
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 23 August 2023
Sec. Endocrinology of Aging
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1195888
This article is part of the Research Topic Serum Uric Acid, Vascular Aging, and Endocrine Comorbidities View all 12 articles
 Ho Suk Kang1†
Ho Suk Kang1† Na-Eun Lee2†
Na-Eun Lee2† Dae Myoung Yoo2
Dae Myoung Yoo2 Kyeong Min Han2
Kyeong Min Han2 Ji Yeon Hong3
Ji Yeon Hong3 Hyo Geun Choi4,5
Hyo Geun Choi4,5 Hyun Lim1
Hyun Lim1 Joo-Hee Kim6
Joo-Hee Kim6 Ji Hee Kim7
Ji Hee Kim7 Seong-Jin Cho8
Seong-Jin Cho8 Eun Sook Nam8
Eun Sook Nam8 Ha Young Park9
Ha Young Park9 Nan Young Kim10
Nan Young Kim10 Sung Uk Baek11
Sung Uk Baek11 Joo Yeon Lee11
Joo Yeon Lee11 Mi Jung Kwon12*
Mi Jung Kwon12*Objective: Accumulating evidence from other countries indicates potential associations between gout and cardiovascular diseases; however, the associations of gout with cardiovascular diseases, particularly stroke, ischemic heart disease, and heart failure, remain ambiguous in the Korean population. We hypothesized that individuals with gout are at a higher likelihood of stroke, ischemic heart disease, or heart failure. This study expands upon previous research by ensuring a comparable baseline between patient and control groups and analyzing 16 years of data derived from an extensive healthcare database.
Methods: We selected 22,480 patients with gout and 22,480 control individuals from the Korean National Health Insurance Service-Health Screening Cohort database (2002–2019), and matched them at a 1:1 ratio according to sex, age, income, and residence. A Cox proportional hazard model with weighted overlap was employed to examine the relationship between gout and the risk of stroke, ischemic heart disease, or heart failure after adjustment for several covariates.
Results: The incidences of stroke, ischemic heart disease, or heart failure in participants with gout were slightly higher than those in controls (stroke: 9.84 vs. 8.41 per 1000 person-years; ischemic heart disease: 9.77 vs. 7.15 per 1000 person-years; heart failure: 2.47 vs. 1.46 per 1000 person-years). After adjustment, the gout group had an 11% (95% confidence interval [CI] = 1.04–1.19), 28% (95% CI = 1.19–1.37), or 64% (95% CI = 1.41–1.91) higher likelihood of experiencing stroke, ischemic heart disease, or heart failure, respectively, than the control group.
Conclusion: The present findings suggest that individuals with gout in the Korean population, particularly those aged ≥ 60 years, were more likely to have stroke, ischemic heart disease, or heart failure.
Gout, a serious systemic and metabolic disorder causing joint inflammation, has been demonstrated to be associated with high uric acid levels (1). The accumulation of monosodium urate crystals in the joints could contribute to the development of comorbidities, such as obesity, insulin resistance, hypertension, kidney problems, and hyperlipidemia (1, 2). Gout is more prevalent in males than in females; the overall prevalence is 2.9–4.5 and peaks in individuals aged ≥ 80 years (3, 4). In recent years, the prevalence of gout has increased worldwide owing to an aging population, obesity, and metabolic diseases (5, 6). The prevalence rate of gout increased 5.15-fold from 0.39% in 2002 to 2.01% in 2015 in Korea, which resulted in a yearly average rise of 10.8% in health insurance costs related to gout (7). This is a larger increase compared with that in other countries; the prevalence of gout in the UK, USA, and Taiwan increased 1.64-times between 1997 and 2012 (5), 1.4-fold between 1988–1994 and 2007–2008 (3), and 1.12-fold between 2005 and 2010, respectively (4, 8). Owing to the increasing elderly population and lifestyle changes that have occurred in recent years, gout and its related problems have emerged as a noteworthy health concern in Korea (7).
Cardiovascular diseases (CVDs) are the principal source of mortality globally, representing approximately one-third of all fatalities (9). Particularly, CVDs are the main cause of death in East Asian countries, such as Taiwan, Singapore, Japan, and Korea, behind cancer (9). In Korea, a majority (70%), a large proportion (41%), and nearly a fifth (19%) of the adults have at least one, two or more, and three or more risk factors for CVDs, respectively, such as hypertension, diabetes, hypercholesterolemia, obesity, and smoking (10). Findings from recent experimental and epidemiologic studies suggest an elevated risk of CVDs associated with gout (11–13), which has drawn particular attention. The breakdown of purines yields serum uric acid, resulting in an inflammatory state and heightened risk of CVDs (14–16). Inflammation caused by monosodium urate crystals could potentially activate inflammasome pathways, cause gout attacks in joints, and result in deposits of coronary plaques, thereby contributing to the excessive cardiovascular risk in gout (17, 18), similar to the mechanism of action of cholesterol crystals (19). In addition, research has identified a correlation between gout and an increased probability of myocardial infarction (20, 21), stroke (22, 23), and atrial fibrillation (24), all of which are part of the broader category of cardiac conditions (25, 26). A cohort study revealed that gout was associated with a 1.49-fold greater risk of overall CVDs (95% confidence interval [CI]=1.44–1.53), without considering potential confounding factors, such as lifestyle, body mass index (BMI), alcohol intake, and smoking (11). Moreover, a meta-analysis has demonstrated an association of uric acid-lowering agents with decreased risk of myocardial infarction (27). Since the elderly have more comorbidities, such as CVDs and metabolic diseases, the effects of these systemic disorders on CVDs are anticipated to become more prominent (1). Indeed, these CVDs caused by gout have emerged as a major public health concern, which needs to be urgently addressed to reach the health goal of disease prevention.
