- 1Department of General Internal Medicine 1, Kawasaki Medical School, Okayama, Japan
- 2Department of Diabetic Medicine, Kurashiki Central Hospital, Kurashiki, Japan
- 3Department of Diabetes, Endocrinology and Metabolism, Kawasaki Medical School, Kurashiki, Japan
Although diabetic neuropathy is a well-known cause of gastrointestinal motility disorders, it is rare that diabetic neuropathy brings about esophageal obstruction. Here, we report a case with Type 3C diabetes mellitus (DM) lasting over 15 years and repeated esophageal obstruction resulting in chicken-meat-induced esophageal obstruction and candidiasis. This case highlights the importance of management of DM to prevent the development of complications such as diabetic neuropathy and associated symptoms.
Introduction
Diabetic neuropathy can lead to various impairments in sensory, motor, and autonomic functions, and it is a common complication of diabetes mellitus (DM). The gastrointestinal system is one of the main systems affected by diabetic neuropathy, with up to 75% of patients with DM reporting some kind of long-term gastrointestinal symptoms, including esophageal symptoms (1). The pathogenesis of gastrointestinal dysfunction of diabetic autonomic neuropathy is complex and involves an increase in oxidative stress, changes in gut bacteria, decrease in growth factors, changes in cell signaling pathways, and dysfunction of the endothelium (2).
Poorly glycemic control over a long period of time can lead to autonomic neuropathy, which can cause gastrointestinal motility and sensory disturbances. Type 3C DM can also become a factor for poor glycemic control and may require insulin preparation, similar to type 1 DM, depending on the extent and location of removal of the pancreas (3).
Here, we report a case with Type 3C DM lasting over 15 years and repeated esophageal obstruction resulting in chicken-meat-induced esophageal obstruction and candidiasis. This case highlights the importance of the management of DM to prevent the development of complications such as diabetic neuropathy and associated symptoms.
Case description
A 72-year-old Japanese male visited the emergency room with symptoms of food obstruction and general fatigue. He had consumed chicken roughly one week prior to the visit and had neglected his symptoms, which worsened over time. Despite having experienced similar symptoms twice in the past at the age of 69 and 71, however, those symptoms improved within a few days. Therefore, he waited to see if his condition would improve for one week. The patient was diagnosed with Type 3C DM at the age of 55, after undergoing a two-thirds pancreatectomy for acute pancreatitis due to excessive drinking (details were unknown). His was treated with 14 units of insulin aspart and 5 units of insulin degludec, along with 0.9 mg/day of voglibose for Type 3C DM management, 20 mg/day of olmesartan and 5 mg/day of amlodipine for hypertension, and 2.5 mg/day of rosuvastatin for dyslipidemia. His height, body weight, and body mass index (BMI) were 152.5 cm, 47.9 kg, and 20.6 kg/m2, respectively. As shown in Table 1, the patient suffered from severe dehydration, electrolyte imbalance, and renal dysfunction, despite relatively good DM control due to their inability to consume food. He had symptoms of numbness in his legs and the Achilles reflex was absent mainly due to peripheral neuropathy. Additionally, he had symptoms of hypoglycemia unawareness, and orthostatic hypotension mainly due to autonomic neuropathy. He repeatedly suffered from constipation as a lower gastrointestinal motility disorder. The coefficient of variation of R-R intervals (CVR-R) showed autonomic neuropathy as follows: CVR-R(rest), 1.8; CVR-R (breath), 1.9, although we could not examine the nerve conduction studies. Schellong test was positive: blood pressure decreased from 157/98 mmHg (rest) to 126/84 mmHg (3 minutes). He had simple diabetic retinopathy and stage 3 diabetic nephropathy with overt proteinuria.
