
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Endocrinol. , 13 July 2023
Sec. Adrenal Endocrinology
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1204851
This article is part of the Research Topic Advances in Diagnostics and Management of Adrenal Tumors View all 24 articles
Pheochromocytoma/paraganglioma (PPGL) are neuroendocrine tumors that frequently produce and release catecholamines. Catecholamine excess can manifest in several cardiovascular syndromes, including cardiomyopathy. PPGL-induced cardiomyopathies occur in up to 11% of cases and are most often associated with an adrenal pheochromocytoma (90%) and rarely with a paraganglioma derived from the sympathetic ganglia (10%). PPGL-associated cardiomyopathies can be chronic or acute, with takotsubo cardiomyopathy being the most often reported. These two types of PPGL-induced cardiomyopathy seem to have different pathophysiological backgrounds. Acute catecholaminergic stress inundates myocardial β-adrenoceptors and leads to left ventricle stunning and slight histological apoptosis. In chronic cardiomyopathy, prolonged catecholamine exposure leads to extended myocardial fibrosis, inflammation, and necrosis, and ultimately it causes dilated cardiomyopathy with a low ejection fraction. Sometimes, especially in cases associated with hypertension, hypertrophic cardiomyopathy can develop. The prognosis appears to be worse in chronic cases with a higher hospital mortality rate, higher cardiogenic shock rate at initial presentation, and lower left ventricular recovery rate after surgery. Therefore, establishing the correct diagnosis at an early stage of a PPGL is essential. This mini-review summarizes current data on pathophysiological pathways of cardiac damage caused by catecholamines, the clinical presentation of PPGL-induced cardiomyopathies, and discusses treatment options.
In recent years, we have witnessed remarkable progress in understanding the rare type of neuroendocrine tumors that produce catecholamines, which arise from chromaffin cells within the adrenal medulla (pheochromocytoma) and extra-adrenal sympathetic ganglia (paraganglioma), collectively referred to as pheochromocytoma-paraganglioma (PPGL). According to the 5th series of the World Health Organization (WHO) Classification of Endocrine and Neuroendocrine Tumors, the term paraganglioma is used for both neoplasms, while pheochromocytoma is classified as an “intra-adrenal paraganglioma” (1). The annual incidence of PPGL increased during the past two decades, reaching an annual incidence of approximately 8 cases per million, mainly due to the increase in the number of imaging studies conducted in clinical practice (2). Despite higher awareness among clinicians and widely used imaging procedures, there are still PPGL cases that remain undetected, often leading to the fatal consequences of catecholamines excess, mainly cardiovascular complications (3).
The effect of supraphysiological levels of epinephrine and norepinephrine on the heart and vessels highly depend on the secretory profile of the PPGL (adrenergic vs. noradrenergic phenotype, episodic via. continuous release) (4). The most common, yet alone nonspecific sign of PPGL: hypertension, among other factors, may lead to myocardial hypoxia with various clinical manifestations: acute (takotsubo, ischemic) and chronic (dilated, hypertrophic) cardiomyopathy (5–8). Catecholamine-induced cardiomyopathy in PPGL (CICMPP) is potentially fatal, but an uncommon complication, with a prevalence of 8–11% of patients with a PPGL (9, 10). Although CICMPP is rarely the initial manifestation of PPGL, it is obligatory to rule out PPGL in patients with heart failure and paroxysmal symptoms: profuse diaphoresis, headaches, pallor, tremor, palpitations, and episodic hypertension, but also hyperglycemia in young patients with normal body mass index (BMI) (4, 10–12).
This review summarizes the pathophysiology of CICMPP, risk factors, the clinical presentation of takotsubo, dilated and hypertrophic CICMPP, treatment options, and future perspectives regarding recent advances in PPGL.
To understand the mechanisms leading to the diverse clinical manifestations of CICMPP, it is essential to comprehend how catecholamines affect the cardiovascular system. Catecholamines (epinephrine, norepinephrine, and dopamine) are tyrosine-derived hormones, and neurotransmitters synthesized predominantly in the adrenal medulla, sympathetic nerves, and brain (13, 14). Norepinephrine and epinephrine bind to adrenoceptors, while the effect of dopamine on adrenoceptors is negligible, but in high levels, dopamine may lead to hypotension mainly due to interaction with dopamine receptors present in mesenteric and renal vascular beds (15–17).
Adrenoceptors are a family of transmembrane G protein–linked receptors. There are two main types of adrenoceptors: α and β, which are further divided into nine subtypes: α1A, α1B, α1D, α2A, α2B, α2C, β1, β2, and β3. Binding the physiological ligands (norepinephrine and epinephrine) to adrenoceptors result in G protein–mediated transduction of the signal, activation of second messengers or ion channels, evoking a response in the cell, highly dependent on the type of adrenoceptor and target tissue (18).
α1-adrenoceptors are mostly found in vascular smooth muscle but are also present in the myocardium (subtype α1A) (19, 20). α1-adrenoceptors signal transduction leads to protein kinase C (PKC) activation, 1,4,5-inositol triphosphate production, and intracellular calcium flow (6, 19). This results in smooth muscle contraction, and increased cardiac output, while persistent α1-adrenoceptors stimulation leads to the hypertrophic phenotype (6, 19). α2-adrenoceptors can be found in vascular smooth muscle distally from the sympathetic nerve (mainly α-2B subtype), leading to vasoconstriction, while presynaptic α2-adrenoceptors inhibit norepinephrine release resulting in the reduction of the sympathetic stress response (20, 21). β1-adrenoceptors are expressed in the sinoatrial node, atrioventricular node, and cardiomyocytes, resulting in calcium-mediated increased contractility, heart rate, and enhanced conduction of electrical stimulus (20). In the cardiovascular system, stimulation of β2-adrenoceptors, through inhibition of cAMP production, leads to vasodilatation and relaxation of the myocardium (20, 22).
Norepinephrine has a stronger affinity to α-adrenoceptors than β-adrenoceptors, leading to increased cardiac output and vasoconstriction, which manifests as elevated blood pressure (6). Early response to norepinephrine includes a rise in heart rate, but activation of the baroreflex by the increased blood pressure causes the heart rate to decrease (6). Considering the affinity of norepinephrine to adrenoceptors and the typically continuous pattern of catecholamine release, persistent hypertension and arrhythmias are part of the clinical presentation (22–24). Epinephrine binds to all major adrenoceptors: α1, α2, β1, and β2 (20). At low concentrations, epinephrine is selective for β2-adrenoceptors. The ability to stimulate peripheral β2-adrenoceptors manifests in patients with a PPGL secreting epinephrine as hypotension, once α1-adrenoceptors are pharmacologically blocked (20, 22, 25, 26). At higher concentrations, epinephrine stimulates α-adrenoceptors resulting in vasoconstriction (20). Epinephrine at higher concentrations also has a strong affinity for β1-adrenoceptor, which results in positive inotropic, chronotropic, and dromotropic effects (20). Typically, a PPGL secreting epinephrine in an episodic release pattern is often experienced by the patients as tachyarrhythmias (22, 25, 26). Furthermore, life-threatening, excessive amounts of catecholamines lead to vasoconstriction (including coronary arteries), myocardial ischemia, and necrosis (27). Conditions (e.g., hyperthyroidism, hypercortisolism, hypokalemia, hypocalcemia) that increase the expression and sensitivity of the adrenoceptor amplify the devastating impact of catecholamine excess (3, 28–30).
