- 1The First Clinical Medical College, Beijing University of Chinese Medicine, Beijing, China
- 2Department of Nephropathy, Beijing Hospital of Traditional Chinese Medicine, Capital Medical University, Beijing, China
- 3Center for Evidence-Based Medicine, Capital Institute of Pediatrics, Beijing, China
- 4Henan University of Chinese Medicine, Zhengzhou, China
Objective: We aimed to explore the association between serum complements and kidney function of diabetic kidney disease (DKD) in Chinese patients.
Methods: This is a retrospective study involving 2,441 participants. DKD was diagnosed according to the Kidney Disease: Improving Global Outcomes (KDIGO) categories. Participants were classified as stages G1-G5 by KDIGO glomerular filtration rate (GFR) categories. Effect sizes are expressed as odds ratio (OR) with 95% confidence interval (CI).
Results: After balancing age, gender, systolic blood pressure (SBP), hemoglobin A1c (HbA1C), serum triglyceride (TG), and urinary albumin-to-creatinine ratio (UACR) between the G2-G5 and control groups, per 0.1 g/L increment in serum complement C3 was significantly associated with a 27.8% reduced risk of DKD at G5 stage (OR, 95% CI, P: 0.722, 0.616-0.847, <0.001) relative to the G1 stage. Conversely, per 0.1 g/L increment in serum complement C4 was associated with an 83.0-177.6% increased risk of G2-G5 stage (P<0.001). Serum complement C1q was not statistically significant compared to controls at all stages prior to or after propensity score matching.
Conclusions: Our results indicate that high concentrations of serum C4 were associated with the significantly elevated risk of kidney function deterioration across all stages, and reduced serum C3 levels with an increased risk of DKD stage G5.
Introduction
Due to the high prevalence of diabetes, diabetic kidney disease (DKD) has drawn growing concerns, as it emerges as the foremost cause of end-stage kidney disease (ESKD) (1, 2). Despite significant progress in preventive care for diabetic individuals, the incidence of ESKD decreased at the slowest rate compared to complications such as lower limb amputation, acute myocardial infarction, stroke, and hyperglycemic crisis. It is worth noting that the number of deaths attributed to DKD increased by 94% (3). These observations underscore the urgent need to develop novel biomarkers capable of identifying individuals at high risk of deteriorating kidney function in DKD. Further, there is a pressing need to explore new agents that can directly target the pathways that contribute to the development of DKD.
It is widely recognized that various factors are involved in the onset and progression of DKD, but precise mechanisms underlying this disease have not been fully elucidated. It is a challenging task when diabetic individuals progress to the moderate or late stage of DKD. The complement system is a fundamental component of innate immunity and is involved in adaptive immunity, contributing to the clearance of immune complexes, cellular debris, and apoptotic cells. Nevertheless, inadequate regulation or excessive activation of the complement system can create a vicious cycle between the complement system, inflammatory cells, and tissue damage (4). Previous studies have mainly focused on the relationship between nephropathy and complements in membranous nephropathy (MN), IgA nephropathy, membranous proliferative glomerulonephritis (MPGN), and other conditions, and studies examining the involvement of the complement system in DKD have emerged in recent years, with compelling evidence demonstrating the activation of the complement system in DKD (5–9). While serum complements C3, C4, and C1q are widely available in clinical practice, studies examining the relationship between serum complements and kidney function in DKD patients are sparse in the literature and inadequate in sample sizes. To the best of our knowledge, this is the first study to simultaneously explore the association between three serum complements (C3, C4, and C1q) and kidney function in DKD patients. Therefore, to enhance clinical application, we examined the relationship between kidney function and serum complement levels in 2,441 patients with DKD and established a nomogram that can be applicable in clinical practice.
Methods
Study design
This retrospective study was approved by the Ethics Committee of Beijing Hospital of Traditional Chinese Medicine, Capital Medical University (2020BL02-062), in accordance with the principles of the Helsinki Declaration. As this study was retrospective in nature and all participants’ information was anonymized and de-identified prior to analysis, and the need for written informed consent was waived by the Committee.
Inclusion criteria
All study participants, ranging in age from 18 to 80 years, were hospitalized for DKD at the Beijing Hospital of Traditional Chinese Medicine Affiliated to Capital Medical University from January 2011 to January 2021.
Exclusion criteria
Initially, 4,903 inpatients with DKD were included. Among these, 2,462 patients were excluded for the following reasons: 1) undergoing dialysis or kidney transplantation; 2) having a definitive diagnosis of non-DKD; 3) experiencing diabetic ketoacidosis, hyperosmolar hyperglycemia coma, or other acute diabetic complications; 4) being complicated with severe primary diseases of the respiratory, digestive, blood systems, or other serious infections, or being found to have malignant tumors; 5) lacking necessary information, including serum creatinine, urinary albumin to creatinine ratio (UACR), and serum complement (C3, C4, C1q).
Ultimately, total 2,441 eligible patients with DKD were enrolled in the analysis, comprising 1,471 men and 970 women. Of these, 821 patients with an estimated glomerular filtration rate (eGFR) > 90 ml/min/1.73m2 were designated as the control group, while the remaining patients were classified as the case group.
Diagnosis of DKD
The 2012 KDIGO clinical practice guidelines provide a clear definition of DKD, which is characterized by an UACR ≥30 mg/g, or an eGFR <60 mL/min/1.73 m2 in the absence of signs or symptoms indicating other primary causes of kidney damage (10). The eGFR was calculated using the 2009 CKD Epidemiology Collaboration (CKD-EPI) equation (11).
