- 1Department of Thyroid & Parathyroid Surgery, West China Hospital, Sichuan University, Chengdu, China
- 2Department of Surgery, The University of Hong Kong-Shenzhen Hospital, Shenzhen, China
Background: Tumor multifocality is frequently observed in papillary thyroid carcinoma (PTC). However, the maximum tumor diameter (MTD), currently utilized in various staging schemes, might not accurately indicate the level of aggressiveness exhibited by multifocal tumors. We aimed to investigate the relationship between total tumor diameter (TTD) and clinicopathological features of papillary thyroid carcinoma.
Methods: Retrospective data analysis was done on 1936 individuals who underwent complete thyroidectomy for PTC. Patients were classified into subgroups according to unilateral multifocality, central lymph node metastasis (CLNM) and lateral lymph node metastasis (LLNM). The relationships of clinicopathological features among these groups were analyzed.
Results: Unilateral multifocality was observed in 117 patients. The clinicopathological features of the unilateral multifocal PTC were similar to the unifocal PTC with approximate TTD. The unilateral multifocality played no independent role in CLNM and LLNM. Moreover, the efficiency of TTD in predicting CLNM and LLNM was significantly higher than that of MTD.
Conclusion: In the case of unilateral multifocal PTC, TTD is a more accurate indicator of the biological characteristics of the tumor than MTD.
Introduction
Thyroid cancer is the ninth most common cancer worldwide, and its prevalence has increased significantly over the last 40 years (1). In 2020, there were 586,202 new cases of thyroid cancer, with papillary thyroid carcinoma (PTC) accounting for more than 80% of all thyroid cancers (1). In general, PTC is regarded as a disease with a favorable prognosis. However, some PTCs with specific clinicopathological characteristics are aggressive and have poor prognoses (2). Tumor multifocality is a frequent observation in PTC, occurring in 18–87% of documented cases (3). Multifocality in thyroid cancer is the concurrent existence of multiple tumor foci within the thyroid gland (4). Depending on where the tumors are, the meaning of multifocality can be broken down into unilateral multifocality (two or more lesions in the same lobe), bilateral multifocality (two or more lesions in the same lobe and one or more lesions in the opposite lobe), or bilaterality (one lesion in each lobe). And a report showed that unilateral multifocality and bilaterality could be two different multifocal entities in patients with PTC (5).
Multiple studies have shown that multifocality is associated with high-risk characteristics of PTC, such as an aggressive histologic subtype, extrathyroidal extension, lymph node involvement, distant metastasis, and recurrence (6, 7). However, the current staging system still defines the size of multifocal tumors in the same manner as that of unifocal tumors, concentrating on the maximum tumor diameter (MTD) (8). In recent years, many studies have also reported the concept of total tumor diameter (TTD) (9–11). Some demonstrated that TTD could reflect a tumor’s biological characteristics and aggressiveness more accurately than MTD (12).
However, the previous researchers took the unilateral multifocality and bilaterality as the same multifocality in their studies (5, 11). The purpose of the present study was to evaluate the association between TTD and unilateral multifocal PTC using the propensity score matching (PSM) method to provide an updated and more comprehensive assessment of the risk for unilateral multifocal PTC.
Methods
Patients
Data for pathologically confirmed PTC patients who underwent thyroid surgery in the Department of Thyroid Surgery, West China Hospital (Chengdu, China) between 2013 and 2018 were collected and reviewed. 2322 PTC Patients who were > 18 years old and underwent the first-time total thyroidectomy with central lymph nodes dissection were enrolled. After excluding PTC with bilateral multifocality (260 cases,11.2%) and bilaterality (126 cases,5.4%), 117 unilateral multifocal cases and 1819 unifocal cases were involved in the study. The collected patient data included age, gender, MTD, TTD, central lymph node metastasis (CLNM), lateral lymph node metastasis (LLNM), capsular invasion, gross extrathyroidal extension (gETE), and so on. The medical ethics committee of West China Hospital, Sichuan University approved the study. The patients in the study provided informed consent. All methods were carried out following relevant regulations and guidelines.
Definition
In the study, MTD was referred to as either the primary tumor focus for unifocal PTC or the diameter of the greatest tumor focus in multifocal PTC. TTD was defined as the sum of each tumor lesion’s diameter within a lobe. Unilateral multifocality was defined as two or more lesions in the same lobe. CLNM was defined as tumor metastasis in level VI cervical lymph nodes. Based on the postoperative pathological diagnosis, LLNM was defined as tumor metastasis in lymph nodes of levels II, III, IV, and V in the neck region. Based on the postoperative pathology diagnosis, the capsular invasion was defined as a tumor involving the capsule but not penetrating it. gETE was characterized as the tumor penetrating the thyroid capsule, and invading surrounding tissues or organs, for example, esophagus, trachea and recurrent laryngeal nerve (RLN).
Study design and grouping
A total of 1936 patients were involved in the study. First, the above data was divided into two groups according to the number of tumor lesions, the unilateral multifocal PTC group and the unifocal PTC group. PSM was performed based on age, sex, body mass index (BMI), nodular goiter (NG), Hashimoto’s thyroiditis (HT), capsule invasion, gETE, and TTD or MTD to minimize the effect of confounders on the outcomes between these groups. Then the data was divided into another two groups: the with CLNM group and the without CLNM group. PSM was performed based on age, sex, BMI, NG, HT, capsule invasion, gETE, TTD and MTD. Moreover, the data was divided according to have LLNM or not. And the same PSM as CLNM was performed.
Surgical strategy
All patients enrolled in the study underwent total thyroidectomy. The central lymph nodes were routinely dissected. Patients found to have cervical lymph node metastases after undergoing fine-needle aspiration biopsy or preoperative imaging were candidates for lateral lymph node dissection. This diagnosis was verified by the intraoperative frozen sections.
Statistical analysis
SPSS 26.0 software was applied to the data analysis. Continuous variables (confirmed by the Kolmogorov-Smirnov test) with non-normal distribution were presented as the median. Differences between continuous variables were examined using the Mann–Whitney U test. Categorical variables were presented as absolute value. Pearson’s χ2 test or Fisher exact test was used to investigate the heterogeneity between categorical variables. Univariate and multivariate logistic regression analyses were used to identify the risk factors for CLNM and LLNM. The receiver operating characteristic (ROC) curve was plotted and compared the area under the curve (AUC) values. R software (R Core Team, Version 4.1.2, Vienna, Austria) with the “matchit” package was used to perform the PSM. The nearest neighbor algorithm was used as the matching method, with the caliper value set to 0.02. A P value of < 0.05 was considered statistically significant.
Results
Comparison of baseline characteristics between the unilateral multifocal PTC group and the unifocal PTC group
A total of 1936 eligible patients were enrolled and categorized into the unilateral multifocal PTC group (n =117) and the unifocal PTC group (n = 1819). Demographic and clinicopathological characteristics of the patients were presented in Table 1. Before PSM, the unilateral multifocal PTC group had larger TTD (16.00[12.50-23.00]vs10.00[8.00-15.00], P<0.001). CLNM (62.4%vs48.9%, P=0.005) and LLNM (31.6%vs20.2%, P=0.003) were reported more frequently in the unilateral multifocal PTC group than in the unifocal PTC group. The patients with unilateral multifocal PTC were more likely to have NG (66.7%vs52.9%, P=0.009).