Acute, uncontrolled release of catecholamines in PPGL causes hyperstimulation of β1-adrenoceptors, increasing heart rate and contractility (31). Furthermore, it significantly raises myocardial oxygen demand, especially when combined with coronary artery spasms due to the activation of α1-adrenoceptors, exposing the myocardium to hypoxia (31). Catecholamine surge also leads to microvascular alterations combined with calcium overload, which according to Wittstein et al., may contribute to reversible coronary vasoconstriction, as observed in patients with takotsubo CICMPP (6, 32).
Myocardial cytosolic and mitochondrial calcium overload, one of the most prominent features of persistent catecholamine excess, promotes oxidative stress and mitochondrial permeability, leading to cell death (33). Mitochondrial calcium excess promotes hydrogen peroxide synthesis during oxidative deamination of catecholamines (31). The production of superoxide anion radicals is also enhanced by α1-adrenoceptor stimulation: they are products of nicotinamide adenine dinucleotide phosphate (NADPH) reactions (34). Another process that contributes to catecholamine-induced oxidative stress in cardiomyocytes is auto-oxidation of catecholamines producing highly reactive, toxic, and unstable “aminochromes” and inactive, more stable “aminolutins” which can be measured in the plasma (6, 35, 36). The reaction is accelerated by oxygen free radicals and various enzymes, e.g., myeloperoxidase, cytochrome oxidase (6). The toxicity of oxidized catecholamines was proven in the study of Yates et al., in which perfusion with oxidized isoproterenol in isolated rat hearts was found to induce ultrastructural mitochondrial damage in cardiomyocytes — the phenomenon was not observed when fresh isoproterenol was used (37). Furthermore, adrenochrome (50 mg/L) infusion of isolated rat hearts ceased contractile activity in 30 minutes, whereas epinephrine or metanephrine infusions had a positive impact on cardiac contractile activity (38). However, studies concerning the role of “aminochromes” in CICMPP are still missing.
Chronic exposure to catecholamine excess leads to desensitization of β-adrenoceptors (39). Desensitization occurs through the phosphorylation of the adrenoceptor by the G protein–coupled receptor kinase (GRK) and binding to the protein called β-arrestin2. GRK2/β-arrestin2 complexes promote β1-adrenoceptor uncoupling and internalization (40). Furthermore, high catecholamine stress causes β2-adrenoceptors to switch from the Gs to Gi signaling pathway, leading to decreased cardiac contractility (41). Interestingly, not only is the density of β2/β1-adrenoceptors higher at the apex than at the base, but also apical adrenoceptors show higher sensitivity to catecholamines than those at the base (41). The apex–base gradient of β-adrenoceptors and described switch from Gs to Gi of β2-adrenoceptors (also mainly observed in the apex) explain the impaired regional contractility and typical clinical presentation of takotsubo cardiomyopathy: apical hypokinesia and ballooning (41). The negative inotropic effect depends on β2-adrenoceptor phosphorylation by both protein kinase A (PKA) and GRKs (42). It is noteworthy that L41Q GRK5 polymorphism is associated with enhanced desensitization and impaired β-adrenoceptor response, and it is more prevalent among patients with takotsubo cardiomyopathy (43).
Given the fact that one in ten patients with PPGL will develop CICMPP, a potentially fatal complication, it is essential to identify and closely monitor predisposed individuals. The results of the study by Wang et al. on 50 patients with CICMPP and 152 patients with PPGL without diagnosed CICMPP, identified five risk factors for CICMPP, namely maximum resting heart rate ≥ 115 per minute, maximum resting systolic blood pressure ≥ 180 mmHg, blood glucose ≥ 8.0 mmol/L, 3 or more reported symptoms (headache, sweating, hypertension, hypotension, palpitation, chest pain, dyspnea, impaired tip perfusion, syncope, nausea, and vomiting), and early onset ≤ 40 years (44). Interestingly, in the study by Zhao et al., among patients with PPGL, female sex, paroxysmal symptoms, PPGL secreting more than one catecholamine, and higher white blood cell and platelet counts were significantly more prevalent in patients developing cardiovascular complications (45). The link between the higher cardiovascular risk in patients with PPGL and increased platelet count may be explained by catecholamine-mediated modulation of platelet function and aggregation via stimulation of dopaminergic and α2-adrenoceptors expressed on platelets, since the activation of platelets is crucial in the pathogenesis of various cardiovascular diseases, e.g., hypertension and atherosclerosis (46–51). Genetic biomarkers may also help to determine the risk of CICMPP. In the recent study by Amar et al., the α2-adrenoceptor variant (alpha 2CDel322–325) was more prevalent among patients with CICMPP (52).
Excess of catecholamines, apart from other stress factors, may lead to acute, reversible left ventricular wall motion abnormalities (LVWMA) with a regional or circumferential pattern extending beyond the coronary artery supply, named after the Japanese fishing pot used to catch octopus — takotsubo cardiomyopathy (53–56). LVWMA in takotsubo cardiomyopathy results in characteristic left ventricle ballooning during systole (56). LVWMA may affect the apical, mid-apical, mid-ventricular, mid-basal, and basal segments of the left ventricle (57). The clinical presentation of takotsubo CICMPP does not differ from acute coronary syndrome: patients usually report chest and/or abdominal pain, dyspnea, and the majority of patients also experience symptoms that should raise the suspicion of PPGL (e.g., palpitations, profuse sweating, headache) (53). The most common electrocardiogram (ECG) changes in takotsubo CICMPP include ST-elevation myocardial infarction (STEMI)–like changes (more than one-third of the patients), ST segment depression, T-wave inversion, and QT-prolongation (53, 58). Although elevated troponin concentrations were observed in 71–95% of patients with takotsubo cardiomyopathy, the peak values in myocardial infarction biomarkers are usually lower compared to patients with acute coronary syndrome and not proportional to left ventricle impairment (53, 58, 59).
On echocardiography, apical ballooning, hypokinesia, akinesia, dyskinesia of apical segments, and the occasional hypokinetic mid-segments, are the classic presentation of takotsubo cardiomyopathy (6, 59). However, it occurs also in reversed (inverted) form when basal segments are akinetic, while the apex is hyperkinetic (6, 59–61). The latter phenotype is rare, yet more prevalent in patients with takotsubo CICMPP than in the overall group of takotsubo cardiomyopathy patients: 28.8% vs. 2.2% in the meta-analysis presented by Y-Hassan et al. (62). Moreover, a global pattern is more frequently present in takotsubo CICMPP (62). Compared to the overall takotsubo cardiomyopathy group, takotsubo CICMPP was characterized by higher complication rates: 68.2% vs. 21.8% (i.e., heart failure, pulmonary edema, and cardiogenic shock) and recurrence rate, whereas mortality was reported in 4% of cases and did not differ between the groups (62). In the takotsubo CICMPP group, death occurred in 7% of men and 2.7% of women (p = 0.35) (62). Mortality increased significantly during the recurrence of takotsubo CICMPP (11%, 2/18) (62).
