- 1Wuhan Children’s Hospital (Wuhan Maternal and Child Healthcare Hospital), Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China
- 2Wuhan Wuchang Hospital, Wuchang Hospital Affiliated to Wuhan University of Science and Technology, Wuhan University of Science and Technology, Wuhan, Hubei, China
- 3Department of Pediatrics, Wuhan Children’s Hospital (Wuhan Maternal and Child Healthcare Hospital), Affiliated Hospital of Jianghan University, Jianghan University, Wuhan, Hubei, China
- 4Institute of Integrated Traditional Chinese and Western Medicine, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China
Objective: This study aimed to determine the clinical characteristics of obese pediatric non-alcoholic fatty liver disease (NAFLD) in central China and verify the applicability of some known risk factors for pediatric NAFLD before late puberty.
Methods: This was a retrospective case–control study. A total of 1,029 inpatients at Wuhan Children’s Hospital before the late puberty stage were enrolled in the study, including 815 children with obesity (non-NAFLD group) and 214 children with obesity and NAFLD (NAFLD group) diagnosed by liver ultrasound. Subgroup analyses were performed according to sex and puberty. The anthropometric indices and laboratory test data of these 1,029 children were sorted. After intergroup comparison, a logistic regression model was used to determine the risk factors for pediatric NAFLD. Significant risk factors for NAFLD were further tested using receiver operating characteristic (ROC) curves to evaluate their ability to predict an early diagnosis of NAFLD.
Results: The NAFLD group had a mean age of 11.03 ± 1.66, with 11.18 ± 1.66 and 10.27 ± 1.45 years for male and female children, respectively (p < 0.05 and p < 0.01, respectively). Even subdivided by both sex and puberty, raised body mass index (BMI), homeostatic model-insulin resistance, triglycerides, alanine transaminase (ALT), aspartate aminotransferase (AST), and gamma-glutamyl transferase (γ-GT) were still found in the non-NAFLD and NAFLD groups (p < 0.05 and p < 0.01, respectively). The results of logistic regression analysis showed that BMI (odds ratio [OR], 1.468;95% confidence interval [CI], 1.356-1.590; p<0.001) and ALT (OR, 1.073;95%CI, 1.060-1.087; P<0.001) were two most independent risk factors for NAFLD. The maximal OR for BMI was 1.721 (95% CI, 1.336–2.217). In the female group, the maximal OR of ALT was found to be 1.104 (95% CI, 1.061–1.148). Age and thyroid-stimulating hormone (TSH) and γ-GT levels were also risk factors, but they appeared only in some groups. The results of the ROC analysis showed that ALT was a better predictor of pediatric NAFLD than BMI. The maximum area under the ROC curve in six of the nine groups belongs to ALT.
Conclusions: BMI, ALT, and age are risk factors for NAFLD in children with obesity before late puberty. BMI had the greatest exposure risk for NAFLD, and ALT had the highest predictive value for the diagnosis of NAFLD. At the stratified level, for exposure risk, age was specific to the male sex, TSH was specific to the early puberty stage, and γ-GT was specific to the female sex plus the prepuberty stage. On a stratified level, for the female sex, even with age stratification, BMI rather than ALT has a better ability for the diagnosis of NAFLD.
1 Introduction
Not only in developed countries but also in China, non-alcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease in children and adolescents (1–5). Recent data report a prevalence of NAFLD of 3%–10% in children worldwide and 3.4% in China (1–5). Pediatric NAFLD is defined as chronic liver steatosis (fat content in the hepatocytes >5%) in children and adolescents aged <18 years, which is a metabolic stress liver injury closely related to insulin resistance and genetic susceptibility. However, the clinical pathological syndrome of chronic fatty deposition in the liver caused by alcohol and other definite pathogenic factors should be excluded (3). The spectrum of pediatric NAFLD includes non-alcoholic fatty liver (NAFL), non-alcoholic steatohepatitis, related liver fibrosis, and cirrhosis (3).
Most pediatric patients with NAFLD are in the NAFL stage and are easily ignored owing to the lack of clinical symptoms. The development and progression of the disease in childhood lead to an increased risk of long-term morbidity, especially cardiovascular diseases (6). Therefore, reliable forecasting factors are necessary for early screening. Obesity is a leading risk factor for pediatric NAFLD. The prevalence of NAFLD in children with obesity and those who are overweight has increased to 50%–80% (7, 8). Male sex is also a risk factor for NAFLD in both children and adults (9, 10). Additionally, the prevalence of NAFLD in children and adolescents increases with age, showing a growth rate of 0.7% in children 2 to 4 years old to 7% in teenagers (6).
However, epidemiological data from China are mostly from the Yangtze River Delta region. Therefore, the first aim of this study was to determine the clinical characteristics of pediatric obese patients with NAFLD in Central China. Owing to the influence of age on the prevalence rate, there were relatively few cases in the pre- and early puberty stages. Thus, the secondary aim of this study was to verify the applicability of known risk factors for pediatric NAFLD before late puberty.
2 Materials and methods
2.1 Study population
This cross-sectional study collected inpatient clinical data from the Department of Endocrinology and Metabolism at Wuhan Children’s Hospital between January 2014 and December 2021. Based on the database of patient files in the Wuhan Children’s Hospital, 305 patients aged between 7.17 and 16.58 years were diagnosed with both obesity and NAFLD and were discharged during the observation period. Simultaneously, during the aforementioned period, through random sampling, 1,233 patients aged between 3.00 and 17.17 years were discharged and diagnosed with obesity but without NAFLD. After excluding patients in the late puberty stage and those with incomplete data, the clinical data of 214 children with obesity and NAFLD (the NAFLD group) were collected, and those of 815 children with obesity and without NAFLD (the non-NAFLD group) were randomly selected and analyzed retrospectively. Meanwhile, subgroup analysis was performed according to sex and puberty: <10.65 (P50) years as male prepuberty, between 10.65 (P50) and 14.83 (P50) years as male early puberty, <9.65 (P50) years as female prepuberty, and between 9.65 (P50) and 12.91 (P50) years as female early puberty (11).
The inclusion criteria for pediatric obesity were based on the body mass index (BMI) classification criteria for overweight and obesity screening of Chinese children and adolescents according to age and sex (12). The inclusion criteria for pediatric NAFLD were based on an imaging diagnosis using ultrasonography (2, 13).
The exclusion criteria were patients with secondary obesity (such as hypothyroidism, Cushing’s disease, or hypothalamic obesity) and patients with other specific causes of fatty liver: a) genetic-metabolic causes: Turner syndrome, α- and β-oxidation defects, low β lipoproteinemia, Wilson’s disease, alpha-1-antitrypsin (AAT) deficiency, abetalipoproteinemia or hypobetalipoproteinemia, citrin deficiency, fatty acid metabolism disorder, congenital disorders of glycosylation, cystic fibrosis, Shwachman-Diamond syndrome, familial hyperlipoproteinemias, glycogen storage disease (types I, VI, and IX), fructosemia, porphyria cutanea tarda, mitochondrial and peroxisomal defects, organic acidosis, and homocystinuria; b) general or systemic causes: thyroid disorders, celiac disease, diabetes mellitus type 1, hepatitis C, hypothalamic–pituitary disorders, inflammatory bowel disease, autoimmune liver disease, protein-calorie malnutrition, rapid weight loss, and anorexia nervosa; c) drugs or chemicals causes: estrogens, corticosteroids, diltiazem, nifedipine, cocaine, ethanol, methotrexate, valproate, pesticides, vitamin A, zidovudine, and human immunodeficiency virus treatments; secondary obesity caused by heredity or endocrine diseases, etc.
This study was approved by the Medical Ethics Committee of the Wuhan Children’s Hospital (Approval No. 2022R051-E01).
2.2 Measurements
Pediatricians in the endocrinology department performed all the anthropometric and blood pressure measurements.
The height was measured to the nearest 0.1 cm. Similarly, body weight was measured to the nearest 0.1 kg. BMI was calculated as body weight (kg) divided by height (m) squared.
A mercury sphygmomanometer was used to measure systolic blood pressure (SBP; mmHg) and diastolic blood pressure (DBP; mmHg) in the right upper arm after resting for at least 10 min. Blood pressure was assessed three times at 2-minute intervals, and the mean of these three measurements was calculated. The blood pressure classification of children was based on their sex, age, and height (14, 15): normal, SBP and DBP <P90; hypertension, SBP or DBP between P95 and P99; and severe hypertension, SBP or DBP >P99.
2.3 Laboratory tests
Blood samples were collected after fasting overnight for 10 hours. All blood samples were processed, refrigerated, and transported to the clinical laboratory of Wuhan Children’s Hospital and analyzed as soon as possible. All clinical analyses were performed in our clinical laboratory, which is certified by the China National Accreditation Service for Conformity Assessment.
The serum concentrations of general biochemistry tests were measured using the Cobas c 702 analyzer (Roche, Mannheim, Germany), such as triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), fasting plasma glucose, and liver chemistry, for instance, alanine transaminase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (γ-GT), alkaline phosphatase (ALP), albumin (ALB), and globulin (GLB). Routine blood tests were performed using the XN-3000 instrument (SYSMEX, Kobe, Japan). These parameters included white blood cells (WBC), red blood cells (RBC), hemoglobin (Hb), platelet count, neutrophils (NEU), lymphocytes (LYM), and monocytes (MONO). Fasting insulin was examined using an IMMULITE 2000 XPi Immunoassay (Siemens, Munich, Germany), and glycated hemoglobin (HbA1c) was measured using a D-10 glycosylated hemoglobin meter (Bio-Rad, Hercules, CA, USA). Thyroid function tests, including serum triiodothyronine (T3), thyroxine (T4), thyroid-stimulating hormone (TSH), free T3 (FT3), and free T4 (FT4) levels, were performed using the ADVIA Centaur XP (Siemens, Germany).
Insulin resistance was assessed using a homeostatic model (homeostasis model assessment insulin resistance index (HOMA-IR)). The formula for HOMA-IR was fasting insulin multiplied by fasting plasma glucose divided by 22.5.
2.4 Sonographic examination
Sonographic examinations were performed using ultrasound (EPIQ5, Philips Healthcare, Bothell, WA, USA) by two trained pediatric sonographers. The patients were instructed to fast for at least 8 hours before the examination. We performed a comprehensive and systematic scan of the liver in the supine and left lateral decubitus positions. The characteristics of enhanced echo in the front field of the liver (“bright liver”), attenuation of echo in the far field, and unclear display of intrahepatic duct structure were classified as NAFLD (3, 13, 16).