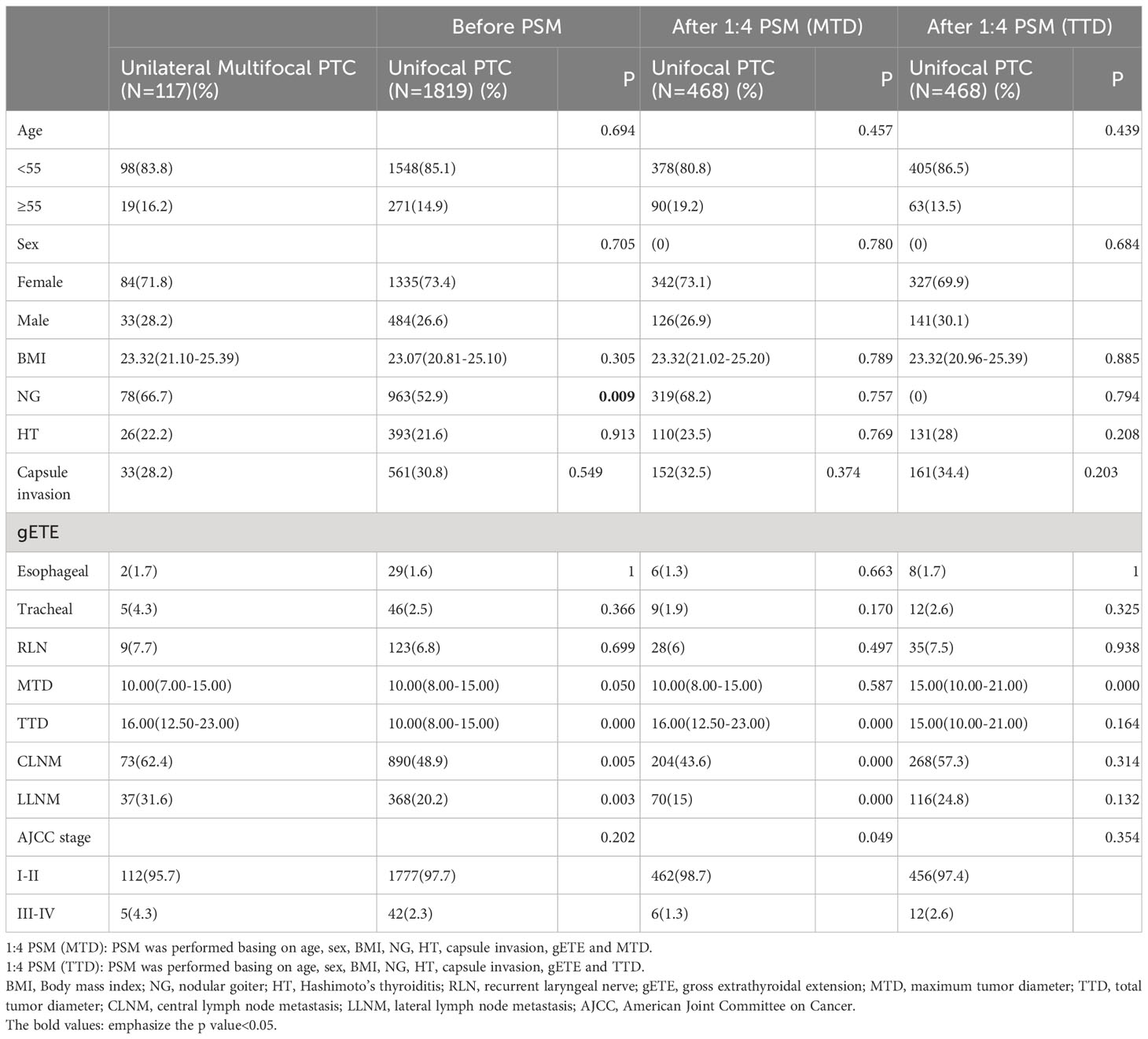
Table 1 Comparison of baseline characteristics between the unilateral multifocal PTC group and the unifocal PTC group.
After PSM was performed basing on age, sex, BMI, NG, HT, capsule invasion, gETE and MTD, significant differences between the unilateral multifocal PTC group and the unifocal PTC group in the rate of CLNM (62.4%vs43.6%, P<0.001) and LLNM (31.6%VS15.0%, P<0.001) still existed. And the unilateral multifocal PTC group had a higher proportion with AJCC stage III-IV (4.3%vs2.3%, P=0.049). After PSM was performed basing on age, sex, BMI, NG, HT, capsule invasion, gETE and TTD, differences between the unilateral multifocal PTC group and the unifocal PTC group disappeared in all characteristics except MTD (10.00[8.00-15.00]vs15.00[10.00-21.00], P<0.001). (Table 1).
Comparison of baseline characteristics between the without LNM (lymph node metastasis) group and the with LNM group
Table 2 listed the demographic and clinicopathological traits of the patients. CLNM occurred more frequently in male patients (30.9%vs22.5%, P<0.001), patients aged less than 55 years (87.9% vs 82.2%, P = 0.001), patients with lower BMI (22.89[20.70-25.00]vs 23.24[20.97-25.20], P<0.001). NG was reported less frequently in the With CLNM group (47.8%vs59.7%, P<0.001). While unilateral multifocality was reported more frequently in the With CLNM group (7.6%vs4.5%, P=0.005). There was a higher proportion of gETE in the With CLNM group (Esophageal 2.6% vs0.6%, P=0.001; Tracheal 3.7%vs1.5%, P=0.003; RLN 9.2%vs4.4%, P<0.001). The group with CLNM had larger MTD (13.00[10.00-20.00]vs10.00[7.00-14.00], P<0.001) and TTD (14.00[10.00-20.00]vs10.00[7.00-15.00], P<0.001). After PSM was performed basing on age, sex, BMI, NG, HT, capsule invasion, gETE, MTD and TTD, differences between the Without CLNM group and With CLNM group in the incidence of unilateral multifocality disappeared.