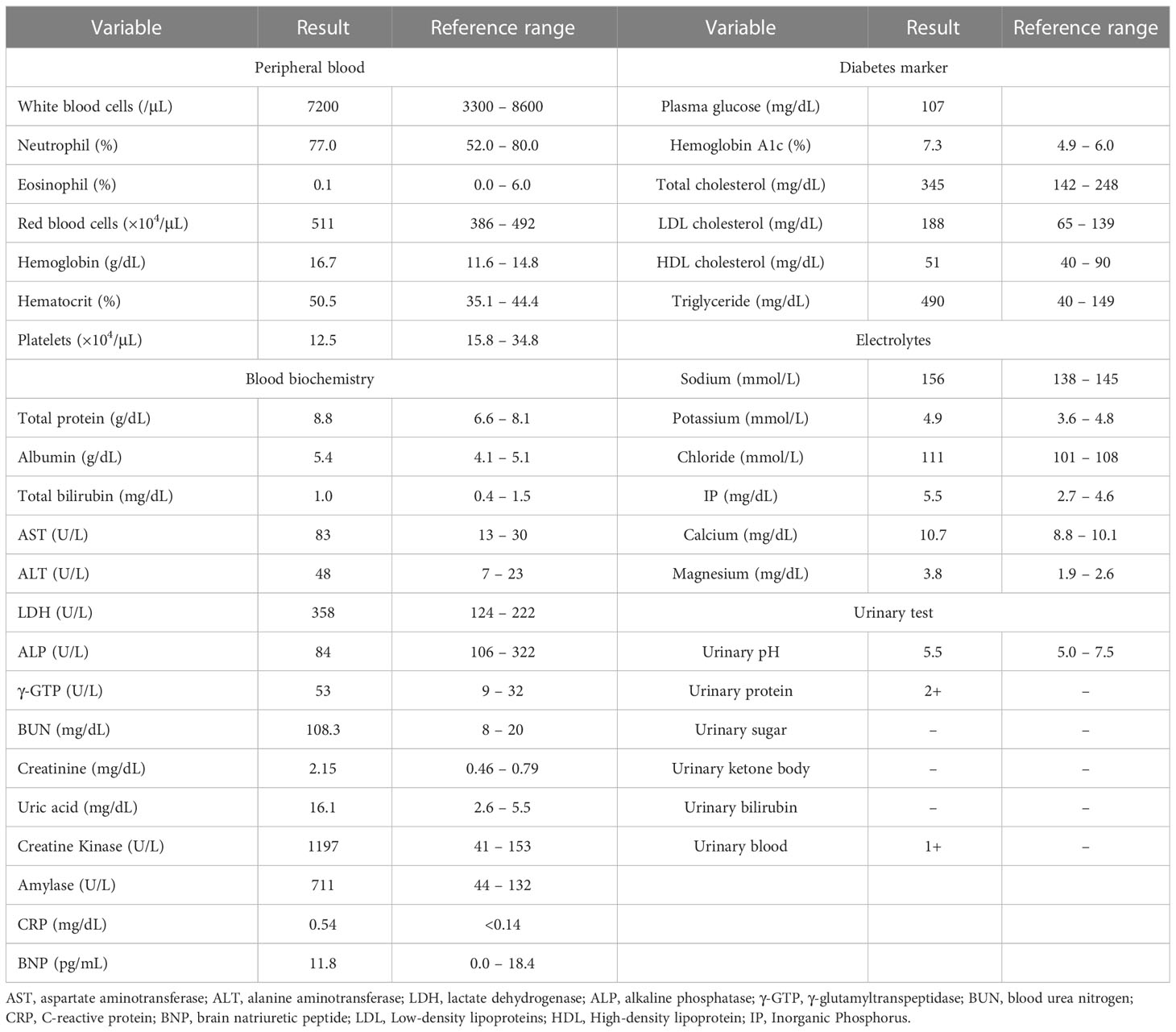
On chest computed tomography, a foreign body was detected in the middle esophagus (Figure 1; upper panels) and it was noted that the space was narrowed with the expansion of the upper lesion of the esophagus. Additionally, expansion was also observed in the lower esophagus (Figure 1; lower panels). Ultrasound examination revealed a high-echoic mass in the cervical side of the esophagus, which was suspected to be food residue of approximately 1 cm in length (Figure 2). Given the patient’s reported symptoms, an upper gastrointestinal endoscopy was performed to evaluate the possible presence of esophageal stricture and a foreign body (Figure 3, upper panels). The endoscopy revealed circumferential esophageal candidiasis at the entrance and upper part of the esophagus, with the middle portion being completely obstructed by a piece of chicken meat and other food debris. The chicken meat was successfully crushed with endoscopic forceps and removed from the esophagus by pushing it into the stomach. In addition, the gastroenterologist suspected eosinophilic esophagitis and performed a biopsy at the time of follow-up endoscopy. However, there were no findings indicating the presence of eosinophilic infiltration.