Stages of DKD
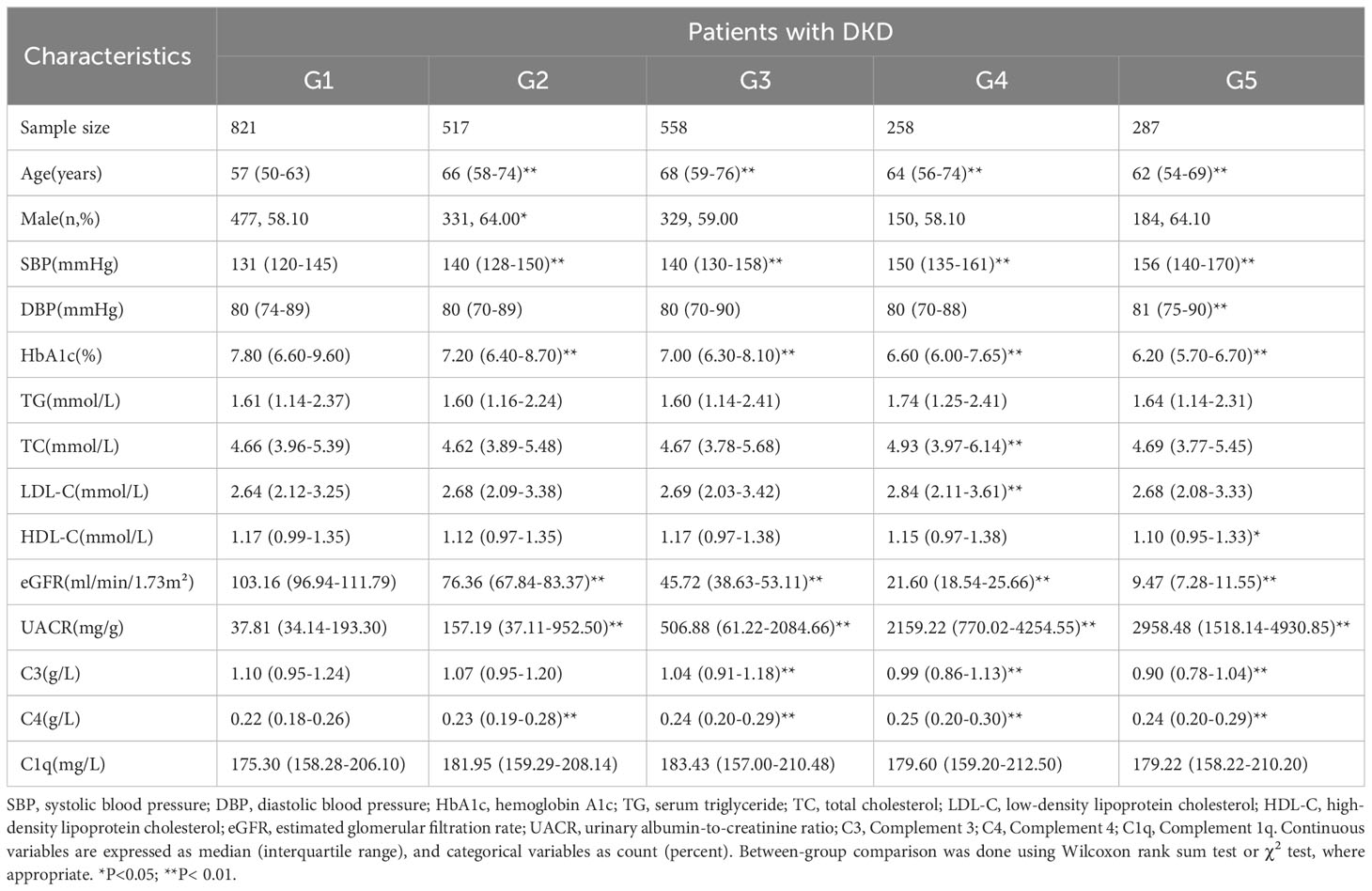
The KDIGO risk categories comprise two primary components: persistent albuminuria and GFR. The GFR categories are classified as follows: G1 (GFR in ml/min/1.73m2: ≥ 90), G2 (60-89), G3 (45-59), G3b (30-44), G4 (15-29), and G5 (≤ 15 or treatment by dialysis). Due to limited sample sizes, the G3a and G3b categories were combined into a single category, namely G3. The persistent albuminuria categories are classified as A1 (UACR in mg/g: ≤ 30), A2 (30-299), and A3 (≥ 300) (10). In the present study, patients with DKD were categorized into five stages based on the KDIGO GFR categories, including G1 (n=821), G2 (n=517), G3 (n=558), G4 (n=258), and G5 (n=287).
Clinical and biochemical indices
Data utilized in this study were extracted from the scientific research sharing platform (Yidu Cloud Research Collaboration Platform) of Beijing Hospital of Traditional Chinese Medicine affiliated to Capital Medical University. Standardized questionnaires were administered to all study participants to collect demographics and medical histories.
The definition of diabetes mellitus was based on the American Diabetes Association’s Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021 (12). Hypertension was defined as systolic blood pressure (SBP) of 140 mmHg or greater, diastolic blood pressure (DBP) of 90 mmHg or greater, or use of antihypertensive medication (13).
Laboratory tests were conducted on serum samples obtained by venipuncture from patients after an 8-hour fast. Serum was separated immediately after centrifugation within 1 hour of blood sampling. Serum complements were measured using immunotransmission turbidimetry, including serum C3, C4, and C1q. The reference ranges for serum C3, C4, and C1q were 0.75-1.40 (g/L), 0.10-0.40 (g/L), and 159.0-233.0 mg/L, respectively.
