
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 25 July 2023
Sec. Adrenal Endocrinology
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1199875
This article is part of the Research TopicSubclinical Hormonal Activity in Adrenal TumorsView all 5 articles
 Hongyuan Zhu1†
Hongyuan Zhu1† Luming Wu1†
Luming Wu1† Tingwei Su1
Tingwei Su1 Lei Jiang1
Lei Jiang1 Weiwei Zhou1
Weiwei Zhou1 Yiran Jiang1
Yiran Jiang1 Cui Zhang1
Cui Zhang1 Xu Zhong1
Xu Zhong1 Weiqing Wang1,2*
Weiqing Wang1,2*Context: Adrenal incidentaloma (AI) is commonly discovered on cross-sectional imaging. Mild autonomous cortisol secretion is the most common functional disorder detected in AI.
Objective: To delineate the association between radiological characteristics of benign adrenocortical tumors and hypothalamus-pituitary-adrenal (HPA) axis.
Methods: In the study, 494 patients diagnosed with benign unilateral adrenocortical tumors were included. Mild autonomous cortisol secretion (MACS) was diagnosed when cortisol after 1mg-dexamethasone suppression test (1-mg DST) was in the range of 1.8-5ug/dl. Non-functional adrenocortical tumor (NFAT) was diagnosed as cortisol following 1-mg DST less than 1.8ug/dL. We performed Logistics regression and causal mediation analyses, looking for associations between radiological characteristics and the HPA axis.
Results: Of 494 patients, 352 (71.3%) with NFAT and 142 (28.7%) with MACS were included. Patients with MACS had a higher tumor diameter, thinner contralateral adrenal gland, and lower plasma ACTH and serum DHEAS than those with NFAT. ACTH (OR 0.978, 0.962-0.993) and tumor diameter (OR 1.857, 95%CI, 1.357-2.540) were independent factors associated with decreased serum DHEAS (all P<0.05). ACTH was also associated with decreased contralateral adrenal diameter significantly (OR 0.973, 95%CI, 0.957-0.988, P=0.001). Causal mediation analysis showed ACTH mediated the effect significantly for the association between 1-mg DST results and DHEAS level (Pmediation<0.001, proportion=22.3%). Meanwhile, we found ACTH mediated 39.7% of the effects of 1-mg DST on contralateral adrenal diameter (Pmediation=0.012).
Conclusions: Patients with MACS had thinner contralateral adrenal glands and disturbed HPA axes compared with NFAT. ACTH may partially be involved in mediating the mild autonomous cortisol secretion to DHEAS and the contralateral adrenal gland.
With the development and widespread use of advanced technology, incidentally discovered adrenal tumors have become common in clinical practice. The prevalence of adrenal incidentalomas (AIs) has been reported to be 0.4% to 7.3% among adults, and it is higher in the elderly, reaching nearly 10% (1–4).
Most AIs are benign and asymptomatic and are often considered non-functional tumors. However, they could actually produce cortisol in amounts insufficient for leading to clinically apparent symptoms, known as mild autonomous cortisol secretion (MACS). According to the updated AI guidelines of the European Society of Endocrinology (5), MACS is defined as failure to suppress serum cortisol sufficiently after a 1-mg overnight dexamethasone suppression test (1-mg DST) in the absence of the classical signs or symptoms of overt Cushing’s syndrome. A post-dexamethasone cortisol level ≤1.8 µg/dL is considered non-functional, and levels above1.8 µg/dL indicate MACS. This mild cortisol excess is associated with adverse cardiometabolic consequences and related mortality (6–12).
There have been few studies on the relationship between radiological features of the adrenal adenomas or adrenal glands and the hypothalamus-pituitary-adrenal (HPA) axis (13–17). Although the relationship between radiological characteristics and cortisol secretion has been previously described, these studies did not evaluate the association between radiological features and the HPA axis, especially in patients with MACS (18, 19).
In this study, we aim to describe the clinical and radiological characteristics of patients with MACS and NFAT. To further assess the association, we performed logistic regression and causal mediation analysis.
This was a retrospective observational study. Patients with adrenal tumors were consecutively treated in the Department of Endocrine and Metabolic Diseases, Ruijin Hospital, from 2010 to 2020. All patients undergo a comprehensive evaluation aimed at assessing the functional status and potential malignancy of the adrenal lesion.
(Supplementary Figure 1). We selected 494 patients on the basis of the following exclusion criteria: (i) age younger than 18 years or adrenal tumor diameter <1cm; (ii) any adrenal hormone disorder, including adrenal insufficiency, congenital adrenal hyperplasia, primary aldosteronism, Cushing’s syndrome, and phaeochromocytoma; (iii) bilateral or unilateral multiple adrenal tumors; (iv) adrenocortical carcinoma: (v) patients with a history of malignant disease; (vi) non-adenoma tumors; (vii) 1-mg DST>5ug/dl. Patients who were using any medications known to affect steroid synthesis or metabolism, as well as those with pre-existing chronic kidney disease and liver cirrhosis, were also excluded prior to the administration of the 1-mg DST. Benign adrenocortical tumor was diagnosed using attenuation criteria <10 Hounsfield units (HU) in unenhanced computed tomography (CT) or stringent washout criteria when delayed contrast CT was available (absolute washout > 60% and/or relative washout > 40%). Finally, 494 patients with diagnosed benign adrenocortical tumors that had a 1-mg DST less than 5ug/dL were included (Figure 1). According to the latest updated guidelines (5), NFAT (n=352) was defined as cortisol following 1-mg DST less than 1.8ug/dL and MACS (n=142) was defined as cortisol following 1-mg DST in the range of 1.8-5.0ug/dL.
