
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 27 July 2023
Sec. Reproduction
Volume 14 - 2023 | https://doi.org/10.3389/fendo.2023.1193826
This article is part of the Research Topic Exploring the Optimal Endometrial Preparation Protocol for Frozen-Thawed Embryo Transfer View all 12 articles
Objective: The administration of progesterone before transfer in hormone replacement treatment (HRT) is crucial for the clinical outcomes of frozen-thawed embryo transfer (FET), but the optimal duration of progesterone remains controversial. This study aimed to investigate the effect of the duration of progesterone administration on the clinical outcomes of FET cycles.
Methods: This prospective cohort study included 353 artificial FET cycles conducted at a reproductive medicine center between April and October 2021. The FET cycles were stratified into four groups based on the duration of progesterone supplementation before the procedure and the embryonic development stage: group P3 (73 patients) received intramuscular progesterone for 3 days and group P4 (87 patients) for 4 days before Day 3 frozen embryo transfer, group P5 (70 patients) for 5 days and group P6 (123 patients) for 6 days before frozen blastocyst transfer. This trial was performed using one or two vitrified embryo(s) when the endometrial thickness reached 7 mm after estrogen supplementation in an artificial cycle. The primary outcome was clinical pregnancy, and secondary outcomes included biochemical pregnancy, implantation, early pregnancy loss, and live births.
Results: There were no significant differences in the demographic and clinical characteristics between the groups. No significant difference was observed in the clinical pregnancy rates between groups: 23/73 (31.5%) in group P3 vs 28/87 (32.2%) in group P4 (P = 0.927). Compared to group P5 (41/70, 58.6%), the clinical pregnancy rate was not significantly different in group P6 (77/123, 62.6%, P = 0.753). There was no significant difference in the implantation rates between groups: 33/136 (24.3%) in group P3 vs 34/166 (20.5%) in group P4 (P = 0.431), and 62/133 (46.6%) in group P5 vs 107/231 (46.3%) in group P6 (P = 0.956). The duration of progesterone supplementation (mean: 3.5 ± 0.5 days; range:3–4 days) before Day 3 frozen embryo transfer did not impact clinical pregnancy (odds ratio [OR] 1.048; 95% confidence interval [CI], 0.518–2.119). The duration of progesterone administration (mean: 5.6 ± 0.5 days; range:5–6 days) before frozen blastocyst transfer may not affect clinical pregnancy (OR 1.339; 95% CI, 0.717–2.497).
Conclusion: There may be no significant correlation between the duration of progesterone supplementation and pregnancy outcomes in artificial FET cycles, although the clinical pregnancy rate was higher when progesterone supplementation was extended for one day before FET.
Due to recent developments in clinical practice and laboratory technology, embryo cryopreservation has become a central component of assisted reproductive technology (ART). Improved laboratory technology has contributed to an increased number of embryos available for in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) cycles. The vitrification method, improved embryo survival rates after thawing (1, 2), and implementation of a single embryo transfer policy to reduce multiple pregnancies without reducing the cumulative delivery rate (3), have boosted the number of FET cycles performed worldwide. A freeze-all strategy is now adopted for a growing number of indications, including the prevention of ovarian hyperstimulation syndrome, preimplantation genetic testing (PGT), progesterone elevation in the late follicular phase, endometrial abnormality, embryo–endometrium asynchrony, egg freezing, and fertility preservation, and so on (4–11). As a result, the proportion of autologous frozen embryo transfers has increased dramatically worldwide over the last decade, which is of great significance in improving the clinical outcomes of FET (12).
Despite the emerging importance of the FET cycle, optimal endometrial preparation before it remains unclear. Although several randomized trials have examined the effects of different cycle regimens on FET, there is still no evidence for a single optimal endometrial priming protocol for FET cycles (13–16). Hormone replacement therapy (HRT) is currently the most widely administered therapy due to its wide range of applications, low cycle cancellation rate, and no requirement for frequent follow-ups (17). Patients can participate in the HRT program irrespective of their ovarian functional status and menstrual irregularities (18). Several studies have suggested that HRT cycles are comparable to natural cycles in terms of pregnancy, miscarriage, and live birth rates (14–16). Most reproductive medicine centers primarily use HRT in endometrial preparation for various reasons. In artificial FET cycles, estrogen and progesterone are sequentially implemented to synchronize the embryo transfer with the window of implantation (WOI) (19). Supplementation of estrogen before FET promotes endometrial thickening, and estrogen is continued as a daily supplementation of progesterone, which is initiated a few days before scheduled embryo transfer. There are two significant factors for successful implantation and pregnancy, namely euploid embryos with developmental potential and a synchronous endometrium (20).
In contrast to estrogen, progesterone appears to be a significant determinant of the WOI because the endometrial WOI is confined to the stenosis interval in the luteal phase (19, 21). Endometrial WOI was first characterized by studies applying hormone preparations in recipients with donor oocytes, indicating that endometrial receptivity is significantly reduced when the frozen embryo is administered prior to or after this critical period (22, 23). Therefore, determining the optimal duration of progesterone exposure before FET is crucial for maximizing the success of ART (24). No clear and definite conclusion regarding the superiority of one protocol over another can be drawn thus far. Most researchers maintain that a stable clinical pregnancy rate can be obtained by transferring the 3rd day (Day 3) embryos with progesterone supplementation for 3 days and blastocysts with progesterone administration for 5 days in the FET cycle (25, 26). However, there are always some unavoidable factors that lead to the incomplete synchronous development of the embryos and endometrium (27, 28). Although some previous studies have indicated that appropriately delaying the transfer time may improve the outcomes of FET (29), another data did not show any difference in clinical pregnancy rates between protocols with or without prolonged progesterone supplementation before FET (30).