Creatinine concentrations were determined using the enzymatic method, while urine microalbumin was determined using the immunoturbidimetric method. Serum triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) concentrations were measured using an automated biochemical analyzer. HbA1c was determined using high-performance liquid chromatography.
All tests were conducted twice before reporting and were performed by trained laboratory staff at the Beijing Hospital of Traditional Chinese Medicine, Capital Medical University.
Statistical analysis
All statistical analyses were performed using STATA version 16 (StataCorp, College Station, TX, USA). Normally distributed continuous variables are presented as mean (standard deviation), skewed continuous variables as median (interquartile range), and categorical variables as count (percent). Differences between groups were evaluated using the χ2 test for categorical variables and the t test or Wilcoxon rank sum test for continuous variables. The association between complements and stages of kidney function in diabetes mellitus, before and after adjusting for confounders, was analyzed using logistic regression analysis, with effect sizes reported as odds ratio (OR) with 95% confidence interval (95% CI). To control for potential bias in group-based equivalents, propensity score matching (PSM) was employed. Age, gender, SBP, HbA1C, TG, and UACR were a priori balanced in PSM analysis. Age, SBP and UACR differed significantly among groups in unadjusted model. As reported, DKD progression differed in men and women (14). It is well-established that optimal glycemic control can reduce the risk of DKD progression (15, 16). Furthermore, published literature supports an association between TG levels and DKD progression (17, 18). The calibration was evaluated using the Akaike Information Criterion (AIC), Bayesian Information Criterion (BIC), and -2 log likelihood ratio tests. The discrimination was assessed by net reclassification improvement (NRI) and integrated differential improvement (IDI). A nomogram was constructed using the “RMS” package in the R programming environment (version 3.5.2). The statistical significance was set at a p-value of less than 0.05.
Results
Baseline characteristics
Table 1 presents the baseline characteristics of the study participants. The G2-G5 stages were observed to be older and had higher SBP and lower HbA1C levels compared to the G1 stage. Serum C3 was significantly lower in G3-G5 stages compared to the control group (P<0.01), whereas serum C4 levels were significantly higher in G2-G5 compared to the G1 group (P<0.01). However, no statistically significant difference was observed in serum C1q levels at any stage (P>0.05).
Serum complements and DKD stages
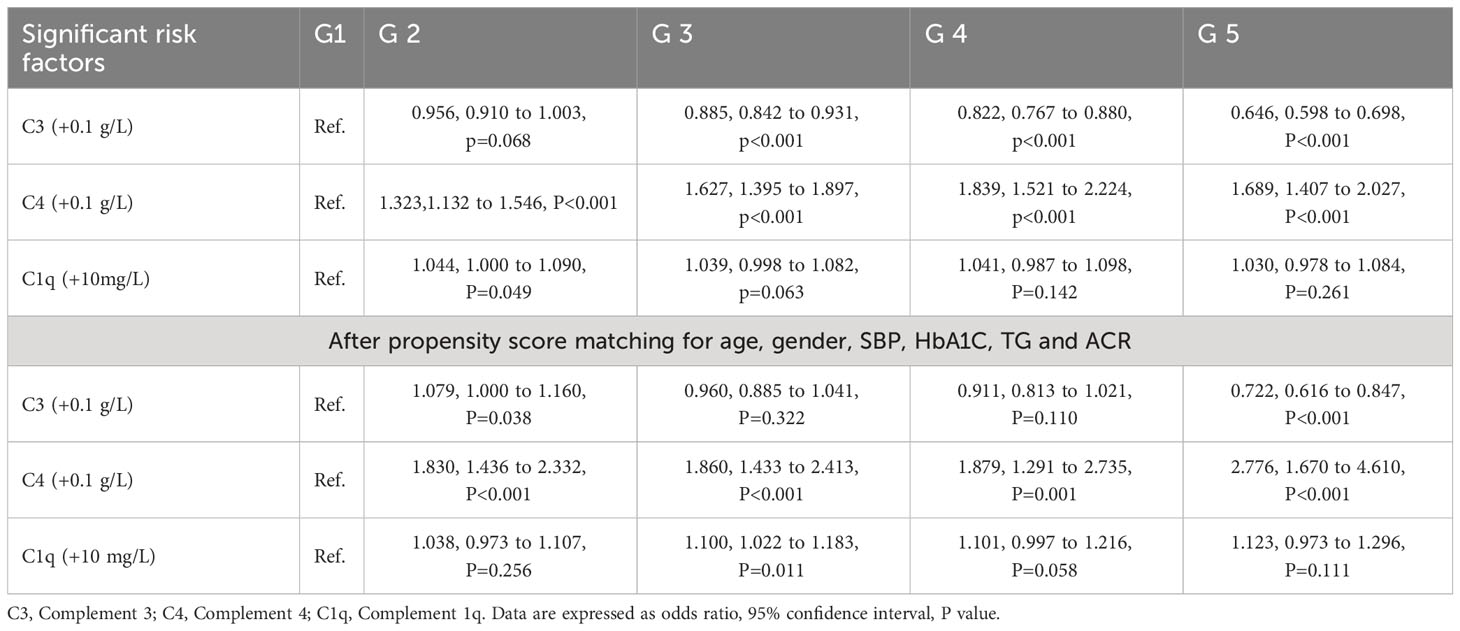
Table 2 displays the correlation between serum C3, C4, C1q at G2-G5 stages in patients with type 2 diabetes before and after PSM. Using the Bonferroni correction, a P-value less than 0.05/12 was considered significant. After balancing covariates including age, gender, SBP, HbA1C, TG, and UACR between the G2-G5 and control groups, per 0.1 g/L increment in serum C3 was significantly associated with a 27.8% reduced risk of DKD at G5 stage (OR, 95% CI, P: 0.722, 0.616-0.847, <0.001) relative to the G1 stage. Conversely, per 0.1 g/L increment in serum C4 was associated with an 83.0-177.6% increased risk of G2-G5 stage (P<0.001). Serum C1q was not statistically significant at all stages before or after PSM.