Longstanding hypertension in undetected PPGL may lead to left ventricle outflow tract obstruction: a hallmark of hypertrophic CICMPP. Patients with hypertrophic cardiomyopathy usually report exertional dyspnea and fatigue with or without chest pain or presyncope (6, 63). In advanced stages, patients may experience orthopnea and/or fluid retention with peripheral/pulmonary edema (63). The typical symptoms of patients with hypertrophic CICMPP may also be complemented with PPGL-suggestive symptoms, namely profuse sweating, and palpitations (64, 65). ECG alterations often meet the criteria of left ventricle hypertrophy (64). Echocardiography shows systolic anterior motion of the anterior mitral valve leaflet, increased left ventricle outflow tract gradient with persisted ejection fraction (EF), and septal and posterior wall hypertrophy (64, 66, 67).
Interestingly, the results of the study by Dobrowolski et al. prove that subclinical impairment of systolic function in PPGL patients was independent of the presence of left ventricle hypertrophy (LVH) (68). In the abovementioned study, the assessment included a global longitudinal strain (GLS) — a parameter derived from two-dimensional speckle-tracking echocardiography — allowing to assess the function of longitudinally-oriented subendocardial fibers, which are the most susceptible to ischemia and wall stress. GLS more accurately reflects intrinsic myocardial function and early systolic dysfunction than EF (68, 69). The patients with PPGL had lower GLS (median 17.2%) than in the control group, while EF did not differ significantly (68). Early systolic dysfunction was confirmed in the meta-analysis, including 252 patients with PPGL, speckle tracking echocardiography (STE) revealed worse GLS in the pooled PPGL group when compared to the control group (−17.3 ± 1.2 vs. −20.0 ± 0.6), differences in EF were not observed between the groups (70). In the study by Dobrowolski et al., the adrenergic biochemical phenotype was associated with worse systolic function and nonsignificantly higher left ventricle mass index compared to BP-matched controls, indicating that apart from pressure overload, epinephrine per se may contribute to LVH (68). Experimental findings suggested the role of catecholamines in the induction of protein synthesis (71). Both systolic and diastolic alterations in patients with PPGL reversed significantly after curative surgery (68).
Patients with dilated cardiomyopathy typically experience symptoms of progressive systolic dysfunction, often after a latent period when they are clinically asymptomatic (72, 73). Interestingly, in the analysis by Zhang et al., among all CICMPP cases, PPGL associated with genetic syndromes or metastatic PPGL were found predominantly in patients with dilated PPGL (23% of cases, i.e., multiple endocrine neoplasia type 2, neurofibromatosis type 1, and von Hippel-Lindau syndrome) (72). However, the abovementioned retrospective study has several limitations, including incomplete data regarding imaging findings (e.g., missing echocardiographic parameters leading to the categorization of 14 cases under unspecified cardiomyopathy) and the results of molecular analysis (e.g., SDHB mutations were not included) (72). In dilated CICMPP, ECG may be normal, although alterations ranging from T wave changes and left bundle branch block to disturbances in atrioventricular conduction may occur (73). Echocardiography reveals increased left ventricle end-diastolic volumes or diameters (> 2 Standard Deviations (SDs) from normal) with global systolic dysfunction not attributable to ischemic or valvular disease (73). Cardiovascular magnetic resonance (CMR) shows left ventricle dilatation, and it is also of use to rule out inflammatory processes, to assess rest stress myocardial perfusion, myocardial perfusion, iron, and fat deposition, and aortic distensibility in CICMPP (73, 74). The key to the successful management of dilated CICMPP is complete PPGL resection, which leads to the improvement of EF and lower mortality rate: death occurred in 4% (2/52) of patients who underwent surgical resection of a PPGL and 22% (2/9) of patients not treated surgically (72). The importance of precise screening and prompt diagnosis is highlighted by two case reports of heart transplants undertaken before the diagnosis of PPGL was established (75, 76).
The catecholamine binding affinities to adrenoceptors and pathophysiologic mechanisms leading to different subtypes of CICMPP are presented in Figure 1.
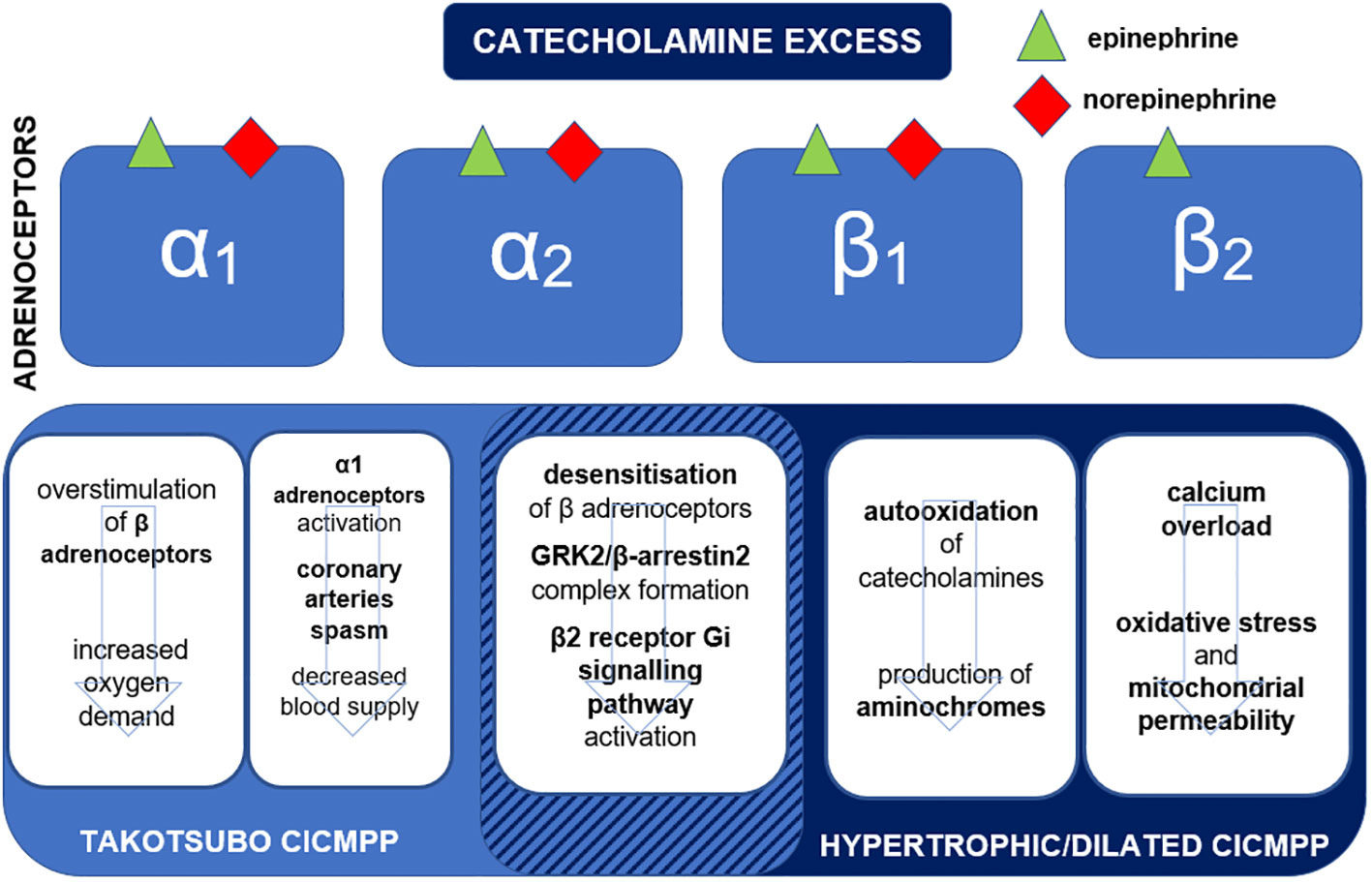
Figure 1 Binding affinities of epinephrine and norepinephrine to adrenoceptors and mechanisms leading to the development of subtypes of catecholamine-induced cardiomyopathy in pheochromocytoma-paraganglioma (CICMPP). Gi, inhibiting G protein; GRK, G protein–coupled receptor kinase.