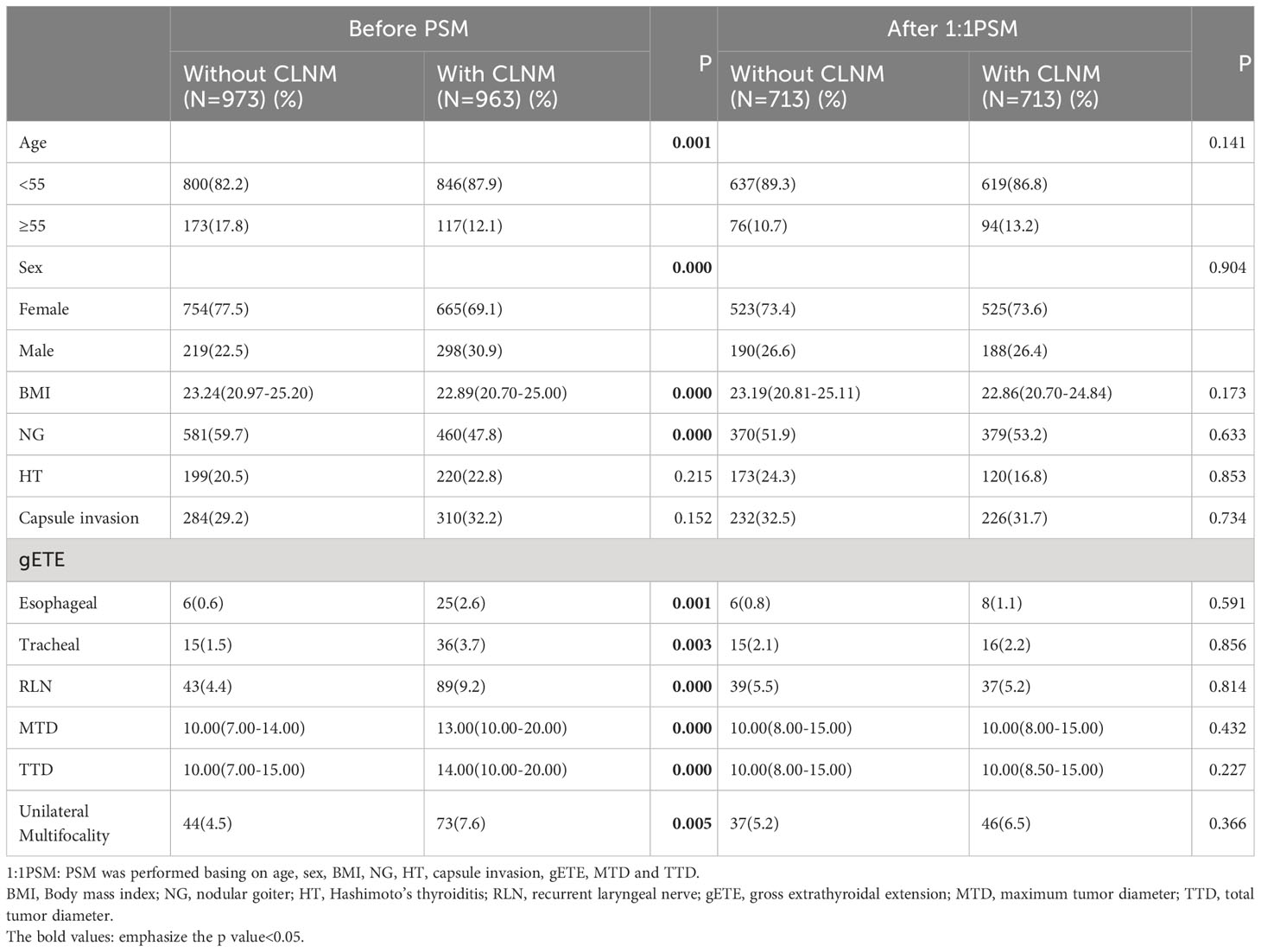
Table 2 Comparison of baseline characteristics between the Without CLNM group and the With CLNM group.
Male patients (32.3%vs25.2%, P=0.004), patients without NG (43.7%vs56.4%, P<0.001), patients with gETE (Esophageal 5.2% vs0.7%, P<0.001; Tracheal 6.7%vs1.6%, P<0.001; RLN 14.1%vs4.9%, P<0.001), patients with larger MTD (15.00[10.00-25.00]vs 10.00[7.00-15.00], P<0.001) or TTD (16.00[10.00-25.00]vs 10.00[8.00-15.00], P<0.001), patients with unilateral multifocal PTC (9.1%vs5.2%, P=0.003) were more likely to have LLNM (Table 3). After PSM was performed basing on age, sex, BMI, NG, HT, capsule invasion, gETE, MTD and TTD, differences in the incidence of unilateral multifocality between the groups With and Without LLNM vanished.
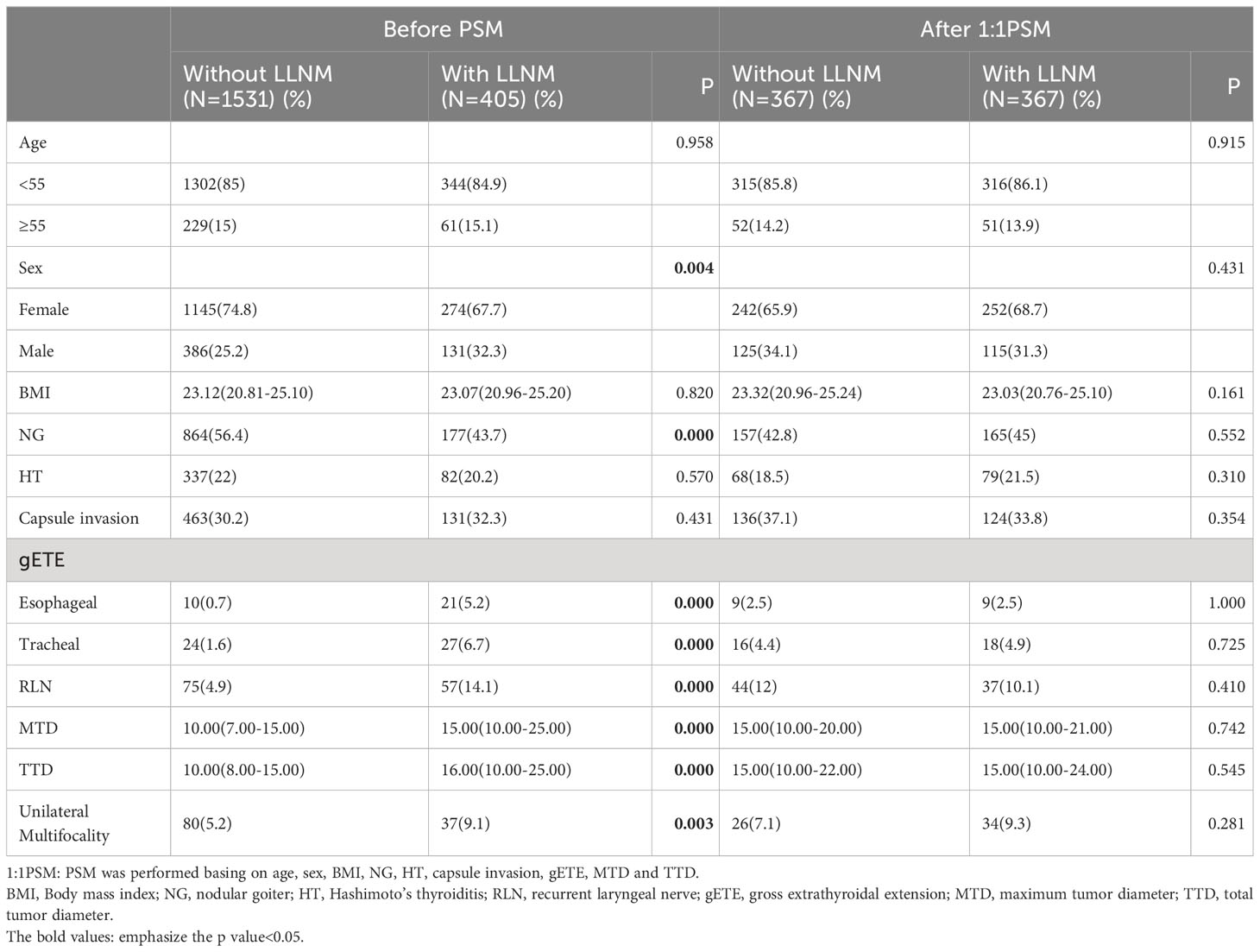
Table 3 Comparison of baseline characteristics between the Without LLNM group and the With LLNM group.
