- 1Department of Nephrology, Xuanwu Hospital, Capital Medical University, Beijing, China
- 2The National Clinical Research Center for Geriatric Disease, Xuanwu Hospital, Capital Medical University, Beijing, China
Background: The potential relationship between Klotho and cognitive function is limited and controversial. This study aimed to quantify the association of Klotho and cognitive impairment in chronic kidney disease (CKD) patients with albuminuria.
Methods: Serum Klotho was measured by enzyme-linked immunosorbent assay. Patients with urine albumin to creatinine ratio (UACR) > 30mg/g from the National Health and Nutrition Survey (NHANES) 2011-2014 were divided into 4 groups according to the quartile of Klotho. Cognitive function was examined using the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD), Digit Symbol Substitution Test (DSST), and Animal Fluency Test. The relationship between Klotho and cognitive function was analyzed by multivariable regression and subgroup analysis.
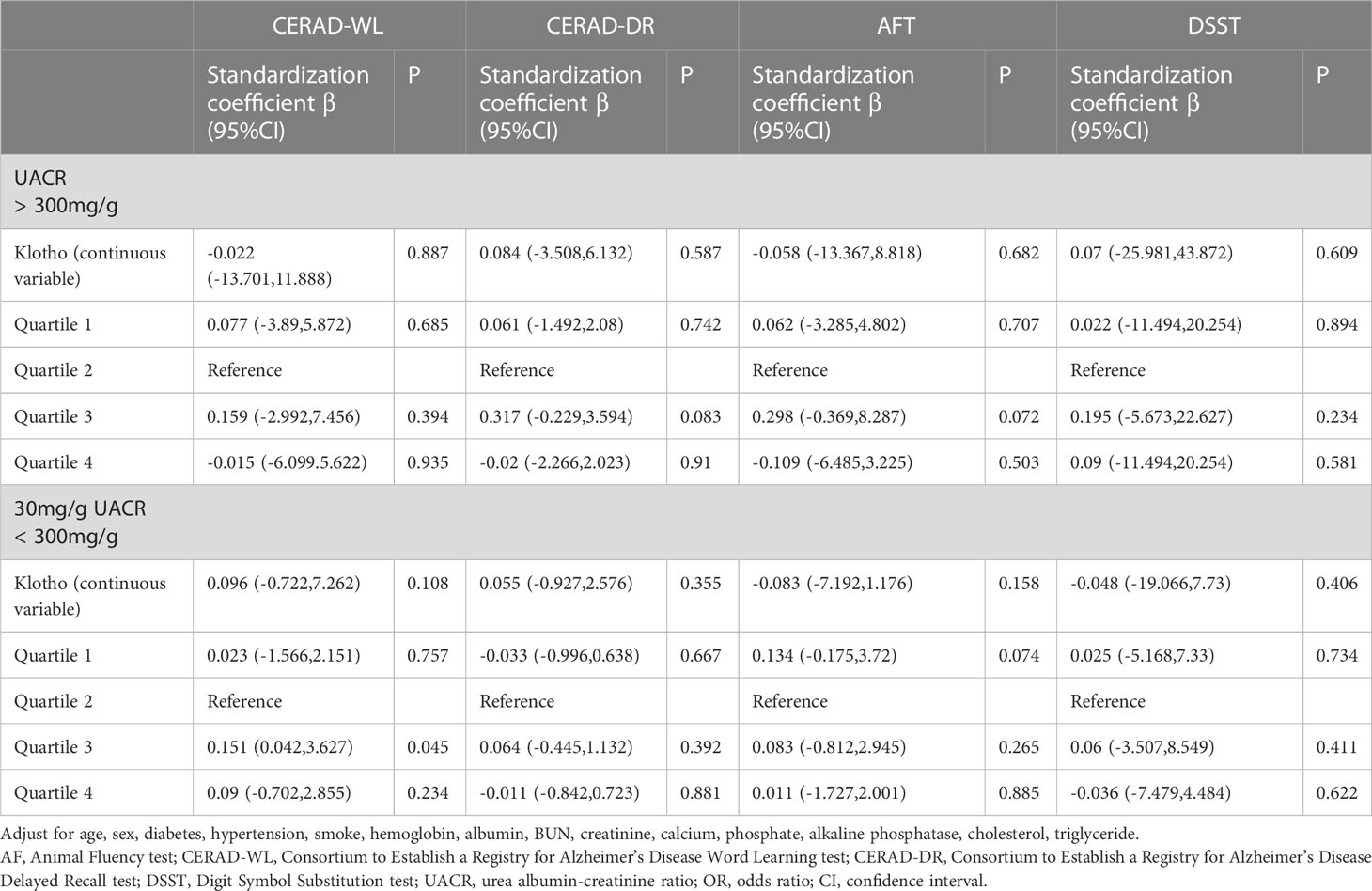
Results: Among 368 CKD patients with albuminuria, we found that Klotho was negatively associated with creatinine, and positively associated with hemoglobin, and estimated glomerular filtration rate. No significant linear relationship was showed between Klotho (as a continuous variable) and cognitive function. When regarded Klotho as a category variable, patients in the quartile 3 group were at a better cognitive performance for CEARD-word learning subset and DSST, especially in the CKD patients with 30 mg/g < UACR <300 mg/g, but not in participants with UACR > 300 mg/g.
Conclusions: The increased Klotho was associated with an increased cognitive function in CKD patients with microalbuminuria. Further studies are needed to demonstrate whether Klotho may be a beneficial biomarker of cognitive health and neurodegeneration.
1 Introduction
Chronic kidney disease (CKD) is defined as abnormal kidney structures and function that affects health and lasts for at least 3 months, characterized by the decrease in glomerular filtration rate (GFR) and the increase in urinary albumin (1). The presence of albuminuria was defined as urine albumin-to-creatinine ratio (UACR) ≥ 30 mg/g. Evidence has reported associations between an increased risk of cognitive impairment and CKD (2, 3).
Three forms of Klotho protein have been identified, including full-length transmembrane Klotho (m-Klotho), soluble Klotho (s-Klotho), and secreted Klotho (4). mKlotho is a single-pass transmembrane protein, and can be shed into sKlotho or secreted Klotho by proteases or alternative mRNA splicing. Klotho has pathophysiological association with various disease, including malignancies, vascular calcification, cardiovascular disease, and renal fibrosis (5–8). Various studies have demonstrated that systemic Klotho levels are downregulated in CKD, indicating a risk for CKD progression (9, 10).