As for the lack of evidence regarding to the optimal duration of progesterone administration, there is still much more to be learned about endometrial preparation and synchronization to maximize endometrial function and receptivity, and select the best time for embryo transfer (31). This study assessed whether the duration of progesterone administration before FET influenced the clinical outcomes of HRT of during the FET cycle.
This prospective study was conducted at the Reproductive Medical Center of the Affiliated Hospital of Southwest Medical University. This study included patients who underwent FET in an artificial cycle from April 2021 to October 2021 and were allocated to one of four groups as soon as the endometrial thickness reached 7 mm after estrogen supplementation. One or two embryo(s) were transferred according to Health Commission legislation. The inclusion criteria were patients aged 22−45 years and on hormone replacement cycles. The exclusion criteria included known allergic reactions to progesterone products, uptake of an experimental medicine within 30 days before study initiation, and cancellation of FET for various reasons. The cycles were stratified into four groups based on the duration of progesterone supplementation before embryo transfer and the embryonic development stage. The cycles were assigned to groups P3 or group P5 from April 2021 to June 2021. The cycles were assigned to Groups P4 and P6 from July 2021 to October 2021. Patients in groups P3 and P4 were administered intramuscular progesterone for 3 and 4 days, respectively, before frozen-thawed Day 3 cleavage-stage embryo transfer. Patients in groups P5 and P6 were separately supplemented with intramuscular progesterone for 5 and 6 days, respectively, before the frozen-thawed blastocyst transfer. This trial was performed using one or two vitrified-warmed embryo(s) when the endometrial thickness reached 7 mm after estrogen supplementation in an artificial cycle. This study was approved by the Institutional Review Board of the hospital. Written informed consent was obtained from all participants.
All cycles were performed using the same artificial protocol. Oral estradiol valerate (Progynova, BayerSchering Pharma AG, Germany) was administered on the second or third day of the menstrual cycle after verifying that the patients were in the early proliferative phase of the menstrual cycle. Typically, 3 mg of estradiol valerate was prescribed twice daily. If the endometrial thickness was <7 mm 14 days later, patients were required to comply with a step-up protocol that involved the addition of vaginal estrogen supplementation (Femoston, Abbott Healthcare Products; 1 mg once daily), to the oral administration of estradiol valerate, based on the physician’s preference and experience. Serum estradiol and progesterone levels were determined on the day before the initiation of progesterone treatment.
Intramuscular progesterone (60 mg once a day) was initiated, supplemented with oral dydrogesterone (10 mg thrice a day) when the endometrial thickness was 7 mm. Embryo transfer was performed days 4, 5, 6 and 7 of progesterone exposure in groups P3, P4, P5 and P6 respectively. No more than two embryos were transferred in any FET cycles. All the embryos were thawed on the morning of the transfer. Embryos were evaluated according to the conventional classification system currently used in our IVF laboratory: number of blastomeres, degree of cytoplasmic fragmentation and the equality of blastomeres (32). Embryos with seven to nine cells on Day 3 and with no multinucleation, cytoplasmic fragmentation, or less than 10% fragmentation were considered good-quality embryos. The blastocyst morphology was evaluated according to the Gardner and Schoolcraft grading system (33). A morphological quality score was assigned to the blastocysts at the moment of transfer; blastocysts with inner cell mass/trophectoderm (ICM/TE) score types AA, AB, or BA were considered good-quality embryos (30). The daily estrogen and progesterone protocol was continued if there was a negative pregnancy test result after FET. If pregnancy was achieved, hormone administration was continued until approximately 11–12 weeks of gestation.
The independent variable of interest was the duration of progesterone exposure, defined as the number of days from the initiation of progesterone treatment to the completion of embryo transfer. To evaluate whether the duration of progesterone administration before FET affected the clinical outcomes, the primary outcome of the study was clinical pregnancy, which was defined as the presence of one or more gestational sac(s) with an embryonic pole indicating the fetal heartbeat on transvaginal ultrasound at 7 weeks of gestation. Secondary outcomes included biochemical pregnancy, rate of implantation, live births, early pregnancy loss, and miscarriage.
Fourteen days after embryo transfer, having a serumβ-hCG level of >5 IU/L was considered a biochemical pregnancy. The implantation rate was determined by dividing the number of intrauterine sacs inspected using transvaginal ultrasound divided by the number of embryos transferred. Live birth was defined as the delivery of viable infant(s) at ≥24 gestational weeks. Early pregnancy loss was considered when no gestational sac was observed even after a serum β-hCG ≥ 5 mIU/mL or loss occurring after the presence of an intrauterine gestational sac was confirmed.
Normally distributed measurement data are presented as the “mean ± standard”; data that is not normally distributed are presented as “median (range).” The Shapiro- Wilk (SW) test was used to determine whether the continuous variables were normally distributed. Statistical comparisons between the two groups were performed using the Mann-Whitney U test. Categorical variables were described as frequencies or percentages, and comparisons between groups were performed using Pearson’s chi-squared test or Fisher’s exact test. All statistical analyses were performed using IBM SPSS Statistics for Windows (version 19.0; IBM, Corp., Armonk, NY, USA. Statistical significance was set at P < 0.05.