2.5 Other investigation contents
The birth weight (kg) of each infant was recorded. The delivery mode of the patient’s mother (natural or cesarean section) was also recorded.
2.6 Statistical analysis
IBM SPSS Statistics for Windows, version 21.0 (IBM Corp., Armonk, NY, USA), was used to perform the statistical analyses. Continuous variables in accordance with the normal distribution were represented as the mean ± standard deviation. Continuous variables that were not normally distributed were presented as medians (25% quartiles, 75% quartiles). Categorical variables were presented as numbers. A two-tailed t-test was used to compare continuous variables with a normal distribution across groups. The rank sum test was used to compare continuous variables that were not normally distributed across groups. The chi-square (χ2) test was used to compare categorical variables across groups. Risk factor analysis was performed using binary logistic regression analysis. A multiple logistic regression analysis was conducted to determine the significant predictors after controlling for all variables. Candidate risk factors for NAFLD were further tested by discrimination analysis using receiver operating characteristics (ROC). A p-value of <0.05 was considered statistically significant.
3 Results
3.1 Patient characteristics
Data from a total of 1,029 inpatients, including children and adolescents with obesity before the late puberty stage (581 male and 448 female patients; mean age, 9.99 ± 1.80 years), were collected. Based on liver ultrasound findings, 1,029 patients were divided into two groups: 815 patients in the non-NAFLD group with 402 male and 413 female patients (mean age, 9.50 years [range, 8.50–10.92]) and 214 in the NAFLD group with 179 male and 35 female patients (mean age, 11.17 years [range, 9.90–12.17]) (Table 1).
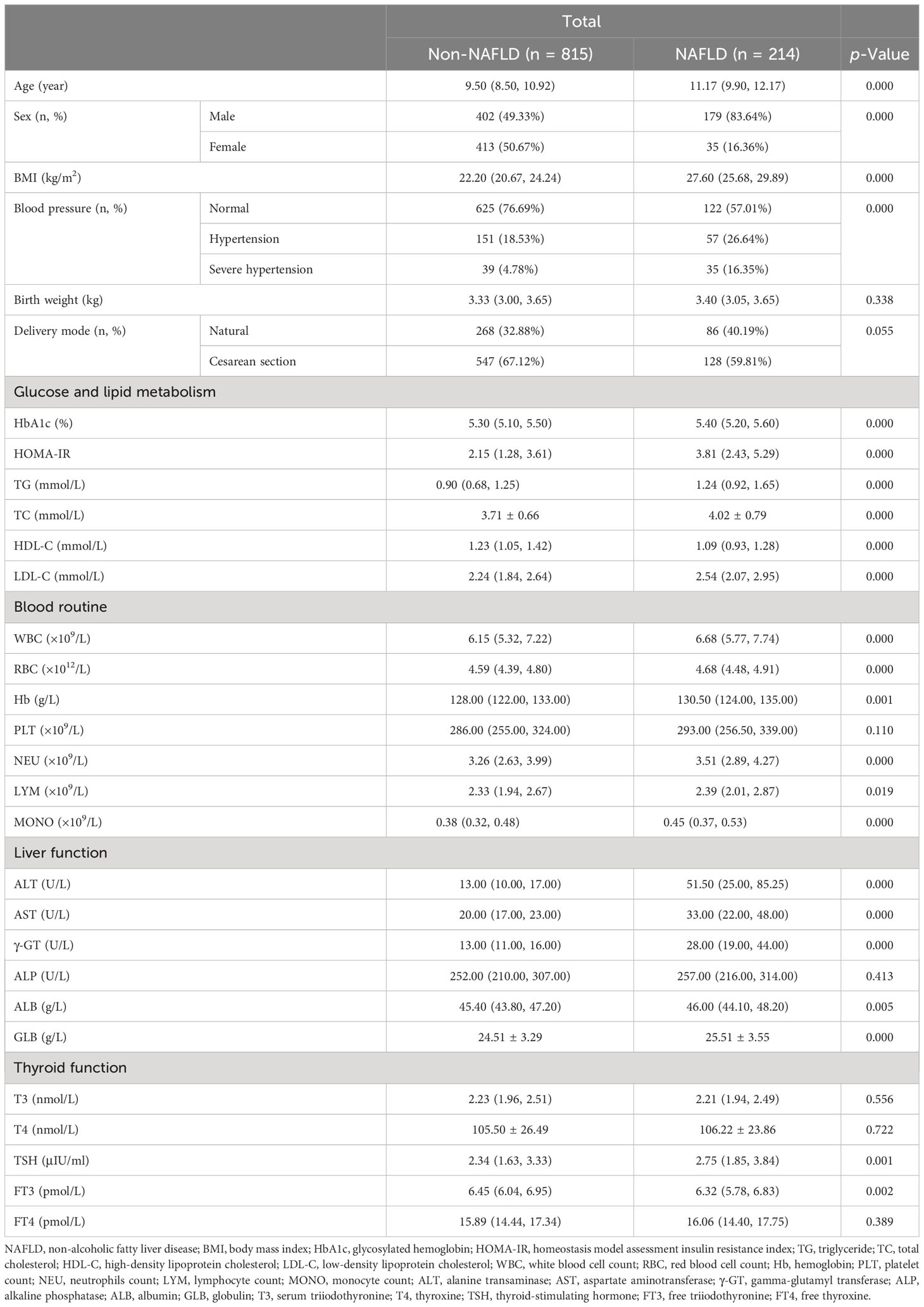
Table 1 Clinical characteristics in hospitalized children with obesity before the late puberty stage with or without NAFLD.
All patients were further divided by sex and puberty. First, 581 male and 448 female patients were divided into the non-NAFLD group (male vs. female: n = 402, mean age, 10.53 ± 1.80 years, vs. n = 413, mean age, 8.93 ± 1.26 years) and the NAFLD group (male vs. female: n = 179, mean age, 11.18 ± 1.66 years, vs. n = 35, mean age, 10.27 ± 1.45 years; Table 2). Second, 573 patients in the prepuberty stage were divided into the non-NAFLD group (n = 504; male vs. female: n [%], 201 [39.88] vs. 303 [60.12]) and the NAFLD group (n = 69; male vs. female: n [%], 57 [82.61] vs. 12 [17.39]), while 456 patients in the early puberty stage were divided into the non-NAFLD group (n = 311; male vs. female: n [%], 201 [64.63] vs. 110 [35.37]) and the NAFLD group (n = 145; male vs. female: n [%], 122 [84.14] vs. 23 [15.86]) (Table 3). Third, 258 male and 315 female patients in the prepuberty stage were divided into the non-NAFLD (n = 201 and 303, respectively) and NAFLD (n = 57 and 12, respectively) groups. Also, 323 male and 133 female patients in the early puberty stage were divided into the non-NAFLD (n = 201 and 110, respectively) and NAFLD (n = 122 and 23, respectively) groups (Tables 4, 5).
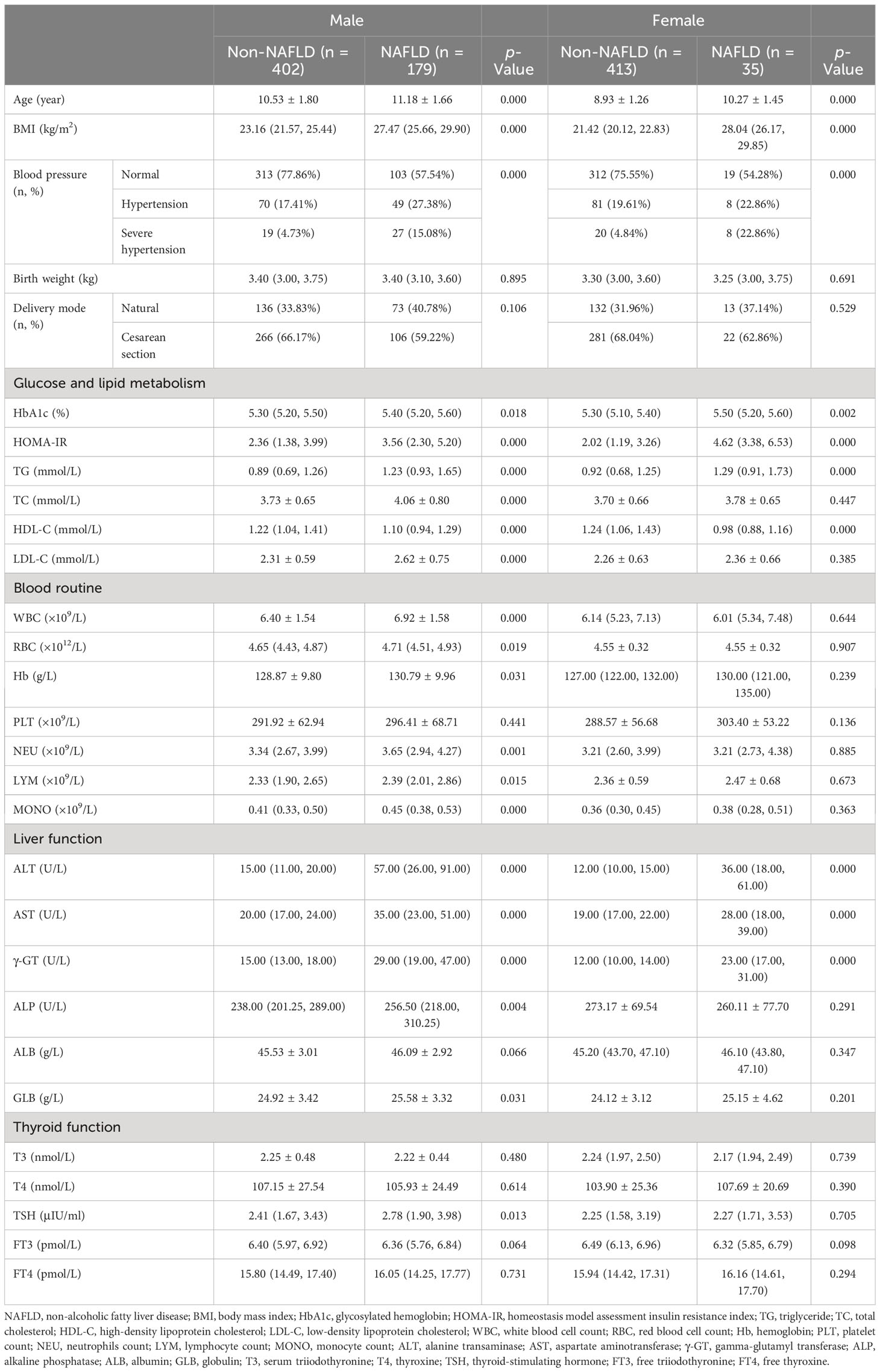
Table 2 Clinical characteristics in hospitalized children with obesity before the late puberty stage with or without NAFLD (divided by sex).
