
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 26 June 2023
Sec. Clinical Diabetes
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1207045
This article is part of the Research Topic Practice Innovation and Outcome Evaluation in Diabetes View all 13 articles
Objective: Serum neurofilament light chain (sNfL) level, which is a biomarker indicative of neuroaxonal damage and cognitive impairment, has been reported in several neurological diseases. There has been a lack of studies on the association between sNfL levels and prediabetes in adolescents. This study investigated whether sNfL levels were higher in adolescents with prediabetes undergoing elective orthopedic surgery.
Methods: The sNfL level was measured in 149 adolescents aged from 12 to 18 years who underwent elective orthopedic surgery at the Hunan Children’s Hospital (18 with and 131 without prediabetes). We evaluated the association between prediabetes and sNfL level after adjusting for age, sex, and triglycerides using a multivariable linear regression model.
Results: The prevalence of prediabetes in adolescents was 12.08%. Univariate logistic regression analysis showed that prediabetes was related to sNfL. In multivariate logistic regression analysis, the association between prediabetes with sNfL levels remained significant after adjustment for age, sex, and triglyceride. The relationship between the two was further visualized by a smoothed curve.
Conclusions: Prediabetes is associated with a higher sNfL. Further large-scale and prospective studies are needed to verify the clinical application of sNfL as a monitoring biomarker for adolescent prediabetes in adolescents and to evaluate the performance of sNfL in predicting the incidence of neuropathy and cognitive dysfunction in adolescents with prediabetes.
Due to increasing obesity, diseases previously almost exclusively found in adults, such as prediabetes and type-II diabetes(T2D), are now frequently diagnosed among adolescents (1). Prediabetes is a term that generally refers to an intermediate state of abnormal glycemia. In the United States, the National Health and Nutrition Examination Surveys have shown that the prevalence of prediabetes among adolescents is approximately 18.0% (2). Meanwhile, prediabetes is a pressing clinical and public health issue, as studies have reported that the highest risk of diabetes, major adverse cardiovascular events (MACE), and chronic kidney disease occur in individuals with prediabetes (3, 4). As a precursor of T2D, prediabetes is more severe in adolescents than in adults because the accelerated period of progression from prediabetes to T2D (5, 6) could lead to an early onset of complications and adverse events that affect patients’ quality of life and long-term outcomes. The increasing prevalence of prediabetes in adolescents has now become one of the major public health concerns worldwide (7).
Neurofilaments are considered major cytoskeletal components of neurons and are classified into light, medium, and heavy chains according to the size of the proteins. Neurofilament proteins are enriched in axons, simultaneously providing mechanical support and maintaining axon homeostasis (8, 9). Neurofilaments can be released from damaged or diseased axons in significant amounts into the blood and cerebrospinal fluid (CSF), and therefore their elevated levels are often used as potential biomarkers to indicate various neurological diseases (10, 11). CSF is the most frequently used biofluid for measuring neurofilament light chain (NfL) levels in neurodegenerative diseases, but because of the invasiveness of lumbar puncture as well as the pain and distress associated with CSF collection, it is impractical to obtain CSF from adolescents, who require strict instruction (12). The application of novel highly sensitive analytical methods has made it possible to measure low levels of NfL in blood with high accuracy and reproducibility. As several studies have demonstrated close correlations between CSF and sNfL (13, 14), it is practical to study sNfL in a wide range of neurological disorders. However, to properly explain sNfL levels in relation to disease states, it is also important to consider factors relevant to this protein change. Recent studies have shown that sNfL may be affected by some factors such as age, systolic blood pressure (SBP), and body mass index (BMI) (15–17).
Traditionally, neuropathy has been considered a microvascular complication that occurs in patients with a long history of diabetes. More recently, however, it has been reported that neuropathic complications may develop as early as the time of diagnosis of diabetes mellitus (18–20). The prevalence of documented neuropathy in individuals with prediabetes is approximately 11%-25% (18). Prediabetes has been increasingly recognized as an important factor leading to neuropathy. In particular, emerging data and epidemiologic studies support that prediabetes is a risk factor for mild cognitive impairment (21–23).
