- 1Metabolic, Nutrition, and Exercise Research (MiNER) Laboratory, Department of Kinesiology, The University of Texas at El Paso, El Paso, TX, United States
- 2Muscle Molecular Physiology Laboratory, Department of Kinesiology, The University of Texas at El Paso, El Paso, TX, United States
- 3Southwest Plastic Surgery, El Paso, TX, United States
- 4Department of Mathematical Sciences, The University of Texas at El Paso, El Paso, TX, United States
Background: Physical inactivity increases the risk for metabolic diseases such as obesity and type 2 diabetes. Neuromuscular electrical stimulation (NMES) is an effective method to induce muscle contraction, particularly for populations with physical impairments and/or metabolic diseases. However, its effectiveness to improve glycemic control is unclear. This review aimed to determine the effectiveness of NMES on glycemic control.
Methods: Electronic search consisted of MEDLINE (PubMed), EMBASE, Cochrane Library, Google Scholar, and Web of Science to identify studies that investigated the effects of NMES on glycemic control for this systematic review. The meta-analysis consists of the studies designed as randomized controlled trials. Effect sizes were calculated as the standardized mean difference (SMD) and meta-analysis was conducted using a random-effects model.
Results: Thirty-five studies met the inclusion criteria for systematic review and of those, nine qualified for the meta-analysis. Existing evidence suggested that NMES effectively improves glycemic control predominantly in middle-aged and elderly population with type 2 diabetes, obesity, and spinal cord injury. The meta-analysis is comprised of 180 participants and reported that NMES intervention lowered fasting blood glucose (SMD: 0.48; 95% CI: 0.17 to 0.78; p=0.002; I²=0%). Additional analysis using the primary measures reported by each study to indicate glycemic control (i.e., OGTT, HOMA-IR, and fasting glucose) also confirmed a significant effect of NMES on improving glycemic control (SMD: 0.41; 95% CI, 0.09 to 0.72; p=0.01; I²=11%). NMES protocol varied across studies and requires standardization.
Conclusion: NMES could be considered as a therapeutic strategy to improve glycemic control in populations with physical impairments and/or metabolic disorders.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42020192491.
Introduction
Physical inactivity increases risk for metabolic diseases such as insulin resistance, obesity, type 2 diabetes (T2D) and is the fourth leading risk factor for death worldwide (1–6). Physical inactivity-related diseases contribute to a heavy economic burden through direct health care related expenditures, indirect productivity costs, lifetime disease burdens, as well as premature mortality (7). Adhering to the Centers for Disease Control and Prevention recommended physical activity guidelines (150 min/week) could avert the increasing cases of metabolic disorders such as diabetes and may decrease the financial burden on the health care system (8, 9). A prominent metabolic disorder evident in diabetes is the loss of glycemic control capability (10). It is well established that muscle contraction through endurance and resistance exercise is effective in improving insulin sensitivity in all populations (11–13). Muscle contraction induced by electrical stimulation in human muscle cells (in-vitro) as well as in isolated rat skeletal muscle have been shown to upregulate glucose uptake (14, 15). Therefore, as an alternative therapeutic approach, the potential to improve glycemic control by inducing muscle contractions through electrical stimulation is of particular interest to populations who are less likely or unable to perform regular physical activity or who are insulin resistant.
Neuromuscular electrical stimulation (NMES) is an alternate strategy to induce involuntary contraction of skeletal muscle via depolarization of the motor axons and nerves being stimulated through an electrical current (16–19). NMES has been widely used across the field of rehabilitation to prevent muscle loss, regain muscle mass and function, and to improve motor learning and exercise performance in individuals with spinal cord injury (SCI), stroke, sport-related injuries, as well as individuals with metabolic diseases (20–26). Specifically, previous studies have established the effectiveness of NMES in preventing muscle loss, improving skeletal muscle mass, power, and work capacity following SCI (23, 27), improving muscle strength in rodents and humans (17, 24, 25), and both knee function and quadricep strength following cruciate ligament reconstruction in humans (25). Use of NMES has also been reported to increase skeletal muscle cross sectional area and capillary number per type IIA and IIB muscle fibers (27–31). Voluntary muscle contraction recruits motor units in an orderly fashion established as the size principle (32, 33), and recruits’ type I fibers under low intensity exercise (33, 34). NMES on the other hand, has been shown to recruit motor units in a reverse pattern. Electrical stimulation preferentially recruits motor units within the spatial area of the electrode and also motor units with greater excitability, larger axonal diameter, and lower resistance against external electrical stimulation (18, 35). It has been suggested that an increase in glucose uptake with involuntary muscle contraction induced by electrical stimulation is due in part to the preferential activation of glycolytic type II fibers (28, 36, 37). This is also supported by rodent studies reporting preferential recruitment of axons with larger diameters and fibers that are more reliant on glycolytic metabolism through NMES (38–42). It has been shown that spatial muscle recruitment with NMES is associated with electrode placement, stimulating the motor neuron branches that are in the proximity of the electrical current being delivered (43). Conversely, muscle fiber type determines the recruited motor units in voluntary contraction (43). Electrical stimulation leads to activation of glycogenolysis and anaerobic glycolysis as the major source of ATP production (44). Translocation of glucose transporter (GLUT-4) to muscle membrane (39–41, 45) and increase in glucose uptake (46–48) have been reported with muscle contraction that uses an insulin independent glucose uptake pathway. An increased accumulation of lactate (44, 49) and whole-body carbohydrate utilization (44, 50, 51) have been reported during electrical stimulation. Similarly, a higher Pi/PCr ratio and lower intracellular pH causing early fatigue (exaggerated metabolic demand) have been reported during high frequency electrically stimulated muscle contraction compared to voluntary contraction (34, 43).
Skeletal muscle, being the primary site for insulin stimulated glucose uptake, plays an important role in glycemic control and regulation of whole-body glucose metabolism (45, 48). Electrically inducing skeletal muscle contractions as a way to improve glucose utilization has been used in a surgical setting to acutely prevent a hyperglycemic response during preoperative anesthesia (52). However, existing literature that assessed the effectiveness of NMES in improving glycemic control and metabolic health is not conclusive. This gap in knowledge is due to highly variable NMES protocols used (frequency, duration, and length of intervention), population studied, variable testing methods used to access glycemic control, and lack of control group in several studies. Therefore, the primary purpose of this comprehensive systematic review and meta-analysis was to evaluate the existing evidence to determine the effectiveness of NMES as an alternative therapeutic approach to improve glycemic control. As improvements in glycemic control have often been connected to whole body substrate utilization and lean mass, we have therefore also explored the existing literature to determine the effects of NMES on substrate utilization and body composition.
Methods
Electronic search strategy and eligibility criteria
This systematic review and meta-analysis were performed in accordance with the Cochrane Collaboration (53) and Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines (54). The protocol of the study was registered on International Prospective Register of Systematic Review (PROSPERO) (CRD42020192491). Randomized controlled trials that evaluated the effects of NMES on glycemic control and/or insulin sensitivity were included. A computerized search was performed on MEDLINE (PubMed), EMBASE, Cochrane Library, Google Scholar, and Web of science to identify all potential literature. Various combinations of keywords and mesh words relating to neuromuscular electrical stimulation were used in the search (See Table, Supplementary Table 1, which illustrates the keyword/meshword search strategies used). References of selected studies were further reviewed to include any additional studies that may not have been found through search terms. The search was not restricted to any geographical region, gender, or population, but was restricted to studies published in English language and conducted on human subjects.
Study selection
In the initial search, four researchers (MS, MG, AM, SS) independently located and reviewed all articles by title and abstract text to ensure that the following inclusion criteria were met for the systematic review: 1) studies administered neuromuscular electrical stimulation on skeletal muscle, 2) articles reported data from original research and did not include secondary data, 3) articles reported glycemic control data and 4) studies were conducted in human subjects. Studies that met inclusion criteria for the systematic review were then considered for meta-analysis if the following additional criteria were met: 1) studies were conducted with a placebo or equivalent control group and 2) articles presented both pre and post NMES intervention data for primary outcome measures with mean and standard deviation or standard error of mean values. All reviewers assessed the selected articles and collectively resolved any discrepancies for initial inclusion. After the potential articles were identified based on the initial criteria, a full text review of all articles was performed before proceeding to data extraction.
Data collection/extraction
Authors independently extracted all relevant data needed for both systematic review and meta-analysis. Extracted data included characteristics of participants (age, gender, body mass index (BMI), and health status), sample size, intervention type (acute or chronic), anatomical location of NMES application, NMES application protocols (frequency, intensity, duration, and length of intervention), testing methods used to assess glycemic control, and effects of NMES on glycemic control (acute and chronic effects of NMES), substrate utilization, and body composition. Meta-analysis was limited to analyzing the effects of NMES on glycemic control using only longitudinal studies that met inclusion criteria (n=9). Due to the limited number of studies that met the inclusion criteria, it was not achievable to conduct a meta-analysis to determine the effects of NMES on substrate utilization (n=1) and body composition (n=4). Following the data extraction phase, all reviewers verified entered data to confirm the accuracy.
Risk of bias and quality assessment
Reviewers independently assessed the risk of bias for the studies included in meta-analysis using the Cochrane Collaboration’s Risk of Bias tool (RoB2) (55). Studies were assessed for the following criteria: random sequence generation, allocation concealment, blinding participants, blinding of outcome assessment, incomplete data reporting, and selective reporting.