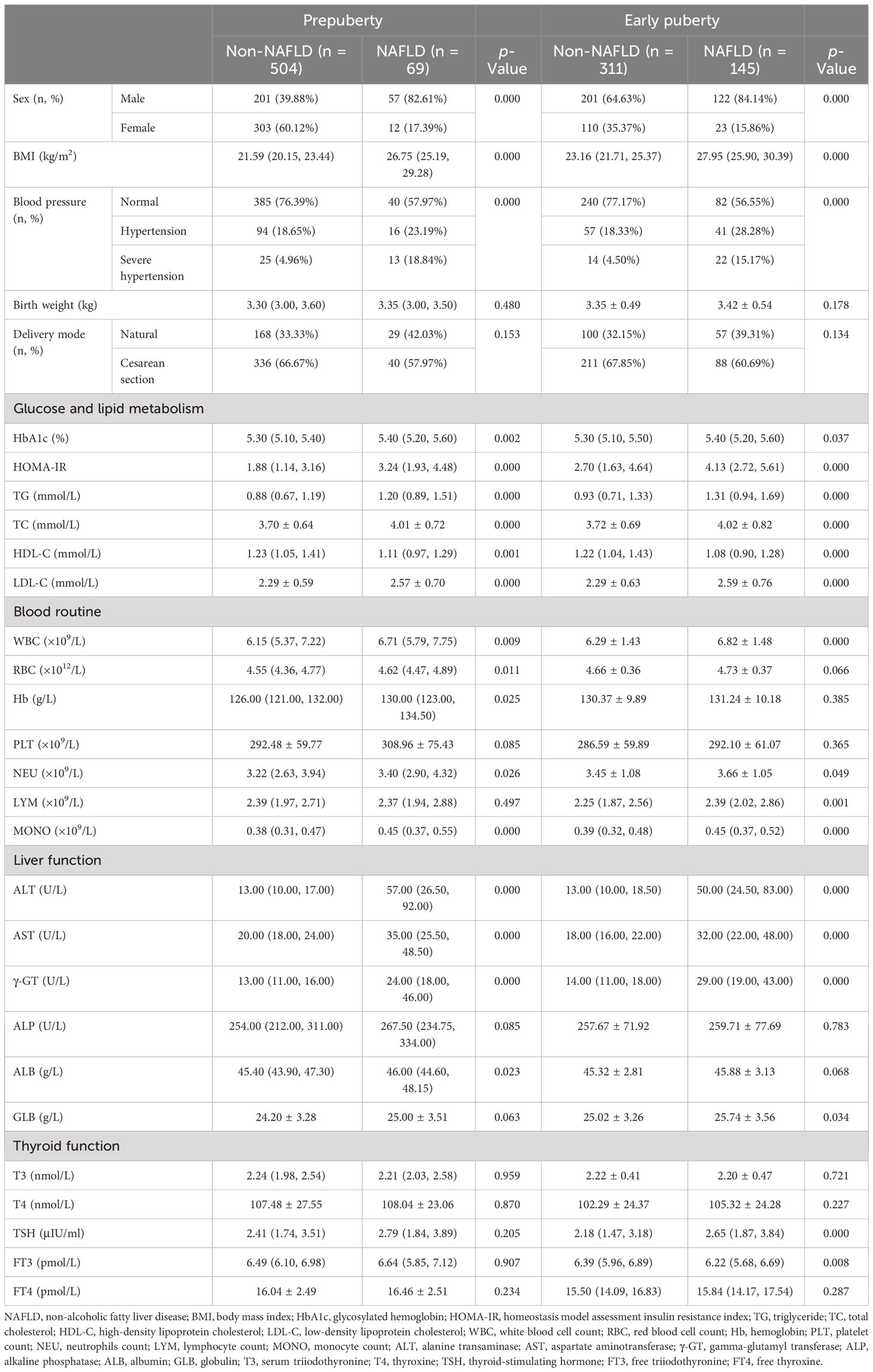
Table 3 Clinical characteristics in hospitalized children with obesity before the late puberty stage with or without NAFLD (divided by puberty).
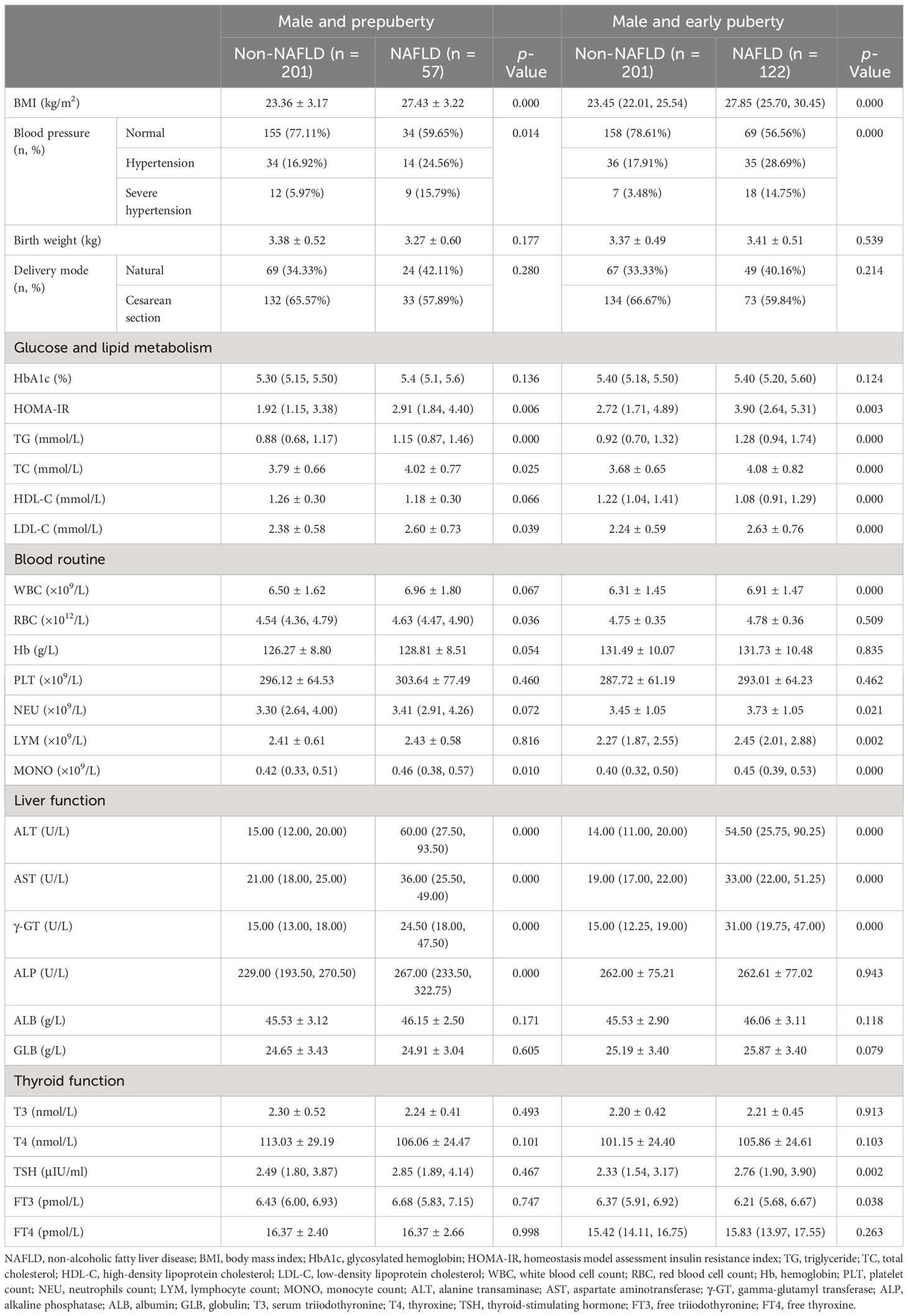
Table 4 Clinical characteristics in hospitalized male children with obesity before the late puberty stage with or without NAFLD (subdivided by puberty).