Risk factors for CLNM and LLNM
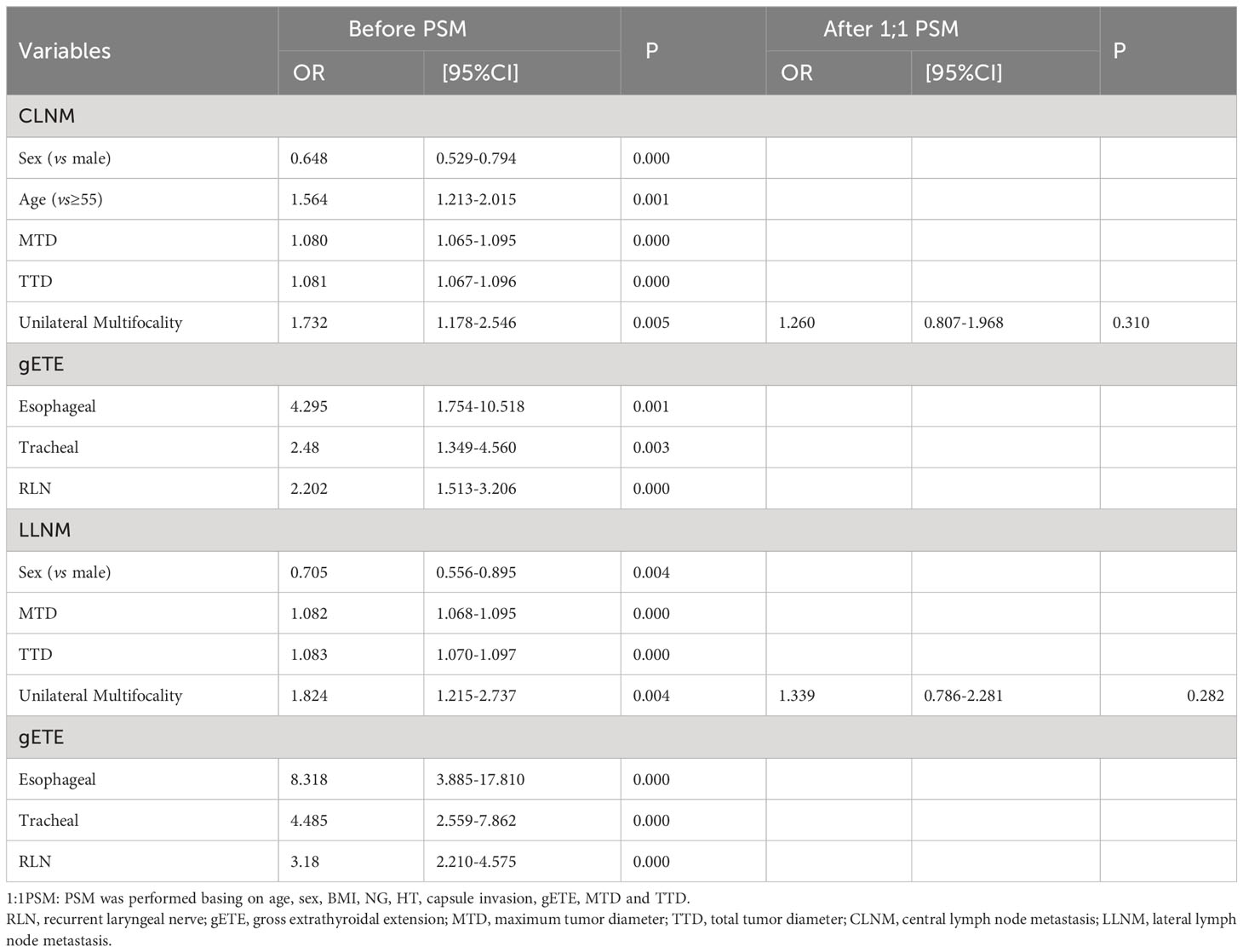
We used both univariate and multivariate analyses to obtain the risk factors for CLNM and LLNM. Univariate analysis identified 8 variables (Sex, Age, MTD, TTD, Esophageal extension, Tracheal extension, RLN extension and Unilateral multifocality) linked with CLNM, but the relationship among unilateral multifocality and CLNM was lost after PSM was performed basing on age, sex, BMI, NG, HT, capsule invasion, gETE, MTD and TTD (Table 4). Univariate regression analysis indicated that LLNM was significantly associated with sex, MTD, TTD, unilateral multifocality, esophageal extension, tracheal extension and RLN extension. The correlation between unilateral multifocality and LLNM was no longer present after PSM was performed basing on age, sex, BMI, NG, HT, capsule invasion, gETE, MTD and TTD (Table 4).
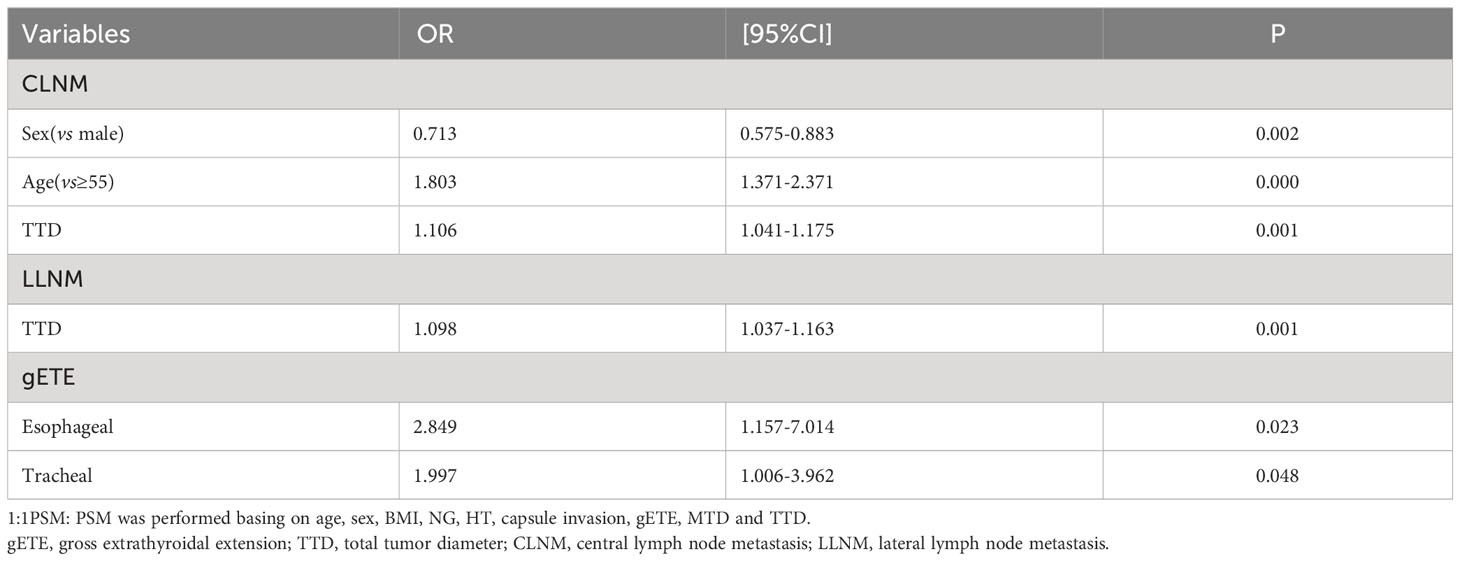
Multivariate analysis found 3 variables (Sex, Age, TTD), which were risk factors of CLNM, and 3 variables (TTD, Esophageal extension, Tracheal extension), which were risk factors of LLNM (Table 5).