Prediction accuracy assessment
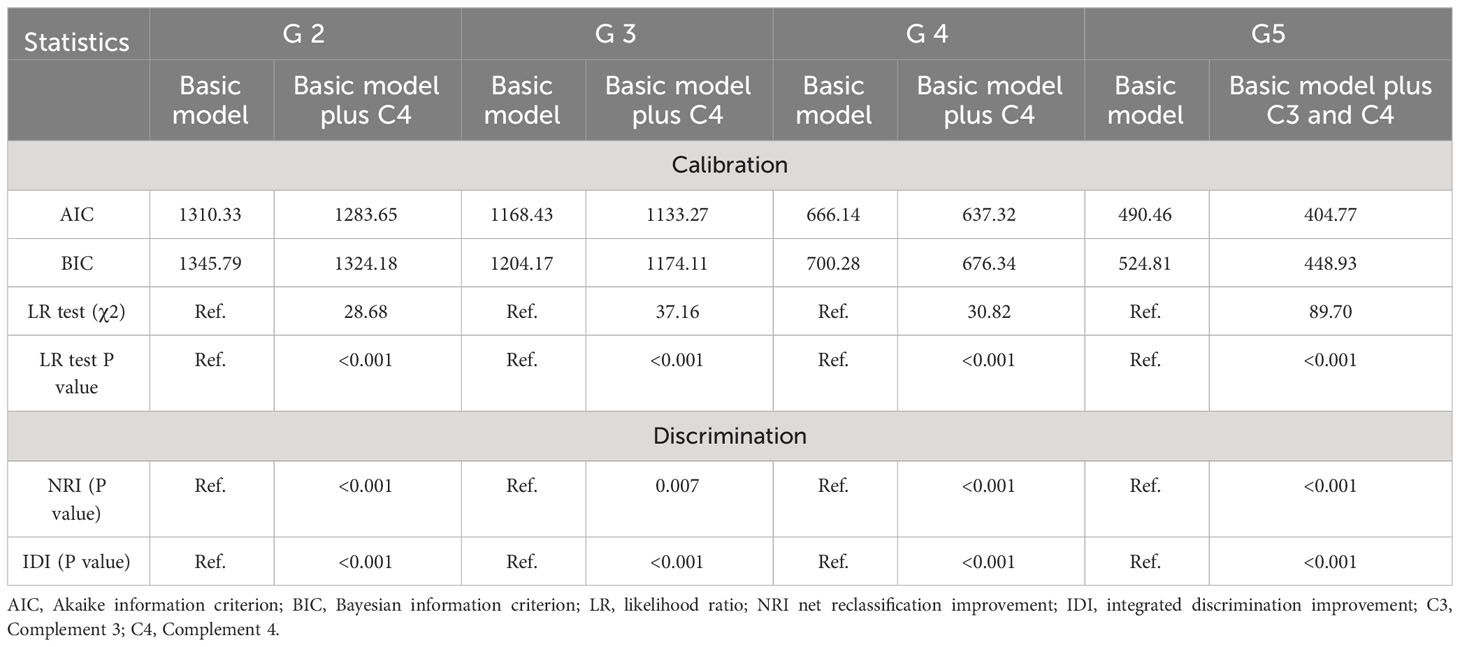
Table 3 illustrates the prediction accuracy achieved by separately adding serum complements to the basic model (including age, gender, SBP, HbA1C,TG and UACR). Using logistic regression analysis after PSM, only C4 was added to the basic model in G2-G4 stages, whereas both C3 and C4 were added to the basic model in G5 stage. Calibration analysis showed that the addition of complements to the basic model resulted in a reduction in both AIC and BIC statistics by more than 10 for each stage, and the likelihood ratio test indicated a statistically significant difference with added complements for all stages. Discrimination analysis using both NRI and IDI showed a significant improvement in prediction accuracy upon the addition (19).
