
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 20 September 2023
Sec. Cardiovascular Endocrinology
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1211954
This article is part of the Research Topic Cardiovascular Diseases Related to Diabetes and Obesity, volume III View all 11 articles
 Chen Die Yang1†
Chen Die Yang1† Jia Wei Chen2†
Jia Wei Chen2† Jin Wei Quan2†
Jin Wei Quan2† Xin Yi Shu2
Xin Yi Shu2 Shuo Feng1
Shuo Feng1 Muladili Aihemaiti2
Muladili Aihemaiti2 Feng Hua Ding1
Feng Hua Ding1 Wei Feng Shen1,2
Wei Feng Shen1,2 Lin Lu1,2
Lin Lu1,2 Rui Yan Zhang1
Rui Yan Zhang1 Xiao Qun Wang1,2*
Xiao Qun Wang1,2*Background: A substantial portion of heart failure (HF) patients adherent to guideline-directed medical therapies have experienced improved ejection fraction (EF), termed HFimpEF. Glycemic variability (GV) has emerged as a critical cardiometabolic factor. However, the relation between long-term GV and the incidence of HFimpEF is still unclear.
Methods: A total of 591 hospitalized HF patients with reduced EF (HFrEF, EF≤ 40%) admitted from January 2013 to December 2020 were consecutively enrolled. Repeat echocardiograms were performed at baseline and after around 12 months. The incidence of HFimpEF, defined as (1) an absolute EF improvement ≥10% and (2) a second EF > 40% and its association with long-term fasting plasma glucose (FPG) variability were analyzed.
Results: During a mean follow-up of 12.2 ± 0.6 months, 218 (42.0%) patients developed HFimpEF. Multivariate analysis showed FPG variability was independently associated with the incidence of HFimpEF after adjustment for baseline HbA1c, mean FPG during follow-up and other traditional risk factors (odds ratio [OR] for highest vs. lowest quartile of CV of FPG: 0.487 [95% CI 0.257~0.910]). Evaluation of GV by alternative measures yielded similar results. Subgroup analysis revealed that long-term GV was associated with HFimpEF irrespective of glycemic levels and diabetic conditions.
Conclusions: This study reveals that greater FPG variability is associated with compromised development of HFimpEF. A more stable control of glycemic levels might provide favorable effects on myocardial functional recovery in HF patients even without diabetes.
Heart failure (HF) is a prevalent clinical syndrome with high mortality and morbidity. With the development of guideline-directed medical treatment and device therapy, a substantial proportion of HF patients with reduced ejection fraction (EF, HFrEF) have experienced improved left ventricular (LV) EF, thereafter termed HF with recovered or improved EF (HFimpEF) (1–5). Compared with other types of HF, HFimpEF possesses distinct pathophysiological characteristics, clinical manifestations, and better prognosis (4–7). In the 2022 AHA/ACC/HFSA Guideline for the Management of HF (8), HFimpEF was thus proposed as a new classification of HF. The process of myocardial functional improvement is coordinately driven by adaptive molecular change, metabolic profile alteration, improved cardiomyocyte contractility and LV geometric restoration (7, 9, 10). However, the predisposing factors for HFimpEF are still under investigation.
Glycemic variability (GV) refers to fluctuations in glucose levels within-days or over months to years. GV has been recognized as a critical risk factor for diabetic macrovascular and microvascular complications (11–14), and adverse cardiovascular events even in patients without diabetes (15–21). In the setting of acute HF, elevated in-hospital GV confers higher risk of both short-term and long-term mortality in addition to classic glucose metrics (22, 23). The adverse impact of long-term glucose fluctuations on clinical outcomes has also been confirmed in chronic HF patients (24, 25). Nevertheless, the impact of GV on myocardial recovery in failing hearts is still unclear. In the present study, we analyzed the relationship between long-term GV and the incidence of HFimpEF.
We consecutively enrolled 951 patients diagnosed with HFrEF (EF ≤ 40%) on hospitalization between January 2013 and December 2020 in Shanghai Ruijin Hospital. A total of 78 patients comorbid with renal failure requiring hemodialysis (n=32), diseases requiring steroid therapy (n=13), malignant tumor (n=9), heart transplantation (n=1) and in-hospital death (n=23) were excluded. The enrolled patients were routinely followed up and underwent repeat echocardiograms at around 12-month ( ± 1 month). During follow-up, there were 49 patients who died for any reason within 13 months from the index admission date and thus were excluded. Another 74 subjects were also excluded due to loss to echocardiogram follow-up for any other reason. Given that the development of HFimpEF was the primary endpoint, patients who received the follow-up echocardiogram at around 12-month but died thereafter were not excluded from the analysis. For calculation of long-term GV, subjects (n=231) without at least three fasting plasma glucose (FPG) measurements with ≥3 months apart were further excluded (Figure 1).
