological evidence have supported the oligometastatic state (21–23), but a consensus on the concept of oligometastatic disease has yet to be reached. Most clinical trials and the Society of Clinical Oncology agreed on the definition of 1 – 3 or 1 – 5 metastatic lesions (24). However, recent studies have shown that oligometastasis is no longer a definition but a strategy most likely to benefit from radical local therapy (25). For instance, Damanakis et al. (26) performed a retrospective analysis of 128 patients with metastatic PDAC and found that low CA19-9 levels (< 1000 U/mL), effective systemic treatment, and single-organ metastases were favorable prognostic factors for this disease. In addition, improved survival was observed in patients with single-organ metastatic PDAC than in those with multiple-organ metastasis (4 vs.2 months).
Selamir et al. (15) reported that 20 patients with synchronous or metachronous OPanc received definitive radiotherapy (median biologically effective dose 10 is 100 Gy) for all lesions and had significantly prolonged survival compared to 21 patients who did not (42 vs.18 months, p = 0.003). The current study found that definitive radiotherapy can delay systemic chemotherapy, and 17 (85%) SABR-treated patients had 6 or more months of off chemotherapy. A recent SEER data analysis of 259 patients with PDCA with liver metastases concluded that cancer-directed surgery significantly prolongs median OS by 5 - 10 months (21). These studies indicated that patients with OPanc would likely benefit from the LCT. Likewise, patients with single-organ metastatic PDAC who received chemotherapy combined with LCT in the present study had extended median OS by approximately 57% compared to those who underwent chemotherapy alone (11 vs.7 months, p < 0.001).
In this study, LCT (HR = 0.62, 95% CI:0.55 - 0.69, p < 0.001) was an independent, favorable prognostic factor for OS in patients with single-organ metastatic PDAC. Notably, 31 patients who underwent surgery in combination with radiotherapy had the best survival outcomes than those who received surgery or radiotherapy alone (17 months, p < 0.001). Therefore, it was postulated that patients with better prognoses would likely receive LCT for primary tumor and metastasis lesions, which aligned with previous reports (6–10). Moreover, surgery patients had significantly better survival outcomes than radiotherapy (14 vs. 9 months, p < 0.001). This finding may be linked to the patient selection in this study, who are generally in good condition with stable metastatic disease, high chances of recovery, lower tumor burden, and a high compliance rate (27). However, these data were unavailable in the SEER database to be analyzed in this study. Meanwhile, in the whole group of patients with single-organ metastatic PDAC, patients who received chemotherapy alone had better overall survival than those who received radiotherapy alone (7 vs.2 months, log-rank test, p < 0.001). Based on the results of this and other studies, patients with oligometastatic PDAC were more likely to benefit from radical surgery or radical radiotherapy to the primary tumor and all visible metastatic sites. To achieve the goal of a radical cure for all residual lesions, multi-modality LCT of patients with oligometastatic PDAC requires an optimal combination of radiotherapy and surgery. Moreover, patients with fewer metastatic lesions and metastatic sites more amenable to radical treatment are more likely to benefit from LCT.
Earlier studies suggested that the primary tumor site impacts the prognosis of patients with OPanc. For example, Yang et al. (28) reported that patients with liver oligometastatic pancreatic body/tail ductal adenocarcinoma who underwent resection of the primary tumor and liver metastases exhibited improved prognosis than patients who received chemotherapy alone (16.8 vs. 8 months, p = 0.003). The primary tumor site directly affects the surgical decision and the possibility of R0 resection in patients with PDAC. In this study, 153 (62.2%) patients with single-organ metastatic pancreatic cancer who underwent surgery had primary tumors located in the head of the pancreas, 78 (31.7%) in the pancreatic body/tail, and 15 (6.1%) in other sites. Meanwhile, there were no significant differences in the survival outcomes between groups (p = 0.82) and all the LCT groups (p = 0.32). Univariate and multivariate Cox regression analyses demonstrated that the primary tumor location was not a prognostic factor that may be related to highly selected patients. Earlier studies reported that tumor size was an independent prognostic factor for OS in patients with PDAC (29–31). For instance, Xu et al. (30) conducted a retrospective analysis of 221 patients with clearly resectable pancreatic cancer, and tumor size (≥ 6 cm) was a significant variable related to OS. The 7th edition of the American Joint Committee on Cancer (AJCC) Cancer Staging Manual classified tumor size by 2 cm, and the updated 8th edition classified the size of pancreatic cancer into three levels. The current study showed no differences in survival between T-stage (p = 0.48), but there was a significant difference in survival between patients with < 5 cm and ≥ 5 cm tumor sizes after the tumor reclassification (9 vs.7 months, p = 0.0017). This survival benefit may be attributed to the lower tumor burden; patients with smaller tumor sizes were more likely to receive LCT. Further analysis found that 467 (73.5%) patients with primary tumors <5 cm in the LCT group had better OS compared to 142 (24.4%) patients ≥ 5 cm (12 vs. 9 months). However, the results did not exhibit statistical significance (p = 0.31).