Interaction of serum complements
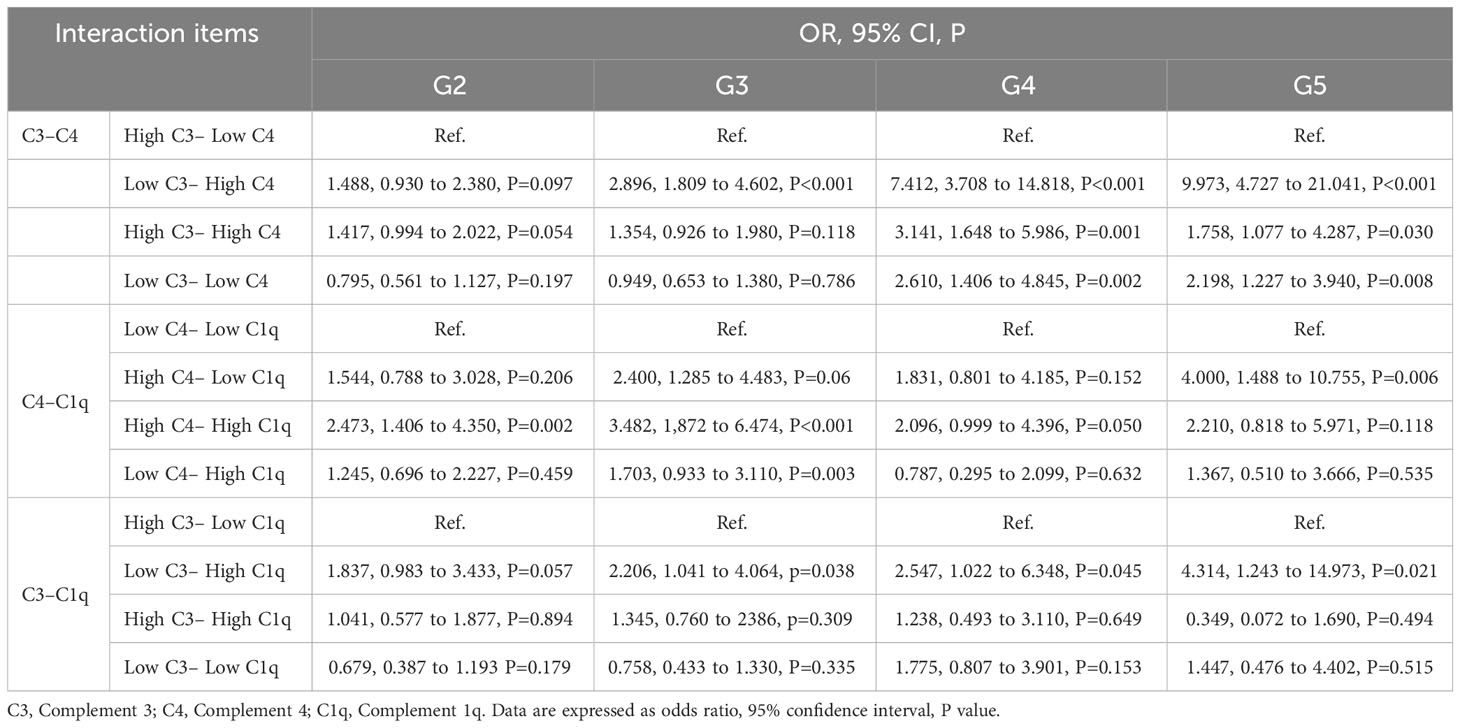
Table 4 presents the effect-size estimates for the interaction between complement and DKD kidney function. To synthesize 12 new variables, we binarized C3, C4, and C1q based on the median values of all study participants and divided them into high and low groups. To prevent false positives, we considered P<0.05/12 statistically significant according to the Bonferroni correction. According to the results, the combination of low C3 and high C4 demonstrated the maximum effect-size estimate in the G4-G5 stage (OR=7.412 and 9.973, 95%CL: 3.708-14.818 and 4.727-21.041, P<0.001).
Nomogram prediction model
To facilitate practical applications, we developed a nomogram prediction model for the DKD stages G2-G5, as depicted in Figure 1. Significant factors in the nomogram model were selected by forward logistic regression analyses at a significance level of 0.4%. The accuracy of the four nomogram models was satisfactory, with the C index values of 0.784, 0.876, 0.922, and 0.977 (all P<0.001).
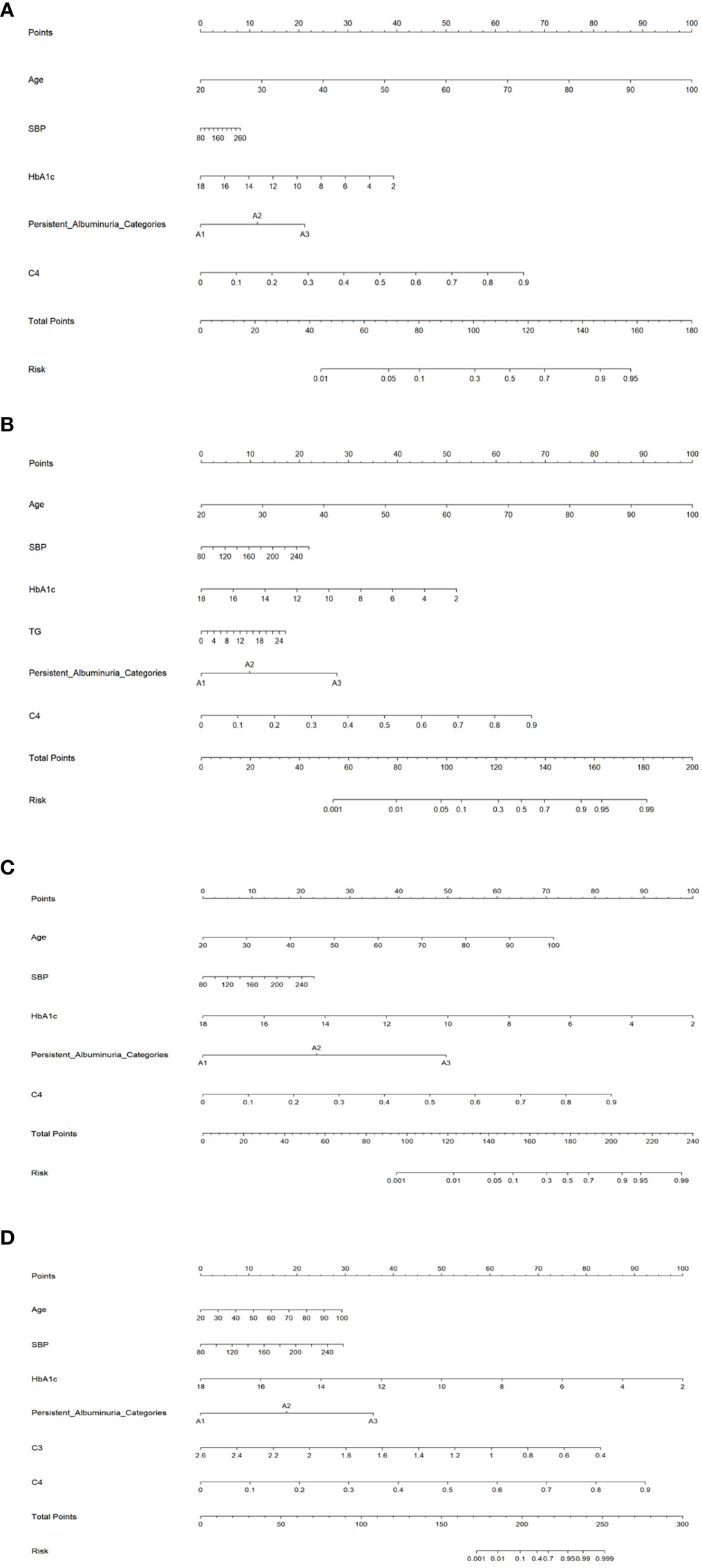
Figure 1 Nomogram prediction models for the G2 (A), G3 (B), G4 (C), and G5 (D) stages. SBP, systolic blood pressure; HbA1c, hemoglobin A1c; TG, serum triglyceride; C3, Complement 3; C4, Complement 4.