Data analysis
The meta-analysis was carried out to determine the effects of NMES on glycemic control, the primary outcome measure of the study. Continuous outcomes were reported as the mean difference (MD) and standardize mean difference (SMD) from pre to post treatment in each group with 95% confidence interval (95% CI). Random effect models were used to combine data in Review Manager (version 5.3). The statistical heterogeneity among studies was tested using I² statistics. I² values 25-50% were considered indicative of low heterogeneity, 50-75% were considered moderate heterogeneity and values above 75% were considered to have a high degree of heterogeneity. A p value < 0.05 was considered statistically significant.
Results
Study selection
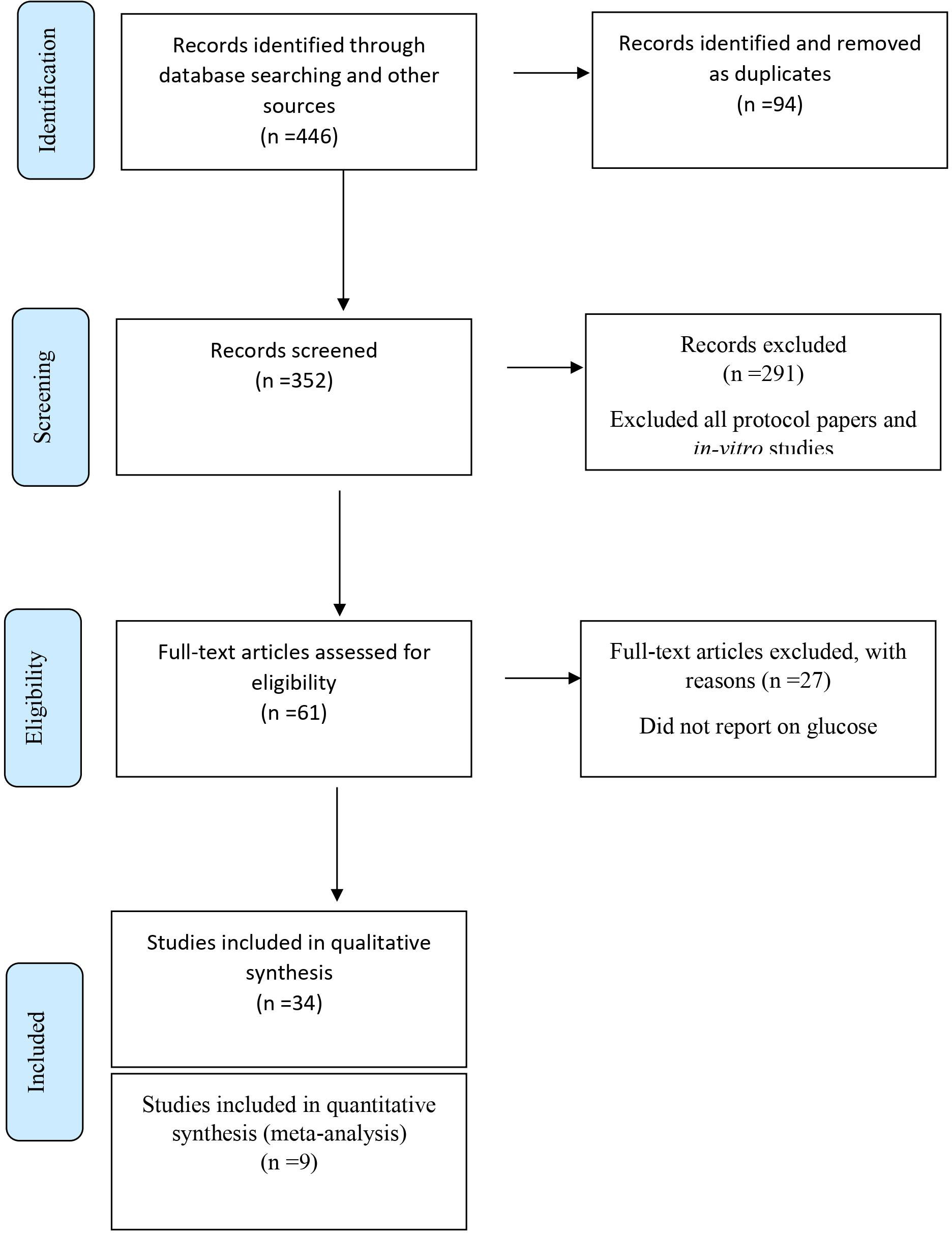
The PRISMA flow diagram details the database search results along with all exclusion rationale (Figure 1). Of the 446 original identified studies through the database search, 385 studies were excluded for having been identified as a duplicate, protocol paper, in-vitro, or animal studies. Of the remaining 61 studies, 27 studies were removed after a thorough full-text assessment revealed that studies did not report on primary outcome measures. The remaining 34 studies met the inclusion criteria for the systematic review, while nine randomized controlled longitudinal studies met inclusion criteria for the meta-analysis.
Population characteristics
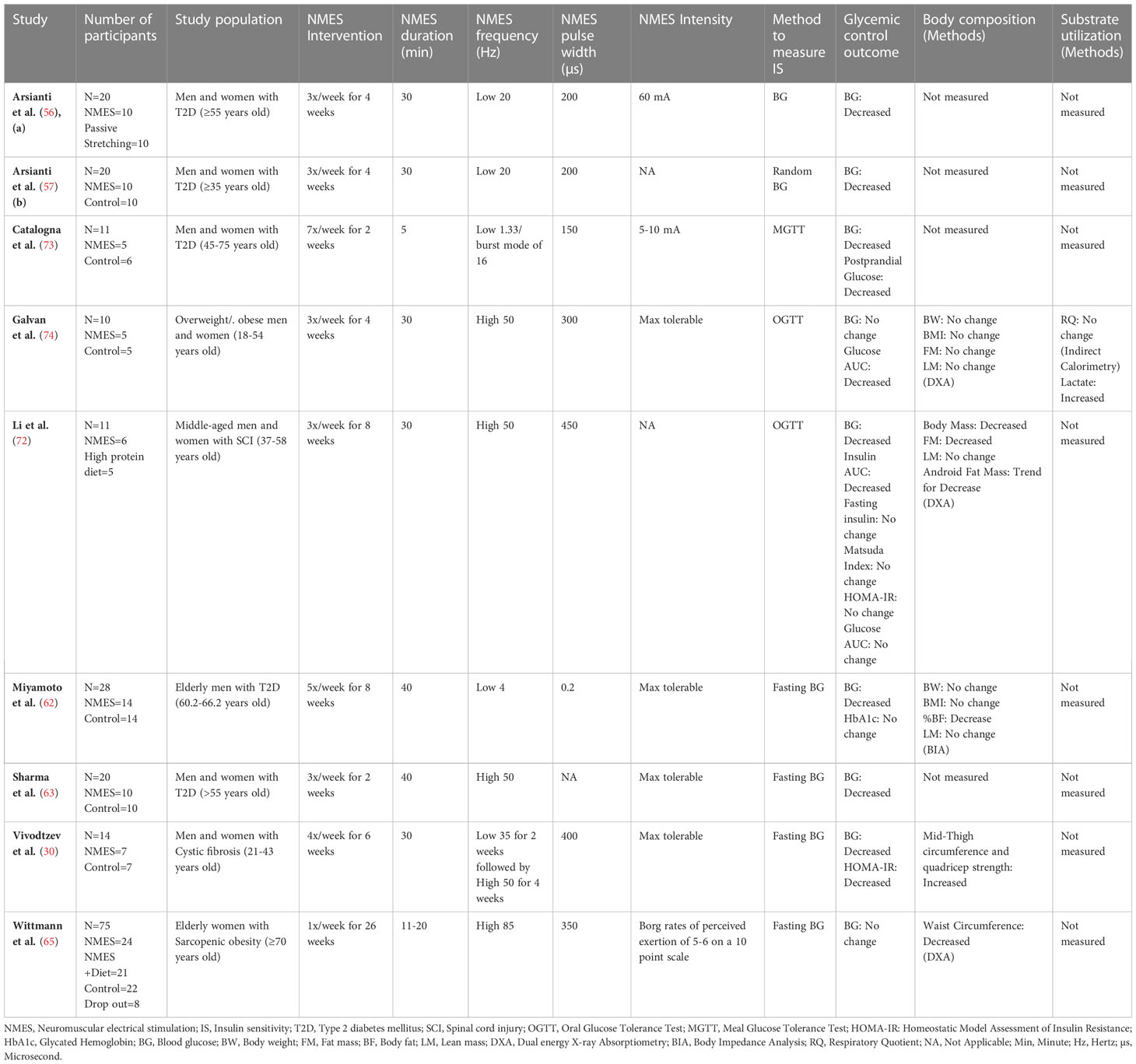
Population characteristics of the reviewed studies are described in Table, Supplementary Table 2. The 34 studies in this systematic review were conducted on healthy individuals (n=7) and populations with obesity (n=3), T2D (n=14), SCI (n=9), and cystic fibrosis (n=1). Data from these 34 studies consisted of a total of 527 young, middle age, and elderly healthy weight, obese, population with T2D or SCI where sample size in each intervention study ranged from 5-75. Among all included studies, 20 studies included male and female subjects whereas 12 studies were conducted on only male subjects and two studies were conducted on only female subjects. Nine randomized controlled studies included in the meta-analysis consisted of a total of 180 young healthy weight, obese, population with T2D or SCI where sample size in each intervention ranged from 9-46. In the meta-analysis a total of 91 participants were allocated to NMES group while 89 participants were allocated to control/placebo group.