The incidence and mortality rates of stroke have decreased, while the mortality and hospitalization rates of heart failure and ischemic heart disease have increased in Korea (9). The trends may vary depending on the type of CVDs and gout (9). Nevertheless, few studies have investigated the potential relationship between gout and stroke, ischemic heart disease, or heart failure in the Korean population. Moreover, merely one study has explored uric acid-lowering agent-associated abnormalities in different CVDs, and the findings are conflicting (28). Examining the effect of gout on each CVD is essential since CVDs are a varied group of diseases with diverse causes but similar vascular risks (25). Therefore, additional studies considering the potential mutual confounding factors are necessary.
We hypothesized that individuals with gout might be more likely to have stroke, ischemic heart disease, and heart failure. This study builds on earlier work (28) by carefully balancing the baseline characteristics between the patient and control cohorts and examining 16 years of data compiled from a comprehensive healthcare database.
This study was approved by the ethics committee of Hallym University (2019-10-023), and the need for written informed consent was waived by the Institutional Review Board. All the analyses were performed in accordance with the rules and regulations of the ethics committee of Hallym University.
The Korean National Health Insurance Service-Health Screening Cohort (KNHIS-HSC) database provides Korean population-based and longitudinal information for research needs, which is selected in an arbitrary manner (29, 30). Since 1999, the KNHIS has been providing obligatory health insurance to nearly all Koreans. The KNHIS-HSC comprises anonymous and de-identified data and information. The diagnostic codes used in the KNHIS-HSC are based on the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM).
This longitudinal follow-up study retrospectively examined a gout group and a control group without a history of gout to determine the effect of gout on the probability of experiencing a stroke, ischemic heart disease, or heart failure. Participants with ICD-10 code M10 (gout) were identified from a total of 514,866 participants aged ≥ 40 years with 895,300,177 medical claim codes who had at least two clinic visits from 2002 to 2019 (n=27,313). To select participants who were diagnosed with gout for the first time, those who were diagnosed with osteoporosis in 2002 (1-year washout period, n=2470), did not have any documents of blood pressure level (n=1), or were diagnosed with stroke, ischemic heart disease, or heart failure before their gout diagnosis (n=2362) were all excluded.
Participants in the control group (n=487,553) did not match those in the gout group from 2002 to 2019, and 13,809 individuals with ICD-10 code M10 (gout) were excluded from the control group.
Matching was performed using a 1:1 ratio in terms of sex, age, economic status, and residence region to reduce variations in the basic demographic and medical characteristics between gout and comparison groups. To avoid selection bias, individuals without gout were randomly selected in numerical order. The index date for each participant with gout was the day when her/his gout diagnosis (M10) was initially logged in the medical insurance database. The index date for a control participant was the same as that for the matched participant with gout. All the participants in the comparison groups who died or had cardiovascular diseases before the index date were excluded. In total, 451,264 control participants were eliminated in the matching process. Finally, 22,480 participants with gout were paired with 22,480 control participants (Figure 1).
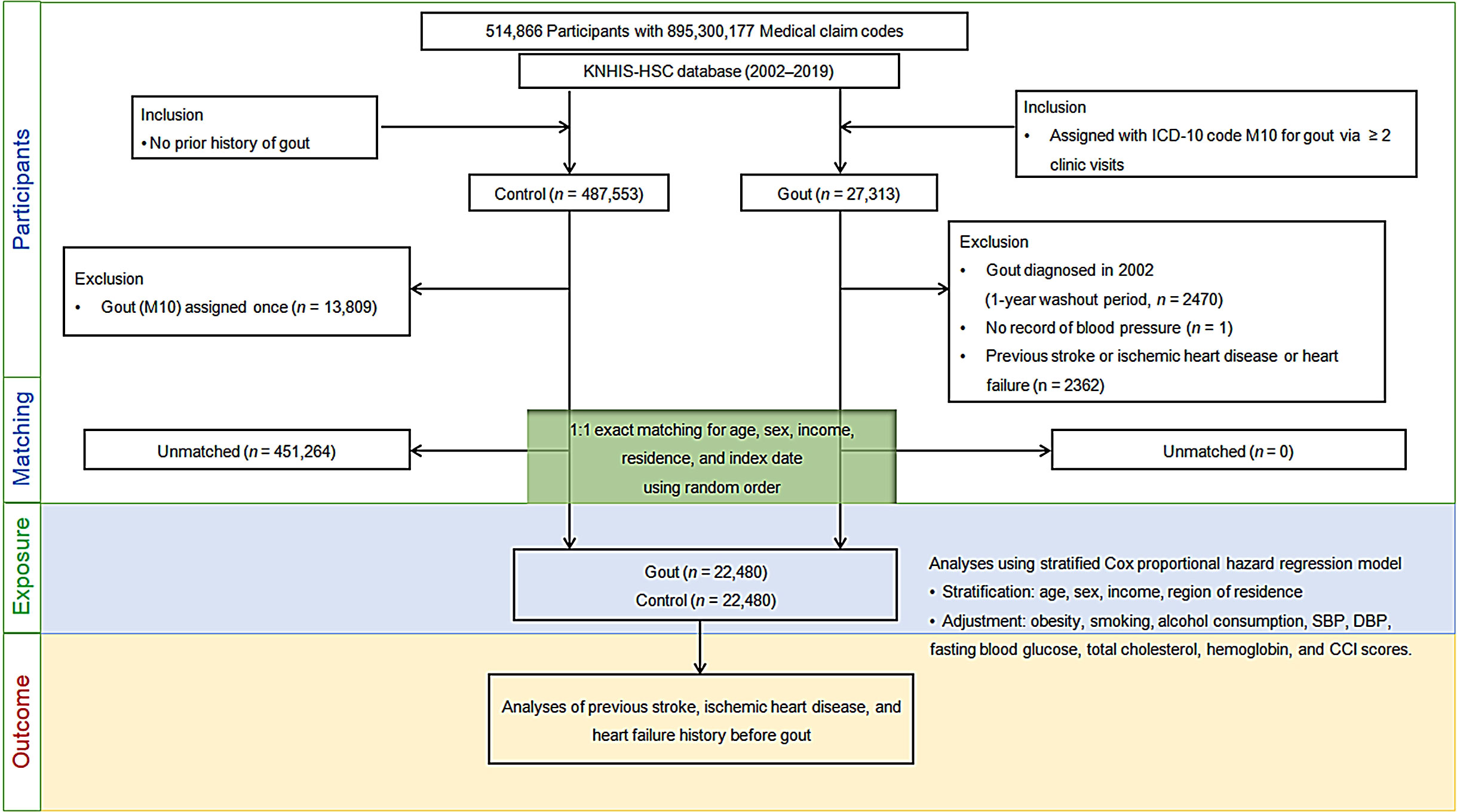
Figure 1 Flow of participant selection. Of the total 514,866 participants, 22,480 individuals with gout were paired with age, sex, financial status, and residential area-matched 22,480 controls. ICD-10, International classification of disease-10; CCI, Charlson comorbidity index; SBP, Systolic blood pressure; DBP, Diastolic blood pressure.