Discussion
The primary aim of this study was to investigate the relationship between serum complements and kidney function levels in patients with DKD. To our knowledge, this was the first study that has explored this relation using the KDIGO GFR categories. Our results indicate that high concentrations of serum C4 were associated with the significantly elevated risk of kidney function deterioration across all stages. Whereas reduced serum C3 levels were associated to an increased risk of DKD stage G5. These results suggest that serum complements may serve as valuable indicators for worsening kidney function in patients with type 2 diabetes.
A substantial body of evidence supports the involvement of the complement system in DKD progression. Among these components, C3, a crucial element of the three complement activation pathways, is well evidenced in support of its contributing role. C3 deposits in the glomeruli of DN mice from early stages, and levels of C3a and the C3a receptor (C3aR) are elevated (20). The inflammatory response and T cell adaptive immunity in C3aR knockout mice are significantly suppressed, which alleviates diabetic kidney injury (20). Treatment of DN mice with C3aR antagonists increases podocyte density and maintains their phenotype, thereby limiting proteinuria and glomerular damage (21). Patients with C3 deposition exhibit higher interstitial fibrosis and tubular atrophy (IFTA) scores and a greater proportion of global sclerosis compared to those without C3 deposition, and complement deposition incidence is higher in advanced DN than in early DN (20, 22). Additionally, it has been discovered that C3a and C5a receptor antagonists can alleviate glomerular fibrosis in DKD patients by improving the endothelial-myofibroblast transition by inhibiting the Wnt/β-catenin signaling pathway (23). C4d deposits in both glomeruli and arterioles, and C1q deposits in both glomerular hili and arterioles are increased compared to the non-DN group. Among them, glomerular C4d deposition correlates with DN severity, while others do not (24). Furthermore, patients with C4c plus C3c and C1q deposits experience heavier proteinuria than those with C4c plus only one of the deposits or those with DN without C4c deposits (25). These studies collectively suggest that the complement system is implicated in the development of renal pathologies in diabetic patients and is a promising target for inhibiting DKD progression.
Several studies have explored the correlation between serum complement and DKD. A recent study found that decreased serum C3 levels and increased serum C4 levels are significantly associated with more severe kidney dysfunction and worse renal outcomes in DN patients (26), which aligns with our findings. However, a relevant exploration of serum C1q was not performed in this study. While there was no significant difference in serum C4 between DN patients with and without C4c deposition, and the median GFR of the higher serum C4 group was lower, but the difference in GFR between the two groups did not achieve statistical significance, possibly due to the insufficient sample size (25). Regarding the plausible mechanisms of serum complements in patients with DKD, many publications related to various forms of renal dysfunction reported that circulatory C3 level decreased and renal tissue deposition of C3 increased, which may be related to abnormal activation of complement C3 leading to the excessive consumption and cleavage of C3 (26–29). Interestingly, it has been shown that not all DN patients had C3 deposition and that C3 deposition was significantly more in late DN than early DN, suggesting that complement activation may be an aggravating factor rather than a causative factor (27, 29). In our study, low serum C3 had the most significant association with the G5 stage of DKD, supporting the above argument. While that the increment in serum C4 may be affected by other factors than renal C4 deposition, such as widespread micro-inflammation in patients with DKD (27). However, the involvement of C1q may be relatively small.
Another notable finding of this study is that serum complements may interact with each other to increase the risk of renal deterioration in DKD, particularly in DKD stages 4-5, where low serum C3 and high serum C4 demonstrate a significant synergistic interaction, far exceeding other combinations. We must acknowledge that our findings are preliminary, and the complex intrinsic associations between complements warrant further exploration.
To facilitate clinical application, we developed a nomogram prediction model for kidney function grading in DKD based on statistical significance and conventional attributes. Importantly, the model exhibits decent prediction accuracy, which can assist in clinical decision-making and personalized management of DKD.