Klotho is detected mainly in distal convoluted tubules of the kidney and choroid plexus in the brain (11), in addition, it is also expressed in the cerebral cortex and cerebellum (12). In a study including subjects aging 39 to 83, α-Klotho in the cerebrospinal fluid may be an important biomarker of neurodegeneration (13). The cerebrospinal fluid Klotho concentrations are lower in Alzheimer’s disease (14), probably leading to a poor synaptic and cognitive functions (15). Besides, the relationship between cognitive functions and Klotho was also suggested in schizophrenia (16), Alzheimer’s disease (17, 18), and multiple system atrophy (19). In humans, a genetic variant of Klotho gene is independently associated with enhanced cognition (20). However, the prognostic value of Klotho for declining of cognition in CKD patients with proteinuria was still under debate.
Therefore, the purpose of this study was to evaluate the association between serum Klotho concentrations and cognitive dysfunction in CKD patients with different level of albuminuria.
2 Methods
2.1 Study population
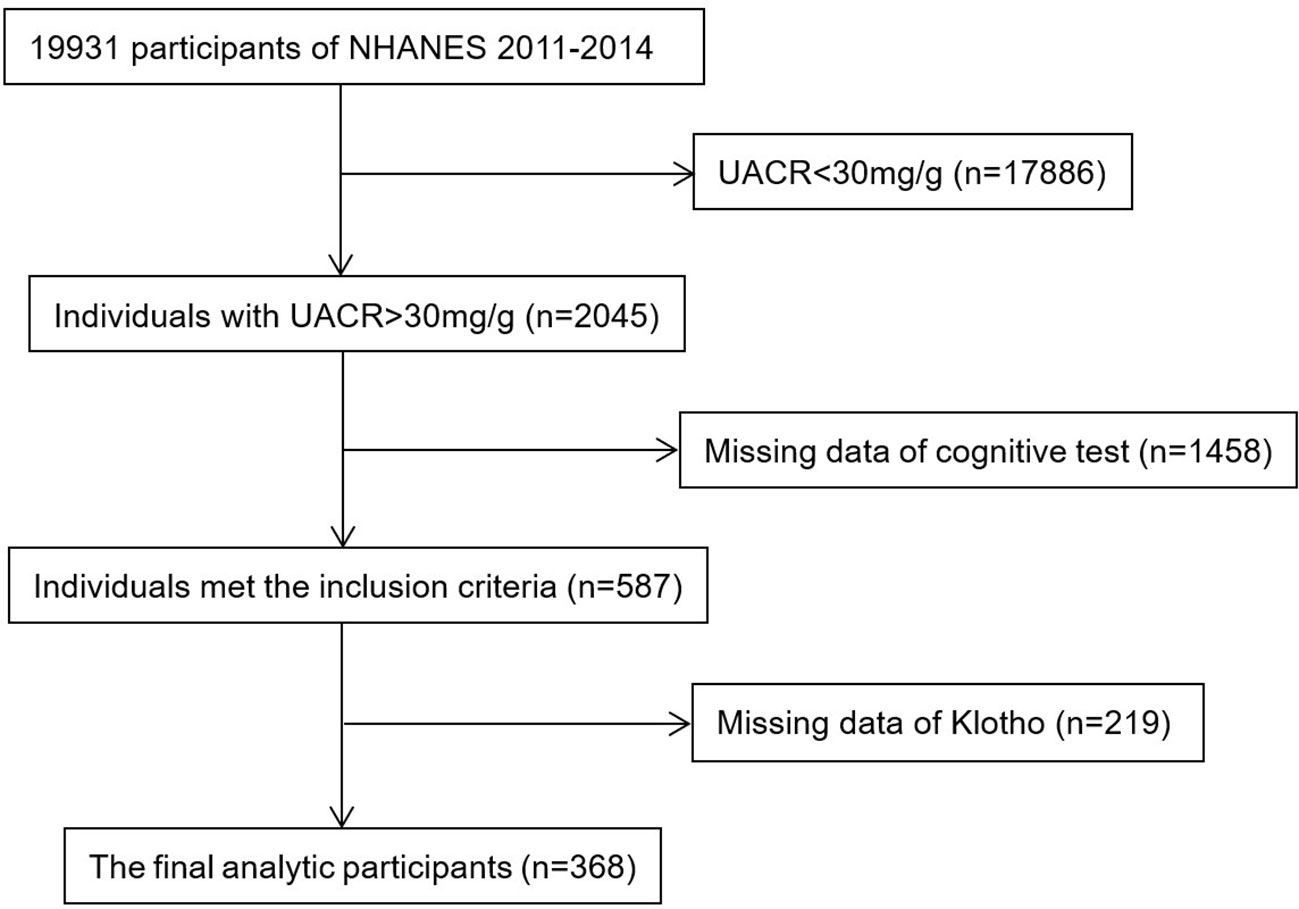
NHANES was conducted biannually in the United States to provide a representative sample of the civilian, non-institutionalized U.S. population (https://www.cdc.gov/nchs/nhanes/about_nhanes.htm). Cognitive Function Tests were measured in two cycles of NHANES (2011–2012 and 2013–2014) for the older population. During this inclusion screening process, 2045 participants with UACR > 30mg/g were available for analysis. We excluded 1458 and 219 participants when missing data of cognitive tests and serum Klotho concentration, respectively. Finally, there were 368 participants in our analysis, and the detailed flow chart was shown in Figure 1. The CKD stages were defined with eGFR and/or evidence of kidney damage according to the KDIGO guidelines: stage 1, eGFR ≧ 90 mL/min/1.73 m2 with UACR ≥ 30 mg/g; stage 2, eGFR 60–89 mL/min/1.73 m2 with ACR ≥ 30 mg/g; stage 3, eGFR 30–59 mL/min/1.73 m2; stage 4, eGFR 15–29 mL/min/1.73 m2; stage 5, eGFR < 15 mL/min/1.73 m2 (21). NHANES was performed by the Centers for Disease Control and Prevention (CDC), the National Center for Health Statistics (NCHS) Ethics Review Board authorized the study, and all participants gave their informed permission.
2.2 Cognitive performance test
NHANES performed a series of assessments for cognitive performance among participants aged 60 years or older, including Consortium to Establish a Registry for Alzheimer’s Disease Word List Learning Test (CERAD-WL), the CERAD Word List Recall Test (CERAD-DR), the Animal Fluency test (AFT) and the Digit Symbol Substitution Test (DSST).
CERAD is designed to assess immediate and delayed learning of new verbal information in the memory sub-domain (22). CERAD consisted of three consecutive learning trials and a delayed recall. The AFT examined categorical verbal fluency, a component of executive function, which was applied to distinguish between persons who have normal cognitive functioning and those who have mild cognitive impairment or more severe forms of cognitive impairment (23). Participants were given a minute to name as many animals as they could in a minute. DSST relied on the integrity of executive function, processing speed, attention, spatial perception, and visual scanning (24). Participants had two minutes to match the symbols corresponding to numbers.