Overall, 353 FET cycles were performed with HRT were analyzed in this study, including FET of cleavage embryos or blastocysts. All FET cycles were stratified into four groups (P3, P4, P5, and P6) according to the embryonic development stage and the duration of progesterone administration before FET. At the time of analysis, groups P3, P4, P5 and P6 were compared.
Comparison of baseline demographic and cycle characteristics based on the duration of progesterone administration before frozen-thawed Day 3 cleavage-stage embryo transfer. The baseline characteristics are presented in Table 1. There were no significant differences in the baseline characteristics between groups P3 and P4. Comparison of baseline demographic and cycle characteristics according to the duration of progesterone exposure before frozen blastocyst transfer was performed. There were no significant differences in demographic and clinical characteristics between groups P5 and P6 (Table 2).
Pregnancy outcomes stratified into two groups according to the duration of progesterone administration prior to frozen-thawed Day 3 cleavage-stage embryo transfer are listed in Table 3. No significant differences were observed between groups in the rates of clinical pregnancy, biochemical pregnancy, implantation, live birth, early pregnancy loss, and miscarriage. The pregnancy outcomes stratified into two groups according to the duration of progesterone exposure before frozen blastocyst transfer are listed in Table 4. There were no significant differences between groups in the rates of clinical pregnancy, biochemical pregnancy, implantation, live birth, early pregnancy loss rate, or miscarriage.
Baseline demographics and cycle characteristics were compared between patients who achieved clinical pregnancy after frozen-thawed Day 3 cleavage-stage embryo transfer (Table 5). Patients who achieved pregnancy after the transfer had a mean duration of 3.5 ± 0.5 days (range: 3–4 days) of progesterone administration before FET. After controlling for age, body mass index, endometrial thickness at transfer, whether the embryo had a morphology grade of ≥730, and the number of days of progesterone administration, the odds of achieving a clinical pregnancy were not modified (odds ratio [OR] 1.048; 95% confidence interval [CI], 0.518–2.119; P = 0.897), Table 5).
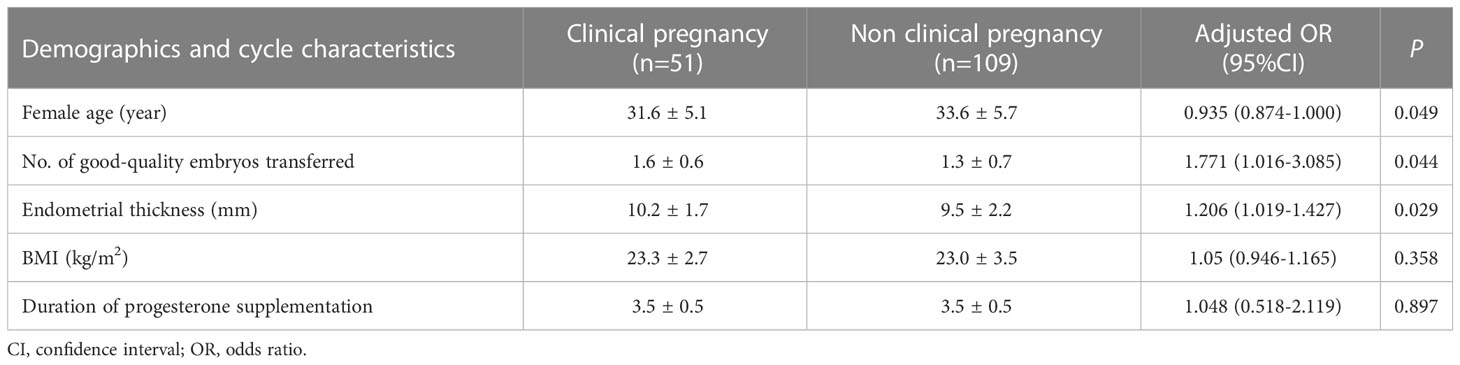
Table 5 A comparison of baseline demographic and characteristics according to whether patients achieved a clinical pregnancy after frozen day 3 cleavage embryo transfer.
The baseline demographics and cycle characteristics were compared between patients who achieved clinical pregnancy after frozen blastocyst transfer (Table 6). Patients who achieved pregnancy after the transfer had a mean duration of 5.6 ± 0.5 days (range: 5–6 days) of progesterone administration before FET. After controlling for age, body mass index, endometrial thickness at transfer, embryonic day of development at freezing, whether the embryo had a morphological grade of 3BB or better, and the number of days of progesterone administration, the odds of achieving clinical pregnancy were not modified (OR 1.339; 95% CI, 0.717–2.497; P = 0.36), Table 6).