It is worth mentioning that patients with advanced DKD often show heterogeneity in glycemic control. In our study, HbA1c in the G5 group of DKD was significantly better than that in the G3 and G4 groups, and the main reason may be that the low GFR of DKD patients in the G5 group led to the decreased ability of the kidneys to expel insulin and hypoglycemic drugs, and the decrease of renal gluconogenesis (30, 31). Also, assessing HbA1c control in patients with advanced DKD may be biased by abnormalities in blood haemoglobin (30).
Several limitations of this study must be acknowledged. First, the cross-sectional design constrains the inference of any temporal relationship and cannot be used to establish a causal relationship between serum complements and kidney function in DKD. Second, all study participants were recruited from a single center, which may have led to population stratification and necessitates additional external validation. Furthermore, certain participant characteristics, such as obesity and insulin secretion levels, were not available, which may confound the association.
Conclusion
Despite these limitations, our study, with its large sample size, indicates that serum C4 can serve as a biomarker of kidney function deterioration in DKD, serum C3 as a biomarker of advanced DKD, and that there may be a synergistic effect between serum C4 and C3 in worsening kidney function in DKD. At present, numerous studies are being conducted on inhibitors of complement components (such as C1, C3, C5, and C5aR1), and the inhibition of pathological complement activation has emerged as a potential therapy to prevent DKD progression. Therefore, we establish background data to further investigate the role of complements in the pathogenesis of DKD and to explore their potential as an adjuvant therapy to control DKD progression.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The Ethics Committee of Beijing Hospital of Traditional Chinese Medicine, Capital Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because This study was retrospective in nature and all participants’ information was anonymized and de-identified prior to analysis.
Author contributions
Y-XW and W-JZ planned and designed the study. W-QN directed its implementation and drafted the protocol. Y-FW, YM, G-MZ, ZC, CS, X-GZ, Y-ZC, and M-DW contributed to data acquisition. Y-FW, M-CL and Y-LW obtained statutory and ethics approvals. M-CL and J-LL conducted statistical analyses. W-JZ, W-QN and Y-XW did the quality control. M-CL and J-LL wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Capital’s Funds for Health Improvement and Research, China (2020–3–2235) and Capital’s Funds for Health Improvement and Research, China (2022–4–1162). Additionally, it was also supported by the Scientific Research Cultivation Program of Beijing Municipal Hospitals, China [PZ2022022].
Acknowledgments
The authors are grateful to all the patients who participated in this study and to all those who help them to gather patients’ information.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med (2019) 380(24):2295–306. doi: 10.1056/NEJMoa1811744
2. Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol (2017) 12(12):2032–45. doi: 10.2215/CJN.11491116
3. Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet (2012) 380(9867):628. doi: 10.1016/S0140-6736(12)61728-0
4. Ricklin D, Reis ES, Lambris JD. Complement in disease: a defence system turning offensive. Nat Rev Nephrol (2016) 12(7):383–401. doi: 10.1038/nrneph.2016.70
5. Flyvbjerg A. The role of the complement system in diabetic nephropathy. Nat Rev Nephrol (2017) 13(5):311–8. doi: 10.1038/nrneph.2017.31
6. Budge K, Dellepiane S, Yu SM, Cravedi P. Complement, a therapeutic target in diabetic kidney disease. Front Med (Lausanne) (2021) 7:599236. doi: 10.3389/fmed.2020.599236
7. Tang SCW, Yiu WH. Innate immunity in diabetic kidney disease. Nat Rev Nephrol (2020) 16(4):206–22. doi: 10.1038/s41581-019-0234-4
8. Pelletier K, Bonnefoy A, Chapdelaine H, Pichette V, Lejars M, Madore F, et al. Clinical value of complement activation biomarkers in overt diabetic nephropathy. Kidney Int Rep (2019) 4(6):797–805. doi: 10.1016/j.ekir.2019.03.004
9. Li MR, Sun ZJ, Chang DY, Yu XJ, Wang SX, Chen M, et al. C3c deposition predicts worse renal outcomes in patients with biopsy-proven diabetic kidney disease in type 2 diabetes mellitus. J Diabetes (2022) 14(4):291–7. doi: 10.1111/1753-0407.13264
10. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl (2013) 3:1–150.