Study design and methods used to measure primary outcome
Study characteristics and testing methods used to measure glycemic control, substrate utilization, and body composition in all studies included in this systematic review are outlined in Table, Supplementary Table 2. Thirteen studies reported on acute effects and 22 studies reported on chronic effects of NMES, with one of those studies reporting on both acute and chronic effects of NMES. Studies reported one or multiple measures of glycemic control/insulin sensitivity which included the fasting blood glucose (n=17) (30, 50–52, 56–68), fasting insulin (n=11) (23, 31, 50–52, 60, 61, 66, 69–71), homeostatic model assessment index (HOMA-IR) (n=4) (50, 52, 60, 70), Matsuda index (n=1) (72), oral glucose tolerance test (OGTT) (n=11) (23, 29, 31, 45, 69–75), meal glucose tolerance test (MGTT) (n=1) (73), HbA1c (n=5) (50, 58, 64, 69, 76), and hyperinsulinemic euglycemic clamp (n=6) (69, 71, 75–78). Among all longitudinal studies that met the inclusion criteria for meta-analysis (N=9) reported fasting blood glucose before and after NMES intervention. Additionally, other relevant insulin sensitivity measures were also reported. This includes fasting insulin (n=4) (30, 72, 73, 79), HOMA-IR (n=3) (30, 72, 79), Matsuda index (n=1) (72), MGTT (n=1) (73), OGTT (n=2) (72, 74), Glucose area under the curve (AUC) (n=3) (72, 74, 79), Insulin area under the curve (AUC) (n=2) (72, 79), and Glycated Hemoglobin (HbA1c) (n=2) (30, 62).
Overview of neuromuscular electrical stimulation protocol
The NMES protocols used in included studies are outlined in Table, Supplementary Table 2. This includes information reported on length of NMES interventions, number of sessions, duration of sessions, frequency, and intensity. NMES frequency below 50 Hz has generally been considered as a low frequency (45, 49, 77), and a frequency of 50 Hz or above is considered as high frequency (49, 70, 74, 80, 81) in existing literature. Therefore, alongside presenting the specific frequency reported in articles, we have also reported frequency as “low” or “high”. Twenty-three studies used low frequency, eight studies used high frequency, two studies reported using both low and high frequency, while one study did not specify the selected frequency for NMES application. Although most studies reported on frequency and duration, NMES intensity was inconsistently reported across the studies. Most of the studies reported intensity as up to maximum tolerable levels (n=14), whereas some studies reported a range from 5-140 mA (n=8). Maximum tolerable intensity level usually varies from one person to another (82). One study reported intensity by oxygen consumption, and eleven studies did not report on NMES intensity. Most studies ranged from 2-8 weeks in duration of NMES intervention. Among the nine studies included in meta-analysis, four studies used low frequency, four studies used high frequency, while one study used both low and high frequency for NMES application. Four studies reported intensity as up to maximum tolerable level, one reported intensity at 60 mA, one study reported intensity at 5-10 mA, and three studies did not report NMES intensity. Duration of NMES session and length of NMES intervention also varied among studies included in meta-analysis. Majority of the studies reported session times ranging from 5-40 minutes with the most common intervention duration lengths of 2-8 weeks.
Risk of bias
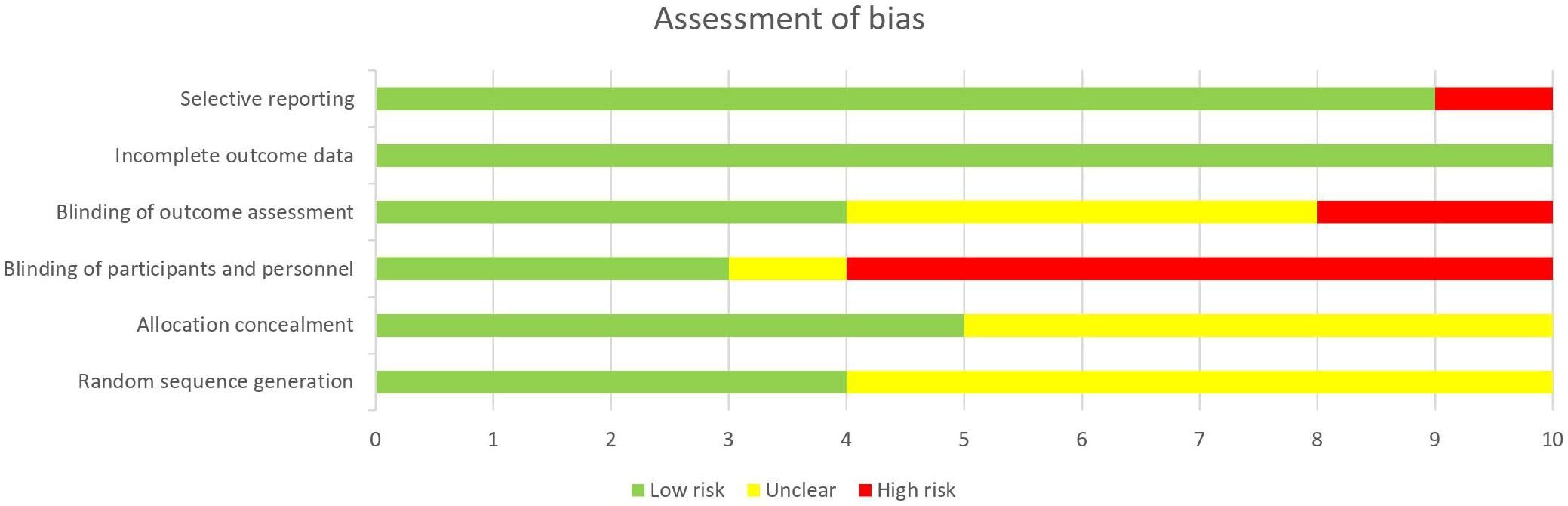
Figure 2 summarizes the assessment of quality and risk of bias of the studies. All reviewers used the Cochrane Collaboration’s risk of bias (RoB) tool to evaluate each study for risk of bias. Risk of bias assessment reported an overall outcome of low to moderate.
Outcome of included studies
Acute effects of NMES on glycemic control
Among 34 studies included in this systematic review, 13 studies investigated acute effects of NMES on glycemic control (including a study reporting both acute and chronic effects) in populations with hyperglycemia and T2D (n=5), obesity (n=1), as well as in a healthy population (n=7). For the population with Obesity or T2D, all studies reported NMES being effective at acutely improving glycemic control. In a healthy population, five (52, 67, 68, 77, 78) out of the seven studies reported an improvement in glycemic control. Overall, six studies (45, 50–52, 61, 66) reported a significant decrease in blood glucose and three studies reported an increase in glucose disposal measured during hyperinsulinemic euglycemic clamp (76–78) with acute application of NMES. Overall, present evidence strongly indicates increased glucose utilization during NMES application.
Chronic effects of NMES on glycemic control
There were 22 longitudinal studies that investigated the chronic effects of NMES on glycemic control were included in this systematic review. Except for four studies that investigated young adult population (30, 59, 69, 74), all studies were conducted in middle-aged and elderly men and women. Majority of the studies (n=16) reported improvement in glycemic control measured by various methods including fasting blood glucose (56–58, 60, 62, 63), OGTT (23, 29, 31, 72, 79), MGTT (73), HbA1c (64, 70), and hyperinsulinemic euglycemic clamp (75, 76), while two studies reported no changes in glycemic control as measured by fasted blood glucose (65), and hyperinsulinemic euglycemic clamp (71).
Meta-analysis
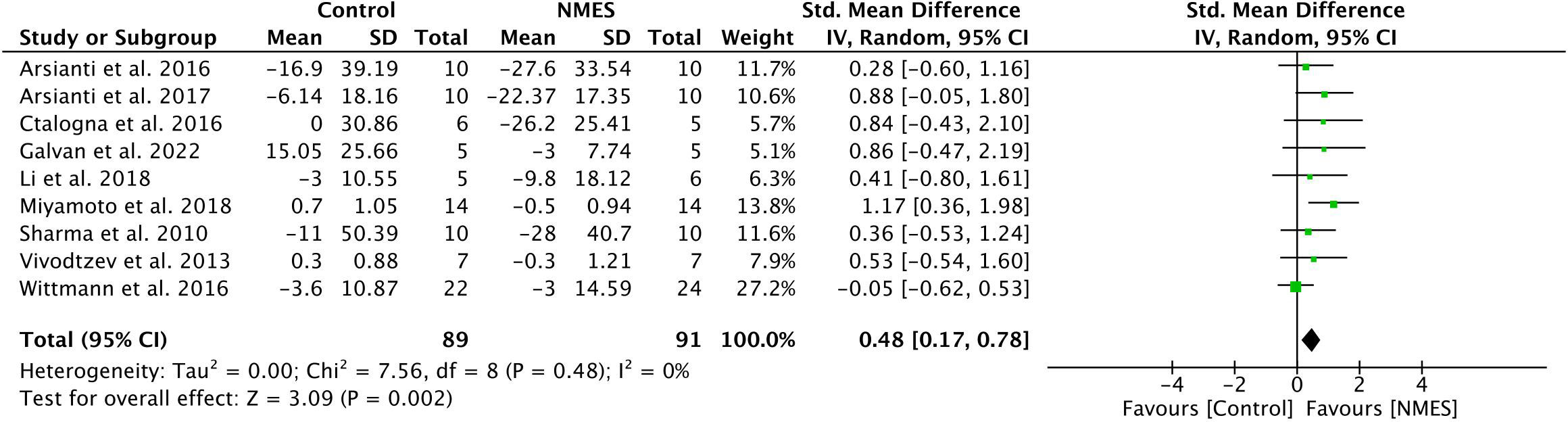
Nine longitudinal studies met the inclusion criteria for a meta-analysis to examine the effectiveness of NMES on glycemic control, measured by fasting blood glucose (Table 1). The meta-analysis determined a significant effect of NMES on lowering fasting blood glucose (SMD: 0.48; 95% CI: 0.17 to 0.78; p=0.002; I²=0%) (Figure 3). The methods used to assess glycemic control varied among studies. Two studies (72, 74) used OGTT, one study used HOMA-IR (30) whereas rest of the studies used blood glucose (56, 57, 62, 63, 65, 73) to assess glycemic control. Additionally, one study (79) that met all the inclusion criteria and reported glycemic control measured by OGTT but did not report fasting glucose level before and after the NMES intervention. Therefore, we performed additional analysis (n=10) using the data from primary measures reported by each study to indicate glycemic control (i.e., OGTT, HOMA-IR, and fasting glucose). This analysis also indicated a significant effect of NMES on improving glycemic control (SMD: 0.41; 95% CI: 0.09 to 0.72; p=0.01; I²= 11%) (See Figure, Supplementary Figure 1, a forest plot indicating effects of NMES on glycemic control).
