- 1Department of Gynecology and Obstetrics, The Second Hospital of Jilin University, Changchun, China
- 2Department of Gastrointestinal Surgery, The Second Hospital of Jilin University, Changchun, China
- 3Department of Operating Room, The Second Hospital of Jilin University, Changchun, China
- 4Department of Anesthesiology, The Second Hospital of Jilin University, Changchun, China
Lactoferrin (Lf) is a multifunctional protein that is synthesized endogenously and has various biological roles including immunological regulation, antibacterial, antiviral, and anticancer properties. Recently, research has uncovered Lf’s critical functions in bone remodeling, where it regulates the function of osteoblasts, chondrocytes, osteoclasts, and mesenchymal stem cells. The signaling pathways involved in Lf’s signaling in osteoblasts include (low density lipoprotein receptor-related protein – 1 (LRP-1), transforming growth factor β (TGF-β), and insulin-like growth factor – 1 (IGF-1), which activate downstream pathways such as ERK, PI3K/Akt, and NF-κB. These pathways collectively stimulate osteoblast proliferation, differentiation, and mineralization while inhibiting osteoclast differentiation and activity. Additionally, Lf’s inhibitory effect on nuclear factor kappa B (NF-κB) suppresses the formation and activity of osteoclasts directly. Lf also promotes chondroprogenitor proliferation and differentiation to chondrocytes by activating the mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) and phosphoinositide 3-kinase/protein kinase B(PI3K/Akt)signaling pathways while inhibiting the expression of matrix-degrading enzymes through the suppression of the NF-κB pathway. Lf’s ability to stimulate osteoblast and chondrocyte activity and inhibit osteoclast function accelerates fracture repair, as demonstrated by its effectiveness in animal models of critical-sized long bone defects. Moreover, studies have indicated that Lf can rescue dysregulated bone remodeling in osteoporotic conditions by stimulating bone formation and suppressing bone resorption. These beneficial effects of Lf on bone health have led to its exploration in nutraceutical and pharmaceutical applications. However, due to the large size of Lf, small bioactive peptides are preferred for pharmaceutical applications. These peptides have been shown to promote bone fracture repair and reverse osteoporosis in animal studies, indicating their potential as therapeutic agents for bone-related diseases. Nonetheless, the active concentration of Lf in serum may not be sufficient at the site requiring bone regeneration, necessitating the development of various delivery strategies to enhance Lf’s bioavailability and target its active concentration to the site requiring bone regeneration. This review provides a critical discussion of the issues mentioned above, providing insight into the roles of Lf in bone remodeling and the potential use of Lf as a therapeutic target for bone disorders.
1 Introduction
Several hormones and cytokines play a crucial role in bone metabolism; some of these have become therapies for osteoporosis. For example, estrogen has been used for many years in hormone replacement therapy to prevent and treat osteoporosis in postmenopausal women (1). Parathyroid hormone (PTH) and calcitonin are also approved for the treatment of osteoporosis and have been shown to increase bone density and reduce fracture risk (2). In addition to hormones, cytokines such as receptor activator of nuclear factor-kappa B ligand (RANKL) and sclerostin have also been identified as potential targets for osteoporosis therapy. Denosumab, a monoclonal antibody that targets RANKL, and romosozumab, a monoclonal antibody that inhibits sclerostin, have both been approved for the treatment of osteoporosis and have demonstrated significant benefits in increasing bone density and reducing fracture risk (3, 4). Endogenous factors naturally occur in the body and therefore have a lower risk of side effects and toxicity. Lactoferrin (Lf) is an endogenous protein in plasma that directly impacts bone cells and modulates bone metabolism (5), making it an attractive candidate for future research for positioning it as a therapeutic target for metabolic bone diseases.
Lf is an iron-binding glycoprotein required for several biological functions, including immune function, antimicrobial activity, and tissue repair. Human serum Lf range from 0.2 to 0.5μg/ml, mostly from neutrophils (6). There is no agreement among researchers on whether there are differences in plasma Lf levels between males and females (6).Lf is remarkably resistant to proteolytic degradation by enzymes such as trypsin, allowing it to be partially resistant to digestion in the gut, likely due to glycan-dependent resistance. The iron-saturated form, holo lactoferrin, is even more resistant to proteolysis than the iron-free form, apo lactoferrin, because the iron ion stabilizes its structure, making it less vulnerable to degradation (7). Resistance to proteolytic degradation enables Lf to be absorbed by the body, making it a significant nutrient for neonatal growth.
In recent years, there has been growing interest in the potential role of Lf in skeletal homeostasis, particularly in maintaining bone health and treating bone-related disorders such as osteoporosis. Studies have shown that Lf is expressed in bone cells, including osteoblasts and osteoclasts, and can modulate bone metabolism by promoting osteoblast differentiation and mineralization, inhibiting osteoclast activity, and regulating bone remodeling (5, 8, 9). The anti-inflammatory (10) and antioxidant (11) effects of Lf could contribute to its salutary effects on bone health. Given the beneficial effects of Lf on bone cells, it has therapeutic potential for treating metabolic bone disorders such as postmenopausal osteoporosis. Lf has also been the subject of extensive research in orthopedics. In recent years, there has been growing interest in using Lf-based therapies to treat various orthopedic conditions, such as fractures, osteoporosis, and implant-associated infections. A promising area of emerging research involves the targeted delivery of Lf to bones through drug delivery methods, in order to leverage its multiple beneficial effects on skeletal health. Expression of Lf receptors on the surface of osteoblasts, which are responsible for bone formation, allows specific targeting of this protein to the bone. Several preclinical and limited clinical research that would be discussed subsequently suggests that Lf has therapeutic promise in metabolic bone disorders and orthopedic applications. The focus of this narrative review is to examine and analyze the interplay between Lf and various cellular and molecular factors involved in maintaining bone health, as well as to assess the potential therapeutic benefits of using Lf-derived molecules for treating metabolic bone diseases and orthopedic conditions.
2 An overview of varied signaling by Lf
Lf has been shown to interact with various receptors and molecules, including CD14 (12), LDL receptor-related protein-1 (LRP-1/CD91) (13, 14), intelectin-1 (omentin-1) (15), Toll-like receptors 2 and 4 (TLR4) (16), cytokine receptor 4, and heparan sulfate proteoglycans (HSPGs) (17). CD14 is a glycosylphosphatidylinositol-anchored protein that acts as a co-receptor for toll-like receptor 4 (TLR4), a receptor recognizing bacterial lipopolysaccharides (LPS) (18). Lf has been shown to bind to CD14 and enhance the recognition of LPS by TLR4, leading to the activation of immune responses (19). LRP-1/CD91 is a multifunctional cell-surface receptor involved in various biological processes, such as endocytosis, cell signaling, and cell migration (20). Lf has been shown to bind to LRP-1/CD91 and regulate the internalization and degradation of the receptor (13). Intelectin-1 (omentin-1) is a lectin-like protein involved in various biological processes, including inflammation, cell adhesion, and angiogenesis. Lf has been shown to bind to intelectin-1 and regulate its biological functions (15). TLR4 is a receptor that recognizes various microbial components, such as LPS, and activates immune responses. Lf has been shown to activate TLR4 to enhance the production of pro-inflammatory cytokines (19). Cytokine receptor 4 (CXCR4) is a G protein-coupled receptor (GPCR) involved in various biological processes, such as cell migration, proliferation, and survival. Lf has been shown to bind to CXCR4 and regulate its signaling pathways (21). HSPGs are cell-surface and extracellular matrix macromolecules involved in various biological processes, such as cell adhesion, migration, and signaling. Lf has been shown to bind to HSPGs and regulate their biological functions, such as cell adhesion and migration (17). These interactions are critical in the innate immune system and other biological processes, such as inflammation, cell adhesion, migration, proliferation, and differentiation.
3 The regulation of bone cells by Lf and its associated signaling mechanisms
3.1 Mesenchymal stem cells (MSC)
In adult mammals, MSCs make up approximately 0.01% to 0.1% of the nucleated cells of bone marrow (22–24). Bone marrow MSCs can differentiate to osteoblasts, adipocytes and chondrocytes. In adult marrow, aging or altered metabolic conditions such as diabetes cause greater adipocyte differentiation over osteoblast differentiation leading to bone loss. Estrogen and PTH are two hormones that support increased osteogenic differentiation of bone marrow MSCs and concomitantly inhibit adipogenic differentiation thus favoring bone formation (25). In human bone marrow-derived MSCs, Lf treatment has been shown to suppress H2O2-derived reactive oxygen species (ROS) levels that likely inhibited senescence, and apoptosis of these cells. Moreover, the antiapoptotic effect of Lf in MSC involved inhibition of caspase-3 and activation of AKT activation (26). In MSCs obtained from adipose tissue, Lf showed mitogenic as well as pro-osteogenic effect demonstrated by the upregulation of Runx2 and ALP, and has the potential for bone tissue engineering applications (27). Indeed, incorporating Lf into biodegradable matrices containing hydroxyapatite, can enhance their osteogenic properties when applied to human MSCs (28, 29). However, there are no studies investigating whether Lf inhibits adipogenic differentiation of bone marrow-derived MSC which could have shed light on how Lf regulates the fate of MSCs, particularly their differentiation into osteoblasts and adipocytes.
3.2 Osteoblasts
Lf has been shown to regulate several molecular pathways in osteoblasts responsible for bone formation and remodeling. Several signaling pathways are involved in Lf’s actions in osteoblast proliferation, differentiation, and survival. The proximal signaling events identified for these actions include low-density lipoprotein receptor-related protein 1 (LRP1), IGF-1R, and TGFβ receptor pathways. LRP1 is a transmembrane receptor that can promote endocytosis of Lf (13). LRP1 can also produce cytoplasmic membrane-bound vesicles in osteoblasts, essential for the intracellular transport of proteins and other molecules. Lf has been shown to activate the extracellular signal-regulated kinase (ERK) pathway in osteoblasts through LRP1. Activation of the ERK pathway can stimulate osteoblast differentiation and bone formation, and Lf-mediated activation of this pathway may contribute to its osteogenic effects. On the other hand, Lf, through the PI3K/Akt pathway that is independent of LRP-1, inhibits osteoblast apoptosis has been reported (30).