11. Casal MA, Ivy SP, Beumer JH, Nolin TD. Effect of removing race from glomerular filtration rate-estimating equations on anticancer drug dosing and eligibility: a retrospective analysis of National Cancer Institute phase 1 clinical trial participants. Lancet Oncol (2021) 22(9):1333–40. doi: 10.1016/S1470-2045(21)00377-6
12. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2021. Diabetes Care (2021) 44(Suppl 1):S15–33. doi: 10.2337/dc21-S002
13. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the american college of cardiology/american heart association task force on clinical practice guidelines. Hypertension (2017) 71(6):e13–e115. doi: 10.1161/HYP.0000000000000065
14. Fernandez-Fernandez B, Mahillo I, Sanchez-Rodriguez J, Carriazo S, Sanz AB, Sanchez-Niño MD, et al. Gender, albuminuria and chronic kidney disease progression in treated diabetic kidney disease. J Clin Med (2020) 9(6):1611. doi: 10.3390/jcm9061611
15. Ueki K, Sasako T, Okazaki Y, Miyake K, Nangaku M, Ohashi Y, et al. Multifactorial intervention has a significant effect on diabetic kidney disease in patients with type 2 diabetes. Kidney Int (2021) 99(1):256–66. doi: 10.1016/j.kint.2020.08.012
16. Ruospo M, Saglimbene VM, Palmer SC, De Cosmo S, Pacilli A, Lamacchia O, et al. Glucose targets for preventing diabetic kidney disease and its progression. Cochrane Database Syst Rev (2017) 6(6):CD010137. doi: 10.1002/14651858.CD010137.pub2
17. Yun KJ, Kim HJ, Kim MK, Kwon HS, Baek KH, Roh YJ, et al. Risk factors for the development and progression of diabetic kidney disease in patients with type 2 diabetes mellitus and advanced diabetic retinopathy. Diabetes Metab J (2016) 40(6):473–81. doi: 10.4093/dmj.2016.40.6.473
18. Zhang B, Wan Y, Zhou X, Zhang H, Zhao H, Ma L, et al. Characteristics of serum metabolites and gut microbiota in diabetic kidney disease. Front Pharmacol (2022) 13:872988. doi: 10.3389/fphar.2022.872988
19. Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology (2010) 21(1):128–38. doi: 10.1097/EDE.0b013e3181c30fb2
20. Li XQ, Chang DY, Chen M, Zhao MH. Deficiency of C3a receptor attenuates the development of diabetic nephropathy. BMJ Open Diabetes Res Care (2019) 7(1):e000817. doi: 10.1136/bmjdrc-2019-000817
21. Morigi M, Perico L, Corna D, Locatelli M, Cassis P, Carminati CE, et al. C3a receptor blockade protects podocytes from injury in diabetic nephropathy. JCI Insight (2020) 5(5):e131849. doi: 10.1172/jci.insight.131849
22. Jiang S, Di D, Jiao Y, Zou G, Gao H, Li W, et al. Complement deposition predicts worsening kidney function and underlines the clinical significance of the 2010 renal pathology society classification of diabetic nephropathy. Front Immunol (2022) 13:868127. doi: 10.3389/fimmu.2022.868127
23. Li L, Chen L, Zang J, Tang X, Liu Y, Zhang J, et al. C3a and C5a receptor antagonists ameliorate endothelial-myofibroblast transition via the Wnt/β-catenin signaling pathway in diabetic kidney disease. Metabolism (2015) 64(5):597–610. doi: 10.1016/j.metabol.2015.01.014
24. Bus P, Chua JS, Klessens CQF, Zandbergen M, Wolterbeek R, van Kooten C, et al. Complement activation in patients with diabetic nephropathy. Kidney Int Rep (2017) 3(2):302–13. doi: 10.1016/j.ekir.2017.10.005
25. Duan S, Sun L, Nie G, Chen J, Zhang C, Zhu H, et al. Association of glomerular complement C4c deposition with the progression of diabetic kidney disease in patients with type 2 diabetes. Front Immunol (2020) 11:2073. doi: 10.3389/fimmu.2020.02073
26. Zhang J, Wang Y, Zhang R, Li H, Han Q, Guo R, et al. Implication of decreased serum complement 3 in patients with diabetic nephropathy. Acta Diabetol (2018) 55(1):31–9. doi: 10.1007/s00592-017-1060-4
27. Dong J, Peng T, Gao J, Jia X, Yan G, Wang Y. A pilot and comparative study between pathological and serological levels of immunoglobulin and complement among three kinds of primary glomerulonephritis. BMC Immunol (2018) 19(1):18. doi: 10.1186/s12865-018-0254-z
28. Kelly KJ, Liu Y, Zhang J, Dominguez JH. Renal C3 complement component: feed forward to diabetic kidney disease. Am J Nephrol (2015) 41(1):48–56. doi: 10.1159/000371426
29. Huang Y, Xu J, Wu X, Chen X, Bai X, Zhuang Y, et al. High expression of complement components in the kidneys of type 2 diabetic rats with diabetic nephropathy. Front Endocrinol (Lausanne) (2019) 10:459. doi: 10.3389/fendo.2019.00459
30. Ling J, Ng JKC, Chan JCN, Chow E. Use of continuous glucose monitoring in the assessment and management of patients with diabetes and chronic kidney disease. Front Endocrinol (Lausanne) (2022) 13:869899. doi: 10.3389/fendo.2022.869899
Keywords: serum complement, complement C3, complement C4, diabetic kidney disease, diabetes mellitus, nomogram
Citation: Liu M-c, Li J-l, Wang Y-f, Meng Y, Zheng G-m, Cai Z, Shen C, Wang M-d, Zhu X-g, Chen Y-z, Wang Y-l, Zhao W-j, Niu W-q and Wang Y-x (2023) Association between serum complements and kidney function in patients with diabetic kidney disease. Front. Endocrinol. 14:1195966. doi: 10.3389/fendo.2023.1195966
Received: 29 March 2023; Accepted: 30 October 2023;
Published: 17 November 2023.
Edited by:
Nehal Mohsen Elsherbiny, Mansoura University, EgyptReviewed by:
Manish Mishra, Mercer University School of Medicine, United StatesShivani Sharma, Medical University of the Americas – Nevis, United States
Copyright © 2023 Liu, Li, Wang, Meng, Zheng, Cai, Shen, Wang, Zhu, Chen, Wang, Zhao, Niu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wen-jing Zhao, emhhb3dlbmppbmdAYmp6aG9uZ3lpLmNvbQ==; Wen-quan Niu, bml1d2VucXVhbl9zaGNuQDE2My5jb20=; Yao-xian Wang, dGNtd3l4MTIzQDEyNi5jb20=
†These authors share first authorship
 Meng-chao Liu
Meng-chao Liu Jia-lin Li2†
Jia-lin Li2† Yue-fen Wang
Yue-fen Wang Yuan Meng
Yuan Meng Meng-di Wang
Meng-di Wang Wen-jing Zhao
Wen-jing Zhao Yao-xian Wang
Yao-xian Wang