2.3 Measurement of serum soluble Klotho
All samples were stored at -80°C until they were analyzed. Klotho concentrations were analyzed by a commercially available enzyme-linked immunosorbent assay (ELISA) kit produced by IBL International, Japan. The details about laboratory methodology as well as the quality assurance and monitoring are discussed by NHANES.
2.4 Covariates
Covariates, such as age, sex (male and female), educational level (less than high school, high school, and higher than high school), smoking status, drinking status, and questionnaire findings (self-reported physician diagnosis of hypertension, and diabetes), laboratory data (white blood cell, hemoglobin, albumin, blood urea nitrogen (BUN), creatinine, calcium, phosphate, alkaline phosphatase (ALP), cholesterol and triglyceride) were gathered by uniform interviews, physical and laboratory testing, and questionnaires administered by medical personnel.
2.5 Statistical analysis
All data were analyzed by SPSS statistics 23. To describe normal variables, we used mean ± standard deviation (SD), and the median (interquartile range) was for non-normally distributed variables. Categoric variables were described by percentage. Patients were divided into four groups according to the level of Klotho. Kruskal–Wallis test was used to analyze the difference between continuous variables, while Chi-square test was used between classified variables. Serum Klotho concentrations were logarithmic transformed due to its skew distribution. Pearson correlation analysis was performed to explore the association between various covariates and Klotho. We further used multivariable linear regression models to examine the relationship between each cognitive test score and Klotho. The relationship between log-transformed (Klotho) and each cognitive scores was evaluated by restricted cubic spline models fitted for linear regression models with 3 knots at the 10th, 50th, and 90th percentiles. The level of significance was defined as P < 0.05.
3 Results
3.1 Population characteristics
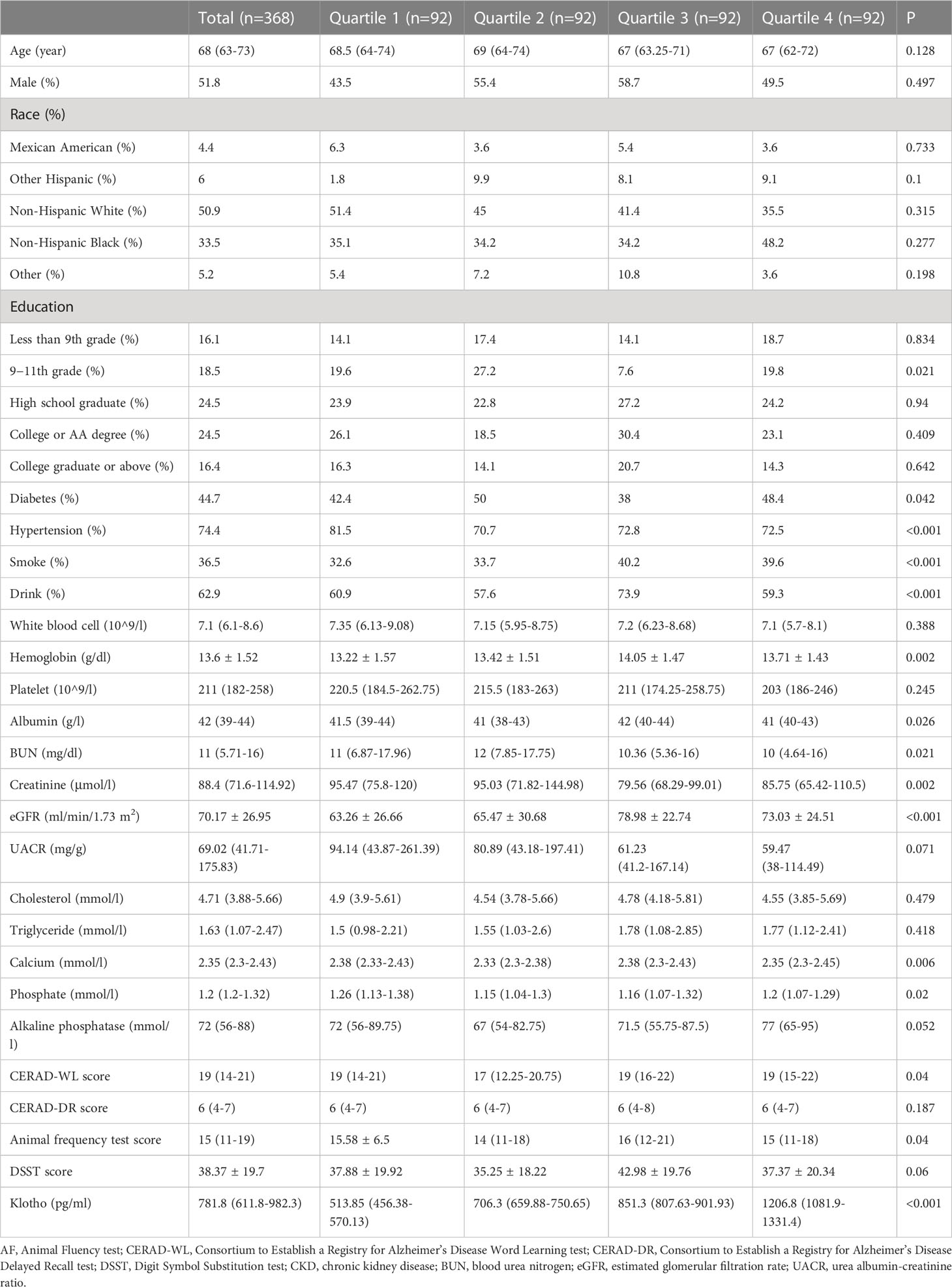
Table 1 showed the characteristics of the patients divided by quartile of serum Klotho levels. The prevalence of diabetes, hypertension, smoking status, drinking status, and the level of hemoglobin, albumin, BUN, creatinine, eGFR, calcium and phosphate were significantly different among four groups. In addition, the value of serum Klotho were different among the four groups and the quartile 2 group had the lowest cognitive value.
Among 1458 participants without cognition tests, 340 participants are aged 60 years and above. The characteristics of the older participants without cognition tests are summarized in Supplementary file Table 1. Compared with the included participants, the excluded participants were more likely to be older, other race/ethnicity, and had poorer residual renal function.