Insulin-like growth factor 1-Insulin-like growth factor 1 receptor (IGF-1-IGF-1R)signaling plays a vital role in regulating bone metabolism, and a decline in IGF-1 has been implicated in age-related bone loss. Lf could address the decline in IGF-1 that occurs with aging by upregulating IGF-1 and IGF-1R in osteoblasts, which improved their viability under apoptotic stimulus (31). In senescent osteoblasts derived from SAMP6 mice (senescence-accelerated mouse-prone 6), an established model of accelerated aging that display several age-related phenotypes, including osteoporosis, sarcopenia, and cognitive decline, Lf enhanced the activity of the IGF1-Akt-mechanistic target of rapamycin (mTOR)signaling pathway. As a consequence of activating the IGF-1R-mediated osteogenic effect, Lf significantly attenuated the progression of osteoporosis due to senility (32). The suppression of senescent proteins, including p16 and p21, and oxidative injury through the upregulation of antioxidant enzyme activity through IGF-1R signaling likely attenuated the senescent-induced bone loss by Lf in SAMP6 model (32). Furthermore, Lf promoted the formation of osteoblasts from adipose tissue-derived stem cells (ADSCs) by activating the PI3K/AKT and IGF-R1 signaling pathways (33). Thus, it appears that to promote Lf’s osteogenic function, which includes osteoblast development from stem cells, osteoblast maturation, and osteoblast survival, the IGF-1-IGF-1R signaling is an effector arm. In bone marrow stromal cells (BMSCs), Lf and its digests activated the PI3K/AKT and ERK signaling pathways and promoted the expression of osteoblast-specific genes, such as runt-related transcription factor 2 (Runx2), alkaline phosphatase (ALP), and osteocalcin (OCN), while suppressing the expression of adipocyte-specific genes, such as peroxisome proliferator-activated receptor gamma(PPARγ)and fatty acid-binding protein 4 (FABP4). However, whether, IGF-1-IGF-1R signaling mediated the effect of Lf and its digests has not been studied (34).
Lf also activated the canonical TGF-β signaling pathway involving smad 2 via the TGF-β receptor II (TβRII), as demonstrated by the upregulation of osteogenic genes including Runx2, osterix, and collagen type I (35). Both canonical and noncanonical TGF-β signaling pathways were involved in the Lf-induced osteogenic activity of C3H10T1/2 MSCs. Lf treatment increased the phosphorylation of Smad2/3 and p38 MAP kinase, indicating the activation of canonical TGF-β signaling in the osteogenic differentiation of C3H10T1/2 cells. Lf also induced the phosphorylation of ERK1/2 in C3H10T1/2 cells, indicating the activation of noncanonical TGF-β signaling in the osteogenic differentiation of the cells (36).
From the preceding discussion, it appears that Lfsignaling through LRP1, IGF-1R, and TGFβ receptor trigger a cascade of events in osteoblasts that result in the activation of several downstream pathways, including ERK1, PI3K, Akt. mTOR and smad2/3 that promote osteoblast growth, survival, and differentiation (for a schematic illustration, refer to Figure 1).
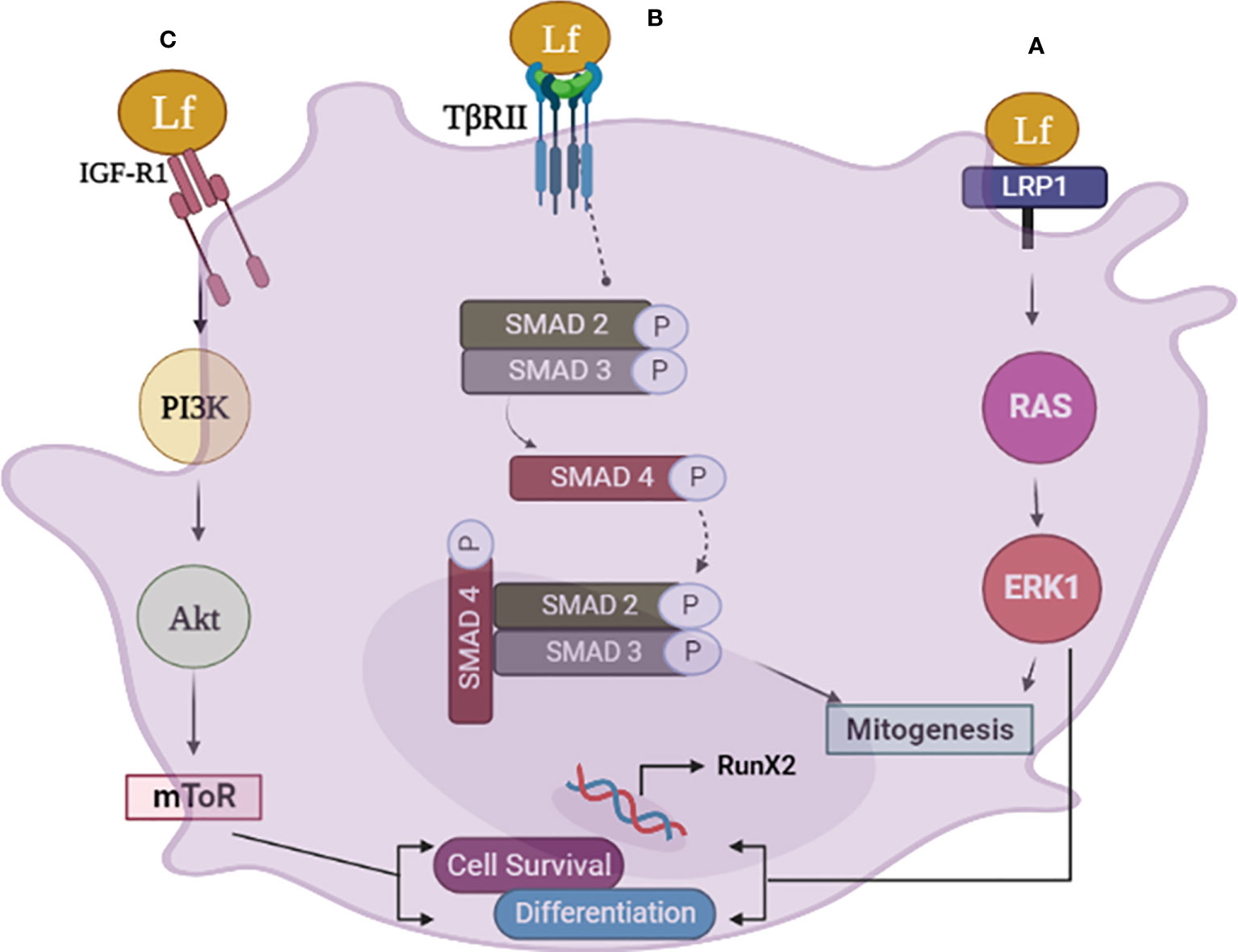
Figure 1 The schematic diagram illustrates the various pathways involved in lactoferrin (Lf) signaling in osteoblasts. (A) Lfsignaling via LRP1 in osteoblasts leads to mitogenesis and differentiation through the RAS-MAPK pathway. Lf binds to LRP1, triggering the activation of ERK, which promotes osteoblast differentiation and bone formation. The activation of ERK also induces mitogenesis in osteoblasts. (B) Lf can also signal through the TGFb receptor II (TβRII) in osteoblasts, activating smad 2, 3. This pathway results in the upregulation of osteogenic genes and promotes osteoblast differentiation. (C) Lfsignaling via the IGF-1 receptor in osteoblasts activates the PI3K/Akt and mTOR pathway, promoting osteoblast differentiation and survival independently of LRP1. This pathway promotes the survival of osteoblasts by inhibiting apoptosis. Image is made using the Biorender Software.
There are reports of additional signaling events elicited by Lf in osteoblasts besides the three receptors described above. For example, Lf stimulates the proliferation of osteoblasts through the upregulation of prostaglandin-endoperoxide synthase 2 (Ptgs2) (the enzyme encoding COX2) and NFATc1 activities. Inhibiting either COX2 or NFATc1 activity blocked the mitogenic effect of Lf in osteoblasts (37). Lf can also regulate gene expression by modulating long non-coding RNAs (lncRNAs). Knockdown of a specific lncRNA (RP11-509I15.3) that was upregulated by Lf treatment impaired osteogenic differentiation of rat BMSCs, suggesting that this lncRNA has roles in mediating the osteogenic effects of Lf (36).
In osteoblasts, Lf can inhibit the NF-κB signaling pathway, which is involved in the regulation of inflammatory responses, and is involved in osteoclast differentiation and bone resorption (38). Activation of NF-κB in osteoblasts results in the increased production of RANKL over OPG, which favors enhanced osteoclastogenesis. E2 negatively regulates RANKL, and its serum levels are increased after menopause (39). Consequently, denosumab, a human antibody against RANKL, is an approved therapy for postmenopausal osteoporosis (3). In the animal model of osteoporosis achieved by OVX, Lf suppressed the OVX-induced increases in RANKL: OPG ratio (38). Lf’s anti-oxidant/anti-inflammatory action appears to mediate this effect, although other regulatory mechanisms need to be investigated further.