Comparison of prediction efficiency of TTD and MTD for CLNM and LLNM
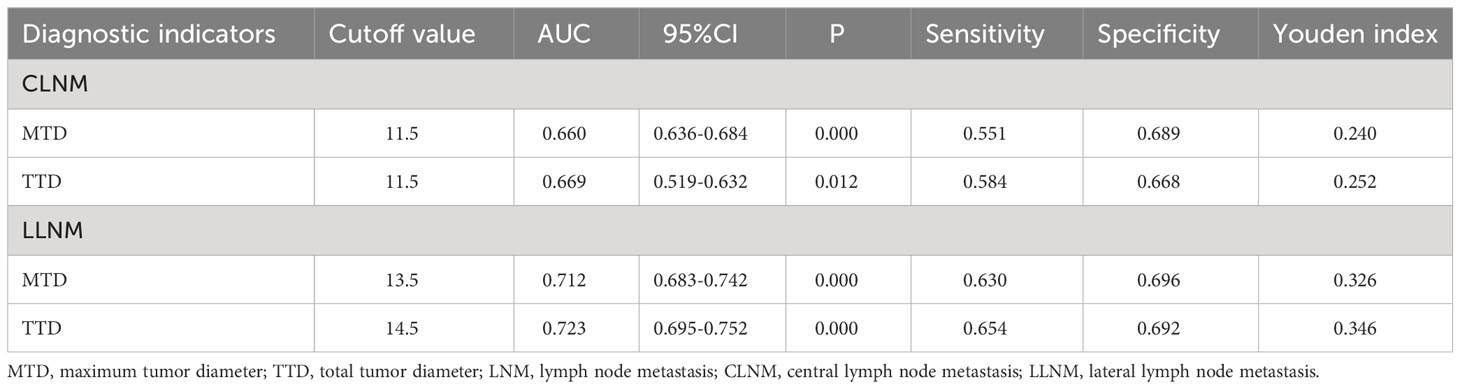
The respective calculated AUC values of 0.660(MTD) and 0.669(TTD) were statistically significantly different (P=0.005) (Table 6), suggesting that TTD may be a better predictor of CLNM for unilateral multifocal PTC (Figure 1). Meanwhile, the computed AUC values of 0.712(MTD) and 0.723(TTD), respectively, differed statistically(p=0.011) (Table 6), showing that for unilateral multifocal PTC, TTD may be a stronger predictor of LLNM (Figure 2).
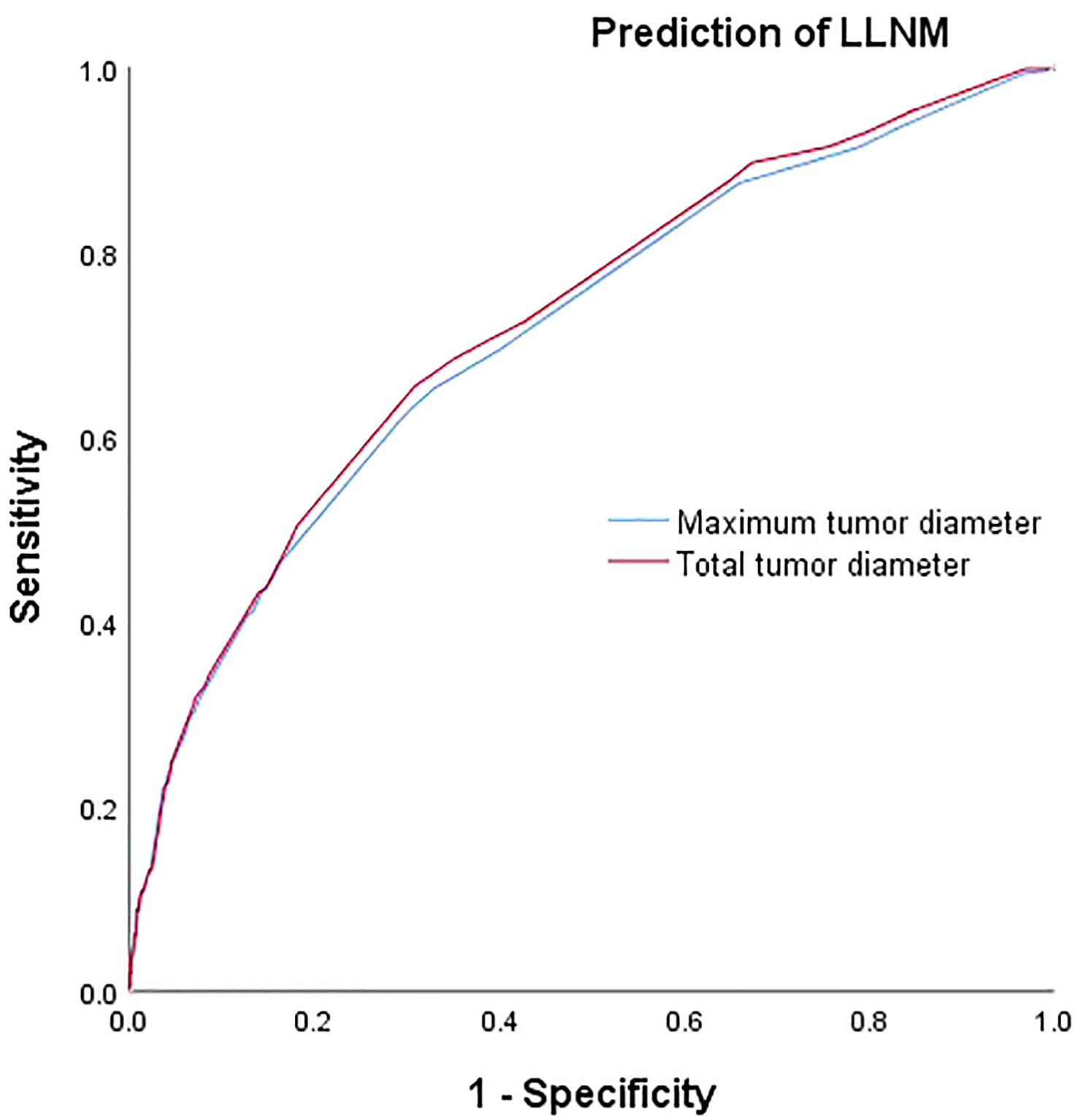
Figure 1 Comparison of prediction efficiency of TTD and MTD for CLNM. AUC values of 0.660(MTD) and 0.669(TTD) differed statistically (P=0.005). MTD, maximum tumor diameter; TTD, total tumor diameter; CLNM, central lymph node metastasis.