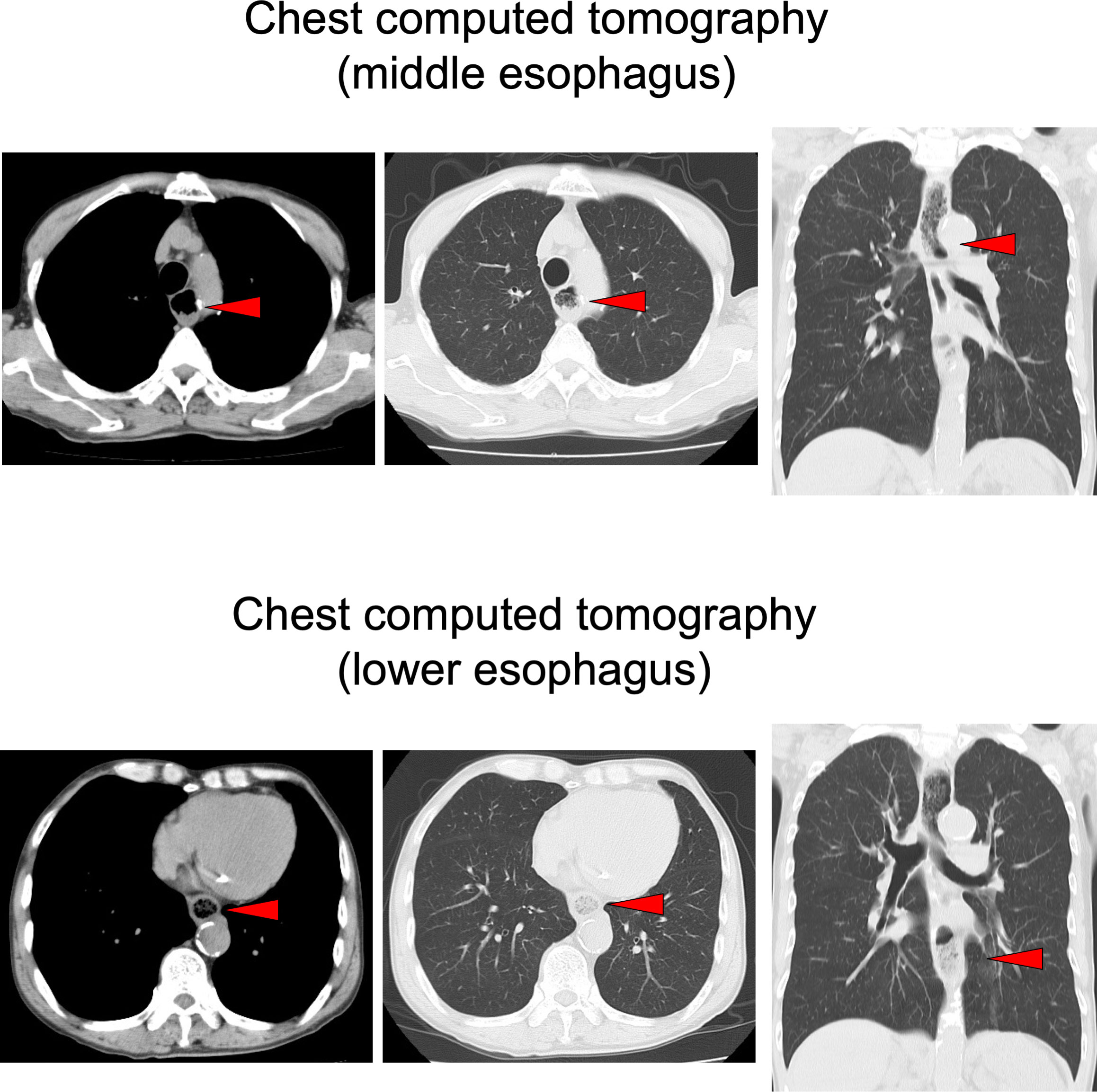
Figure 1 Chest computed tomography in the emergency room. A foreign body (red allow head) was detected in the middle esophagus (upper panels) and it was noted that the space was narrowed with the expansion of the upper lesion of the esophagus. Expansion of the esophagus was also observed in the lower esophagus (lower panels).