Effect of NMES on substrate utilization
To our knowledge seven studies have reported the acute effects of NMES on substrate utilization measured by Respiratory Quotient (RQ) or Respiratory Exchange Ratio (RER), oxygen consumption (VO2), and lactate production. All these studies indicate increased glucose utilization during NMES application as measured by increased RQ (50, 51, 77, 78, 83, 84), increased lactate level (50, 51, 61, 77, 78) or elevated oxygen utilization (50, 51, 77, 78, 83) (see Table, Supplementary Table 2). Additionally, three studies reported on energy expenditure during NMES. Two of these studies reported an increase (69, 84), and one study reported no change (76) in energy expenditure. Only one study, to our knowledge, investigated the chronic effects of NMES on substrate utilization (74) and reported no change in resting substrate utilization and energy expenditure after four weeks of NMES.
Body composition
Among all studies that met inclusion criteria for this systematic review, nine studies reported on body composition parameters at baseline and at the end of the NMES intervention (see Table, Supplementary Table 2). Six studies (23, 58, 72, 74, 75, 79) used dual-energy X-ray absorptiometry (DXA), and three studies (62, 64, 69) used bio-electrical impedance assessment (BIA) to assessed body composition. No significant changes in body composition were reported in majority of the studies (64, 69, 74, 79). Two studies (58, 60) reported a significant reduction in total body weight and body fat without any changes in lean body mass after 8 and 12 weeks of NMES intervention. One study reported significant increase in body mass and lean muscle mass (23) and one study (62) reported a significant decrease in body fat without any change in body weight and lean mass after NMES treatment. One study (72) that combined NMES with exercise reported a significant decrease in body mass and fat mass, as well as a trend for decrease in android fat mass.
Discussion
The aim of this systematic review and meta-analysis was to investigate the effects of NMES on glycemic control. Based on the existing evidence and meta-analysis, we conclude that acute application of NMES is effective in improving glucose utilization, while chronic use of NMES is effective in improving glycemic control particularly in populations with physical impairments and/or metabolic disorders such as T2D, obesity, and/or SCI.
NMES is an alternate strategy to induce skeletal muscle contraction and has been widely used in rehabilitation settings to prevent muscle atrophy and loss of muscular strength (21, 23, 27, 29, 85). Equivalent to the effect of an action potential seen in voluntary muscle contraction, the electrical current from NMES also results in changes to the membrane potential, which in turn releases calcium and initiates the signaling cascade leading to skeletal muscle contraction. The energy requiring mechanism that is seen in voluntary muscle contraction is also seen with the use of NMES, thus implying a possible beneficial effect by its potential to increase energy expenditure (69). Elevated ATP utilization has been demonstrated in skeletal muscle after intermittent NMES application (86). Interestingly, a greater anaerobic ATP turnover has been reported with electrically induced contractions, compared to equivalent voluntary contractions of the soleus and gastrocnemius muscles (87). Several studies indicated use of glycolytic sources as major substrates during NMES induced muscle contractions (49, 56–58, 73, 74). Increased glucose uptake has been shown with electrical stimulation using cell culture model (88) as well as in isolated rat skeletal muscle (47).
Downregulation of insulin dependent glucose uptake pathway has often been reported in upstream signaling molecules such as insulin receptor substrate 1, phosphatidylinositol 3-kinase, and Akt phosphorylation (89) with little to no impact on GLUT4 content and GLUT4 translocation in population with insulin resistance and T2D (90). Increase in GLUT4 content and GLUT4 translocation to cell membrane are well established mechanisms to increase glucose uptake during exercise in healthy and people with T2D (90, 91). Muscle contraction induced by electrical stimulation has also been shown to effectively increase AMP-activated protein kinase-α, Ca+2/calmodulin-dependent protein kinase II CaMKII and Akt phosphorylation (89), upregulate GLUT4 content and translocation (31), deplete muscle glycogen (80), increase glucose uptake from peripheral circulation (45, 50–52, 61, 66, 77, 78) and increase whole body glucose utilization (50, 51, 61, 74, 77, 78). Therefore, NMES seems to have great potential to be used as an effective alternate strategy to improve glycemic control especially in physically inactive population with insulin resistance and T2D via insulin independent glucose uptake pathway.
Acute effects of NMES on glycemic control
Existing evidence strongly suggests that NMES application utilizes carbohydrates as fuel and acutely increases glucose utilization in healthy, T2D, and SCI populations. Several studies reported a decrease in blood glucose level (45, 50–52, 59, 61, 66–68, 76–78), an increase in whole body glucose utilization as measured by rise in RQ (50, 51, 77, 78), increased lactate production (50, 51, 67, 77, 78), and elevated glucose uptake as measured by hyperinsulinemic euglycemic clamp (76–78) with NMES application. In addition to an increased glucose disposal rate, Hamada et al. demonstrated maintaining this elevated glucose uptake for at least 90 minutes following the cessation of NMES application, which also showed a greater requirement for glucose during the poststimulation period (77). Other studies, however, did not observe any significant acute effect of NMES on postprandial glucose level among healthy active participants (69, 83, 84). Combining whole body NMES with voluntary exercise has also shown an additive effect in elevating RQ and increasing blood lactate concentration (92). Holzer et al. however reported no significant additive effect of whole body NMES with resistance training on postprandial glucose measured by continuous glucose monitor (CGM) (93), a device. Additionally, increased Akt phosphorylation which is associated with contraction-induced GLUT4 translocation signaling mechanism (89) and recruitment of predominately glycolytic type II muscle fibers (36) have also been reported during NMES. These findings suggest the potential of NMES to maintain and enhance the function of type II muscle fibers in glucose uptake independent of insulin signaling. Further studies are needed to fully elucidate the exact mechanism by which NMES impacts blood glucose levels and glucose disposal rates in both healthy individuals and people with dysglycemia. Most of the above-mentioned studies reported increases in glucose utilization regardless of NMES intensity (low or high) and duration.
Effectiveness of NMES on glucose utilization was evident in healthy as well as population with T2D. However, studies in young and healthy/obese populations are limited and it is unknown if NMES could acutely affect blood glucose levels in these populations. Most studies in this review used low frequency stimulation. Only three of the studies; Poole et al. (50 Hz) (69), Wall et al. (60 Hz) (66) and Guzman et al. out of the 13 studies applied high stimulation frequencies. Wall et al. reported a decrease in blood glucose with no change in insulin level while Poole et al. study showed no significant change in neither blood glucose nor glucose disposal rate, with both studies involving small sample sizes of 6 and 5 respectively. Guzman et al. reported a significant hypoglycemic effect of 5 Hz contrary to higher frequencies (10 Hz and 50 Hz). Future investigation should determine optimum frequency, intensity, and duration for NMES that could be beneficial for populations with hyperglycemia and insulin resistance.
Chronic effects of NMES on glycemic control
To the best of our knowledge, this is the first systematic review with meta-analysis to investigate the effectiveness of NMES on chronic glycemic control. The majority of the studies in this systematic review indicated acute and chronic improvements in glycemic control after NMES interventions. Meta-analysis with randomized controlled trials strongly suggests that NMES can be used effectively as an alternative strategy to improve chronic glycemic control. It should be noted that longitudinal studies that met inclusion criteria for the meta-analysis, were limited to populations with obesity, T2D, SCI, and cystic fibrosis. Future investigation should focus on studying the effectiveness of NMES in normoglycemic populations. Methods used to assess glycemic control also varied across the studies. However, all but one study (79), reported on fasting blood glucose before and after the NMES intervention. A significant decrease in fasting blood glucose was reported by all studies, with the exception of Wittman et al. (65) and Galvan et al. (74). This lack of improvement in the Wittman et al. study may be due to the NMES parameters prescribed which included treatment once a week for 26 weeks at an inconsistent intensity. Furthermore, the elderly female participants in this study had sarcopenic obesity which is characterized by a progressive loss of muscle mass and a high fat mass which may explain why an improvement in glycemic control was not reported in this particular population. Galvan et al. study was conducted in people without diabetes with a small sample size which might be the reason why no improvement in fasting blood glucose was observed. Their study however, showed improvement in glucose tolerance. Specific NMES protocol should be further investigated to determine the effectiveness of NMES in this special population. Studies that met the inclusion criteria for meta-analysis, also used various methods to measure insulin sensitivity (OGTT, HOMA-IR, and fasting glucose). It is unknown whether the methods used to assess insulin sensitivity in the studies impacted the ability to detect changes in glycemic control brought about by NMES. However, as our analysis strongly suggest an impact of NMES on reducing fasting glucose, it can be expected that the effectiveness can be confirmed using more sensitive methods to measure insulin sensitivity. With the exception of Wittman et al. (65), all studies in the meta-analysis reported favoring NMES as an effective intervention in improving glycemic control, regardless of frequency (low or high), varied session times, duration and intensity. Therefore, NMES intervention was shown to be an effective alternative strategy to improve glycemic control in population with metabolic diseases and mobility limitations.