We examined the number of fresh diagnoses for stroke, ischemic heart disease, and heart failure based on ICD-10 labels in both gout and comparison groups from the index date to the end of 2019.
The study specified gout as a condition that had been either diagnosed or managed at least twice using ICD-10 codes (M10), as described previously (31, 32).
We included only the participants who had been hospitalized for at least two days or who had died owing to any of the following diseases: stroke (ICD-10 codes I60–I69), ischemic heart disease (I20–I25), or heart failure (I50). This selection criterion has been reported previously (33).
The 10 age groups ranged from 40–44 to ≥85 years in 5-year increments. Income classes were classified into 5 categories, with classes 1 and 5 having the lowest and highest salaries, respectively. The region of residence was classified as urban (Seoul, Busan, Daegu, Incheon, Gwangju, Daejeon, and Ulsan) and rural (Gangwon, Gyeongsangnam, Chungcheongbuk, Jeollabuk, Chungcheongnam, Jeollanam, Gyeongsangbuk, Gyeonggi, and Jeju) areas (34). In addition, participants were classified into current smokers, past smokers, or non-smokers based on smoking status. Alcohol consumption was divided into two categories based on consumption frequency (<1 time a week or ≥1 time a week). Obesity was classified into five categories as <18.5 (underweight), ≥ 18.5 to < 23 (normal), ≥ 23 to < 25 (overweight), ≥ 25 to < 30 (obese I), and ≥ 30 (obese II) using BMI (kg/m2) based on the Asia-Pacific criteria and the Western Pacific Regional Office 2000 (35).
Hemoglobin (g/dL), systolic blood pressure (SBP, mmHg), fasting blood glucose (mg/dL), diastolic blood pressure (DBP, mmHg), and total cholesterol (mg/dL) were also measured. The Charlson Comorbidity Index (CCI) was used to quantify the burden of 17 comorbidities (acute myocardial infarction, congestive heart failure, peripheral vascular disease, cerebral vascular accident, dementia, pulmonary disease, connective tissue disorder, peptic ulcer, liver disease, diabetes, diabetes complications, paraplegia, renal disease, cancer, metastatic cancer, severe liver disease, and HIV) (36, 37). The CCI calculated for these comorbidities was summed as the continuous variable (0 [no comorbidities] to 29 [multiple comorbidities]) (36, 37). Cerebrovascular disease, acute myocardial infarction, and congestive heart failure were excluded when calculating the CCI score (36, 37).
Categorical data are expressed as percentages, and continuous data were summarized as means and standard deviations; the standardized difference was employed to compare the rate of general characteristics between the cohort sets. We employed propensity score overlap weighting to maintain the covariate balance and optimal sample size to diminish the probability of intergroup bias. We employed multivariable logistic regression to calculate the propensity score and subsequently used these scores to calculate the overlap weighting. The participants with gout were weighted using the probability of the propensity score, whereas the control participants were weighted with the probability of 1- propensity score, ranging from 0 to 1. The standardized differences before and after weighting were compared to examine the difference in general characteristics between gout and control groups. To assess the accuracy of matching, absolute standardized differences of the covariates before and after matching were compared, with ≤ 0.20 being considered an appropriate balance. The Cox proportional hazard model was used to adjust covariates with an absolute standardized difference of > 0.20 (38).
The Kaplan–Meier analysis and the log-rank test were employed to compare the collective risk of stroke, ischemic heart disease, and heart failure between gout and control groups. A Cox proportional hazard regression model with an overlap weight was used to determine the hazard ratios (HRs) and 95% CIs of gout in relation to stroke, ischemic heart disease, and heart failure. Both the crude (simple) and adjusted (for obesity, smoking, alcohol consumption, SBP, DBP, fasting blood glucose, total cholesterol, hemoglobin, and CCI scores) results were generated. The analyses were stratified based on factors, such as age, sex, income, and region of residence. Subgroup analyses were performed based on age (< 60 and ≥ 60 years) or sex (males and females). Statistical assessments were performed using two-tailed tests, and p-values of <0.05 were considered statistically significant. All the analyses were conducted using the SAS 9.4 software (SAS Institute Inc., Cary, NC, USA).
This study included 22,480 people diagnosed with gout between 2003 and 2019 and an equivalent number of age, sex, income, and region of residence-matched comparison participants. Table 1 summarizes the baseline characteristics of both groups before and after an overlap-weighting adjusted PS matching procedure. The two groups were exactly alike in terms of demographic characteristics (standardized difference=0), except for the proportion of obese participants; the gout group had a higher percentage of obese participants than the control group (69.16% vs. 60.15%). However, after the overlap weighting adjustment process, the standardized differences of all the covariates were minimized, and the two groups were balanced (standardized difference ≤0.2).
In gout and control groups, stroke, ischemic heart disease, and heart failure occurred with incidence rates of 9.84 vs. 8.41, 9.77 vs. 7.15, and 2.47 vs. 1.46 per 1000 person-years, respectively; all the differences were statistically significant. The Cox proportional hazard analysis revealed that those with gout were 1.11, 1.28, and 1.64 times more likely to experience subsequent stroke, ischemic heart disease, and heart failure, respectively, than controls in the adjusted models (95% CI=1.04–1.19, p=0.003; 95% CI= 1.19–1.37, p<0.001; and 95% CI=1.41–1.91, p<0.001, respectively) over 16 years, after considering the demographic, lifestyle, and medical factors (Table 2).