There is a lack of studies on prediabetes in adolescents. This study was designed to identify the main contributors to the sNfL level in perioperative adolescents and to assess whether a higher sNfL level was related to adolescents with prediabetes in elective orthopedic surgery. The current data analyzed in our study were obtained from Hunan Children’s Hospital.
The data for this study were collected from the electronic medical records (2021-2022) of the Hunan Children’s Hospital. This retrospective study was approved by the Ethics Committee of Hunan Children’s Hospital [No. HCHLL-20230-43]. Informed consent was waived due to the observational nature of the study.
This study contained anonymous demographic, medical, surgical, and laboratory information from adolescents undergoing elective orthopedic surgery in the Department of Anesthesiology. All adolescent patients (aged from 12 to 18 years old) had a random preoperative plasma glucose or hemoglobin A1c. Exclusion criteria were patients undergoing emergency surgery, age <12 years old, ASA grade VI, no sNfL data, or no plasma glucose. The present analysis was exempted due to the deidentified dataset of the study. The study was conducted following a pre-specified protocol and statistical plan that was not disclosed prior to data analysis. This manuscript was prepared according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
Patient data were collected. This included age, sex, and body mass index (BMI), calculated as weight in kilograms divided by height in meters squared.
Laboratory methods were used to measure hemoglobin A1c(HbA1c), plasma glucose, high-density lipoprotein (HDL), systolic blood pressure (SBP), sNfL, cholesterol, and triglyceride levels. The single point insulin sensitivity estimator (SPISE) was calculated using the following formula: [600* HDL^0.185/(TG^0.2* BMI^1.338)] (24).
Prediabetes was defined in accordance with the American Diabetes Association criteria when any of the following conditions were met: (1) hemoglobin A1c (HbA1c) level≥5.7% and <6.5% (25); and (2) a random plasma glucose≥100 mg/dL and <160mg/dL (26).
Frozen serum was thawed on ice and then spun prior to NfL measurement using the Simoa HD-X Analyzer (Quanterix) and Single Molecule Array (Simoa) technology according to the manufacturer’s instructions. The assay for these samples had a lower limit of detection of 0.038 pg/mL, a lower limit of quantitation (LLOQ) of 0.174 pg/mL, a dynamic range of 0–2,000 pg/mL, and a coefficient of variation of 7% at the LLOQ. Measurements were performed in duplicate and lots were completed by an investigator blinded to all clinical data, including outcome measures. Samples with a between-measurement coefficient of variation >20% were repeated according to standard practice.
All the analyses were conducted using IBM SPSS (version 22.0) and R (version 4.12.0) software. Data were presented as median (M) and interquartile range (IQR). The Wilcoxon rank-sum test was used to compare continuous variables with non-normal distributions, and the chi-squared test was used to compare the composition ratio of classified data. The association between prediabetes and sNfL level was modeled using multivariate linear regression analysis. The selection of covariates to be included in the model was based on data from previous studies in which age, sex, prediabetes, and triglycerides were found to be associated with sNfL level. A two-tailed value of P<0.05 was considered statistically significant.
The process of patient selection was shown in Figure 1. A total of 149 patients were eligible for analysis, including 131 participants without prediabetes and 18 patients with prediabetes (12.08%). All the adolescents were grouped according to whether they had prediabetes before surgery. The overall characteristics of the study population were shown in Table 1. The median [IQR] sNfL of adolescents with and without prediabetes before surgery was 13.500 mmol/L and 9.750 mmol/L, respectively, with a statistically significant difference (P=0.029). Other factors including gender, age, BMI, cholesterol, triglycerides, HDL, SBP, and SPISE were not significantly different between the two groups.