3.3 Osteoclasts
Bone marrow cells are a heterogeneous population that includes osteoclast precursors, osteoblasts, and other cell types. Lipopolysaccharide (LPS), a component of the outer membrane of gram-negative bacteria, stimulates osteoclastogenesis (the formation of bone-destroying cells) by activating the RANKL signaling pathway in bone marrow cells. In mouse bone marrow cells, Lf inhibited LPS-induced osteoclastogenesis dose-dependently (9). When human peripheral CD14+ monocyte and macrophage cells were induced to differentiate into osteoclasts by a cocktail of macrophage colony-stimulating factor (M-CSF) and RANKL, Lf suppressed the expression of genes and proteins involved in osteoclast differentiation and activity such as TRAP and cathepsin K.
Overall, Lf regulates osteoclast function via two different mechanisms. Firstly, it lowers the RANKL/OPG ratio by influencing osteoblastic cells (9) and so suppressing osteoclastogenesis. Secondly, through its anti-inflammatory activity, Lf directly reduces osteoclastogenesis by blocking the downstream signaling that occurs when RANKL binds to RANK. Furthermore, by scavenging free radicals, Lf can limit the formation of ROS that are implicated in osteoclastogenesis (for a schematic illustration of osteoclast regulation by Lf, refer to Figure 2). The functional outcome of these suppressive effects was the inhibition of the resorption of bones by Lf ex vivo (40). Inhibition of osteoclastogenesis by Lf may have therapeutic potential for preventing bone loss associated with infectious diseases, periodontitis, and other inflammation-related diseases such as RA besides postmenopausal osteoporosis (41, 42).
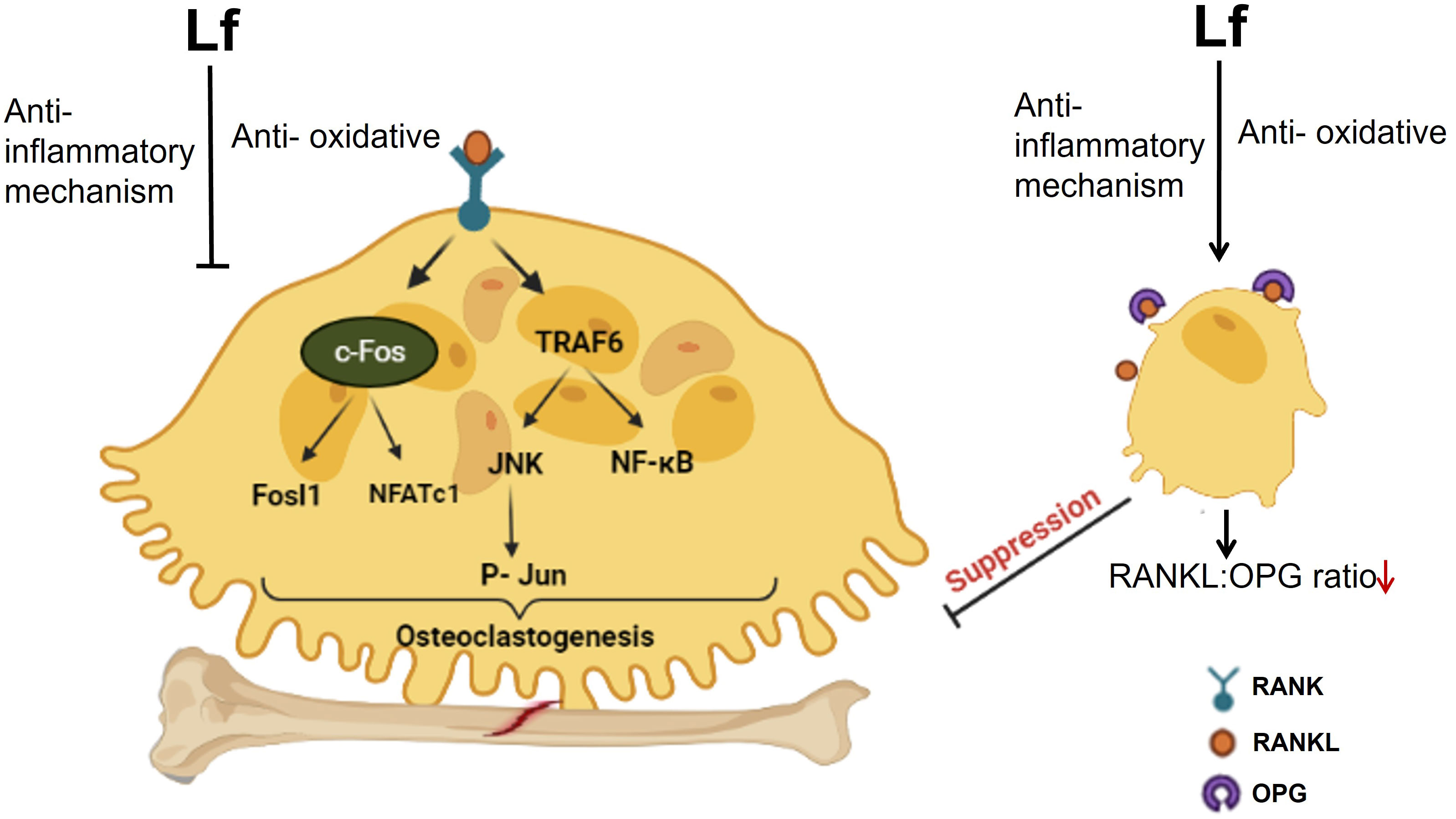
Figure 2 The schematic diagram illustrates the regulation of osteoclast function by Lf. Lf inhibits osteoclastogenesis in two ways. Firstly, it lowers the RANKL/OPG ratio by acting on osteoblastic cells. Secondly, it directly inhibits osteoclastogenesis through its anti-inflammatory effect, which prevents downstream signaling following RANKL binding to RANK. Moreover, Lf’s ability to scavenge free radicals also inhibits the generation of ROS involved in osteoclastogenesis. Image is made using the Biorender Software.
3.4 Chondrocyte
Lf has a pro-survival effect in chondrocytes. Lf was found to prevent the programmed cell death of chondrocytes induced by interleukin-1 beta (IL-1β), a cytokine known to contribute to osteoarthritis (OA) development. Lf inhibited chondrocyte apoptosis by activating the protein kinase B (AKT1) pathway, which leads to the activation of the cAMP response element-binding protein 1 (CREB1) transcription factor. CREB1 plays a crucial role in regulating cell survival and has been shown to protect chondrocytes from apoptosis. When OA was induced in mice by destabilization of the medial meniscus (DMM) surgery in the knee joint, Lf significantly reduced cartilage degradation in the knee joints of the mice compared to the control group, as indicated by histological and immunohistochemical analyses (43).
Additionally, the Lf-treated mice showed significantly reduced chondrocyte apoptosis in the cartilage tissues of the knee joints, as indicated by TUNEL staining (43). Lf also protected chondrocytes from dexamethasone (Dex)-induced apoptosis by preventing the Dex-induced down-regulation of ERK1/2 and up-regulation of proteins involved in apoptosis, including FAS, FASL, and caspase 3 (44). By activating ERK1/2, Lf appears to preferentially increase the expression of BMP7, compared to BMP2 or BMP4 in chondrocytes (45). An increase in BMP7 expression in chondrocytes is generally considered beneficial, as it can enhance the ability of these cells to maintain and repair the cartilage matrix by promoting the proliferation, differentiation, and survival of these cells, as well as the synthesis of extracellular matrix proteins, such as collagen and proteoglycans. In the ATDC5 chondroprogenitor cell line, Lf inhibited their hypertrophic differentiation by inhibiting the expression of hypertrophic markers such as collagen X and ALP (46). Hypertrophic differentiation of chondrocytes leads to chondrocyte enlargement during the formation of bone, which is required for bone development. During bone development, chondrocytes undergo hypertrophic differentiation and contribute to the formation of mineralized bone tissue. However, excessive hypertrophic differentiation of chondrocytes can lead to cartilage breakdown and ultimately contribute to the progression of joint diseases such as OA.
4 Conservation of bone mass by Lf
When Lf was given to adult rats, it resulted in a significant increase in the rate at which minerals were deposited on the bone surface (mineral apposition rate), which is an indicator of osteoblast activity, and an increase in the overall amount of bone formed (cumulative bone formation rate) in skull bone (5). Lf supplementation was found to upregulate vitamin D receptor in both osteoblasts and kidneys, leading to improved BMD in both vitamin D deficient and vitamin D normal mice (47), highlighting its significance in maintaining appropriate vitamin D signaling necessary for optimal bone health. In SAMP6 mice, a model of aging and senescence, which are associated with decreased bone mass and increased risk of osteoporosis, Lf (2 g/kg/day) alleviated the adverse effects of age-related bone loss (32). Dietary supplementation or gavage administration of Lf prevented the loss of bone mass and strength in OVX mice and rats (38, 48–50). Femur is a critical bone for weight-bearing and movement, and the preservation of bone mass and strength in this bone by Lf is important for maintaining overall bone health and reducing the risk of fractures (49, 50). Thus it is conceivable that Lf may find therapeutic application in several disease conditions, including osteoporosis, chronic kidney disease, celiac disease, and vitamin D deficiency, where decreased bone formation rate is one of the reasons for bone loss over time.
Bone turnover markers (BTMs) are molecules released during the process of bone remodeling that include markers of bone resorption (β-CTx and NTx) and markers of bone formation (BALP, P1NP, osteocalcin, etc.) indicating the rate of bone turnover. BTMs are used as biomarkers in clinical trials for osteoporosis and related conditions, as they reflect changes in bone metabolism and can predict the risk of fractures. These markers are typically higher in OVX condition, where estrogen deficiency leads to increased bone resorption and decreased bone formation, resulting in osteoporosis. Higher levels of resorption markers and lower levels of formation markers are associated with bone loss and increased risk of fractures. Lf has been demonstrated to reduce the increases in bone resorption markers, such as CTX and NTx, induced by OVX. In addition, Lf increased markers of bone formation, such as osteocalcin and BSALP, compared to OVx animals (38, 49, 51). BTMs are useful in assessing the efficacy of drugs that aim to modify bone turnover, such as antiresorptive and anabolic agents. Given that Lf has been shown to affect BTMs in preclinical studies, including reducing bone resorption markers and increasing bone formation markers, it is a promising candidate for further investigation in clinical trials.