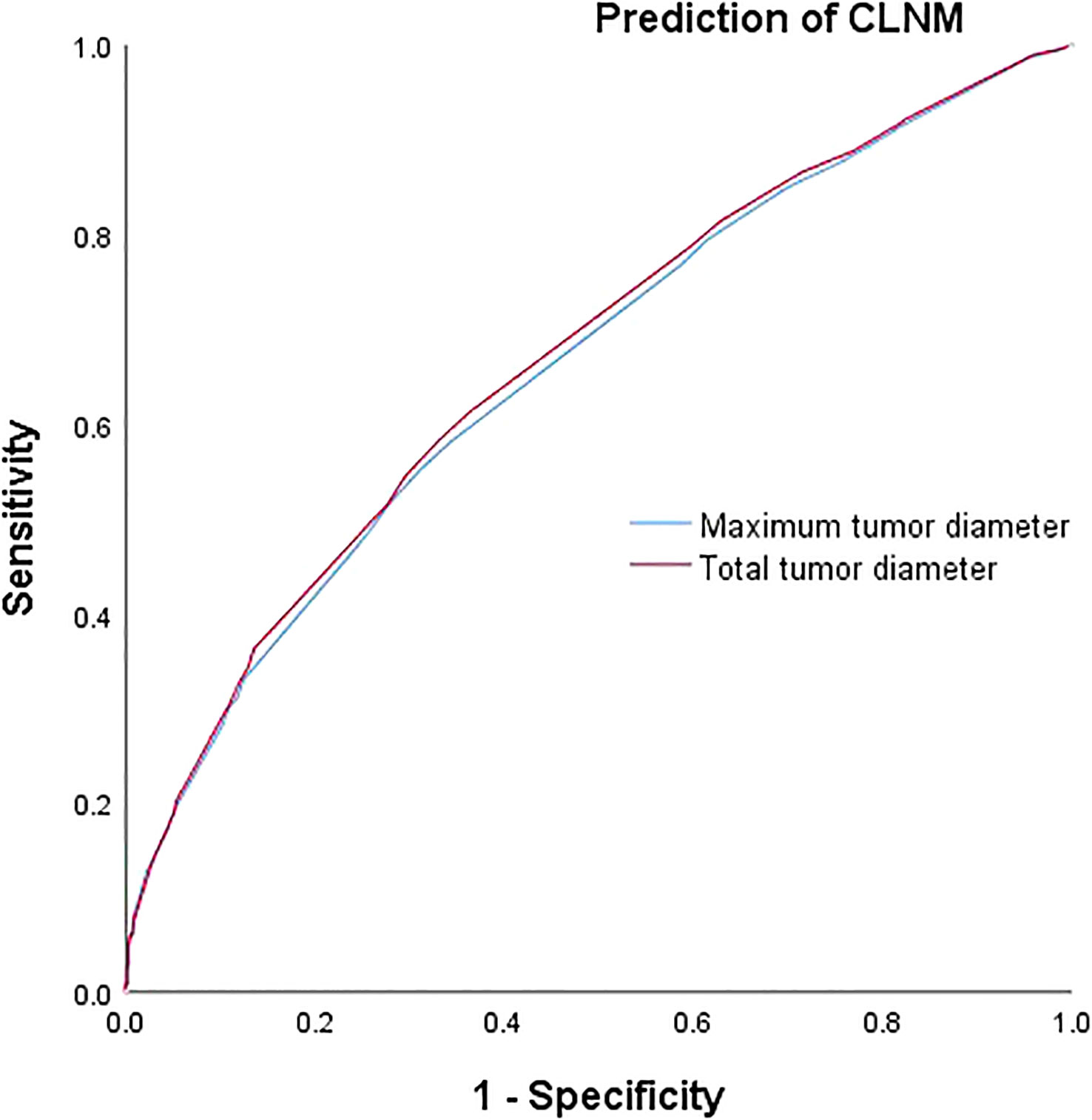
Figure 2 Comparison of prediction efficiency of TTD and MTD for LLNM. AUC values of 0.712(MTD) and 0.723(TTD) differed statistically (P=0.011). MTD, maximum tumor diameter; TTD, total tumor diameter; LLNM, lateral lymph node metastasis.
Discussion
The impact of TTD in multifocal tumors has been reported on diseases like breast cancer and kidney cancer (13–15). Additionally, TTD’s significance in thyroid cancer has been documented in prior researches. They found that TTD was associated with lymph node metastasis and may represent the tumor biological traits and aggressiveness more accurately than MTD (9, 11, 12, 16). However, the researchers treated unilateral multifocality and bilaterality as the same multifocality types. The current study used a PSM method to investigate the association between TTD and clinicopathological characteristics in unilateral multifocal PTC based on thyroid cancer patients in our center.
The AJCC TNM classification determines the primary tumor (T) stage according to the largest tumor size and ETE (17), even in the multifocal PTC. The impact of other smaller tumor foci on aggressiveness has not been well investigated. Evaluating the largest tumor size and neglecting the smaller foci in multifocal tumors may underestimate the clinicopathologic aspects of malignancies, impacting tumor staging and treatment. In our study, we did two different types of PSM, matching the MTD and TTD respectively, to explore which diameter could represent the tumor biological characteristics and aggressiveness more accurately. We found that the lymph node metastasis rate had significant differences between unilateral multifocal PTC and unifocal PTC after PSM matching the MTD. However, no difference was found after PSM matching the TTD. In other words, the tumor biological traits of the unilateral multifocal PTC were similar to the unifocal PTC with approximate TTD. This result was identical to the previous study (11), which mainly focused on papillary thyroid microcarcinoma (PTMC), showing that micro-PTC tended to behave like the macro-PTC once the TTD exceeded 1.0 cm.
LNM was common among PTC patients. According to earlier research, LNM was present in 30% to 90% of PTC patients, particularly in the central region (18–20), which was one of the major factors that contributed to postoperative recurrence. Although reoperation could still result in a favorable prognosis, patients were more probably to suffer surgical complications (21, 22). Therefore, it was crucial for the patient’s overall prognosis that the lymph nodes were managed at the initial surgery. Because the unique anatomical position limited the diagnostic ability of the current imaging techniques (23), the clinical lymph nodes negative (cN0) was unreliable. Whether prophylactic central lymph nodes dissection (PCLND) was appropriate for cN0 PTC patients was still up for debate. PCLND at the time of the initial surgery, according to Guo et al. (21, 24), may have advantages, including avoiding the significant risk of long-term hypoparathyroidism and RLN damage associated with repeat surgery. Yoon et al. (25), on the other hand, came to the conclusion that the chance of developing permanent hypocalcemia may rise and parathyroid hormone levels may be dramatically lowered following PCLND. In order to direct therapeutic decision-making, it was helpful to identify high-risk variables for LNM. In our study, TTD was significantly correlated with CLNM and LLNM. Also, the ability of TTD to predict CLNM and LLNM was much better than that of MTD in PTC, showing that TTD might be a better predictor of LNM for unilateral multifocal PTC.