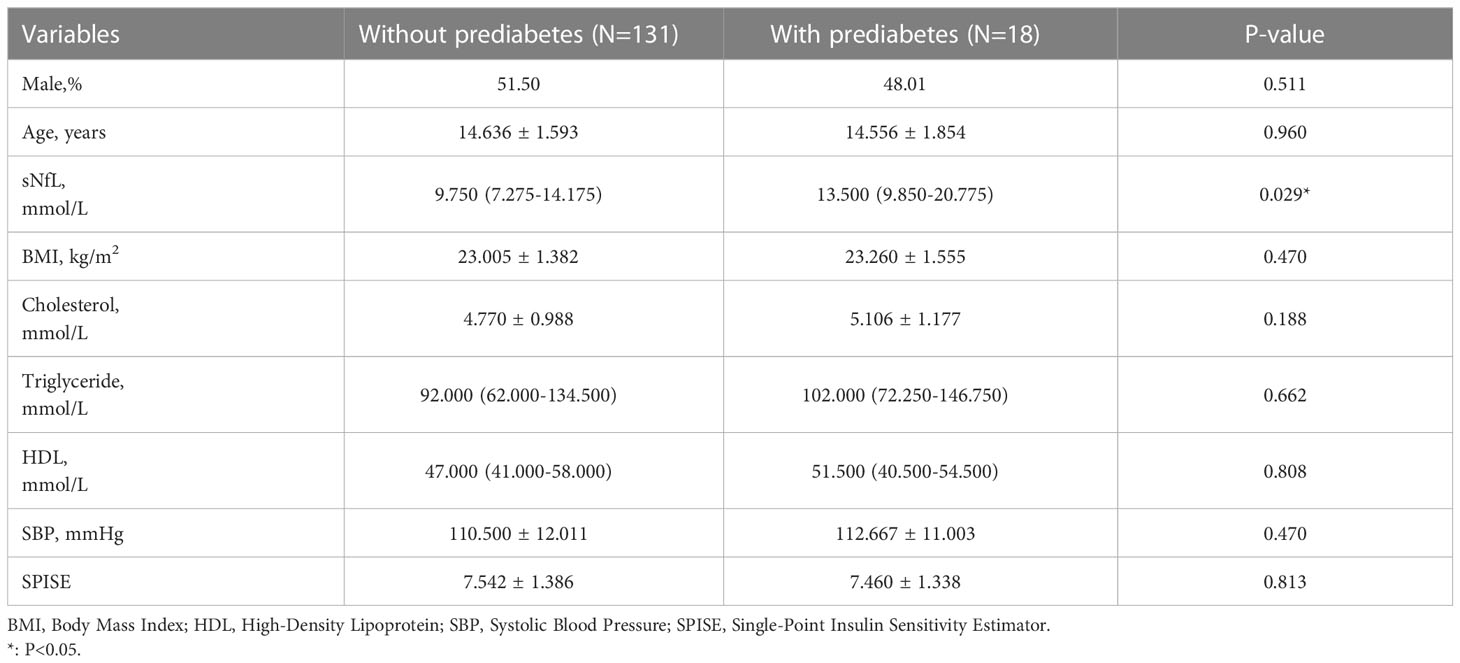
Table 1 Characteristics of participants according to quartiles of serum neurofilament light chain (sNfL) levels.
As shown in Table 2, when considering the entire cohort, the univariable analysis showed that prediabetes (RR, 4.147; 95%CI, 0.584-7.710, P=0.024) was associated with sNfL. Apart from prediabetes, other factors, such as gender, age, BMI, cholesterol, triglycerides, HDL, SBP, and SPISE, were irrelevant to sNfL. In Figure 2, a smoothed curve was applied to visualize the relationship between sNfL and the prevalence of prediabetes in adolescents, indicating that the prevalence of prediabetes was correlated with sNfL.
The association between prediabetes and sNfL level was modeled using multivariate linear regression analysis. The results were shown in Table 3. Multiple regression analysis revealed that prediabetes (RR, 4.172; 95%CI, 0.602-0.742, P=0.023) was positively correlated with sNfL independent of other factors in all patients.
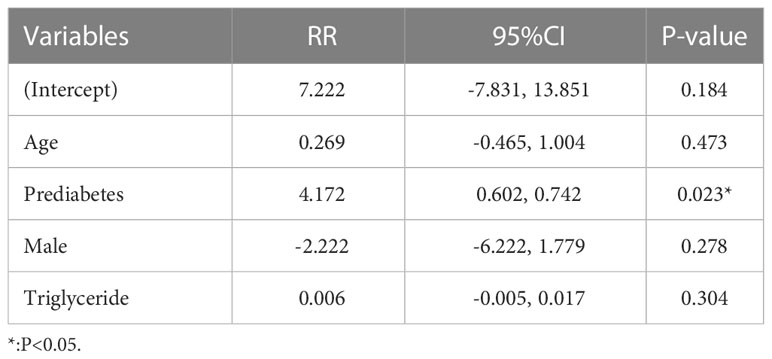
Table 3 Multivariable linear regression model evaluating predictors of serum neurofilament light chain (sNfL) levels in the studied population.
