
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Endocrinol., 10 August 2023
Sec. Reproduction
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1198437
This article is part of the Research TopicFunctional Acquired Hypogonadotropic Hypogonadism in MalesView all 5 articles
According to World Health Organization estimates, 5% of the adult population worldwide suffers from depression. In addition to the affective, psychomotor and cognitive symptoms which characterize this mood disorder, sexual dysfunction has been frequently reported among men suffering from depression. The most common sexual manifestations are decreased libido, erectile dysfunction and orgasmic disorder. In addition, epidemiological studies have documented a reduction of testosterone concentrations in men with depression and, for these reasons, depressive disorders appear as one possible cause of male functional hypogonadism. Moreover, some largely used antidepressant medications can cause or worsen sexual complaints, thus depression and its treatments rise several andrological-relevant issues. The other way round, men with hypogonadism can manifest depressed mood, anxiety, insomnia, memory impairment which, if mild, may respond to testosterone replacement therapy (TRT). However, the prevalence of functional hypogonadism in depression, and of depressive symptoms in hypogonadal men, is not known. Severe depressive symptoms do not respond to TRT, while the effect of treating major depression on functional hypogonadism, has not been investigated. Overall, the clinical relevance of each condition to the other, as well as the physiopathological underpinnings of their relationship, are still to be clarified. The present review summarizes current evidence on the influence of testosterone on mood and of depression on the hypothalamic-pituitary-testis axis; the clinical association between male hypogonadism and depression; and the reciprocal effects of respective treatments.
Male hypogonadism is a clinical and biochemical syndrome associated with low levels of testosterone (1–3). While the most specific symptoms are the sexual ones, i.e. low libido, erectile dysfunction, diminished frequency of morning erections (4) and orgasmic disorders (5), other non-specific manifestations are included in the syndrome, like fatigue, cognitive impairment and depressed mood (1, 3).
Depressive symptoms have been reported in 35-50% of male patients with hypogonadism in cross-sectional studies (6–8). Hypogonadism at baseline has been associated with development of depressive disorders in some short (9) and long term longitudinal studies (10), even if negative results have been reported as well (11). With the aim of characterizing their emotional state, Lašaite and colleagues compared the scores obtained in questionnaires on mood, quality of life and cognitive functioning by 34 young hypogonadal men and 34 age-matched healthy controls (12). In this way, authors identified higher levels of depression, fatigue, confusion and inactivity among patients with hypogonadism compared to healthy controls. Interestingly, the social and psychological domains of quality of life and some cognitive functions were impaired too. Likewise, young patients affected with congenital hypogonadotropic hypogonadism, displayed difficulty in emotional role, reduced vitality and a higher prevalence of depression compared to age-matched controls, as assessed by Short Form 36 and the Beck Depression Inventory (13). Overall, evidence suggests that low testosterone may cause depressed mood in at least a subset of hypogonadal men.
The other way round, men with depressive disorders can exhibit features consistent with hypogonadism. According to the latest edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), depressive disorders are defined by depressed mood, loss of interest or pleasure in most activities, and a range of symptoms including psychomotor changes with agitation or retardation, low energy, impaired ability to concentrate or make decisions (14). However, besides the affective, psychomotor and cognitive symptoms, depression can bring about vegetative manifestations which encompass unintentional weight loss or gain, insomnia or hypersomnia, and sexual dysfunction.
A recent meta-analysis has reported that sexual dysfunction can be observed in 63% of men with major depressive disorder overall; it manifests with decreased libido in 40% of male patients; erectile dysfunction in 32%; and orgasmic disorder in 35% (15). Furthermore, a systematic review with meta-analysis of case-control studies on male depressive disorder, has reported that patients have significantly lower plasma testosterone concentrations than healthy controls (16). Moreover, depression severity is significantly and inversely associated with levels of bioavailable and free testosterone and dihydrotestosterone (8, 17, 18). Therefore, depression should be considered a comorbid illness leading to a condition of functional hypogonadism, i.e. hypogonadism due to potentially reversible disruption of the hypothalamic–pituitary–testis (HPT) axis functioning (1).
Some epidemiologic clues further suggest association between sex steroids and mood. Depression shows different prevalence and features between men and women: the prevalence of affective disorders in women is twice as high as in men (19), and male depressed patients display lower stress tolerance, worse impulse control, a higher prevalence of antisocial behaviour and suicide compared to female subjects (19). Male hypogonadism and depression are age-related, as both conditions have a higher prevalence in older men (20, 21) and may have a reciprocal influence: if, on one hand, sexual issues can result in depression and social withdrawal, on the other hand sexual thinking is affected by psychological disturbances, including depression, in older men (22).
The aim of this review is to explore the physiopathology behind the influence of testosterone on mood and of depression on the HPT axis; to examine the clinical association between male hypogonadism and depression; and to summarize evidence on the effects of testosterone therapy on depression, and of antidepressants on male sexual function.
A primary literature search was performed in PubMed including the following keywords: “male hypogonadism”, “testosterone”, “sexual symptoms”, “sexual dysfunction”, “depression”, “mood”, using the Boolean functions AND/OR. The search was restricted to English-language studies published up to December 2022. The reference lists of the identified papers were also scrutinized for further pertinent studies.
Narrative reviews, systematic reviews, human observational studies, clinical trials and animal studies were included. If it was not clear from the abstract whether the study contained relevant data, the full text was retrieved. The eligibility criteria for selection were as follows: 1) animal studies investigating the behavioural response to HPT hormones, and/or their expression and effects in cerebral areas related to mood disorders; 2) human studies investigating the relationship between endogenous HPT hormones’ levels, androgen therapy, sexual dysfunction, depressive symptoms, antidepressant medications, and activity of cerebral areas related to mood disorders as assessed by morphologic or functional studies.
An in-depth description of the HPT axis functioning and regulation is beyond the purpose of this review. Shortly, the preoptic area and the infundibular nucleus of the hypothalamus contain neurons producing gonadotropin releasing hormone (GnRH) (23). They project to the median eminence, so that GnRH is secreted into portal circulation to reach the anterior pituitary. GnRH is released in a pulsatile pattern and determines the pulsatile secretion of gonadotropins, luteinizing hormone (LH) and follicle stimulating hormone (FSH), into systemic circulation (23). Finally, LH acts on testis where it stimulates Leydig cells to produce testosterone (24).
Even though GnRH neurons have intrinsic electrical pulsatility, their activity is synchronized by an external hypothalamic pulse generator which encompasses the kisspeptin-neurokinin B-dynorphin pathway mainly, but also glutamatergic cells and other neurons (23). Kisspeptin neurons are located in the preoptic area and the infundibular nucleus of the hypothalamus. In the latter, Kisspeptin neurons co-express neurokinin B and dynorphin and are called KNDy neurons. Through the (stimulatory) neurokinin B receptor and the (inhibitory) kappa opioid peptide receptor, KNDy neurons regulate kisspeptin secretion in an autologous fashion (23).
Kisspeptin is the most potent GnRH secretagogue currently known. However, other mediators influence the GnRH-gonadotrope axis, including gamma-amino butyric acid (GABA), vasoactive intestinal polypeptide, vasopressin, catecholamines, nitric oxide, neurotensin, gonadotropin-inhibitory hormone (GnIH)/RFamide related peptide-3 (RFRP-3) (23). In mammals, RFRP-3 neurons have been identified in the hypothalamic dorsomedial nucleus, and they act by inhibiting GnRH- and kisspeptin-secreting cells. Through these complex interactions, a multitude of stimuli can modulate the HPT axis, including stress, inflammation, opioid drugs (23).
As stated above, depressive disorders can be associated with functional hypogonadotropic hypogonadism. Many aspects of depression may contribute to this outcome, including weight loss, disrupted sleep, hyperactivity of the hypothalamus-pituitary-adrenal axis, and psychomotor retardation.
In some patients, depression entails loss of appetite which results in inadequate energy intake and weight loss. In this setting, HPT axis dysfunction stems at least in part from reduction in circulating levels of leptin (25). Leptin is an anorexigenic adipokine, the levels of which drop in response to starvation. It acts in different brain regions including the hypothalamus (26), where 40% of Kisspeptin neurons express leptin receptor (23). The loss of GnRH pulsatility in food deprivation results from the lack of leptin stimulation on kisspeptin neurons (25).
Sleep disorders, i.e. difficulty initiating or maintaining sleep, with or without hypersomnolence, are common in depression, where insomnia prevalence exceeds 80% (27). Moreover, longitudinal studies suggest that insomnia is a risk factor for depression, as it is associated with depressive symptom severity, lower rates of remission, higher risk of relapse, suicidal thoughts and self-injury (28). On the other hand, sleep disturbances can impact testosterone concentrations and sexual symptoms. In a study on shift workers, authors distinguished participants who suffered from shift work sleep disorder, i.e. a circadian rhythm disorder characterized by excessive day-time sleepiness, from participants who did not (29). The former were found to have worse hypogonadal symptoms and lower testosterone levels than shift workers without shift work sleep disorder and day-time workers. Authors conclude that poor sleep habits may contribute to hypogonadal symptoms severity. In another study analysing association between sex hormones concentration and self-rated health and life satisfaction in elderly men, lower free testosterone concentrations were significantly associated with sleeping problems (30). Among 48 male patients treated at a men’s health clinic for andrological problems, 32 (67%) were classified as poor sleepers according to the Pittsburgh Sleep Quality Index (31).
Serum cortisol concentrations are significantly increased in patients with depressive disorder, as a result of hypothalamus-pituitary-adrenal axis hyperactivity (32). Cortisol but also upstream factors like corticotropin releasing hormone and vasopressin, exert negative effects at both pituitary and hypothalamic levels (23). Cortisol treatment reduced pulse frequency of GnRH measured in ovine pituitary portal blood (33). Both the hypothalamic corticotropin releasing hormone and corticosterone downregulated the expression of Kisspeptin gene in rats (34). Administration of hydrocortisone to eumenorrheic women reduced LH pulse frequency, an outcome which most likely resulted from interference with GnRH pulsatile release (35). Male patients with endogenous hypercortisolism present hypogonadotropic hypogonadism, which generally resolves upon hypercortisolism remission (36). Moreover, in patients affected with Cushing’s syndrome, a blunted response of gonadotropins to exogenous GnRH administration has been documented, a finding which points to a direct inhibitory effect of excess cortisol at the pituitary level (36, 37).
Psychomotor retardation is a central, complex feature of depressive disorders which encompasses modifications in individual motility, mental activity, and speech (38). As far as motility is concerned, psychomotor retardation impairs gross and fine motor activity, eye movements, facial movements (38). Together with other symptoms of depression like loss of interest in most activities and low energy, psychomotor retardation contributes to physical inactivity in depressed subjects (39). Actually, evidence on the effects of physical exercise on testosterone levels is controversial (40). While some studies show that testosterone levels rise acutely after training, others support a long-term effect; however, reduction and no variation of testosterone levels have been reported in relation to physical exercise as well (40). This variability can be ascribed to numerous factors which influence the relationship between physical activity and testosterone production, like the type of exercise (endurance or resistance), training intensity and duration, subjects’ features (age, body weight, sedentary lifestyle) (40).