Consistent with the preclinical studies in OVX animals, where Lf reduced bone resorption markers, and increased bone formation markers, a study in healthy postmenopausal women reported that milk ribonuclease-enriched Lf supplementation resulted in a significant increase in osteocalcin and BSALP, and a decrease in β-CTx compared with the placebo control (52). Monitoring BTMs during clinical trials with Lf could provide insight into its mechanism of action and effectiveness in improving human bone health.
5 Effects of Lf in fracture healing
Fractures of long bones, especially large, comminuted, segmental, transverse, or open, are difficult to heal and have a high risk of non-union (failure to heal), especially in osteoporotic conditions (53).Nonunions require revision surgery that involves removing any hardware (such as screws or plates) used to stabilize the bone during the initial surgery and then using bone grafting to promote proper bone healing. When nonunions are suspected, BMPs (BMP-2/-7) are applied locally to the fracture site to stimulate the growth of new bone tissue and promote healing. However, BMPs (BMP-2/-7) are typically reserved for more complex or difficult-to-heal fractures and are not considered a first-line treatment for most fractures (54). Moreover, a high amount of BMP-2 in the graft can stimulate the local production of noggin, a protein that inhibits BMP-2 activity (55). This can lead to a negative feedback loop in which the efficacy of BMP-2 in promoting bone healing is diminished. As a result, bone growth promoters such as Lf could be evaluated for systemic delivery to reduce the rate of nonunions in large fractures.
The primary process involved in fracture healing is endochondral ossification. In this process, MSCs differentiate into chondrocytes that form a cartilage template, which is then mineralized. Blood vessels invade the calcified cartilage and bring osteoblasts that deposit new bone tissue, while osteoclasts break down and remodel the newly formed bone (56). Osteoporotic conditions can delay fracture healing by promoting excessive bone resorption and delaying the formation of nascent bone. This can lead to weaker callus formation and reduced bone strength at the fracture site, making it more prone to re-injury. Our preceding discussion described that Lf has salutary effects in osteoblasts, chondrocytes, and osteoclasts that support its use in nonunions by acting as a systemic bone growth promoter.
In the laboratory setting, the rabbit tibia is a commonly used model for studying long bone defects and fracture healing. The rabbit tibia offers several advantages as a model for studying bone healing, including its similar size and anatomy to human long bones and its weight-bearing. The unilateral tibial osteodistraction model is an animal model for studying bone regeneration and is often used to evaluate potential therapies for bone defects and fractures (57). In the osteodistraction model, a small cut (osteotomy) is made in the tibia, and an external fixator device is attached to the bone on either side of the osteotomy. The device is then slowly adjusted over time, causing the bone ends to gradually separate and new bone tissue to form in the gap between them. The osteodistraction technique can be used to study the effects of mechanical loading and other factors on bone formation and healing. Oral Lf was found to promote bone regeneration during distraction osteogenesis in rabbit tibia by increasing OPG to RANKL ratio, inhibiting the bone resorption rate (58). Another large bone defect model that is difficult to heal is a surgically created critical-sized defect. This defect is too large to heal on its own and is used in research to mimic open tibial fractures in humans that require orthopedic reconstructive procedures (59). By creating a critical-sized bone defect in the rabbit tibia, it is possible to study various interventions, such as bone grafting, growth factors, and tissue engineering, to promote bone regeneration and healing and to develop new treatments for orthopedic injuries and disorders in humans. An 18 amino acid peptide (RKVRGPPVSCIKRDSPIQ) from the N-terminus of the N-lobe of human Lf called LP2 stimulated bone regeneration in the critical-sized defect in rabbits by increasing the production of BMP-2 in osteoblasts. Additionally, the LP2 peptide had an anti-osteoclastogenic effect by enhancing the ratio of OPG to RANKL in osteoblasts (60). These data suggest that the upregulation of OPG is the critical mechanism underlying the healing of long bone fracture by Lf.
6 Designing therapeutic peptides from Lf FOR treating bone diseases
Lf is a large and multifunctional protein. Hence, small peptides made from Lf are useful for therapeutic purposes because they allow for more efficient delivery and targeting of specific biological functions. Furthermore, smaller peptides are less immunogenic than bigger proteins, lowering the risk of unfavorable immune responses and adverse effects. Smaller peptides are also more likely to penetrate tissues and reach their target cells or molecules, increasing their bioavailability and efficacy (61).
Positively charged amino acid segments, such as those containing arginine, lysine, and histidine, are often preferred for the design of bioactive peptides because they can interact with negatively charged molecules in biological systems. These positively charged amino acids, in particular, can generate electrostatic interactions with negatively charged cell membranes and other macromolecules, resulting in various biological effects. Moreover, positively charged amino acid segments can facilitate the uptake of peptides into cells, as some cellular uptake mechanisms are known to be selective for peptides with positively charged residues. This enhanced cellular uptake can increase the bioavailability and efficacy of peptides. Thus, the N-terminal region of Lf has become a focal point for designing peptides with potential therapeutic applications. Lactoferricin (62–64) and lactoferrampin (65, 66) deserve special mention because these have undergone extensive research for their anti-microbial effect. Both are cationic and α-helical peptides with a hydrophobic N-terminus and a hydrophilic C-terminus that are stable over a wide pH and temperature range (67).Lactoferricin has potent antimicrobial activity against a broad range of bacteria, fungi, and viruses, and lactoferrampin has broad-spectrum activity against bacteria, fungi, and protozoa (62–66). Given the anti-microbial property of Lf may be considered in the treatment of osteomyelitis, a bone infection commonly caused by Staphylococcus aureus. In this regard, a human Lf-derived peptide in both prophylactic and therapeutic modes significantly reduced severity of osteomyelitis in a rabbit model evident from improved microbiological, radiological and histological scores compared to the placebo group. Strikingly, the effect of the peptide was on a par with gentamicin (68, 69). The rabbit model of osteomyelitis mimics the progression of human disease because the long bones of rabbits are physiologically similar to humans - both species having Haversian remodeling. Hence, the findings of the reports showing the mitigation of osteomyelitis by Lf-derived peptide in the rabbit model of the disease holds potential translational value for human applications.
The anti-microbial mechanism of Lf-derived peptides could also be useful in affording protection against cartilage degradation. For example, lactoferricin inhibits the effects of IL-1 and fibroblast growth factor 2, which are known to cause cartilage degradation (70). Lactoferricin also induces the production of interleukin-11 (IL-11), an anti-inflammatory cytokine, which then activates the STAT3 signaling pathway to up-regulate the expression of TIMP-1 in human adult articular chondrocytes. The up-regulation of TIMP-1 expression by IL-11 may be a secondary cellular response after IL-11 induction by lactoferricin via the ERK-AP-1 axis (71). Together, these reports suggest that lactoferricin may have a dual mechanism of action in reducing inflammation in human articular cartilage by both inducing the production of anti-inflammatory cytokines and inhibiting the production of pro-inflammatory cytokines. These reports also underscore the potential of lactoferricin as a therapeutic agent for the treatment of inflammatory joint diseases such as OA. However, the effect of lactoferricin on metabolic bone diseases such as postmenopausal osteoporosis has not been investigated. As discussed in the preceding section, by suppressing inflammatory mediators including TNFα, IL-1β and IL-6, Lf/Lf-derived peptides also inhibit osteoclast formation and activity. In this regard, the effect of lactoferricin and other Lf-derived peptides on osteoclast formation and function, and inhibition of bone resorption in vivo has not been investigated.
Two Lf-derived peptides have been shown to have potential effects on osteoblast function. LFP-C, a 9-amino acid peptide was isolated from Lf hydrolysates by pepsin digestion enhanced osteoblast differentiation and mineralization and increased the expression of genes involved in bone formation (72). LP2 is another osteogenic peptide derived from human Lf. This synthetic peptide has a self-assembling property and assumes nanoglobular structures owing to which it spontaneously aggregate and form stable, spherical structures, without the need for external assembly factors or scaffolds. LP2, when systemically administered, demonstrated osteogenic and anti-resorptive effects in maintaining bone mass and strength in OVX rats by activating p38 MAPK and BMP-2 production and increasing OPG production, and in rabbits with a critical-sized defect in the tibia, it led to faster healing of the defect than control (60). For various Lf-derived peptides and their functions, refer to Table 1. Taken together, it appears that Lf-derived peptides hold great promise as potential therapeutic agents for the treatment of orthopedic and metabolic bone diseases.
7 Delivery of Lf to the bone
Although Lf is present in serum, its active concentration may not be present at the site requiring bone regeneration. Bone regeneration is a complex process that requires the presence of various growth factors and biomolecules at the site of injury, and the levels of these factors can vary depending on the location and extent of the injury. Delivering Lf to the site of bone regeneration can ensure that it is present in sufficient quantities to promote bone growth and regeneration. Additionally, Lf delivery strategies can protect it from degradation and provide sustained release over time, further enhancing its effectiveness. Biocompatibility of Lf, i.e. non-toxicity to cells and tissue makes it an attractive target for delivery to the bone to promote bone growth, reduce inflammation, and prevent infections. Effective delivery of Lf to the site of bone regeneration has been achieved mostly through hydrogels, which protect it from degradation and enable sustained release over time.