The early diagnosis, confirmed by biochemical tests (elevated free or fractionated plasma or urine metanephrines), followed by localization of the lesion and complete PPGL resection is the mainstay of the successful treatment of patients with CICMPP. The analysis by Zhang et al. showed that PPGL resection was associated with an improved EF in 96% of CICMPP cases (72). Prior to surgery, α-adrenoceptor blockade should be initiated for 7–14 days (77). Some studies favor α1-selective over nonselective α-adrenoceptor blockers due to lower preoperative diastolic pressure, lower intraoperative heart rate, and better postoperative outcome (77, 78). However, the results of the randomized controlled PRECIST trial showed no differences between phenoxybenzamine and doxazosin in the duration of blood pressure being outside the target range during operation, but phenoxybenzamine was more efficient in preventing intraoperative hemodynamic instability (79). The addition of metyrosine (tyrosine hydroxylase inhibitor) should be considered for patients at high risk of catecholamine surge (e.g., with symptomatic, multifocal, or metastatic disease intolerance of α-adrenoceptor blockers or when difficult surgery of PPGL encroaching neighboring vascular structures is anticipated) (80). However, the availability of metyrosine is limited (11).
Once the α-adrenoceptor blockade is assured and the target heart rate (of 60–70 bpm seated and 70–80 bpm standing) is not achieved, a β-adrenoceptor blocker should be initiated (not earlier than 3–4 days after initiation of an α-adrenoceptor blocker) (22, 77). Selective β1-adrenoceptor blockers are favored (since β2-adrenoceptor blockade may result in hypertension). In the emergency setting, intravenous fast-acting esmolol or alternatively metoprolol may allow to optimally react to hemodynamic alterations (3). Among oral β-adrenoceptor blockers, metoprolol succinate (controlled-release) or atenolol are preferred (3). The choice of β-adrenoceptor blockers in the treatment of CICMPP should also include subtype-specific recommendations: preferred non-vasodilating β-adrenoceptor blockers (atenolol, metoprolol, bisoprolol) in hypertrophic cardiomyopathy (81).
The preoperative aim is a blood pressure of less than 130/80 mm Hg while seated and greater than 90 mm Hg systolic while standing (77). If blood pressure is not optimally controlled calcium channel blockers may be added (77). If heart failure is confirmed, angiotensin-converting enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARBs) are a part of pharmacological therapy. There are also novel, potential therapeutic options for patients with PPGL cardiomyopathy and heart failure, namely sodium-glucose cotransporter 2 inhibitors (SGLT2i) and angiotensin receptor/neprilysin inhibitor (ARNI). Multiple trials proved their efficacy in patients with heart failure, but in PPGL data are limited and further studies are needed (82, 83). Before the resection of PPGL, a high-sodium diet and fluid intake should be assured to prevent severe hypotension after PPGL removal (77). Preoperative assessment should also include an electrocardiogram and echocardiogram, which may identify the features of CICMPP (84).
Patients with PPGL are more prone to develop acute cardiovascular complications. In case of hypertensive crisis, intravenous administration of phentolamine, sodium nitroprusside, or nicardipine should be initiated. Once the state of the patient is stable, titration of the phenoxybenzamine dosage can be initiated to reach the target blood pressure (85). Fluid status should be monitored, diuretics are to be avoided unless the patient has fluid congestion, and even then, administered judiciously (6). In the management of hemodynamic instability, vasoactive amines are often administered, but their efficacy may be limited due to sympathetic receptor down-regulation, and they may even exacerbate PPGL-induced cardiac dysfunction (6, 86–88). If hypotension persists despite pharmacological treatment, mechanical circulatory support (MCS) may be needed. In the systematic review of 62 patients with severe systolic dysfunction (median left ventricular ejection fraction (LVEF) of 16% (range 5–32%)) requiring extracorporeal life support (ECLS) due to intractable pheochromocytoma crisis, full recovery of left ventricle function (LVEF >50%) was observed in most patients and 54 (87%) of 62 reported cases survived (89). Also, there are reports of successful left ventricular assist device (LVAD) use in PPGL-induced heart failure and perioperative management (90–92). An intra-aortic balloon pump (IABP) has been used for unresponsive patients but was not effective (93).
The general pharmacological management of hypertensive crisis, hypotension/cardiac shock, and the most common tachyarrhythmias in PPGL are summarized in Figure 2.