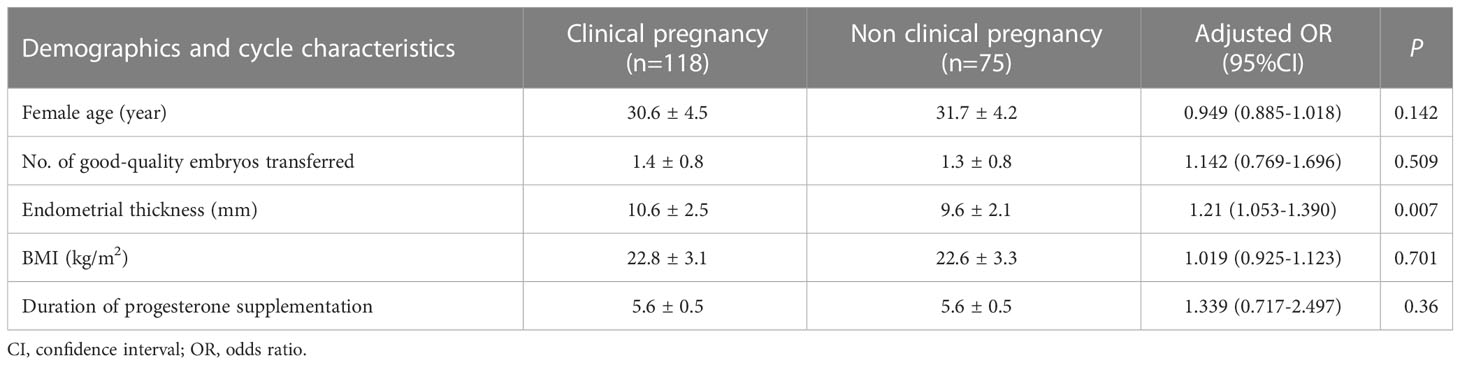
Table 6 A comparison of baseline demographic and characteristics according to whether patients achieved a clinical pregnancy after frozen blastocyst transfer.
HRT for FET cycles includes the sequential administration of exogenous estrogen and progesterone to imitate the physiological hormonal exposure of the endometrium in a normal menstrual cycle and to accurately time the transfer of the thawed embryo to the receptive endometrium (19, 34). In HRT cycles, progesterone is initiated to promote the final phase of endometrial preparation prior to embryo transfer (28, 31). Empirically, progesterone supplementation is usually initiated 3 days before the embryo transfer with excellent pregnancy rates in artificially prepared cycles (35). Most reproductive medicine centers believe that a stable clinical pregnancy rate can be obtained by administering the protocol of transferring the Day 3 embryos with progesterone administration for 3 days and transferring blastocysts with progesterone administration for 5 days in HRT cycles (25, 26, 35). Some reproductive medicine centers still consider that a similar or even higher clinical pregnancy rates can be achieved by prolonging the program by one day (29, 30). These scholars maintained that prolonging the time of endometrial transformation using progesterone can diminish uterine contractions, improve endometrial receptivity, and foreshorten the period between development and implantation after embryo transplantation in the FET cycle (29).
However, the hypothesis that prolonging the duration of progesterone administration could increase the pregnancy rates has not yet been confirmed (29, 30, 36, 37). Better implantation outcomes can be obtained by prolonging the exposure of progesterone to the endometrium during HRT cycles, which prolongs the endometrial receptivity period by increasing the interaction between the embryo and endometrium (38). However, this assumption was not fully confirmed in the present study. Although the clinical pregnancy rates of FET Day 3 embryos on the fifth day of progesterone administration were higher than those on the fourth day of progesterone administration, there was no statistically significant difference between the two groups. This research showed that transferring vitrified-warmed Day 3 cleavage stage embryos on the fifth day of progesterone administration may not increase pregnancy rates compared to transferring on the fourth day of progesterone administration. In addition, this study demonstrated that transferring vitrified-thawed blastocysts on the seventh day of progesterone supplementation may not improve the pregnancy rates compared to transferring blastocysts on the sixth day of progesterone supplementation. To our knowledge, this is the first prospective trial comparing the effects of different duration of progesterone administration before FET. Although various studies have indicated a trend toward better pregnancy outcomes with shorter progesterone supplementation, no definite conclusion has been reached (39–43). Furthermore, it has been proposed that WOI switches approximately 48 h after the initiation of progesterone and is sustained for at least 4 days (44). This study showed that similar pregnancy outcomes were achieved with or without extending progesterone by one day, which may indicate that WOI may be longer than estimated (40, 42). Previous studies have shown that delayed endometrial development during the luteal phase occurs in approximately 25% of the general population. Thus, delaying the day of embryo transfer is feasible (36, 37). Perhaps, the extended day is also theoretically covered by the implantation window (29). This viewpoint seems to have been recognized in the present study.
The hypothesis that prolonging the duration of progesterone administration could diminish the early pregnancy loss rate has not yet been confirmed (42). This hypothesis was not verified in the present study. A higher risk of early pregnancy loss may occur when the duration of progesterone administration before transplantation is shorter than embryonic age (42). Thus, extended progesterone administration before transfer is recommended (45). Implantation after the normal endometrial receptivity period is closely associated with early pregnancy loss (46). A prolonged window of endometrial receptivity may account for the delayed implantation of severely damaged embryos and the subsequent early pregnancy loss (47). A higher incidence of early pregnancy loss was not detected in between group P3, which was administered progesterone for 3 days, and P4, which was administered progesterone for 4 days before transferring vitrified-warmed embryos at the third day cleavage stage, This was not observed in either group P5, which was administered progesterone for 5 days, or P6, which was administered progesterone for 6 days before transferring vitrified-thawed blastocysts, which may be because the duration of progesterone administration was no shorter than the age of the embryo (42). According to these data, there may also be no difference in the rate of early pregnancy loss between the two groups with or without prolonged progesterone administration, which may be due to adaptation to the normal period of endometrial receptivity (46). In contrast, Day 3 embryos of group P4 were transferred on the fifth day of progesterone supplementation, and blastocysts of group P6 were transferred on the seventh day of progesterone supplementation, which might have been too long, although no evidence exists that longer progesterone supplementation had a negative impact on success rates (43).