Interestingly, somatization has been suggested as a factor potentially determining or exacerbating sexual symptoms (41). In a retrospective study analysing 2833 patients complaining sexual symptoms, Fanni et al. observed that patients scoring higher on a “somatized anxiety symptoms” scale, were older and more obese, and reported unhealthy lifestyle and a lower education, along with sexual impairment, with a higher frequency. A significant association between the “somatized anxiety symptoms” score and low testosterone levels was observed too. Therefore, we suggest that somatization could be one more mechanism underlying sexual dysfunction in depressed patients.
Finally, neuronal circuits involved in the physiopathology of mood disorders, may be directly responsible for HPT axis deregulation. However, to the best of our knowledge, data on this intriguing hypothesis are not currently available.
Hormones of the HPT axis, i.e. Kisspeptin, GnIH/RFRP-3, GnRH, gonadotropins, testosterone and estradiol, act on cerebral areas involved in the pathogenesis of mood disorders, like the hippocampus and the limbic system, particularly the amygdala (42, 43).
Neuronal fibers containing kisspeptin and GnIH/RFRP are found not only in the hypothalamus, but also in amygdala, hippocampus, habenula, periaqueductal gray, and ventral tegmental area in mammals (44). Consistently, Kisspeptin receptors expression has been documented in several regions of human brain, which include hippocampus and amygdala (45).
The dysfunction in serotoninergic neurotransmission has been implicated in mood disorders (46). In zebrafish, kisspeptin has been found to interact with the serotonergic system during a substance-evoked alarm experiment (47). Administration of kisspeptin in male mice during a forced swimming test resulted in antidepressive-like effects, which were at least in part mediated by the interaction with α2-adrenergic and 5-hydroxytryptamine-type 2 (5-HT2) serotonergic receptors (48).
In rodents, administration of GnRH agonist exerts antidepressant effect, and anxiolytic effect comparable to diazepam, whereas GnRH antagonist increases anxiety levels (49, 50). These observations were confirmed in castrated animals, ruling out the involvement of peripheral sex hormones in the anxiolytic and antidepressant actions of GnRH.
Animal studies have demonstrated that the preoptic area, the hypothalamus and the amygdala are targets of potent, non-aromatizable androgens (51, 52). Additionally, androgens influence dopaminergic neurotransmission to the caudate putamen, nucleus accumbens and amygdala in rats (53). Apostolinas and colleagues showed that androgen receptor expression is found in medial amygdala, preoptic area, lateral ventral septum, and stria terminalis, and is modulated by testosterone levels (54). Moreover, testosterone may exert neuroprotective effects: indeed, Sarchielli and colleagues showed that testosterone administration reduced high-fat diet induced hypothalamic inflammation in an animal model of metabolic syndrome (55).
The conversion of testosterone by the enzyme aromatase results in the production of estrogen, which acts in target tissues through estrogen receptors α and β. Biegon et al. studied aromatase distribution in the healthy human brain (56): distribution volume values were measured using positron emission tomography (PET) with the radiolabeled aromatase inhibitor [N-methyl-11C] vorozole. High aromatase expression was observed in the thalamus and the amygdala. Using in situ hybridization histochemistry on post-mortem specimens, Osterlund and colleagues identified human cerebral areas containing ribonucleic acid (mRNA) of estrogen receptors (57). The highest expression of estrogen receptor α was found in the amygdala and hypothalamus, while that of estrogen receptor β in the temporal cortex, claustrum, thalamus and hippocampus. In particular, estrogen has been shown to act on serotonergic neurons in rats (58) and primates (59) and reduce anxiety- and depression-like behaviors in females (60). Therefore, testosterone may be involved in mood disorders’ physiopathology through the conversion in estrogen and the subsequent action on estrogen receptors in the limbic system.
It has to be mentioned that the nervous system not only responds to sex steroids released by testicles into circulation, but it has its own steroidogenic activity which leads to production of testosterone and estrogen as well (61). Neurosteroids activate the classical nuclear receptors (androgen receptor and estrogen receptors α and β), but some of them may act through different pathways. For instance, estradiol also binds the membrane G protein coupled estrogen receptor (GPER) (62), while other steroid metabolites may activate non-classical steroid receptors, including dopamine 1 receptor, GABA receptors A and B, serotonin type 3 receptors, both at synaptic and extra-synaptic sites (61). To which extent neurosteroids interact with peripherally synthetized sex steroids to influence mood, is still to be clarified.
Voxel based morphometry has been used to study the relationship between testosterone levels and grey and white matter volumes in humans. A significantly positive correlation has been identified for grey matter volume in the hippocampus, amygdala, hypothalamus and mammilary bodies (63, 64). Animal studies have shown that androgen deprivation causes a reduction in synaptic density in the hippocampus, which recovers after testosterone replacement (65).
Functional studies using functional magnetic resonance imaging (fMRI) or PET have found an association between testosterone levels and activation of distinct brain regions (66–68). In a study on young boys affected with familial male precocious puberty, patients with early excess testosterone secretion displayed more intense activation of the hippocampus when shown fearful faces compared to age-matched healthy controls (69). Derntl et al. performed fMRI in 21 healthy men during an emotion recognition protocol to detect amygdala activation (70). A positive correlation was found between testosterone concentrations and amygdala response to fearful and angry facial expressions. Hence, authors conclude that testosterone affects threat-related amygdala activation. In another study employing fMRI to assess amygdala activation in response to threat-related stimuli, Manuck et al. reported that reactivity in the ventral amygdala of adult men was inversely correlated with number of CAG repeats in the androgen receptor-encoding gene; conversely, activation of dorsal amygdala correlated positively with salivary testosterone, but not with number of CAG repeats (71). The latter finding suggests that testosterone effects in dorsal amygdala may even be mediated by non-genomic or androgen receptor-independent pathways.
Comninos et al. used a combination of functional studies and psychometric analyses to assess effects of kisspeptin in 29 healthy young men (72). Kisspeptin administration enhanced brain activity in limbic areas in response to sexual stimuli, and this finding correlated with psychometric measures of reward, drive, sexual aversion and mood, in particular with attenuation of negative mood. When participants were shown negative-evoked visual stimuli like images of car crashes or terminal patients, kisspeptin enhanced activity of prefrontal cortex (72), which has a role in reducing fear and anxiety in response to negative stimuli (73).
Many clues suggest that HPT axis hormones, neurotransmission and cerebral areas involved in mood disorders, can influence each other. Depression symptoms and physiopathological aspects like weight loss, sleep disorders, activation of the hypothalamus-pituitary-adrenal axis, and physical inactivity can lead to functional hypogonadism.
The other way round, testosterone and other hormones of the HPT axis can act on a multitude of cerebral areas and modulate neurotransmission, including the ones involved in mood disorders. Indeed, neuro-hormonal fibers, hormone receptors and metabolic enzymes are largely distributed in the central nervous system. Morphologic and functional studies have confirmed the effects of sexual hormones in cerebral regions of interest. Table 1 summarizes current evidence on these aspects.
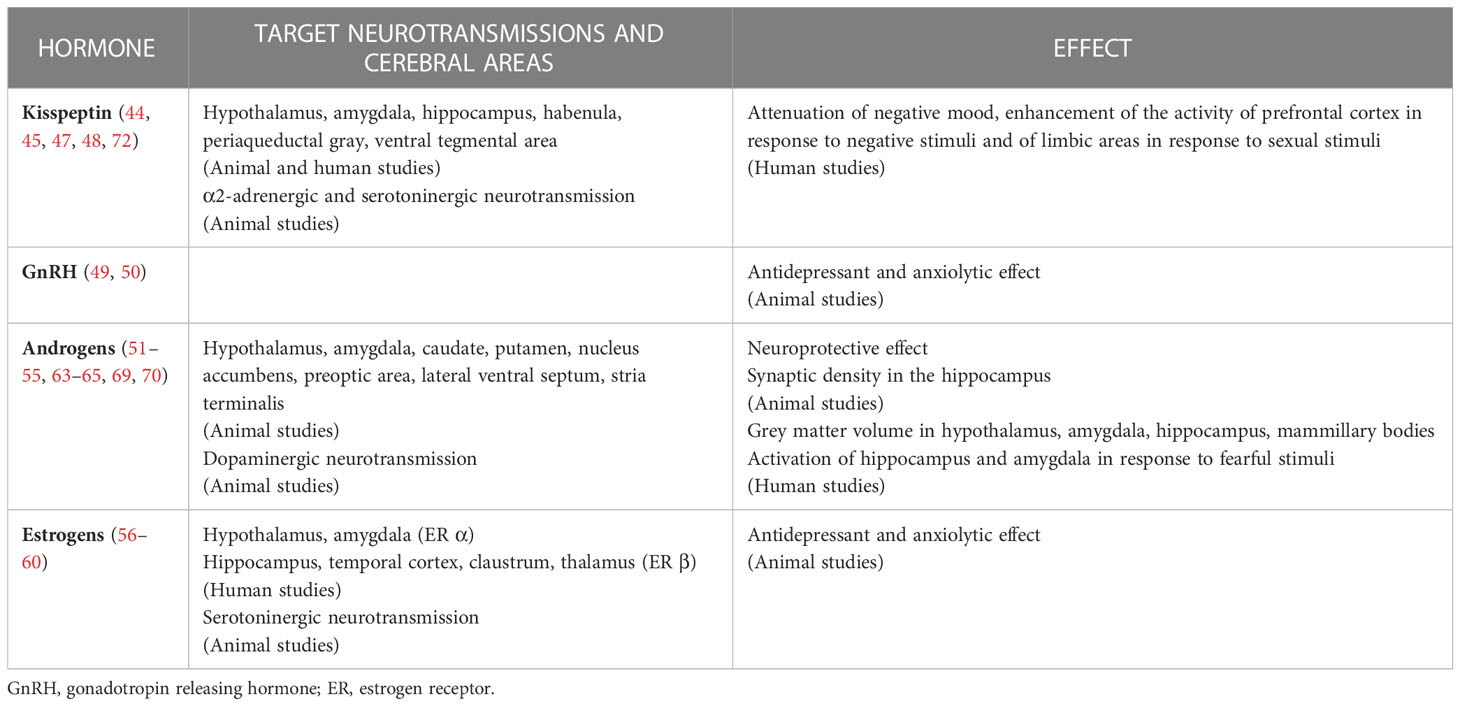
Table 1 Summary of current evidence on the effects of hypothalamus-pituitary-testis axis hormones in central nervous system.
However, the modulation of mood, as well as that of HPT axis functioning, are highly complex and integrated. Many other factors contribute to the tuning of these two systems, so that the reciprocal influence is hard to be clearly quantified. Observational and intervention studies can help define the clinical relevance of either alteration, and are summarized below.