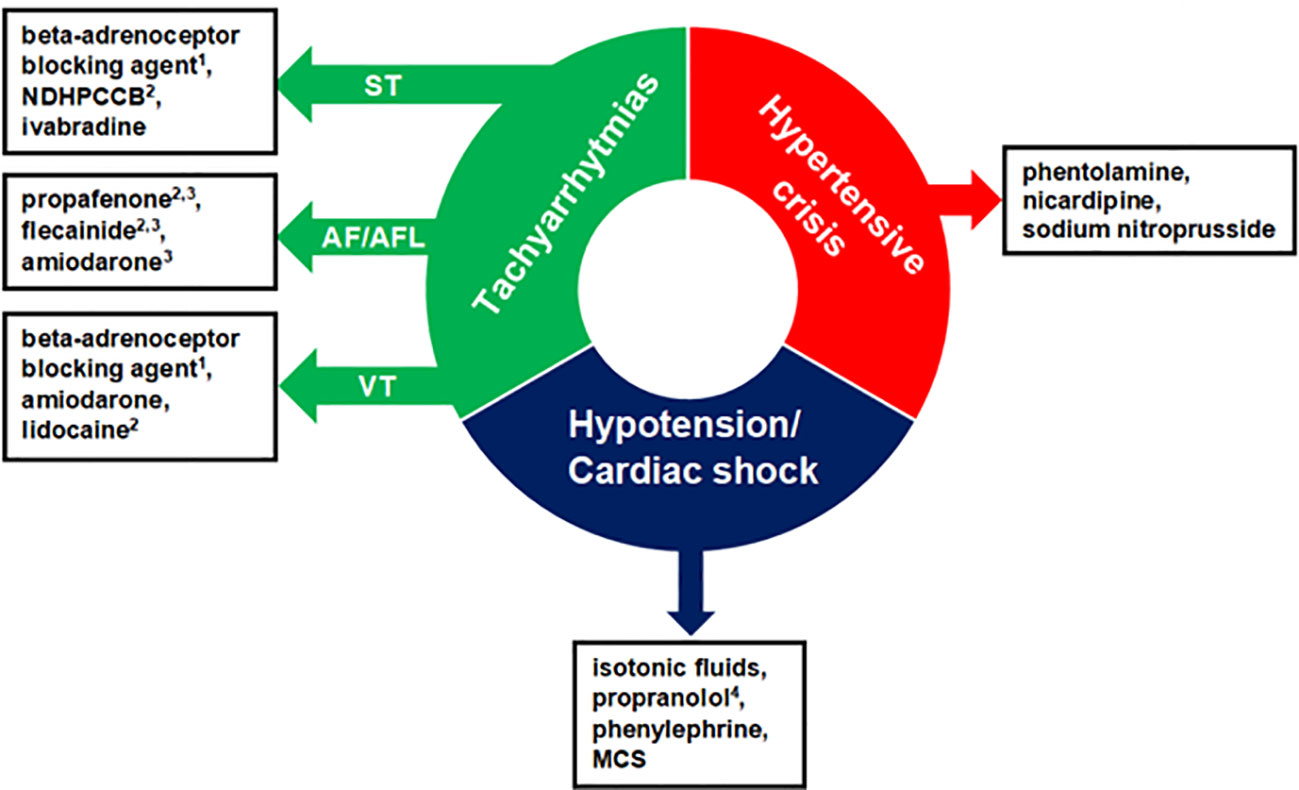
Figure 2 Pharmacological management of acute cardiovascular complications in patients with pheochromocytoma-paraganglioma. The order of presented drugs does not correspond to first-, second-, and third-line therapy and the choice of administered drugs should be individualized. 1With alpha-adrenoceptor blockade, otherwise, it can precipitate a hypertensive crisis. 2Antiarrhythmic agent not recommended in systolic dysfunction. 3Used to restore sinus rhythm. 4In case of hypotension and suspected beta2-adrenoceptor overstimulation. NDHPCCB, Nondihydropyridine Calcium Channel Blockers; ST, Sinus Tachycardia; AF/AFL, Atrial Fibrillation/Atrial Flutter; VT, Ventricular Tachycardia; MCS, Mechanical Circulatory Support.
CICMPP is a potentially fatal complication of PPGL, but there are still patients with CICMPP not diagnosed early enough. Thus, it is essential to broaden awareness about the clinical course and adequate management of CICMPP among clinicians and underline the importance of accurate cardiac assessment of PPGL patients.
Currently, the management of CICMPP is based on the guidelines for PPGL treatment, recommended general cardiological interventions, published case series, and few systematic reviews or meta-analyses. Dedicated guidelines of CICMPP management addressing specific features of this rare entity and integrating novel advances in pharmacotherapy and MCS could help to optimally treat patients with CICMPP.
Probably the ongoing progress in genetics and metabolomics in PPGL, completed by integration of the results using artificial intelligence, may contribute to a better understanding of the diverse effects of catecholamine excess on the cardiovascular system, identify predisposing factors (also among asymptomatic carriers of pathogenic mutations in genes predisposing to PPGL development), biomarkers, and establish the prognosis.
MG-C provided the idea for the manuscript. AS, PG, and MG-C reviewed the relevant literature and drafted the manuscript. AS drafted the figures. MG-C and PG reviewed critically the manuscript. All authors have made substantial contributions to the manuscript and have read and approved the final version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Mete O, Asa SL, Gill AJ, Kimura N, de Krijger RR, Tischler A. Overview of the 2022 who classification of paragangliomas and pheochromocytomas. Endocr Pathol (2022) 33(1):90–114. doi: 10.1007/s12022-022-09704-6
2. Berends AMA, Buitenwerf E, de Krijger RR, Veeger N, van der Horst-Schrivers ANA, Links TP, et al. Incidence of pheochromocytoma and sympathetic paraganglioma in the Netherlands: a nationwide study and systematic review. Eur J Intern Med (2018) 51:68–73. doi: 10.1016/j.ejim.2018.01.015
3. Nazari MA, Rosenblum JS, Haigney MC, Rosing DR, Pacak K. Pathophysiology and acute management of tachyarrhythmias in pheochromocytoma: jacc review topic of the week. J Am Coll Cardiol (2020) 76(4):451–64. doi: 10.1016/j.jacc.2020.04.080
4. Pacak K. New biology of pheochromocytoma and paraganglioma. Endocr Pract (2022) 28(12):1253–69. doi: 10.1016/j.eprac.2022.09.003
5. Soltani A, Pourian M, Davani BM. Does this patient have pheochromocytoma? a systematic review of clinical signs and symptoms. J Diabetes Metab Disord (2015) 15:6. doi: 10.1186/s40200-016-0226-x
6. Santos JRU, Brofferio A, Viana B, Pacak K. Catecholamine-induced cardiomyopathy in pheochromocytoma: how to manage a rare complication in a rare disease? Horm Metab Res (2019) 51(7):458–69. doi: 10.1055/a-0669-9556
7. Zuber SM, Kantorovich V, Pacak K. Hypertension in pheochromocytoma: characteristics and treatment. Endocrinol Metab Clin North Am (2011) 40(2):295–311. doi: 10.1016/j.ecl.2011.02.002
8. Kobayashi Y, Kobayashi Y, Hirohata A. Pheochromocytoma found in takotsubo cardiomyopathy patients. J Invasive Cardiol (2014) 26(6):E76–7.
9. Park JH, Kim KS, Sul JY, Shin SK, Kim JH, Lee JH, et al. Prevalence and patterns of left ventricular dysfunction in patients with pheochromocytoma. J Cardiovasc Ultrasound (2011) 19(2):76–82. doi: 10.4250/jcu.2011.19.2.76
10. Giavarini A, Chedid A, Bobrie G, Plouin PF, Hagege A, Amar L. Acute catecholamine cardiomyopathy in patients with phaeochromocytoma or functional paraganglioma. Heart (2013) 99(19):1438–44. doi: 10.1136/heartjnl-2013-304073
11. Lenders JWM, Kerstens MN, Amar L, Prejbisz A, Robledo M, Taieb D, et al. Genetics, diagnosis, management and future directions of research of phaeochromocytoma and paraganglioma: a position statement and consensus of the working group on endocrine hypertension of the European society of hypertension. J Hypertens (2020) 38(8):1443–56. doi: 10.1097/HJH.0000000000002438
12. La Batide-Alanore A, Chatellier G, Plouin PF. Diabetes as a marker of pheochromocytoma in hypertensive patients. J Hypertens (2003) 21(9):1703–7. doi: 10.1097/00004872-200309000-00020
13. Eisenhofer G, Kopin IJ, Goldstein DS. Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol Rev (2004) 56(3):331–49. doi: 10.1124/pr.56.3.1
14. Schulz C, Eisenhofer G, Lehnert H. Principles of catecholamine biosynthesis, metabolism and release. Front Horm Res (2004) 31:1–25. doi: 10.1159/000074656
15. Contreras F, Fouillioux C, Bolivar A, Simonovis N, Hernandez-Hernandez R, Armas-Hernandez MJ, et al. Dopamine, hypertension and obesity. J Hum Hypertens (2002) 16 Suppl 1:S13–7. doi: 10.1038/sj.jhh.1001334
16. Goldberg LI. Cardiovascular and renal actions of dopamine: potential clinical applications. Pharmacol Rev (1972) 24(1):1–29.