The Kaplan–Meier analysis and the log-rank test indicated that individuals with gout had a greater likelihood of experiencing stroke, ischemic heart disease, and heart failure than those in the non-gout group (all p <0.0001; Figures 2A–C).
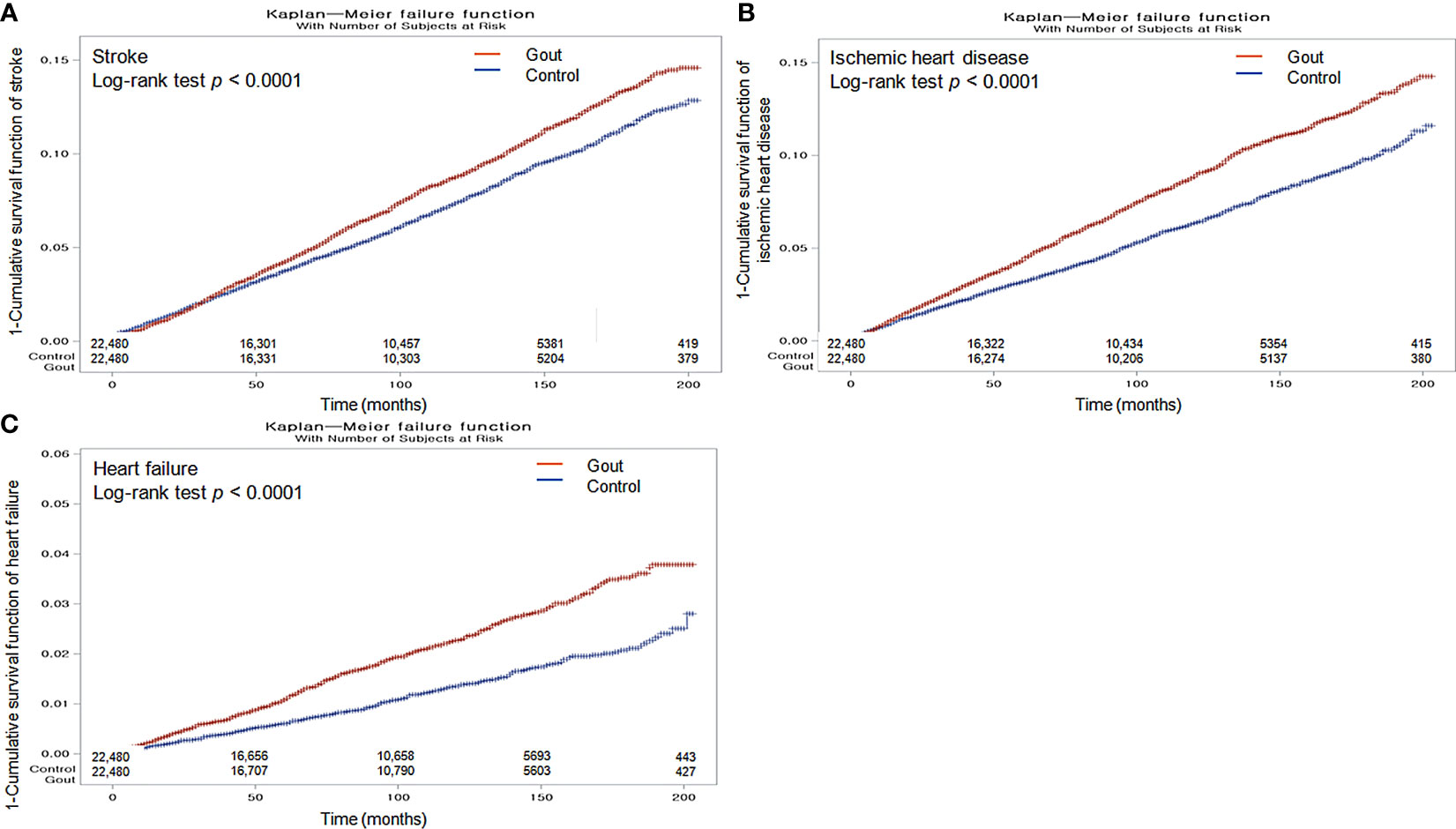
Figure 2 Kaplan-Meier probability of the incidence of stroke (A), ischemic heart disease (B), and heart failure (C) in gout and the control populations within 16 years of the index date.
We further categorized the cohorts by sex and age to ascertain the potential relationship between gout and further incidence of stroke, ischemic heart disease, and heart failure. Stratification analyses showed that the threat of subsequent stroke was prominently augmented in participants with gout aged ≥ 60 years (HR=1.10, 95% CI=1.01–1.20, p=0.022), as well as in male (HR=1.09, 95% CI=1.01–1.18, p=0.036) and female (HR=1.22, 95% CI=1.03–1.44, p=0.018) patients (Figure 3A, Supplementary Table 1).