Effects of NMES on substrate utilization and body composition
Although the primary purpose of this review was to determine effects of NMES on glycemic control, we also explored the effects of NMES on whole body substrate utilization and body composition. A greater reliance on whole body fat oxidation and metabolic flexibility has been well established with insulin sensitivity (94). RER has been shown to increase with exercise intensity and has been referred to as an indirect method of revealing the oxidative capacity of skeletal muscle (68, 93). Lower RER values and higher oxidative metabolism has been observed in trained males when compared to untrained males at similar submaximal workloads (67). Given skeletal muscle is the largest site for insulin stimulated glucose uptake and has been associated with insulin sensitivity (23, 29, 79), we aimed to determine if NMES is also effective in improving whole body substrate utilization and body composition. There were only six studies that reported on whole body substrate oxidation during acute use of NMES, measured by indirect calorimetry (50, 51, 61, 74, 77, 78). These studies indicated increase in whole body carbohydrate utilization during NMES. Two other studies reported an increase in whole body carbohydrate utilization using a Douglas Bag method (84) while Cohen et al. showed an additive effect of NMES with blood flow restriction on carbohydrate utilization (83). An increased whole body oxygen uptake measured by RER has been noted at the onset of low frequency electrical stimulation, followed by an immediate return to resting levels at the termination of stimulation (77, 78). There is lack of longitudinal studies that assessed effects of chronic use of NMES on whole body substrate utilization. To the best of our knowledge, there is only a pilot study that investigated the chronic effects of NMES on whole body substrate utilization and reported no effects from NMES (74). Effects of chronic use of NMES on body composition is also limited. Most studies indicated that there was either no change in body composition or no gain in lean muscle mass (62, 72, 74).. NMES has been shown to increase lean body mass, as well as muscle force and strength when stimulated at a frequency of at least 50 Hz in patients with SCI (23). Overall, the limited data indicates no change in body composition after NMES use with some indication of improvement in muscle mass in patients with SCI. Future studies should investigate long term effects of NMES sessions on substrate utilization and body composition to understand if the NMES induced improvement in glycemic control can be achieved independent of concurrent improvements in substrate utilization and/or muscle mass. Future studies should also consider evaluating the effects of NMES on other cardiovascular health aspects such as blood flow, breathing rate, oxygen saturation, heart rate, and blood pressure to fully understand the implication of NMES on various components of cardiovascular health.
Recommended NMES protocol to improve glycemic control
Although our findings strongly suggest the effectiveness of NMES to improve glycemic control, a specific recommendation of NMES protocol has not been established. Lack of randomized controlled trials along with varied study populations make this challenging to determine an effective recommendation. Present literature indicates both low and high intensity NMES with varied frequency has been effective in acutely increasing glucose utilization as well as improving insulin sensitivity. While considering the NMES protocol, it is important to recognize safety and comfort of the individuals, and the target population (e.g., insulin resistant or having physical limitations to perform physical activities etc.). The side effects and discomforts with NMES use are not clearly described in most studies. While some studies indicated that participants did not report significant pain with NMES use (45, 95), physical discomfort, pain, or low subjective tolerance were reported in few studies (96–99). This is particularly seen with high frequency and high intensity, which is also known to increase muscle fatigue (100). A higher NMES intensity has been shown to result in a greater glucose uptake when compared to lower intensities (45). Significant correlations between stimulation intensity and blood glucose levels revealed that the contraction intensity substantially contributes to acute glucose metabolism (45). Poor tolerability is often explained as a limiting factor in many previous studies. Majority of the reviewed studies used a maximum tolerable intensity, which indicates a subjective intensity that varied across studies and participants. The option for the participants to select a maximum tolerability has been shown to lead to better compliance but less consistency with the given intervention. Tolerability and compliance should be considered when developing an NMES intervention. Therefore, it has been suggested that a low frequency NMES can be employed in attempt to minimize subject discomfort while still achieving efficiency and largely activating glycolytic type II muscle fibers that substantially utilize carbohydrate and glycogen, while improving insulin sensitivity (36, 45, 76, 78). Jabbour et al., reported a significant decrease in glucose concentrations after an acute (1 hour) session of low frequency NMES (8 Hz) in a middle-aged population with T2D and reported to be tolerable by all participants (45). Several studies that used low frequency NMES, reported increases in glucose utilization with variable NMES intensities (29, 31, 45, 50–52, 56–59, 61, 62, 70, 73, 75–79). These findings are also supported by Joubert et al., 2015 (76), demonstrating that after a single 25-minute session of low frequency (35 Hz) NMES, a significant increase was reported in glucose uptake as measured by the hyperinsulinemic euglycemic clamp in a population with T2D. On the other hand, when a chronic high frequency protocol was applied to individuals with T2D, no significant changes to glucose uptake was reported. It was also noted that participants were unable to tolerate NMES intensities above 40 mA (approximately 10% of maximum voluntary contraction) (69). In rehabilitation settings, NMES is commonly used to aid in completion of functional tasks and therefore intensity of NMES varies in general practice. Previous studies adjusted the treatment intensity to achieve the desired outcome of visible muscle contractions, cadence count, or amount of oxygen consumption (23, 50, 70, 79). Present literature also supports the improvement in glycemic control when high frequency NMES was used in populations with T2D or SCI. High frequency NMES has been shown to be effective in majority of the studies (23, 30, 59, 63, 64, 66, 72, 74), except for two studies (65, 69) that have not only used a low intensity, but also used NMES only once a week, which might explain the lack of improvements in glycemic control reported by these two studies. Moreover, one of these studies utilized the Borg rates of perceived exertion of 5-6 on a 10-point scale that relied on the participants rating/selecting their treatment intensity and could have led to inconsistencies and varied intensities throughout the study (65). As previously mentioned, the other study utilized a 40 mA intensity which is equivalent to ~10% of a maximum voluntary contraction (69). Limited studies have specifically investigated the role of NMES frequency and intensity on glycemic control. Most of the studies that reported improvement in glycemic control have used a duration of 20-30 minutes a session, between 2-3 times per week and 4-8 weeks NMES intervention (see Table, Supplementary Table 2).
Taken together, the present evidence suggests that regardless of frequency and intensity used, NMES is effective in improving glycemic control. NMES used between 2-3/week with a minimum duration of 2 weeks seems to effectively improve glycemic control in populations with insulin resistance or those who are unable to adhere to regular exercise or physical activity.
Clinical implications
The main clinical applications for NMES have focused on rehabilitation for muscle strengthening, maintenance of muscle mass and strength during prolonged periods of immobilization, selective muscle training, and control of edema (21). Using these same endpoints, NMES is being used for body contouring in aesthetic medicine to selectively increase muscle mass and tone in muscle groups of the body (66, 84). NMES is also being used to increase blood flow in the extremities to avoid blood clots and promote wound healing (83). Understanding that NMES can improve glycemic control, it has a potential to be incorporated into the multidimensional (diet, exercise, behavior modification, and pharmaceuticals) treatment of obesity, metabolic syndrome, and type 2 diabetes as a complement or when traditional exercise modalities are not feasible. Further research will be necessary to determine the ideal treatment protocols.
Our study is not without limitations. First, our meta-analysis is limited by small number of randomized controlled trials that has been conducted to determine the effects of NMES on glycemic control. However, this is the first comprehensive review that has reviewed all existing literature to address the effectiveness of NMES on improving insulin sensitivity. The systematic review with meta-analysis strongly indicates the effectiveness of NMES in improving glycemic control and insulin sensitivity. Second, two studies included in this meta-analysis incorporated exercise in addition to NMES treatment (72, 79). However, the outcome does not change when those two studies were excluded from the analysis. Third, most of the studies that utilized NMES were predominantly conducted in population with T2D and SCI. Therefore, present evidence may not be translatable to all population. Future studies should investigate the effectiveness of NMES in healthy population.
In summary, this is the first comprehensive systematic review with meta-analysis to determine the effects of NMES on glycemic control. Our analysis strongly suggests that NMES can effectively improve glycemic control, mainly in population with T2D and those incapable of doing regular traditional exercises (people with SCI). Present literature is not adequate to conclude the effects of NMES on substrate utilization or body composition. Our results strongly suggest the promising potential of NMES use as an alternative therapeutic to improve glycemic control. This systematic review and meta-analysis will serve as a groundwork for many future studies that employ the use of NMES and its evidence-based effectiveness on improving glycemic control in populations with impairments in glycemic control and/or physical limitations.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author contributions
MS, MG, AM, and SS did the initial search and selected articles for the study. JA did additional search. All reviewers assessed the selected articles for inclusion. All authors contributed to the writing and revision of the article and approved the submitted version.
Funding
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health under award number R01DK132430 (SB). This work was also supported by Grant 5U54MD007592 from the National Institutes on Minority Health and Health Disparities (NIMHD), of the National Institutes of Health (NIH).
Acknowledgments
The authors would like to acknowledge the librarian from the University of Texas at El Paso Jacob Galindo for assisting with the collection process of articles, accessing the articles, and reference formatting.
Conflict of interest
Author FA was employed by the company Southwest Plastic Surgery.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1222532/full#supplementary-material
References
1. Must A, Spadano J, Coakley EH, Field AE, Colditz GD, William H. The disease burden associated with overweight and obesity. JAMA (1999) 282(16):1523–9. doi: 10.1001/jama.282.16.1523
2. Burton BT, Foster WR, Hirsch J, Van Itallie TB. Health implications of obesity: an NIH Consensus Development Conference. Int J Obes (1986) 10(1):155–76.