Multiple studies (32–34) reported that age is an important prognostic factor for patients with PDAC. For instance, Van Dongen et al. (35) conducted a population-based cohort study using data from the Netherlands Cancer Registry involving 10298 patients diagnosed with PDAC. The findings suggested that patients < 60 years mostly underwent surgery (22 vs. 14%, p < 0.001), frequently agreed to chemotherapy, and had enhanced OS (6.9 vs. 3.3 months, p < 0.001) than older patients. Meanwhile, the present study demonstrated that patients aged < 65 years were associated with better survival outcomes in univariate and multivariate analyses (HR = 0.83, 95% CI:0.73 - 0.94, p = 0.003) and improved OS compared to older patients (10 vs. 8 months, p = 0.002). This survival benefit may be attributed to the better health status of younger patients, less underlying disease, higher tolerance to chemotherapy, better immune function, and more likely to receive LCT than older patients.
In this study, only 52.6% (n = 334) of the 653 patients who received chemotherapy and LCT were younger than 65. However, further investigations were not possible due to limited data. According to the literature, the lung is an uncommon site for distant pancreatic cancer metastasis and may define a distinct biological subgroup (36). Kruger et al. (37) performed a multicenter retrospective study in PDAC with lung metastasis. They reported that the median survival time of 115 patients with PDAC and lung metastasis was 20 months, and the most favorable prognosis (28 months) occurred in 66 patients with metachronous lung metastases whom previous primary tumors underwent radical surgery. In this study, the most common site of metastatic disease was the liver (88.3%), followed by the lung (10.0%), bone (1.6%), and brain (0.1%). Patients with lung metastasis had longer median OS and better prognoses (12 months, p = 0.002) compared to other single-organ metastasis (liver, bone, and brain) in the whole group. Notably, patients with lung metastasis who received LCT had better OS compared to those who underwent chemotherapy alone (16 vs.10 months, p < 0.001). At the same time, patients with lung metastasis receiving LCT also had longer median OS (16 months, p < 0.001) compared to other single-organ metastasis (liver, bone, and brain) in the LCT group. Of these patients, 56 (57.7%) received radiotherapy, 36 (37.1%) received surgery, and 5 (5.2%) received a combination of radiation therapy and surgery.
While the SEER database provided a large patient population and long-term survival data, this study had several limitations that should be acknowledged. Firstly, the SEER database lacks complete, detailed information on patients’ general condition, tumor diagnosis, and treatment. For instance, it was challenging to discern between actual patients with oligometastatic disease due to the lack of data on the number of metastatic sites. Similarly, distinguishing synchronous from metachronous oligometastatic disease was difficult due to the lack of information on the interval between primary tumor diagnosis and the appearance of metastases. Furthermore, these diseases had different oligometastatic statuses with varying prognoses and responses to anti-cancer treatment. Third, the SEER database did not specify the radiotherapy doses and regimens or systemic treatment cycles. Nonetheless, the treatment regimens in this study were significantly homogenous as the samples covered a window from 2010 to 2019.