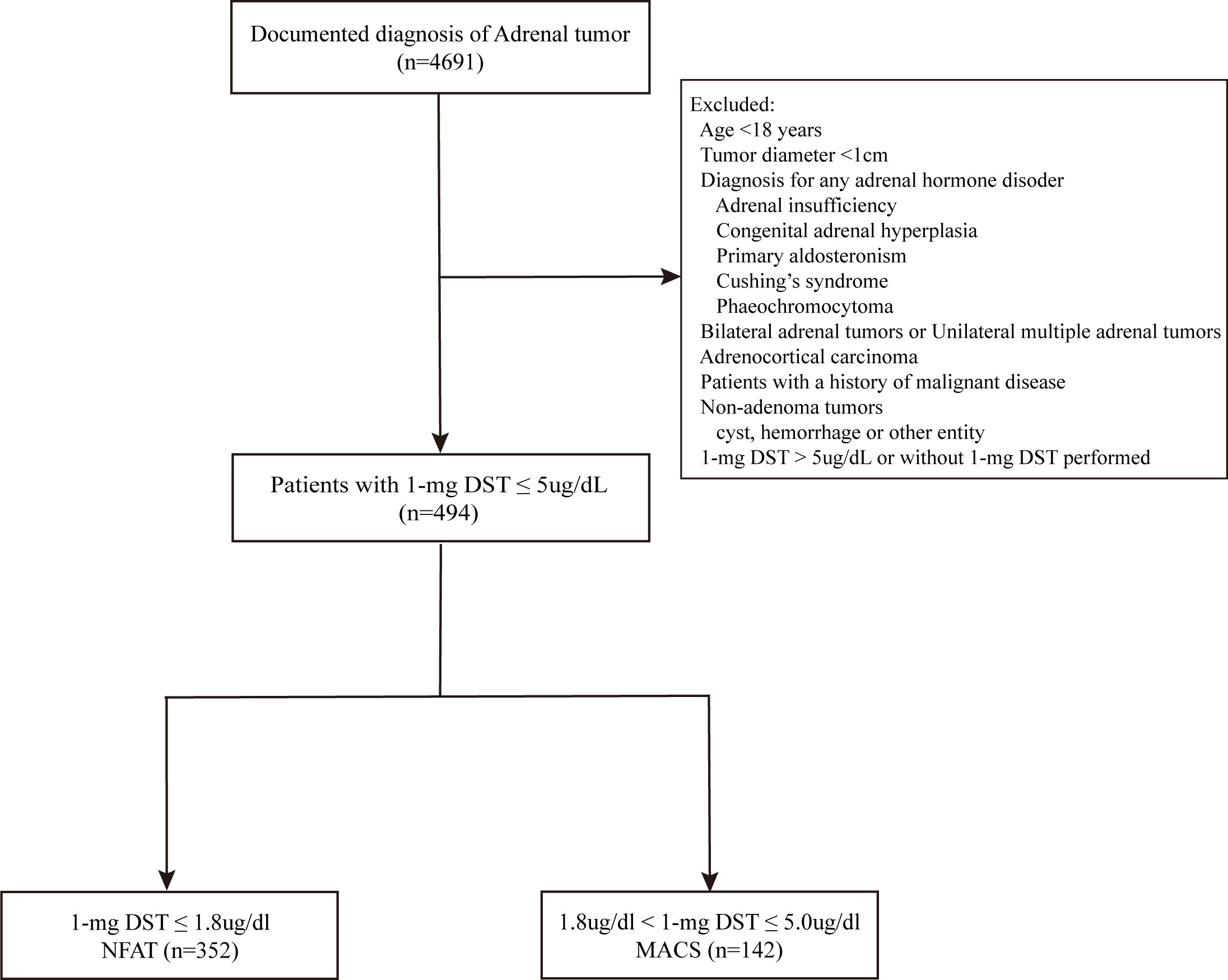
Figure 1 Flowchart of the study. 1-mg DST, 1-mg overnight dexamethasone-suppression test; NFAT, nonfunctional adrenocortical tumor; MACS, mild autonomous cortisol secretion.
All patients gave their informed consent for participation. This study was approved by the ethics committee of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine.
All blood samples were detected in a College of American Pathologists (NO. 7217913)-accredited laboratory. We performed a 1mg overnight dexamethasone suppression test (DST) by administering 1mg of dexamethasone at 24:00, with measurement of cortisol the following morning (between 08:00 and 09:00 hours). Serum cortisol and 24h-UFC were measured by using an Access Immunoassay System (A Beckman Coulter Corp, Fullerton, CA, USA). Plasma ACTH levels were measured by using an ELSA-ACTH immunoradiometric assay method (Cisbio Bioassays, Codolet, France). The intraassay and interassay coefficients of variation were 6.1% and 5.3% for ACTH. The normal range for ACTH is 12-78 pg/ml. Serum-free testosterone (T) and androstenedione (AD) were measured by radioimmunoassay. Other sex hormones, such as dehydroepiandrosterone sulfate (DHEAS), SHBG, and T were measured by using a chemiluminescence immunoassay (Abbott Laboratories, Abbott Park, IL).