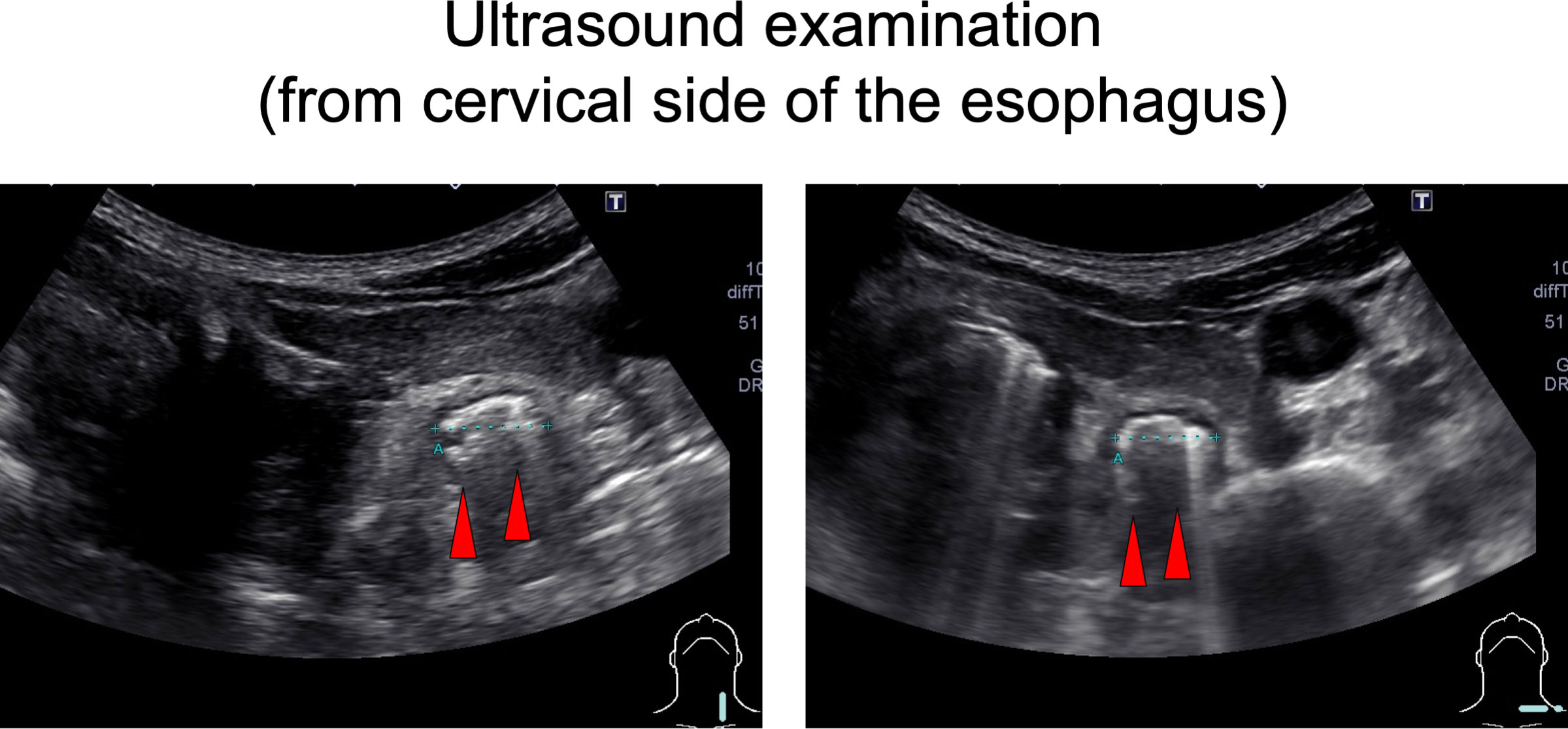
Figure 2 Ultrasound examination. An approximately 1 cm long, high echoic mass was detected in the cervical side of the esophagus (a red arrowhead).