3.2 Correlated factors for Klotho
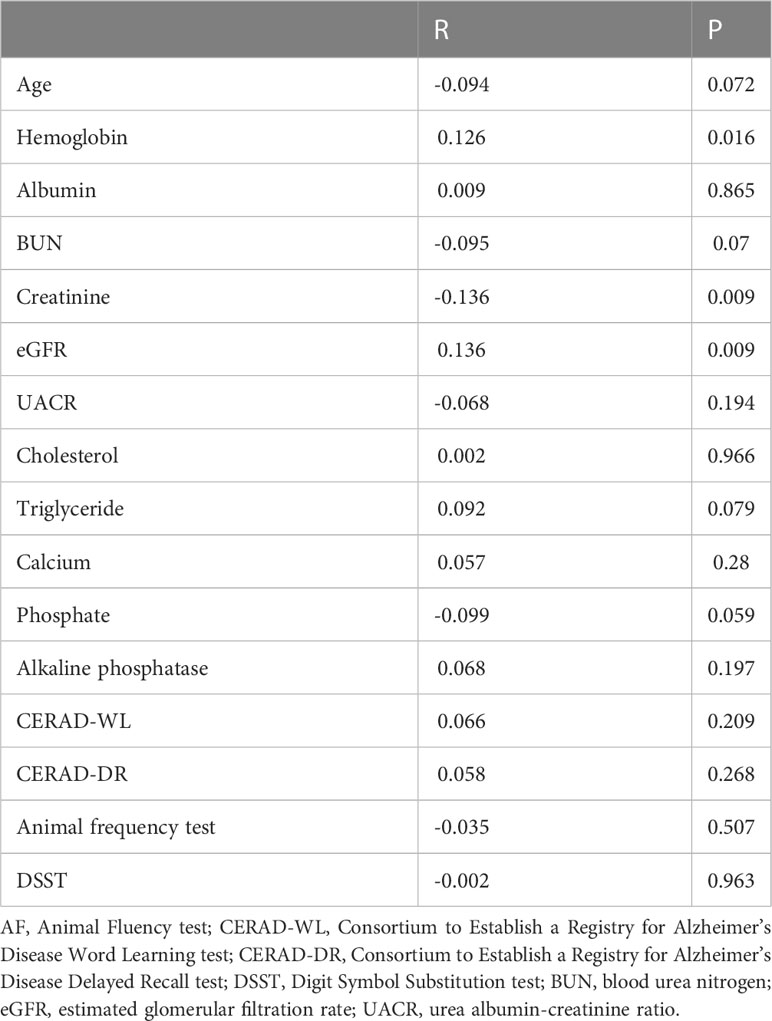
As shown in Table 2, positive association between hemoglobin, while negative association between creatinine and Klotho was observed. However, in our Pearson correlation analysis, we did not find any significant relationship between each cognitive test and serum Klotho (shown as a continuous variable).
3.3 Klotho concentrations and performance on cognitive tests
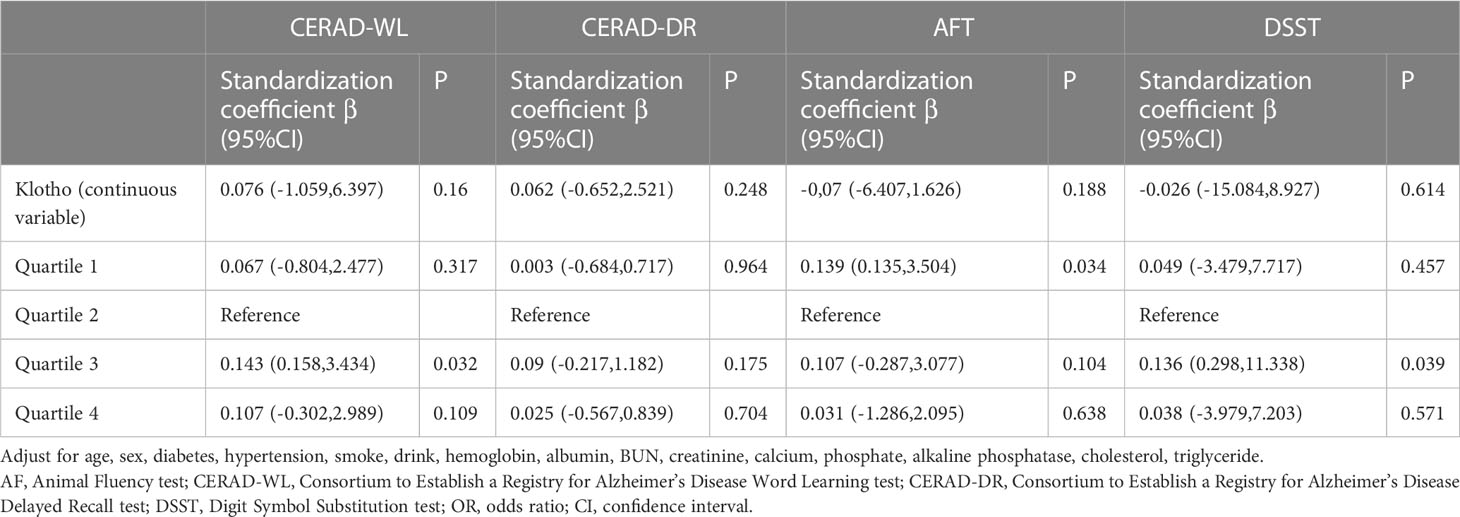
Higher level of serum Klotho were significantly associated with lower odds of cognitive impairment in patients with UACR > 30mg/g. Comparing to the quartile 2 group, patients in the quartile 3 group had higher scores of CERAD-WL and DSST. However, patients with a higher quartile of Klotho were not with a better cognitive function assessed by AFT. The associations between cognitive function test scores and Klotho are shown in Table 3, and there was no significant association between each cognitive test and serum Klotho regarded as a continuous variable. A non-linear association was found between Klotho and DSST score (shown in Figure 2).