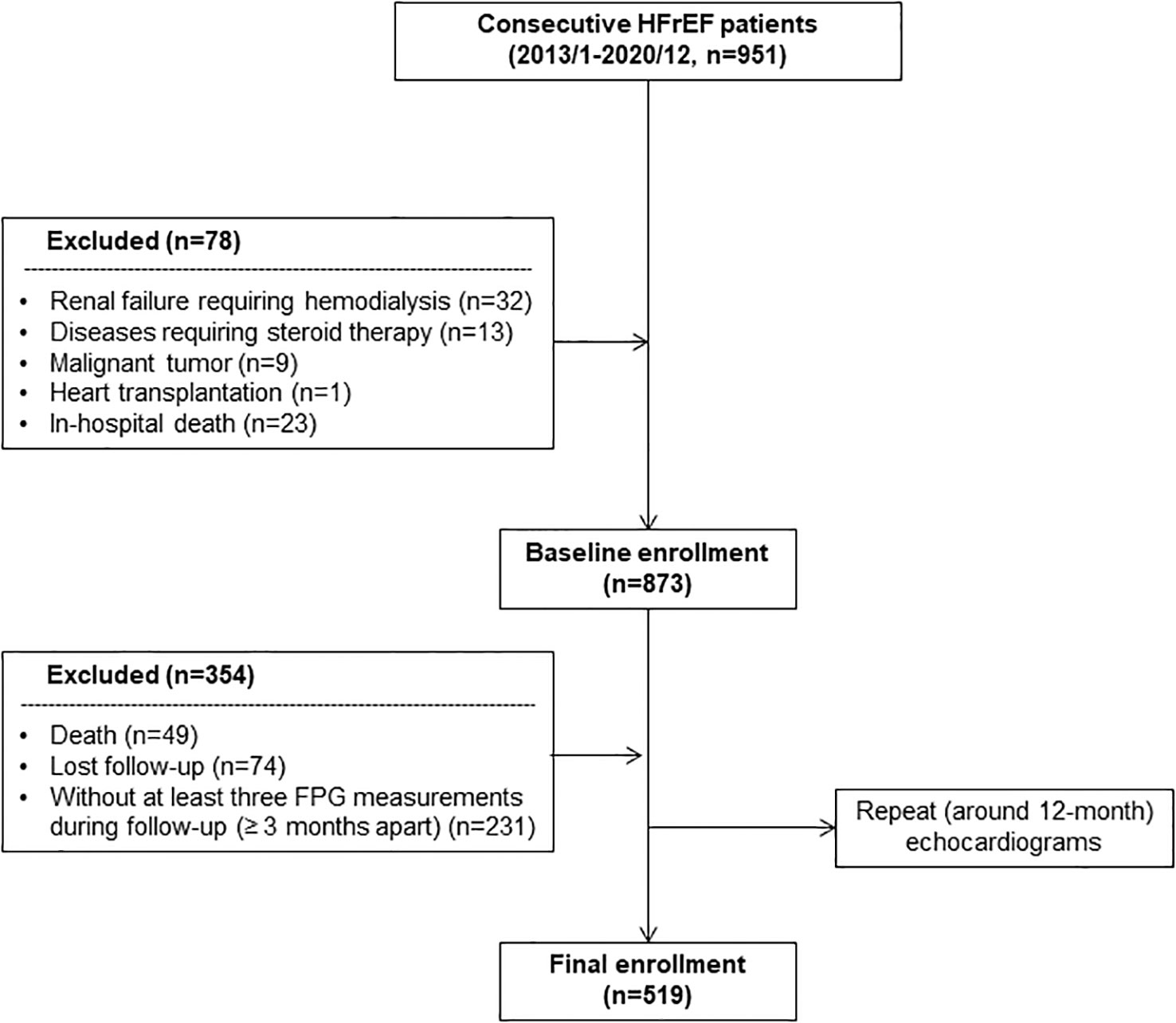
Figure 1 Flowchart of patient enrollment. FPG, fasting plasma glucose; HFrEF, heart failure with reduced ejection fraction.
The primary outcome was the development of HFimpEF, which was diagnosed based on follow-up echocardiogram according to the universal HFimpEF definition (26): (1) an absolute EF improvement ≥10% and (2) a second EF > 40%.
This study complies with the Declaration of Helsinki. The study protocol was approved by Shanghai Ruijin Hospital ethics committee, and written informed consent was obtained from all participants.
Detailed information of medical history and lifestyle was obtained using a standard questionnaire by trained physicians on admission. Body mass index (BMI) was calculated as weight/height2 (kilograms per square meter). Body surface area (BSA) was calculated by Stevenson’s formula: 0.0061 × height + 0.0128 × weight - 0.1529 (27). Hypertension was diagnosed according to the seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure (BP; JNC 7) (28). The diagnosis of diabetes was made according to the criteria of American Diabetes Association (29). Ischemic etiology was diagnosed based on medical history survey, examination by coronary computed tomography angiography (CTA) or coronary angiogram.
All the blood samples were drawn after overnight fasting. Plasma glucose, insulin, liver and renal function, total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, and N-terminal pro-B-type natriuretic peptide (NT-proBNP) were assessed (HITACHI 912 Analyzer, Roche Diagnostics, Germany). The estimated glomerular filtration rate (eGFR) was computed using the Chronic Kidney Disease Epidemiology Collaboration equation (30). Blood HbA1c was measured using ion-exchange high performance liquid chromatography with Bio-rad Variant Hemoglobin Testing System (Bio-Rad Laboratories, USA).
Comprehensive transthoracic echocardiography was performed using a commercially available system (Vivid-I, GE Healthcare, Milwaukee, WI). The sonographers were blinded to this study. Two-dimensional echocardiography and Doppler flow imaging were recorded from standard parasternal and apical transducer positions.
EF was calculated using the modified Simpson’s biplane technique. The LV length was measured in an apical 4-chamber view. To facilitate application of clinical normality cut points, LV end-diastolic volume (EDV) and end-systolic volume (ESV) were indexed by BSA calculated at the study time point. LV mass was estimated from M-mode measurements by the formula: LV mass = , and was indexed by BSA, where EDD is LV end-diastolic diameter, IVST is interventricular septal thickness, PWT is LV posterior wall thickness.
Long-term GV was measured during follow-up period for ≥ 3 times with at least 3-month intervals. The mean and variability of FPG were calculated. FPG variability was primarily defined as intraindividual coefficient of variation (CV) of FPG across visits, which was calculated as the standard deviation (SD) divided by the mean value. The alternative variability of FPG includes: 1) average successive variability (ASV), which was defined as the average absolute difference between successive values and 2) the variability independent of the mean (VIM), which was calculated by the equation as previously reported (19): VIM=100×SD/meanβ, where β is the regression coefficient based on natural logarithm of SD on natural logarithm of mean of the study population. FPG variability was calculated both as continuous and categorical variables grouped by quartiles of CV, ASV or VIM.
A case-control study was nested into the HF cohort to examine the association between GV and the development of HFimpEF. Each case (HFimpEF) was matched by 1 control (persistent HFrEF) randomly sampled from the cohort members based on sex, age ( ± 2 years) and duration of echocardiogram follow-up. Meanwhile, GV was treated as a dichotomized variable by fusing the original quartile 1~2 as stable glycemic control and quartile 3~4 as unstable glycemic control of all the 3 GV measures (CV, ASV, VIM). A total of 200 case-control pairs were matched for the final analysis.