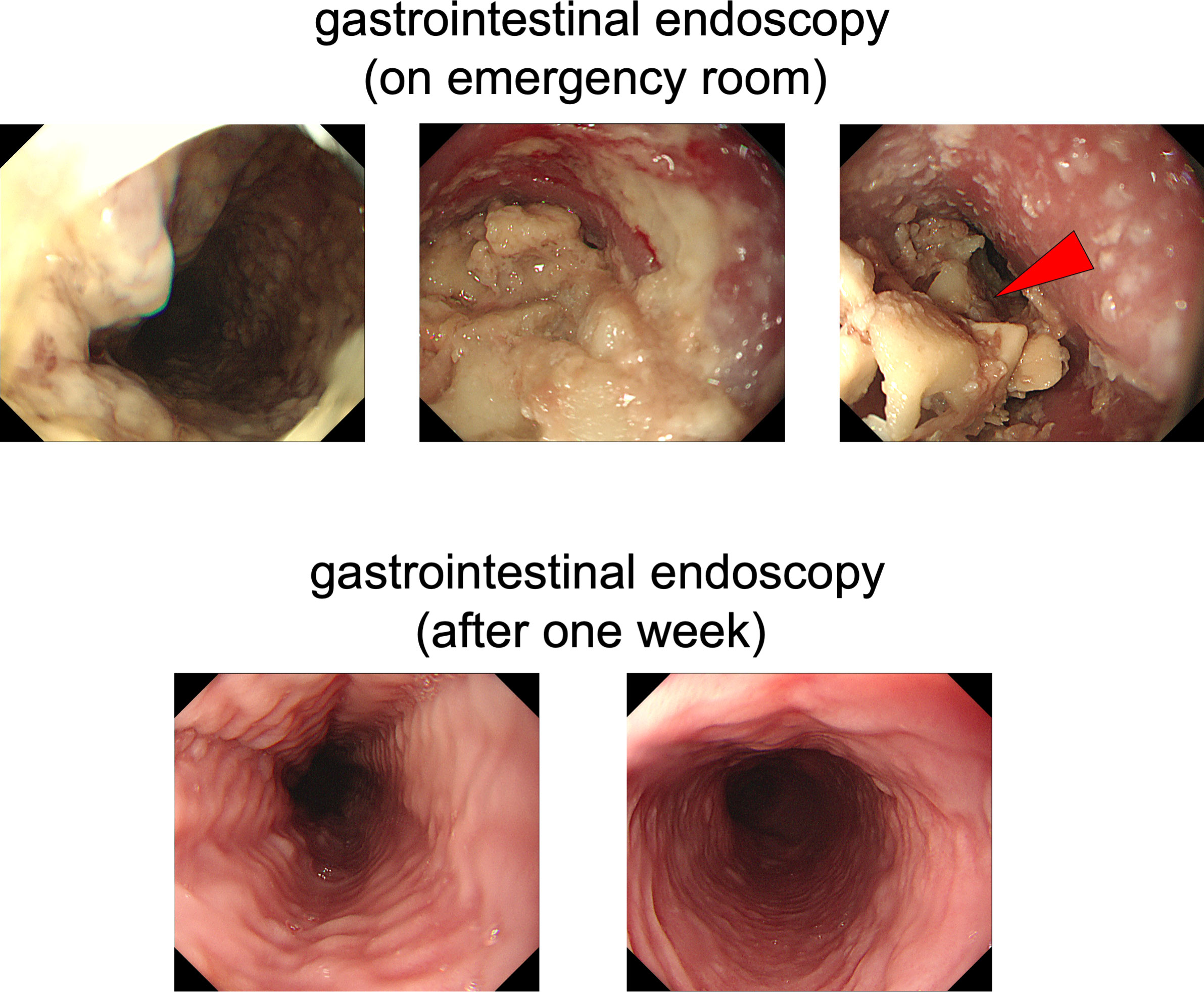
Figure 3 Upper gastrointestinal endoscopy. In the upper panels, circumferential esophageal candidiasis was observed at the entrance and upper part of the esophagus. The middle part of the esophagus was completely obstructed by a piece of chicken meat and other foodstuffs. In the lower panels, a follow-up upper gastrointestinal endoscopy was performed one week later and conducted without any complications.
Following one day of fasting, the patient was able to gradually resume diet, and after a week, a follow-up endoscopy was performed without any specific incident (Figure 3, lower panels), leading to discharge. Since he was a very fast eater, we performed nutritional guidance for him to eat slowly and chew his food well. Finally, he was placed in a facility that provided meals because he was elderly and lived alone, and after then, no similar symptoms were observed.