As shown in Table 4, various variables were divided into subgroups, which were subjected to regression analysis to investigate the relationship between the subgroup variables and sNfL using sensitivity analysis. We found that low cholesterol (RR, 10.585; 95%CI, 3.616-17.554, P=0.005), low triglyceride (RR, 13.124; 95%CI, 6.160-20.089, P<0.001), and high HDL (RR, 9.593; 95%CI, 2.964, 16.222, P=0.006) were associated with sNfL.
In the present study, we evaluated the association between a higher sNfL level and prediabetes in adolescents undergoing elective orthopedic surgery. According to the American Diabetes Association criteria, the prevalence of prediabetes in adolescents in our study was 12.08%. The current univariate analysis showed a relation between prediabetes and sNfL. After adjustment for age, gender, and triglyceride, the results showed that the association between prediabetes and sNfL level was still significant. The smoothed curve further visualized the relationship between the two. Our findings demonstrated showed a higher sNfL level in adolescents who had prediabetes, providing neurochemical evidence for subclinical axonal damage and underlying cognitive impairment in prediabetes adolescents. Such a finding could help improve interventions to promote the reversion of prediabetic states to normal glucose tolerance.
Although several studies have examined sNfL levels in patients with various neurological disorders, to the best of our knowledge, this was the first study to report the association of sNfL levels with prediabetes in adolescents. Importantly, the association between prediabetes and sNfL remained significant after adjustment for sex, age, and triglycerides. Previous studies have shown a positive association between sNfL levels and age (27, 28), as a marked increase in sNfL levels has been found to be associated with older age, particularly in those over 60 years of age (29). Our results did not over 60 years of age. Similarly, there was no independent association between sex and sNfL in our study, which was generally over 60 years of age studies (30, 31).
Neurofilaments are released into the extracellular space following neuroaxonal damage, and we found that prediabetes was associated with a higher sNfL. Our study was the first to validate a higher sNfL as a potential biomarker of mild nerve axon damage or milder peripheral neuropathy in prediabetic adolescents. It was well known that neuropathy is one of the major causes of complications in the diabetic population, however, but some studies reported that peripheral neuropathy may develop in humans with prediabetes before overt hyperglycemia (19, 32, 33), suggesting that peripheral nerve injury may occur in the early stages of the disease with milder glycemic dysregulation. In general, prediabetic neuropathy is milder than diabetic neuropathy and primarily affects nerve fibers that mediate sensory function (34, 35). Currently, there is increasing scientific evidence indicating that sNfL is correlated with neurodegenerative diseases (36) and peripheral neuropathies in both humans and animals (37–40). Some studies have observed that diabetes is associated with sNfL (41–43) and that individuals recently diagnosed with diabetes provide new evidence that a higher sNfL is related to diabetic sensorimotor polyneuropathy and peripheral nerve dysfunction (44). Our findings extend previous observations by demonstrating the association between sNfL and prediabetes in adolescents. A recent study showed that the level of sNfL is elevated 6 years before the clinical onset of multiple sclerosis (MS), implying that damage to nerve axons has already begun during the long prodromal period before the diagnosis of MS (45). Similar to this study, we showed that in an intermediate state of abnormal glycemia, that is, prediabetes, started to appear potential or minor nerve axon damage began to appear before the diagnosis of diabetes based on the changes in sNfL.
By correlating sNfL with adolescent prediabetes, our study indirectly demonstrated that there may be an underlying cognitive impairment in adolescent prediabetes. Previous studies have shown that performance in some domains of cognitive function appears to be impaired in patients at early stages of the disease, including prediabetes (46, 47). In addition, prediabetic status or progression to the diabetic phase may promote the reversion of mild cognitive impairment (MCI) to normal cognition (48). Current evidence increasingly supports the use of sNfL level as a biomarker for cognitive dysfunction (49–52). Our study found that measuring sNfL in adolescents with prediabetes could help identify young people at risk of developing cognitive impairment. Therefore, it was necessary to measure sNfL in prediabetic adolescents to provide an early opportunity to reverse the prediabetic state to a normal blood glucose state and prevent more serious cognitive impairment.