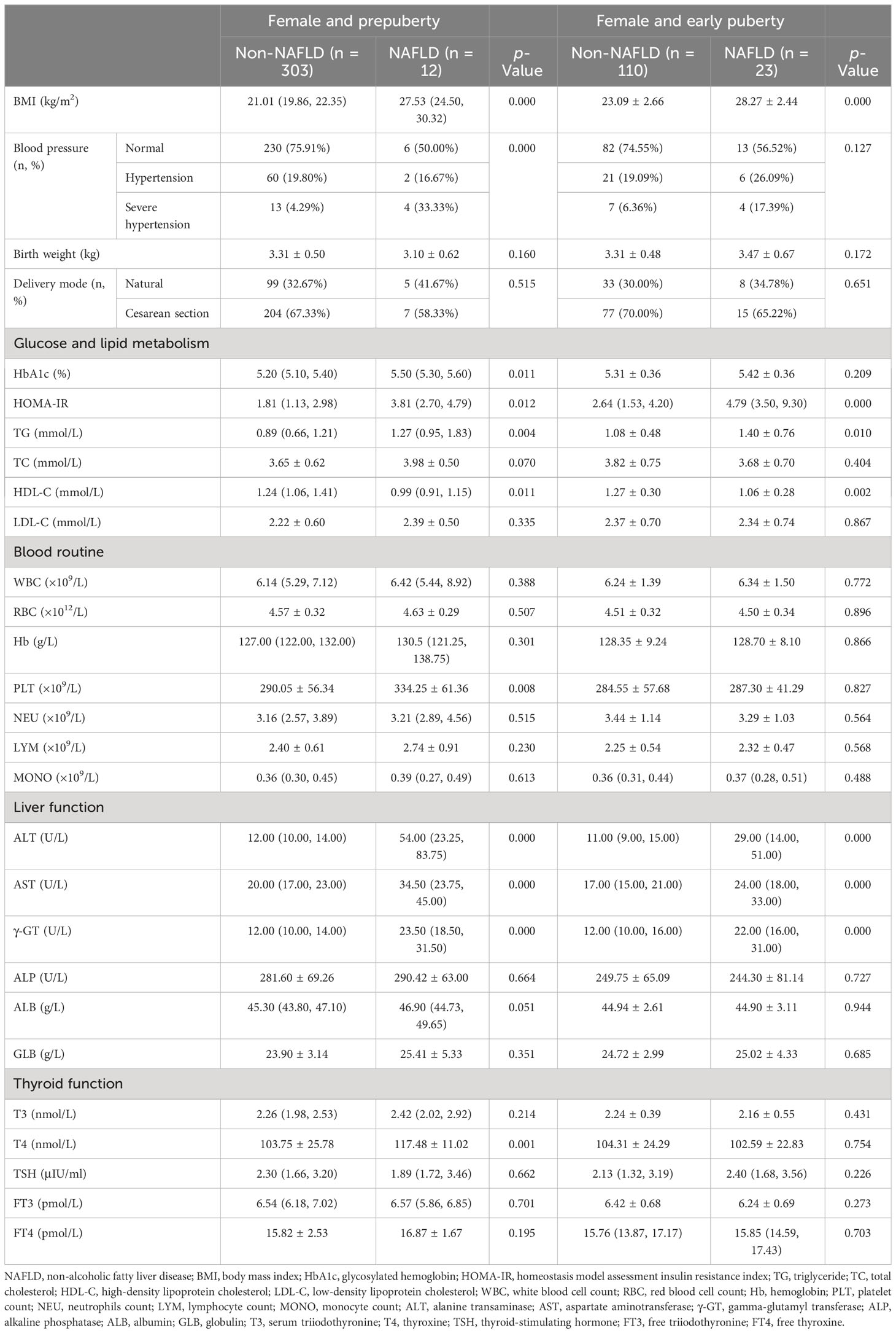
Table 5 Clinical characteristics in hospitalized female children with obesity before the late puberty stage with or without NAFLD (subdivided by puberty).
3.2 Intergroup comparison of clinical characteristics
As shown in Table 1, the sex distribution was different between the two groups (p < 0.01). Male children with obesity revealed a significantly higher prevalence of NAFLD. Additionally, significant and progressive increases in age, BMI, HbA1c, HOMA-IR, TG, TC, LDL-C, WBC, RBC, Hb, NEU, LYM, MONO, ALT, AST, γ-GT, ALB, GLB, and TSH levels were found in the non-NAFLD and NAFLD groups (p < 0.05 and p < 0.01, respectively). Moreover, the incidence of hypertension was significantly higher whereas HDL-C and FT3 levels were significantly lower in children with obesity and NAFLD than in children with obesity and without NAFLD (p < 0.01).
As shown in Table 2, for both male and female children, similar to the total, a significant and progressive increase in age, BMI, the incidence of hypertension, HbA1c, HOMA-IR, TG, ALT, AST, and γ-GT and a significant decrease in HDL-C were found in the non-NAFLD and NAFLD groups (p < 0.05 and p < 0.01, respectively). Moreover, in male children, there was a significant and progressive increase in TC, LDL-C, WBC, RBC, Hb, NEU, LYM, MONO, GLB, and TSH in the non-NAFLD and NAFLD groups (p < 0.05 and p < 0.01, respectively). However, for both male and female children, the ALB and FT3 levels were not different between the two groups (p > 0.05), whereas the ALP level was significantly higher in male patients with obesity and NAFLD than in male children with obesity alone (p > 0.01).
As shown in Table 3, for both pre- and early puberty, consistent with the total, a significant and progressive increase in the prevalence of male children, BMI, the incidence of hypertension, HbA1c, HOMA-IR, TG, TC, LDL-C, WBC, NEU, MONO, ALT, AST, and γ-GT and a significant decrease in HDL-C were found in the non-NAFLD and NAFLD groups (p < 0.05 and p < 0.01, respectively). However, only in the prepuberty stage, as with the total, was a significant and progressive increase in RBC, Hb, and ALB found in the non-NAFLD and NAFLD groups (p < 0.05 and p < 0.01, respectively). Moreover, only in early puberty, as with total, were a significant and progressive increase in LYM, GLB, and TSH and a significant decrease in FT3 found in the non-NAFLD and NAFLD groups (p < 0.05 and p < 0.01, respectively).
As shown in Tables 4, 5, even when subdivided by both sex and puberty, a significant and progressive increase in BMI, HOMA-IR, TG, ALT, AST, and γ-GT was still found in the non-NAFLD and NAFLD groups (p < 0.05 and p < 0.01, respectively). However, a significant increase in ALP was found in the non-NAFLD and NAFLD groups only in male children at the prepuberty stage (p < 0.01). Moreover, only in female children at the prepuberty stage was a significant elevation in platelet count and T4 levels found in the non-NAFLD and NAFLD groups (p < 0.01).
3.3 Identification of risk factors for NALFD in children with obesity
From total to stratification, all candidate variables were first included in the binary logistic regression analysis during the primary screening for potential risk factors. Next, multiple logistic regression was performed to determine independent risk factors.
As presented in Table 6 and Figure 1, age (OR, 1.346; 95% CI, 1.166–1.553; p < 0.001), BMI (OR, 1.468; 95% CI, 1.356–1.590; p < 0.001), and ALT level (OR, 1.073; 95% CI, 1.060–1.087; p < 0.001) were found to be three independent risk factors for NAFLD. However, HOMA-IR and Hb had no logistic regression relevance for pediatric NAFLD owing to their negative beta values.
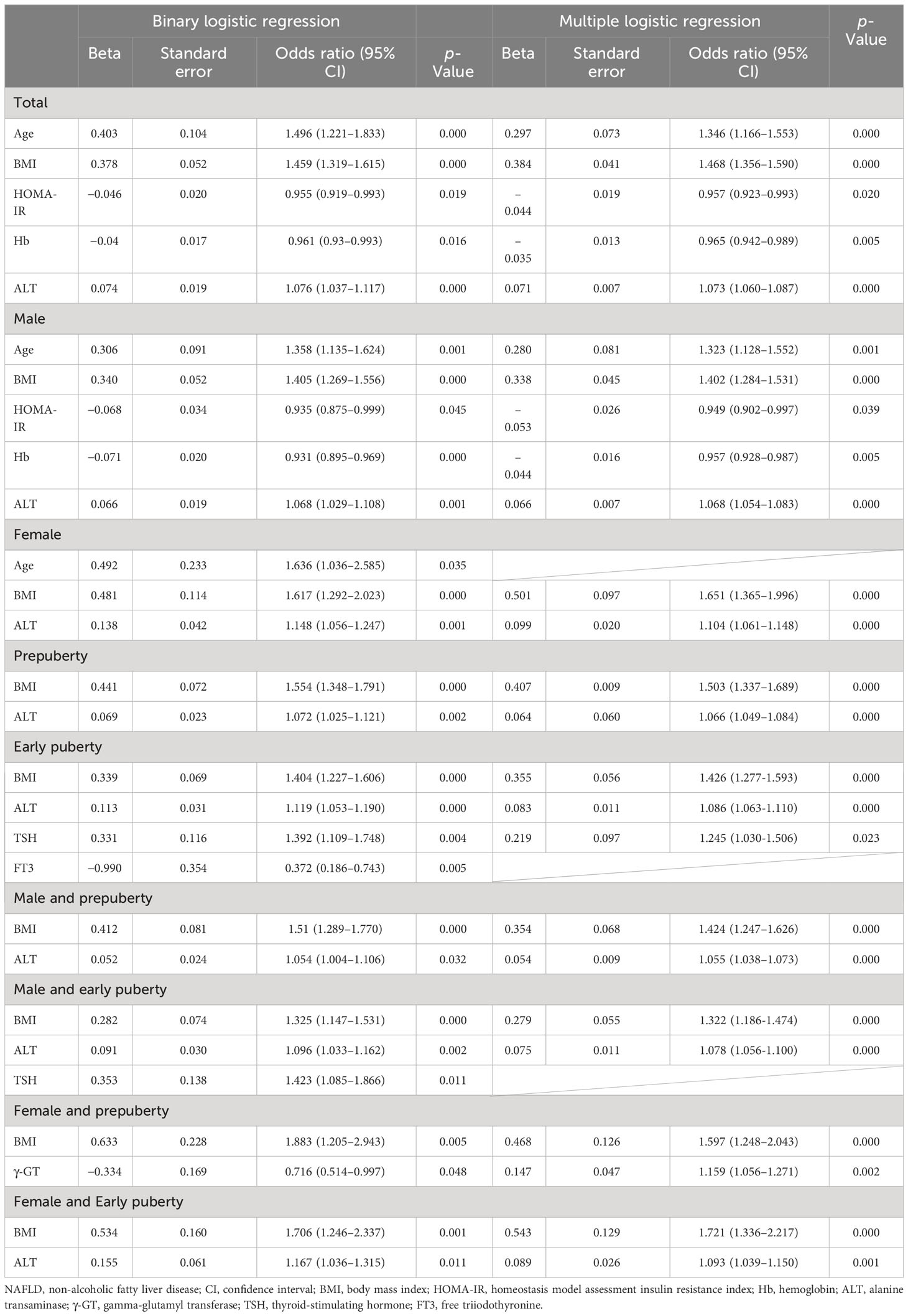
Table 6 Risk factors of NAFLD in hospitalized children with obesity before the late puberty stage from logistic regression.