As stated above, depressive disorders can be associated with HPT axis dysfunction, however research into the relationship between plasma testosterone levels and major depression is complicated by etiologic and diagnostic uncertainties (74).
Three important epidemiological studies assessed the association between plasma testosterone levels and depressive symptoms, leading to controversial results: the Massachusetts Male Aging Study (MMAS) (75), the Veterans’ Experience Study (76) and the Rancho Bernardo Study (17). The MMAS was a population-based survey of 1709 men aged 40-70 years (75). Participants were required to fill a self-reported depression questionnaire, the Center for Epidemiologic Studies Depression Scale (CES-D), and to provide a morning blood sample for testosterone measurement. The statistical analysis showed no association between serum testosterone levels and CES-D-diagnosed depression. The Veterans’ Experience Study investigated a sample of 4393 veterans who served the U.S. military (mean age 37 years) (76). Participants were administered the Diagnostic Interview Schedule and provided morning blood samples for testosterone assessment. Plasma testosterone level was weakly but significantly correlated with depression, mania, and anxiety. Finally, in the Rancho Bernardo Study (17), 856 adult residents of a Californian community aged 50-89 years were enrolled in a 10-year follow-up study. They completed the Beck Depression Inventory and had a morning blood sample drawn for hormone assays. The study showed a significant inverse correlation between Beck Depression Inventory score and bioavailable, but not total, testosterone levels, pointing out that men with lower free testosterone levels had more severe depressive symptoms.
Other observational cross-sectional studies compared mean testosterone levels of depressed men with those of nondepressed controls (77, 78). Findings from such studies showed conflicting results (79). McIntyre et al. (80) assessed and compared total testosterone (TT) and bioavailable testosterone (BT) levels in two groups of middle-aged men (40-65 years): untreated subjects meeting DSM-4 criteria for a major depressive episode (N 44), and a matched nondepressed control group (N 50). Mean BT and TT levels were lower in the depressed group compared to the control one. Biochemical hypogonadism (i.e., BT level ≤ 70 ng/dL or TT level ≤ 350 ng/dL) was also more prevalent among depressed men than in nondepressed controls (34% versus 6%; 61% versus 14%, respectively). Giltay et al. (18) carried out a prospective 2-year study of testosterone levels in men with major depressive disorder as defined by the DSM-4 criteria compared with age matched healthy controls with mean age 70.5 ± 7.3 years. The study showed that the depressed group had lower mean testosterone levels than non-depressed controls after correction for age, level of education, body mass index, physical activity, smoking status, alcohol use, number of chronic diseases, and androgen-affecting medication. Of the 5.4% of subjects with total testosterone level lower than 230 ng/dL, 90% met criteria for major depressive disorder. These data underline the association between low testosterone levels and depression, demonstrating the presence of a graded risk of depression based on testosterone levels (81).
In contrast with the former considerations, however, other cross-sectional studies showed no difference in testosterone levels between depressed men and healthy controls (82–85).
Increased levels of estradiol were observed in depressed men in a case-control study by Fischer and colleagues (16). However, association of estradiol levels with depressive symptoms was confirmed in female, but not male, patients with HIV infection (86). Moreover, depressed mood showed no improvement after a short course of estradiol treatment in older men receiving androgen deprivation therapy (87).
In conclusion, the relationship between depression and sex steroids’ levels needs to be clarified yet. Evidence is limited and controversial, but seems to suggest an association, although weak, between low levels of testosterone and depressive symptoms in males (88).
According to the World Health Organization, sexual health is defined as a state of physical, emotional, mental, and social well-being related to sexuality. It is not merely an absence of disease, dysfunction, or infirmity, and it requires a positive and respectful approach to sexuality and sexual relationship (89). Typical manifestations of sexual dysfunction in men comprise erectile dysfunction, ejaculatory disorders, particularly premature ejaculation, low libido and orgasmic difficulty (90, 91). Moreover, erectile dysfunction is the main sexual problem associated with mental health and quality of life in males (92).
Several reports have suggested that erectile function is affected negatively by depression (91, 93, 94). Data obtained from the MMAS were revised in an analytic model by Araujo et al. (95) and showed that erectile dysfunction was associated with depressive symptoms, after controlling for potential confounders, with an odds ratio of 1.82. The authors concluded that the relationship between depressive symptoms and erectile dysfunction in middle-aged men is robust and independent of aging and para-aging confounders.
Takao and colleagues (96) looked at the prevalence of erectile dysfunction, assessed by the International Index of Erectile Function 5, among 87 Japanese patients with functional hypogonadism, 34 of which were diagnosed as having depression by the Mini International Neuropsychiatric Interview. They found that International Index of Erectile Function 5 scores of depressed patients were significantly lower than those of non-depressed patients.
In a multicenter study carried out in Brazil, Italy, Japan, and Malaysia, depression was shown to be associated with erectile dysfunction in a graded manner, and men with erectile dysfunction were 2.09 times more likely to have depression (97).
Many studies have documented an association between depression and sexual function. Howell et al. compared a population of depressed men by means of the Derogatis Sexual Functioning Inventory, a retrospective questionnaire on sexual function, with a group of age-matched healthy controls (98). They found that depressed men had lower sexual desire, a poorer self-image, and less sexual satisfaction, despite no significant difference in the frequency of sexual episodes.
Rizvi et al. carried out a study on 44 untreated depressed males and 50 age-matched healthy controls (78). Both populations had blood samples drawn to determine morning levels of total testosterone, sexual function outcomes measured using the Sex Effects Scale and depression severity assessed with the Hamilton Rating Scale for Depression-17 item. 27.9% of men were defined as hypogonadal and among these, men with major depressive disorder had lower scores on all domains of sexual function, especially orgasm and desire, compared to hypogonadal healthy controls. Multiple linear regression analyses revealed that depression status was the main factor influencing sexual function.
Some clinical manifestations of hypogonadism in males may overlap with those typical of major depression, like anxiety, insomnia, memory impairment and reduced cognitive function (88). However, it is not known what proportion of hypogonadal men meet criteria for major depression (74).
Korenman et al. conducted a retrospective study on 186 male hypogonadal men (mean testosterone values < 10.4 nmol/L) with mean age 18-40 years to test the hypothesis of a high prevalence of depression in young men with functional hypogonadism (99). They compared their demographic factors, other diagnoses and treatments with those of 3 different populations: 1) general population, 2) a population of 930 controls matched for age, BMI and alcohol use, 3) 404 controls with normal testosterone determinations and no hypogonadism diagnosis. Depression, defined as either an International Classification of Diseases, Ninth Revision (ICD-9) diagnosis, or treatment with an antidepressant medication, was found in 22.6% of cases vs 6.6% of the general population (P < 0.001). The matched controls had a depression rate of 13.4% (P < 0.002). Controls with normal testosterone determinations had a depression rate of 16.8% (P = 0.121).
Studies by Burris et al. (100), Wang et al. (101, 102), and Lašaite et al. (103) reported on mood in young men with functional hypogonadism compared to men with normal testosterone levels, showing a significant association between hypogonadal state and depressive symptoms.
Male patients undergoing androgen deprivation therapy for prostate cancer constitute an interesting clinical model to study the effects of testosterone deficiency. A greater increase in Beck Depression Inventory score has been observed in patients receiving androgen deprivation therapy compared to those treated with prostatectomy only (104). Variation in Beck Depression Inventory was proportional to severity of pre-existing erectile dysfunction, increase in body mass index and reduction of testosterone concentrations. A systematic review of clinical studies reporting on incidence of depression among individuals exposed to androgen deprivation therapy compared to non-exposed patients, found that androgen deprivation therapy conferred a 41% increased risk of depression (relative risk 1.41; 95% confidence interval 1.18–1.70; p<0.001) (105).
In conclusion, these association studies document a high incidence of depressive symptoms in male hypogonadism, and underline the importance of investigating these symptoms in young hypogonadal men.
The association between depression, testosterone levels and sexual symptoms in males is difficult to assess, due to numerous confounding factors, such as medical conditions, obesity, smoking, alcohol use, diet, and stress. Overall, current evidence shows that these conditions are linked and influence each other bidirectionally, with each factor reinforcing the other. However, additional studies are needed to investigate the relationship of testosterone levels with psychiatric symptoms.
Although there is not enough evidence at this time to recommend routine monitoring for plasma testosterone levels in patients with major depressive disorder, clinicians must be sensibilized on this topic and should keep in mind the potential link between these conditions.
As described above, a mutual relationship between depression and sexual symptoms has been suggested by several studies. From this perspective, medications able to improve depressive disorders could also lead to an improvement in sexual symptoms. However, psychotropic drugs can negatively affect sexual function and behaviour. Eventually, knowledge of biology of the sexual response in humans is crucial to understand the potential effects of these drugs.
Sexual response includes 3 phases: libido or desire, arousal - resulting in erections in men and genital lubrication and swelling in women – and orgasm (106, 107). Many neurotransmitters (eg, dopamine, serotonin, acetylcholine, nitric oxide, noradrenaline) as well as hormones (prolactin, testosterone, oestrogen) are involved at various levels in this process (106–108).
The mesolimbic system plays a primary role in sexual motivation and dopamine is the most important neurotransmitter in this context. In fact, dopamine activation of the nucleus accumbens and medial preoptic hypothalamic region is fundamental for sexual interest (106). On the other hand, serotonin determines at this level an inhibitory effect on libido, in particular with involvement of the hippocampus and amygdala (109). It is noteworthy that central effects of serotoninergic system vary according to the activation of different receptor subtypes: in particular, receptors 5-HT2 and 5-HT3 have an inhibiting influence on sexual activity, whereas the activation of 5-HT1A stimulates sexual response.
Sexual arousal involves mesolimbic structures and peripheral autonomic sympathetic and parasympathetic nervous system. The entire process is influenced by several neurotransmitters, including dopamine, serotonin, acetylcholine and noradrenaline. Furthermore, nitric oxide, a mediator of vasodilation, also plays a relevant role in the erectile tissues (106, 109, 110).
Finally, orgasm and ejaculation are mediated through hypothalamus and peripheral sympathetic nervous system where serotonin, prolactin and noradrenaline contribute to the regulation of this phase (106).
Antidepressant drugs can affect all 3 phases of normal sexual response in males by reducing libido, causing arousal disturbance or delaying orgasm. However, the rate and severity of sexual symptoms vary among antidepressants according to different central and autonomic actions (111).
First-generation antidepressant [tricyclic antidepressants (TCAs) and Monoamine oxidase inhibitors (MAOIs)] such as antidepressants with serotoninergic activity [selective serotonin reuptake inhibitors (SSRIs) and serotonin/norepinephrine reuptake inhibitors (SNRIs)] are frequently associated with sexual dysfunction (111–113).
A meta-analysis including double-blind, single-blind, open-label, cross-sectional and retrospective studies reported a rate of treatment-emergent sexual symptoms ranging from 25 to 80%, with the highest risk observed for SSRIs citalopram, fluoxetine, paroxetine, sertraline and for the SNRI venlafaxine (110).