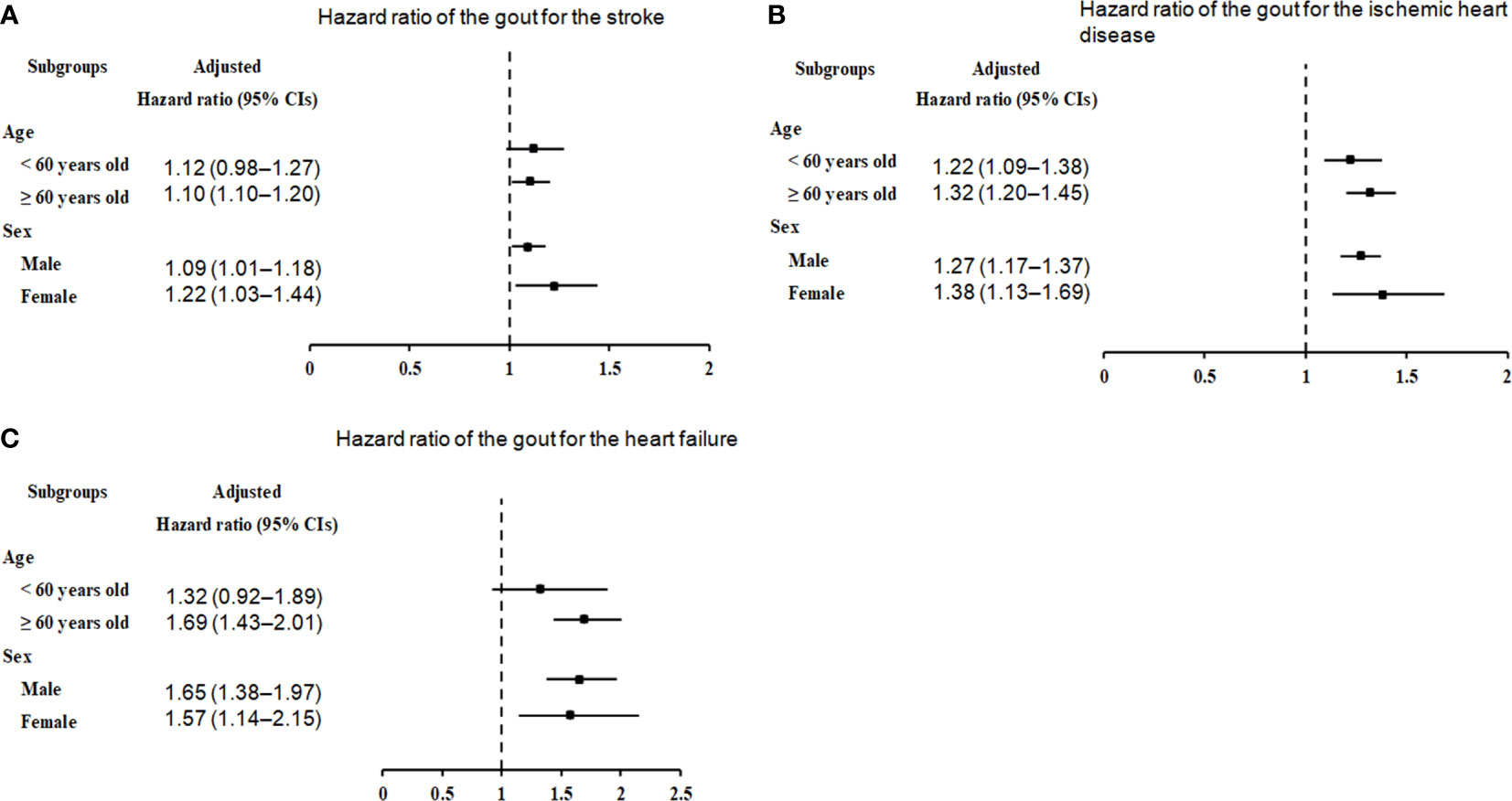
Figure 3 Forest plots for hazard ratios (95% confidence intervals [CI]) for the probability of stroke (A), ischemic heart disease (B), and heart failure (C) based on age and sex.
In the subgroup analyses by ischemic heart disease (Figure 3B, Supplementary Table 2), irrespective of age (< 60 years: HR=1.22, 95% CI=1.09–1.38, p<0.001; ≥ 60 years: HR=1.32, 95% CI=1.20–1.45, p<0.001) and sex (males: HR=1.27, 95% CI=1.17–1.37, p<0.001; males: HR=1.38, 95% CI=1.13–1.69, p=0.002), the correlation between gout and ischemic heart disease remained conspicuous.
In the subgroup analyses by heart failure (Figure 3C; Supplementary Table 3), the presence of gout was associated with an unremarkable but steady increase in the probability of heart failure in individuals aged ≥ 60 years (HR=1.69, 95% CI=1.43–2.01, p<0.001), as well as in men (HR=1.65, 95% CI=1.38–1.97, p<0.001) or women (HR=1.57, 95% CI=1.14–2.15, p=0.005).
Based on a large sample of Korean adults, this longitudinal study, considering the demographic data and pre-existing medical conditions, demonstrated that individuals with gout were slightly more likely to experience stroke, ischemic heart disease, or heart failure over the 16 years of observation. We identified a small but statistically-significant increased incidence of stroke, ischemic heart disease, and heart failure among individuals with gout compared with those without (stroke: 9.84 vs. 8.41 per 1000 person-years; ischemic heart disease: 9.77 vs. 7.15 per 1000 person-years; and heart failure: 2.47 vs. 1.46 per 1000 person-years). The weighted Cox proportional hazard analysis, adjusted for factors including age, sex, economic status, anemia, hypertension, hyperlipidemia, hyperglycemia, obesity, smoking, alcohol consumption, and comorbidities, also confirmed that individuals with gout were 11% (95% CI=1.04–1.19), 28% (95% CI=1.19–1.37), and 64% (95% CI=1.41–1.91) more susceptible to stroke, ischemic heart disease, and heart failure, respectively, than those without gout. This study with a long-term follow-up period provides further evidence that gout is an independent risk factor for CVDs, as demonstrated in a previous study (39). Thus, individuals with gout should be provided with extra information and training to ensure that they are aware of the potential risks of CVDs.
Our findings are consistent with those of a long-term survey that employed the National Health Insurance Research Database of Taiwan, which has a health insurance plan similar to that of Korea; 15,690 patients with gout and 246,210 controls were included in that survey, and the overall risk of heart diseases, including ischemic heart disease and heart failure, and the risk of stroke in the gout group were respectively 1.57 (95% CI=1.52–1.63) and 1.32 (95% CI=1.27–1.38) times higher than those in the control group (11). A study of 5713 Black and White men and women aged ≥ 65 years in the USA found a 1.97-fold greater risk (95% CI=1.22–3.19) of heart failure in the gout group than in the control group, but the risk of stroke was similar in both groups (odds ratio=0.83, 95% CI=0.48–1.43) (40). The discrepancies could be attributed to variations in the country settings, factors adjusted in the analysis, definitions of outcomes, underlying conditions, study sample, and populations involved in each study.