3. World Health Organization. Obesity and Overweight (2020). Available at: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
4. Centers For Disease Control and Prevention. Insulin resistance and diabetes: Centers For Disease Control and Prevention (2021). Available at: https://www.cdc.gov/diabetes/basics/insulin-resistance.html.
5. Myers A, Gibbons C, Finlayson G, Blundell J. Associations among sedentary and active behaviours, body fat and appetite dysregulation: investigating the Myth of physical inactivity and obesity. Br J Sports Med (2017) 51(21):1540–4. doi: 10.1136/bjsports-2015-095640
6. Díaz-Martínez X, Petermann F, Leiva AM, Garrido-Méndez A, Salas-Bravo C, Martínez MA, et al. Association of physical inactivity with obesity, diabetes, hypertension and metabolic syndrome in the Chilean population. Rev Medíca Chile (2018) 146(5):585–95. doi: 10.4067/s0034-98872018000500585
7. Ding D, Lawson KD, Kolbe-Alexander TL, Finkelstein EA, Katzmarzyk PT, Van Mechelen W, et al. The economic burden of physical inactivity: a global analysis of major non-communicable diseases. Lancet (British edition) (2016) 388(10051):1311–24. doi: 10.1016/S0140-6736(16)30383-X
8. Centers For Disease Control and Prevention. Physical Activity-Why it matters (2020). Available at: https://www.cdc.gov/physicalactivity/about-physical-activity/why-it-matters.html.
9. Zhao M, Veeranki SP, Magnussen CG, Xi B. Recommended physical activity and all cause and cause specific mortality in US adults: prospective cohort study. BMJ (2020) 370:m2031. doi: 10.1136/bmj.m2031
10. Dansa M, Rodrigues VHP, Oliveira TR. 2 - Blood glucose regulation in patients with type 1 diabetes by means of output-feedback sliding mode control. In: Azar AT, editor. Control Applications for Biomedical Engineering Systems. Academic Press (2020). p. 25–54. doi: 10.1016/B978-0-12-817461-6.00002-0
11. Gao J, Gulve EA, Holloszy JO. Contraction-induced increase in muscle insulin sensitivity: requirement for a serum factor. Am J Physiol - Endocrinol And Metab (1994) 266(2):186–92. doi: 10.1152/ajpendo.1994.266.2.E186
12. Polly AH, Lorraine AN, May MC, John OH. Increased GLUT-4 translocation mediates enhanced insulin sensitivity of muscle glucose transport after exercise. J Appl Physiol (1998) 85(4):1218–22. doi: 10.1152/jappl.1998.85.4.1218
13. Eric Arthur G. Exercise and glycemic control in diabetes: benefits, challenges, and adjustments to pharmacotherapy. Phys Ther (2008) 88(11):1297–321. doi: 10.2522/ptj.20080114
14. Nikolić N, Skaret Bakke S, Tranheim Kase E, Rudberg I, Flo Halle I, Rustan AC, et al. Electrical pulse stimulation of cultured human skeletal muscle cells as an in vitro model of exercise. PLoS One (2012) 7(3):e33203. doi: 10.1371/journal.pone.0033203
15. Santos JM, Benite-Ribeiro SA, Queiroz G, Duarte JA. The interrelation between aPKC and glucose uptake in the skeletal muscle during contraction and insulin stimulation. Cell Biochem Funct (2014) 32(8):621–4. doi: 10.1002/cbf.3081
16. Bergquist A, Clair J, Lagerquist O, Mang C, Okuma Y, Collins D. Neuromuscular electrical stimulation: implications of the electrically evoked sensory volley. Eur J Appl Physiol (2011) 111(10):2409–26. doi: 10.1007/s00421-011-2087-9
17. Samiee F, Zarrindast M-R. Effect of electrical stimulation on motor nerve regeneration in sciatic nerve ligated-mice. Eur J Trans Myology (2017) 27(3):6488. doi: 10.4081/ejtm.2017.6488
18. Blair EA, Erlanger J. A comparison of the characteristics of axons through their individual electrical responses. Am J Physiol (1933) . p:524–64. doi: 10.1152/ajplegacy.1933.106.3.524
19. Collins DF. Central contributions to contractions evoked by tetanic neuromuscular electrical stimulation. Exercise Sport Sci Rev (2007) 35(3):102–9. doi: 10.1097/jes.0b013e3180a0321b
20. Doucet BM, Lam A, Griffin L. Neuromuscular electrical stimulation for skeletal muscle function. Focus: Biomed Eng (2012) 85(2):201–15.
21. Lake DA. Neuromuscular electrical stimulation. An overview and its application in the treatment of sports injuries. Sports Med (Auckland NZ) (1992) 13(5):320–36. doi: 10.2165/00007256-199213050-00003
22. Sheffler LR, Chae J. Neuromuscular electrical stimulation in neurorehabilitation. Muscle Nerve (2007) 35(5):562–90. doi: 10.1002/mus.20758
23. Griffin L, Decker MJ, Hwang JY, Wang B, Kitchen K, Ding Z, et al. Functional electrical stimulation cycling improves body composition, metabolic and neural factors in persons with spinal cord injury. J Electromyography Kinesiology (2008) 19(4):614–22. doi: 10.1016/j.jelekin.2008.03.002
24. Cabric M, Appell HJ. Effect of electrical stimulation of high and low frequency on maximum isometric force and some morphological characteristics in men. Int J Sports Med (1987) 8(4):256–60. doi: 10.1055/s-2008-1025665
25. Fitzgerald GK, Piva SR, Irrgang JJ. A modified neuromuscular electrical stimulation protocol for quadriceps strength training following anterior cruciate ligament reconstruction. J Orthopaedic Sports Phys Ther (2003) 33(9):492–501. doi: 10.2519/jospt.2003.33.9.492
26. Paz P, Oliveira TR, Pino AV, Fontana AP. Model-free neuromuscular electrical stimulation by stochastic Extremum seeking. IEEE Trans Control Syst Technol (2020) 28(1):238–53. doi: 10.1109/TCST.2019.2892924
27. Dudley GA, Castro MJ, Rogers S, Apple JDF. A simple means of increasing muscle size after spinal cord injury: a pilot study. Eur J Appl Physiol Occup Physiol (1999) 80(4):394–6. doi: 10.1007/s004210050609
28. Thériault R, Boulay MR, Thériault G, Simoneau JA. Electrical stimulation-induced changes in performance and fiber type proportion of human knee extensor muscles. Eur J Appl Physiol Occup Physiol (1996) 74(4):311–7. doi: 10.1007/BF02226926
29. Mahoney ET, Bickel CS, Elder C, Black C, Slade JM, Apple D, et al. Changes in skeletal muscle size and glucose tolerance with electrically stimulated resistance training in subjects with chronic spinal cord injury. Arch Phys Med Rehabil (2005) 86(7):1502–4. doi: 10.1016/j.apmr.2004.12.021
30. Vivodtzev I, Decorte N, Wuyam B, Gonnet N, Durieu IM, Levy PM, et al. Benefits of neuromuscular electrical stimulation prior to endurance training in patients with cystic fibrosis and severe pulmonary dysfunction. Chest (2013) 143(2):485–93. doi: 10.1378/chest.12-0584
31. Chilibeck PD, Bell G, Jeon J, Weiss CB, Murdoch G, MacLean I, et al. Functional electrical stimulation exercise increases GLUT-1 and GLUT-4 in paralyzed skeletal muscle. Metabolism (1999) 48(11):1409–13. doi: 10.1016/S0026-0495(99)90151-8
32. Henneman E, Somjen G, Carpenter DO. Functional significance of cell size in spinal motorneurons. J Neurophysiol (1965) 28(3):560–80. doi: 10.1152/jn.1965.28.3.560
33. Milner-Brown HS, Stein RB, Yemm R. The orderly recruitment of human motor units during voluntary isometric contractions. J Physiol (1973) 230(2):359–70. doi: 10.1113/jphysiol.1973.sp010192
34. Vanderthommen M R, Gilles R, Carlier P, Ciancabilla F, Zahlan O, Sluse F, et al. Human muscle energetics during voluntary and electrically induced isometric contractions as measured by 31P NMR spectroscopy. Int J Sports Med (1999) 20(5):279–83. doi: 10.1055/s-2007-971131
35. Feiereisen P, Duchateau J, Hainaut K. Motor unit recruitment order during voluntary and electrically induced contractions in the tibialis anterior. Exp Brain Res (1997) 114(1):117–23. doi: 10.1007/pl00005610
36. Sinacore DR, Delitto A, King DS, Rose SJ. Type II fiber activation with electrical stimulation: a preliminary report. Phys Ther (1990) 70(7):416–22. doi: 10.1093/ptj/70.7.416
37. Hennig R, Lømo T. Firing patterns of motor units in normal rats. Nature (1985) 314(6007):164–6. doi: 10.1038/314164a0
38. Roy D, Jóhannsson E, Bonen A, Marette A. Electrical stimulation induces fiber type-specific translocation of GLUT-4 to T tubules in skeletal muscle. Am J Physiology: Endocrinol Metab (1997) 273(4):E688. doi: 10.1152/ajpendo.1997.273.4.E688
39. Johannsson E, Jensen J, Gundersen K, Dahl HA, Bonen A. Effect of electrical stimulation patterns on glucose transport in rat muscles. Am J Physiol - Regulatory Integr Comp Physiol (1996) 271(2):426–31. doi: 10.1152/ajpregu.1996.271.2.R426