First-generation antidepressants increase central availability of serotine and noradrenalin and exert an anticholinergic action. Serotoninergic activity in turn interferes with dopaminergic signal in the mesolimbic system and can also lead to an increase of prolactin levels with subsequent hypogonadotropic hypogonadism (114–116).
SSRIs and SNRIs can induce sexual dysfunction through multiple mechanisms. As described above, activation of 5-HT2 receptors results in the central inhibition of sexual circuits likely due to decreased dopaminergic transmission (110). Inhibition of peripheral autonomic sympathetic and parasympathetic nervous systems can also contribute to the onset of sexual side effects. Finally, SSRIs can decrease production of nitric oxide by interfering with nitric oxide synthetase (111, 113, 117). Among SNRIs, the balance between the degree of serotonin and norepinephrine reuptake inhibition changes the impact of these drugs on sexual function (namely, the greater the inhibition of serotonin reuptake, the greater the incidence of sexual effects). Accordingly, in their meta-analysis Serretti and coll. found that venlafaxine, a potent serotonin reuptake inhibitor, is the SNRI with the highest risk of dysfunction (110).
Conversely, other antidepressants such as agomelatine (an analogue of melatonin which combines 5HT2C antagonism with melatonin receptors 1 and 2 agonism) (118), amineptine and bupropion (both dopamine reuptake inhibitors), nefazodone (a 5-HT2 receptor antagonist) and mirtazapine (an antagonist of central α2-adrenergic, 5-HT2 and 5-HT3 receptors) did not show a higher risk with respect to placebo (110).
Another meta-analysis including data from 63 studies (58 randomized controlled trials, 5 observational studies) with more than 26,000 patients confirmed that second-generation antidepressants are related to an increased risk of sexual dysfunction. Furthermore, male-specific analysis showed the highest risk with sertraline and paroxetine. Conversely, bupropion showed a statistically significantly lower risk of dysfunction than other second-generation antidepressants (119).
In a recent systematic review and meta-analysis considering only randomized controlled studies, Trinchieri and coll. evaluated the effect of second-generation antidepressants on the male sex cycle (113). The analysis included 22 studies and showed an overall increased odds ratio (OR) for decreased libido (OR=1.89), erectile dysfunction (OR=2.28) and ejaculatory dysfunction (OR=7.31) in patients treated with antidepressants compared with placebo, thus confirming the results of previous studies.
Interestingly, subgroup analysis for SSRIs and SNRIs showed that both classes of antidepressants were associated with higher OR of decreased libido and ejaculatory dysfunction with respect to placebo. Conversely, SNRIs but not SSRIs presented higher OR of erectile dysfunction, suggesting a specific effect of SNRIs on this phase of sexual cycle possibly related to dose-dependent noradrenaline-mediated vasoconstriction at peripheral sympathetic level (113).
Very recently, Winter and coll. performed a systematic literature search, including case studies, case reports, and clinical trials with the aim to assess the sexual impact of antidepressant treatments in patients with major depressive disorders (116). In addition to confirming the association between sexual dysfunction and therapy with SSRIs, SNRIs, TCAs and MAOI phenelzine, the study focused on some antidepressants having a more favourable profile on sexual function. Among these, vortioxetine (a 5-HT3, 5-HT7 and 5-HT1D receptor antagonist, 5-HT1B receptor partial agonist, 5-HT1A receptor agonist and serotonin transporter inhibitor) and vilazodone (a serotonin transporter inhibitor, 5-HT1A partial agonist with low affinity for 5-HT1D, 5-HT2A and 5-HT2C receptors), both approved for the treatment of major depressive disorders, were characterized by a low risk of sexual dysfunction, in particular at low doses. Finally, as previously described, the prevalence of sexual disorders in patients treated with bupropion and mirtazapine was similar to placebo (116).
Atypical (or second-generation) antipsychotics (AAPs) are frequently prescribed as augmenting agents in patients with major depression in case of incomplete clinical response to monotherapy with antidepressants (116). Similarly to the latter, also AAPs can induce sexual disorders - in particular erectile dysfunction, ejaculation disorders and reduced intensity of orgasm - with an overall prevalence rate of 54% (109). AAPs-induced sexual dysfunction is mainly related to the antagonism of these drugs for D2 and 5-HT2A receptors in the brain. In addition to harming the previously described direct central effects of dopamine in the mesolimbic area, blocking D2 receptors in the tuberoinfundibular pathway also increases prolactin levels, thus leading to an increase of opioid and GABA levels as well as a reduction of testosterone levels resulting in hypogonadotropic hypogonadism. Other possible mechanisms of action of AAPs include central alpha-adrenergic, anticholinergic and antihistaminergic effects, which can induce sedation and reduce peripheral vasodilatation (109, 113). Of note, the peripheral effect on adrenergic system, through blocking of apha1-adrenergic receptors, account for the onset of priapism, another sexual dysfunction frequently reported during treatment with these drugs, especially olanzapine and risperidone (120, 121). Meta-analytic studies showed that among AAPs, those most affecting prolactin secretion, such as risperidone, olanzapine and clozapine were associated with a higher risk of sexual side effects (40-60%), whereas quetiapine, aripiprazole, ziprasidone and perphenazine were associated with a lower risk (16-27%) (122, 123). In particular, risperidone (characterized by a high affinity for D2, α1- and α2-adrenergic receptors) and aripiprazole (partial D2 agonist with lower affinity for α1-adrenergic receptors) seem to be AAPs with greater and lesser risk of sexual dysfunction, respectively (113, 124).
Figure 1 summarizes the effects of antidepressant and antipsychotic drugs on sexual response.
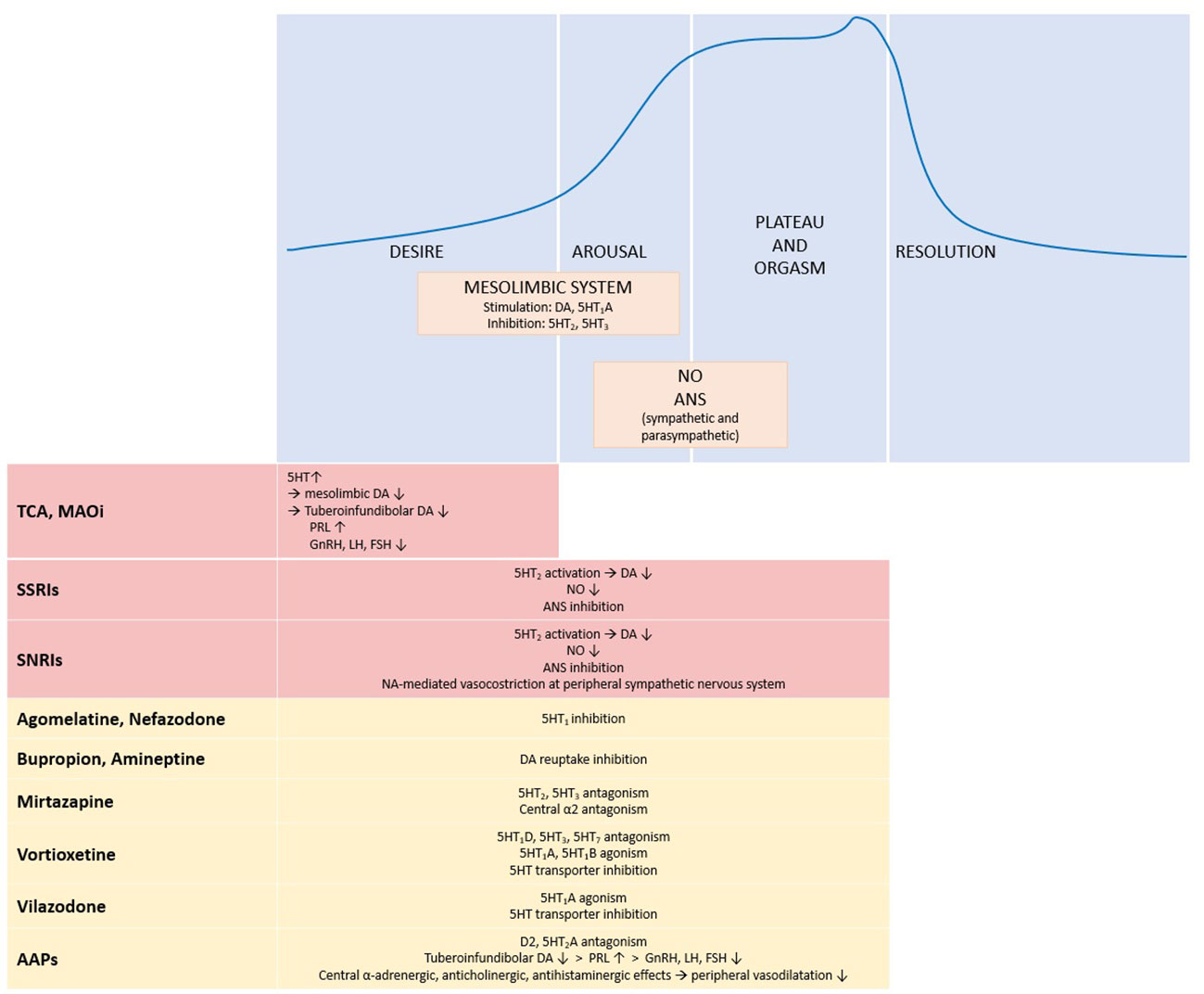
Figure 1 Physiology of sexual response and effects of antidepressant and antipsychotic drugs. The upper panel shows the four phases of sexual response and the main systems and neurotransmitters involved. The lower panel summarizes the effects of antidepressant and antipsychotic drugs on the different phases of the sexual response cycle: red boxes stand for inhibitory effects, yellow boxes for neutral effect. ↑, increase; ↓, decrease; 5HT, serotonin; 5HT1, serotonin receptor 1; 5HT1A, serotonin receptor 1A; 5HT1B, serotonin receptor 1B; 5HT1D, serotonin receptor 1D; 5HT2, serotonin receptor 2; 5HT3, serotonin receptor 3; 5HT7, serotonin receptor 7; α2, α2 adrenergic receptor; AAPs, atypical antipsychotics; ANS, autonomic nervous system; DA, dopamine; D2, dopamine receptor 2; FSH, follicle stimulating hormone; GnRH, gonadotropin releasing hormone; LH, luteinizing hormone; MAOi, monoamine oxidase inhibitors; NA, norepinephrine; NO, nitric oxide; PRL, prolactin; SNRIs, serotonin/norepinephrine reuptake inhibitors; SSRI, selective serotonin reuptake inhibitors; TCA, tricyclic antidepressants.
To date, only few placebo-controlled randomized clinical trials (RCTs) aimed to describe the effect of TRT on depressive symptoms have been performed.