Our study had several strengths. It was the first study of the relationship between sNfL and prediabetes in adolescents, and such a selection of the study population allowed analyses of associations without the confounding effect of age-related comorbidities. In addition, sNfL levels were measured with a highly reproducible method, and serum samples from cases and controls were analyzed simultaneously in a blinded and randomized fashion, effectively avoiding potential artifactual differences in sNfL concentrations.
Several limitations should also be acknowledged. First, the retrospective design made it impossible for us to know the predictive value of sNfL, and the retrospective study implies the possibility of missing data. Second, we lacked cognitive function tests and objective measurements of sensorimotor neuropathy, so more studies are needed to thoroughly illustrate the relevant contents. Finally, although several variables were adjusted in the analysis, we cannot completely exclude the possibility of residual confounding.
In conclusion, our study showed that the association between prediabetes and sNfL was significant even after adjustment for several covariates. A higher serum NFL level was associated with prediabetes in adolescents. It also suggested the possibility of potential neurological damage and cognitive impairment in prediabetic adolescents. Further large-scale and prospective studies are needed to verify the clinical application of sNfL as a monitoring biomarker for prediabetes, and we encourage future studies to evaluate the performance of sNfL in predicting the incidence of neuropathy and cognitive dysfunction in adolescents with prediabetes.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Hunan Children’s Hospital. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
ZC contributed to the conception and design of the study. T-CP performed data collection and statistical analysis. L-PW collated and interpreted the results and wrote the first draft of the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the Project of Health and Health Commission of Hunan Province (grant number 20200712).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Dingle E, Brar PC. Prediabetes in obese adolescents. Clin Pediatr (Phila) (2017) 56:115–6. doi: 10.1177/0009922816681138
2. Andes LJ, Cheng YJ, Rolka DB, Gregg EW, Imperatore G. Prevalence of prediabetes among adolescents and young adults in the united states, 2005-2016. JAMA pediatrics (2020) 174:e194498. doi: 10.1001/jamapediatrics.2019.4498
3. Warren B, Pankow JS, Matsushita K, Punjabi NM, Daya NR, Grams M, et al. Comparative prognostic performance of definitions of prediabetes: a prospective cohort analysis of the atherosclerosis risk in communities (ARIC) study. Lancet Diabetes Endocrinol (2017) 5:34–42. doi: 10.1016/S2213-8587(16)30321-7
4. Yahyavi SK, Snorgaard O, Knop FK, Schou M, Lee C, Selmer C, et al. Prediabetes defined by first measured HbA(1c) predicts higher cardiovascular risk compared with HbA(1c) in the diabetes range: a cohort study of nationwide registries. Diabetes Care (2021) 44:2767–74. doi: 10.2337/dc21-1062
5. Tabak AG, Herder C, Rathmann W, Brunner EJ, Kivimaki M. Prediabetes: a high-risk state for diabetes development. Lancet (2012) 379:2279–90. doi: 10.1016/S0140-6736(12)60283-9
6. Galderisi A, Giannini C, Weiss R, Kim G, Shabanova V, Santoro N, et al. Trajectories of changes in glucose tolerance in a multiethnic cohort of obese youths: an observational prospective analysis. Lancet Child Adolesc Health (2018) 2:726–35. doi: 10.1016/S2352-4642(18)30235-9