Stratification analyses by age and sex showed that men and women aged ≥ 60 years with gout were more likely to experience stroke, heart failure, and ischemic heart disease. However, the heightened risk of ischemic heart disease was evident regardless of age and sex; the finding indicates that gout might increase the risk of ischemic heart disease at an earlier age than that of stroke or heart failure, and patients with gout should be carefully monitored for ischemic heart disease. Moreover, this study demonstrated that males and females with gout had a similar risk of stroke (9% and 22% higher, respectively), ischemic heart disease (27% and 38% higher, respectively), or heart failure (65% and 57% higher, respectively) in the overall age group. Consistently, two Taiwanese studies also found that gout-related comorbidities were associated with an elevated risk of stroke in both sexes (22, 23). However, another Taiwanese study concluded that females had a significantly greater likelihood of overall CVDs than males, with an odds ratio of 2.06 (95% CI=1.94–2.20) for females versus an odds ratio of 1.26 (95% CI=1.21–1.31) for males (11).
Individuals with a tendency of increased alcohol intake, high BMI, elevated blood pressure, high total cholesterol level, proteinuria, and high uric acid levels in the Korean population are reportedly at a high risk of developing gout (1). The same risk factors shared between gout and CVDs might be a potential source of confusion. The pathophysiological associations of gout with CVDs are yet to be fully understood; however, genetic, lifestyle, and environmental factors, together with other unidentified elements, are presumably involved. Changes in the genes related to inflammasomes and their molecular associates could probably impact hyperuricemia and the risk of developing gout, as well as other conditions, such as metabolic syndrome and CVDs (41). Genome-wide association studies indicate that genetically predisposed elevated serum urate concentrations could potentially result in an elevated risk of gout, ischemic heart disease, or heart failure (26), with a notable percentage of cardiovascular risk being possibly derived from pleiotropic genes controlling xanthin oxidase activity and urate formation (26). Additionally, chronic inflammation and interleukin (IL)-1β pathways, as well as C-reactive protein, all of which are hallmarks of gout, may be associated with the pathogenesis of CVDs (42, 43). Upon leukocyte activation, endothelial dysfunction and other common pathogenic mechanisms, NLRP3 inflammasome activation, and IL-1β production are promoted in individuals with risk factors for CVDs (44–46). Moreover, research has corroborated that inflammation speeds up aging and therefore increases the chances of heart issues in gout, and telomere length, a risk factor for gout, is an autonomous element linked to the higher recurrence rate and CVD risk (12). Thus, upregulation of these inflammatory pathways in individuals with gout could, at least in part, explain the increased risk of CVDs.
The integrity of this study is primarily based on the comprehensive and nationwide population data that have been adapted to consider socioeconomic standing, potential lifestyle-related hazards, and associated health problems. Second, to limit selection bias and improve the precision of the study, two balanced groups (22,480 participants with gout and 22,480 participants without gout) were paired according to their likelihood of having gout, which could imitate randomized experiments. Despite the high prevalence of gout in men and the elderly, a balanced distribution of age and sex was possible by equally matching 22,480 individuals with gout against the same number of non-gout participants. The heterogeneity in terms of sex differences likely reflects distinctions in the original characteristics of the research groups (20). We found that both men and women with gout were more likely to suffer from stroke, ischemic heart disease, and heart failure. Third, the data gathered from all medical and clinic services in Korea enabled a compilation of complete medical histories throughout the study, thereby enhancing the generalizability and reliability of the research results. Additionally, this 16-year follow-up study is one of the most extensive longitudinal studies on the association between gout and CVDs.
Our findings should be interpreted with a few limitations in mind. First, because this study only included Korean nationals and relied on diagnosis codes, some confounding factors might not have been taken into consideration. Second, no data regarding the family history, personal genetics, or diets for gout or heart conditions were included in the KNHIS-HSC database. Creatinine, glomerular filtration rate, or urate lowering therapies or diuretics were not provided in this study (47, 48); thus, these data were not considered in this study.
In summary, our study demonstrated that individuals with gout in the Korean population, particularly those aged ≥ 60 years, were more likely to have stroke, ischemic heart disease, or heart failure. The findings suggest that individuals diagnosed with gout should receive additional information and training regarding the potential hazards of CVDs.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
HK and N-EL: investigation, writing—original draft, and review & editing. MK: funding acquisition, writing—original draft, and review & editing. DY, KH, JH, HC, and HL: methodology. J-HK, JK, S-JC, and EN: formal analysis. HP: software. NK, SB, and JL: project administration. All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed. All authors have read and agreed to the published version of the manuscript.
This research was supported by Hallym University Research Fund. The APC was funded by Hallym University Research Fund.