Radiologic characteristics of the adrenal tumor and contralateral adrenal were evaluated in patients with unenhanced or pre-contrast CT images. The widths of the contralateral adrenal limb were defined as the mean of the maximum width of the limbs perpendicular to the long axis (medial and lateral) (18, 20). The diameters of the tumor were measured as the maximum width of the tumor. The CT scans were assessed by two different investigators who were not given information about the hormonal results. If there was a disagreement on measurements, investigators would re-evaluate the CT scans together and measure them again. All measurements were performed with a standard window setting for abdominal CT scans at Ruijin Hospital (width: 200 HU, level: 40 HU).
Continuous variables were presented as the medians (interquartile ranges, 25%-75%), and categorical variables were displayed as percentages. Variables were tested for normal distribution using the Kolmogorov-Smirnov test. Normally distributed variables were analyzed by One-way ANOVA, and non-normal distributions variables were analyzed by the Mann-Whitney U test. Categorical variables were analyzed by the Chi-square test. A logistic regression model was performed with unadjusted and adjusted models (age and sex) assessing odds ratios for the variables associated with decreased DHEAS and contralateral diameter.
Causal mediation analysis was performed by using the R package mediation (version 4.5.0). Consistent with the previous study (21), relationship groups that met the following criteria were defined as significant causal relationships: (1) total effect is significant (Ptotal effect<0.05), (2) mediated proportion>10%, and (3) indirect effect is significant (Pmediation<0.05). Moreover, we performed a sensitivity analysis using multiple imputation techniques to address missing data.
Statistical analysis was performed using SPSS (v.24: SPSS, Inc., Chicago, IL) and R 4.1.3 software. A two-sided P value < 0.05 was considered statistically significant.
A total of 494 patients diagnosed with unilateral benign adrenocortical tumor were included in the study. According to the morning cortisol following 1-mg DST, 352 (71.3%) patients had nonfunctional adrenocortical tumors (NFAT), and 142 (28.7%) had mild autonomous cortisol secretion (MACS) and lacked the distinctive clinical features of overt cortisol excess. Women represented 54.0% of the patients included in the study, and the female predominance was mainly pronounced in MACS (65.5%) (Table 1). Patients with MACS were older than those with NFAT at the time of adrenal tumor diagnosis (57 years vs 55 years, p=0.004).
Serum cortisol (8am, 0am) and SHBG were significantly higher in MACS. In contrast, MACS had lower levels of plasma ACTH and androgens (serum DHEAS, T, and FT). To adjust for the potential impact of age and gender on DHEAS level, we utilized the DHEAS ratio and found that it was significantly decreased in the MACS group. Meanwhile, we found MACS had higher tumor diameter and thinner contralateral adrenal gland diameter than NFAT. However, no difference was observed for 24h-UFC between the two groups. In addition, the prevalence of cardiometabolic disease (including diabetes, hypertension, and dyslipidemia) did not differ between patients with NFAT and those with MACS.
We further presented the clinical and endocrine characteristics of different gender groups (Supplementary Table 1). Consistent with the total patients, MACS showed similar HPA axis changes in both groups. However, males showed significantly distinct radiological features; there was a similar trend in the female group but without statistically significant differences.
Significantly lower serum DHEAS and contralateral diameters were observed in patients with MACS. Grouping according to the quartiles, we found 1-mg DST and plasma ACTH may be associated with serum DHEAS and contralateral diameter (Figure 2).