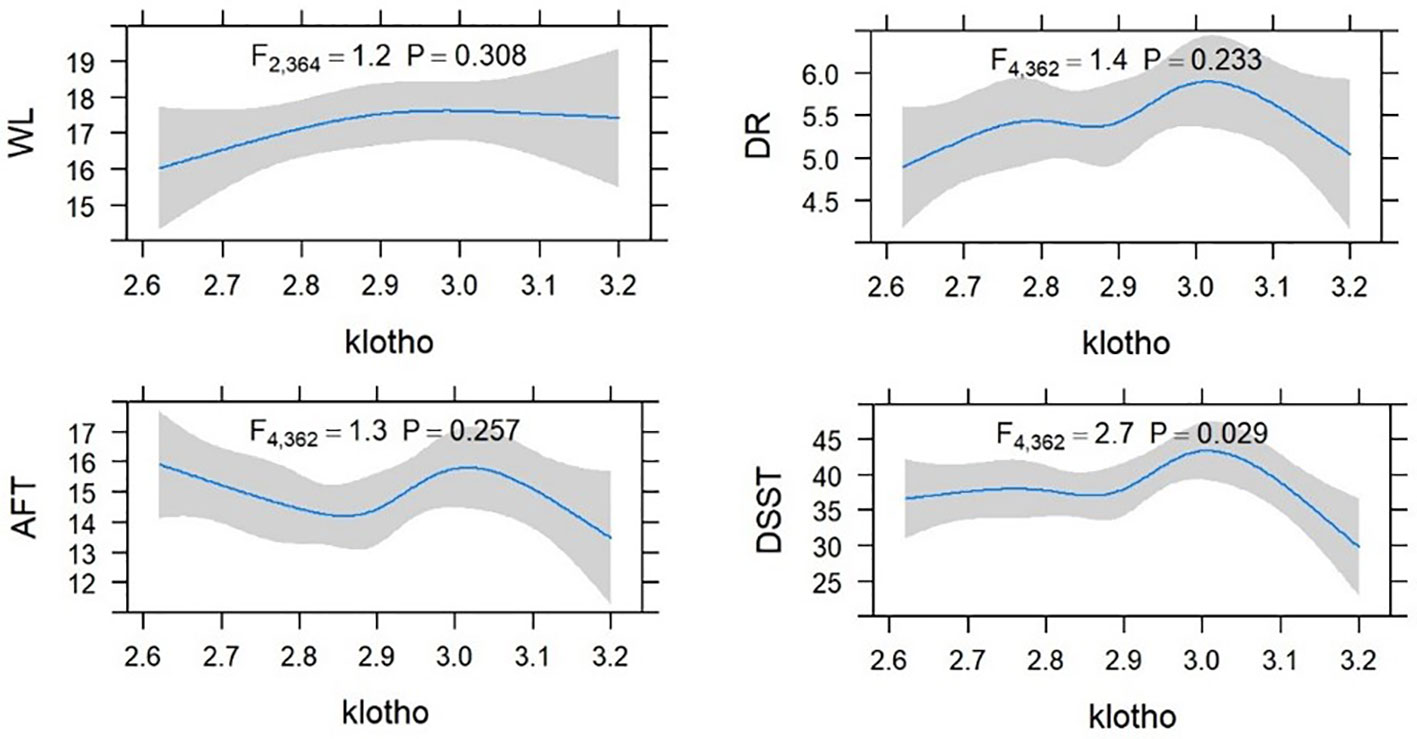
Figure 2 Restricted cubic spline (RCS) plot of the association between Klotho and cognitive function in CKD.
3.4 Serum Klotho and cognitive performance across different level of albuminuria
Subgroup analyses were performed to demonstrate how the relations between Klotho and cognitive performance varied across different level of UACR. In patients with UACR ranging from 30 to 300mg/g, only under the CERAD-WL test, patients in the quartile 3 group of serum Klotho had a better cognitive test score comparing to quartile 2. At the same time, there was nonsignificant difference between each cognitive test and Klotho levels in patients with UACR > 300mg/g (shown in Table 4).
4 Discussion
In the current study, the relationship between serum Klotho and cognitive dysfunction in CKD patients with albuminuria was analyzed. We found that a relatively higher level of Klotho was significantly associated with a better cognition function. However, in CKD patients with UACR > 300mg/g, the association of Klotho and cognition function was slight. Our results probably presented a non-linear association of Klotho and cognitive impairment in CKD.
CKD is a critical health burden worldwide. Both low eGFR and albuminuria are both independent risk factors for cognitive impairment (25, 26). Cognitive impairment is common in end-stage renal disease patients (27, 28). The pathophysiology of CKD-related cognitive impairment was multifactorial, including vascular disease, cerebrovascular disease, the toxicity of uremic toxins, depression, sleep disturbance, anemia, and so on (2). However, the relationship and pathophysiological mechanism of Klotho on poor cognitive function has not been fully elucidated.
The Klotho gene exists in three paralogs: αKlotho (referred to as Klotho here), βKlotho, and γKlotho (29). The extracellular domain of mKlotho can be shed constitutively by proteases and yields sKlotho (30). Increasing evidence showed that a close association between Klotho and kidney diseases (31, 32). Besides, the relationship between Klotho and cognitive functions has been explored in general older adults (33) and various diseases, containing schizophrenia (16, 34), Alzheimer’s disease (17) and cerebrovascular diseases (35). Klotho is probably a potential key player in the metabolic coupling between neurons and astrocytes (36). A growing number of studies also examined the association of Klotho with adverse outcomes (37–39). However, whether Klotho in CKD patients could affect cognitive function is still inconclusive.
In our study, we collected and analyzed relevant data from NHANES 2011-2014. As a result, we found patients with albuminuria in the quartile 3 group obtained better results in cognition-related tests, when comparing to the quartile 2 group. Our finding also showed that the serum Klotho concentration was negatively correlated with age and creatinine, and positively associated with eGFR, which was consistent with previous findings (40, 41). Although an inverse relationship between serum α-Klotho and proteinuria was shown in CKD patients (42), the association was not significant in our study.
The mechanism underlying low serum Klotho levels increasing the risk of cognitive impairment is multifactorial. Klotho was reported to protect against fibrosis, apoptosis, and autophagy (43–45). Reduced circulating Klotho levels are also associated with enhanced oxidative stress and inflammation (46). Upregulated Klotho levels could inhibit oxidative stress and inflammation by suppressing insulin/IGF-1 signaling and nuclear factor-κB (47, 48). Cognitive dysfunction associated with Klotho has been demonstrated before (49, 50). Serum and cerebrospinal fluid protein levels of α-Klotho were positively correlated, and both of them were strongly correlated with cognition scores (13). Additionally, circulating Klotho concentration is even higher in cerebrospinal fluid than in serum regardless of KL-VS genotype (51). The variation in Klotho gene is associated with bigger brain volume and better cognitive function in older adults (52). In CKD rats, the expression of α-Klotho significantly declined in the hippocampus, which might affect the cognitive function (53). Furthermore, elevating Klotho in mice enhanced synaptic plasticity, enriched synaptic receptor subunit for learning and memory, and intrinsic connectivity in fronto-parietal (20, 54). In another nephrectomized induced cognitive impairment rodent model, Klotho levels decreased in frontal cortex but not in hippocampus, accompanying with an increase in NF-κB and tumor necrosis factor-alpha levels (55).