Continuous variables were presented as median (interquartile range [IQR]) or mean ± SD, and categorical data were summarized as frequencies (percentages). Normal distribution of continuous variables was evaluated by Shapiro-Wilk test. For normally distributed variables, differences in quartiles of FPG variability and subgroup analysis were performed by one-way analysis of variance (ANOVA) followed by post hoc Bonferroni correction. For non-normally distributed continuous variables, differences were analyzed by Mann-Whitney U test or Kruskal-Wallis test. Differences in categorical variables were analyzed by χ2 test. Univariate logistic regression analysis was performed to identify predictors for HFimpEF. Afterwards, multivariate regression models were constructed to interrogate the association between FPG variability and HFimpEF. In model 1, age and sex were adjusted. In model 2, additional adjustment was performed with HF etiology, BP, BMI, and history of diabetes. In model 3, we further adjusted HbA1c, renal function, mean FPG levels during follow-up and baseline EDV index. In model 4, cardiac resynchronizing therapy (CRT) and medical therapies including beta-blockers, angiotensin converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARB), angiotensin receptor-neprilysin inhibitors (ARNI), spironolactones as well as sodium-glucose cotransporter 2 (SGLT2) inhibitors were additionally adjusted. FPG variability was analyzed both as continuous and categorical variables in univariate and multivariate regression models. The association between GV and HFimpEF in the nested case-control study was analyzed by conditional logistic regression.
All statistical analyses were performed using the R statistical package v.4.0.3 (R Project for Statistical Computing, Vienna, Austria). A 2-tailed P<0.05 was considered statistically significant.
A total of 519 HFrEF patients were finally enrolled in this study. The mean age was 61.3 ± 12.7 years with 80.3% male patients. Among these subjects, 30.1% were with diabetes (n=156). There were 53.2% of HFrEF patients with an ischemic etiology, and 83.3% of them were diagnosed based on coronary CTA or angiogram during the index admission. The mean number of intrapersonal FPG tests was 5.34 ± 2.47 times. The mean FPG level during follow-up was 6.61 ± 1.99 mmol/L, and CV, ASV, VIM of FPG during follow-up were 0.162 [IQR 0.093~0.268], 1.190 [IQR 0.568~2.235] and 0.641 [IQR 0.408~0.968], respectively. Correlation analyses showed that GV indices such as CV and ASV of FPG were positively correlated to mean FPG levels (CV: Pearson’s r = 0.56, P<0.001; ASV: Pearson’s r= 0.73, P<0.001), whereas no correlation was found between VIM of FPG and mean FPG levels (Pearson’s r= -0.04, P=0.348).
After dividing these patients into 4 groups based on quartiles of CV of FPG, we found subjects with higher GV tended to be elder, more frequently with diabetes and ischemic HF etiology, and with higher levels of baseline HbA1c, FPG as well as NT-proBNP. Subjects in the highest quartile were more frequently on anti-platelet therapy. There was no significant difference in sex, BMI, smoking habits, history of hypertension, atrial fibrillation, New York Heart Association (NYHA) grades, lipid profiles, renal function, CRT implantation and other therapies between the 4 quartiles (Table 1).
In addition, documented hypoglycemic event defined as FPG< 2.8 mmol/L during follow-up was compared. In our study, 3.3% of the subjects suffered hypoglycemic episodes, which was more frequent in higher quartiles of CV of FPG (0 vs. 0 vs. 2.3% vs. 10.8%, P<0.003).
LV geometric and functional parameters at baseline and around 12-month follow-up were compared in subjects with different quartiles of CV of long-term FPG (Table 2). At baseline, there was no significant difference in LV function and volumes. During the follow-up, EF was improved from 32.38% ± 5.28% to 42.12% ± 10.17% (P<0.001) and LV volumes were restored in the overall population. However, the trend towards EF improvement was markedly impaired with increasing FPG variability (P=0.003). LV reverse remodeling was also attenuated in patients with high FPG variability (ΔEDV index: P=0.004; ΔESV index: P<0.001).
After 12.2 ± 0.6 months, 218 (42.0%) patients developed HFimpEF and another 301 (58.0%) patients remained HFrEF.
Univariate regression analysis (Supplementary Table 1) revealed that predictors for HFimpEF were younger age, non-diabetes, non-ischemic etiology, higher BP, lower HbA1c levels, lower EDV index and use of SGLT2 inhibitors. The 3 measures of FPG variability (CV, ASV and VIM) were all inversely associated with HFimpEF either when treated as continuous or categorical variables.
Multivariate regression analysis (Table 3) showed that different measures of FPG variability were persistently associated with the development of HFimpEF after adjustment for age and sex (Model 1), clinical characteristics (Model 2), renal function, baseline HbA1c, mean FPG control levels, LV volumes (Model 3) and treatment regimens (Model 4). In the full adjustment model (Model 4), patients with highest quartile of CV of FPG corresponded to a 51.3% (OR: 0.487 [95% CI 0.257~0.910]) decreased likelihood of HFimpEF as compared to the lowest quartile. Similar findings were also observed when these measures of FPG variability were treated as continuous variables (Supplementary Table 2).
Furthermore, subgroup analysis (Figure 2) demonstrated interaction terms were non-significant across subgroups of sex, age, BMI, FPG levels, the presence of diabetes and ischemic etiology, indicating the associations between FPG variability and HFimpEF were similar among these subgroups. Especially, the association kept significant irrespective of diabetic conditions and mean FPG levels.