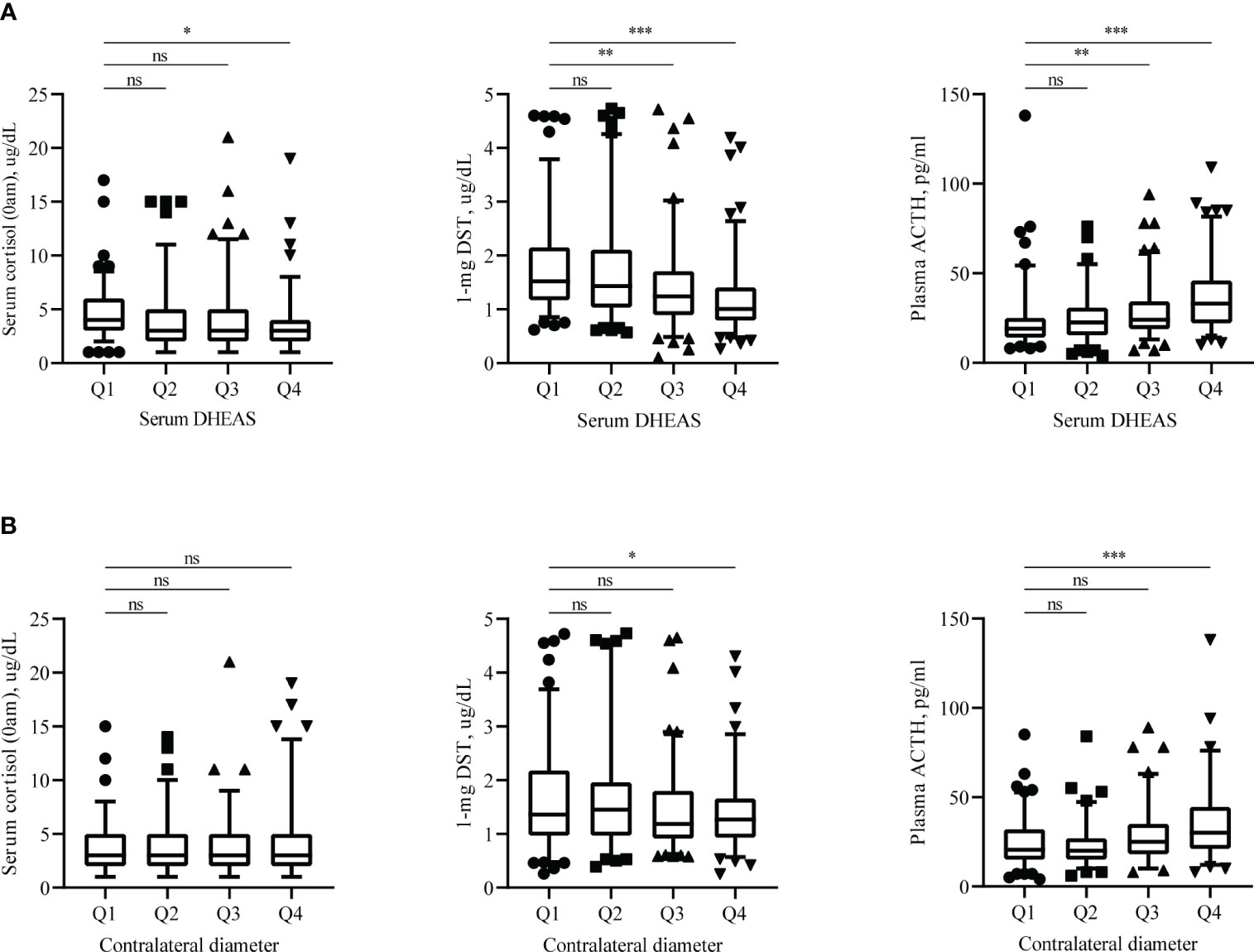
Figure 2 Endocrine assessments results. Distribution of serum cortisol (0am), plasma ACTH and 1-mg DST according to serum DHEAS (A) and contralateral adrenal diameter (B). Endocrine hormones measured in these patients are shown as scatters, with boxes representing median and 5%-95% range. DHEAS = Dehydroepiandrosterone sulfate; 1-mg DST, 1mg overnight dexamethasone-suppression test. Q1: 0-25%, Q2: 25-50%, Q3: 50-75%, Q4:75-100%. ns: not statistically significant (P >0.05), *P < 0.05, **P < 0.01, and ***P < 0.001.
To find the independent factors, we redivided the total patients into two new groups by the median of the serum DHEAS level or contralateral diameter and constructed logistic regression models [odds ratio (OR) and 95%CI]. In the multivariable logistic regression model adjusted for age and gender, we found tumor diameter (per 1-cm increase, OR 1.857 (1.357-2.540), P<0.001) was negatively associated and plasma ACTH was positively associated with decreased DHEAS, with OR (per 1-unit increase, 0.978 (0.962-0.993), P=0.006) (Table 2). About the decreased contralateral diameter, the univariable analysis showed that plasma ACTH and 1-mg DST were significantly associated with decreased contralateral diameter (Table 3). However, only ACTH was significantly associated with decreased contralateral diameter in multivariable logistic regression model (per 1-unit increase, OR 0.973 (0.957-0.988), P=0.001).
In the subgroup analysis of MACS group, we found plasma ACTH was associated with decreased DHEAS in multivariable logistic regression model, with OR (per 1-unit increase, 0.937 (0.888-0.988), P=0.016) (Supplementary Table 2). However, there was no significant variable associated with decreased contralateral diameter (Supplementary Table 3).
We first performed the causal mediation analysis on the linkages between tumor diameter, cortisol (including cortisol (8am, and 0am), and morning cortisol after the 1-mg DST) and ACTH. Significant causal mediation linkages of 1-mg DST were observed (Pmediation=0.042, proportion=24.2%, Supplementary Table 4). To further investigate the concrete relationship, a causal mediation analysis between 1-mg DST and DHEAS was performed. We found that ACTH mediated the effects significantly (Pmediation <0.001, proportion=22.3%, Figure 3A), and contralateral adrenal diameter mediated 9.5% of the effects (Pmediation =0.013, Figure 3B). The plasma ACTH mediated 39.7% of the effects of 1-mg DST on contralateral adrenal diameter (Pmediation =0.012, Figure 3C). The contralateral adrenal diameter was shown to mediate the effects of ACTH on DHEAS significantly (Pmediation <0.001, proportion=12.7%, Figure 3D). Additionally, the sensitive analysis showed ACTH mediated 16.2% of the effects between 1-mg DST and DHEAS and 34.0% of the effects between 1-mg DST and contralateral adrenal diameter (both Pmediation <0.05, Supplementary Figures 2A, C). The contralateral adrenal diameter mediated 10.9% of the effects between 1-mg DST and DHEAS and 18.3% of the effects between ACTH and DHEAS (Supplementary Figures 2B, D).