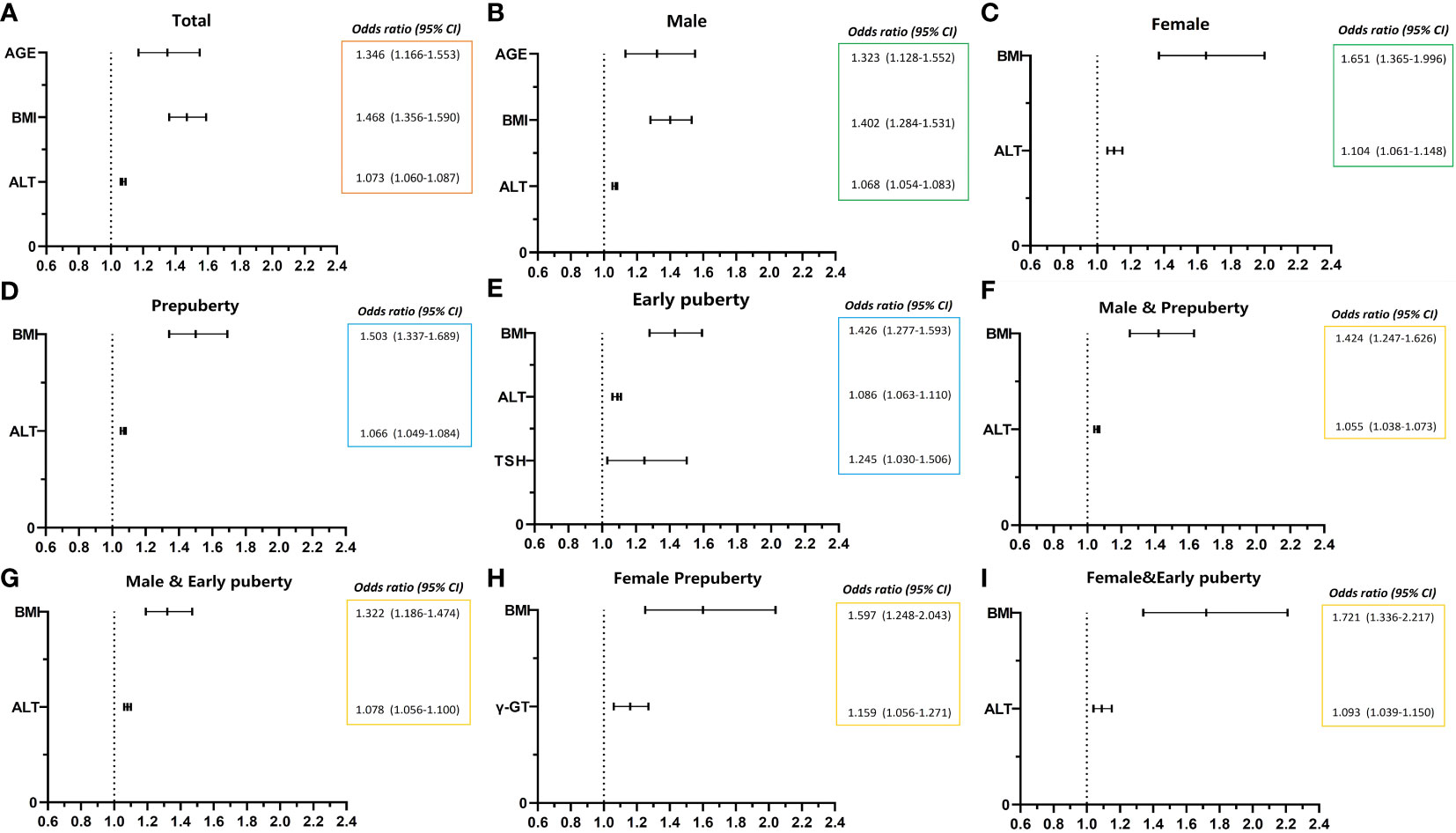
Figure 1 Risk factors of NAFLD of obese children before late puberty stage from logistic regression. NAFLD, nonalcoholic fatty liver disease. CI, confidence interval. BMI, body mass index. HOMA-IR, homeostasis model assessment insulin resistance index. HGB, hemoglobin. ALT, alanine transaminase. γ-GT, gamma glutamyl transferase. TSH, thyroid-stimulating hormone. (A) level of total; (B, C) stratified by gender; (D, E) stratified by puberty; F–I, stratified by both gender and puberty.
As presented in Table 6 and Figure 1, for male patients, age (OR, 1.323; 95% CI, 1.128–1.552; p < 0.01), BMI (OR, 1.402; 95% CI, 1.284–1.531; p < 0.001), and ALT level (OR, 1.068; 95% CI, 1.054–1.083; p < 0.001) were also found to be independent risk factors for NAFLD. However, in female patients, BMI (OR, 1.651; 95% CI, 1.365–1.996; p < 0.001) and ALT level (OR, 1.104; 95% CI, 1.061–1.148; p < 0.001) were found to be two independent risk factors for NAFLD, and age was no longer found to be an independent risk factor for NAFLD.
As presented in Table 6 and Figure 1, BMI (OR, 1.503; 95% CI, 1.337–1.689; p < 0.001) and ALT (OR, 1.066; 95% CI, 1.049–1.084; p < 0.001) at the prepuberty stage were found to be two independent risk factors for NAFLD. Additionally, in early puberty, BMI (OR, 1.426; 95% CI, 1.277–1.593; p < 0.001) and ALT (OR, 1.086; 95% CI, 1.063–1.110; p < 0.001) were still found to be two independent risk factors for NAFLD. Additionally, during early puberty, the TSH level (OR, 1.245; 95% CI, 1.030–1.506; p < 0.05) was also an independent risk factor for NAFLD.
As presented in Table 6 and Figure 1, after being graded by sex and puberty at the same time, BMI (male and prepuberty: OR, 1.424; 95% CI, 1.247–1.626; p < 0.001; male and early puberty: OR, 1.322; 95% CI, 1.186–1.474; p < 0.001; female and prepuberty: OR, 1.597; 95% CI, 1.248–2.043; p < 0.001; female and early puberty: OR, 1.721; 95% CI, 1.336–2.217; p < 0.01) remained an independent risk factor for NAFLD. Meanwhile, after being graded by both sex and puberty, ALT (male and prepuberty: OR, 1.055; 95% CI, 1.038–1.073; p < 0.001; male and early puberty: OR, 1.078; 95% CI, 1.056–1.100; p < 0.001; female and early puberty: OR, 1.093; 95% CI, 1.039–1.150; p < 0.001) remained an independent risk factor for NAFLD, except in prepuberty female children. Nevertheless, for prepuberty female children, γ-GT (OR, 1.159; 95% CI, 1.056–1.271; p < 0.01) was found to be a unique independent risk factor for NAFLD.
3.4 Evaluation of risk factors for NALFD in children with obesity
Independent risk factors were selected from the logistic regression analysis. A ROC curve analysis was performed to further evaluate the predictive ability of the independent risk factors for the early diagnosis of pediatric NAFLD.
As illustrated in Table 7 and Figure 2, the ROC curve analysis of NAFLD in children with obesity before the late puberty stage revealed that BMI was a significant predictor. BMI presented optimal area under the ROC curve (AUROC) in three groups (female, 0.929–0.979; female and prepuberty, 0.921–0.998; female and early puberty, 0.865–0.970) and presented better AUROC in the other six groups (total, 0.861–0.907; male, 0.789–0.859; prepuberty, 0.880–0.973; early puberty, 0.802–0.880; male and prepuberty, 0.775–0.880; male and early puberty, 0.764–0.861).
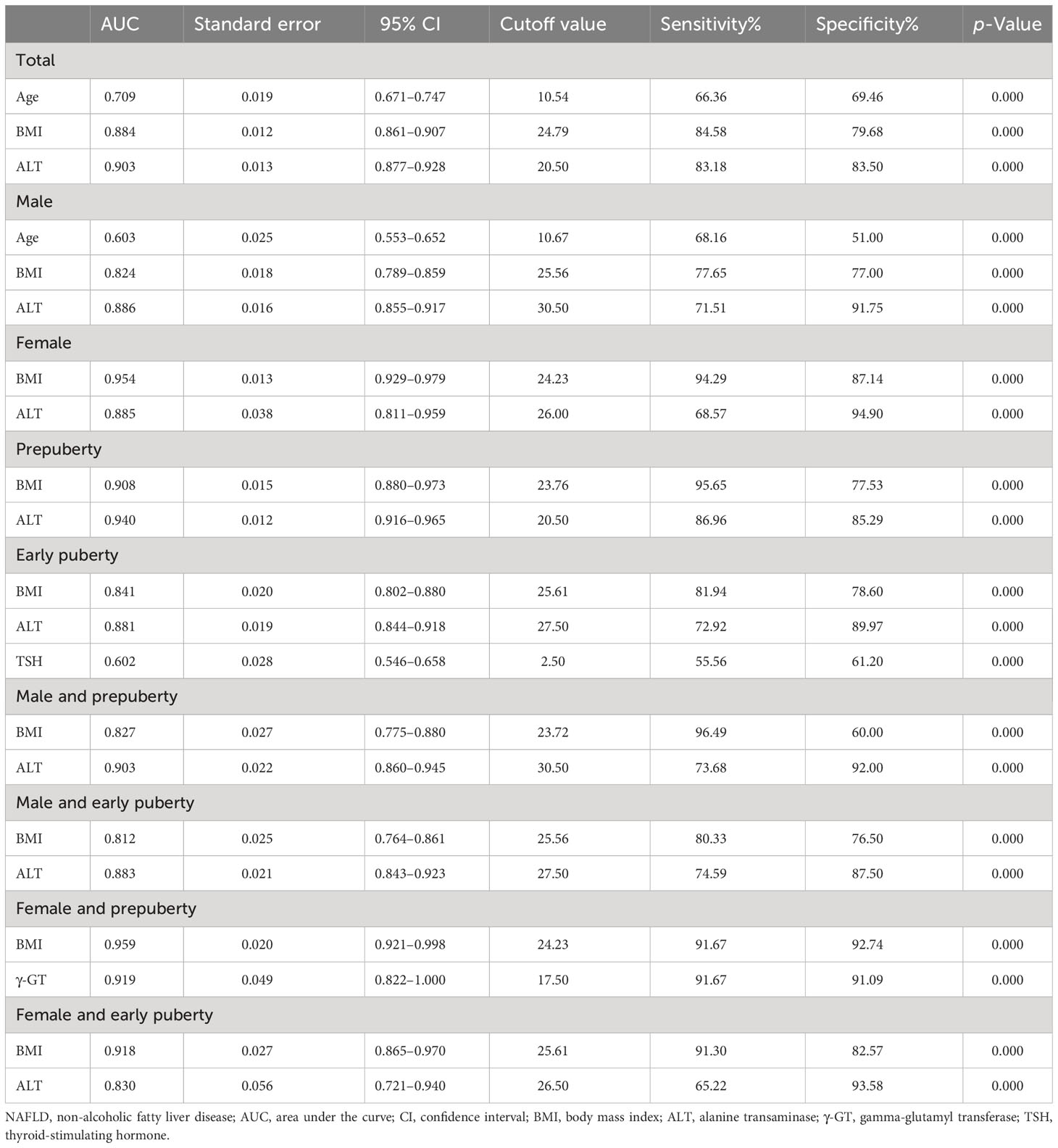
Table 7 Receiver operating characteristic curve of risk factors to predict the occurrence of NAFLD in hospitalized children with obesity before the late puberty stage.