Based on these results, it may be concluded that enhanced flexibility would be possible when programming vitrified-warmed embryos transfer after progesterone administration, because no differences were observed between the duration of progesterone administration ≥1 day of embryonic age as far as clinical pregnancy, implantation, live birth, and early pregnancy loss rates are concerned (29, 30, 36, 37). This study demonstrated that there may be no correlation between the developmental stage of the blastocyst and clinical pregnancy in the FET cycle in day 5 embryos or day 6 blastocysts when an equal duration of progesterone supplementation was employed, considering that the duration of progesterone supplementation for day 5 and day 6 blastocysts was equal before FET (48, 49). In the light of this result, it could be concluded that clinical pregnancy may not be related to the developmental stage of blastocysts, but rather to the quality of the embryo, as morphologically high-quality day 5 and 6 blastocysts resulted in similar pregnancy outcomes, possibly due to the lack of difference between high-quality embryos on days 5 and 6 regarding aneuploidy (48, 49). However, this assumption may be challenged because other data from some scholars indicated that a blastocyst on day 6 has diverse developmental potential and a specific synchronization between the embryo and endometrium with a different WOI compared with a blastocyst on day 5 (50). On the basis of these results, programming the transfer of a vitrified-warmed blastocyst on day 6 requires further investigation, since these “delayed” blastocysts may encounter different and possibly narrower WOI compared with day 5 embryos. Consequently, the optimal duration of progesterone administration remains controversial.
In addition, this study discovered that blastocysts might have higher pregnancy outcomes in FET cycles compared with that of cleavage-stage embryos (51), which might be related to differences in intimal thickness between them. Additionally, differences in age and the number of good-quality embryos transferred may be related to these pregnancy outcomes as well. An explanation for these findings may be that blastocysts undergo a re-selection procedure that spans the 8-cell-stage developmental block and eliminates embryos with poor developmental potential compared to cleavage embryos (52–54). Moreover, this study found that a stable clinical pregnancy may have no correlation with the number of good-quality embryos, indicating the feasibility of single blastocyst transfer, effectively reducing the incidence of ovarian hyper stimulation syndrome, multiple birth rates, and fetal and maternal risks while ensuring relatively higher pregnancy and live birth rates (55–57). In addition, the current trend in assisted reproduction may become a more feasible approach in extending the duration of in vitro embryo culture to obtain more developed embryos, determining the implantation window for these mature embryos, and further improving the success rate of assisted reproduction (29). Single embryo transfer (SET) may be safer than double embryo transfer and as effective as single blastocyst transfer if patients are thoroughly informed of the reasons for the proposition (55, 56). The question may not be whether to apply SET, but how to apply it in terms of patient selection, patient-centered counselling, and coverage of treatment (3).
The data also displayed that the pregnancy outcome of embryos at the cleavage stage might be related to the woman’s age, while the blastocyst pregnancy outcome might be uncorrelated with it (53). It is possible that the aneuploidy rate of oocytes increases with age and then undergoes an embryo developmental block or poor developmental potential (58). However, blastocysts could have better quality and higher euploidy because of a re-selection procedure that spans developmental blocks and eliminates embryos with poor developmental potential compared to that of cleavage embryos (52–54). These results indicate that the difference in pregnancy outcomes between the transfer of blastocysts and day 3 cleavage embryos may largely depend on the quality of the embryos transferred (59).
This study had similar or different results from those of other studies, possibly due to epidemiological variables, limitations of its design, a high level of heterogeneity in the study populations, and insufficient sample size. Although this study adjusted for multiple potential confounding factors, caution should be taken when considering the results as a basis for definitive policy due to the prospective design of a single-center study with a limited sample size, as well as possible differences in research protocol and clinical performance. Consequently, confirmation of these findings in a multi-center study with a large sample size and a more rigorous research design is warranted.
In summary, although the results of this study need to be confirmed in multicenter randomized trials, they suggest that obtaining as many high-quality blastocysts as possible and programming single blastocyst transplantation deserved consideration. Although the optimal duration of progesterone supplementation remains to be clarified, it is recommended to transfer embryos on days 3 and 5 with a degree of flexibility. However, there may be different and possibly narrower WOI for the blastocyst on the sixth day of vitrification. Further research is required to optimize FET protocols and distinguish other contributing factors that may affect pregnancy outcomes after transferring frozen-thawed embryos.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Affiliated Hospital of Southwest Medical University Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
LL and HZ: conceptualization and writing-original draft prepatation. JH and XS: data curation DL and GH: revising the manuscript critically for important intellectual content. All authors contributed to the article and approved the submitted version.
The study was supported by a project of the Science Foundation of Affiliated Hospital of Southwest Medical University (no. 20014).