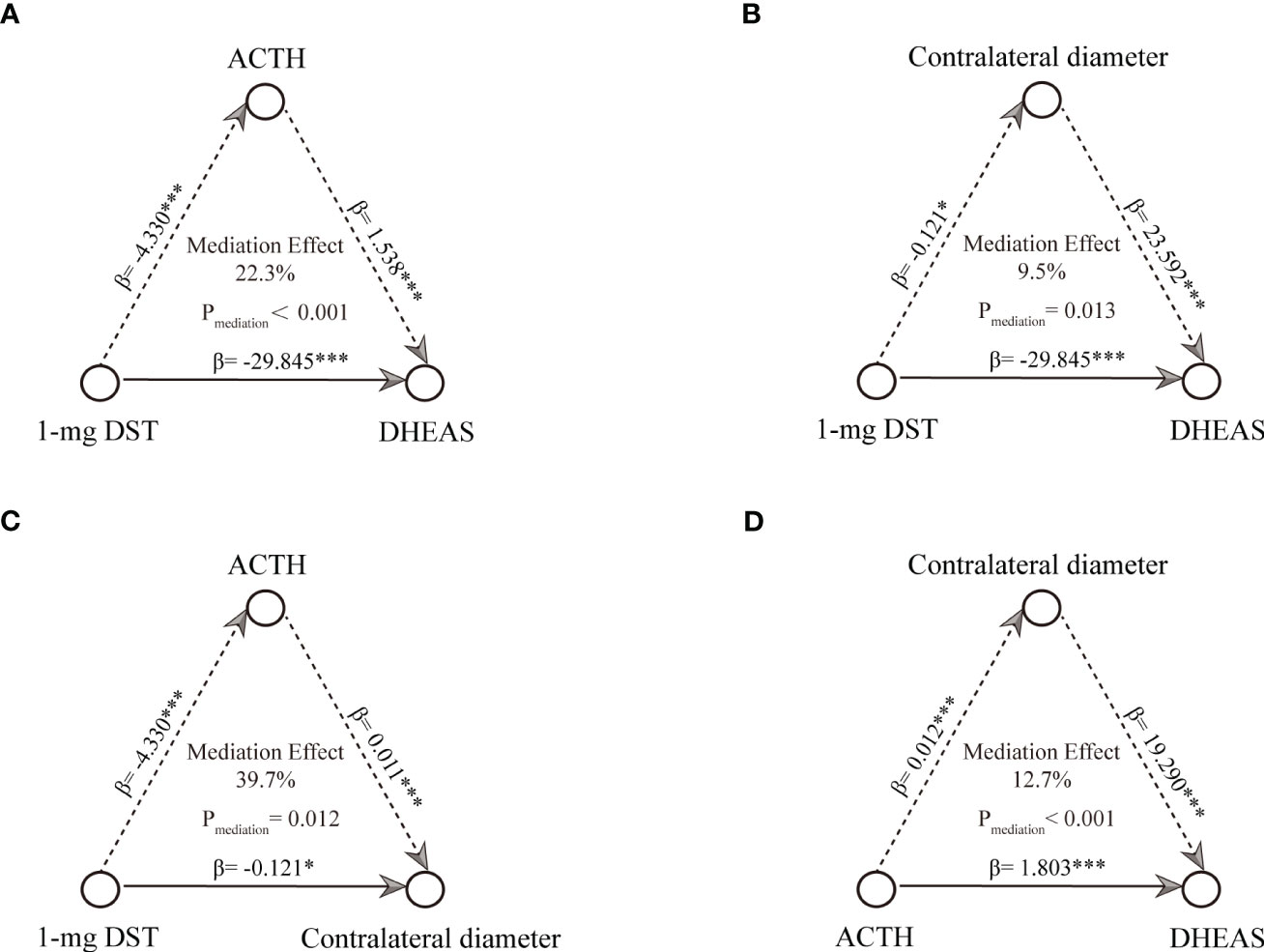
Figure 3 Causal mediation analysis among radiological characteristics, 1-mg DST and endocrine assessments. (A) mediation linkages between ACTH and 1-mg DST contributed DHEAS. (B) mediation linkages between contralateral diameter and 1-mg DST contributed DHEAS. (C) mediation linkages between ACTH and 1-mg DST contributed contralateral diameter. (D) mediation linkages between contralateral diameter and ACTH contributed DHEAS. *P < 0.05, **P < 0.01, and ***P < 0.001.
In the present study, we found patients with MACS had a lower serum DHEAS and contralateral diameter than those with NFAT. Multivariable logistic regression models found ACTH and tumor diameter were independent factors associated with decreased serum DHEAS levels. For the decreased contralateral diameter, ACTH was significantly associated. Furthermore, significant mediation linkages for association of 1-mg DST and DHEAS through ACTH were observed, which is consistent with the hypothesis that low DHEAS levels can reflect chronically suppressed HPA axis (22). Plasma ACTH played a mediation role in the estimated effect between 1-mg DST and DHEAS and contralateral diameter.