Interestingly, in our subgroup analysis according to the different level of UACR, we only found a significant relationship between higher Klotho level and better cognitive performance on CERAD-WL test in patients with microalbuminuria (30mg/g < UACR < 300mg/g), but not in patients with UACR > 300mg/g. A probable non-linear association of Klotho and cognitive dysfunction was also presented in this article. In a study including patients with diabetic kidney disease, a positive correlation was found between Klotho and reduction of eGFR, and the tubular injury marker (56). Other previous studies documented significantly higher levels of Klotho with CKD (57) or even nonsignificant relationship between residual renal function and serum Klotho (40, 58). Klotho is also produced by extra-renal organs (59). Previous study revealed a key role for local vascular Klotho as an endogenous inhibitor of vascular calcification, demonstrating the vascular territory as another source of serum soluble Klotho (60). The opposite finding probably suggested that a reduction of tubular excretion of sKlotho due to a severe proximal tubule injury, which might lead to an increasing in serum Klotho concentrations. A heavy proteinuria might reflect an aggravated renal tubule damage and poor renal function. In our study, we found that the association of Klotho and cognitive function was not significant in patients with UACR > 300mg/g. Patients with macroalbuminuria probably had a higher risk of other comorbidities, hospitalization and mortality (61, 62). Our results infer that the accumulating Klotho due to a severe kidney damage could not benefit for a better cognitive function. Although the interaction of albuminuria and Klotho on cognition is inconclusive, an early detection and comprehensive evaluation of Klotho in different stage of CKD is essential. More research is warranted to confirm the predictive value of Klotho on cognition for older CKD patients.
Several limitations should still be considered. Although several covariates were adjusted to remove potential confounding factors, we could not exclude some other residual confounding. Our study did not include enough information of other comorbidities. Moreover, this is an observational study, the association of Klotho and cognition could not be interpreted as causality. Additionally, we only explore the association of serum Klotho and cognition by ELISA, the level of Klotho protein in tissues is unknown and unpredicted. The diagnostic sensitivity and specificity of Klotho ELISA kit could be affected by the freshness of serum samples. Finally, our results may not be expanded to the participants under the age of 60. The excluded patients without cognition scores may have ethnic, and renal function differences, leading to select bias. Further research is needed to elucidate the explore potential mechanisms of Klotho and cognition function in patients with different age and stage of CKD.
5 Conclusion
Overall, our results suggest that a relatively higher level of serum Klotho may positively correlate with a better cognitive performance in CKD patients with albuminuria. Our study proposed that the detection of Klotho should be taken into consideration in the early stage of kidney damage, and clinicians should incorporate Klotho detection into routine assessments for older CKD patients aiming at cognitive enhncement.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by The study was approved by the NCHS Research Ethics Review Board (Continuation of Protocol #2011–17). The patients/participants provided their written informed consent to participate in this study.
Author contributions
AZ contributed to the study concept and design. JZ contributed to data collection. JZ, and AZ contributed to the statistical analysis. JZ contributed to the original draft. AZ contributed to the review draft. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Xuanwu Hospital Huizhi talent leader training program to Aihua Zhang.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1215977/full#supplementary-material
References
1. Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, et al. Prevalence of chronic kidney disease in the United States. Jama (2007) 298(17):2038–47. doi: 10.1001/jama.298.17.2038
2. Drew DA, Weiner DE, Sarnak MJ. Cognitive impairment in CKD: pathophysiology, management, and prevention. Am J Kidney Dis (2019) 74(6):782–90. doi: 10.1053/j.ajkd.2019.05.017
3. Berger I, Wu S, Masson P, Kelly PJ, Duthie FA, Whiteley W, et al. Cognition in chronic kidney disease: a systematic review and meta-analysis. BMC Med (2016) 14(1):206. doi: 10.1186/s12916-016-0745-9
4. Xu Y, Sun Z. Molecular basis of Klotho: from gene to function in aging. Endocr Rev (2015) 36(2):174–93. doi: 10.1210/er.2013-1079
5. Semba RD, Cappola AR, Sun K, Bandinelli S, Dalal M, Crasto C, et al. Plasma Klotho and cardiovascular disease in adults. J Am Geriatr Soc (2011) 59(9):1596–601. doi: 10.1111/j.1532-5415.2011.03558.x
6. Sachdeva A, Gouge J, Kontovounisios C, Nikolaou S, Ashworth A, Lim K, et al. Klotho and the treatment of human malignancies. Cancers (Basel) (2020) 12(6):1665. doi: 10.3390/cancers12061665
7. Doi S, Zou Y, Togao O, Pastor JV, John GB, Wang L, et al. Klotho inhibits transforming growth factor-beta1 (TGF-beta1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J Biol Chem (2011) 286(10):8655–65. doi: 10.1074/jbc.M110.174037
8. Hu MC, Shi M, Zhang J, Quiñones H, Griffith C, Kuro-o M, et al. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol (2011) 22(1):124–36. doi: 10.1681/ASN.2009121311
9. Shimamura Y, Hamada K, Inoue K, Ogata K, Ishihara M, Kagawa T, et al. Serum levels of soluble secreted α-Klotho are decreased in the early stages of chronic kidney disease, making it a probable novel biomarker for early diagnosis. Clin Exp Nephrol (2012) 16(5):722–9. doi: 10.1007/s10157-012-0621-7
10. Buchanan S, Combet E, Stenvinkel P, Shiels PG. Klotho, aging, and the failing kidney. Front Endocrinol (Lausanne) (2020) 11:560. doi: 10.3389/fendo.2020.00560