We thank the survey participants and all members involved in this study for their painstaking efforts in conducting the data collection.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Nagy ZP, Shapiro D, Chang CC. Vitrification of the human embryo: a more efficient and safer in vitro fertilization treatment. Fertil Steril (2020) 113(2):241–7. doi: 10.1016/j.fertnstert.2019.12.009
2. Chang CC, Shapiro DB, Nagy ZP. The effects of vitrification on oocyte quality. Biol Reprod (2022) 106(2):316–27. doi: 10.1093/biolre/ioab239
3. Mejia RB, Capper EA, Summers KM, Ten Eyck P, Van Voorhis BJ. Elective transfer of one embryo is associated with a higher cumulative live birth rate and improved perinatal outcomes compared to the transfer of two embryos with in vitro fertilization. F S Rep (2020) 2(1):50–7. doi: 10.1016/j.xfre.2020.10.011
4. Singh B, Reschke L, Segars J, Baker VL. Frozen-thawed embryo transfer: the potential importance of the corpus luteum in preventing obstetrical complications. Fertil Steril (2020) 113(2):252–7. doi: 10.1016/j.fertnstert.2019.12.007
5. Mizrachi Y, Horowitz E, Farhi J, Raziel A, Weissman A. Ovarian stimulation for freeze-all IVF cycles: a systematic review. Hum Reprod Update (2020) 26(1):118–35. doi: 10.1093/humupd/dmz037
6. Abe M, Yamamoto Y, Noguchi H, Tamura K, Aoki H, Takeda A. Is a freeze-all strategy necessary for all embryo transfers : fresh embryo transfer without progesterone elevation results in an equivalent pregnancy rate to cryopreserved embryo transfer. J Med Invest (2022) 69(3.4):224–9. doi: 10.2152/jmi.69.224
7. Munné S, Kaplan B, Frattarelli JL, Child T, Nakhuda G, Shamma FN, et al. Preimplantation genetic testing for aneuploidy versus morphology as selection criteria for single frozen-thawed embryo transfer in good-prognosis patients: a multicenter randomized clinical trial. Fertil Steril (2019) 112(6):1071–1079.e7. doi: 10.1016/j.fertnstert.2019.07.1346
8. Yang Y, Zhu D, Wang Q, Ma C, Li D, Wang J, et al. Frozen embryo transfer in the menstrual cycle after moderate-severe ovarian hyperstimulation syndrome: a retrospective analysis. BMC Pregnancy Childbirth (2022) 22(1):907. doi: 10.1186/s12884-022-05239-0
9. Racca A, Santos-Ribeiro S, De Munck N, Mackens S, Drakopoulos P, Camus M, et al. Impact of late-follicular phase elevated serum progesterone on cumulative live birth rates: is there a deleterious effect on embryo quality? Hum Reprod (2018) 33(5):860–8. doi: 10.1093/humrep/dey031
10. Groenewoud E, Cohlen BJ, Maclon N. Programming the endometrium for deferred transfer of cryopreserved embryos: hormone replacement versus modified natural cycles. Fertil Steril (2018) 109:768–74. doi: 10.1016/j.fertnstert.2018.02.135
11. Maheshwari A, Bell JL, Bhide P, Brison D, Child T, Chong HY, et al. Elective freezing of embryos versus fresh embryo transfer in IVF: a multicentre randomized controlled trial in the UK (E-freeze). Hum Reprod (2022) 37(3):476–87. doi: 10.1093/humrep/deab279
12. Sciorio R, Esteves SC. Clinical utility of freeze-all approach in ART treatment: a mini-review. JCryobiology (2020) 92:9–14. doi: 10.1016/j.cryobiol.2019.11.041
13. Chada A, Hipp HS. 'Let'rozole it grow: endometrial preparation methods for frozen-thawed embryo transfer. Fertil Steril (2022) 118(4):699–700. doi: 10.1016/j.fertnstert.2022.08.003
14. Ghobara T, Gelbaya TA, Ayeleke RO. Cycle regimens for frozen-thawed embryo transfer. Cochrane Database Syst Rev (2017) 7:Cd003414. doi: 10.1002/14651858.CD003414.pub3
15. Wang B, Zhu Q, Wang Y. Pregnancy outcomes after different cycle regimens for frozen-thawed embryo transfer: a retrospective study using propensity score matching. Front Med (Lausanne) (2020) 7:327. doi: 10.3389/fmed.2020.00327
16. Wang B, Zhang J, Zhu Q, Yang X, Wang Y. Effects of different cycle regimens for frozen embryo transfer on perinatal outcomes of singletons. Hum Reprod (2020) 35(7):1612–22. doi: 10.1093/humrep/deaa093
17. Yu J, Chen P, Luo Y, Lv M, Lou L, Xiao Q, et al. GnRH-agonist pretreatment in hormone replacement therapy improves pregnancy outcomes in women with male-factor infertility. Front Endocrinol (Lausanne) (2022) 13:1014558. doi: 10.3389/fendo.2022.1014558
18. Li SJ, Zhang YJ, Chai XS, Nie MF, Zhou YY, Chen JL, et al. Letrozole ovulation induction: an effective option in endometrial preparation for frozen–thawed embryo transfer. Arch Gynecol Obstet (2014) 289(3):687–93. doi: 10.1007/s00404-013-3044-0
19. Sekhon L, Feuerstein J, Pan S, Overbey J, Lee JA, Briton-Jones C, et al. Endometrial preparation before the transfer of single, vitrified-warmed, euploid blastocysts: does the duration of estradiol treatment influence clinical outcome? Fertil Steril (2019) 111(6):1177–1185.e3. doi: 10.1016/j.fertnstert.2019.02.024