AIs are commonly discovered during abdominal imaging. Although these incidentally discovered adrenal masses are mostly benign and asymptomatic, MACS account for approximately 12-30% of Ais (4, 7, 23). Several studies had suggested that MACS may be associated with an increased risk for bone fracture (24, 25), cardiovascular disease and mortality. However, a recent study also found no elevated risk of hypertension, diabetes, and dyslipidemia in patients with MACS (1-mg DST: 1.8-5.0ug/dL) compared with NFAT (12). Although, dexamethasone is a potent synthetic glucocorticoid, which could suppress corticotropin secretion in normal subjects (26), the cutoff value for the serum cortisol level after 1-mg DST to make a diagnosis of mild autonomous cortisol excess is controversial. A level of more than 1.8 ug/dL has high sensitivity (95 to 100%) but low specificity (60 to 80%), whereas a level of more than 5.0ug/dL has lower sensitivity (86%) but higher specificity (92 to 97%) (27).
DHEAS could reflect a chronically suppressed HPA axis due to the relatively stable levels throughout the day (28). Multiple studies have assessed the diagnostic accuracy of DHEAS in confirmed MACS (29–31). A recent study by Liu et al. suggested that a single measurement of DHEAS is beneficial for identifying MACS (30); even circulating DHEA(S) levels decline with age and are associated with gender (32). However, they used a level of more than 1.8ug/dL of low-dose DST (LDDST) to diagnose MACS, which may overestimate the findings. The reason why the differences in DHEAS in our study were not more obvious may be related to the various diagnostic criteria and individuals included.
Adrenal tumors with overt Cushing’s syndrome presented with atrophy of the remaining contralateral adrenal gland due to suppressed ACTH production. Based on this pathophysiological phenomenon, several studies regarding the association between radiological characteristics and endocrine hormones have been made. Kong et al. found a negative linear correlation between limb width of the contralateral adrenal gland and cortisol levels after DST in patients with unilateral adrenal adenomas (18). Another study showed the significance of the volume of adrenal adenomas and contralateral adrenal glands associated with cortisol secretion (19). In our study, we found that patients with MACS had thinner contralateral adrenal compared with NFAT. Plasma ACTH was significantly associated with a decreased contralateral diameter in total patients, although there was no such association in the subgroup analysis of the MACS group. This may be related to the smaller number of patients in the MACS group, and the exact duration time of the HPA axis changes is unknown due to the nature of retrospective cross-sectional studies. Prospective studies are needed to evaluate how long it takes for changes in the HPA axis to have an effect on adrenal limb width.
Our study has several strengths. First, we comprehensively demonstrated the radiological and clinical characteristics of patients with MACS. The difference between MACS and NFAT indicated that clinical follow-up is essential. Second, we performed the causal mediation analysis to further investigate the association between radiological features and the HPA axis. This analysis was able to provide quantitative evidence supporting the role of ACTH as a mediator in the association between mild autonomous cortisol secretion and DHEAS and the contralateral adrenal gland. Third, this study is based on a relatively larger cohort in data regarding the association between radiological characteristics and hormone functionality. Large-scale analyses may give a high level of statistical power to detect effects.
This study also has several limitations. First, the study was cross-sectional and could not evaluate the association between radiologic features and the prognosis of MACS. Second, the cardiovascular morbidity in our cohort was not significantly different between two groups. Though this was in accordance with another recent large cohort, given the aim of our study, the fact that we did not include patients with bilateral tumors who had higher risks of morbidity may also account for this. Third, different types of CT protocols (abdomen or adrenal) were included in the study, which may affect diameter measurements. However, the difference could be minimal since the scan slice interval of each CT protocol was similar between 3 and 5 mm. The measurement is taken as the maximum width of the adrenal gland in the transverse plane rather than continuous measurements along the longitudinal axis. Therefore, the difference in slice interval may have a minimal impact on the measurement results.
In conclusion, our study demonstrated that patients with MACS had a higher tumor diameter, thinner contralateral adrenal diameter, and disturbed HPA axis. ACTH may partially be involved in mediating the mild autonomous cortisol secretion to the DHEAS and contralateral adrenal gland. The association between radiological features and the HPA axis indicated prospective studies are needed to assess risks in this population.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ruijin Hospital, Shanghai Jiao Tong University School of Medicine. The patients/participants provided their written informed consent to participate in this study.
WW conceptualized the study. TS, WZ, YJ, and XZ designed the study. LJ and CZ collected the data. HZ and LW performed the analysis and drafted the article. All authors contributed to critical revisions and final approval of the article.
This work was supported by the National Key Research and Development Program of China (2021YFC2501600, 2021YFC2501603); the Shanghai Shenkang Hospital Development Center (SHDC2020CR2002A and SHDC2020CR6015); Natural Science Foundation of Shanghai (22ZR1439100); and National Natural Science Foundation of China (82170797).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1199875/full#supplementary-material
Supplementary Figure 1 | The schematic diagram of the brief evaluation process of patients with adrenal incidentaloma in our center.