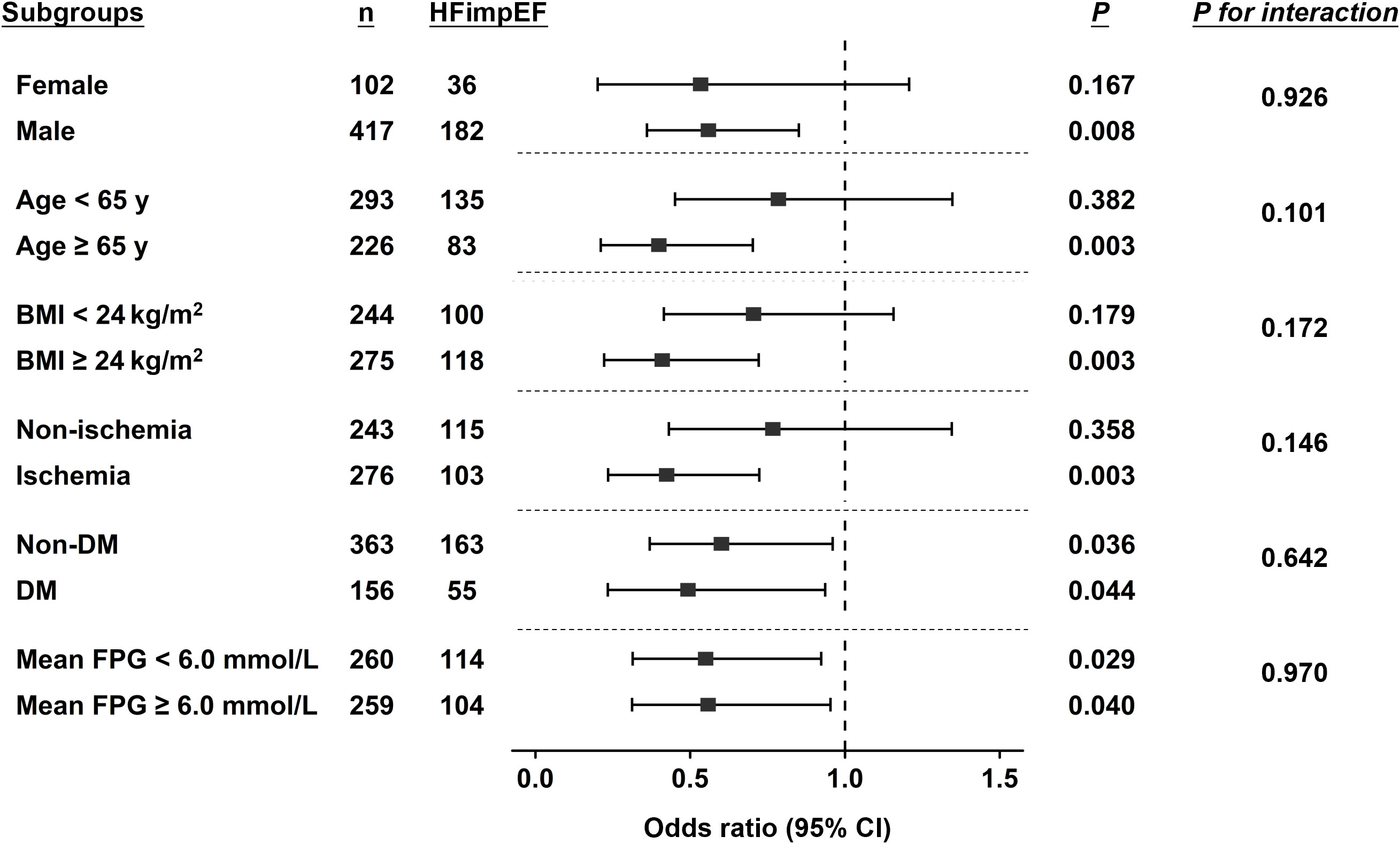
Figure 2 Subgroup analysis by forest plot. Forest plot shows the association between VIM of long-term FPG and incidence of HFimpEF in different subgroups and the significance of the corresponding interaction terms. The dashed reference line indicates odds ratio of 1.0. BMI, body mass index; DM, diabetes mellitus; FPG, fasting plasma glucose; HFimpEF, heart failure with improved ejection fraction; VIM, variability independent of the mean.
Given that diabetic patients, especially those with poor glycemic control, usually have greater glycemic fluctuations, the association between GV and HFimpEF was verified by excluding patients with baseline HbA1c > 8% or on insulin treatment (Supplementary Table 3). We found CV and VIM of FPG persisted to significantly associate with HFimpEF in both models after multivariable adjustment, suggesting that GV was associated with HFimpEF even when patients with poor glycemic control were excluded. Furthermore, a nested case-control study was conducted by matching HFimpEF and persistent HFrEF patients in the cohort at 1:1 ratio (Supplementary Table 4). Consistently, we found patients with high GV (quartile 3~4 had significantly lower probability of HFimpEF than those with low GV (quartile 1~2) assessed by 3 different GV measures (Supplementary Table 5).
The major findings of the present study are that HF patients with higher long-term GV are less likely to experience LV functional improvement. Long-term GV is an independent risk factor for the development of HFimpEF.
Hyperglycemia increases risk of physical impairment (31), coronary heart disease (32), heart failure (33), peripheral artery disease (34) and stroke (35), irrespective of diabetic conditions. Long-term poor glycemic control marked by high HbA1c was associated with higher risk of all-cause mortality and hospitalization for patients with cardiovascular disease (36–39). Recent studies also found that acute hyperglycemic status reflected by stress hyperglycemia ratio predicted adverse outcomes in patients with nonobstructive coronary arteries (40), coronary chronic total occlusion (41) and acute coronary syndrome (42).
Apart from mean glycemic levels, existing evidence reveals that GV, no matter short-term or long-term, is an independent risk factor for the incidence of HF. Of note, both FPG variability and HbA1c variability represent variability of glycemic control levels but comprise different aspects of dysregulated glycemic homeostasis. On one hand, HbA1c, representing a weighted mean glucose level over the preceding 2-3 months, is usually more stable than FPG and thus has less variability (43). On the other hand, HbA1c is an integrated assessment reflecting both FPG and postprandial plasma glucose (PPG) levels (44). A Korean nationwide population-based study revealed that over a median follow-up of 5.3 years, the risk of HF increased by 15% (HR: 1.15 [95% CI 1.10~1.20]) in subjects with the highest quartile of FPG variability compared to those with the lowest quartile (45). A number of diabetic cohort studies demonstrated that higher long-term HbA1c variability valued by different measures was independently associated with increased risk of HF (46–48). In non-diabetic patients, GV accessed by mean amplitude of glycemic excursions (MAGE) was also related to incident HF after myocardial infarction (49). Furthermore, GV has been recognized as a significant predictor for major adverse cardiovascular events (MACE) independent of mean glycemic control levels and conventional risk factors both in diabetic and non-diabetic HF patients (22–25, 50).