Author HC was employed by the company M.D. Analytics.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1195888/full#supplementary-material
1. Lee CH, Sung NY, Lee J, Bae SC. Factors associated with gout in South Koreans: analysis using the National Health Insurance Corporation and the National Health Screening Exam databases. Clin Rheumatol (2013) 32:829–37. doi: 10.1007/s10067-013-2183-9
3. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum (2011) 63:3136–41. doi: 10.1002/art.30520
4. Kuo CF, Grainge MJ, See LC, Yu KH, Luo SF, Zhang W, et al. Epidemiology and management of gout in Taiwan: a nationwide population study. Arthritis Res Ther (2015) 17:13. doi: 10.1186/s13075-015-0522-8
5. Kuo CF, Grainge MJ, Mallen C, Zhang W, Doherty M. Rising burden of gout in the UK but continuing suboptimal management: a nationwide population study. Ann Rheum Dis (2015) 74:661–7. doi: 10.1136/annrheumdis-2013-204463
6. Peng L, Jing J, He S, Wang J, Gao X, Wang T. The role of lipid traits in mediating the effect of body mass index on serum urate. Front Endocrinol (Lausanne) (2022) 13:938891. doi: 10.3389/fendo.2022.938891
7. Park JS, Kang M, Song J-S, Kim HS, Lee CH. Trends of gout prevalence in South Korea based on medical utilization: A national health insurance service database (2002∼2015). J Rheum Dis (2020) 27:174–81. doi: 10.4078/jrd.2020.27.3.174
8. Kuo CF, Grainge MJ, Zhang W, Doherty M. Global epidemiology of gout: prevalence, incidence and risk factors. Nat Rev Rheumatol (2015) 11:649–62. doi: 10.1038/nrrheum.2015.91
9. Kim HC. Epidemiology of cardiovascular disease and its risk factors in Korea. Glob Health Med (2021) 3:134–41. doi: 10.35772/ghm.2021.01008
10. Lee HH, Cho SMJ, Lee H, Baek J, Bae JH, Chung WJ, et al. Korea heart disease fact sheet 2020: analysis of nationwide data. Korean Circ J (2021) 51:495–503. doi: 10.4070/kcj.2021.0097
11. Liang CW, Islam MM, Poly TN, Yang HC, Jack Li YC. Association between gout and cardiovascular disease risk: A nation-wide case-control study. Joint Bone Spine (2019) 86:389–91. doi: 10.1016/j.jbspin.2018.06.011
12. Vazirpanah N, Kienhorst LBE, Van Lochem E, Wichers C, Rossato M, Shiels PG, et al. Patients with gout have short telomeres compared with healthy participants: association of telomere length with flare frequency and cardiovascular disease in gout. Ann Rheum Dis (2017) 76:1313–9. doi: 10.1136/annrheumdis-2016-210538
13. Wandell P, Carlsson AC, Sundquist J, Sundquist K. The association between gout and cardiovascular disease in patients with atrial fibrillation. SN Compr Clin Med (2019) 1:304–10. doi: 10.1007/s42399-019-0043-x
14. Scanu A, Luisetto R, Ramonda R, Spinella P, Sfriso P, Galozzi P, et al. Anti-inflammatory and hypouricemic effect of bioactive compounds: molecular evidence and potential application in the management of gout. Curr Issues Mol Biol (2022) 44:5173–90. doi: 10.3390/cimb44110352
15. Kim JH, Kwon MJ, Choi HG, Lee SJ, Kim SW, Kim JH, et al. The association between hyperuricemia and cardiovascular disease history: A cross-sectional study using KoGES HEXA data. Med (Baltimore) (2022) 101:e32338. doi: 10.1097/MD.0000000000032338
16. Maloberti A, Biolcati M, Ruzzenenti G, Giani V, Leidi F, Monticelli M, et al. The role of uric acid in acute and chronic coronary syndromes. J Clin Med (2021) 10(20):4750. doi: 10.3390/jcm10204750
17. Braga TT, Forni MF, Correa-Costa M, Ramos RN, Barbuto JA, Branco P, et al. Soluble uric acid activates the NLRP3 inflammasome. Sci Rep (2017) 7:39884. doi: 10.1038/srep39884
18. Braga TT, Davanso MR, Mendes D, De Souza TA, De Brito AF, Cruz MC, et al. Sensing soluble uric acid by Naip1-Nlrp3 platform. Cell Death Dis (2021) 12:158. doi: 10.1038/s41419-021-03445-w
19. Hammer HB, Rollefstad S, Semb AG, Jensen G, Karoliussen LF, Terslev L, et al. Urate crystal deposition is associated with inflammatory markers and carotid artery pathology in patients with intercritical gout: results from the NOR-Gout study. RMD Open (2022) 8(2):e002348. doi: 10.1136/rmdopen-2022-002348
20. Kuo CF, Yu KH, See LC, Chou IJ, Ko YS, Chang HC, et al. Risk of myocardial infarction among patients with gout: a nationwide population-based study. Rheumatol (Oxford) (2013) 52:111–7. doi: 10.1093/rheumatology/kes169
21. Singh JA, Cleveland JD. Gout and the risk of myocardial infarction in older adults: a study of Medicare recipients. Arthritis Res Ther (2018) 20:109. doi: 10.1186/s13075-018-1606-z
22. Huang HC, Chiang HP, Hsu NW, Huang CF, Chang SH, Lin KC. Differential risk group of developing stroke among older women with gouty arthritis: A latent transition analysis. Eur J Clin Invest (2019) 49:e13090. doi: 10.1111/eci.13090
23. Huang CF, Liu JC, Huang HC, Chuang SY, Chen CI, Lin KC. Longitudinal transition trajectory of gouty arthritis and its comorbidities: a population-based study. Rheumatol Int (2017) 37:313–22. doi: 10.1007/s00296-016-3634-9
24. Liu CH, Huang SC, Yin CH, Huang WC, Chen JS, Chen YS, et al. Atrial fibrillation risk and urate-lowering therapy in patients with gout: A cohort study using a clinical database. Biomedicines 11 (2022) 11(1):59–68. doi: 10.3390/biomedicines11010059
25. Clarson LE, Chandratre P, Hider SL, Belcher J, Heneghan C, Roddy E, et al. Increased cardiovascular mortality associated with gout: a systematic review and meta-analysis. Eur J Prev Cardiol (2015) 22:335–43. doi: 10.1177/2047487313514895
26. Li X, Meng X, He Y, Spiliopoulou A, Timofeeva M, Wei WQ, et al. Genetically determined serum urate levels and cardiovascular and other diseases in UK Biobank cohort: A phenome-wide mendelian randomization study. PloS Med (2019) 16:e1002937. doi: 10.1371/journal.pmed.1002937