7. American Diabetes A. 3. prevention or delay of type 2 diabetes: standards of medical care in diabetes-2020. Diabetes Care (2020) 43:S32–S6. doi: 10.2337/dc20-S003
8. Gaetani L, Blennow K, Calabresi P, Di Filippo M, Parnetti L, Zetterberg H. Neurofilament light chain as a biomarker in neurological disorders. J Neurol Neurosurg Psychiatry (2019) 90:870–81. doi: 10.1136/jnnp-2018-320106
9. Yuan A, Rao MV, Veeranna, Nixon RA. Neurofilaments and neurofilament proteins in health and disease. Cold Spring Harb Perspect Biol (2017) 9. doi: 10.1101/cshperspect.a018309
10. Khalil M, Teunissen CE, Otto M, Piehl F, Sormani MP, Gattringer T, et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol (2018) 14:577–89. doi: 10.1038/s41582-018-0058-z
11. Bittner S, Oh J, Havrdova EK, Tintore M, Zipp F. The potential of serum neurofilament as biomarker for multiple sclerosis. Brain (2021) 144:2954–63. doi: 10.1093/brain/awab241
13. Novakova L, Zetterberg H, Sundstrom P, Axelsson M, Khademi M, Gunnarsson M, et al. Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology (2017) 89:2230–7. doi: 10.1212/WNL.0000000000004683
14. Kuhle J, Barro C, Disanto G, Mathias A, Soneson C, Bonnier G, et al. Serum neurofilament light chain in early relapsing remitting MS is increased and correlates with CSF levels and with MRI measures of disease severity. Mult Scler (2016) 22:1550–9. doi: 10.1177/1352458515623365
15. Manouchehrinia A, Piehl F, Hillert J, Kuhle J, Alfredsson L, Olsson T, et al. Confounding effect of blood volume and body mass index on blood neurofilament light chain levels. Ann Clin Transl Neurol (2020) 7:139–43. doi: 10.1002/acn3.50972
16. Benkert P, Meier S, Schaedelin S, Manouchehrinia A, Yaldizli O, Maceski A, et al. Serum neurofilament light chain for individual prognostication of disease activity in people with multiple sclerosis: a retrospective modelling and validation study. Lancet Neurol (2022) 21:246–57. doi: 10.1016/S1474-4422(22)00009-6
17. Akamine S, Marutani N, Kanayama D, Gotoh S, Maruyama R, Yanagida K, et al. Renal function is associated with blood neurofilament light chain level in older adults. Sci Rep (2020) 10:20350. doi: 10.1038/s41598-020-76990-7
18. Papanas N, Vinik AI, Ziegler D. Neuropathy in prediabetes: does the clock start ticking early? Nat Rev Endocrinol (2011) 7:682–90. doi: 10.1038/nrendo.2011.113
19. Smith AG, Singleton JR. Impaired glucose tolerance and neuropathy. Neurologist (2008) 14:23–9. doi: 10.1097/NRL.0b013e31815a3956
20. Rota E, Quadri R, Fanti E, Isoardo G, Poglio F, Tavella A, et al. Electrophysiological findings of peripheral neuropathy in newly diagnosed type II diabetes mellitus. J Peripher Nerv Syst (2005) 10:348–53. doi: 10.1111/j.1085-9489.2005.00046.x
21. Park J, Choi S, Kim R. Association between prediabetes and cognitive function in parkinson's disease. Brain Behav (2023) 13:e2838. doi: 10.1002/brb3.2838
22. Sundermann EE, Thomas KR, Bangen KJ, Weigand AJ, Eppig JS, Edmonds EC, et al. Prediabetes is associated with brain hypometabolism and cognitive decline in a sex-dependent manner: a longitudinal study of nondemented older adults. Front Neurol (2021) 12:551975. doi: 10.3389/fneur.2021.551975
23. Sanchez-Gomez A, Diaz Y, Duarte-Salles T, Compta Y, Marti MJ. Prediabetes, type 2 diabetes mellitus and risk of parkinson's disease: a population-based cohort study. Parkinsonism Relat Disord (2021) 89:22–7. doi: 10.1016/j.parkreldis.2021.06.002