20. Álvarez M, Gaggiotti-Marre S, Martínez F, Coll L, García S, González-Foruria I, et al. Individualised luteal phase support in artificially prepared frozen embryo transfer cycles based on serum progesterone levels: a prospective cohort study. Hum Reprod (2021) 36(6):1552–60. doi: 10.1093/humrep/deab031
21. Ruiz-Alonso M, Valbuena D, Gomez C, Cuzzi J, Simon C. Endometrial receptivity analysis (ERA): data versus opinions. Hum Reprod Open (2021) 2021(2):hoab011. doi: 10.1093/hropen/hoab011
22. Lessey BA, Young SL. What exactly is endometrial receptivity? Fertil Steril (2019) 111(4):611–7. doi: 10.1016/j.fertnstert.2019.02.009
23. Navot D, Bergh PA, Williams M, Garrisi GJ, Guzman I, Sandler B, et al. An insight into early reproductive processes through the in vivo model of ovum donation. J Clin Endocrinol Metab (1991) 72(2):408–14. doi: 10.1210/jcem-72-2-408
24. Franasiak JM, Ruiz-Alonso M, Scott RT, Simon C. Both slowly developing embryos and a variable pace of luteal endometrial progression may conspire to prevent normal birth in spite of a capable embryo. Fertil Steril (2016) 105(4):861–6. doi: 10.1016/j.fertnstert.2016.02.030
25. Mumusoglu S, Polat M, Ozbek IY, Bozdag G, Papanikolaou EG, Esteves SC, et al. Preparation of the endometrium for frozen embryo transfer: a systematic review. Front Endocrinol (Lausanne) (2021) 12:688237. doi: 10.3389/fendo.2021.688237
26. Yang X, Bu Z, Hu L. Live birth rate of frozen-thawed single blastocyst transfer after 6 or 7 days of progesterone administration in hormone replacement therapy cycles: a propensity score-matched cohort study. Front Endocrinol (Lausanne) (2021) 12:706427. doi: 10.3389/fendo.2021.706427
27. Glujovsky D, Pesce R, Fiszbajn G, Sueldo C, Hart RJ, Ciapponi A. Endometrial preparation for women undergoing embryo transfer with frozen embryos or embryos derived from donor oocytes. Cochrane Database Syst Rev (2010) 20(1):CD006359. doi: 10.1002/14651858.CD006359.pub2
28. Mackens S, Santos-Ribeiro S, van de Vijver A, Racca A, Van Landuyt L, Tournaye H, et al. Frozen embryo transfer: a review on the optimal endometrial preparation and timing. Hum Reprod (2017) 32(11):2234–42. doi: 10.1093/humrep/dex285
29. Xu M, Yan Y, Shen X, Sun H, Yan G, Kong N, et al. Prolonging the time of progesterone supplementation to improve the pregnancy outcomes of single day 6 blastocyst transfer in frozen-thawed cycles: study protocol for a randomized controlled trial. Trials (2022) 23(1):1024. doi: 10.1186/s13063-022-07013-1
30. Roelens C, Santos-Ribeiro S, Becu L, Mackens S, Van Landuyt L, Racca A, et al. Frozen-warmed blastocyst transfer after 6 or 7 days of progesterone administration: impact on live birth rate in hormone replacement therapy cycles. Fertil Steril (2020) 114(1):125–32. doi: 10.1016/j.fertnstert.2020.03.017
31. Bulletti C, Bulletti FM, Sciorio R, Guido M. Progesterone: the key factor of the beginning of life. Int J Mol Sci (2022) 23(22):14138. doi: 10.3390/ijms232214138
32. Herbemont C, Sarandi S, Boujenah J, Cedrin-Durnerin I, Sermondade N, Vivot A, et al. Should we consider day-2 and day-3 embryo morphology before day-5 transfer when blastocysts reach a similar good quality? Reprod BioMed Online (2017) 35(5):521–8. doi: 10.1016/j.rbmo.2017.07.014
33. Gardner DK, Schoolcraft WB. Culture and transfer of human blastocysts. Curr Opin Obstet Gynecol (1999) 11(3):307–11. doi: 10.1097/00001703-199906000-0013
34. Boomsma CM, Kavelaars A, Eijkemans MJ, Amarouchi K, Teklenburg G, Gutknecht D, et al. Cytokine profiling in endometrial secretions: a non-invasive window on endometrial receptivity. Reprod BioMed Online (2009) 18(1):85–94. doi: 10.1016/S1472-6483(10)60429-4
35. Mizrachi Y, Weissman A, Rozen G, Rogers PAW, Stern C, Polyakov A. Timing of progesterone luteal support in natural cryopreserved embryo transfer cycles: back to basics. Reprod BioMed Online (2022) 45(1):63–8. doi: 10.1016/j.rbmo.2022.03.021
36. Murray MJ, Meyer WR, Zaino RJ, Lessey BA, Novotny DB, Ireland K, et al. A critical analysis of the accuracy, reproducibility, and clinical utility of histologic endometrial dating in fertile women. Fertil Steril (2004) 81(5):1333–43. doi: 10.1016/j.fertnstert.2003.11.030
37. Diaz-Gimeno P, Ruiz-Alonso M, Blesa D, Bosch N, Martinez-Conejero JA, Alama P, et al. The accuracy and reproducibility of the endometrial receptivity array is superior to histology as a diagnostic method for endometrial receptivity. Fertil Steril (2013) 99(2):508–17. doi: 10.1016/j.fertnstert.2012.09.046
38. Saupstad M, Freiesleben NC, Skouby SO, Andersen LF, Knudsen UB, Petersen KB, et al. Preparation of the endometrium and timing of blastocyst transfer in modified natural cycle frozen-thawed embryo transfers (mNC-FET): a study protocol for a randomised controlled multicentre trial. BMJ Open (2019) 9(12):e031811. doi: 10.1136/bmjopen-2019-031811