Supplementary Figure 2 | The sensitive analysis of causal mediation analysis among radiological characteristics, 1-mg DST and endocrine assessments. (A) mediation linkages between ACTH and 1-mg DST contributed DHEAS. (B) mediation linkages between contralateral diameter and 1-mg DST contributed DHEAS. (C) mediation linkages between ACTH and 1-mg DST contributed contralateral diameter. (D) mediation linkages between contralateral diameter and ACTH contributed DHEAS. *P < 0.05, **P < 0.01, and ***P < 0.001.
1. Young WF Jr. Clinical practice. The incidentally discovered adrenal mass. N Engl J Med (2007) 356(6):601–10. doi: 10.1056/NEJMcp065470
2. Davenport C, Liew A, Doherty B, Win HH, Misran H, Hanna S, et al. The prevalence of adrenal incidentaloma in routine clinical practice. Endocrine (2011) 40(1):80–3. doi: 10.1007/s12020-011-9445-6
3. Kloos RT, Gross MD, Francis IR, Korobkin M, Shapiro B. Incidentally discovered adrenal masses. Endocr Rev (1995) 16(4):460–84. doi: 10.1210/edrv-16-4-460
4. Fassnacht M, Arlt W, Bancos I, Dralle H, Newell-Price J, Sahdev A, et al. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol (2016) 175(2):G1–G34. doi: 10.1530/EJE-16-0467
5. Fassnacht M, Tsagarakis S, Terzolo M, Tabarin A, Sahdev A, Newell-Price J, et al. European Society of Endocrinology Clinical Practice Guidelines on the management of adrenal incidentalomas, in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol (2023). doi: 10.1093/ejendo/lvad066
6. Debono M, Bradburn M, Bull M, Harrison B, Ross RJ, Newell-Price J. Cortisol as a marker for increased mortality in patients with incidental adrenocortical adenomas. J Clin Endocrinol Metab (2014) 99(12):4462–70. doi: 10.1210/jc.2014-3007
7. Di Dalmazi G, Vicennati V, Garelli S, Casadio E, Rinaldi E, Giampalma E, et al. Cardiovascular events and mortality in patients with adrenal incidentalomas that are either non-secreting or associated with intermediate phenotype or subclinical Cushing's syndrome: a 15-year retrospective study. Lancet Diabetes Endocrinol (2014) 2(5):396–405. doi: 10.1016/S2213-8587(13)70211-0
8. Morelli V, Palmieri S, Lania A, Tresoldi A, Corbetta S, Cairoli E, et al. Cardiovascular events in patients with mild autonomous cortisol secretion: analysis with artificial neural networks. Eur J Endocrinol (2017) 177(1):73–83. doi: 10.1530/EJE-17-0047
9. Patrova J, Kjellman M, Wahrenberg H, Falhammar H. Increased mortality in patients with adrenal incidentalomas and autonomous cortisol secretion: a 13-year retrospective study from one center. Endocrine (2017) 58(2):267–75. doi: 10.1007/s12020-017-1400-8
10. Sbardella E, Minnetti M, D'Aluisio D, Rizza L, Di Giorgio MR, Vinci F, et al. Cardiovascular features of possible autonomous cortisol secretion in patients with adrenal incidentalomas. Eur J Endocrinol (2018) 178(5):501–11. doi: 10.1530/EJE-17-0986
11. Kjellbom A, Lindgren O, Puvaneswaralingam S, Londahl M, Olsen H. Association between mortality and levels of autonomous cortisol secretion by adrenal incidentalomas : A cohort study. Ann Intern Med (2021) 174(8):1041–9. doi: 10.7326/M20-7946
12. Prete A, Subramanian A, Bancos I, Chortis V, Tsagarakis S, Lang K, et al. Cardiometabolic disease burden and steroid excretion in benign adrenal tumors : A cross-sectional multicenter study. Ann Intern Med (2022) 175(3):325–34. doi: 10.7326/M21-1737
13. Yener S, Secil M, Demir O, Ozgen Saydam B, Yorukoglu K. Chemical shift magnetic resonance imaging could predict subclinical cortisol production from an incidentally discovered adrenal mass. Clin Endocrinol (Oxf) (2018) 88(6):779–86. doi: 10.1111/cen.13587
14. Mosconi C, Vicennati V, Papadopoulos D, Dalmazi GD, Morselli-Labate AM, Golfieri R, et al. Can imaging predict subclinical cortisol secretion in patients with adrenal adenomas? A CT predictive score. AJR Am J Roentgenol (2017) 209(1):122–9. doi: 10.2214/AJR.16.16965
15. Olsen H, Nordenstrom E, Bergenfelz A, Nyman U, Valdemarsson S, Palmqvist E. Subclinical hypercortisolism and CT appearance in adrenal incidentalomas: a multicenter study from Southern Sweden. Endocrine (2012) 42(1):164–73. doi: 10.1007/s12020-012-9622-2
16. Golden SH, Malhotra S, Wand GS, Brancati FL, Ford D, Horton K. Adrenal gland volume and dexamethasone-suppressed cortisol correlate with total daily salivary cortisol in African-American women. J Clin Endocrinol Metab (2007) 92(4):1358–63. doi: 10.1210/jc.2006-2674