In our data, 62.4% of unilateral multifocal PTC presented with CLNM and 31.6% presented with LLNM, significantly higher than unifocal PTC (48.9%&20.2%). Other researches showed the same results (26, 27). In a report by Lombardi et al., multifocal illness increased cervical lymph node recurrence 17.9-fold (28). In another study, the rate of LNM for patients with multifocal PTCs was 55.6%, while it was only 28.6% for those with unifocal illnesses (29). However, the association between LNM and multifocality remained controversial. According to certain studies, multifocality was not independently related to LNM (30). Many other studies have focused on the risk factors of LNM for multifocal PTC patients. For example, Zheng WH proposed that multifocality should be evaluated when selecting patients for PCLND (31). Some reports showed that multifocality was an independent risk factor for CLNM and LLNM (32, 33). As found in previous studies (33–36), our univariate analyses revealed an association between multifocality and LNM. However, on multivariate adjustment for classic clinicopathological risk factors, including MTD and TTD, the association between unilateral multifocality and LNM was lost in PTC. The result demonstrated that unilateral multifocality played no independent role in LNM.
Once, it was thought that multifocality in PTC was caused by the lymphatic spread of tumor cells from one malignant clone. This theory was backed by the fact that tumor cells were often found in lymphatic vessels and that lymph node metastases happened often. But others had the opposite ideas. Bansal et al. found that as many as 60% of multifocal PTCs may have multiple sources, and these origins were generally found in various lobes. The same mutation status was typically shared by multifocality within the same lobe, though (37, 38). A clinical research also showed that unilateral multifocality differed from bilaterality (5). Unlike earlier studies (5, 11), we only included unilateral multifocal PTC in our study to remove the distraction of different types of multifocality and used PSM method to provide an updated and more comprehensive assessment of the risk for unilateral multifocal PTC.
There were some limitations in the study. The inclusion of patients based on their characteristics resulting in a selection bias in this retrospective analysis. Despite the fact that we used PSM, the results could still be influenced by selection bias. Moreover, the participant sample of unilateral multifocal PTC was small. Our data could be hampered by the small sample size. Conducting more validation studies with a bigger cohort and long-term follow-up is necessary.
Conclusion
In conclusion, the current study offers fresh evidence of the connection between TTD and PTC. TTD can more accurately reflect the biological characteristics and aggressiveness of unilateral multifocal PTC than MTD.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by The medical ethics committee of West China Hospital, Sichuan University. The studies were conducted in accordance with the local legislation and institutional requirements.
Author contributions
Author Contributions (I) Conception and design: A-PS, Z-JW (II) Administrative support: A-PS (III) Provision of study materials or patients: A-PS, Z-JW (IV) Collection and assembly of data: Z-JW, Z-WC, B-YX, HG (V) Data analysis and interpretation: Z-JW, MA. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Kim SH, Roh JL, Gong G, Cho KJ, Choi SH, Nam SY, et al. Differences in the recurrence and survival of patients with symptomatic and asymptomatic papillary thyroid carcinoma: an observational study of 11,265 person-years of follow-up. Thyroid (2016) 26:1472–9. doi: 10.1089/thy.2016.0238
3. Qu N, Zhang L, Ji QH, Zhu YX, Wang ZY, Shen Q, et al. Number of tumor foci predicts prognosis in papillary thyroid cancer. BMC Cancer (2014) 14:914. doi: 10.1186/1471-2407-14-914
4. Kuhn E, Teller L, Piana S, Rosai J, Merino MJ. Different clonal origin of bilateral papillary thyroid carcinoma, with a review of the literature. Endocr Pathol (2012) 23:101–7. doi: 10.1007/s12022-012-9202-2
5. Cai J, Fang F, Chen J, Xiang D. Unilateral multifocality and bilaterality could be two different multifocal entities in patients with papillary thyroid microcarcinoma. BioMed Res Int (2020) 2020:9854964. doi: 10.1155/2020/9854964
6. Vuong HG, Duong UNP, Pham TQ, Tran HM, Oishi N, Mochizuki K, et al. Clinicopathological risk factors for distant metastasis in differentiated thyroid carcinoma: A meta-analysis. World J Surg (2018) 42:1005–17. doi: 10.1007/s00268-017-4206-1
7. Qu N, Zhang L, Lu ZW, Ji QH, Yang SW, Wei WJ, et al. Predictive factors for recurrence of differentiated thyroid cancer in patients under 21 years of age and a meta-analysis of the current literature. Tumour Biol (2016) 37:7797–808. doi: 10.1007/s13277-015-4532-6
8. Perrier ND, Brierley JD, Tuttle RM. Differentiated and anaplastic thyroid carcinoma: Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin (2018) 68:55–63. doi: 10.3322/caac.21439
9. Zhao Q, Ming J, Liu C, Shi L, Xu X, Nie X, et al. Multifocality and total tumor diameter predict central neck lymph node metastases in papillary thyroid microcarcinoma. Ann Surg Oncol (2013) 20:746–52. doi: 10.1245/s10434-012-2654-2
10. Liu C, Wang S, Zeng W, Guo Y, Liu Z, Huang T. Total tumour diameter is superior to unifocal diameter as a predictor of papillary thyroid microcarcinoma prognosis. Sci Rep (2017) 7:1846. doi: 10.1038/s41598-017-02165-6
11. Feng JW, Pan H, Wang L, Ye J, Jiang Y, Qu Z. Total tumor diameter: the neglected value in papillary thyroid microcarcinoma. J Endocrinol Invest (2020) 43:601–13. doi: 10.1007/s40618-019-01147-x
12. Jiang KC, Lin B, Zhang Y, Zhao LQ, Luo DC. Total tumor diameter is a better indicator of multifocal papillary thyroid microcarcinoma: A propensity score matching analysis. Front Endocrinol (2022) 13:974755. doi: 10.3389/fendo.2022.974755
13. Karakas Y, Dizdar O, Aksoy S, Hayran M, Altundag K. The effect of total size of lesions in multifocal/multicentric breast cancer on survival. Clin Breast Cancer (2018) 18:320–7. doi: 10.1016/j.clbc.2017.11.002
14. Cabioglu N, Ozmen V, Kaya H, Tuzlali S, Igci A, Muslumanoglu M, et al. Increased lymph node positivity in multifocal and multicentric breast cancer. J Am Coll Surg (2009) 208:67–74. doi: 10.1016/j.jamcollsurg.2008.09.001
15. Siracusano S, Novara G, Antonelli A, Artibani W, Bertini R, Carini M, et al. Prognostic role of tumour multifocality in renal cell carcinoma. BJU Int (2012) 110:E443–8. doi: 10.1111/j.1464-410X.2012.11121.x
16. Tam AA, Ozdemir D, Ogmen BE, Faki S, Dumlu EG, Yazgan AK, et al. Should multifocal papillary thyroid carcinomas classified as T1a with a tumor diameter sum of 1 to 2 centimeters be reclassified as T1b? Endocr Pract (2017) 23:526–35. doi: 10.4158/EP161488.OR
17. Chun YS, Pawlik TM, Vauthey JN. 8th edition of the AJCC cancer staging manual: pancreas and hepatobiliary cancers. Ann Surg Oncol (2018) 25:845–7. doi: 10.1245/s10434-017-6025-x
18. Podnos YD, Smith D, Wagman LD, Ellenhorn JD. The implication of lymph node metastasis on survival in patients with well-differentiated thyroid cancer. Am Surg (2005) 71:731–4. doi: 10.1177/000313480507100907
19. Zaydfudim V, Feurer ID, Griffin MR, Phay JE. The impact of lymph node involvement on survival in patients with papillary and follicular thyroid carcinoma. Surgery (2008) 144:1070–7. doi: 10.1016/j.surg.2008.08.034
20. Caron NR, Clark OH. Papillary thyroid cancer: surgical management of lymph node metastases. Curr Treat Options Oncol (2005) 6:311–22. doi: 10.1007/s11864-005-0035-9
21. Guo K, Wang Z. Risk factors influencing the recurrence of papillary thyroid carcinoma: a systematic review and meta-analysis. Int J Clin Exp Pathol (2014) 7:5393–403.