Discussion
In this report, we show a case of repeated esophageal obstruction in a patient with Type 3C DM complicated by advanced and severe diabetic neuropathy. Although diabetic neuropathy is a well-known cause of gastrointestinal motility disorders, including gastroparesis, diarrhea, and constipation in intestinal enteropathy (4), it is rare that diabetic neuropathy brings about esophageal obstruction. Despite the limited attention given to esophageal motor disorders in the complications of DM, their reported prevalence is as high as 63%, surpassing that of gastroparesis (5).
Esophageal symptoms of diabetic neuropathy, including abnormal peristalsis, spontaneous contractions, and impaired lower esophageal sphincter tone, can lead to symptoms such as heartburn and dysphagia (6, 7). These symptoms have been reported in 5.4% of DM patients and are commonly attributed to an esophageal motor disorder (8). The pathophysiology of esophageal symptoms in DM patients is complex and multi-factorial, and is thought to involve a combination of hyperglycemia, autonomic neuropathy, biomechanical and sensory alterations of the esophagus, presbyesophagus, and psychiatric comorbidities (9, 10). The identification of esophageal dysfunction in DM patients is clinically significant, as it has been linked to delayed transit of food and oral medications (11, 12).
Several studies have indicated that patients with type 1 DM have a lower incidence of gastrointestinal symptoms in comparison to the general population (13). This may be attributed to esophageal hypo-sensitivity due to long-standing diabetic neuropathy. Additionally, it has been established that esophageal transit is delayed in approximately half of patients with long-standing DM (14). Type 3C DM, which is similar to type 1 DM, may result in poor sensitivity to neurological symptoms as a result of continued poor glycemic control and progressive complications.
There is a limitation in this case reports. Esophageal candidiasis may cause esophageal stricture. Esophageal candidiasis has been recognized more frequently in immunocompromised hosts, as the result of the examination of esophagogastric endoscopy (15). While esophageal research in diabetics focuses mainly on motility dysfunction, DM patients are at a higher risk for a number of other esophageal disorders (16). It has been reported that in several cases underlying diabetes was the only predisposing factor for esophageal candidiasis (17–19). However, since follow-up endoscopy showed the improvement of esophageal candidiasis without antifungal drugs, we think the main pathology of this patient was gastrointestinal motility disorders induced by diabetic neuropathy. We discussed the possibility of esophageal achalasia or gastroparesis in this case with the gastroenterologist; however, based on imaging examination and clinical time course, we finally diagnosed this subject as esophageal obstruction by diabetic neuropathy. In addition, according to the patient, he drank alcohol only occasionally after the onset of acute pancreatitis, but esophageal stricture in this case may have been caused by alcoholic neuropathy.
Taken together, we should bear in mind that impaired esophageal transit is a complication of severe diabetic neuropathy in patients with DM. Furthermore, if severe diabetic neuropathy persists in the condition of continued poorly controlled DM for a long period of time, it can lead to esophageal obstruction with limited subjective symptoms.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
TA researched data and wrote the manuscript. KaK, HT and TK researched data and contributed to the discussion. KoK and HK reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Krishnan B, Babu S, Walker J, Walker AB, Pappachan JM. Gastrointestinal complications of diabetes mellitus. World J Diab (2013) 4:51–63. doi: 10.4239/wjd.v4.i3.51
2. Yarandi SS, Srinivasan S. Diabetic gastrointestinal motility disorders and the role of enteric nervous system: current status and future directions. Neurogastroenterol Motil. (2014) 26:611–24. doi: 10.1111/nmo.12330
3. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care (2013) 36(Suppl 1):S67–74. doi: 10.2337/dc13-S067
4. Azpiroz F, Malagelada C. Diabetic neuropathy in the gut: pathogenesis and diagnosis. Diabetologia (2016) 59:404–8. doi: 10.1007/s00125-015-3831-1
5. Gustafsson RJ, Littorin B, Berntorp K, Frid A, Thorsson O, Olsson R, et al. Esophageal dysmotility is more common than gastroparesis in diabetes mellitus and is associated with retinopathy. Rev Diabetes Stud (2011) 8:268–75. doi: 10.1900/RDS.2011.8.268
6. Rayner CK, Samsom M, Jones KL, Horowitz M. Relationships of upper gastrointestinal motor and sensory function with glycemic control. Diabetes Care (2001) 24:371–81. doi: 10.2337/diacare.24.2.371
7. Monreal-Robles R, Remes-Troche JM. Diabetes and the esophagus. Curr Treat Options Gastroenterol (2017) 15:475–89. doi: 10.1007/s11938-017-0153-z
8. Bytzer P, Talley NJ, Leemon M, Young LJ, Jones MP, Horowitz M. Prevalence of gastrointestinal symptoms associated with diabetes mellitus: a population-based survey of 15,000 adults. Arch Intern Med (2001) 161:1989–96. doi: 10.1001/archinte.161.16.1989
9. Zhao J, Frøkjaer JB, Drewes AM, Ejskjaer N. Upper gastrointestinal sensory-motor dysfunction in diabetes mellitus. World J Gastroenterol (2006) 12:2846–57. doi: 10.3748/wjg.v12.i18.2846