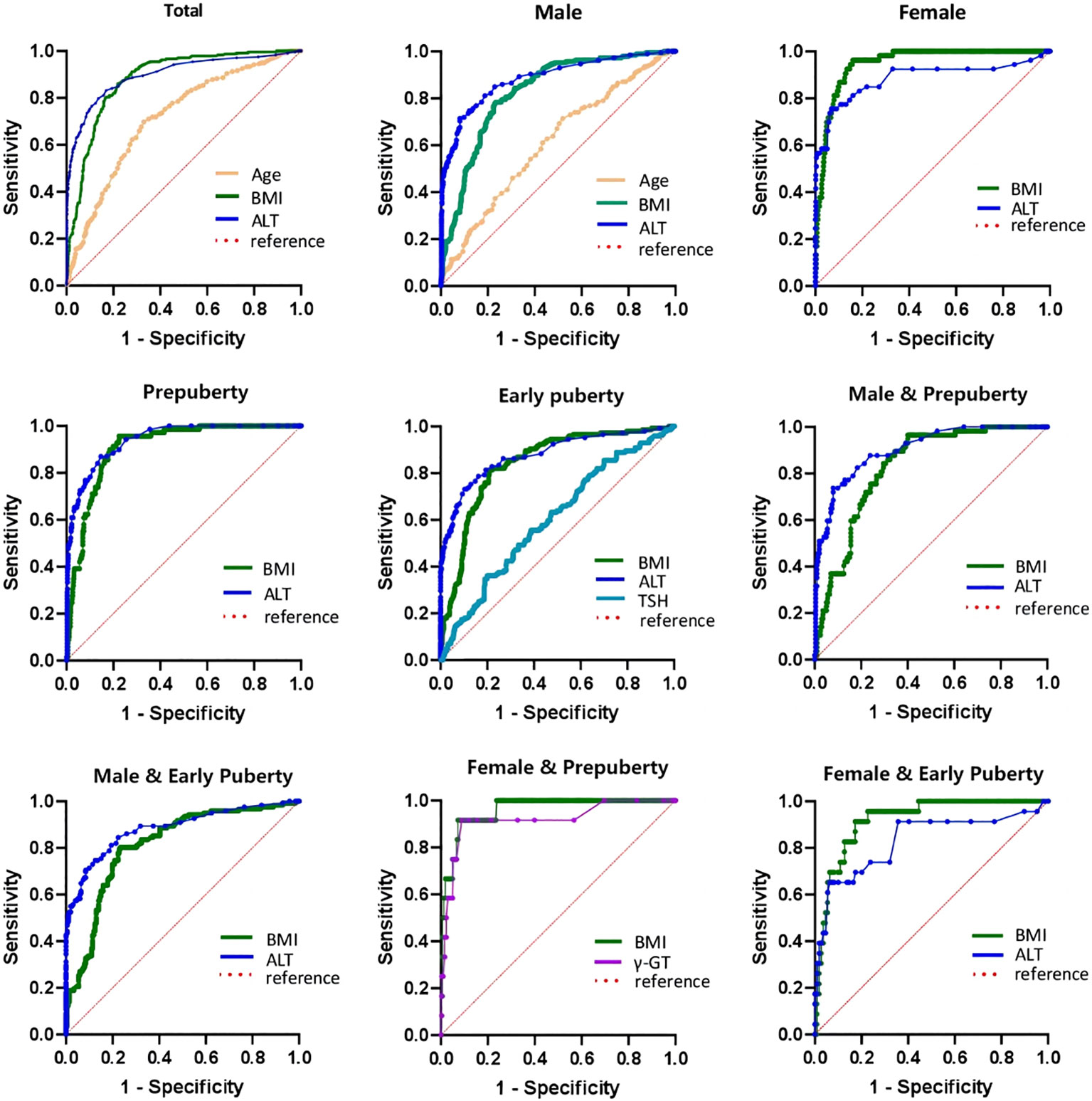
Figure 2 ROC curve of risk factors of NAFLD of obese children before late-puberty stage (Total and Stratification levels). ROC, receiver operating characteristics. NAFLD, nonalcoholic fatty liver disease. BMI, body mass index. HOMA-IR, homeostasis model assessment insulin resistance index. HGB, hemoglobin. ALT, alanine transaminase. γ-GT, gamma glutamyl transferase. TSH, thyroid-stimulating hormone.
As illustrated in Table 7 and Figure 2, excluding female children at the prepuberty stage, the ROC curve analysis demonstrated that ALT was another significant predictor. ALT presented optimal AUROC in six groups (total, 0.877–0.928; male, 0.855–0.917; prepuberty, 0.916–0.965; early puberty, 0.844–0.918; male and prepuberty, 0.860–0.945; male and early puberty, 0.843–0.923).
As illustrated in Table 7 and Figure 2, three other significant predictors were distinctive to certain groups: age for the total group and male group, TSH for the early puberty group, and γ-GT for the female and prepuberty groups. Age showed good AUROC in the total group, 0.709 (0.671–0.747); in contrast, γ-GT showed remarkable AUROC in the female and prepuberty groups, 0.919 (0.822–1.000).
4 Discussion
In this study, we targeted children with obesity because of the global NAFLD epidemic (1). Although teenagers have a higher risk of NAFLD than young children, the children suffering the most from NAFLD are aged 10–13 years (17, 18). Italian case reports suggest a lower age of onset (10–11 years) (19–21). Therefore, in the current study, we chose those aged before the late puberty stage from among the target participants and conducted a retrospective case analysis to evaluate the adaptability of some known risk factors for NAFLD in children in central China.
The present study reported that pediatric NAFLD is more likely to occur in older children and male children; pediatric NAFLD has clinical characteristics of elevated BMI and increased incidence of hypertension; pediatric NAFLD has alterations in biochemical characteristics of glucose and lipid metabolisms (low HDL-C, high TG, TC, LDL-C, and HbA1c), routine blood investigations (high WBC, NEU, LYM, MONO, RBC, and Hb), liver function tests (high ALT, AST, γ-GT, ALB, and GLB), and thyroid function tests (low FT3 and high TSH). The majority of the above significant factors have been reported and accepted (6, 22–28). However, the correlation between the prevalence of pediatric NAFLD and blood cell levels is unclear because there is little research on this topic. In this study, after logistic regression analysis, age remained significant for male children rather than female children. The specific cutoff is 10.67 years, supporting a peak onset age of 10–11 years (19–21).
Furthermore, stratified analysis was performed to verify the aforementioned significant variables at the total level. First, BMI, HOMA-IR, TG, ALT, AST, and γ-GT levels were always statistically significant regardless of stratification. Second, the female children tested negative for alterations in TC, LDL-C, and all thyroid function tests. Third, all thyroid function tests were negative for alterations in the patients at the prepuberty stage. Fourth, HbA1c levels were negative when subdivided according to puberty in male children. Fifth, the incidence of hypertension and HbA1c were negative in female children in the early puberty stage.
It has been demonstrated that female children are more sensitive to TG than cholesterol. This finding is in accordance with those of 29–32, who reported that one of the components related to the association between NAFLD and metabolic syndrome risk factors in female children was hypertriglyceridemia. Our results also showed that biomarkers reflecting hypothyroidism may not be sensitive to female sex and age at the prepuberty stage. Thyroid hormones influence all major metabolic pathways. Thyroid dysfunction, especially hypothyroidism, has been associated with IR, dyslipidemia, and obesity, all of which play important roles in the development of NAFLD (29). Pacifico et al. and Kaltenbach et al. reported that increased TSH levels could be an important risk factor for the development and progression of NAFLD in children and adolescents with obesity; however, in contrast to those of our study, these results did not change after adjusting for sex and stage of puberty (29, 33). In this study, after logistic regression analysis, TSH only remained significant at the early puberty stage, and the specific cutoff was 2.5 U/ml, which is in the range of 2.31–3.22 U/ml, as suggested in the study of Kaltenbach et al. (33).
The results of the logistic regression analysis indicated that BMI played a dominant role in all risk factors because it maintained significance for both the total and all stratified levels. Overall, the risk of NAFLD in children with obesity increased by 46.8% for every unit increase in BMI. After stratification, the OR of BMI of the four groups (female, prepuberty, female and prepuberty, and female and early puberty) exceeded the total level, and the maximal OR was found in the female and early puberty group, 1.721 (95% CI, 1.336–2.217), which means that for obese female children before late puberty, BMI might be more crucial for risk exposure to NAFLD. The sex differences in the prevalence of NAFLD may be due to the fat distribution and the protective effects of estrogen (21). In a study by Cohen et al. (34), BMI was significantly higher in female children than in male children; Alkassabany YM et al. also reported no significant correlation between gender and NAFLD (35). However, in our study, the BMI cutoff value for female children was lower than for male children (24.23 vs. 25.56).
This study also revealed that ALT is important because of its high frequency of emergence. Overall, the risk of NAFLD in children with obesity increased by 7.3% for every unit increase in ALT. After stratification, four groups (female, early puberty, male and early puberty, and female and early puberty) showed a higher OR, and the female group showed the maximal OR of 1.104 (95% CI, 1.061–1.148), which means that in the early puberty age stage, ALT might be a necessary risk factor for NAFLD, especially in female children. This finding is in accordance with those of many previous studies (27, 31, 35–39). Villanueva-Ortega et al. suggested that although ALT is the only predictor of NAFLD in male children, it does not have the same diagnostic efficiency as it does in female patients (27). Wiegand et al. found that the association of significantly elevated ALT with age supports the hypothesis that an increase in sex hormones at the prepuberty stage may be important in the predisposition to pediatric NAFLD (35). In addition, the results of the ROC analysis showed that ALT was a better predictor of pediatric NAFLD than BMI. The North American guidelines use an ALT of >52 U/L for male children and an ALT of >44 U/L for female children, and the European guidelines use an elevated ALT of >45 U/L for both sexes (40, 41), but different from them, the cutoff value in our study was 30.5 U/L and 26 U/L for male and female children, respectively.