17. Jose PA, Eisner GM, Felder RA. Regulation of blood pressure by dopamine receptors. Nephron Physiol (2003). 95(2):19–27 doi: 10.1159/000073676
18. Docherty JR. The pharmacology of alpha(1)-adrenoceptor subtypes. Eur J Pharmacol (2019) 855:305–20. doi: 10.1016/j.ejphar.2019.04.047
19. Brodde OE, Michel MC. Adrenergic and muscarinic receptors in the human heart. Pharmacol Rev (1999) 51(4):651–90.
20. Gordan R, Gwathmey JK, Xie LH. Autonomic and endocrine control of cardiovascular function. World J Cardiol (2015) 7(4):204–14. doi: 10.4330/wjc.v7.i4.204
21. Giovannitti JA Jr., Thoms SM, Crawford JJ. Alpha-2 adrenergic receptor agonists: a review of current clinical applications. Anesth Prog (2015) 62(1):31–9. doi: 10.2344/0003-3006-62.1.31
22. Gupta G, Pacak K, Committee AAS. Precision medicine: an update on Genotype/Biochemical phenotype relationships in Pheochromocytoma/Paraganglioma patients. Endocr Pract (2017) 23(6):690–704. doi: 10.4158/EP161718.RA
23. Pacak K. Preoperative management of the pheochromocytoma patient. J Clin Endocrinol Metab (2007) 92(11):4069–79. doi: 10.1210/jc.2007-1720
24. Eisenhofer G, Huynh TT, Elkahloun A, Morris JC, Bratslavsky G, Linehan WM, et al. Differential expression of the regulated catecholamine secretory pathway in different hereditary forms of pheochromocytoma. Am J Physiol Endocrinol Metab (2008) 295(5):E1223–33. doi: 10.1152/ajpendo.90591.2008
25. Kantorovich V, Pacak K. A new concept of unopposed beta-adrenergic overstimulation in a patient with pheochromocytoma. Ann Intern Med (2005) 142(12 Pt 1):1026–8. doi: 10.7326/0003-4819-142-12_part_1-200506210-00023
26. Baxter MA, Hunter P, Thompson GR, London DR. Phaeochromocytomas as a cause of hypotension. Clin Endocrinol (Oxf) (1992) 37(3):304–6. doi: 10.1111/j.1365-2265.1992.tb02326.x
27. Rona G. Catecholamine cardiotoxicity. J Mol Cell Cardiol (1985) 17(4):291–306. doi: 10.1016/s0022-2828(85)80130-9
28. Motulsky HJ, Insel PA. Adrenergic receptors in man: direct identification, physiologic regulation, and clinical alterations. N Engl J Med (1982) 307(1):18–29. doi: 10.1056/NEJM198207013070104
29. Ciaraldi TP, Marinetti GV. Hormone action at the membrane level. viii. adrenergic receptors in rat heart and adipocytes and their modulation by thyroxine. Biochim Biophys Acta (1978) 541(3):334–46. doi: 10.1016/0304-4165(78)90193-9
30. Leon AS, Abrams WB. The role of catecholamines in producing arrhythmias. Am J Med Sci (1971) 262(1):9–13. doi: 10.1097/00000441-197107000-00002
31. Costa VM, Carvalho F, Bastos ML, Carvalho RA, Carvalho M, Remiao F. Contribution of catecholamine reactive intermediates and oxidative stress to the pathologic features of heart diseases. Curr Med Chem (2011) 18(15):2272–314. doi: 10.2174/092986711795656081
32. Wittstein IS, Thiemann DR, Lima JA, Baughman KL, Schulman SP, Gerstenblith G, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med (2005) 352(6):539–48. doi: 10.1056/NEJMoa043046
33. Khan MU, Cheema Y, Shahbaz AU, Ahokas RA, Sun Y, Gerling IC, et al. Mitochondria play a central role in nonischemic cardiomyocyte necrosis: common to acute and chronic stressor states. Pflugers Arch (2012) 464(1):123–31. doi: 10.1007/s00424-012-1079-x
34. Amin JK, Xiao L, Pimental DR, Pagano PJ, Singh K, Sawyer DB, et al. Reactive oxygen species mediate alpha-adrenergic receptor-stimulated hypertrophy in adult rat ventricular myocytes. J Mol Cell Cardiol (2001) 33(1):131–9. doi: 10.1006/jmcc.2000.1285
35. Dhalla NS. Formation of aminochrome leads to cardiac dysfunction and sudden cardiac death. Circ Res (2018) 123(4):409–11. doi: 10.1161/CIRCRESAHA.118.313416
36. Dhalla KS, Ganguly PK, Rupp H, Beamish RE, Dhalla NS. Measurement of adrenolutin as an oxidation product of catecholamines in plasma. Mol Cell Biochem (1989) 87(1):85–92. doi: 10.1007/BF00421086
37. Yates JC, Dhalla NS. Induction of necrosis and failure in the isolated perfused rat heart with oxidized isoproterenol. J Mol Cell Cardiol (1975) 7(11):807–16. doi: 10.1016/0022-2828(75)90132-7
38. Yates JC, RE B, Dhalla NS. Ventricular dysfunction and necrosis produced by adrenochrome metabolite of epinephrine: relation to pathogenesis of catecholamine cardiomyopathy. Am Heart J (1981) 102(2):210–21. doi: 10.1016/s0002-8703(81)80012-9
39. Kassim TA, Clarke DD, Mai VQ, Clyde PW, Mohamed Shakir KM. Catecholamine-induced cardiomyopathy. Endocr Pract (2008) 14(9):1137–49. doi: 10.4158/EP.14.9.1137
40. Kumar A, Pappachan JM, Fernandez CJ. Catecholamine-induced cardiomyopathy: an endocrinologist's perspective. Rev Cardiovasc Med (2021) 22(4):1215–28. doi: 10.31083/j.rcm2204130
41. Paur H, Wright PT, Sikkel MB, Tranter MH, Mansfield C, O'Gara P, et al. High levels of circulating epinephrine trigger apical cardiodepression in a Beta2-adrenergic Receptor/Gi-dependent manner: a new model of takotsubo cardiomyopathy. Circulation (2012) 126(6):697–706. doi: 10.1161/CIRCULATIONAHA.112.111591
42. Liu R, Ramani B, Soto D, De Arcangelis V, Xiang Y. Agonist dose-dependent phosphorylation by protein kinase a and G protein-coupled receptor kinase regulates Beta2 adrenoceptor coupling to G(I) proteins in cardiomyocytes. J Biol Chem (2009) 284(47):32279–87. doi: 10.1074/jbc.M109.021428
43. Spinelli L, Trimarco V, Di Marino S, Marino M, Iaccarino G, Trimarco B. L41q polymorphism of the G protein coupled receptor kinase 5 is associated with left ventricular apical ballooning syndrome. Eur J Heart Fail (2010) 12(1):13–6. doi: 10.1093/eurjhf/hfp173
44. Wang Y, Yu X, Huang Y. Predictive factors for catecholamine-induced cardiomyopathy in patients with pheochromocytoma and paraganglioma. Front Endocrinol (Lausanne) (2022) 13:853878. doi: 10.3389/fendo.2022.853878
45. Zhao L, Meng X, Mei Q, Fan H, Liu Y, Zhou X, et al. Risk factors for cardiac complications in patients with pheochromocytoma and paraganglioma: a retrospective single-center study. Front Endocrinol (Lausanne) (2022) 13:877341. doi: 10.3389/fendo.2022.877341
46. Ricci A, Bronzetti E, Mannino F, Mignini F, Morosetti C, Tayebati SK, et al. Dopamine receptors in human platelets. Naunyn Schmiedebergs Arch Pharmacol (2001) 363(4):376–82. doi: 10.1007/s002100000339
47. Anfossi G, Trovati M. Role of catecholamines in platelet function: pathophysiological and clinical significance. Eur J Clin Invest (1996) 26(5):353–70. doi: 10.1046/j.1365-2362.1996.150293.x