Hydrogels are cross-linked polymer networks that can absorb large amounts of water while maintaining their three-dimensional structure and have a similar mechanical strength and elasticity to natural tissues (78). Lf can be added to the hydrogel during the synthesis process or can be incorporated after the hydrogel is formed. Once implanted at the site of bone regeneration, the hydrogel can slowly release Lf, providing sustained delivery over time. Additionally, the hydrogel can provide a matrix for cell attachment and proliferation, promoting bone growth and regeneration. Hydrogels can also be functionalized with specific chemical groups to enhance the delivery of Lf with other growth factors.
Gelatin hydrogels are hydrophilic and biodegradable, and they are commonly used in biomedical applications due to their biocompatibility and ability to mimic the extracellular matrix of natural tissues. When the release of Lf from a gelatin hydrogel was sustained for 28 days, it promoted the proliferation and differentiation of osteoblasts. In a rat femoral defect model, the Lf-releasing gelatin hydrogel resulted in bone regeneration. The newly formed bone showed good integration with the surrounding bone tissue and no signs of inflammation or necrosis (79).
Poloxamer hydrogels are a class of hydrogels made up of a triblock copolymer of poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) that can exist as a liquid at low temperatures but form a gel at body temperature (80). However, poloxamer hydrogels are non-biodegradable and have a relatively low mechanical strength compared to gelatin hydrogels. Besides thermal reversibility, poloxamer hydrogels have good biocompatibility and low toxicity, making them suitable for various biomedical applications as injectable gel formulations. Poloxamer hydrogels loaded with Lf can sustain the release of Lf for up to 21 days, which promotes bone regeneration. This sustained release formulation of Lf enhanced the osteogenic differentiation of rat MSCs and improved mechanical strength compared to the non-loaded hydrogels. In a rat calvarial defect model, this formulation promoted bone regeneration and new bone formation, and the newly formed bone tissue showed no signs of inflammation or necrosis (81).
Chitin/PLGA-CaSO4 hydrogel has distinct advantages over other hydrogels, such as osteogenic and angiogenic activity, a porous structure, good biocompatibility, sol-gel transition at body temperature, and controlled release of bioactive molecules. The chitin/PLGA-CaSO4 hydrogel loaded with Lf and substance P significantly promoted bone regeneration and new bone formation in the calvarial bone defect model compared to the hydrogel alone or hydrogel loaded with Lf or substance P alone. As substance P has bone regenerative action and improves bone healing, it was included with Lf, which resulted in synergistic effects on bone regeneration and improved the therapeutic efficacy of the hydrogel. The combination of Lf and substance P enhanced the osteogenic and angiogenic activity of the hydrogel, as evidenced by increased expression of osteogenic and angiogenic markers in vitro and in vivo (82). The findings of this study suggest that combining Lf with other osteogenic agents, such as teriparatide, could potentially enhance the overall bone regeneration response. Figure 3 describes various strategies to improve the delivery of Lf or Lf-derived peptides to the bone to accelerate critical-sized bone defects that are observed in comminuted fractures in humans.
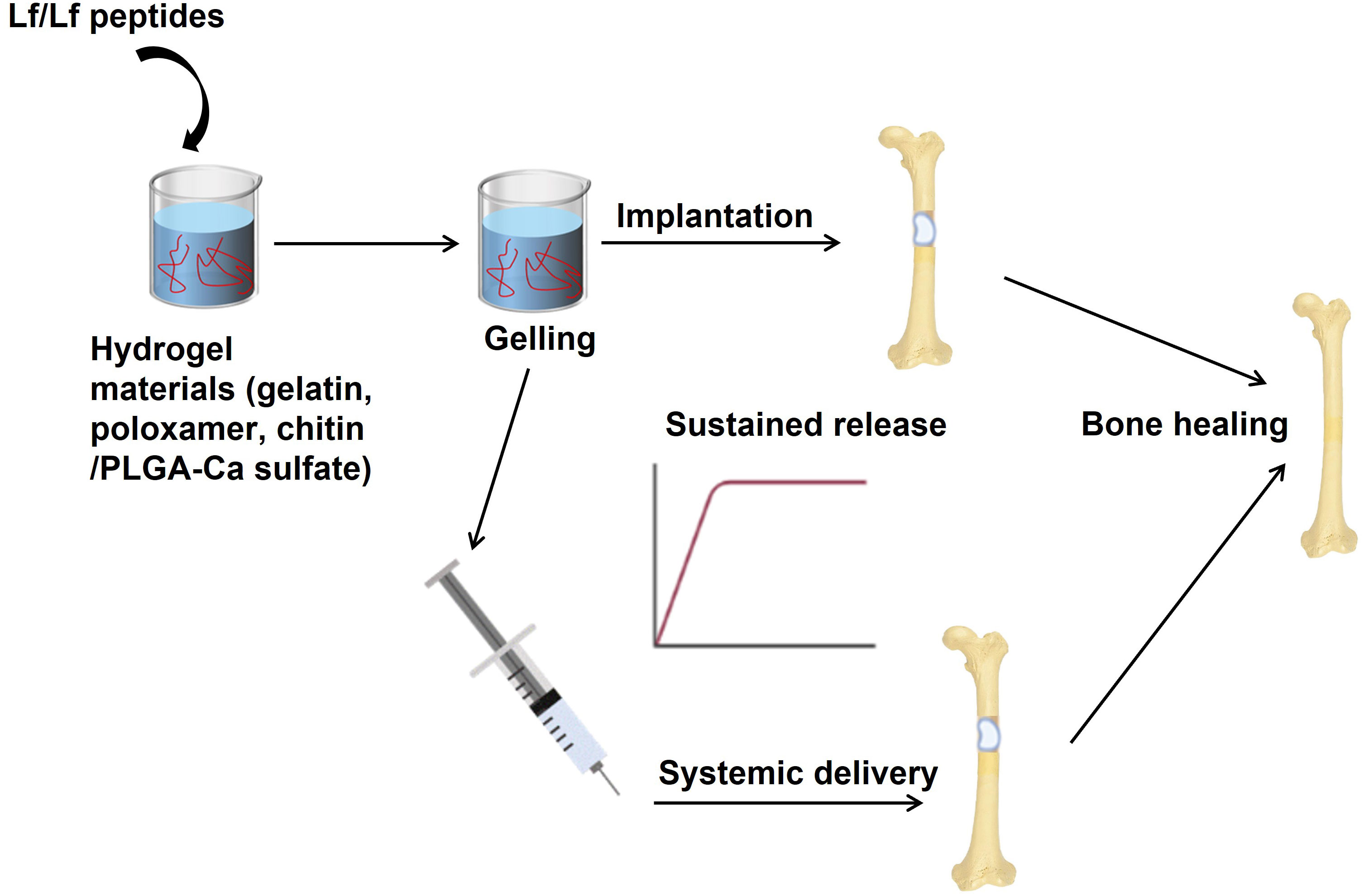
Figure 3 Strategies to improve the delivery of Lf or Lf-derived peptides to the bone. Hydrogels have been used in preclinical models of critical-sized bone defects (mimicking non-unions) as sustained-release formulations to deliver high amounts of Lf to bones. In these models, Lf has been used for local delivery of Lf as implants at the site of the bone defect. This approach ensures Lf’s sustained release, which can stimulate bone regeneration and repair. In addition, systemic delivery of Lf through hydrogels has also been explored. In this case, hydrogels containing Lf are injected into the bloodstream, allowing for the controlled release of Lf over time. These approaches have shown promising results in promoting bone regeneration in critical-sized bone defects. Image is made using the Biorender Software.
8 Summary & future research
This review covered the roles of Lf bone remodeling and resorption, bone healing, and regeneration. LRP1, IGF-1R, and TGFβ receptor pathways have been identified as the proximal signaling events involved in the actions of Lf in osteoblasts. The downstream events of these proximal signaling pathways lead to the activation of ERK1/2, Akt, PI3K/Akt, MAPK, and SMAD pathways. These signaling pathways promote proliferation, migration, survival, differentiation, and extracellular matrix formation in osteoblasts, which are important for tissue repair and regeneration. Lf inhibits osteoclast differentiation and activity by suppressing NF-κB signaling, inducing OPG expression, and downregulating RANKL expression, thereby modulating key regulatory pathways involved in osteoclastogenesis and bone resorption. Lf also modulates matrix metalloproteinase activity, which is involved in bone remodeling. Lf’s actions on chondrocytes involve activating multiple signaling pathways, including Akt, CREB, ERK, and BMP7, which promote chondrogenesis, cell survival, and cartilage formation. Regulation of these signaling events in bone cells by Lf contributes to tissue repair and regeneration and inhibits bone loss in osteoporosis. By improving bone mineral density and reducing the risk of bone loss, Lf may help prevent fractures and other osteoporosis-related complications. In addition, further investigation is needed to elucidate the exact mechanisms of action of Lf on bone metabolism, including the role of Lf receptors in bone cells.
The limitations of current osteoporosis therapies are that they tend to have a one-sided approach that either inhibits bone resorption or stimulates bone formation. For example, bisphosphonates, which are one of the most commonly used osteoporosis drugs, inhibit bone resorption but do not stimulate bone formation. On the other hand, anabolic agents such as teriparatide and abaloparatide stimulate bone formation but have no effect on bone resorption. Therefore, there is a need for a therapy that can inhibit bone resorption as well as stimulate bone formation, providing a dual benefit for osteoporosis patients. Estrogen was once the only therapy that could inhibit bone resorption and stimulate bone formation. However, significant cancer and cardiovascular risks associated with estrogen use in postmenopausal women have resulted in its discontinuation. Therefore, there is currently no therapy that can provide the dual benefit of estrogen without the associated risks (for additional details, refer to Figure 4). Lf has the potential to fill the void left by estrogen as a therapy that can inhibit bone resorption and stimulate bone formation in osteoporosis patients. As an endogenously produced protein that has a good safety profile, low toxicity, and availability make it an attractive candidate for further investigation as a therapeutic agent for osteoporosis. However, further research is needed to fully understand its mechanisms of action and to determine its optimal dose and delivery route for therapeutic use.