24. Correa-Burrows P, Matamoros M, de Toro V, Zepeda D, Arriaza M, Burrows R. A single-point insulin sensitivity estimator (SPISE) of 5.4 is a good predictor of both metabolic syndrome and insulin resistance in adolescents with obesity. Front Endocrinol (Lausanne) (2023) 14:1078949. doi: 10.3389/fendo.2023.1078949
25. Morris DH, Khunti K, Achana F, Srinivasan B, Gray LJ, Davies MJ, et al. Progression rates from HbA1c 6.0-6.4% and other prediabetes definitions to type 2 diabetes: a meta-analysis. Diabetologia (2013) 56:1489–93. doi: 10.1007/s00125-013-2902-4
26. American Diabetes A. 5. prevention or delay of type 2 diabetes: standards of medical care in diabetes-2018. Diabetes Care (2018) 41:S51–S4. doi: 10.2337/dc18-S005
27. Disanto G, Barro C, Benkert P, Naegelin Y, Schädelin S, Giardiello A, et al. Serum neurofilament light: a biomarker of neuronal damage in multiple sclerosis. Ann neurol (2017) 81:857–70. doi: 10.1002/ana.24954
28. Fitzgerald KC, Sotirchos ES, Smith MD, Lord HN, DuVal A, Mowry EM, et al. Contributors to serum NfL levels in people without neurologic disease. Ann neurol (2022) 92:688–98. doi: 10.1002/ana.26446
29. Khalil M, Pirpamer L, Hofer E, Voortman MM, Barro C, Leppert D, et al. Serum neurofilament light levels in normal aging and their association with morphologic brain changes. Nat Commun (2020) 11:812. doi: 10.1038/s41467-020-14612-6
30. Lee EH, Kwon HS, Koh SH, Choi SH, Jin JH, Jeong JH, et al. Serum neurofilament light chain level as a predictor of cognitive stage transition. Alzheimer's Res Ther (2022) 14:6. doi: 10.1186/s13195-021-00953-x
31. Sjölin K, Aulin J, Wallentin L, Eriksson N, Held C, Kultima K, et al. Serum neurofilament light chain in patients with atrial fibrillation. J Am Heart Assoc (2022) 11:e025910. doi: 10.1161/JAHA.122.025910
32. Ziegler D, Rathmann W, Dickhaus T, Meisinger C, Mielck A, Group KS. Prevalence of polyneuropathy in pre-diabetes and diabetes is associated with abdominal obesity and macroangiopathy: the MONICA/KORA augsburg surveys S2 and S3. Diabetes Care (2008) 31:464–9. doi: 10.2337/dc07-1796
33. Singleton JR, Smith AG, Russell JW, Feldman EL. Microvascular complications of impaired glucose tolerance. Diabetes (2003) 52:2867–73. doi: 10.2337/diabetes.52.12.2867
34. Papanas N, Ziegler D. Prediabetic neuropathy: does it exist? Curr Diabetes Rep (2012) 12:376–83. doi: 10.1007/s11892-012-0278-3
35. Divisova S, Vlckova E, Hnojcikova M, Skorna M, Nemec M, Dubovy P, et al. Prediabetes/early diabetes-associated neuropathy predominantly involves sensory small fibres. J Peripher Nerv Syst (2012) 17:341–50. doi: 10.1111/j.1529-8027.2012.00420.x
36. Gordon BA. Neurofilaments in disease: what do we know? Curr Opin Neurobiol (2020) 61:105–15. doi: 10.1016/j.conb.2020.02.001
37. Altmann P, De Simoni D, Kaider A, Ludwig B, Rath J, Leutmezer F, et al. Increased serum neurofilament light chain concentration indicates poor outcome in Guillain-barre syndrome. J Neuroinflammation (2020) 17:86. doi: 10.1186/s12974-020-01737-0
38. Bischof A, Manigold T, Barro C, Heijnen I, Berger CT, Derfuss T, et al. Serum neurofilament light chain: a biomarker of neuronal injury in vasculitic neuropathy. Ann Rheum Dis (2018) 77:1093–4. doi: 10.1136/annrheumdis-2017-212045
39. Fukami Y, Iijima M, Koike H, Yamada S, Hashizume A, Katsuno M. Association of serum neurofilament light chain levels with clinicopathology of chronic inflammatory demyelinating polyneuropathy, including NF155 reactive patients. J Neurol (2021) 268:3835–44. doi: 10.1007/s00415-021-10537-2
40. Meregalli C, Fumagalli G, Alberti P, Canta A, Chiorazzi A, Monza L, et al. Neurofilament light chain: a specific serum biomarker of axonal damage severity in rat models of chemotherapy-induced peripheral neurotoxicity. Arch Toxicol (2020) 94:2517–22. doi: 10.1007/s00204-020-02755-w