48. Tschuor C, Asmis LM, Lenzlinger PM, Tanner M, Harter L, Keel M, et al. In vitro norepinephrine significantly activates isolated platelets from healthy volunteers and critically ill patients following severe traumatic brain injury. Crit Care (2008) 12(3):R80. doi: 10.1186/cc6931
49. Amadio P, Zara M, Sandrini L, Ieraci A, Barbieri SS. Depression and cardiovascular disease: the viewpoint of platelets. Int J Mol Sci (2020) 21(20):7560. doi: 10.3390/ijms21207560
50. Ouvina SM, La Greca RD, Zanaro NL, Palmer L, Sassetti B. Endothelial dysfunction, nitric oxide and platelet activation in hypertensive and diabetic type ii patients. Thromb Res (2001) 102(2):107–14. doi: 10.1016/s0049-3848(01)00237-7
51. Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA (2002) 287(19):2570–81. doi: 10.1001/jama.287.19.2570
52. Amar J, Brunel J, Cardot Bauters C, Jacques V, Delmas C, Odou MF, et al. Genetic biomarkers of life-threatening pheochromocytoma-induced cardiomyopathy. Endocr Relat Cancer (2022) 29(5):267–72. doi: 10.1530/ERC-21-0373
53. YH S. Clinical features and outcome of pheochromocytoma-induced takotsubo syndrome: analysis of 80 published cases. Am J Cardiol (2016) 117(11):1836–44. doi: 10.1016/j.amjcard.2016.03.019
54. YH S, Falhammar H. Pheochromocytoma- and paraganglioma-triggered takotsubo syndrome. Endocrine (2019) 65(3):483–93. doi: 10.1007/s12020-019-02035-3
55. Dote K, Sato H, Tateishi H, Uchida T, Ishihara M. [Myocardial stunning due to simultaneous multivessel coronary spasms: a review of 5 cases]. J Cardiol (1991) 21(2):203–14.
56. YH S, Tornvall P. Epidemiology, pathogenesis, and management of takotsubo syndrome. Clin Auton Res (2018) 28(1):53–65. doi: 10.1007/s10286-017-0465-z
57. YH S, De Palma R. Contemporary review on the pathogenesis of takotsubo syndrome: the heart shedding tears: norepinephrine churn and foam at the cardiac sympathetic nerve terminals. Int J Cardiol (2017) 228:528–36. doi: 10.1016/j.ijcard.2016.11.086
58. Gagnon N, Mansour S, Bitton Y, Bourdeau I. Takotsubo-like cardiomyopathy in a Large cohort of patients with pheochromocytoma and paraganglioma. Endocr Pract (2017) 23(10):1178–92. doi: 10.4158/EP171930.OR
59. Ghadri JR, Wittstein IS, Prasad A, Sharkey S, Dote K, Akashi YJ, et al. International expert consensus document on takotsubo syndrome (Part ii): diagnostic workup, outcome, and management. Eur Heart J (2018) 39(22):2047–62. doi: 10.1093/eurheartj/ehy077
60. Tagawa M, Nanba H, Suzuki H, Nakamura Y, Uchiyama H, Ochiai S, et al. Ventricular rhythm and hypotension in a patient with pheochromocytoma-induced myocardial damage and reverse takotsubo cardiomyopathy. Intern Med (2015) 54(18):2343–9. doi: 10.2169/internalmedicine.54.4732
61. Di Valentino M, Balestra GM, Christ M, Raineri I, Oertli D, Zellweger MJ. Inverted takotsubo cardiomyopathy due to pheochromocytoma. Eur Heart J (2008) 29(6):830. doi: 10.1093/eurheartj/ehm449
62. YH S, Falhammar H. Clinical features, complications, and outcomes of exogenous and endogenous catecholamine-triggered takotsubo syndrome: a systematic review and meta-analysis of 156 published cases. Clin Cardiol (2020) 43(5):459–67. doi: 10.1002/clc.23352
63. Maron BJ, Desai MY, Nishimura RA, Spirito P, Rakowski H, Towbin JA, et al. Management of hypertrophic cardiomyopathy: jacc state-of-the-Art review. J Am Coll Cardiol (2022) 79(4):390–414. doi: 10.1016/j.jacc.2021.11.021
64. Wani A, Adil A, Gardezi SAA, Jain R, Galazka P, Waples MJ, et al. Pheochromocytoma presenting as hypertrophic obstructive cardiomyopathy. JAMA Cardiol (2021) 6(8):974–6. doi: 10.1001/jamacardio.2021.0944
65. Alamri A, Oriez C, Brenier M, Voican A, Banu I, Mourad JJ, et al. A case of late-detected pheochromocytoma in a young patient with resistant hypertension and hypertrophic cardiomyopathy. Am J Hypertens (2020). doi: 10.1093/ajh/hpaa126
66. Jacob JL, da Silveira LC, de Freitas CG, Centola CA, Nicolau JC, Lorga AM. Pheochromocytoma with echocardiographic features of obstructive hypertrophic cardiomyopathy. a case report. Angiology (1994) 45(11):985–9. doi: 10.1177/000331979404501113
67. Huddle KR, Kalliatakis B, Skoularigis J. Pheochromocytoma associated with clinical and echocardiographic features simulating hypertrophic obstructive cardiomyopathy. Chest (1996) 109(5):1394–7. doi: 10.1378/chest.109.5.1394
68. Dobrowolski P, Januszewicz A, Klisiewicz A, Gosk-Przybylek M, Peczkowska M, Kabat M, et al. Left ventricular structural and functional alterations in patients with Pheochromocytoma/Paraganglioma before and after surgery. JACC Cardiovasc Imaging (2020) 13(12):2498–509. doi: 10.1016/j.jcmg.2020.07.017
69. Joseph G, Zaremba T, Johansen MB, Ekeloef S, Heiberg E, Engblom H, et al. Echocardiographic global longitudinal strain is associated with infarct size assessed by cardiac magnetic resonance in acute myocardial infarction. Echo Res Pract (2019) 6(4):81–9. doi: 10.1530/ERP-19-0026
70. Cuspidi C, Gherbesi E, Faggiano A, Sala C, Carugo S, Grassi G, et al. Targeting left ventricular mechanics in patients with Pheochromocytoma/Paraganglioma: an updated meta-analysis. Am J Hypertens (2023) 36(6):333–340. doi: 10.1093/ajh/hpad006
71. Prejbisz A, Lenders JW, Eisenhofer G, Januszewicz A. Cardiovascular manifestations of phaeochromocytoma. J Hypertens (2011) 29(11):2049–60. doi: 10.1097/HJH.0b013e32834a4ce9
72. Zhang R, Gupta D, Albert SG. Pheochromocytoma as a reversible cause of cardiomyopathy: analysis and review of the literature. Int J Cardiol (2017) 249:319–23. doi: 10.1016/j.ijcard.2017.07.014
73. Schultheiss HP, Fairweather D, Caforio ALP, Escher F, Hershberger RE, Lipshultz SE, et al. Dilated cardiomyopathy. Nat Rev Dis Primers (2019) 5(1):32. doi: 10.1038/s41572-019-0084-1
74. Mavrogeni S, Markousis-Mavrogenis G, Markussis V, Kolovou G. The emerging role of cardiovascular magnetic resonance imaging in the evaluation of metabolic cardiomyopathies. Horm Metab Res (2015) 47(9):623–32. doi: 10.1055/s-0035-1555913
75. Wilkenfeld C, Cohen M, Lansman SL, Courtney M, Dische MR, Pertsemlidis D, et al. Heart transplantation for end-stage cardiomyopathy caused by an occult pheochromocytoma. J Heart Lung Transplant (1992) 11(2 Pt 1):363–6.