17. Huayllas MKP, Sirineni GK, Smith LM, Gallagher JC, Singh RJ, Netzel BC, et al. Correlation between size and function of unilateral and bilateral adrenocortical nodules: an observational study. AJR Am J Roentgenol (2020) 214(4):800–7. doi: 10.2214/AJR.19.21753
18. Kong SH, Kim JH, Shin CS. Contralateral adrenal thinning as a distinctive feature of mild autonomous cortisol excess of the adrenal tumors. Eur J Endocrinol (2020) 183(3):325–33. doi: 10.1530/EJE-20-0301
19. Olmos R, Mertens N, Vaidya A, Uslar T, Fernandez P, Guarda FJ, et al. Discriminative capacity of volumetry by CT scan to identify autonomous cortisol secretion in incidental adrenal adenomas. J Clin Endocrinol Metab (2022) 107(5):e1946-e53. doi: 10.1210/clinem/dgac005
20. Vincent JM, Morrison ID, Armstrong P, Reznek RH. The size of normal adrenal glands on computed tomography. Clin Radiol (1994) 49(7):453–5. doi: 10.1016/S0009-9260(05)81739-8
21. Wang S, Li M, Lin H, Wang G, Xu Y, Zhao X, et al. Amino acids, microbiota-related metabolites, and the risk of incident diabetes among normoglycemic Chinese adults: Findings from the 4C study. Cell Rep Med (2022) 3(9):100727. doi: 10.1016/j.xcrm.2022.100727
22. Xing Y, Edwards MA, Ahlem C, Kennedy M, Cohen A, Gomez-Sanchez CE, et al. The effects of ACTH on steroid metabolomic profiles in human adrenal cells. J Endocrinol (2011) 209(3):327–35. doi: 10.1530/JOE-10-0493
23. Mantero F, Terzolo M, Arnaldi G, Osella G, Masini AM, Ali A, et al. A survey on adrenal incidentaloma in Italy. Study Group on Adrenal Tumors of the Italian Society of Endocrinology. J Clin Endocrinol Metab (2000) 85(2):637–44. doi: 10.1210/jc.85.2.637
24. Morelli V, Eller-Vainicher C, Palmieri S, Cairoli E, Salcuni AS, Scillitani A, et al. Prediction of vertebral fractures in patients with monolateral adrenal incidentalomas. J Clin Endocrinol Metab (2016) 101(7):2768–75. doi: 10.1210/jc.2016-1423
25. Salcuni AS, Morelli V, Eller Vainicher C, Palmieri S, Cairoli E, Spada A, et al. Adrenalectomy reduces the risk of vertebral fractures in patients with monolateral adrenal incidentalomas and subclinical hypercortisolism. Eur J Endocrinol (2016) 174(3):261–9. doi: 10.1530/EJE-15-0977
26. Nugent CA, Macdiarmid WD, Nelson AR, Tyler FH. Rate of adrenal cortisol production in response to maximal stimulation with ACTH. J Clin Endocrinol Metab (1963) 23:684–93. doi: 10.1210/jcem-23-7-684
27. Kebebew E. Adrenal incidentaloma. N Engl J Med (2021) 384(16):1542–51. doi: 10.1056/NEJMcp2031112
28. Klinge CM, Clark BJ, Prough RA. Dehydroepiandrosterone research: past, current, and future. Vitam Horm (2018) 108:1–28. doi: 10.1016/bs.vh.2018.02.002
29. Dennedy MC, Annamalai AK, Prankerd-Smith O, Freeman N, Vengopal K, Graggaber J, et al. Low DHEAS: A sensitive and specific test for the detection of subclinical hypercortisolism in adrenal incidentalomas. J Clin Endocrinol Metab (2017) 102(3):786–92. doi: 10.1210/jc.2016-2718
30. Liu MS, Lou Y, Chen H, Wang YJ, Zhang ZW, Li P, et al. Performance of DHEAS as a screening test for autonomous cortisol secretion in adrenal incidentalomas: A prospective study. J Clin Endocrinol Metab (2022) 107(5):e1789-e96. doi: 10.1210/clinem/dgac072
31. Yener S, Yilmaz H, Demir T, Secil M, Comlekci A. DHEAS for the prediction of subclinical Cushing's syndrome: perplexing or advantageous? Endocrine (2015) 48(2):669–76. doi: 10.1007/s12020-014-0387-7
Keywords: contralateral adrenal, nonfunctional adrenocortical tumor, mild autonomous cortisol secretion, hypothalamus-pituitary-adrenal (HPA) axis, causal mediation analysis
Citation: Zhu H, Wu L, Su T, Jiang L, Zhou W, Jiang Y, Zhang C, Zhong X and Wang W (2023) Association between contralateral adrenal and hypothalamus-pituitary-adrenal axis in benign adrenocortical tumors. Front. Endocrinol. 14:1199875. doi: 10.3389/fendo.2023.1199875
Received: 04 April 2023; Accepted: 04 July 2023;
Published: 25 July 2023.
Edited by:
Renata Świątkowska-Stodulska, Medical University of Gdansk, PolandReviewed by:
Ivana Kraljevic, University Hospital Centre Zagreb, CroatiaCopyright © 2023 Zhu, Wu, Su, Jiang, Zhou, Jiang, Zhang, Zhong and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiqing Wang, d3Fpbmd3NjFAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.