40. Kugelberg E, Edstrom L. Differential histochemical effects of muscle contractions on phosphorylase and glycogen in various types of fibres: relation to fatigue. J Neurology Neurosurg Psychiatry (1968) 31(5):415–23. doi: 10.1136/jnnp.31.5.415
41. Edgerton VR, Barnard RJ, Peter JB, Simpson DR, Gillespie CA. Response of muscle glycogen and phosphorylase to electrical stimulation in trained and nontrained guinea pigs. Exp Neurol (1970) 27(1):46–56. doi: 10.1016/0014-4886(70)90200-1
42. Solomonow M. External control of the neuromuscular system. IEEE Trans Biomed Eng (1984) BME-31(12):752–63. doi: 10.1109/TBME.1984.325235
43. Vanderthommen M, Depresseux J, Dauchat L, Degueldre C, Croisier J, Crielaard J. Blood flow variation in human muscle during electrically stimulated exercise bouts. Arch Phys Med Rehabil (2002) 83(7):936–41. doi: 10.1053/apmr.2002.33226
44. Hultman E, Sjöholm H. Energy metabolism and contraction force of human skeletal muscle in situ during electrical stimulation. J Physiol (1983) 345(1):525–32. doi: 10.1113/jphysiol.1983.sp014994
45. Jabbour G, Belliveau L, Probizanski D, Newhouse I, McAuliffe J, Jakobi J, et al. Effect of low frequency neuromuscular electrical stimulation on glucose profile of persons with type 2 diabetes: A pilot study. Diabetes Metab J (2015) 39(3):264–7. doi: 10.4093/dmj.2015.39.3.264
46. Goodyear LJ, Hirshman MF, Horton ES. Exercise-induced translocation of skeletal muscle glucose transporters. Am J Physiol - Endocrinol And Metab (1991) 261(6):795–9. doi: 10.1152/ajpendo.1991.261.6.E795
47. Goodyear LJ, King PA, Hirshman MF, Thompson CM, Horton ED, Horton ES. Contractile activity increases plasma membrane glucose transporters in absence of insulin. Am J Physiol - Endocrinol And Metab (1990) 258(4):667–72. doi: 10.1152/ajpendo.1990.258.4.E667
48. Lund S, Holman GD, Schmitz O, Pedersen O. Contraction stimulates translocation of glucose transporter GLUT4 in skeletal muscle through a mechanism distinct from that of insulin. Proc Natl Acad Sci (1995) 92(13):5817–21. doi: 10.1073/pnas.92.13.5817
49. Hultman E, Spriet LL. Skeletal muscle metabolism, contraction force and glycogen utilization during prolonged electrical stimulation in humans. J Physiol (1986) 374(1):493–501. doi: 10.1113/jphysiol.1986.sp016093
50. Miyamoto T, Fukuda K, Watanabe K, Hidaka M, Moritani T. Gender difference in metabolic responses to surface electrical muscle stimulation in type 2 diabetes. J Electromyography Kinesiology (2014) 25(1):136–42. doi: 10.1016/j.jelekin.2014.06.013
51. Miyamoto T, Fukuda K, Kimura T, Matsubara Y, Tsuda K, Moritani T. Effect of percutaneous electrical muscle stimulation on postprandial hyperglycemia in type 2 diabetes. Diabetes Res Clin Pract (2012) 96(3):306–12. doi: 10.1016/j.diabres.2012.01.006
52. Man KM, Man S, Shen JL, Law KS, Chen SL, Liaw WJ, et al. Transcutaneous electrical nerve stimulation on ST36 and SP6 acupoints prevents hyperglycaemic response during anaesthesia: a randomised controlled trial. Eur J Anaesthesiology (2011) 28(6):420–6. doi: 10.1097/EJA.0b013e32833fad52
53. Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of interventions. Version 6.0. (2019), updated July 2019) ed: Cochrane. (Chichester (UK): John Wiley & Sons).
54. Moher D, Liberati A, Tetzlaff J, Altman DG, Group* P. Preferred reporting items for systematic reviews and meta-analysis: the PRISMA statement. Ann Intern Med (2009) 151(4):264–9. doi: 10.1136/bmj.b2535
55. Higgins JP, Altman DG, Gotzche PC, Junim P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk bias in randomized trials. BMJ (2011) 343:d5928. doi: 10.1136/bmj.d5928
56. Arsianti RW, Parman DH, Lesmana H. Comparison electrical stimulation and passive stretching for blood glucose control type 2 diabetes mellitus patients. AIP Conf Proc (2018) 1945:020005. doi: 10.1063/1.5030227
57. Arsianti RW, Parman DH, Lesmana H, Taufiqqurohman M. Effect of electrical stimulation in lower extremity as physical exercise in type 2 diabetes mellitus patients. Indonesian Biomed J (2018) 10(1):62–5. doi: 10.18585/inabj.v10i1.353
58. Giggins OM, Crowe L, Coughlan GF, Caulfield B. Neuromuscular electrical stimulation exercise: a potential alternative to conventional exercise in the management of type 2 diabetes. Br J Diabetes (2017) 17(2):46. doi: 10.15277/bjd.2017.127
59. Guzmán-González B, Llanos P, Calatayud J, Maffiuletti NA, Cruz-Montecinos C. Effect of neuromuscular electrical stimulation frequency on postprandial glycemia, current-related discomfort, and muscle soreness. A crossover study. Appl Physiology Nutrition Metab (2019) 44(8):1–6. doi: 10.1139/apnm-2018-0801
60. Jeon JY, Hettinga D, Steadward RD, Wheeler GD, Bell G, Harber V. Reduced plasma glucose and Leptin after 12 weeks of functional electrical stimulation–rowing exercise training in spinal cord injury patients. Arch Phys Med Rehabil (2010) 91(12):1957–9. doi: 10.1016/j.apmr.2010.08.024
61. Kimura T, Matsumoto K, Kameda N, Tanaka S, Hayashi T, Moritani T. Percutaneous electrical muscle stimulation attenuates postprandial hyperglycemia in obese and pre-obese Japanese men. Int J Sport Health Sci (2010) 8:1–6. doi: 10.5432/ijshs.20090033
62. Miyamoto T, Iwakura T, Matsuoka N, Iwamoto M, Takenaka M, Akamatsu Y, et al. Impact of prolonged neuromuscular electrical stimulation on metabolic profile and cognition-related blood parameters in type 2 diabetes: A randomized controlled cross-over trial. Diabetes Res Clin Pract (2018) 142:37–45. doi: 10.1016/j.diabres.2018.05.032
63. Sharma D, Shenoy S, Singh J. Effect of electrical stimulation on blood glucose level and lipid profile of sedentary type 2 diabetic patients. Int J Diabetes Developing Countries (2010) 30(4):194. doi: 10.4103/0973-3930.70859
64. Van Buuren F, Horstkotte D, Mellwig KP, Fründ A, Vlachojannis M, Bogunovic N, et al. Electrical myostimulation (EMS) improves glucose metabolism and oxygen uptake in type 2 diabetes mellitus patients—Results from the EMS study. Diabetes Technol Ther (2015) 17(6):413–9. doi: 10.1089/dia.2014.0315
65. Wittmann K, Sieber C, Stengel S, Kohl M, Freiberger E, Jakob F, et al. Impact of whole body electromyostimulation on cardiometabolic risk factors in older women with sarcopenic obesity: the randomized controlled FORMOsA-sarcopenic obesity study. Clin Interventions Aging (2016) 11:1697–706. doi: 10.2147/cia.S116430
66. Wall BT, Dirks ML, Verdijk LB, Snijders T, Hansen D, Vranckx P, et al. Neuromuscular electrical stimulation increases muscle protein synthesis in elderly type 2 diabetic men. Am J Physiol Endocrinol Metab (2012) 303(5):E614–23. doi: 10.1152/ajpendo.00138.2012
67. Hoshiai M, Ochiai K, Tamura Y, Tsurumi T, Terashima M, Tamiya H, et al. Effects of whole-body neuromuscular electrical stimulation device on hemodynamics, arrhythmia, and sublingual microcirculation. Heart Vessels (2021) 36(6):844–52. doi: 10.1007/s00380-020-01755-1
68. Hioki M, Kanehira N, Koike T, Saito A, Takahashi H, Shimaoka K, et al. Effect of electromyostimulation on intramyocellular lipids of the vastus lateralis in older adults: a randomized controlled trial. BMC Musculoskeletal Disord (2021) 22(1):1–569. doi: 10.1186/s12891-021-04456-6
69. Poole RB, Harrold CP, Burridge JH, Byrne CD, Holt RIG. Electrical muscle stimulation acutely mimics exercise in neurologically intact individuals but has limited clinical benefits in patients with type 2 diabetes. Diabetes Obes Metab (2005) 7(4):344–51. doi: 10.1111/j.1463-1326.2004.00400.x
70. Erickson ML, Ryan TE, Backus D, McCully KK. Endurance neuromuscular electrical stimulation training improves skeletal muscle oxidative capacity in individuals with motor-complete spinal cord injury. Muscle Nerve (2017) 55(5):669–75. doi: 10.1002/mus.25393
71. Mohr T, Dela F, Handberg A, Biering-Sorensen FIN, Galbo H, Kjaer M. Insulin action and long-term electrically induced training in individuals with spinal cord injuries. Med Sci Sports Exercise (2001) 33(8):1247–52. doi: 10.1097/00005768-200108000-00001
72. Li J, Polston KFL, Eraslan M, Bickel CS, Windham ST, McLain AB, et al. A high-protein diet or combination exercise training to improve metabolic health in individuals with long-standing spinal cord injury: a pilot randomized study. Physiol Rep (2018) 6(16):e13813–n/a. doi: 10.14814/phy2.13813