In summary, we conclude that BMI, ALT, and age are risk factors for NAFLD in children with obesity before the late puberty stage. BMI had the greatest exposure risk for NAFLD, and ALT had the highest predictive value for the diagnosis of NAFLD. At the stratified level, for exposure risk, age was specific to the male sex, TSH was specific to the early puberty stage, and γ-GT was specific to the female sex plus the prepuberty stage. On a stratified level, for the female sex, even with age stratification, BMI rather than ALT has a better ability to diagnose NAFLD.
In the end, we are aware of the limitation of the present study. First, some well-known risk factors for pediatric NAFLD were missing, such as lifestyle (dietary factors and physical inactivity), ethnicity, genetic factors, and maternal factors (metabolic disease, metabolic level during pregnancy, breastfeeding, etc.) (42, 43). Second, there is a popular study about the new biomarkers to monitor NAFLD, such as Sirtuin 1, CK-18, and more genetic and epigenetic factors (42, 44, 45). Due to retrospective nature of the studies, no data were available. Third, more accurate diagnostic methods for pediatric NAFLD, such as nuclear magnetic resonance and liver biopsy, were not selected. Fourth, the design of the control group could be more comprehensive, including healthy children, overweight and non-obese patients, patients in late puberty, and non-alcoholic fatty hepatitis (NASH) patients. Fifth, since the global coronavirus disease-2019 pandemic occurred during the data collection period, it is not clear whether this event had an impact on the disease.
4.1 Future perspective
The debate existed for many years that Calls to rename NAFLD to Metabolic [Dysfunction-] Associated Liver Disease (MAFLD) (46–48) has existed for many years. The Chinese Society of Hepatology (CSH) endorsed the proposal of changing the term NAFLD to MAFLD in 2021 (49). Clinical diagnosis of MAFLD would be based upon hepatic steatosis (liver biopsy, imaging, or blood biomarker evidence) and at least one of three criteria (obesity/overweight, type 2 diabetes (T2D), or evidence of at least two metabolic abnormalities). We have reviewed our data, the diagnosis is a basic one. However, this a retrospective study, and the design of this article predates the renaming statement for nonalcoholic fatty liver disease. We can consider that using the new nomenclature in future studies for MAFLD would benefit the patient’s understanding and management of the disease and be better for identifying significant hepatic diseases. Moreover, so far, the discussion primarily addressed adult patients and now awaits pediatric contribution (47).
To this date, there is no pharmacological drug registered for the treatment of pediatric NAFLD (49). Proposed pharmacological therapies include insulin sensitizers, probiotics, anti-inflammatory agents, lipid-lowering drugs, and vitamins. Recently, using natural products as an alternative approach in the treatment of NAFLD has drawn increasing attention. Among the most studied substances in the literature, the following molecules were chosen: spirulina, oleuropein, garlic, berberine, resveratrol, curcumin, ginseng, glycyrrhizin, coffee, cocoa powder, epigallocatechin-3-gallate, and bromelain (48, 50, 51). However, there are many examples of hepatotoxicity induced by herbal remedies (42, 52, 53), and the causes are different, such as impurities, batch-to-batch variability, misidentification and/or labeling, and different source of used production materials. That said, some herbal products should be consumed with caution due to their hepatotoxicity.
It is noteworthy that the excess visceral adipose tissue (VAT) may be more important than BMI (54). Obese persons with excess VAT, or abdominal obesity, are at higher risk for metabolic syndrome components than those whose fat is located predominantly in the lower body subcutaneously. However, our research lacks data on waist circumference and visceral fat, so it cannot be compared with BMI, which will be an important point in our next research.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Medical Ethics Committee of the Wuhan Children’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from another research group. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
LishZ and XC conceived and designed the study. LinlZ, PC, LingZ, WY, XY, YW, XX, and SY collected the clinical data. LishZ, LinlZ, and HM performed the data statistics. LishZ, LinlZ, WY, HX, and HQ wrote the paper. LishZ, LingZ, and HD reviewed and edited the manuscript. All authors read and approved the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (No. 82074249), the Wuhan Municipal Health Commission (WX20B13), and the Traditional Chinese Medicine Funding of the Health Commission of Hubei Province (ZY2019M006).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Younossi Z, Tacke F, Arrese M, Chander Sharma B, Mostafa I, Bugianesi E, et al. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology (2019) 69(6):2672–82. doi: 10.1002/hep.30251
2. Li J, Zou B, Yeo YH, Feng Y, Xie X, Lee DH, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999-2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol (2019) 4(5):389–98. doi: 10.1016/S2468-1253(19)30039-1
3. Zhou XL, Fu JF. Expert consensus on diagnosis and treatment of nonalcoholic fatty liver disease in children. Zhongguo Shi Yong Er Ke Za Zhi (2018) 33(7):487–92. doi: 10.19538/j.ek2018070602
4. Vos MB, Abrams SH, Barlow SE, Caprio S, Daniels SR, Kohli R, et al. NASPGHAN clinical practice guideline for the diagnosis and treatment of nonalcoholic fatty liver disease in children: recommendations from the expert committee on NAFLD (ECON) and the north american society of pediatric gastroenterology, hepatology and nutrition (NASPGHAN). J Pediatr Gastroenterol Nutr (2017) 64(2):319–34. doi: 10.1097/MPG.0000000000001482
5. Nobili V, Alisi A, Newton KP, Schwimmer JB. Comparison of the phenotype and approach to pediatric vs adult patients with nonalcoholic fatty liver disease. Gastroenterology (2016) 150(8):1798–810. doi: 10.1053/j.gastro.2016.03.009
6. Nobili V, Alisi A, Valenti L, Miele L, Feldstein AE, Alkhouri N. NAFLD in children: new genes, new diagnostic modalities and new drugs. Nat Rev Gastroenterol Hepatol (2019) 16(9):517–30. doi: 10.1038/s41575-019-0169-z
7. Scapaticci S, D’Adamo E, Mohn A, Chiarelli F, Giannini C. Non-alcoholic fatty liver disease in obese youth with insulin resistance and type 2 diabetes. Front Endocrinol (Lausanne) (2021) 12:639548. doi: 10.3389/fendo.2021.639548
8. Anderson EL, Howe LD, Jones HE, Higgins JP, Lawlor DA, Fraser A. The prevalence of non-alcoholic fatty liver disease in children and adolescents: A systematic review and meta-analysis. PloS One (2015) 10(10):e0140908. doi: 10.1371/journal.pone.0140908
9. Sharma V, Coleman S, Nixon J, Sharples L, Hamilton-Shield J, Rutter H, et al. A systematic review and meta-analysis estimating the population prevalence of comorbidities in children and adolescents aged 5 to 18 years. Obes Rev (2019) 20(10):1341–9. doi: 10.1111/obr.12904
10. Liu J, Mu C, Li K, Luo H, Liu Y, Li Z. Estimating global prevalence of metabolic dysfunction-associated fatty liver disease in overweight or obese children and adolescents: systematic review and meta-analysis. Int J Public Health (2021) 66:1604371. doi: 10.3389/ijph.2021.1604371
11. Ballestri S, Nascimbeni F, Baldelli E, Marrazzo A, Romagnoli D, Lonardo A. NAFLD as a sexual dimorphic disease: role of gender and reproductive status in the development and progression of nonalcoholic fatty liver disease and inherent cardiovascular risk. Adv Ther (2017) 34(6):1291–326. doi: 10.1007/s12325-017-0556-1
12. Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics (2006) 118(4):1388–93. doi: 10.1542/peds.2006-1212
13. Pu JQ, Zhang JW, Chen RM, Maimaiti M, Luo JS, Chen SK, et al. Survey of height and weight of children and adolescents at different Tanner stages in urban China. Zhonghua Er Ke Za Zhi (2021) 59(12):1065–73. doi: 10.3760/cma.j.cn112140-20210525-00453
14. Group of China Obesity Task Force. Body mass index reference norm for screening overweight and obesity in Chinese children and adolescents. Zhonghua Liu Xing Bing Xue Za Zhi (2004) 25(2):97–102.
15. Di Sessa A, Cirillo G, Guarino S, Marzuillo P, Miraglia Del Giudice E. Pediatric non-alcoholic fatty liver disease: current perspectives on diagnosis and management. Pediatr Health Med Ther (2019) 10:89–97. doi: 10.2147/PHMT.S188989
16. Fan H, Yan YK, Mi J. Updating blood pressure references for Chinses children aged 3-17 in years. Zhonghua Gao Xue Ya Za Zhi (2017) 25(5):428–35. doi: 10.16439/j.cnki.1673-7245.2017.05.009
17. Mi J. Blood pressure references for Chinses children aged 3-17 in years. Zhongguo Er Tong Bao Jian Za Zhi (2010) 18(6):534.