10. Careyva B, Stello B. Diabetes mellitus: management of gastrointestinal complications. Am Fam Phys (2016) 94:980–6.
11. Lloyd CE, Matthews KA, Wing RR, Orchard TJ. Psychosocial factors and complications of IDDM. The Pittsburgh Epidemiology of Diabetes Complications Study. VIII. Diabetes Care (1992) 15:166–72. doi: 10.2337/diacare.15.2.166
12. Kong MF, Horowitz M, Jones KL, Wishart JM, Harding PE. Natural history of diabetic gastroparesis. Diabetes Care (1999) 22:503–7. doi: 10.2337/diacare.22.3.503
13. Maleki D, Locke GR 3, Camilleri M, Zinsmeister AR, Yawn BP, Leibson C, et al. Gastrointestinal tract symptoms among persons with diabetes mellitus in the community. Arch Intern Med (2000) 160:2808–16. doi: 10.1001/archinte.160.18.2808
14. Annese V, Bassotti G, Caruso N, De Cosmo S, Gabbrielli A, Modoni S, et al. Gastrointestinal motor dysfunction, symptoms, and neuropathy in noninsulin-dependent (type 2) diabetes mellitus. J Clin Gastroenterol (1999) 29:171–7. doi: 10.1097/00004836-199909000-00014
15. Frokjaer JB, Andersen SD, Ejskjaer N, Funch-Jensen P, Drewes AM, Gregersen H. Impaired contractility and remodeling of the upper gastrointestinal tract in diabetes mellitus type-1. World J Gastroenterol (2007) 13:4881–90. doi: 10.3748/wjg.v13.i36.4881
16. Macêdo DP, Oliveira NT, Farias AM, Silva VK, Wilheim AB, Couto FM, et al. Esophagitis caused by Candida guilliermondii in diabetes mellitus: first reported case. Med Mycol (2010) 48(6):862–5. doi: 10.3109/13693780903582614
17. Underwood JA, Williams JW, Keate RF. Clinical findings and risk factors for Candida esophagitis in outpatients. Dis Esophagus (2003) 16:66–9. doi: 10.1046/j.1442-2050.2003.00305.x
18. Takasawa H, Takahashi Y, Abe M, Osame K, Watanabe S, Hisatake T, et al. An elderly case of type 2 diabetes which developed in association with oral and esophageal candidiasis. Intern Med (2007) 46:387–90. doi: 10.2169/internalmedicine.46.1898
Keywords: esophageal obstruction, type 3C diabetes mellitus, esophageal candidiasis, diabetic neuropathy, gastrointestinal motility disorders
Citation: Koyama K, Anno T, Takenouchi H, Kimura T, Kaku K and Kaneto H (2023) Case Report: Repeated esophageal obstruction in a patient with type 3C diabetes mellitus. Front. Endocrinol. 14:1225385. doi: 10.3389/fendo.2023.1225385
Received: 19 May 2023; Accepted: 10 July 2023;
Published: 28 July 2023.
Edited by:
Habib Yaribeygi, Semnan University of Medical Sciences, IranReviewed by:
Takahisa Deguchi, Kagoshima University, JapanYukihiro Fujita, Shiga University of Medical Science, Japan
Copyright © 2023 Koyama, Anno, Takenouchi, Kimura, Kaku and Kaneto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Takatoshi Anno, YW5uby10QHVtaW4uYWMuanA=
 Katsumasa Koyama
Katsumasa Koyama Takatoshi Anno
Takatoshi Anno Haruka Takenouchi1
Haruka Takenouchi1 Tomohiko Kimura
Tomohiko Kimura Hideaki Kaneto
Hideaki Kaneto