41. Uyar M, Lezius S, Buhmann C, Potter-Nerger M, Schulz R, Meier S, et al. Diabetes, glycated hemoglobin (HbA1c), and neuroaxonal damage in parkinson's disease (MARK-PD study). Mov Disord (2022) 37:1299–304. doi: 10.1002/mds.29009
42. Ciardullo S, Muraca E, Bianconi E, Cannistraci R, Perra S, Zerbini F, et al. Diabetes mellitus is associated with higher serum neurofilament light chain levels in the general US population. J Clin Endocrinol Metab (2023) 108:361–7. doi: 10.1210/clinem/dgac580
43. Sotirchos ES, Fitzgerald KC, Singh CM, Smith MD, Reyes-Mantilla M, Hersh CM, et al. Associations of sNfL with clinico-radiological measures in a large MS population. Ann Clin Transl Neurol (2023) 10:84–97. doi: 10.1002/acn3.51704
44. Maalmi H, Strom A, Petrera A, Hauck SM, Strassburger K, Kuss O, et al. Serum neurofilament light chain: a novel biomarker for early diabetic sensorimotor polyneuropathy. Diabetologia (2023) 66:579–89. doi: 10.1007/s00125-022-05846-8
45. Bjornevik K, Munger KL, Cortese M, Barro C, Healy BC, Niebuhr DW, et al. Serum neurofilament light chain levels in patients with presymptomatic multiple sclerosis. JAMA Neurol (2020) 77:58–64. doi: 10.1001/jamaneurol.2019.3238
46. Ruis C, Biessels GJ, Gorter KJ, van den Donk M, Kappelle LJ, Rutten GE. Cognition in the early stage of type 2 diabetes. Diabetes Care (2009) 32:1261–5. doi: 10.2337/dc08-2143
47. Yaffe K, Blackwell T, Kanaya AM, Davidowitz N, Barrett-Connor E, Krueger K. Diabetes, impaired fasting glucose, and development of cognitive impairment in older women. Neurology (2004) 63:658–63. doi: 10.1212/01.WNL.0000134666.64593.BA
48. Makino K, Lee S, Bae S, Chiba I, Harada K, Katayama O, et al. Diabetes and prediabetes inhibit reversion from mild cognitive impairment to normal cognition. J Am Med Directors Assoc (2021) 22:1912–8.e2. doi: 10.1016/j.jamda.2021.02.033
49. Edwards K, Kamath A, O'Connor J, Kamath V, Whidden C, Button A, et al. Serum neurofilament light chain levels correlate strongly with cognitive status in patients with relapsing and progressive multiple sclerosis. In: MULTIPLE SCLEROSIS JOURNAL: SAGE. 55 CITY ROAD, LONDON EC1Y 1SP, ENGLAND: PUBLICATIONS LTD 1 OLIVERS YARD (2019). p. NP6–8.
50. Jakimovski D, Zivadinov R, Ramanthan M, Hagemeier J, Weinstock-Guttman B, Tomic D, et al. Serum neurofilament light chain level associations with clinical and cognitive performance in multiple sclerosis: a longitudinal retrospective 5-year study. Multiple sclerosis (Houndmills Basingstoke England) (2020) 26:1670–81. doi: 10.1177/1352458519881428
51. Kuhle J, Kropshofer H, Barro C, Meinert R, Haring D, Leppert D, et al. Neurofilament light chain levels in blood are predictive of cognitive impairment in patients with secondary progressive multiple sclerosis. Eur J Neurol (2019) 92(15):666.
Keywords: prediabetes, adolescents, sNfL, cognitive dysfunction, retrospective cohort study
Citation: Chen Z, Wu L-P and Peng T-C (2023) Prediabetes is associated with a higher serum neurofilament light chain level in adolescents. Front. Endocrinol. 14:1207045. doi: 10.3389/fendo.2023.1207045
Received: 17 April 2023; Accepted: 06 June 2023;
Published: 26 June 2023.
Edited by:
Chunjiang Wang, Central South University, ChinaReviewed by:
Chao Deng, HaiKou People’s Hospital, ChinaCopyright © 2023 Chen, Wu and Peng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tuo-Chao Peng, MjU4MDE0ODExQHFxLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.