Attributed to advanced guideline-directed medical and device therapies, 10%~52% of HF patients have experienced myocardial recovery and developed HFimpEF (1–5). Of note, the specific definition of HFimpEF varies according to different guidelines or clinical studies. The proposed universal definition of HFimpEF (51) put forward a requirement of ≥10-point increase from baseline EF in addition to the criteria of a baseline EF ≤40% and a follow-up measurement > 40% as stated in the 2022 AHA/ACC/HFSA guideline (8). In this study, we adopted the universal definition since a 10-point increase in EF guarantees actual myocardial functional improvement and minimizes the impact by interobserver and intraobserver measurement variabilities.
We recently showed that glucose metabolic disorders reflected by hyperglycemia or insulin resistance are associated with compromised development of HFimpEF (52, 53). However, to our knowledge, the relationship between GV and HFimpEF remains unknown. In accordance with previous reports, 42.0% of hospitalized HF patients in this study developed HFimpEF during 12-month follow-up. Besides, we for the first time demonstrated that LV functional improvement accompanied by reverse remodeling was prominently compromised with increasing long-term FPG variability. Multivariate regression analysis showed that long-term FPG variability was independently associated with the incidence of HFimpEF, even after adjustment for baseline HbA1c as well as mean FPG levels during follow-up. These findings were also confirmed by the nested case-control study. Furthermore, subgroup analysis revealed the association between FPG variability and HFimpEF persisted significant irrespective of the presence of diabetes and mean FPG control levels. In addition, we assessed FPG variability by different measures including CV, ASV and VIM. CV and ASV are relatively simple and more feasible in clinical practice, whereas VIM is calculated based on logarithmic curve fitting to eliminate its correlation with mean FPG. We revealed that all these measures of FPG variability yielded similar findings. These data jointly support the notion that GV per se plays a negative role in the development of HFimpEF through mechanisms independent of glycemic levels.
Noteworthy, although only a small proportion of patients were on SGLT2 inhibitors (n=32, 6.2%) since the medication has not been introduced to our center until the second half of 2019, the univariate analysis exhibited a positive association between the use of SGLT2 inhibitors and HFimpEF. SGLT2 inhibitors have pleiotropic cardio-protective effects through modulating renin-angiotensin-aldosterone system, shifting energy substrate, and attenuating systemic inflammatory status (54, 55). Given the promising results from DAPA-HF (56) and EMPEROR-Reduced trials (57), SGLT2 inhibitors have become a cornerstone of HFrEF treatment. Resent trails revealed that SGLT2 inhibitors also improve outcomes of patients with HF with preserved EF, no matter with or without diabetes (58–60). Existing evidence suggested that SGLT2 inhibitors facilitate LV reverse remodeling and diastolic function (61–64). Our results further implied that SGLT2 inhibitors may exert favorable effects on myocardial functional recovery, which certainly awaits further confirmation in prospectively designed clinical studies.
Based on existing clinical and basic studies, several potential mechanisms might account for the negative impact of GV on myocardial recovery. First, dramatic glycemic oscillation promotes oxidative stress in the myocardium, thereby leading to mitochondrial damage, endothelial dysfunction, inflammatory response and finally myocardial fibrosis (65–68). Second, greater GV is presumably associated with more hypoglycemic episodes. In our study, 3.3% of the subjects suffered hypoglycemic event, which was only observed in patients with higher GV. Established evidence has displayed that hypoglycemia stimulates sympathetic nervous system, thus increasing cardiac preload, arrhythmia, inflammation and thereby posing deleterious effects on myocardium (69–71). Third, patients with marked glycemic oscillation tend to have poor compliance to medical treatments, thus attenuating the beneficial effects of pharmacological therapies on myocardial recovery.
Our findings should be interpreted in the context of the following limitations. First, this study is a retrospective analysis based on prospectively collected data from a single center, and the result is potentially subject to selection bias. Second, hypoglycemic episodes were not analyzed and adjusted in the multivariate analysis since they were only documented from long-term FPG values owing to the study design and thus probably underestimated. Third, hospitalization for HF is associated with worsening of EF, which may to some extent affect our findings. Finally, prospective studies are warranted to analyze the causal link between GV and occurrence of HFimpEF.
In conclusion, our findings suggest that greater long-term GV is associated with compromised development of HFimpEF. A more stable control of glycemic levels might provide favorable effects on myocardial recovery in HF patients even without diabetes.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Ruijin Hospital, Shanghai Jiao-Tong University School of Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
CY, JC and XW performed study design, data analysis and data interpretation. CY and XW performed manuscript writing. CY, JC, JQ, XS, SF, MA and XW performed data collection. CY, JQ, FD, WS, LL, RZ and XW performed manuscript revision. All authors contributed to the article and approved the submitted version.