27. Wan H, Zeng L, Xiao R, Tang X, Shu Y, Shen S. Colchicine linked with risk reduction for myocardial infarction in gout patients: systematic review and meta-analysis. Z Rheumatol (2022) 81:501–6. doi: 10.1007/s00393-022-01232-2
28. Jeong H, Choi E, Suh A, Yoo M, Kim B. Risk of cardiovascular disease associated with febuxostat versus allopurinol use in patients with gout: a retrospective cohort study in Korea. Rheumatol Int (2023) 43:265–81. doi: 10.1007/s00296-022-05222-0
29. Kim SY, Min C, Oh DJ, Choi HG. Tobacco smoking and alcohol consumption are related to benign parotid tumor: A nested case-control study using a national health screening cohort. Clin Exp Otorhinolaryngol (2019) 12:412–9. doi: 10.21053/ceo.2018.01774
30. Kwon MJ, Kang HS, Kim JH, Kim JH, Kim SH, Kim NY, et al. Association between statin use and gastric cancer: A nested case-control study using a national health screening cohort in Korea. Pharm (Basel) (2021) 14(12):1283–99. doi: 10.3390/ph14121283
31. Kim JW, Kwak SG, Lee H, Kim SK, Choe JY, Park SH. Prevalence and incidence of gout in Korea: data from the national health claims database 2007-2015. Rheumatol Int (2017) 37:1499–506. doi: 10.1007/s00296-017-3768-4
32. Kwon MJ, Park JY, Kim SG, Kim JK, Lim H, Kim JH, et al. Potential association of osteoporosis and not osteoporotic fractures in patients with gout: A longitudinal follow-up study. Nutrients (2022) 15(1):134–148. doi: 10.3390/nu15010134
33. Choi S, Kim K, Kim SM, Lee G, Jeong SM, Park SY, et al. Association of obesity or weight change with coronary heart disease among young adults in South Korea. JAMA Intern Med (2018) 178:1060–8. doi: 10.1001/jamainternmed.2018.2310
34. Kwon MJ, Kim JH, Kim JH, Cho SJ, Nam ES, Choi HG. The occurrence of alzheimer's disease and parkinson's disease in individuals with osteoporosis: A longitudinal follow-up study using a national health screening database in Korea. Front Aging Neurosci (2021) 13:786337. doi: 10.3389/fnagi.2021.786337
35. Who/Iaso/Iotf. The Asia-Pacific perspective: redefining obesity and its treatment. Sydney, Australia: Health Communications Australia Pty Limited (2000).
36. Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol (2011) 173:676–82. doi: 10.1093/aje/kwq433
37. Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care (2005) 43:1130–9. doi: 10.1097/01.mlr.0000182534.19832.83
38. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med (2009) 28:3083–107. doi: 10.1002/sim.3697
39. Janssens HJ, Arts PG, Schalk BW, Biermans MC. Gout and rheumatoid arthritis, both to keep in mind in cardiovascular risk management: A primary care retrospective cohort study. Joint Bone Spine (2017) 84:59–64. doi: 10.1016/j.jbspin.2015.12.003
40. Colantonio LD, Saag KG, Singh JA, Chen L, Reynolds RJ, Gaffo A, et al. Gout is associated with an increased risk for incident heart failure among older adults: the REasons for Geographic And Racial Differences in Stroke (REGARDS) cohort study. Arthritis Res Ther (2020) 22:86. doi: 10.1186/s13075-020-02175-2
41. De Lima JD, De Paula AGP, Yuasa BS, De Souza Smanioto CC, Da Cruz Silva MC, Dos Santos PI, et al. Genetic and epigenetic regulation of the innate immune response to gout. Immunol Invest (2023) 52(3):1–34. doi: 10.1080/08820139.2023.2168554
42. Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature (2006) 440:237–41. doi: 10.1038/nature04516
43. Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation (2002) 105:1135–43. doi: 10.1161/hc0902.104353
44. Interleukin-6 Receptor Mendelian Randomisation Analysis C, Swerdlow DI, Holmes MV, Kuchenbaecker KB, Engmann JE, Shah T, et al. The interleukin-6 receptor as a target for prevention of coronary heart disease: a mendelian randomisation analysis. Lancet (2012) 379:1214–24. doi: 10.1016/S0140-6736(12)60110-X
45. Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature (2010) 464:1357–61. doi: 10.1038/nature08938
46. Pasceri V, Cheng JS, Willerson JT, Yeh ET. Modulation of C-reactive protein-mediated monocyte chemoattractant protein-1 induction in human endothelial cells by anti-atherosclerosis drugs. Circulation (2001) 103:2531–4. doi: 10.1161/01.CIR.103.21.2531
47. Russo E, Viazzi F, Pontremoli R, Barbagallo CM, Bombelli M, Casiglia E, et al. Serum uric acid and kidney disease measures independently predict cardiovascular and total mortality: the uric acid right for heart health (URRAH) project. Front Cardiovasc Med (2021) 8:713652. doi: 10.3389/fcvm.2021.713652
48. Maloberti A, Bombelli M, Facchetti R, Barbagallo CM, Bernardino B, Rosei EA, et al. and Working Group on Uric Acid C R O T I S O H, Relationships between diuretic-related hyperuricemia and cardiovascular events: data from the URic acid Right for heArt Health study. J Hypertens (2021) 39:333–40. doi: 10.1097/HJH.0000000000002600
Keywords: gout, stroke, ischemic heart disease, heart failure, cardiovascular diseases, longitudinal follow-up study, nationwide health insurance research database
Citation: Kang HS, Lee N-E, Yoo DM, Han KM, Hong JY, Choi HG, Lim H, Kim J-H, Kim JH, Cho S-J, Nam ES, Park HY, Kim NY, Baek SU, Lee JY and Kwon MJ (2023) An elevated likelihood of stroke, ischemic heart disease, or heart failure in individuals with gout: a longitudinal follow-up study utilizing the National Health Information database in Korea. Front. Endocrinol. 14:1195888. doi: 10.3389/fendo.2023.1195888
Received: 29 March 2023; Accepted: 13 July 2023;
Published: 23 August 2023.
Edited by:
Yijun Hu, Guangdong Provincial People’s Hospital, ChinaReviewed by:
Aleksandra Klisic, Primary Health Care Center Podgorica, MontenegroCopyright © 2023 Kang, Lee, Yoo, Han, Hong, Choi, Lim, Kim, Kim, Cho, Nam, Park, Kim, Baek, Lee and Kwon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mi Jung Kwon, bXVsYW5rQGhhbm1haWwubmV0; bXVsYW5rOTlAaGFsbHltLm9yLmty
†These authors contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.