Chemotherapy agents and radiotherapy protocols change over time with drugs and technological advancements. Conroy et al. (38) reported that FOLFIRINOX was associated with a survival advantage (median OS) in clinical trials compared to gemcitabine (11.1 vs. 6.8 months, p < 0.001). Moreover, a phase III randomized controlled trial (39) demonstrated that nab-paclitaxel in combination with gemcitabine significantly improved the OS of patients with metastatic pancreatic cancer than gemcitabine alone (8.5 vs. 6.7 months, p < 0.001). Meanwhile, Selamir et al. (15) reported that 20 patients with OPanc who received SABR for all lesions had significantly prolonged survival compared to 21 patients who did not (42 vs. 18 months, p = 0.003). In the present study, the SEER analysis did not include the serum level CA 19-9, an important prognostic and monitoring indicator for patients with PDAC (40). An earlier study reported that a preoperative CA19-9 level of ≥ 100 U/ml significantly predicts poor prognosis after cancer surgery (41).
Information on the sequence of local therapy and systemic treatment is essential in predicting the optimal timing of LCT interventions for patients with metastatic pancreatic cancer. Based on previous randomized controlled clinical studies (7, 9, 10), systemic treatment followed by definitive local therapy for all lesions is highly beneficial for patients with oligometastatic disease. The initial systemic treatment may lead to stable or responsive disease, but the remaining tumors may contain treatment-resistant malignant cells that the maintenance systemic therapy does not eliminate. Stable disease is the first prerequisite for surgery or definitive radiotherapy to all residual tumor sites. However, suppose the patient has known presumably symptomatic brain metastases or a series of bone-related events that seriously affect the quality of life. In that case, it is necessary to consider the timing of local therapy. Additionally, the SEER database was unavailable for information relating to underlying diseases, insurance information, and smoking status. These factors can potentially influence patients’ survival and treatment decisions. Lastly, the SEER database does not include data on the treatments for recurrences or progression that could contribute to the decision-making for palliative patients and those who have undergone chemotherapy and radiation.
There is increasing promising evidence of the clinical benefits of LCT in oligometastatic disease. However, mechanism research and prospective studies on oligometastatic PDAC are sparse. The differential between non-oligometastatic PDAC and true oligometastatic PDAC is a real challenge for physicians. Identification of the true oligometastatic state plays a vital role in clinical decision-making, which is significant in determining the therapeutic option. In addition, tumor immunotherapy has revolutionized cancer treatment and has established itself as an important pillar of oncological therapy. Shi Y et al. (42) conducted a study on the ferroptosis regulator and its association with the immune microenvironment and programmed cell death ligand 1 (PD-L1) in pancreatic adenocarcinoma, and it was found that FANCD2 could be effective for prognostic recognition, immune efficacy evaluation, and mRNA vaccine for patients with pancreatic adenocarcinoma. Meanwhile, Wu L et al. (43) summarized preclinical and clinical studies regarding the synergic effect of radiotherapy combined with immune checkpoint inhibitors, especially SBRT irradiation to the tumor. Moreover, Bauml JM et al. (44) reported that after locally ablative therapy for oligometastatic non-small cell lung cancer, pembrolizumab improved PFS with no reduction in quality of life (median PFS: 19.1 months).
5 Conclusion
The SEER database analysis in this study suggested that patients with single-organ metastatic pancreatic cancer (liver, lung, bone, brain) who received chemotherapy combined with LCT may prolong their OS more than chemotherapy alone. Nevertheless, further studies should be conducted to support LCT and the optimal combination of systemic treatment and LCT as treatment options for this disease and identify patients who would benefit most from the treatment.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical review and approval were waived for this study due to the data being publicly available and anonymous.
Author contributions
(I) Conception and design: YW, XH. (II) Administrative support: DH, JZ. (III) Provision of study materials or patients: GR, CL, XW, BF. (IV)Collection and assembly of data: XH, HQL, HP, HFL, JS. (V) Data analysis and interpretation: XH, HQL, XK. (VI) Manuscript writing: XH, DH. All authors contributed to the article and approved the submitted version.
Funding
School Clinical Research Program of Air Force Military Medical University (No. 2021LC2227) and Capital Health Development Research Program (No. 2022-2-5121).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021 [published correction appears in CA Cancer J Clin. 2021 Jul;71(4):359]. CA Cancer J Clin (2021) 71(1):7–33. doi: 10.3322/caac.21654
2. Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet (2011) 378(9791):607–20. doi: 10.1016/S0140-6736(10)62307-0
3. Vanek P, Urban O, Zoundjiekpon V, Falt P. Current Screening Strategies for Pancreatic Cancer. Biomedicines (2022) 10(9):2056. doi: 10.3390/biomedicines10092056
4. American Cancer Society Cancer Statistics 2021 Report. J Nucl medicine: Off publication Soc Nucl Med (2021) 62(3):12N.
5. Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol (1995) 13(1):8–10. doi: 10.1200/JCO.1995.13.1.8
6. Chalkidou A, Macmillan T, Grzeda MT, Peacock J, Summers J, Eddy S, et al. Stereotactic ablative body radiotherapy in patients with oligometastatic cancers: a prospective, registry-based, single-arm, observational, evaluation study. Lancet Oncol (2021) 22(1):98–106. doi: 10.1016/S1470-2045(20)30537-4
7. Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomized, phase 2, open-label trial. Lancet (2019) 393(10185):2051–8. doi: 10.1016/S0140-6736(18)32487-5
8. Tang C, Msaouel P, Hara K, et al. Definitive radiotherapy in lieu of systemic therapy for oligometastatic renal cell carcinoma: a single-arm, single-center, feasibility, phase 2 trial. Lancet Oncol (2021) 22(12):1732–9. doi: 10.1016/S1470-2045(21)00528-3
9. Gomez DR, Tang C, Zhang J, Blumenschein Jr Hernandez GR M, Lee JJ, et al. Local consolidative therapy vs. maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer: long-term results of a multi-institutional, Phase II, Randomized Study. J Clin Oncol (2019) 37(18):1558–65. doi: 10.1200/JCO.19.00201
10. Gomez DR, Blumenschein GR Jr, Lee JJ, Hernandez M, Ye R, Camidge DR, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomized, controlled, phase 2 study. Lancet Oncol (2016) 17(12):1672–82. doi: 10.1016/S1470-2045(16)30532-0
11. Seufferlein T, Bachet JB, Van Cutsem E, Rougier P, Guidelines Working Group ESMO. Pancreatic adenocarcinoma: ESMO-ESDO Clinical Practice Guidelines for diagnosis, treatment, and follow-up. Ann Oncol (2012) 23. doi: 10.1093/annonc/mds224
12. Paulson AS, Tran Cao HS, Tempero MA, Lowy AM. Therapeutic advances in pancreatic cancer [published correction appears in Gastroenterology. Gastroenterology (2013) 144(6):1316–26. doi: 10.1053/j.gastro.2013.01.078
13. Crippa S, Bittoni A, Sebastiani E, Partelli S, Zanon S, Lanese A, et al. Is there a role for surgical resection in patients with pancreatic cancer with liver metastases responding to chemotherapy? Eur J Surg Oncol (2016) 42(10):1533–9. doi: 10.1016/j.ejso.2016.06.398
14. Wright GP, Poruk KE, Zenati MS, Steve J, Bahary N, Hogg ME, et al. Primary tumor resection following favorable response to systemic chemotherapy in stage iv pancreatic adenocarcinoma with synchronous metastases: a bi-institutional analysis. J Gastrointest Surg (2016) 20(11):1830–5. doi: 10.1007/s11605-016-3256-2
15. Elamir AM, Karalis JD, Sanford NN, Polanco PM, Folkert MR, Porembka MR, et al. Ablative Radiation Therapy in Oligometastatic Pancreatic Cancer to Delay Polyprogression, Limit Chemotherapy, and Improve Outcomes. Int J Radiat Oncol Biol Phys (2022) 114(4):792–802. doi: 10.1016/j.ijrobp.2022.07.019
16. Oweira H, Petrausch U, Helbling D, Schmidt J, Mannhart M, Mehrabi A, et al. Prognostic value of site-specific metastases in pancreatic adenocarcinoma: a surveillance epidemiology and end results database analysis. World J Gastroenterol (2017) 23(10):1872–80. doi: 10.3748/wjg.v23.i10.1872
17. Cao BY, Tong F, Zhang LT, Kang YX, Wu CC, Wang QQ, et al. Risk factors, prognostic predictors, and nomograms for pancreatic cancer patients with initially diagnosed synchronous liver metastasis. World J Gastrointest Oncol (2023) 15(1):128–42. doi: 10.4251/wjgo.v15.i1.128
18. Li Z, Zhang X, Sun C, Li Z, Fei H, Zhao D. Does surgical resection significantly prolong the long-term survival of patients with oligometastatic pancreatic ductal adenocarcinoma? a cross-sectional study based on 18 registries. J Clin Med (2023) 12(2):513. doi: 10.3390/jcm12020513
19. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res (2011) 46(3):399–424. doi: 10.1080/00273171.2011.568786
20. Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat (2011) 10(2):150–61. doi: 10.1002/pst.433
21. Pausch TM, Liu X, Cui J, Wei J, Miao Y, Heger U, et al. Survival benefit of resection surgery for pancreatic ductal adenocarcinoma with liver metastases: a propensity score-matched seer database analysis. Cancers (Basel). (2021) 14(1):57. doi: 10.3390/cancers14010057
22. Yatabe Y, Matsuo K, Mitsudomi T. Heterogeneous distribution of egfr mutations is extremely rare in lung adenocarcinoma. J Clin Oncol (2011) 29:2972–7. doi: 10.1200/JCO.2010.33.3906
23. Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med (2012) 366:883–92. doi: 10.1056/NEJMoa1113205
24. Guckenberger M, Lievens Y, Bouma AB, Collette L, Dekker A, deSouza NM, et al. Characterisation and classification of oligometastatic disease: a european society for radiotherapy and oncology and european organisation for research and treatment of cancer consensus recommendation. Lancet Oncol (2020) 21(1):e18–28. doi: 10.1016/S1470-2045(19)30718-1
25. Jasper K, Stiles B, McDonald F, Palma DA. practical management of oligometastatic non-small-cell lung cancer. J Clin Oncol (2022) 40(6):635–41. doi: 10.1200/JCO.21.01719
26. Damanakis AI, Ostertag L, Waldschmidt D, Kütting F, Quaas A, Plum P, et al. Proposal for a definition of "Oligometastatic disease in pancreatic cancer”. BMC Cancer (2019) 19(1):1261. doi: 10.1186/s12885-019-6448-9
27. Shrikhande SV, Kleeff J, Reiser C, Weitz J, Hinz U, Esposito I, et al. Pancreatic resection for M1 pancreatic ductal adenocarcinoma. Ann Surg Oncol (2007) 14(1):118–27. doi: 10.1245/s10434-006-9131-8
28. Yang J, Zhang J, Lui W, Huo Y, Fu X, Yang M, et al. Patients with hepatic oligometastatic pancreatic body/tail ductal adenocarcinoma may benefit from synchronous resection. HPB (Oxford). (2020) 22(1):91–101. doi: 10.1016/j.hpb.2019.05.015
29. Das R, McGrath K, Seiser N, Smith K, Uttam S, Brand RE, et al. Tumor size differences between preoperative endoscopic ultrasound and postoperative pathology for neoadjuvant-treated pancreatic ductal adenocarcinoma predict patient outcome. Clin Gastroenterol Hepatol (2022) 20(4):886–97. doi: 10.1016/j.cgh.2020.11.041
30. Xu D, Wang J, Liu T, Huang Z, Luo J, Chen Y, et al. Quantitative definitions of pain, CA19-9, and tumor size as high-risk features of resectable pancreatic cancer: a single-center retrospective cohort study. Gland Surg (2021) 10(2):770–9. doi: 10.21037/gs-20-877
31. Pu N, Chen Q, Gan W, Shen Y, Gao S, Habib JR, et al. Lymph node metastatic patterns and survival predictors based on tumor size in pancreatic ductal adenocarcinoma. Adv Ther (2021) 38(8):4258–70. doi: 10.1007/s12325-021-01819-2
32. Ordonez JE, Hester CA, Zhu H, Augustine M, Porembka MR, Wang SC, et al. Clinicopathologic features and outcomes of early-onset pancreatic adenocarcinoma in the united states. Ann Surg Oncol (2020) 27(6):1997–2006. doi: 10.1245/s10434-019-08096-y
33. Alese OB, Jiang R, Shaib W, Wu C, Akce M, Gaines T, et al. Young adults with pancreatic cancer: national trends in treatment and outcomes. Pancreas (2020) 49(3):341–54. doi: 10.1097/MPA.0000000000001502
34. Eguchi H, Yamaue H, Unno M, Mizuma M, Hamada S, Igarashi H, et al. Clinicopathological characteristics of young patients with pancreatic cancer: an analysis of data from pancreatic cancer registry of japan pancreas society. Pancreas (2016) 45(10):1411–7. doi: 10.1097/MPA.0000000000000636