This work was supported by National Natural Science Foundation of China (Grant No. 81870179, 82000369, 82170423), Shanghai Municipal Commission of Health and Family Planning (Grant No. 20194Y0042), Technology Transfer Project of Shanghai Jiao Tong University School of Medicine (Grant No. ZT202103).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1211954/full#supplementary-material
1. Swat SA, Cohen D, Shah SJ, Lloyd-Jones DM, Baldridge AS, Freed BH, et al. Baseline longitudinal strain predicts recovery of left ventricular ejection fraction in hospitalized patients with nonischemic cardiomyopathy. J Am Heart Assoc (2018) 7(20):e09841. doi: 10.1161/jaha.118.009841
2. Agra Bermejo R, Gonzalez Babarro E, López Canoa JN, Varela Román A, Gómez Otero I, Oro Ayude M, et al. Heart failure with recovered ejection fraction: Clinical characteristics, determinants and prognosis. CARDIOCHUS-CHOP registry. Cardiol J (2018) 25(3):353–62. doi: 10.5603/CJ.a2017.0103
3. Punnoose LR, Givertz MM, Lewis EF, Pratibhu P, Stevenson LW, Desai AS. Heart failure with recovered ejection fraction: a distinct clinical entity. J Card Fail Jul (2011) 17(7):527–32. doi: 10.1016/j.cardfail.2011.03.005
4. Kalogeropoulos AP, Fonarow GC, Georgiopoulou V, Burkman G, Siwamogsatham S, Patel A, et al. Characteristics and outcomes of adult outpatients with heart failure and improved or recovered ejection fraction. JAMA Cardiol (2016) 1(5):510–8. doi: 10.1001/jamacardio.2016.1325
5. Basuray A, French B, Ky B, Vorovich E, Olt C, Sweitzer NK, et al. Heart failure with recovered ejection fraction: clinical description, biomarkers, and outcomes. Circulation (2014) 129(23):2380–7. doi: 10.1161/circulationaha.113.006855
6. Wilcox JE, Fang JC, Margulies KB, Mann DL. Heart failure with recovered left ventricular ejection fraction: JACC scientific expert panel. J Am Coll Cardiol (2020) 76(6):719–34. doi: 10.1016/j.jacc.2020.05.075
7. Nijst P, Martens P, Mullens W. Heart failure with myocardial recovery - the patient whose heart failure has improved: What next? Prog Cardiovasc Dis (2017) 60(2):226–36. doi: 10.1016/j.pcad.2017.05.009
8. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. AHA/ACC/HFSA guideline for the management of heart failure: A report of the american college of Cardiology/American heart association joint committee on clinical practice guidelines. Circulation (2022 2022) 145(18):e895–e1032. doi: 10.1161/cir.0000000000001063
9. Kim GH, Uriel N, Burkhoff D. Reverse remodelling and myocardial recovery in heart failure. Nat Rev Cardiol (2018) 15(2):83–96. doi: 10.1038/nrcardio.2017.139
10. Marsico F, Gargiulo P, Marra AM, Parente A, Paolillo S. Glucose metabolism abnormalities in heart failure patients: insights and prognostic relevance. Heart Fail Clin (2019) 15(3):333–40. doi: 10.1016/j.hfc.2019.02.002
11. Cardoso CRL, Leite NC, Moram CBM, Salles GF. Long-term visit-to-visit glycemic variability as predictor of micro- and macrovascular complications in patients with type 2 diabetes: The Rio de Janeiro Type 2 Diabetes Cohort Study. Cardiovasc Diabetol (2018) 17(1):33. doi: 10.1186/s12933-018-0677-0
12. Zhou JJ, Schwenke DC, Bahn G, Reaven P. Glycemic variation and cardiovascular risk in the veterans affairs diabetes trial. Diabetes Care (2018) 41(10):2187–94. doi: 10.2337/dc18-0548
13. Hirakawa Y, Arima H, Zoungas S, Ninomiya T, Cooper M, Hamet P, et al. Impact of visit-to-visit glycemic variability on the risks of macrovascular and microvascular events and all-cause mortality in type 2 diabetes: the ADVANCE trial. Diabetes Care (2014) 37(8):2359–65. doi: 10.2337/dc14-0199
14. Yang CD, Shen Y, Lu L, Yang ZK, Hu J, Zhang RY, et al. Visit-to-visit HbA(1c) variability is associated with in-stent restenosis in patients with type 2 diabetes after percutaneous coronary intervention. Cardiovasc Diabetol (2020) 19(1):133. doi: 10.1186/s12933-020-01111-7
15. Kaze AD, Santhanam P, Erqou S, Ahima RS, Echouffo-Tcheugui JB. Long-term variability of glycemic markers and risk of all-cause mortality in type 2 diabetes: the Look AHEAD study. BMJ Open Diabetes Res Care (2020) 8(2). doi: 10.1136/bmjdrc-2020-001753
16. Huang D, Huang YQ, Zhang QY, Cui Y, Mu TY, Huang Y. Association between long-term visit-to-visit hemoglobin A1c and cardiovascular risk in type 2 diabetes: The ACCORD trial. Front Cardiovasc Med (2021) 8:777233. doi: 10.3389/fcvm.2021.777233
17. Gerbaud E, Darier R, Montaudon M, Beauvieux MC, Coffin-Boutreux C, Coste P, et al. Glycemic variability is a powerful independent predictive factor of midterm major adverse cardiac events in patients with diabetes with acute coronary syndrome. Diabetes Care (2019) 42(4):674–81. doi: 10.2337/dc18-2047
18. Takahashi H, Iwahashi N, Kirigaya J, Kataoka S, Minamimoto Y, Gohbara M, et al. Glycemic variability determined with a continuous glucose monitoring system can predict prognosis after acute coronary syndrome. Cardiovasc Diabetol (2018) 17(1):116. doi: 10.1186/s12933-018-0761-5
19. Echouffo-Tcheugui JB, Zhao S, Brock G, Matsouaka RA, Kline D, Joseph JJ. Visit-to-visit glycemic variability and risks of cardiovascular events and all-cause mortality: The ALLHAT study. Diabetes Care (2019) 42(3):486–93. doi: 10.2337/dc18-1430
20. Ghouse J, Skov MW, Kanters JK, Lind B, Isaksen JL, Blanche P, et al. Visit-to-Visit variability of hemoglobin A(1c) in people without diabetes and risk of major adverse cardiovascular events and all-cause mortality. Diabetes Care (2019) 42(1):134–41. doi: 10.2337/dc18-1396
21. Jang JY, Moon S, Cho S, Cho KH, Oh CM. Visit-to-visit HbA1c and glucose variability and the risks of macrovascular and microvascular events in the general population. Sci Rep (2019) 9(1):1374. doi: 10.1038/s41598-018-37834-7
22. Lazzeri C, Valente S, Chiostri M, D'Alfonso MG, Gensini GF. Prognostic impact of early glucose variability in acute heart failure patients: a pilot study. Int J Cardiol (2014) 177(2):693–5. doi: 10.1016/j.ijcard.2014.09.150
23. Gerbaud E, Bouchard de la Poterie A, Baudinet T, Montaudon M, Beauvieux MC, Lemaître AI, et al. Glycaemic variability and hyperglycaemia as prognostic markers of major cardiovascular events in diabetic patients hospitalised in cardiology intensive care unit for acute heart failure. J Clin Med (2022) 11(6):1549. doi: 10.3390/jcm11061549
24. Dungan KM, Binkley P, Nagaraja HN, Schuster D, Osei K. The effect of glycaemic control and glycaemic variability on mortality in patients hospitalized with congestive heart failure. Diabetes Metab Res Rev (2011) 27(1):85–93. doi: 10.1002/dmrr.1155
25. Gu J, Pan JA, Fan YQ, Zhang HL, Zhang JF, Wang CQ. Prognostic impact of HbA1c variability on long-term outcomes in patients with heart failure and type 2 diabetes mellitus. Cardiovasc Diabetol (2018) 17(1):96. doi: 10.1186/s12933-018-0739-3
26. Bozkurt B, Coats AJ, Tsutsui H, Abdelhamid M, Adamopoulos S, Albert N, et al. Universal definition and classification of heart failure: A report of the heart failure society of america, heart failure association of the european society of cardiology, japanese heart failure society and writing committee of the universal definition of heart failure. J Card Fail (2021) 27(4):387–413. doi: 10.1016/j.cardfail.2021.01.022
27. Stevenson P. Height. weight. surface formula for the estimation of surface area in Chinese subjects. Chin J Physiol (1937) 12:327–30.
28. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr., et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension (2003) 42(6):1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2
29. American Diabetes A. Standards of medical care in diabetes–2012. Diabetes Care (2012) 35 Suppl 1:S11–63. doi: 10.2337/dc12-s011
30. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Internal Med (2009) 150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
31. Pansini A, Lombardi A, Morgante M, Frullone S, Marro A, Rizzo M, et al. Hyperglycemia and physical impairment in frail hypertensive older adults. Front Endocrinol (Lausanne) (2022) 13:831556. doi: 10.3389/fendo.2022.831556
32. Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ (2000) 321(7258):405–12. doi: 10.1136/bmj.321.7258.405
33. Matsushita K, Blecker S, Pazin-Filho A, Bertoni A, Chang PP, Coresh J, et al. The association of hemoglobin a1c with incident heart failure among people without diabetes: the atherosclerosis risk in communities study. Diabetes (2010) 59(8):2020–6. doi: 10.2337/db10-0165
34. Jude EB, Oyibo SO, Chalmers N, Boulton AJ. Peripheral arterial disease in diabetic and nondiabetic patients: a comparison of severity and outcome. Diabetes Care (2001) 24(8):1433–7. doi: 10.2337/diacare.24.8.1433
35. Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke (2001) 32(10):2426–32. doi: 10.1161/hs1001.096194
36. van Melle JP, Bot M, de Jonge P, de Boer RA, van Veldhuisen DJ, Whooley MA. Diabetes, glycemic control, and new-onset heart failure in patients with stable coronary artery disease: data from the heart and soul study. Diabetes Care (2010) 33(9):2084–9. doi: 10.2337/dc10-0286
37. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med (2015) 373(22):2117–28. doi: 10.1056/NEJMoa1504720
38. Cavender MA, Steg PG, Smith SC Jr., Eagle K, Ohman EM, Goto S, et al. Impact of diabetes mellitus on hospitalization for heart failure, cardiovascular events, and death: Outcomes at 4 years from the reduction of atherothrombosis for continued health (REACH) registry. Circulation (2015) 132(10):923–31. doi: 10.1161/CIRCULATIONAHA.114.014796
39. Group AC, Patel A, MacMahon S, Chalmers J, Neal B, Billot L, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med (2008) 358(24):2560–72. doi: 10.1056/NEJMoa0802987
40. Mone P, Lombardi A, Salemme L, Cioppa A, Popusoi G, Varzideh F, et al. Stress hyperglycemia drives the risk of hospitalization for chest pain in patients with ischemia and nonobstructive coronary arteries (INOCA). Diabetes Care (2023) 46(2):450–4. doi: 10.2337/dc22-0783
41. Song Y, Cui K, Yang M, Song C, Yin D, Dong Q, et al. High triglyceride-glucose index and stress hyperglycemia ratio as predictors of adverse cardiac events in patients with coronary chronic total occlusion: a large-scale prospective cohort study. Cardiovasc Diabetol (2023) 22(1):180. doi: 10.1186/s12933-023-01883-8
42. Yang J, Zheng Y, Li C, Gao J, Meng X, Zhang K, et al. The impact of the stress hyperglycemia ratio on short-term and long-term poor prognosis in patients with acute coronary syndrome: Insight from a large cohort study in asia. Diabetes Care (2022) 45(4):947–56. doi: 10.2337/dc21-1526
43. Sherwani SI, Khan HA, Ekhzaimy A, Masood A, Sakharkar MK. Significance of hbA1c test in diagnosis and prognosis of diabetic patients. biomark Insights (2016) 11:95–104. doi: 10.4137/bmi.S38440
44. Ketema EB, Kibret KT. Correlation of fasting and postprandial plasma glucose with HbA1c in assessing glycemic control; systematic review and meta-analysis. Arch Public Health (2015) 73:43. doi: 10.1186/s13690-015-0088-6
45. Kwon S, Lee SR, Choi EK, Lee SH, Han KD, Lee SY, et al. Visit-to-visit variability of metabolic parameters and risk of heart failure: A nationwide population-based study. Int J Cardiol (2019) 293:153–8. doi: 10.1016/j.ijcard.2019.06.035
46. Segar MW, Patel KV, Vaduganathan M, Caughey MC, Butler J, Fonarow GC, et al. Association of long-term change and variability in glycemia with risk of incident heart failure among patients with type 2 diabetes: A secondary analysis of the ACCORD trial. Diabetes Care (2020) 43(8):1920–8. doi: 10.2337/dc19-2541
47. Gu J, Fan YQ, Zhang JF, Wang CQ. Association of hemoglobin A1c variability and the incidence of heart failure with preserved ejection fraction in patients with type 2 diabetes mellitus and arterial hypertension. Hellenic J Cardiol (2018) 59(2):91–7. doi: 10.1016/j.hjc.2017.08.001
48. Parry HM, Deshmukh H, Levin D, Van Zuydam N, Elder DH, Morris AD, et al. Both high and low HbA1c predict incident heart failure in type 2 diabetes mellitus. Circ Heart Fail (2015) 8(2):236–42. doi: 10.1161/circheartfailure.113.000920
49. Mi SH, Su G, Yang HX, Zhou Y, Tian L, Zhang T, et al. Comparison of in-hospital glycemic variability and admission blood glucose in predicting short-term outcomes in non-diabetes patients with ST elevation myocardial infarction underwent percutaneous coronary intervention. Diabetol Metab Syndr (2017) 9:20. doi: 10.1186/s13098-017-0217-1
50. Cunha FM, Cidade-Rodrigues C, Elias C, Oliveira D, Bettencourt P, Lourenço P. Glucose variability predicts 6-month mortality in patients hospitalized with acute heart failure. Intern Emerg Med (2021) 16(8):2121–8. doi: 10.1007/s11739-021-02719-7