In 2009 Zarrouf and coll. published a meta-analysis including 7 RCTs evaluating androgen replacement therapy in different patient populations with DSM-4-defined depressive disorders, ranging from dysthymia/minor depression to major depressive disorder (125). In particular, four trials used intramuscular testosterone therapy, one trial oral dehydroepiandrosterone (DHEA) and two testosterone gel. Moreover, 5 studies included patients with low levels of total testosterone, while 2 evaluated eugonadal subjects. Overall, meta-analysis showed a significant positive impact of androgen therapy on Hamilton Rating Scale for Depression response with respect to placebo. A subgroup analysis confirmed the beneficial effect of both TRT and DHEA on depressive symptoms, while no significant changes in eugonadal subjects were found (125).
A subsequent meta-analysis of Amanatkar and coll. selected 16 RCTs (944 patients) including subjects with and without hypogonadism evaluated for mood disorders through mixed questionnaires (126). Nine trials were performed in hypogonadal patients, 3 trials in subjects with normal testosterone levels, and 4 in both populations. Similarly to the aforementioned work, this meta-analysis also showed in the whole population a positive effect of testosterone on mood compared with placebo. Interestingly, subgroup analysis found significant effects in men younger than 60 years, in hypogonadal patients and in the case of subthreshold depression. On the contrary, testosterone therapy did not result effective in men older than 60 years, in eugonadal population and in patients with major depressive disorder (126).
Finally, in 2018 Walther and coll. summarized in their meta-analysis the results of 27 RCTs (1890 patients) including heterogenous populations with diverse medical conditions and mood disorders (127). While this study confirmed a moderate positive effect of testosterone treatment on depression compared with placebo, it also showed, in contrast to previous work, that initial testosterone status as well as age were not moderators of the effect of testosterone treatment on depressive symptoms.
In a setting where only patients with hypogonadism were considered, Elliott and coll. evaluated the effect of TRT on many outcomes, including quality of life, depression, sexual function, metabolic and adverse events (128). As far as depressive symptoms are concerned, the analysis of 12 RCTs (852 patients) showed that, compared with placebo, treatment with any TRT improved depression. However, no subgroup analysis based on different depressive disorders was carried out.
More recently, Vartolomei and coll. performed a systematic review with the aim to analyze the effect of TRT on depression and depressive symptoms in adult patients affected by late-onset hypogonadism (129). For the analysis, RCTs including at least 20 men with age >30 years and total testosterone levels <350 ng/ml, treated with TRT compared with placebo, were considered. Moreover, the Authors studied the impact of TRT on well-established pre-treatment depressive disorders as primary outcome, and its impact in patients without pre-treatment depression as secondary outcome. Similarly to the meta-analysis described above, TRT showed a positive impact on mood in patients with late-onset hypogonadism with clinical mild depression diagnosed before treatment. Moreover, TRT did not show a significant effect within the complex spectrum of major depressive disorder, in which TRT should probably be included in a multimodal therapeutic approach.
Finally, the same systematic review analysed the role of TRT in patients suffering from late-onset hypogonadism without clinically significant depression before treatment. Despite conflicting results between studies, the overall analysis suggested a beneficial effect of TRT compared with placebo, resulting in a reduction of depressive symptoms. However, clinical implication of this effect must be clarified as well (129).
The effects of TRT on depression have been investigated in few and relatively small-sized RCTs that were heterogeneous for study populations, testosterone doses and formulation, endocrinological and psychiatric eligibility criteria, intervention durations, and outcome ascertainment. Overall, they found TRT beneficial, with significant improvement among those patients with hypogonadism and clinical mild depression. On the other hand, in patients affected by major depression testosterone therapy seems to be ineffective. It is also important to consider that major depressive disorder is a complex disease, in which the neurobiological role of testosterone as well as the safety of TRT need to be further clarified, also considering previous suggestions on the relationship between testosterone levels and the course of bipolar disorders and suicidal behaviour (130).
Taking into account these evidences, recent guidelines recommended against the use of TRT with the sole purpose of improving major depressive symptoms in subjects with hypogonadism (1).
Several observations suggest a bidirectional influence between male hypogonadism and mood regulation, with each factor reinforcing the other. Epidemiological and observational studies highlight that men suffering from depression have lower circulating testosterone levels (17, 75, 76) and higher prevalence and greater severity of sexual symptoms (78, 95, 96, 98). On the other hand, men with hypogonadism can manifest depressive symptoms (6–10) or even receive a diagnosis of major depression (99) more frequently than eugonadal controls. However, the prevalence of functional hypogonadism in major depression, and that of major depression among hypogonadal men, are not currently known, and some studies have not confirmed this association (11, 82–85). It is noteworthy that available studies are largely heterogeneous with regard to population included (age, comorbidities, alcohol or substance abuse), definition of depression (questionnaires, DSM, ICD-9, prescription of antidepressants) and of male hypogonadism (total or bioavailable testosterone concentrations, and/or sexual dysfunction). Furthermore, if depression is a cause of functional hypogonadism, an improvement of sexual symptoms would be expected when patients are treated with anti-depressant medications. However, most psychotropic drugs are known to negatively affect sexual function and behaviour because of their specific mechanism of action.
Under a mechanistic perspective, several cerebral areas, involved in depression physiopathology, express the receptors and respond to HPT axis hormones (45, 54, 57). In vivo studies have shown that circulating levels of sex hormones correlate with grey matter volume, synaptic density (63–65), and functioning of critical regions (66–71). Moreover, animal and human studies have shown that administration of exogenous kisspeptin or GnRH can influence adrenergic and serotoninergic transmission, modulate activity of limbic areas and prefrontal cortex, and exert antidepressant effects (48–50, 72). However, the same areas directly receive neuronal fibers containing kisspeptin and GnIH/RFRP (44), express enzymes which metabolize sex hormones (e.g. aromatase) (56), and may even synthetize steroid hormones locally (so-called neurosteroids) (61). Therefore, the effect of HPT axis hormones on these cerebral circuits may add to local regulation. Moreover, other biological aspects, like polymorphisms of androgen receptor, may further complicate the relationship between circulating levels of sexual hormones and their actual effects on the nervous system.
Overall, some clinical suggestions emerge from available literature. Firstly, sexual dysfunction should be investigated in men suffering from depression. If sexual symptoms are present and reported as relevant to the patient’s quality of life, testosterone levels should be assessed. If a diagnosis of male hypogonadism is established (1–3), we suggest that TRT may be offered to the patient, and other sexual symptoms (e.g. erectile dysfunction) should be managed properly. If sexual complaints develop while on antidepressant treatment, switching to antidepressant or antipsychotic medications with lower risk of sexual dysfunction, could be considered (i.e. bupropion, mirtazapine, vortioxetine, vilazodone, aripiprazole) (116, 124). Conversely, the use of TRT with the sole purpose of improving depressive symptoms is not recommended according to current evidence (1). TRT should be periodically reassessed as, along with resolution of the depressive episode, testosterone discontinuation may be attempted. Moreover, in patients with bipolar disorder, testosterone therapy should be carefully weighed, as we hypothesize that testosterone treatment may increase the risk of manic episodes and suicide attempts (130), and presumably worsen hypersexuality symptoms.
On the other hand, mood disorders, anxiety, insomnia, memory impairment and reduced cognitive function should be evaluated in men diagnosed with hypogonadism before and after starting TRT, in order to assess the response of these symptoms to treatment, and to identify those conditions which deserve a specialist consultation.
Finally, larger longitudinal studies are needed to document changes in HPT axis functioning and sexual symptoms in men during depressive episodes and remission phases, to explore correlation of male hypogonadism with some physiopathological aspects like sleep disruption, hypothalamus-pituitary-adrenal axis hyperactivity, weight loss and psychomotor retardation, and, lastly, to better understand the clinical relevance of male hypogonadism in major depression.
RI, VL and EF conducted the literature search and wrote the manuscript. MA and GM critically revised the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by Ricerca Corrente funds from the Italian Ministry of Health to Fondazione IRCSS Ca' Granda Policlinico and partially supported by grant NET-2018-12365454 from the Italian Ministry of Health and Regione Lombardia.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Isidori AM, Aversa A, Calogero A, Ferlin A, Francavilla S, Lanfranco F, et al. Adult- and late-onset male hypogonadism: the clinical practice guidelines of the Italian Society of Andrology and Sexual Medicine (SIAMS) and the Italian Society of Endocrinology (SIE). J Endocrinol Invest (2022) 45(12):2385–403. doi: 10.1007/s40618-022-01859-7