22. Wei Q, Wu D, Luo H, Wang X, Zhang R, Liu Y. Features of lymph node metastasis of papillary thyroid carcinoma in ultrasonography and CT and the significance of their combination in the diagnosis and prognosis of lymph node metastasis. J BUON (2018) 23:1041–8.
23. Al Afif A, Williams BA, Rigby MH, Bullock MJ, Taylor SM, Trites J, et al. Multifocal papillary thyroid cancer increases the risk of central lymph node metastasis. Thyroid (2015) 25:1008–12. doi: 10.1089/thy.2015.0130
24. Chen Y, Chen S, Lin X, Huang X, Yu X, Chen J. Clinical analysis of cervical lymph node metastasis risk factors and the feasibility of prophylactic central lymph node dissection in papillary thyroid carcinoma. Int J Endocrinol (2021) 2021:6635686. doi: 10.1155/2021/6635686
25. So YK, Seo MY, Son YI. Prophylactic central lymph node dissection for clinically node-negative papillary thyroid microcarcinoma: influence on serum thyroglobulin level, recurrence rate, and postoperative complications. Surgery (2012) 151:192–8. doi: 10.1016/j.surg.2011.02.004
26. Lee SH, Lee SS, Jin SM, Kim JH, Rho YS. Predictive factors for central compartment lymph node metastasis in thyroid papillary microcarcinoma. Laryngoscope (2008) 118:659–62. doi: 10.1097/MLG.0b013e318161f9d1
27. Agarwal S, Agarwal A, Chand G. Incidental papillary microcarcinoma of the thyroid–further evidence of a very low Malignant potential: a retrospective clinicopathologic study with up to 30 years of follow-up. Ann Surg Oncol (2011) 18(Suppl 3):S306. doi: 10.1245/s10434-011-1865-2
28. Lombardi CP, Bellantone R, De Crea C, Paladino NC, Fadda G, Salvatori M, et al. Papillary thyroid microcarcinoma: extrathyroidal extension, lymph node metastases, and risk factors for recurrence in a high prevalence of goiter area. World J Surg (2010) 34:1214–21. doi: 10.1007/s00268-009-0375-x
29. Lim YC, Choi EC, Yoon YH, Kim EH, Koo BS. Central lymph node metastases in unilateral papillary thyroid microcarcinoma. Br J Surg (2009) 96:253–7. doi: 10.1002/bjs.6484
30. Zhou YL, Gao EL, Zhang W, Yang H, Guo GL, Zhang XH, et al. Factors predictive of papillary thyroid micro-carcinoma with bilateral involvement and central lymph node metastasis: a retrospective study. World J Surg Oncol (2012) 10:67. doi: 10.1186/1477-7819-10-67
31. Zheng W, Wang K, Wu J, Wang W, Shang J. Multifocality is associated with central neck lymph node metastases in papillary thyroid microcarcinoma. Cancer Manag Res (2018) 10:1527–33. doi: 10.2147/CMAR.S163263
32. Sessa L, Lombardi CP, De Crea C, Tempera SE, Bellantone R, Raffaelli M. Risk factors for central neck lymph node metastases in micro- versus macro- clinically node negative papillary thyroid carcinoma. World J Surg (2018) 42:623–9. doi: 10.1007/s00268-017-4390-z
33. So YK, Kim MJ, Kim S, Son YI. Lateral lymph node metastasis in papillary thyroid carcinoma: A systematic review and meta-analysis for prevalence, risk factors, and location. Int J Surg (2018) 50:94–103. doi: 10.1016/j.ijsu.2017.12.029
34. Kim KJ, Kim SM, Lee YS, Chung WY, Chang HS, Park CS. Prognostic significance of tumor multifocality in papillary thyroid carcinoma and its relationship with primary tumor size: a retrospective study of 2,309 consecutive patients. Ann Surg Oncol (2015) 22:125–31. doi: 10.1245/s10434-014-3899-8
35. Qu N, Zhang L, Wu WL, Ji QH, Lu ZW, Zhu YX, et al. Bilaterality weighs more than unilateral multifocality in predicting prognosis in papillary thyroid cancer. Tumour Biol (2016) 37:8783–9. doi: 10.1007/s13277-015-4533-5
36. Pyo JS, Sohn JH, Kang G. Detection of tumor multifocality is important for prediction of tumor recurrence in papillary thyroid microcarcinoma: A retrospective study and meta-analysis. J Pathol Transl Med (2016) 50:278–86. doi: 10.4132/jptm.2016.03.29
37. Shattuck TM, Westra WH, Ladenson PW, Arnold A. Independent clonal origins of distinct tumor foci in multifocal papillary thyroid carcinoma. New Engl J Med (2005) 352:2406–12. doi: 10.1056/NEJMoa044190
Keywords: papillary thyroid carcinoma, propensity score matching analysis, total tumor diameter, unilateral multifocal, lymph node metastases
Citation: Wu Z-j, Xia B-y, Chen Z-w, Gong H, Abuduwaili M, Xing Z-c and Su A-p (2023) The value of total tumor diameter in unilateral multifocal papillary thyroid carcinoma: a propensity score matching analysis. Front. Endocrinol. 14:1217613. doi: 10.3389/fendo.2023.1217613
Received: 05 May 2023; Accepted: 16 August 2023;
Published: 06 September 2023.
Edited by:
Malgorzata Gabriela Wasniewska, University of Messina, ItalyReviewed by:
Barbara Maria Jarzab, Maria Skłodowska-Curie National Research Institute of Oncology, PolandFabrizio Consorti, Sapienza University of Rome, Italy
Kyriakos Vamvakidis, Henry Dunant Hospital, Greece
Copyright © 2023 Wu, Xia, Chen, Gong, Abuduwaili, Xing and Su. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: An-ping Su, c3VhbnBpbmdwaW5nQDEyNi5jb20=
 Zhu-juan Wu
Zhu-juan Wu Bao-ying Xia
Bao-ying Xia Zi-wei Chen
Zi-wei Chen Hao Gong
Hao Gong Munire Abuduwaili1
Munire Abuduwaili1 Zhi-chao Xing
Zhi-chao Xing