Figure 4 The schematic diagram shows the effects of Lf in bone remodeling. The bone remodeling cycle comprises several stages, including quiescence, activation & resorption, reversal, and formation. In the activation & resorption stage, osteoclasts are recruited to the bone surface and resorb the old bone. In the reversal stage, osteoblasts are recruited to the bone surface to begin the process of new bone formation. The final stage involves the production of new bone matrix by osteoblasts, which undergoes mineralization. In the normal bone remodeling cycle, there is a balance between bone resorption and bone formation such that the amount of bone that is resorbed is replaced by an equal amount of new bone formation, resulting in the maintenance of a constant bone mass. In osteoporosis, there is an imbalance between bone resorption and formation because osteoclast activity is increased. In contrast, osteoblast activity is decreased, resulting in decreased bone mass and an increased risk of fractures. Lf acts at the activation & resorption phases by the mechanisms described in Figure 2 to inhibit bone resorption. Lf also acts at the reversal and formation stages to stimulate bone formation by the mechanisms described in Figure 1. By these mechanisms, Lf corrects the remodeling cycle and restores bone mass. Osteioid, unmineralized bone matrix; osteon, mineralized bone matrix. Image is made using the Biorender Software.
Since osteogenic and chondrogenic peptides from Lf have already been found, a rational design strategy may be appropriate for developing more such peptides from Lf with improved function. By analyzing the amino acid sequences and structural features of existing osteogenic/chondrogenic peptides, key amino acid residues implicated in their biological activities can be identified and included in the design of novel osteogenic/chondrogenic peptides. Additionally, alanine scanning can be used to confirm the importance of specific amino acid residues identified through rational design and ensure that they are essential for the osteogenic/chondrogenic activity of the peptide. Combining these techniques can provide a more thorough understanding of the structure-function correlations of Lf-derived peptides and aid in generating novel osteogenic/chondrogenic peptides with increased potency and selectivity.
Hydrogels may enhance their therapeutic potential in bone-related applications by protecting Lf from degradation and enabling sustained release. Hence, hydrogels have been utilized as a drug delivery system and discussed here. However, further studies are needed to investigate the potential synergistic effects of combining Lf with other osteogenic agents in these hydrogels. There is also a need to develop hydrogels that mimic the complex mechanical properties of natural bone tissue and that can degrade over time and be replaced by new bone tissue. pH is an important factor during bone remodeling and the formulation of hydrogels because it can affect the solubility, stability, and bioactivity of biomolecules such as Lf. In bone regeneration, the pH of the local environment can affect the activity of bone cells. For example, a slightly acidic environment (pH 6.8-7.2) is beneficial for osteoclast activity required for initiating remodeling, while a slightly alkaline environment (pH 7.4-7.8) is beneficial for osteoblast activity. Therefore, enhancing the efficiency of Lf administration for bone regeneration may require regulating the pH of the local environment and the hydrogel formulation. This can be accomplished by using pH-sensitive hydrogels or by incorporating pH-modulating agents into the hydrogel formulation. Other approaches to more efficiently targeting Lf to bones may include encapsulating it in liposomes or polymeric nanoparticles and functionalizing the nanoparticles with bone-targeting molecules such as bisphosphonates, or conjugating Lf with bone-targeting peptides derived from osteocalcin or bone sialoprotein.
Future research areas for Lf and bone include determining its optimal dose and delivery routes for bone regeneration and osteoporosis. In addition, further investigation is needed to elucidate the exact mechanisms of action of Lf on bone metabolism, including the role of Lf receptors in bone cells. Clinical trials are also necessary to evaluate the safety and efficacy of Lf as an osteoporosis therapy in humans and to investigate its long-term effects on bone density and fracture risk. Additionally, considering its osteogenic and anti-resorptive effects, there is potential use for Lf in combination with osteogenic anti-osteoporosis drugs including teriparatide, abaloparatide or romosozumab, whether in the form of intact Lf, enzymatically digested Lf or a bioactive peptide such as LP2. Combining Lf or suitable Lf-derived peptide with any of the osteogenic drugs could potentially have a synergistic effect in the treatment of osteoporosis. Because Lf or the proteolytic digests have neutraceutical use, it could be conveniently assessed in clinical trials via oral administration. For Lf-derived peptides, however, given their parenteral route of administration, regulatory studies to assess safety, efficacy and optimal dosage are required before their use in humans. Finally, Lf may have therapeutic applications in other bone diseases, such as OA and periodontitis, and further research is needed to explore its potential in these conditions.
Overall, the research on Lf has shown its potential in various aspects of bone remodeling, signaling, fracture healing, peptide synthesis, and Lf delivery to bones. Further research in this area may lead to the development of new treatments for bone-related disorders.
Author contributions
MT, Y-BH, G-YY, J-LL, and C-SH conducted literature search and wrote the manuscript. DT conceptualized the theme of the review and finalized the manuscript. DT takes responsibility for the integrity of the substance described in the review as a whole as ‘guarantor’. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gosset A, Pouilles JM, Tremollieres F. Menopausal hormone therapy for the management of osteoporosis. Best Pract Res Clin Endocrinol Metab (2021) 35(6):101551. doi: 10.1016/j.beem.2021.101551
2. Tella SH, Gallagher JC. Prevention and treatment of postmenopausal osteoporosis. J Steroid Biochem Mol Biol (2014) 142:155–70. doi: 10.1016/j.jsbmb.2013.09.008
3. Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med (2009) 361(8):756–65. doi: 10.1056/NEJMoa0809493
4. Bhattacharyya S, Pal S, Chattopadhyay N. Targeted inhibition of sclerostin for post-menopausal osteoporosis therapy: A critical assessment of the mechanism of action. Eur J Pharmacol (2018) 826:39–47. doi: 10.1016/j.ejphar.2018.02.028
5. Cornish J, Callon KE, Naot D, Palmano KP, Banovic T, Bava U, et al. Lactoferrin is a potent regulator of bone cell activity and increases bone formation in vivo. Endocrinology (2004) 145(9):4366–74. doi: 10.1210/en.2003-1307
7. Brines RD, Brock JH. The effect of trypsin and chymotrypsin on the in vitro antimicrobial and iron-binding properties of lactoferrin in human milk and bovine colostrum. Unusual resistance of human apolactoferrin to proteolytic digestion. Biochim Biophys Acta (1983) 759(3):229–35. doi: 10.1016/0304-4165(83)90317-3
8. Inubushi T, Kosai A, Yanagisawa S, Chanbora C, Miyauchi M, Yamasaki S, et al. Bovine lactoferrin enhances osteogenesis through smad2/3 and P38 mapk activation. J Oral Biosci (2020) 62(2):147–54. doi: 10.1016/j.job.2020.05.001
9. Inubushi T, Kawazoe A, Miyauchi M, Yanagisawa S, Subarnbhesaj A, Chanbora C, et al. Lactoferrin inhibits infection-related osteoclastogenesis without interrupting compressive force-related osteoclastogenesis. Arch Oral Biol (2014) 59(2):226–32. doi: 10.1016/j.archoralbio.2013.11.002
10. Conneely OM. Antiinflammatory activities of lactoferrin. J Am Coll Nutr (2001) 20(5 Suppl):389S–95S. doi: 10.1080/07315724.2001.10719173. discussion 96S-97S.