2. Jayasena CN, Anderson RA, Llahana S, Barth JH, MacKenzie F, Wilkes S, et al. Society for endocrinology guidelines for testosterone replacement therapy in male hypogonadism. Clin Endocrinol (Oxf) (2022) 96(2):200–19. doi: 10.1111/cen.14633
3. Lunenfeld B, Mskhalaya G, Zitzmann M, Corona G, Arver S, Kalinchenko S, et al. Recommendations on the diagnosis, treatment and monitoring of testosterone deficiency in men. Aging Male Off J Int Soc Study Aging Male (2021) 24(1):119–38. doi: 10.1080/13685538.2021.1962840
4. Wu FCW, Tajar A, Beynon JM, Pye SR, Silman AJ, Finn JD, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med (2010) 363(2):123–35. doi: 10.1056/NEJMoa0911101
5. Corona G, Mannucci E, Ricca V, Lotti F, Boddi V, Bandini E, et al. The age-related decline of testosterone is associated with different specific symptoms and signs in patients with sexual dysfunction. Int J Androl (2009) 32(6):720–8. doi: 10.1111/j.1365-2605.2009.00952.x
6. Westley CJ, Amdur RL, Irwig MS. High rates of depression and depressive symptoms among men referred for borderline testosterone levels. J Sex Med (2015) 12(8):1753–60. doi: 10.1111/jsm.12937
7. Makhlouf AA, Mohamed MA, Seftel AD, Niederberger C, Neiderberger C. Hypogonadism is associated with overt depression symptoms in men with erectile dysfunction. Int J Impot Res (2008) 20(2):157–61. doi: 10.1038/sj.ijir.3901576
8. Hintikka J, Niskanen L, Koivumaa-Honkanen H, Tolmunen T, Honkalampi K, Lehto SM, et al. Hypogonadism, decreased sexual desire, and long-term depression in middle-aged men. J Sex Med (2009) 6(7):2049–57. doi: 10.1111/j.1743-6109.2009.01299.x
9. Shores MM, Sloan KL, Matsumoto AM, Moceri VM, Felker B, Kivlahan DR. Increased incidence of diagnosed depressive illness in hypogonadal older men. Arch Gen Psychiatry (2004) 61(2):162–7. doi: 10.1001/archpsyc.61.2.162
10. Ford AH, Yeap BB, Flicker L, Hankey GJ, Chubb SAP, Handelsman DJ, et al. Prospective longitudinal study of testosterone and incident depression in older men: the health in men study. Psychoneuroendocrinology (2016) 64:57–65. doi: 10.1016/j.psyneuen.2015.11.012
11. Ponholzer A, Madersbacher S, Rauchenwald M, Jungwirth S, Fischer P, Tragl KH. Serum androgen levels and their association to depression and Alzheimer dementia in a cohort of 75-year-old men over 5 years: results of the VITA study. Int J Impot Res (2009) 21(3):187–91. doi: 10.1038/ijir.2009.10
12. Lašaitė L, Ceponis J, Preikša RT, Zilaitienė B. Impaired emotional state, quality of life and cognitive functions in young hypogonadal men. Andrologia (2014) 46(10):1107–12. doi: 10.1111/and.12199
13. Aydogan U, Aydogdu A, Akbulut H, Sonmez A, Yuksel S, Basaran Y, et al. Increased frequency of anxiety, depression, quality of life and sexual life in young hypogonadotropic hypogonadal males and impacts of testosterone replacement therapy on these conditions. Endocr J (2012) 59(12):1099–105. doi: 10.1507/endocrj.EJ12-0134
14. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th. Arlington: American Psychiatric Publishing (2013). doi: 10.1176/appi.books.9780890425596.dsm05
15. Gonçalves WS, Gherman BR, Abdo CHN, Coutinho ESF, Nardi AE, Appolinario JC. Prevalence of sexual dysfunction in depressive and persistent depressive disorders: a systematic review and meta-analysis. Int J Impot Res (2022) 35(4):340–9. doi: 10.1038/s41443-022-00539-7
16. Fischer S, Ehlert U, Amiel Castro R. Hormones of the hypothalamic-pituitary-gonadal (HPG) axis in male depressive disorders - A systematic review and meta-analysis. Front Neuroendocrinol (2019) 55:100792. doi: 10.1016/j.yfrne.2019.100792
17. Barrett-Connor E, Von Mühlen DG, Kritz-Silverstein D. Bioavailable testosterone and depressed mood in older men: the Rancho Bernardo study. J Clin Endocrinol Metab (1999) 84(2):573–7. doi: 10.1210/jcem.84.2.5495
18. Giltay EJ, van der Mast RC, Lauwen E, Heijboer AC, de Waal MWM, Comijs HC. Plasma testosterone and the course of major depressive disorder in older men and women. Am J Geriatr Psychiatry Off J Am Assoc Geriatr Psychiatry (2017) 25(4):425–37. doi: 10.1016/j.jagp.2016.12.014
19. Höfer P, Lanzenberger R, Kasper S. Testosterone in the brain: neuroimaging findings and the potential role for neuropsychopharmacology. Eur Neuropsychopharmacol J Eur Coll Neuropsychopharmacol (2013) 23(2):79–88. doi: 10.1016/j.euroneuro.2012.04.013
20. Seftel AD. Male hypogonadism. Part I: epidemiology of hypogonadism. Int J Impot Res (2006) 18(2):115–20. doi: 10.1038/sj.ijir.3901397
21. Zenebe Y, Akele B, W/Selassie M, Necho M. Prevalence and determinants of depression among old age: a systematic review and meta-analysis. Ann Gen Psychiatry (2021) 20(1):55. doi: 10.1186/s12991-021-00375-x
22. Corona G, Rastrelli G, Maseroli E, Forti G, Maggi M. Sexual function of the ageing male. Best Pract Res Clin Endocrinol Metab (2013) 27(4):581–601. doi: 10.1016/j.beem.2013.05.007
23. Marques P, Skorupskaite K, Rozario KS, Anderson RA, George JT. Physiology of gnRH and gonadotropin secretion. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, et al, editors. Endotext. South Dartmouth (MA: MDText.com, Inc (2000). Available at: http://www.ncbi.nlm.nih.gov/books/NBK279070/.
24. O’Donnell L, Stanton P, de Kretser DM. Endocrinology of the male reproductive system and spermatogenesis. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, et al, editors. Endotext. South Dartmouth (MA: MDText.com, Inc (2000). Available at: http://www.ncbi.nlm.nih.gov/books/NBK279031/.
25. Grossmann M, Wittert GA. Dysregulation of the hypothalamic-pituitary-testicular axis due to energy deficit. J Clin Endocrinol Metab (2021) 106(12):e4861–71. doi: 10.1210/clinem/dgab517
26. Dornbush S, Aeddula NR. Physiology, leptin. In: StatPearls. Treasure Island (FL: StatPearls Publishing (2022). Available at: http://www.ncbi.nlm.nih.gov/books/NBK537038/.
27. Stewart R, Besset A, Bebbington P, Brugha T, Lindesay J, Jenkins R, et al. Insomnia comorbidity and impact and hypnotic use by age group in a national survey population aged 16 to 74 years. Sleep (2006) 29(11):1391–7. doi: 10.1093/sleep/29.11.1391
28. Mirchandaney R, Asarnow LD, Kaplan KA. Recent advances in sleep and depression. Curr Opin Psychiatry (2023) 36(1):34–40. doi: 10.1097/YCO.0000000000000837
29. Balasubramanian A, Kohn TP, Santiago JE, Sigalos JT, Kirby EW, Hockenberry MS, et al. Increased risk of hypogonadal symptoms in shift workers with shift work sleep disorder. Urology (2020) 138:52–9. doi: 10.1016/j.urology.2019.10.040
30. Eskelinen SI, Vahlberg TJ, Isoaho RE, Kivelä SL, Irjala KM. Associations of sex hormone concentrations with health and life satisfaction in elderly men. Endocr Pract Off J Am Coll Endocrinol Am Assoc Clin Endocrinol (2007) 13(7):743–9. doi: 10.4158/EP.13.7.743
31. Le HH, Salas RME, Gamaldo A, Billups KL, Dziedzic P, Choi S, et al. The utility and feasibility of assessing sleep disruption in a men’s health clinic using a mobile health platform device: A pilot study. Int J Clin Pract (2018) 72(1). doi: 10.1111/ijcp.12999
32. Sahu MK, Dubey RK, Chandrakar A, Kumar M, Kumar M. A systematic review and meta-analysis of serum and plasma cortisol levels in depressed patients versus control. Indian J Psychiatry (2022) 64(5):440–8. doi: 10.4103/indianjpsychiatry.indianjpsychiatry_561_21
33. Oakley AE, Breen KM, Clarke IJ, Karsch FJ, Wagenmaker ER, Tilbrook AJ. Cortisol reduces gonadotropin-releasing hormone pulse frequency in follicular phase ewes: influence of ovarian steroids. Endocrinology (2009) 150(1):341–9. doi: 10.1210/en.2008-0587
34. Kinsey-Jones JS, Li XF, Knox AMI, Wilkinson ES, Zhu XL, Chaudhary AA, et al. Down-regulation of hypothalamic kisspeptin and its receptor, Kiss1r, mRNA expression is associated with stress-induced suppression of luteinising hormone secretion in the female rat. J Neuroendocrinol (2009) 21(1):20–9. doi: 10.1111/j.1365-2826.2008.01807.x
35. Saketos M, Sharma N, Santoro NF. Suppression of the hypothalamic-pituitary-ovarian axis in normal women by glucocorticoids. Biol Reprod (1993) 49(6):1270–6. doi: 10.1095/biolreprod49.6.1270
36. Luton JP, Thieblot P, Valcke JC, Mahoudeau JA, Bricaire H. Reversible gonadotropin deficiency in male Cushing’s disease. J Clin Endocrinol Metab (1977) 45(3):488–95. doi: 10.1210/jcem-45-3-488
37. Boccuzzi G, Angeli A, Bisbocci D, Fonzo D, Giadano GP, Ceresa F. Effect of synthetic luteinizing hormone releasing hormone (LH-RH) on the release of gonadotropins in Cushing’s disease. J Clin Endocrinol Metab (1975) 40(5):892–5. doi: 10.1210/jcem-40-5-892
38. Bennabi D, Vandel P, Papaxanthis C, Pozzo T, Haffen E. Psychomotor retardation in depression: a systematic review of diagnostic, pathophysiologic, and therapeutic implications. BioMed Res Int (2013) 2013:158746. doi: 10.1155/2013/158746
39. Roshanaei-Moghaddam B, Katon WJ, Russo J. The longitudinal effects of depression on physical activity. Gen Hosp Psychiatry (2009) 31(4):306–15. doi: 10.1016/j.genhosppsych.2009.04.002
40. Riachy R, McKinney K, Tuvdendorj DR. Various factors may modulate the effect of exercise on testosterone levels in men. J Funct Morphol Kinesiol (2020) 5(4):81. doi: 10.3390/jfmk5040081
41. Fanni E, Castellini G, Corona G, Boddi V, Ricca V, Rastrelli G, et al. The role of somatic symptoms in sexual medicine: somatization as important contextual factor in male sexual dysfunction. J Sex Med (2016) 13(9):1395–407. doi: 10.1016/j.jsxm.2016.07.002
42. MacQueen G, Frodl T. The hippocampus in major depression: evidence for the convergence of the bench and bedside in psychiatric research? Mol Psychiatry (2011) 16(3):252–64. doi: 10.1038/mp.2010.80
43. Grogans SE, Fox AS, Shackman AJ. The amygdala and depression: A sober reconsideration. Am J Psychiatry (2022) 179(7):454–7. doi: 10.1176/appi.ajp.20220412