11. Papaconstantinou J. Insulin/IGF-1 and ROS signaling pathway cross-talk in aging and longevity determination. Mol Cell Endocrinol (2009) 299(1):89–100. doi: 10.1016/j.mce.2008.11.025
12. Lim K, Groen A, Molostvov G, Lu T, Lilley KS, Snead D, et al. α-Klotho expression in human tissues. J Clin Endocrinol Metab (2015) 100(10):E1308–18. doi: 10.1210/jc.2015-1800
13. Kundu P, Zimmerman B, Quinn JF, Kaye J, Mattek N, Westaway SK, et al. Serum levels of α-Klotho are correlated with cerebrospinal fluid levels and predict measures of cognitive function. J Alzheimers Dis (2022) 86(3):1471–81. doi: 10.3233/JAD-215719
14. Semba RD, Moghekar AR, Hu J, Sun K, Turner R, Ferrucci L, et al. Klotho in the cerebrospinal fluid of adults with and without Alzheimer's disease. Neurosci Lett (2014) 558:37–40. doi: 10.1016/j.neulet.2013.10.058
15. Dubal DB, Zhu L, Sanchez PE, Worden K, Broestl L, Johnson E, et al. Life extension factor Klotho prevents mortality and enhances cognition in hAPP transgenic mice. J Neurosci (2015) 35(6):2358–71. doi: 10.1523/JNEUROSCI.5791-12.2015
16. Turkmen BA, Yazici E, Erdogan DG, Suda MA, Yazici AB. BDNF, GDNF, NGF and Klotho levels and neurocognitive functions in acute term of schizophrenia. BMC Psychiatry (2021) 21(1):562. doi: 10.1186/s12888-021-03578-4
17. Zhao Y, Zeng CY, Li XH, Yang TT, Kuang X, Du JR. Klotho overexpression improves amyloid-β clearance and cognition in the APP/PS1 mouse model of Alzheimer's disease. Aging Cell (2020) 19(10):e13239. doi: 10.1111/acel.13239
18. Kuang X, Chen YS, Wang LF, Li YJ, Liu K, Zhang MX, et al. Klotho upregulation contributes to the neuroprotection of ligustilide in an Alzheimer's disease mouse model. Neurobiol Aging (2014) 35(1):169–78. doi: 10.1016/j.neurobiolaging.2013.07.019
19. Guo Y, Zhuang XD, Xian WB, Wu LL, Huang ZN, Hu X, et al. Serum Klotho, vitamin D, and homocysteine in combination predict the outcomes of Chinese patients with multiple system atrophy. CNS Neurosci Ther (2017) 23(8):657–66. doi: 10.1111/cns.12711
20. Dubal DB, Yokoyama JS, Zhu L, Broestl L, Worden K, Wang D, et al. Life extension factor Klotho enhances cognition. Cell Rep (2014) 7(4):1065–76. doi: 10.1016/j.celrep.2014.03.076
21. Sun DQ, Jin Y, Wang TY, Zheng KI, Rios RS, Zhang HY, et al. MAFLD and risk of CKD. Metabolism (2021) 115:154433. doi: 10.1016/j.metabol.2020.154433
22. Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology (1989) 39(9):1159–65. doi: 10.1212/wnl.39.9.1159
23. Henry JD, Crawford JR, Phillips LH. Verbal fluency performance in dementia of the Alzheimer's type: a meta-analysis. Neuropsychologia (2004) 42(9):1212–22. doi: 10.1016/j.neuropsychologia.2004.02.001
24. McIntyre RS, Harrison J, Loft H, Jacobson W, Olsen CK. The effects of vortioxetine on cognitive function in patients with major depressive disorder: A meta-analysis of three randomized controlled trials. Int J Neuropsychopharmacol (2016) 19(10):pyw055. doi: 10.1093/ijnp/pyw055
25. Kurella Tamura M, Wadley V, Yaffe K, McClure LA, Howard G, Go R, et al. Kidney function and cognitive impairment in US adults: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Am J Kidney Dis (2008) 52(2):227–34. doi: 10.1053/j.ajkd.2008.05.004
26. Chang J, Hou W, Li Y, Li S, Zhao K, Wang Y, et al. Prevalence and associated factors of cognitive frailty in older patients with chronic kidney disease: a cross-sectional study. BMC Geriatr (2022) 22(1):681. doi: 10.1186/s12877-022-03366-z
27. O'Lone E, Connors M, Masson P, Wu S, Kelly PJ, Gillespie D, et al. Cognition in people with end-stage kidney disease treated with hemodialysis: A systematic review and meta-analysis. Am J Kidney Dis (2016) 67(6):925–35. doi: 10.1053/j.ajkd.2015.12.028
28. Bugnicourt JM, Godefroy O, Chillon JM, Choukroun G, Massy ZA. Cognitive disorders and dementia in CKD: the neglected kidney-brain axis. J Am Soc Nephrol (2013) 24(3):353–63. doi: 10.1681/ASN.2012050536
29. Yu LX, Li SS, Sha MY, Kong JW, Ye JM, Liu QF. The controversy of Klotho as a potential biomarker in chronic kidney disease. Front Pharmacol (2022) 13:931746. doi: 10.3389/fphar.2022.931746
30. Zhong X, Jagarlapudi S, Weng Y, Ly M, Rouse JC, McClure K, et al. Structure-function relationships of the soluble form of the antiaging protein Klotho have therapeutic implications for managing kidney disease. J Biol Chem (2020) 295(10):3115–33. doi: 10.1074/jbc.RA119.012144
31. Thongprayoon C, Neyra JA, Hansrivijit P, Medaura J, Leeaphorn N, Davis PW, et al. Serum Klotho in living kidney donors and kidney transplant recipients: A meta-analysis. J Clin Med (2020) 9(6):1834. doi: 10.3390/jcm9061834
32. Hu MC, Shi M, Zhang J, Addo T, Cho HJ, Barker SL, et al. Renal production, uptake, and handling of circulating αKlotho. J Am Soc Nephrol (2016) 27(1):79–90. doi: 10.1681/ASN.2014101030
33. Linghui D, Simin Y, Zilong Z, Yuxiao L, Shi Q, Birong D. The relationship between serum Klotho and cognitive performance in a nationally representative sample of US adults. Front Aging Neurosci (2023) 15:1053390. doi: 10.3389/fnagi.2023.1053390
34. Xiong JW, Zhan JQ, Luo T, Chen HB, Wan QG, Wang Y, et al. Increased plasma level of longevity protein Klotho as a potential indicator of cognitive function preservation in patients with schizophrenia. Front Neurosci (2020) 14:610. doi: 10.3389/fnins.2020.00610
35. Wei H, Li H, Song X, Du X, Cai Y, Li C, et al. Serum Klotho: a potential predictor of cerebrovascular disease in hemodialysis patients. BMC Nephrol (2019) 20(1):63. doi: 10.1186/s12882-019-1232-2
36. Mazucanti CH, Kawamoto EM, Mattson MP, Scavone C, Camandola S. Activity-dependent neuronal Klotho enhances astrocytic aerobic glycolysis. J Cereb Blood Flow Metab (2019) 39(8):1544–56. doi: 10.1177/0271678X18762700
37. Qian J, Zhong J, Yan M, Cheng P, Shi H, Hao C, et al. Circulating α-Klotho is related to plasma aldosterone and its follow-up change predicts CKD progression. Kidney Blood Press Res (2018) 43(3):836–46. doi: 10.1159/000490138
38. Yang K, Yang J, Bi X, Yu Z, Xiao T, Huang Y, et al. Serum Klotho, cardiovascular events, and mortality in nondiabetic chronic kidney disease. Cardiorenal Med (2020) 10(3):175–87. doi: 10.1159/000506380