11. Mulder AM, Connellan PA, Oliver CJ, Morris CA, Stevenson LM. Bovine lactoferrin supplementation supports immune and antioxidant status in healthy human males. Nutr Res (2008) 28(9):583–9. doi: 10.1016/j.nutres.2008.05.007
12. Rawat P, Kumar S, Sheokand N, Raje CI, Raje M. The multifunctional glycolytic protein glyceraldehyde-3-phosphate dehydrogenase (Gapdh) is a novel macrophage lactoferrin receptor. Biochem Cell Biol (2012) 90(3):329–38. doi: 10.1139/o11-058
13. Grey A, Banovic T, Zhu Q, Watson M, Callon K, Palmano K, et al. The low-density lipoprotein receptor-related protein 1 is a mitogenic receptor for lactoferrin in osteoblastic cells. Mol Endocrinol (2004) 18(9):2268–78. doi: 10.1210/me.2003-0456
14. Ikoma-Seki K, Nakamura K, Morishita S, Ono T, Sugiyama K, Nishino H, et al. Role of lrp1 and erk and camp signaling pathways in lactoferrin-induced lipolysis in mature rat adipocytes. PloS One (2015) 10(10):e0141378. doi: 10.1371/journal.pone.0141378
15. Shin K, Wakabayashi H, Yamauchi K, Yaeshima T, Iwatsuki K. Recombinant human intelectin binds bovine lactoferrin and its peptides. Biol Pharm Bull (2008) 31(8):1605–8. doi: 10.1248/bpb.31.1605
16. Gao CH, Dong HL, Tai L, Gao XM. Lactoferrin-containing immunocomplexes drive the conversion of human macrophages from M2- into M1-like phenotype. Front Immunol (2018) 9:37. doi: 10.3389/fimmu.2018.00037
17. Ji ZS, Mahley RW. Lactoferrin binding to heparan sulfate proteoglycans and the ldl receptor-related protein. Further evidence supporting the importance of direct binding of remnant lipoproteins to hspg. Arterioscler Thromb (1994) 14(12):2025–31. doi: 10.1161/01.atv.14.12.2025
18. Ciesielska A, Matyjek M, Kwiatkowska K. Tlr4 and cd14 trafficking and its influence on lps-induced pro-inflammatory signaling. Cell Mol Life Sci (2021) 78(4):1233–61. doi: 10.1007/s00018-020-03656-y
19. Curran CS, Demick KP, Mansfield JM. Lactoferrin activates macrophages via tlr4-dependent and -independent signaling pathways. Cell Immunol (2006) 242(1):23–30. doi: 10.1016/j.cellimm.2006.08.006
20. Gonias SL, Campana WM. Ldl receptor-related protein-1: A regulator of inflammation in atherosclerosis, cancer, and injury to the nervous system. Am J Pathol (2014) 184(1):18–27. doi: 10.1016/j.ajpath.2013.08.029
21. Takayama Y, Aoki R, Uchida R, Tajima A, Aoki-Yoshida A. Role of cxc chemokine receptor type 4 as a lactoferrin receptor. Biochem Cell Biol (2017) 95(1):57–63. doi: 10.1139/bcb-2016-0039
22. Tewari D, Khan MP, Sagar N, China SP, Singh AK, Kheruka SC, et al. Ovariectomized rats with established osteopenia have diminished mesenchymal stem cells in the bone marrow and impaired homing, osteoinduction and bone regeneration at the fracture site. Stem Cell Rev Rep (2015) 11(2):309–21. doi: 10.1007/s12015-014-9573-5
23. Rebolj K, Veber M, Drobnic M, Malicev E. Hematopoietic stem cell and mesenchymal stem cell population size in bone marrow samples depends on patient's age and harvesting technique. Cytotechnology (2018) 70(6):1575–83. doi: 10.1007/s10616-018-0250-4
24. Li H, Ghazanfari R, Zacharaki D, Lim HC, Scheding S. Isolation and characterization of primary bone marrow mesenchymal stromal cells. Ann N Y Acad Sci (2016) 1370(1):109–18. doi: 10.1111/nyas.13102
25. Wang LT, Chen LR, Chen KH. Hormone-related and drug-induced osteoporosis: A cellular and molecular overview. Int J Mol Sci (2023) 24(6). doi: 10.3390/ijms24065814
26. Park SY, Jeong AJ, Kim GY, Jo A, Lee JE, Leem SH, et al. Lactoferrin protects human mesenchymal stem cells from oxidative stress-induced senescence and apoptosis. J Microbiol Biotechnol (2017) 27(10):1877–84. doi: 10.4014/jmb.1707.07040
27. Ying X, Cheng S, Wang W, Lin Z, Chen Q, Zhang W, et al. Effect of lactoferrin on osteogenic differentiation of human adipose stem cells. Int Orthop (2012) 36(3):647–53. doi: 10.1007/s00264-011-1303-x
28. Montesi M, Panseri S, Iafisco M, Adamiano A, Tampieri A. Effect of hydroxyapatite nanocrystals functionalized with lactoferrin in osteogenic differentiation of mesenchymal stem cells. J BioMed Mater Res A (2015) 103(1):224–34. doi: 10.1002/jbm.a.35170
29. Icriverzi M, Bonciu A, Rusen L, Sima LE, Brajnicov S, Cimpean A, et al. Human mesenchymal stem cell response to lactoferrin-based composite coatings. Mater (Basel) (2019) 12(20). doi: 10.3390/ma12203414
30. Grey A, Zhu Q, Watson M, Callon K, Cornish J. Lactoferrin potently inhibits osteoblast apoptosis, via an lrp1-independent pathway. Mol Cell Endocrinol (2006) 251(1-2):96–102. doi: 10.1016/j.mce.2006.03.002
31. Hou JM, Chen EY, Wei SC, Lin F, Lin QM, Lan XH, et al. Lactoferrin inhibits apoptosis through insulin-like growth factor I in primary rat osteoblasts. Acta Pharmacol Sin (2014) 35(4):523–30. doi: 10.1038/aps.2013.173
32. Chen XW, Li YH, Zhang MJ, Chen Z, Ke DS, Xue Y, et al. Lactoferrin ameliorates aging-suppressed osteogenesis via igf1 signaling. J Mol Endocrinol (2019) 63(1):63–75. doi: 10.1530/JME-19-0003
33. Chang Y, Ping A, Chang C, Betz VM, Cai L, Ren B. Lactoferrin mediates enhanced osteogenesis of adipose-derived stem cells: innovative molecular and cellular therapy for bone repair. Int J Mol Sci (2023) 24(2). doi: 10.3390/ijms24021749
34. Hou JM, Chen EY, Lin F, Lin QM, Xue Y, Lan XH, et al. Lactoferrin induces osteoblast growth through igf-1r. Int J Endocrinol (2015) 2015:282806. doi: 10.1155/2015/282806
35. Li Y, Wang J, Ren F, Zhang W, Zhang H, Zhao L, et al. Lactoferrin promotes osteogenesis through tgf-beta receptor ii binding in osteoblasts and activation of canonical tgf-beta signaling in mc3t3-E1 cells and C57bl/6j mice. J Nutr (2018) 148(8):1285–92. doi: 10.1093/jn/nxy097
36. Xu Y, An JJ, Tabys D, Xie YD, Zhao TY, Ren HW, et al. Effect of lactoferrin on the expression profiles of long non-coding rna during osteogenic differentiation of bone marrow mesenchymal stem cells. Int J Mol Sci (2019) 20(19). doi: 10.3390/ijms20194834
37. Naot D, Chhana A, Matthews BG, Callon KE, Tong PC, Lin JM, et al. Molecular mechanisms involved in the mitogenic effect of lactoferrin in osteoblasts. Bone (2011) 49(2):217–24. doi: 10.1016/j.bone.2011.04.002
38. Hou JM, Xue Y, Lin QM. Bovine lactoferrin improves bone mass and microstructure in ovariectomized rats via opg/rankl/rank pathway. Acta Pharmacol Sin (2012) 33(10):1277–84. doi: 10.1038/aps.2012.83
39. Bord S, Ireland DC, Beavan SR, Compston JE. The effects of estrogen on osteoprotegerin, rankl, and estrogen receptor expression in human osteoblasts. Bone (2003) 32(2):136–41. doi: 10.1016/s8756-3282(02)00953-5
40. Lorget F, Clough J, Oliveira M, Daury MC, Sabokbar A, Offord E. Lactoferrin reduces in vitro osteoclast differentiation and resorbing activity. Biochem Biophys Res Commun (2002) 296(2):261–6. doi: 10.1016/s0006-291x(02)00849-5
41. Yanagisawa S, Nagasaki K, Chea C, Ando T, Ayuningtyas NF, Inubushi T, et al. Oral administration of bovine lactoferrin suppresses the progression of rheumatoid arthritis in an skg mouse model. PloS One (2022) 17(2):e0263254. doi: 10.1371/journal.pone.0263254
42. Kawazoe A, Inubushi T, Miyauchi M, Ishikado A, Tanaka E, Tanne K, et al. Orally administered liposomal lactoferrin inhibits inflammation-related bone breakdown without interrupting orthodontic tooth movement. J Periodontol (2013) 84(10):1454–62. doi: 10.1902/jop.2012.120508
43. Xue H, Tu Y, Ma T, Liu X, Wen T, Cai M, et al. Lactoferrin inhibits il-1beta-induced chondrocyte apoptosis through akt1-induced creb1 activation. Cell Physiol Biochem (2015) 36(6):2456–65. doi: 10.1159/000430206
44. Tu Y, Xue H, Francis W, Davies AP, Pallister I, Kanamarlapudi V, et al. Lactoferrin Inhibits Dexamethasone-Induced Chondrocyte Impairment from Osteoarthritic Cartilage through up-Regulation of Extracellular Signal-Regulated Kinase 1/2 and Suppression of Fasl, Fas, and Caspase 3. Biochem Biophys Res Commun (2013) 441(1):249–55. doi: 10.1016/j.bbrc.2013.10.047
45. Zhang C, Li Y, Tang W, Kamiya N, Kim H. Lactoferrin activates bmp7 gene expression through the mitogen-activated protein kinase erk pathway in articular cartilage. Biochem Biophys Res Commun (2013) 431(1):31–5. doi: 10.1016/j.bbrc.2012.12.111
46. Takayama Y, Mizumachi K. Inhibitory effect of lactoferrin on hypertrophic differentiation of atdc5 mouse chondroprogenitor cells. Biometals (2010) 23(3):477–84. doi: 10.1007/s10534-010-9291-7
47. Li Y, Huang J, Wang J, Ma M, Lu Y, Wang R, et al. Lactoferrin is a potential activator of the vitamin D receptor in its regulation of osteogenic activities in C57bl/6j mice and mc3t3-E1 cells. J Nutr (2021) 151(8):2105–13. doi: 10.1093/jn/nxab105