44. Parhar IS, Ogawa S, Ubuka T. Reproductive neuroendocrine pathways of social behavior. Front Endocrinol (2016) 7:28. doi: 10.3389/fendo.2016.00028
45. Mills EG, Izzi-Engbeaya C, Abbara A, Comninos AN, Dhillo WS. Functions of galanin, spexin and kisspeptin in metabolism, mood and behaviour. Nat Rev Endocrinol (2021) 17(2):97–113. doi: 10.1038/s41574-020-00438-1
46. Kasper S, Tauscher J, Willeit M, Stamenkovic M, Neumeister A, Küfferle B, et al. Receptor and transporter imaging studies in schizophrenia, depression, bulimia and Tourette’s disorder–implications for psychopharmacology. World J Biol Psychiatry Off J World Fed Soc Biol Psychiatry (2002) 3(3):133–46. doi: 10.3109/15622970209150614
47. Nathan FM, Ogawa S, Parhar IS. Kisspeptin1 modulates odorant-evoked fear response via two serotonin receptor subtypes (5-HT1A and 5-HT2) in zebrafish. J Neurochem (2015) 133(6):870–8. doi: 10.1111/jnc.13105
48. Tanaka M, Csabafi K, Telegdy G. Neurotransmissions of antidepressant-like effects of kisspeptin-13. Regul Pept (2013) 180:1–4. doi: 10.1016/j.regpep.2012.08.017
49. Umathe SN, Bhutada PS, Jain NS, Dixit PV, Wanjari MM. Effects of central administration of gonadotropin-releasing hormone agonists and antagonist on elevated plus-maze and social interaction behavior in rats. Behav Pharmacol (2008) 19(4):308–16. doi: 10.1097/FBP.0b013e328308f1fb
50. Umathe SN, Bhutada PS, Jain NS, Shukla NR, Mundhada YR, Dixit PV. Gonadotropin-releasing hormone agonist blocks anxiogenic-like and depressant-like effect of corticotrophin-releasing hormone in mice. Neuropeptides (2008) 42(4):399–410. doi: 10.1016/j.npep.2008.04.005
51. Michael RP, Rees HD. Autoradiographic localization of 3H-dihydrotestosterone in the preoptic area, hypothalamus, and amygdala of a male rhesus monkey. Life Sci (1982) 30(24):2087–93. doi: 10.1016/0024-3205(82)90450-7
52. Handa RJ, Connolly PB, Resko JA. Ontogeny of cytosolic androgen receptors in the brain of the fetal rhesus monkey. Endocrinology (1988) 122(5):1890–6. doi: 10.1210/endo-122-5-1890
53. Creutz LM, Kritzer MF. Mesostriatal and mesolimbic projections of midbrain neurons immunoreactive for estrogen receptor beta or androgen receptors in rats. J Comp Neurol (2004) 476(4):348–62. doi: 10.1002/cne.20229
54. Apostolinas S, Rajendren G, Dobrjansky A, Gibson MJ. Androgen receptor immunoreactivity in specific neural regions in normal and hypogonadal male mice: effect of androgens. Brain Res (1999) 817(1–2):19–24. doi: 10.1016/S0006-8993(98)01180-9
55. Sarchielli E, Comeglio P, Filippi S, Cellai I, Guarnieri G, Marzoppi A, et al. Neuroprotective effects of testosterone in the hypothalamus of an animal model of metabolic syndrome. Int J Mol Sci (2021) 22(4):1589. doi: 10.3390/ijms22041589
56. Biegon A, Kim SW, Alexoff DL, Jayne M, Carter P, Hubbard B, et al. Unique distribution of aromatase in the human brain: in vivo studies with PET and [N-methyl-11C]vorozole. Synap N Y N (2010) 64(11):801–7. doi: 10.1002/syn.20791
57. Osterlund MK, Gustafsson JA, Keller E, Hurd YL. Estrogen receptor beta (ERbeta) messenger ribonucleic acid (mRNA) expression within the human forebrain: distinct distribution pattern to ERalpha mRNA. J Clin Endocrinol Metab (2000) 85(10):3840–6. doi: 10.1210/jcem.85.10.6913
58. Fink G, Sumner B, Rosie R, Wilson H, McQueen J. Androgen actions on central serotonin neurotransmission: relevance for mood, mental state and memory. Behav Brain Res (1999) 105(1):53–68. doi: 10.1016/S0166-4328(99)00082-0
59. Bethea CL, Lu NZ, Gundlah C, Streicher JM. Diverse actions of ovarian steroids in the serotonin neural system. Front Neuroendocrinol (2002) 23(1):41–100. doi: 10.1006/frne.2001.0225
60. Vargas KG, Milic J, Zaciragic A, Wen KX, Jaspers L, Nano J, et al. The functions of estrogen receptor beta in the female brain: A systematic review. Maturitas (2016) 93:41–57. doi: 10.1016/j.maturitas.2016.05.014
61. Giatti S, Garcia-Segura LM, Barreto GE, Melcangi RC. Neuroactive steroids, neurosteroidogenesis and sex. Prog Neurobiol (2019) 176:1–17. doi: 10.1016/j.pneurobio.2018.06.007
62. Hadjimarkou MM, Vasudevan N. GPER1/GPR30 in the brain: Crosstalk with classical estrogen receptors and implications for behavior. J Steroid Biochem Mol Biol (2018) 176:57–64. doi: 10.1016/j.jsbmb.2017.04.012
63. Bramen JE, Hranilovich JA, Dahl RE, Forbes EE, Chen J, Toga AW, et al. Puberty influences medial temporal lobe and cortical gray matter maturation differently in boys than girls matched for sexual maturity. Cereb Cortex N Y N 1991 (2011) 21(3):636–46. doi: 10.1093/cercor/bhq137
64. Neufang S, Specht K, Hausmann M, Güntürkün O, Herpertz-Dahlmann B, Fink GR, et al. Sex differences and the impact of steroid hormones on the developing human brain. Cereb Cortex N Y N 1991 (2009) 19(2):464–73. doi: 10.1093/cercor/bhn100
65. Leranth C, Petnehazy O, MacLusky NJ. Gonadal hormones affect spine synaptic density in the CA1 hippocampal subfield of male rats. J Neurosci Off J Soc Neurosci (2003) 23(5):1588–92. doi: 10.1523/JNEUROSCI.23-05-01588.2003
66. Schöning S, Engelien A, Bauer C, Kugel H, Kersting A, Roestel C, et al. Neuroimaging differences in spatial cognition between men and male-to-female transsexuals before and during hormone therapy. J Sex Med (2010) 7(5):1858–67. doi: 10.1111/j.1743-6109.2009.01484.x
67. Volman I, Toni I, Verhagen L, Roelofs K. Endogenous testosterone modulates prefrontal-amygdala connectivity during social emotional behavior. Cereb Cortex N Y N 1991 (2011) 21(10):2282–90. doi: 10.1093/cercor/bhr001
68. Witte AV, Flöel A, Stein P, Savli M, Mien LK, Wadsak W, et al. Aggression is related to frontal serotonin-1A receptor distribution as revealed by PET in healthy subjects. Hum Brain Mapp (2009) 30(8):2558–70. doi: 10.1002/hbm.20687
69. Mueller SC, Mandell D, Leschek EW, Pine DS, Merke DP, Ernst M. Early hyperandrogenism affects the development of hippocampal function: preliminary evidence from a functional magnetic resonance imaging study of boys with familial male precocious puberty. J Child Adolesc Psychopharmacol (2009) 19(1):41–50. doi: 10.1089/cap.2008.031
70. Derntl B, Windischberger C, Robinson S, Kryspin-Exner I, Gur RC, Moser E, et al. Amygdala activity to fear and anger in healthy young males is associated with testosterone. Psychoneuroendocrinology (2009) 34(5):687–93. doi: 10.1016/j.psyneuen.2008.11.007
71. Manuck SB, Marsland AL, Flory JD, Gorka A, Ferrell RE, Hariri AR. Salivary testosterone and a trinucleotide (CAG) length polymorphism in the androgen receptor gene predict amygdala reactivity in men. Psychoneuroendocrinology (2010) 35(1):94–104. doi: 10.1016/j.psyneuen.2009.04.013
72. Comninos AN, Wall MB, Demetriou L, Shah AJ, Clarke SA, Narayanaswamy S, et al. Kisspeptin modulates sexual and emotional brain processing in humans. J Clin Invest (2017) 127(2):709–19. doi: 10.1172/JCI89519
73. Silvers JA, Wager TD, Weber J, Ochsner KN. The neural bases of uninstructed negative emotion modulation. Soc Cognit Affect Neurosci (2015) 10(1):10–8. doi: 10.1093/scan/nsu016
74. Seidman SN, Walsh BT. Testosterone and depression in aging men. Am J Geriatr Psychiatry (1999) 7(1):18–33. doi: 10.1097/00019442-199902000-00004
75. Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the massachusetts male aging study. J Clin Endocrinol Metab (2002) 87(2):589–98. doi: 10.1210/jcem.87.2.8201
76. Mazur A. Biosocial models of deviant behavior among male army veterans. Biol Psychol (1995) 41(3):271–93. doi: 10.1016/0301-0511(95)05138-4
77. Yesavage JA, Davidson J, Widrow L, Berger PA. Plasma testosterone levels, depression, sexuality, and age. Biol Psychiatry (1985) 20(2):222–5. doi: 10.1016/0006-3223(85)90088-5
78. Rizvi SJ, Kennedy SH, Ravindran LN, Giacobbe P, Eisfeld BS, Mancini D, et al. The relationship between testosterone and sexual function in depressed and healthy men. J Sex Med (2010) 7(2):816–25. doi: 10.1111/j.1743-6109.2009.01504.x
79. Amiaz R, Seidman SN. Testosterone and depression in men. Curr Opin Endocrinol Diabetes Obes (2008) 15(3):278–83. doi: 10.1097/MED.0b013e3282fc27eb
80. Mcintyre R, Mancini D, Eisfeld B, Soczynska J, Grupp L, Konarski J, et al. Calculated bioavailable testosterone levels and depression in middle-aged men. Psychoneuroendocrinology (2006) 31(9):1029–35. doi: 10.1016/j.psyneuen.2006.06.005
81. Smith JB, Rosen J, Colbert A. Low serum testosterone in outpatient psychiatry clinics: addressing challenges to the screening and treatment of hypogonadism. Sex Med Rev (2018) 6(1):69–76. doi: 10.1016/j.sxmr.2017.08.007
82. Levitt AJ, Joffe RT. Total and free testosterone in depressed men. Acta Psychiatr Scand (1988) 77(3):346–8. doi: 10.1111/j.1600-0447.1988.tb05132.x
83. Davies RH, Harris B, Thomas DR, Cook N, Read G, Riad-Fahmy D. Salivary testosterone levels and major depressive illness in men. Br J Psychiatry (1992) 161(5):629–32. doi: 10.1192/bjp.161.5.629
84. Rubin RT, Poland RE, Lesser IM. Neuroendocrine aspects of primary endogenous depression VIII. Pituitary-gonadal axis activity in male patients and matched control subjects. Psychoneuroendocrinology (1989) 14(3):217–29. doi: 10.1016/0306-4530(89)90020-6
85. de Wit AE, Giltay EJ, de Boer MK, Nolen WA, Bosker FJ, Penninx BWJH, et al. Plasma androgens and the presence and course of depression in a large cohort of men. Psychoneuroendocrinology (2021) 130:105278. doi: 10.1016/j.psyneuen.2021.105278
86. Turk MC, Bakker CJ, Spencer SM, Lofgren SM. Systematic review of sex differences in the relationship between hormones and depression in HIV. Psychoneuroendocrinology (2022) 138:105665. doi: 10.1016/j.psyneuen.2022.105665
87. Taxel P, Stevens MC, Trahiotis M, Zimmerman J, Kaplan RF. The effect of short-term estradiol therapy on cognitive function in older men receiving hormonal suppression therapy for prostate cancer. J Am Geriatr Soc (2004) 52(2):269–73. doi: 10.1111/j.1532-5415.2004.52067.x
88. Carnahan RM, Perry PJ. Depression in aging men: the role of testosterone. Drugs Aging (2004) 21(6):361–76. doi: 10.2165/00002512-200421060-00002
89. Sexual and reproductive health and research (SRH) . Available at: https://www.who.int/teams/sexual-and-reproductive-health-and-research/key-areas-of-work/sexual-health/defining-sexual-health.