35. van Dongen JC, van der Geest LGM, de Meijer VE, van Santvoort HC, de Vos-Geelen J, Besselink MG, et al. Age and prognosis in patients with pancreatic cancer: a population-based study. Acta Oncol (2022) 61(3):286–93. doi: 10.1080/0284186X.2021.2016949
36. Guerra F, Barucca V, Coletta D. Metastases or primary recurrence to the lung is related to improved survival of pancreatic cancer as compared to other sites of dissemination. Results of a systematic review with meta-analysis. Eur J Surg Oncol (2020) 46. doi: 10.1016/j.ejso.2020.06.013
37. Kruger SF, Lohneis A, Abendroth A, Berger AW, Ettrich TJ, Waidmann O, et al. Prognosis and tumor biology of pancreatic cancer patients with isolated lung metastases: translational results from the German multicenter AIO-YMO-PAK-0515 study. ESMO Open (2022) 7(1):100388. doi: 10.1016/j.esmoop.2022.100388
38. Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med (2011) 364(19):1817–25. doi: 10.1056/NEJMoa1011923
39. Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med (2013) 369(18):1691–703. doi: 10.1056/NEJMoa1304369
40. Hartwig W, Strobel O, Hinz U, Fritz S, Hackert T, Roth C, et al. CA19-9 in potentially resectable pancreatic cancer: perspective to adjust surgical and perioperative therapy. Ann Surg Oncol (2013) 20(7):2188–96. doi: 10.1245/s10434-012-2809-1
41. Sugiura T, Uesaka K, Kanemoto H, Mizuno T, Sasaki K, Furukawa H, et al. Serum CA19-9 is a significant predictor among preoperative parameters for early recurrence after resection of pancreatic adenocarcinoma. J Gastrointest Surg (2012) 16(5):977–85. doi: 10.1007/s11605-012-1859-9
42. Shi Y, Wang Y, Dong H, Niu K, Zhang W, Feng K, et al. Crosstalk of ferroptosis regulators and tumor immunity in pancreatic adenocarcinoma: novel perspective to mRNA vaccines and personalized immunotherapy. Apoptosis (2023) 28(9-10):1423–35. doi: 10.1007/s10495-023-01868-8
43. Wu L, Zhang Z, Bai M, Yan Y, Yu J, Xu Y, et al. Radiation combined with immune checkpoint inhibitors for unresectable locally advanced non-small cell lung cancer: synergistic mechanisms, current state, challenges, and orientations. Cell Commun Signal (2023) 21(1):119. doi: 10.1186/s12964-023-01139-8
Keywords: oligometastatic, local consolidative therapy, single-organ metastatic pancreatic cancer, radiotherapy, surgery
Citation: Hu X, Hu D, Fu B, Li H, Ren G, Liu H, Song J, Kang X, Wang X, Pang H, Liu C, Zhang J and Wang Y (2023) Survival benefit of local consolidative therapy for patients with single-organ metastatic pancreatic cancer: a propensity score-matched cross-sectional study based on 17 registries. Front. Endocrinol. 14:1225979. doi: 10.3389/fendo.2023.1225979
Received: 20 May 2023; Accepted: 19 October 2023;
Published: 02 November 2023.
Edited by:
Liang Liu, Fudan University, ChinaReviewed by:
Menglin Bai, Shandong University, ChinaLuis Lara-Mejía, National Institute of Cancerology (INCAN), Mexico
Yanlong Shi, Nanjing Medical University, China
Copyright © 2023 Hu, Hu, Fu, Li, Ren, Liu, Song, Kang, Wang, Pang, Liu, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yingjie Wang, V2FuZ3lqOTk5OUAxMjYuY29t
 Xiaolong Hu
Xiaolong Hu Dan Hu2
Dan Hu2 Bowen Fu
Bowen Fu Hongqi Li
Hongqi Li Gang Ren
Gang Ren Jiazhao Song
Jiazhao Song Xiaoli Kang
Xiaoli Kang Haifeng Pang
Haifeng Pang Chen Liu
Chen Liu Yingjie Wang
Yingjie Wang