48. Blais A, Malet A, Mikogami T, Martin-Rouas C, Tome D. Oral bovine lactoferrin improves bone status of ovariectomized mice. Am J Physiol Endocrinol Metab (2009) 296(6):E1281–8. doi: 10.1152/ajpendo.90938.2008
49. Guo HY, Jiang L, Ibrahim SA, Zhang L, Zhang H, Zhang M, et al. Orally administered lactoferrin preserves bone mass and microarchitecture in ovariectomized rats. J Nutr (2009) 139(5):958–64. doi: 10.3945/jn.108.100586
50. Fan F, Shi P, Liu M, Chen H, Tu M, Lu W, et al. Lactoferrin preserves bone homeostasis by regulating the rankl/rank/opg pathway of osteoimmunology. Food Funct (2018) 9(5):2653–60. doi: 10.1039/c8fo00303c
51. Li W, Hu J, Ji P, Zhu S, Zhu Y. Oral administration of bovine lactoferrin accelerates the healing of fracture in ovariectomized rats. J Bone Miner Metab (2020) 38(5):648–57. doi: 10.1007/s00774-020-01105-1
52. Bharadwaj S, Naidu AG, Betageri GV, Prasadarao NV, Naidu AS. Milk ribonuclease-enriched lactoferrin induces positive effects on bone turnover markers in postmenopausal women. Osteoporos Int (2009) 20(9):1603–11. doi: 10.1007/s00198-009-0839-8
53. Giannoudis P, Tzioupis C, Almalki T, Buckley R. Fracture healing in osteoporotic fractures: is it really different? A basic science perspective. Injury (2007) 38(Suppl 1):S90–9. doi: 10.1016/j.injury.2007.02.014
54. Conway JD, Shabtai L, Bauernschub A, Specht SC. Bmp-7 versus bmp-2 for the treatment of long bone nonunion. Orthopedics (2014) 37(12):e1049–57. doi: 10.3928/01477447-20141124-50
55. Gazzerro E, Gangji V, Canalis E. Bone morphogenetic proteins induce the expression of noggin, which limits their activity in cultured rat osteoblasts. J Clin Invest (1998) 102(12):2106–14. doi: 10.1172/JCI3459
56. Einhorn TA, Gerstenfeld LC. Fracture healing: mechanisms and interventions. Nat Rev Rheumatol (2015) 11(1):45–54. doi: 10.1038/nrrheum.2014.164
57. Li Y, Chen SK, Li L, Qin L, Wang XL, Lai YX. Bone defect animal models for testing efficacy of bone substitute biomaterials. J Orthop Translat (2015) 3(3):95–104. doi: 10.1016/j.jot.2015.05.002
58. Li W, Zhu S, Hu J. Bone regeneration is promoted by orally administered bovine lactoferrin in a rabbit tibial distraction osteogenesis model. Clin Orthop Relat Res (2015) 473(7):2383–93. doi: 10.1007/s11999-015-4270-5
59. Sagar N, Pandey AK, Gurbani D, Khan K, Singh D, Chaudhari BP, et al. In-vivo efficacy of compliant 3d nano-composite in critical-size bone defect repair: A six month preclinical study in rabbit. PloS One (2013) 8(10):e77578. doi: 10.1371/journal.pone.0077578
60. Pal S, Sayeed M, Kumar A, Verma DP, Harioudh MK, Verma NK, et al. Self-assembling nano-globular peptide from human lactoferrin acts as a systemic enhancer of bone regeneration: A novel peptide for orthopedic application. ACS Appl Mater Interf (2021) 13(15):17300–15. doi: 10.1021/acsami.1c01513
61. Gray T, Storz G, Papenfort K. Small proteins; big questions. J Bacteriol (2022) 204(1):e0034121. doi: 10.1128/JB.00341-21
62. Bruni N, Capucchio MT, Biasibetti E, Pessione E, Cirrincione S, Giraudo L, et al. Antimicrobial activity of lactoferrin-related peptides and applications in human and veterinary medicine. Molecules (2016) 21(6). doi: 10.3390/molecules21060752
63. Hwang PM, Zhou N, Shan X, Arrowsmith CH, Vogel HJ. Three-dimensional solution structure of lactoferricin B, an antimicrobial peptide derived from bovine lactoferrin. Biochemistry (1998) 37(12):4288–98. doi: 10.1021/bi972323m
64. Ulvatne H, Samuelsen O, Haukland HH, Kramer M, Vorland LH. Lactoferricin B inhibits bacterial macromolecular synthesis in escherichia coli and bacillus subtilis. FEMS Microbiol Lett (2004) 237(2):377–84. doi: 10.1016/j.femsle.2004.07.001
65. Wang WY, Wong JH, Ip DT, Wan DC, Cheung RC, Ng TB. Bovine lactoferrampin, human lactoferricin, and lactoferrin 1-11 inhibit nuclear translocation of hiv integrase. Appl Biochem Biotechnol (2016) 179(7):1202–12. doi: 10.1007/s12010-016-2059-y
66. Haney EF, Nazmi K, Lau F, Bolscher JG, Vogel HJ. Novel lactoferrampin antimicrobial peptides derived from human lactoferrin. Biochimie (2009) 91(1):141–54. doi: 10.1016/j.biochi.2008.04.013
67. Adao R, Nazmi K, Bolscher JG, Bastos M. C- and N-truncated antimicrobial peptides from lfampin 265 - 284: biophysical versus microbiology results. J Pharm Bioallied Sci (2011) 3(1):60–9. doi: 10.4103/0975-7406.76467
68. Stallmann HP, Faber C, Bronckers AL, Nieuw Amerongen AV, Wuisman PI. Osteomyelitis prevention in rabbits using antimicrobial peptide hlf1-11- or gentamicin-containing calcium phosphate cement. J Antimicrob Chemother (2004) 54(2):472–6. doi: 10.1093/jac/dkh346
69. Faber C, Stallmann HP, Lyaruu DM, Joosten U, von Eiff C, van Nieuw Amerongen A, et al. Comparable efficacies of the antimicrobial peptide human lactoferrin 1-11 and gentamicin in a chronic methicillin-resistant staphylococcus aureus osteomyelitis model. Antimicrob Agents Chemother (2005) 49(6):2438–44. doi: 10.1128/AAC.49.6.2438-2444.2005
70. Yan D, Chen D, Shen J, Xiao G, van Wijnen AJ, Im HJ. Bovine lactoferricin is anti-inflammatory and anti-catabolic in human articular cartilage and synovium. J Cell Physiol (2013) 228(2):447–56. doi: 10.1002/jcp.24151
71. Yan D, Kc R, Chen D, Xiao G, Im HJ. Bovine lactoferricin-induced anti-inflammation is, in part, via up-regulation of interleukin-11 by secondary activation of stat3 in human articular cartilage. J Biol Chem (2013) 288(44):31655–69. doi: 10.1074/jbc.M112.440420
72. Shi P, Fan F, Chen H, Xu Z, Cheng S, Lu W, et al. A bovine lactoferrin-derived peptide induced osteogenesis via regulation of osteoblast proliferation and differentiation. J Dairy Sci (2020) 103(5):3950–60. doi: 10.3168/jds.2019-17425
73. Vergis J, Malik SS, Pathak R, Kumar M, Ramanjaneya S, Kurkure NV, et al. Exploiting lactoferricin (17-30) as a potential antimicrobial and antibiofilm candidate against multi-drug-resistant enteroaggregative escherichia coli. Front Microbiol (2020) 11:575917. doi: 10.3389/fmicb.2020.575917
74. Rahman R, Fonseka AD, Sua SC, Ahmad M, Rajendran R, Ambu S, et al. Inhibition of breast cancer xenografts in a mouse model and the induction of apoptosis in multiple breast cancer cell lines by lactoferricin B peptide. J Cell Mol Med (2021) 25(15):7181–9. doi: 10.1111/jcmm.16748
75. van der Kraan MI, Nazmi K, Teeken A, Groenink J, van 't Hof W, Veerman EC, et al. Lactoferrampin, an antimicrobial peptide of bovine lactoferrin, exerts its candidacidal activity by a cluster of positively charged residues at the C-terminus in combination with a helix-facilitating N-terminal part. Biol Chem (2005) 386(2):137–42. doi: 10.1515/BC.2005.017
76. Wang Y, Morton JD, Bekhit AEA, Carne A, Mason SL. Amino acid sequences of lactoferrin from red deer (Cervus elaphus) milk and antimicrobial activity of its derived peptides lactoferricin and lactoferrampin. Foods (2021) 10(6). doi: 10.3390/foods10061305
77. van der Kraan MI, Groenink J, Nazmi K, Veerman EC, Bolscher JG, Nieuw Amerongen AV. Lactoferrampin: A novel antimicrobial peptide in the N1-domain of bovine lactoferrin. Peptides (2004) 25(2):177–83. doi: 10.1016/j.peptides.2003.12.006
78. Vigata M, Meinert C, Hutmacher DW, Bock N. Hydrogels as drug delivery systems: A review of current characterization and evaluation techniques. Pharmaceutics (2020) 12(12). doi: 10.3390/pharmaceutics12121188
79. Takaoka R, Hikasa Y, Hayashi K, Tabata Y. Bone regeneration by lactoferrin released from a gelatin hydrogel. J Biomater Sci Polym Ed (2011) 22(12):1581–9. doi: 10.1163/092050610X517095
80. Russo E, Villa C. Poloxamer hydrogels for biomedical applications. Pharmaceutics (2019) 11(12). doi: 10.3390/pharmaceutics11120671
81. Park YE, Chandramouli K, Watson M, Zhu M, Callon KE, Tuari D, et al. Sustained delivery of lactoferrin using poloxamer gels for local bone regeneration in a rat calvarial defect model. Mater (Basel) (2021) 15(1). doi: 10.3390/ma15010212
Keywords: lactoferrin, bone remodeling, signaling pathways, fracture repair, osteoporosis
Citation: Tian M, Han Y-b, Yang G-y, Li J-l, Shi C-s and Tian D (2023) The role of lactoferrin in bone remodeling: evaluation of its potential in targeted delivery and treatment of metabolic bone diseases and orthopedic conditions. Front. Endocrinol. 14:1218148. doi: 10.3389/fendo.2023.1218148
Received: 06 May 2023; Accepted: 28 July 2023;
Published: 23 August 2023.
Edited by:
Michaela Tencerova, Academy of Sciences of the Czech Republic (ASCR), CzechiaReviewed by:
Naibedya Chattopadhyay, Central Drug Research Institute (CSIR), IndiaAnirudha Karvande, Children’s Hospital of Philadelphia, United States
Copyright © 2023 Tian, Han, Yang, Li, Shi and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dan Tian, dGRhbkBtYWlsLmpsdS5lZHUuY24=
 Miao Tian1
Miao Tian1 Dan Tian
Dan Tian