76. Dalby MC, Burke M, Radley-Smith R, Banner NR. Pheochromocytoma presenting after cardiac transplantation for dilated cardiomyopathy. J Heart Lung Transplant (2001) 20(7):773–5. doi: 10.1016/s1053-2498(00)00233-3
77. Lenders JW, Duh QY, Eisenhofer G, Gimenez-Roqueplo AP, Grebe SK, Murad MH, et al. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab (2014) 99(6):1915–42. doi: 10.1210/jc.2014-1498
78. Prys-Roberts C, Farndon JR. Efficacy and safety of doxazosin for perioperative management of patients with pheochromocytoma. World J Surg (2002) 26(8):1037–42. doi: 10.1007/s00268-002-6667-z
79. Buitenwerf E, Osinga TE, Timmers H, Lenders JWM, Feelders RA, Eekhoff EMW, et al. Efficacy of alpha-blockers on hemodynamic control during pheochromocytoma resection: a randomized controlled trial. J Clin Endocrinol Metab (2020) 105(7):2381–91. doi: 10.1210/clinem/dgz188
80. Gruber LM, Jasim S, Ducharme-Smith A, Weingarten T, Young WF, Bancos I. The role for metyrosine in the treatment of patients with pheochromocytoma and paraganglioma. J Clin Endocrinol Metab (2021) 106(6):e2393–e401. doi: 10.1210/clinem/dgab130
81. Authors/Task Force m, PM E, Anastasakis A, MA B, Borggrefe M, Cecchi F, et al. ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the task force for the diagnosis and management of hypertrophic cardiomyopathy of the European society of cardiology (ESC). Eur Heart J (2014) 35(39):2733–79. doi: 10.1093/eurheartj/ehu284
82. Yu M, Du B, Yao S, Ma J, Yang P. Von Hippel-lindau syndrome with a rare complication of dilated cardiomyopathy: a case report. BMC Cardiovasc Disord (2022) 22(1):489. doi: 10.1186/s12872-022-02913-1
83. Wang FZ, Wei WB, Li X, Huo JY, Jiang WY, Wang HY, et al. The cardioprotective effect of the sodium-glucose cotransporter 2 inhibitor dapagliflozin in rats with isoproterenol-induced cardiomyopathy. Am J Transl Res (2021) 13(9):10950–61.
84. Fagundes GFC, Almeida MQ. Perioperative management of pheochromocytomas and sympathetic paragangliomas. J Endocr Soc (2022) 6(2):bvac004. doi: 10.1210/jendso/bvac004
85. Casey RT, Challis BG, Pitfield D, Mahroof RM, Jamieson N, Bhagra CJ, et al. Management of an acute catecholamine-induced cardiomyopathy and circulatory collapse: a multidisciplinary approach. Endocrinol Diabetes Metab Case Rep (2017) 2017:17–0122. doi: 10.1530/EDM-17-0122
86. Grasselli G, Foti G, Patroniti N, Rona R, Perlangeli MV, Pesenti A. Extracorporeal cardiopulmonary support for cardiogenic shock caused by pheochromocytoma: a case report and literature review. Anesthesiology (2008) 108(5):959–62. doi: 10.1097/ALN.0b013e31816c8a78
87. Dominedo C, D'Avino E, Martinotti A, Cingolani E. A rare pheochromocytoma complicated by cardiogenic shock and posterior reversible encephalopathy syndrome: case report. Eur Heart J Case Rep (2021) 5(2):ytaa513. doi: 10.1093/ehjcr/ytaa513
88. Cohen CD, Dent DM. Phaeochromocytoma and acute cardiovascular death (with special reference to myocardial infarction). Postgrad Med J (1984) 60(700):111–5. doi: 10.1136/pgmj.60.700.111
89. Matteucci M, Kowalewski M, Fina D, Jiritano F, Meani P, Raffa GM, et al. Extracorporeal life support for phaeochromocytoma-induced cardiogenic shock: a systematic review. Perfusion (2020) 35(1_suppl):20–8. doi: 10.1177/0267659120908413
90. Nakamura M, Imamura T, Fukui T, Oshima A, Ueno H, Kinugawa K. Successful management of pheochromocytoma crisis with cardiogenic shock by percutaneous left ventricular assist device. J Cardiovasc Dev Dis (2022) 9(3):71. doi: 10.3390/jcdd9030071
91. Albulushi A, Zolty R, Lowes B, Duhacheck-Stapleman AL, Sandler T, Sullivan JN, et al. Perioperative management of pheochromocytoma resection in a patient with a continuous flow left ventricular assist device. J Saudi Heart Assoc (2020) 32(2):233–5. doi: 10.37616/2212-5043.1026
92. Westaby S, Shahir A, Sadler G, Flynn F, Ormerod O. Mechanical bridge to recovery in pheochromocytoma myocarditis. Nat Rev Cardiol (2009) 6(7):482–7. doi: 10.1038/nrcardio.2009.58
Keywords: pheochromocytoma, paraganglioma, dilated cardiomyopathy, hypertrophic cardiomyopathy, takotsubo cardiomyopathy
Citation: Szatko A, Glinicki P and Gietka-Czernel M (2023) Pheochromocytoma/paraganglioma-associated cardiomyopathy. Front. Endocrinol. 14:1204851. doi: 10.3389/fendo.2023.1204851
Received: 12 April 2023; Accepted: 26 June 2023;
Published: 13 July 2023.
Edited by:
Valentina Morelli, Istituto Auxologico Italiano, ItalyReviewed by:
Bojana Popovic, University of Belgrade, SerbiaCopyright © 2023 Szatko, Glinicki and Gietka-Czernel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alicja Szatko, YWxpY2phLnN6YXRrb0BnbWFpbC5jb20=; Małgorzata Gietka-Czernel, bWFsZ2lldGthQHZwLnBs
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.