18. Vajro P, Lenta S, Socha P, Dhawan A, McKieman P, Baumann U, et al. Diagnosis of nonalcoholic fatty liver disease in children and adolescents: position paper of the ESPGHAN Hepatology Committee. J Pediatr Gastroenterol Nutr (2012) 54(5):700–13. doi: 10.1097/MPG.0b013e318252a13f
19. Goyal NP, Schwimmer JB. The progression and natural history of pediatric nonalcoholic fatty liver disease. Clin Liver Dis (2016) 20(2):325–38. doi: 10.1016/j.cld.2015.10.003
20. Li J, Ren WH. Diagnosis and therapeutic strategies for non-alcoholic fatty liver disease in children. Zhonghua Gan Zang Bing Za Zhi (2020) 28(3):208–12. doi: 10.3760/cma.j.cn501113-20200317-00121
21. Nobili V, Marcellini M, Devito R, Ciampalini P, Piemonte F, Comparcola D, et al. NAFLD in children: a prospective clinical-pathological study and effect of lifestyle advice. Hepatology (2006) 44(2):458–65. doi: 10.1002/hep.21262
22. Valenti L, Alisi A, Galmozzi E, Bartuli A, Del Menico B, Alterio A, et al. I148M patatin-like phospholipase domain-containing 3 gene variant and severity of pediatric nonalcoholic fatty liver disease. Hepatology (2010) 52(4):1274–80. doi: 10.1002/hep.23823
23. Zhou X, Lin X, Chen J, Pu J, Wu W, Wu Z, et al. Clinical spectrum transition and prediction model of nonalcoholic fatty liver disease in children with obesity. Front Endocrinol (Lausanne) (2022) 13:986841. doi: 10.3389/fendo.2022.986841
24. Ward M, Nguyen P, Akintorin S, Arcia R, Soyemi K. Interventions to improve liver enzyme screening testing in obese patients aged <18 years in a public hospital, Chicago, IL, 2017-2018. Pediatr Health Med Ther (2018) 10:1–4. doi: 10.2147/PHMT.S183900
25. Hua MC, Huang JL, Hu CC, Yao TC, Lai MW. Including fibroblast growth factor-21 in combined biomarker panels improves predictions of liver steatosis severity in children. Front Pediatr (2019) 7:420. doi: 10.3389/fped.2019.00420
26. Kim MJ, Lee KJ. Analysis of the dietary factors associated with suspected pediatric nonalcoholic fatty liver disease and potential liver fibrosis: Korean National Health and Nutrition Examination Survey 2014-2017. BMC Pediatr (2020) 20(1):121. doi: 10.1186/s12887-020-02022-y
27. Peña-Vélez R, Garibay-Nieto N, Cal-Y-Mayor-Villalobos M, Laresgoiti-Servitje E, Pedraza-Escudero K, García-Blanco MDC, et al. Association between neck circumference and non-alcoholic fatty liver disease in Mexican children and adolescents with obesity. J Pediatr Endocrinol Metab (2020) 33(2):205–13. doi: 10.1515/jpem-2019-0204
28. Peng L, Wu S, Zhou N, Zhu S, Liu Q, Li X. Clinical characteristics and risk factors of nonalcoholic fatty liver disease in children with obesity. BMC Pediatr (2021) 21(1):122. doi: 10.1186/s12887-021-02595-2
29. Zhang J, Cao J, Xu H, Dong G, Huang K, Wu W, et al. Ferritin as a key risk factor for nonalcoholic fatty liver disease in children with obesity. J Clin Lab Anal (2021) 35(2):e23602. doi: 10.1002/jcla.23602
30. Villanueva-Ortega E, Garcés-Hernández MJ, Herrera-Rosas A, López-Alvarenga JC, Laresgoiti-Servitje E, Escobedo G, et al. Gender-specific differences in clinical and metabolic variables associated with NAFLD in a Mexican pediatric population. Ann Hepatol (2019) 18(5):693–700. doi: 10.1016/j.aohep.2019.04.012
31. Marcinkiewicz K, Horodnicka-Józwa A, Jackowski T, Strączek K, Biczysko-Mokosa A, Walczak M, et al. Nonalcoholic fatty liver disease in children with obesity- observations from one clinical centre in the Western Pomerania region. Front Endocrinol (Lausanne) (2022) 13:992264. doi: 10.3389/fendo.2022.992264
32. Putri RR, Casswall T, Hagman E. Risk and protective factors of non-alcoholic fatty liver disease in paediatric obesity: A nationwide nested case-control study. Clin Obes (2022) 12(2):e12502. doi: 10.1111/cob.12502
33. Pacifico L, Bonci E, Ferraro F, Andreoli G, Bascetta S, Chiesa C. Hepatic steatosis and thyroid function tests in overweight and obese children. Int J Endocrinol (2013) 2013:381014. doi: 10.1155/2013/381014
34. Cohen D, Gonzales-Pacheco D, Myers O. Relationships between alanine aminotransferase, serum triglycerides, body mass index and nonalcoholic fatty liver disease in an outpatient pediatric clinic population. J Pediatr Nurs (2016) 31(2):152–8. doi: 10.1016/j.pedn.2015.10.009
35. Alkassabany YM, Farghaly AG, El-Ghitany EM. Prevalence, risk factors, and predictors of nonalcoholic fatty liver disease among schoolchildren: a hospital-based study in Alexandria, Egypt. Arab J Gastroenterol (2014) 15(2):76–81. doi: 10.1016/j.ajg.2014.05.002
36. Kaltenbach TE, Graeter T, Oeztuerk S, Holzner D, Kratzer W, Wabitsch M, et al. Thyroid dysfunction and hepatic steatosis in overweight children and adolescents. Pediatr Obes (2017) 12(1):67–74. doi: 10.1111/ijpo.12110
37. Wiegand S, Keller KM, Röbl M, L’Allemand D, Reinehr T, Widhalm K, et al. Obese boys at increased risk for nonalcoholic liver disease: evaluation of 16,390 overweight or obese children and adolescents. Int J Obes (Lond) (2010) 34(10):1468–74. doi: 10.1038/ijo.2010.106
38. Greber-Platzer S, Thajer A, Bohn S, Brunert A, Boerner F, Siegfried W, et al. Increased liver echogenicity and liver enzymes are associated with extreme obesity, adolescent age and male gender: analysis from the German/Austrian/Swiss obesity registry APV. BMC Pediatr (2019) 19(1):332. doi: 10.1186/s12887-019-1711-4
39. Gupta N, Jindal G, Nadda A, Bansal S, Gahukar S, Kumar A. Prevalence and risk factors for nonalcoholic fatty liver disease in obese children in rural Punjab, India. J Family Community Med (2020) 27(2):103–8. doi: 10.4103/jfcm.JFCM_287_19
40. Schwimmer JB, McGreal N, Deutsch R, Finegold MJ, Lavine JE. Influence of gender, race, and ethnicity on suspected fatty liver in obese adolescents. Pediatrics (2005) 115(5):e561–5. doi: 10.1542/peds.2004-1832
41. Ezaizi Y, Kabbany MN, Conjeevaram Selvakumar PK, Sarmini MT, Singh A, Lopez R, et al. Comparison between non-alcoholic fatty liver disease screening guidelines in children and adolescents. JHEP Rep (2019) 1(4):259–64. doi: 10.1016/j.jhepr.2019.06.005
42. Di Francia R, Rinaldi L, Cillo M, Varriale E, Facchini G, D’Aniello C, et al. Antioxidant diet and genotyping as tools for the prevention of liver disease. Eur Rev Med Pharmacol Sci (2016) 20(24):5155–63.
43. Salvatore T, Caturano A, Galiero R, Di Martino A, Albanese G, Vetrano E, et al. Cardiovascular benefits from gliflozins: effects on endothelial function. Biomedicines (2021) 9(10):1356. doi: 10.3390/biomedicines9101356
44. Furthner D, Weghuber D, Dalus C, Lukas A, Stundner-Ladenhauf HN, Mangge H, et al. Nonalcoholic fatty liver disease in children with obesity: narrative review and research gaps. Horm Res Paediatr (2022) 95(2):167–76. doi: 10.1159/000518595
45. Martins IJ. Unhealthy nutrigenomic diets accelerate NAFLD and adiposity in global communities. J Mol Genet Med (2015) 9:162. doi: 10.4172/1747-0862.1000162
46. Martins IJ. Sirtuin 1, a diagnostic protein marker and its relevance to chronic disease and therapeutic drug interventions. EC Pharmacol Toxicol (2018) 6(4):209–15.
47. Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol (2020) 73(1):202–9. doi: 10.1016/j.jhep.2020.03.039
48. Eslam M, Sanyal AJ, George J, International Consensus Panel. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology (2020) 158(7):1999–2014.e1. doi: 10.1053/j.gastro.2019.11.312
49. Nan Y, An J, Bao J, Chen H, Chen Y, Ding H, et al. The Chinese Society of Hepatology position statement on the redefinition of fatty liver disease. J Hepatol (2021) 75(2):454–61. doi: 10.1016/j.jhep.2021.05.003
50. Furthner D, Weghuber D, Dalus C, Lukas A, Stundner-Ladenhauf HN, Mangge H, et al. Nonalcoholic fatty liver disease in children with obesity: Narrative review and research gaps. Horm Res Paediatr (2022) 95(2):167–176. doi: 10.1159/000518595
51. Tarantino G, Balsano C, Santini SJ, Brienza G, Clemente I, Cosimini B, et al. It is high time physicians thought of natural products for alleviating NAFLD. Is there sufficient evidence to use them? Int J Mol Sci (2021) 22(24):13424. doi: 10.3390/ijms222413424
52. Tarantino G, Pezzullo MG, di Minno MN, Milone M, Pezzullo LS, Milone M, et al. Drug-induced liver injury due to “natural products” used for weight loss: a case report. World J Gastroenterol (2009) 15(19):2414–7. doi: 10.3748/wjg.15.2414
53. Danan G, Teschke R. RUCAM in drug and herb induced liver injury: the update. Int J Mol Sci (2015) 17(1):14. doi: 10.3390/ijms17010014
Keywords: nonalcoholic fatty liver disease, children, risk factors, obese, prepuberty, early puberty
Citation: Zhou L, Zhang L, Zhang L, Yi W, Yu X, Mei H, Xiao H, Wang Y, Qin H, Xiong X, Yan S, Dong H, Chen P and Chen X (2023) Analysis of risk factors for non-alcoholic fatty liver disease in hospitalized children with obesity before the late puberty stage. Front. Endocrinol. 14:1224816. doi: 10.3389/fendo.2023.1224816
Received: 18 May 2023; Accepted: 04 August 2023;
Published: 31 August 2023.
Edited by:
Hao Mei, University of Mississippi Medical Center School of Dentistry, United StatesReviewed by:
Giovanni Tarantino, University of Naples Federico II, ItalyIan James Martins, University of Western Australia, Australia
Luca Rinaldi, University of Campania Luigi Vanvitelli, Italy
Copyright © 2023 Zhou, Zhang, Zhang, Yi, Yu, Mei, Xiao, Wang, Qin, Xiong, Yan, Dong, Chen and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peng Chen, bG9uZ3RpbmcyMDEwQHllYWgubmV0; Xiaohong Chen, Y3hoZGFpZnVAMTYzLmNvbQ==
 Lishan Zhou
Lishan Zhou Linli Zhang2
Linli Zhang2 Lingling Zhang
Lingling Zhang Wei Yi
Wei Yi Hong Mei
Hong Mei Huan Qin
Huan Qin Xiaoli Xiong
Xiaoli Xiong Suqi Yan
Suqi Yan Xiaohong Chen
Xiaohong Chen