90. Tan HM, Tong SF, Ho CCK. Men’s health: sexual dysfunction, physical, and psychological health—Is there a link? J Sex Med (2012) 9(3):663–71. doi: 10.1111/j.1743-6109.2011.02582.x
91. Makhlouf A, Kparker A, Niederberger CS. Depression and erectile dysfunction. Urol Clin North Am (2007) 34(4):565–74. doi: 10.1016/j.ucl.2007.08.009
92. Lau JTF, Kim JH, Tsui HY. Prevalence of male and female sexual problems, perceptions related to sex and association with quality of life in a Chinese population: a population-based study. Int J Impot Res (2005) 17(6):494–505. doi: 10.1038/sj.ijir.3901342
93. Walia AS, Lomeli L de JM, Jiang P, Benca R, Yafi FA. Patients presenting to a Men’s Health clinic are at higher risk for depression, insomnia, and sleep apnea. Int J Impot Res (2019) 31(1):39–45. doi: 10.1038/s41443-018-0057-z
94. Shabsigh R, Klein LT, Seidman S, Kaplan SA, Lehrhoff BJ, Ritter JS. Increased incidence of depressive symptoms in men with erectile dysfunction. Urology (1998) 52(5):848–52. doi: 10.1016/S0090-4295(98)00292-1
95. Araujo AB, Durante R, Feldman HA, Goldstein I, McKinlay JB. The relationship between depressive symptoms and male erectile dysfunction: cross-sectional results from the massachusetts male aging study. Psychosom Med (1998) 60(4):458–65. doi: 10.1097/00006842-199807000-00011
96. Takao T, Tsujimura A, Okuda H, Yamamoto K, Fukuhara S, Matsuoka Y, et al. Lower urinary tract symptoms and erectile dysfunction associated with depression among Japanese patients with late-onset hypogonadism symptoms. Aging Male (2011) 14(2):110–4. doi: 10.3109/13685538.2010.512374
97. Nicolosi A, Moreira ED, Villa M, Glasser DB. A population study of the association between sexual function, sexual satisfaction and depressive symptoms in men. J Affect Disord (2004) 82(2):235–43. doi: 10.1016/j.jad.2003.12.008
98. Howell J. Assessment of sexual function, interest and activity in depressed men. J Affect Disord (1987) 13(1):61–6. doi: 10.1016/0165-0327(87)90074-7
99. Korenman SG, Grotts JF, Bell DS, Elashoff DA. Depression in nonclassical hypogonadism in young men. J Endocr Soc (2018) 2(11):1306–13. doi: 10.1210/js.2018-00137
100. Burris AS, Banks SM, Carter CS, Davidson JM, Sherins RJ. A longterm, prospective study of the physiologic and behavioral effects of hormone replacement in untreated hypogonadal men. J Androl (1992) 13(4):297–304.
101. Wang C, Cunningham G, Dobs A, Iranmanesh A, Matsumoto AM, Snyder PJ, et al. Long-term testosterone gel (AndroGel) treatment maintains beneficial effects on sexual function and mood, lean and fat mass, and bone mineral density in hypogonadal men. J Clin Endocrinol Metab (2004) 89(5):2085–98. doi: 10.1210/jc.2003-032006
102. Wang C, Alexander G, Berman N, Salehian B, Davidson T, McDonald V, et al. Testosterone replacement therapy improves mood in hypogonadal men–a clinical research center study. J Clin Endocrinol Metab (1996) 81(10):3578–83. doi: 10.1210/jcem.81.10.8855804
103. Lašaitė L, Čeponis J, Preikša RT, Žilaitienė B. Effects of two-year testosterone replacement therapy on cognition, emotions and quality of life in young and middle-aged hypogonadal men. Andrologia (2017) 49(3):e12633. doi: 10.1111/and.12633
104. Shin D, Shim SR, Kim CH. Changes in Beck Depression Inventory scores in prostate cancer patients undergoing androgen deprivation therapy or prostatectomy. PloS One (2020) 15(6):e0234264. doi: 10.1371/journal.pone.0234264
105. Nead KT, Sinha S, Yang DD, Nguyen PL. Association of androgen deprivation therapy and depression in the treatment of prostate cancer: A systematic review and meta-analysis. Urol Oncol (2017) 35(11):664.e1–9. doi: 10.1016/j.urolonc.2017.07.016
106. Clayton AH, Alkis AR, Parikh NB, Votta JG. Sexual dysfunction due to psychotropic medications. Psychiatr Clin North Am (2016) 39(3):427–63. doi: 10.1016/j.psc.2016.04.006
107. Stahl SM. The psychopharmacology of sex, part 2: effects of drugs and disease on the 3 phases of human sexual response. J Clin Psychiatry (2001) 62(3):147–8. doi: 10.4088/JCP.v62n0301
108. Stahl SM. The psychopharmacology of sex, Part 1: Neurotransmitters and the 3 phases of the human sexual response. J Clin Psychiatry (2001) 62(2):80–1. doi: 10.4088/JCP.v62n0201
109. Just MJ. The influence of atypical antipsychotic drugs on sexual function. Neuropsychiatr Dis Treat (2015) 11:1655–61. doi: 10.2147/NDT.S84528
110. Serretti A, Chiesa A. Treatment-emergent sexual dysfunction related to antidepressants: A meta-analysis. J Clin Psychopharmacol (2009) 29(3):259–66. doi: 10.1097/JCP.0b013e3181a5233f
111. Bakr AM, El-Sakka AA, El-Sakka AI. Pharmaceutical management of sexual dysfunction in men on antidepressant therapy. Expert Opin Pharmacother (2022) 23(9):1051–63. doi: 10.1080/14656566.2022.2064218
112. Corona G, Ricca V, Bandini E, Mannucci E, Lotti F, Boddi V, et al. Selective serotonin reuptake inhibitor-induced sexual dysfunction. J Sex Med (2009) 6(5):1259–69. doi: 10.1111/j.1743-6109.2009.01248.x
113. Trinchieri M, Trinchieri M, Perletti G, Magri V, Stamatiou K, Cai T, et al. Erectile and ejaculatory dysfunction associated with use of psychotropic drugs: A systematic review. J Sex Med (2021) 18(8):1354–63. doi: 10.1016/j.jsxm.2021.05.016
114. Voicu V, Medvedovici A, Ranetti AE, Rădulescu FŞ. Drug-induced hypo- and hyperprolactinemia: mechanisms, clinical and therapeutic consequences. Expert Opin Drug Metab Toxicol (2013) 9(8):955–68. doi: 10.1517/17425255.2013.791283
115. Leucht C, Huhn M, Leucht S. Amitriptyline versus placebo for major depressive disorder. Cochrane Database Syst Rev (2012) 12:CD009138. doi: 10.1002/14651858.CD009138.pub2
116. Winter J, Curtis K, Hu B, Clayton AH. Sexual dysfunction with major depressive disorder and antidepressant treatments: impact, assessment, and management. Expert Opin Drug Saf (2022) 21(7):913–30. doi: 10.1080/14740338.2022.2049753
117. Angulo J, Peiró C, Sanchez-Ferrer CF, Gabancho S, Cuevas P, Gupta S, et al. Differential effects of serotonin reuptake inhibitors on erectile responses, NO-production, and neuronal NO synthase expression in rat corpus cavernosum tissue. Br J Pharmacol (2001) 134(6):1190–4. doi: 10.1038/sj.bjp.0704351
118. Stahl SM. Mechanism of action of agomelatine: a novel antidepressant exploiting synergy between monoaminergic and melatonergic properties. CNS Spectr (2014) 19(3):207–12. doi: 10.1017/S1092852914000248
119. Reichenpfader U, Gartlehner G, Morgan LC, Greenblatt A, Nussbaumer B, Hansen RA, et al. Sexual dysfunction associated with second-generation antidepressants in patients with major depressive disorder: results from a systematic review with network meta-analysis. Drug Saf (2014) 37(1):19–31. doi: 10.1007/s40264-013-0129-4
120. Pérez-Iglesias R, Mata I, Martínez-García O, Garcia-Unzueta MT, Amado JA, Valdizán EM, et al. Long-term effect of haloperidol, olanzapine, and risperidone on plasma prolactin levels in patients with first-episode psychosis. J Clin Psychopharmacol (2012) 32(6):804–8. doi: 10.1097/JCP.0b013e318272688b
121. Ginory A, Nguyen M. A case of priapism with risperidone. Case Rep Psychiatry (2014) 2014:241573. doi: 10.1155/2014/241573
122. Serretti A, Chiesa A. A meta-analysis of sexual dysfunction in psychiatric patients taking antipsychotics. Int Clin Psychopharmacol (2011) 26(3):130–40. doi: 10.1097/YIC.0b013e328341e434
123. Hunter RH, Joy CB, Kennedy E, Gilbody SM, Song F. Risperidone versus typical antipsychotic medication for schizophrenia. Cochrane Database Syst Rev (2003) 2):CD000440. doi: 10.1002/14651858.CD000440
124. Jayaram MB, Hosalli P. Risperidone versus olanzapine for schizophrenia. Cochrane Database Syst Rev (2005) 2):CD005237. doi: 10.1002/14651858.CD005237
125. Zarrouf FA, Artz S, Griffith J, Sirbu C, Kommor M. Testosterone and depression: systematic review and meta-analysis. J Psychiatr Pract (2009) 15(4):289–305. doi: 10.1097/01.pra.0000358315.88931.fc
126. Amanatkar HR, Chibnall JT, Seo BW, MaNepalli JN, Grossberg GT. Impact of exogenous testosterone on mood: a systematic review and meta-analysis of randomized placebo-controlled trials. Ann Clin Psychiatry Off J Am Acad Clin Psychiatr (2014) 26(1):19–32.
127. Walther A, Breidenstein J, Miller R. Association of testosterone treatment with alleviation of depressive symptoms in men: A systematic review and meta-analysis. JAMA Psychiatry (2019) 76(1):31–40. doi: 10.1001/jamapsychiatry.2018.2734
128. Elliott J, Kelly SE, Millar AC, Peterson J, Chen L, Johnston A, et al. Testosterone therapy in hypogonadal men: a systematic review and network meta-analysis. BMJ Open (2017) 7(11):e015284. doi: 10.1136/bmjopen-2016-015284
129. Vartolomei MD, Kimura S, Vartolomei L, Shariat SF. Systematic review of the impact of testosterone replacement therapy on depression in patients with late-onset testosterone deficiency. Eur Urol Focus (2020) 6(1):170–7. doi: 10.1016/j.euf.2018.07.006
Keywords: depression, male hypogonadism, testosterone, testosterone replacement therapy (TRT), antidepressants
Citation: Indirli R, Lanzi V, Arosio M, Mantovani G and Ferrante E (2023) The association of hypogonadism with depression and its treatments. Front. Endocrinol. 14:1198437. doi: 10.3389/fendo.2023.1198437
Received: 01 April 2023; Accepted: 21 July 2023;
Published: 10 August 2023.
Edited by:
Richard Ivell, University of Nottingham, United KingdomReviewed by:
Sarah Cipriani, University of Florence, ItalyCopyright © 2023 Indirli, Lanzi, Arosio, Mantovani and Ferrante. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rita Indirli, cml0YS5pbmRpcmxpQHVuaW1pLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.