51. Bozkurt B, Coats A, Tsutsui H. Universal definition and classification of heart failure. J Card Fail (2021) 27(4):387–413. doi: 10.1016/j.cardfail.2021.01.022
52. Yang CD, Aihemaiti M, Quan JW, Chen JW, Shu XY, Ding FH, et al. HbA1c level is associated with the development of heart failure with recovered ejection fraction in hospitalized heart failure patients with type 2 diabetes. Int J Cardiol (2023) 371:259–65. doi: 10.1016/j.ijcard.2022.09.029
53. Yang CD, Pan WQ, Feng S, Quan JW, Chen JW, Shu XY, et al. Insulin resistance is associated with heart failure with recovered ejection fraction in patients without diabetes. J Am Heart Assoc (2022) 11(19):e026184. doi: 10.1161/jaha.122.026184
54. Chen X, Hocher CF, Shen L, Krämer BK, Hocher B. Reno- and cardioprotective molecular mechanisms of SGLT2 inhibitors beyond glycemic control - from bedside to bench. Am J Physiol Cell Physiol (2023). doi: 10.1152/ajpcell.00177.2023
55. Panico C, Bonora B, Camera A, Chilelli NC, Prato GD, Favacchio G, et al. Pathophysiological basis of the cardiological benefits of SGLT-2 inhibitors: a narrative review. Cardiovasc Diabetol (2023) 22(1):164. doi: 10.1186/s12933-023-01855-y
56. McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med (2019) 381(21):1995–2008. doi: 10.1056/NEJMoa1911303
57. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med (2020) 383(15):1413–24. doi: 10.1056/NEJMoa2022190
58. Mone P, Lombardi A, Gambardella J, Pansini A, Macina G, Morgante M, et al. Empagliflozin improves cognitive impairment in frail older adults with type 2 diabetes and heart failure with preserved ejection fraction. Diabetes Care (2022) 45(5):1247–51. doi: 10.2337/dc21-2434
59. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Bohm M, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med (2021) 385(16):1451–61. doi: 10.1056/NEJMoa2107038
60. Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med (2022) 387(12):1089–98. doi: 10.1056/NEJMoa2206286
61. Verma S, Mazer CD, Yan AT, Mason T, Garg V, Teoh H, et al. Effect of empagliflozin on left ventricular mass in patients with type 2 diabetes mellitus and coronary artery disease: The EMPA-HEART CardioLink-6 randomized clinical trial. Circulation (2019) 140(21):1693–702. doi: 10.1161/circulationaha.119.042375
62. Soga F, Tanaka H, Tatsumi K, Mochizuki Y, Sano H, Toki H, et al. Impact of dapagliflozin on left ventricular diastolic function of patients with type 2 diabetic mellitus with chronic heart failure. Cardiovasc Diabetol (2018) 17(1):132. doi: 10.1186/s12933-018-0775-z
63. Matsutani D, Sakamoto M, Kayama Y, Takeda N, Horiuchi R, Utsunomiya K. Effect of canagliflozin on left ventricular diastolic function in patients with type 2 diabetes. Cardiovasc Diabetol (2018) 17(1):73. doi: 10.1186/s12933-018-0717-9
64. Verma S, Garg A, Yan AT, Gupta AK, Al-Omran M, Sabongui A, et al. Effect of empagliflozin on left ventricular mass and diastolic function in individuals with diabetes: An important clue to the EMPA-REG OUTCOME trial? Diabetes Care (2016) 39(12):e212–3. doi: 10.2337/dc16-1312
65. Quagliaro L, Piconi L, Assaloni R, Martinelli L, Motz E, Ceriello A. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD(P)H-oxidase activation. Diabetes (2003) 52(11):2795–804. doi: 10.2337/diabetes.52.11.2795
66. Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA (2006) 295(14):1681–7. doi: 10.1001/jama.295.14.1681
67. Ceriello A, Esposito K, Piconi L, Ihnat MA, Thorpe JE, Testa R, et al. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes (2008) 57(5):1349–54. doi: 10.2337/db08-0063
68. Tsutsui H, Kinugawa S, Matsushima S. Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol (2011) 301(6):H2181–90. doi: 10.1152/ajpheart.00554.2011
69. Dandona P, Chaudhuri A, Dhindsa S. Proinflammatory and prothrombotic effects of hypoglycemia. Diabetes Care (2010) 33(7):1686–7. doi: 10.2337/dc10-0503
70. Stahn A, Pistrosch F, Ganz X, Teige M, Koehler C, Bornstein S, et al. Relationship between hypoglycemic episodes and ventricular arrhythmias in patients with type 2 diabetes and cardiovascular diseases: silent hypoglycemias and silent arrhythmias. Diabetes Care (2014) 37(2):516–20. doi: 10.2337/dc13-0600
71. Skyler JS, Bergenstal R, Bonow RO, Buse J, Deedwania P, Gale EA, et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the american diabetes association and a scientific statement of the american college of cardiology foundation and the american heart association. J Am Coll Cardiol (2009) 53(3):298–304. doi: 10.1016/j.jacc.2008.10.008
Keywords: glycemic variability, heart failure with improved ejection fraction, heart failure with reduced ejection fraction, myocardial recovery, fasting plasma glucose
Citation: Yang CD, Chen JW, Quan JW, Shu XY, Feng S, Aihemaiti M, Ding FH, Shen WF, Lu L, Zhang RY and Wang XQ (2023) Long-term glycemic variability predicts compromised development of heart failure with improved ejection fraction: a cohort study. Front. Endocrinol. 14:1211954. doi: 10.3389/fendo.2023.1211954
Received: 25 April 2023; Accepted: 31 August 2023;
Published: 20 September 2023.
Edited by:
Lu Cai, University of Louisville, United StatesReviewed by:
Aleksandr E. Vendrov, University of Michigan, United StatesCopyright © 2023 Yang, Chen, Quan, Shu, Feng, Aihemaiti, Ding, Shen, Lu, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao Qun Wang, d2FuZ3hxQHNoc211LmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.