73. Catalogna M, Fishman S, Halpern Z, Ben-Shlomo S, Nevo U, Ben-Jacob E. Regulation of glucose dynamics by noninvasive peripheral electrical stimulation in normal and insulin-resistant rats. Metabolism (2016) 65(6):863–73. doi: 10.1016/j.metabol.2016.03.004
74. Galvan MJ, Sanchez MJ, McAinch AJ, Covington JD, Boyle JB, Bajpeyi S. Four weeks of electrical stimulation improves glucose tolerance in a sedentary overweight or obese Hispanic population. Endocrine Connections (2022) 11(2). doi: 10.1530/ec-21-0533
75. Jeon JY, Weiss CB, Steadward RD, Ryan E, Burnham RS, Bell G, et al. Improved glucose tolerance and insulin sensitivity after electrical stimulation-assisted cycling in people with spinal cord injury. Spinal Cord (2002) 40(3):110–7. doi: 10.1038/sj.sc.3101260
76. Joubert M, Metayer L, Prevost G, Morera J, Rod A, Cailleux A, et al. Neuromuscular electrostimulation and insulin sensitivity in patients with type 2 diabetes: the ELECTRODIAB pilot study. Acta Diabetologica (2015) 52(2):285–91. doi: 10.1007/s00592-014-0636-5
77. Hamada T, Sasaki H, Hayashi T, Moritani T, Nakao K. Enhancement of whole body glucose uptake during and after human skeletal muscle low-frequency electrical stimulation. J Appl Physiol (2003) 94(6):2107–12. doi: 10.1152/japplphysiol.00486.2002
78. Hamada T, Hayashi T, Kimura T, Nakao K, Moritani T. Electrical stimulation of human lower extremities enhances energy consumption, carbohydrate oxidation, and whole body glucose uptake. J Appl Physiol (2004) 96(3):911–6. doi: 10.1152/japplphysiol.00664.2003
79. Gorgey AS, Mather KJ, Cupp HR, Gater DR. Effects of resistance training on adiposity and metabolism after spinal cord injury. Med Sci Sports Exercise (2012) 44(1):165–74. doi: 10.1249/MSS.0b013e31822672aa
80. Simoneau JA, Lortie G, Johnson MJ, Boulay MR. Glycogen depletion of human skeletal muscle fibers in response to high-frequency electrical stimulation. Can J Appl Physiol (2003) 28(3):424–33. doi: 10.1139/h03-031
81. Yocheved L, Michal E. Effect of burst frequency and duration of kilohertz-frequency alternating currents and of low-frequency pulsed currents on strength of contraction, muscle fatigue, and perceived discomfort. Phys Ther (2008) 88(10):1167–76. doi: 10.2522/ptj.20080001
82. Oliveira TR, Costa LR, Catunda JMY, Pino AV, Barbosa W, Souza M. Time-scaling based sliding mode control for Neuromuscular Electrical Stimulation under uncertain relative degrees. Med Eng Phys (2017) 44:53–62. doi: 10.1016/j.medengphy.2017.03.001
83. Cohen JN, Kuikman MA, Politis-Barber V, Stairs BE, Coates AM, Millar PJ, et al. Blood flow restriction and stimulated muscle contractions do not improve metabolic or vascular outcomes following glucose ingestion in young, active individuals. J Appl Physiol (1985) (2022) 133(1):75–86. doi: 10.1152/japplphysiol.00178.2022
84. Chen YC, Davies RG, Hengist A, Carroll HA, Perkin OJ, Betts JA, et al. Effects of neuromuscular electrical stimulation on energy expenditure and postprandial metabolism in healthy men. Appl Physiology Nutrition Metab (2022) 47(1):27–33. doi: 10.1139/apnm-2021-0361
85. Castro MJ, Apple DF Jr., Hillegass EA, Dudley GA. Influence of complete spinal cord injury on skeletal muscle cross-sectional area within the first 6 months of injury. Eur J Appl Physiol Occup Physiol (1999) 80(4):373–8. doi: 10.1007/s004210050606
86. Chasiotis D, Bergstrom M, Hultman E. ATP utilization and force during intermittent and continuous muscle contractions. J Appl Physiol (1987) 63(1):167–74. doi: 10.1152/jappl.1987.63.1.167
87. RatkeviAius A, Mizuno M, Povilonis E, Quistorff B. Energy metabolism of the gastrocnemius and soleus muscles during isometric voluntary and electrically induced contractions in man. J Physiol (1998) 507(2):593–602. doi: 10.1111/j.1469-7793.1998.593bt.x
88. Park S, Turner KD, Zheng D, Brault JJ, Zou K, Chaves AB, et al. Electrical pulse stimulation induces differential responses in insulin action in myotubes from severely obese individuals. J Physiol (2019) 597(2):449–66. doi: 10.1113/JP276990
89. Yarar-Fisher C, Bickel CS, Windham ST, McLain AB, Bamman MM. Skeletal muscle signaling associated with impaired glucose tolerance in spinal cord-injured men and the effects of contractile activity. J Appl Physiol (2013) 115(5):756–64. doi: 10.1152/japplphysiol.00122.2013
90. Kennedy JW, Hirshman MF, Gervino EV, Ocel JV, Forse RA, Hoenig SJ, et al. Acute exercise induces GLUT4 translocation in skeletal muscle of normal human subjects and subjects with type 2 diabetes. Diabetes (1999) 48(5):1192–7. doi: 10.2337/diabetes.48.5.1192
91. Knudsen JR, Steenberg DE, Hingst JR, Hodgson LR, Henriquez-Olguin C, Li Z, et al. Prior exercise in humans redistributes intramuscular GLUT4 and enhances insulin-stimulated sarcolemmal and endosomal GLUT4 translocation. Mol Metab (2020) 39:100998. doi: 10.1016/j.molmet.2020.100998
92. Watanabe K, Yoshida T, Ishikawa T, Kawade S, Moritani T. Effect of the combination of whole-body neuromuscular electrical stimulation and voluntary exercise on metabolic responses in human. Front Physiol (2019) 10:291. doi: 10.3389/fphys.2019.00291
93. Holzer R, Schulte-Körne B, Seidler J, Predel HG, Brinkmann C. Effects of acute resistance exercise with and without whole-body electromyostimulation and endurance exercise on the postprandial glucose regulation in patients with type 2 diabetes mellitus: A randomized crossover study. Nutrients (2021) 13(12):4322. doi: 10.1007/s00380-020-01755-1
94. Kelley DE, Goodpaster B, Wing RR, Simoneau JA. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol (1999) 277(6):E1130–E41. doi: 10.1152/ajpendo.1999.277.6.e1130
95. Arsianti RW, Parman DH, Lesmana H, Taufiqqurohman M. Effect of electrical stimulation in lower extremity as physical exercise in type 2 diabetes mellitus patients. Indonesian Biomed J (2018) 10(1):62–5. doi: 10.18585/inabj.v10i1.353
96. Wittmann K, Sieber C, von Stengel S, Kohl M, Freiberger E, Jakob F, et al. Impact of whole body electromyostimulation on cardiometabolic risk factors in older women with sarcopenic obesity: the randomized controlled FORMOsA-sarcopenic obesity study. Clin Interventions Aging (2016) 11:1697–706. doi: 10.2147/cia.S116430
97. Guzmán-González B, Llanos P, Calatayud J, Maffiuletti NA, Cruz-Montecinos C. Effect of neuromuscular electrical stimulation frequency on postprandial glycemia, current-related discomfort, and muscle soreness. A crossover study. Appl Physiology Nutrition Metab (2019) 44(8):1–6. doi: 10.1139/apnm-2018-0801
98. Hioki M, Kanehira N, Koike T, Saito A, Takahashi H, Shimaoka K, et al. Effect of electromyostimulation on intramyocellular lipids of the vastus lateralis in older adults: a randomized controlled trial. BMC Musculoskeletal Disord (2021) 22(1):1–569. doi: 10.1186/s12891-021-04456-6
99. Joubert M, Metayer L, Prevost G, Morera J, Rod A, Cailleux A, et al. Neuromuscular electrostimulation and insulin sensitivity in patients with type 2 diabetes: the ELECTRODIAB pilot study. Acta Diabetologica (2015) 52(2):285–91. doi: 10.1007/s00592-014-0636-5
Keywords: e-stim, NMES, myostimulation, glucose, insulin sensitivity, muscle, metabolic health, substrate utilization
Citation: Sanchez MJ, Mossayebi A, Sigaroodi S, Apaflo JN, Galvan MJ, Min K, Agullo FJ, Wagler A and Bajpeyi S (2023) Effects of neuromuscular electrical stimulation on glycemic control: a systematic review and meta-analysis. Front. Endocrinol. 14:1222532. doi: 10.3389/fendo.2023.1222532
Received: 14 May 2023; Accepted: 12 July 2023;
Published: 31 July 2023.
Edited by:
Roger Gutiérrez-Juárez, National Autonomous University of Mexico, MexicoReviewed by:
Theodore Ciaraldi, University of California, San Diego, United StatesTiago Roux Oliveira, Rio de Janeiro State University, Brazil
Copyright © 2023 Sanchez, Mossayebi, Sigaroodi, Apaflo, Galvan, Min, Agullo, Wagler and Bajpeyi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sudip Bajpeyi, c2JhanBleWlAdXRlcC5lZHU=
 Michael J. Sanchez
Michael J. Sanchez Ali Mossayebi
Ali Mossayebi Solmaz Sigaroodi
Solmaz Sigaroodi Jehu N. Apaflo
Jehu N. Apaflo Michelle J. Galvan1
Michelle J. Galvan1 Kisuk Min
Kisuk Min Francisco J. Agullo
Francisco J. Agullo Amy Wagler
Amy Wagler Sudip Bajpeyi
Sudip Bajpeyi