39. Liu QF, Yu LX, Feng JH, Sun Q, Li SS, Ye JM. The prognostic role of Klotho in patients with chronic kidney disease: A systematic review and meta-analysis. Dis Markers (2019) 2019:6468729. doi: 10.1155/2019/6468729
40. Akimoto T, Yoshizawa H, Watanabe Y, Numata A, Yamazaki T, Takeshima E, et al. Characteristics of urinary and serum soluble Klotho protein in patients with different degrees of chronic kidney disease. BMC Nephrol (2012) 13:155. doi: 10.1186/1471-2369-13-155
41. Liu QF, Ye JM, Yu LX, He AL, Sun Q, He DW, et al. Plasma s-Klotho is related to kidney function and predicts adverse renal outcomes in patients with advanced chronic kidney disease. J Investig Med (2018) 66(3):669–75. doi: 10.1136/jim-2017-000560
42. Hage V, Pelletier S, Dubourg L, Drai J, Cuerq C, Lemoine S, et al. In chronic kidney disease, serum α-Klotho is related to serum bicarbonate and proteinuria. J Ren Nutr (2014) 24(6):390–4. doi: 10.1053/j.jrn.2014.06.009
43. Hum JM, O'Bryan L, Smith RC, White KE. Novel functions of circulating Klotho. Bone (2017) 100:36–40. doi: 10.1016/j.bone.2016.11.025
44. Yuan Q, Ren Q, Li L, Tan H, Lu M, Tian Y, et al. A Klotho-derived peptide protects against kidney fibrosis by targeting TGF-β signaling. Nat Commun (2022) 13(1):438. doi: 10.1038/s41467-022-28096-z
45. Chen K, Sun Z. Autophagy plays a critical role in Klotho gene deficiency-induced arterial stiffening and hypertension. J Mol Med (Berl) (2019) 97(11):1615–25. doi: 10.1007/s00109-019-01841-6
46. Oh HJ, Nam BY, Lee MJ, Kim CH, Koo HM, Doh FM, et al. Decreased circulating Klotho levels in patients undergoing dialysis and relationship to oxidative stress and inflammation. Perit Dial Int (2015) 35(1):43–51. doi: 10.3747/pdi.2013.00150
47. Yamamoto M, Clark JD, Pastor JV, Gurnani P, Nandi A, Kurosu H, et al. Regulation of oxidative stress by the anti-aging hormone Klotho. J Biol Chem (2005) 280(45):38029–34. doi: 10.1074/jbc.M509039200
48. Buendía P, Carracedo J, Soriano S, Madueño JA, Ortiz A, Martín-Malo A, et al. Klotho prevents NFκB translocation and protects endothelial cell from senescence induced by uremia. J Gerontol A Biol Sci Med Sci (2015) 70(10):1198–209. doi: 10.1093/gerona/glu170
49. Torbus-Paluszczak M, Bartman W, Adamczyk-Sowa M. Klotho protein in neurodegenerative disorders. Neurol Sci (2018) 39(10):1677–82. doi: 10.1007/s10072-018-3496-x
50. Cararo-Lopes MM, Mazucanti CHY, Scavone C, Kawamoto EM, Berwick DC. The relevance of α-KLOTHO to the central nervous system: Some key questions. Ageing Res Rev (2017) 36:137–48. doi: 10.1016/j.arr.2017.03.003
51. Gaitán JM, Asthana S, Carlsson CM, Engelman CD, Johnson SC, Sager MA, et al. Circulating Klotho is higher in cerebrospinal fluid than serum and elevated among KLOTHO heterozygotes in a cohort with risk for alzheimer's disease. J Alzheimers Dis (2022) 90(4):1557–69. doi: 10.3233/JAD-220571
52. Yokoyama JS, Sturm VE, Bonham LW, Klein E, Arfanakis K, Yu L, et al. Variation in longevity gene KLOTHO is associated with greater cortical volumes. Ann Clin Transl Neurol (2015) 2(3):215–30. doi: 10.1002/acn3.161
53. Huang M, Li G, Tan J, Huang M, Huang F, Ma R, et al. Effects of chronic kidney disease on cognitive function and α-Klotho expression in hippocampus. Transl Androl Urol (2022) 11(8):1157–68. doi: 10.21037/tau-22-465
54. Yokoyama JS, Marx G, Brown JA, Bonham LW, Wang D, Coppola G, et al. Systemic Klotho is associated with KLOTHO variation and predicts intrinsic cortical connectivity in healthy human aging. Brain Imaging Behav (2017) 11(2):391–400. doi: 10.1007/s11682-016-9598-2
55. Degaspari S, Tzanno-Martins CB, Fujihara CK, Zatz R, Branco-Martins JP, Viel TA, et al. Altered KLOTHO and NF-κB-TNF-α Signaling are correlated with nephrectomy-induced cognitive impairment in rats. PloS One (2015) 10(5):e0125271. doi: 10.1016/j.celrep.2014.03.076
56. Bob F, Schiller A, Timar R, Lighezan D, Schiller O, Timar B, et al. Rapid decline of kidney function in diabetic kidney disease is associated with high soluble Klotho levels. Nefrologia (Engl Ed) (2019) 39(3):250–7. doi: 10.1016/j.nefroe.2019.04.002
57. Devaraj S, Syed B, Chien A, Jialal I. Validation of an immunoassay for soluble Klotho protein: decreased levels in diabetes and increased levels in chronic kidney disease. Am J Clin Pathol (2012) 137(3):479–85. doi: 10.1309/AJCPGPMAF7SFRBO4
58. Seiler S, Wen M, Roth HJ, Fehrenz M, Flügge F, Herath E, et al. Plasma Klotho is not related to kidney function and does not predict adverse outcome in patients with chronic kidney disease. Kidney Int (2013) 83(1):121–8. doi: 10.1038/ki.2012.288
59. Picciotto D, Murugavel A, Ansaldo F, Rosa GM, Sofia A, Milanesi S, et al. The organ handling of soluble Klotho in humans. Kidney Blood Press Res (2019) 44(4):715–26. doi: 10.1159/000501316
60. Lim K, Lu TS, Molostvov G, Lee C, Lam FT, Zehnder D, et al. Vascular Klotho deficiency potentiates the development of human artery calcification and mediates resistance to fibroblast growth factor 23. Circulation (2012) 125(18):2243–55. doi: 10.1161/CIRCULATIONAHA.111.053405
61. Barzilay JI, Buzkova P, Shlipak MG, Bansal N, Garimella P, Mukamal KJ. Hospitalization rates in older adults with albuminuria: the cardiovascular health study. J Gerontol A Biol Sci Med Sci (2020) 75(12):2426–33. doi: 10.1093/gerona/glaa020
Keywords: albuminuria, chronic kidney disease, cognition impairment, Klotho, NHANES
Citation: Zhang J and Zhang A (2023) Relationships between serum Klotho concentrations and cognitive performance among older chronic kidney disease patients with albuminuria in NHANES 2011-2014. Front. Endocrinol. 14:1215977. doi: 10.3389/fendo.2023.1215977
Received: 03 May 2023; Accepted: 07 July 2023;
Published: 25 July 2023.
Edited by:
Mohammed S. Razzaque, Lake Erie College of Osteopathic Medicine, United StatesReviewed by:
Javier Donate-Correa, University Hospital of the Nuestra Señora de Candelaria, SpainDi Xie, Southern Medical University, China
Copyright © 2023 Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aihua Zhang, cm9zZXpoYW5nOTk4QGhvdG1haWwuY29t
 Jialing Zhang
Jialing Zhang Aihua Zhang
Aihua Zhang