39. van de Vijver A, Drakopoulos P, Polyzos NP, Van Landuyt L, Mackens S, Santos-Ribeiro S, et al. Vitrified-warmed blastocyst transfer on the 5th or 7th day of progesterone supplementation in an artificial cycle: a randomized controlled trial. Gynecol Endocrinol (2017) 33(10):783–6. doi: 10.1080/09513590.2017.1318376
40. An BGL, Chapman M, Tilia L, Venetis C. Is there an optimal window of time for transferring single frozen-thawed euploid blastocysts? a cohort study of 1170 embryo transfers. Hum Reprod (2022) 37(12):2797–807. doi: 10.1093/humrep/deac227
41. Fonttis AA, Napolitano R, Borda C. Successful pregnancy and delivery after delaying the initiation of progesterone supplementation in a postmenopausal donor oocyte recipient. Reprod BioMed Online (2004) 9(6):611–3. doi: 10.1016/s1472-6483(10)61769-5
42. van de Vijver A, Polyzos NP, Van Landuyt L, Mackens S, Stoop D, Camus M, et al. What is the optimal duration of progesterone administration before transferring a vitrified-warmed cleavage stage embryo? a randomized controlled trial. Hum Reprod (2016) 31(5):1097–104. doi: 10.1093/humrep/dew045
43. Escriba MJ, Bellver J, Bosch E, Sanchez M, Pellicer A, Remohi J. Delaying the initiation of progesterone supplementation until the day of fertilization does not compromise cycle outcome in patients receiving donated oocytes: a randomized study. Fertil Steril (2006) 86(1):92–7. doi: 10.1016/j.fertnstert.2005.12.048
44. Bartels CB, Ditrio L, Grow DR, O'Sullivan DM, Benadiva CA, Engmann L, et al. The window is wide: flexible timing for vitrified-warmed embryo transfer in natural cycles. Reprod BioMed Online (2019) 39(2):241–8. doi: 10.1016/j.rbmo.2019.04.003
45. Nawroth F, Ludwig M. What is the “ideal” duration of progesterone supplementation before the transfer of cryopreserved-thawed embryos in estrogen/progesterone replacement protocols? Hum Reprod (2005) 20(5):1127–34. doi: 10.1093/humrep/deh762
46. Wilcox AJ, Baird DD, Weinberg CR. Time of implantation of the conceptus and loss of pregnancy. N Engl J Med (1999) 340(23):1796–9. doi: 10.1056/NEJM199906103402304
47. Salker M, Teklenburg G, Molokhia M, Lavery S, Trew G, Aojanepong T, et al. Natural selection of human embryos: impaired decidualization of endometrium disables embryo-maternal interactions and causes recurrent pregnancy loss. PLoS (2010) 5(4):e10287. doi: 10.1371/journal.pone.0010287
48. Shi W, Zhou H, Chen L, Xue X, Shi J. Live birth rate following frozen-thawed blastocyst transfer is higher in high-grade day 6 blastocysts than in low-grade day 5 blastocysts. Front Endocrinol (Lausanne) (2023) 13:1066757. doi: 10.3389/fendo.2022.1066757
49. Niu X, Wang CT, Li R, Haddad G, Wang W. Is day 7 culture necessary for in vitro fertilization of cryopreserved/warmed human oocytes? Reprod Biol Endocrinol (2020) 18(1):4. doi: 10.1186/s12958-020-0565-9
50. Bourdon M, Pocate-Cheriet K, Finet de Bantel A, Grzegorczyk-Martin V, Amar Hoffet A, Arbo E, et al. Day 5 versus day 6 blastocyst transfers: a systematic review and meta-analysis of clinical outcomes. Hum Reprod (2019) 34(10):948–1964. doi: 10.1093/humrep/dez163
51. Wang SS, Sun HX. Blastocyst transfer ameliorates live birth rate compared with cleavage-stage embryos transfer in FreshIn VitroFertilization or intracytoplasmic sperm injection cycles: reviews and meta-analysis. Yonsei Med J (2014) 55(3):815–25. doi: 10.3349/ymj.2014.55.3.815
52. Magli MC, Jones GM, Gras L, Gianaroli L, Korman I, Trounson AO. Chromosome mosaicism in day 3 aneuploid embryos that develop to morphologically normal blastocysts in vitro. Hum Reprod (2000) 15(8):1781–6. doi: 10.1093/humrep/15.8.1781
53. Fernández-Shaw S, Cercas R, Braña C, Villas C, Pons I. Ongoing and cumulative pregnancy rate after cleavage-stage versus blastocyst-stage embryo transfer using vitrification for cryopreservation: impact of age on the results. J Assist Reprod Genet (2015) 32(2):177–84. doi: 10.1007/s10815-014-0387-9
54. Capalbo A, Hoffmann E, Cimadomo D, Maria Ubaldi F, Rienzi L. Human female meiosis revised: new insights into the mechanisms of chromosome segregation and aneuploidies from advanced genomics and time-lapse imaging. Hum Reprod Update (2017) 23(6):706–22. doi: 10.1093/humupd/dmx026
55. Sullivan EA, Wang YA, Hayward I, Chambers GM, Illingworth P, McBain J, et al. Single embryo transfer reduces the risk of perinatal mortality, a population study. Hum Reprod (2012) 27(12):3609–15. doi: 10.1093/humrep/des315
56. Su W, Xu J, Arhin SK, Liu C, Zhao J, Lu X. The feasibility of all-Blastocyst-Culture and single blastocyst transfer strategy in elderly women: a retrospective analysis. BioMed Res Int (2020) 2020:5634147. doi: 10.1155/2020/5634147
57. Mancuso A, Kapfhamer J. With a good quality blastocyst, single embryo transfer remains the best choice. Fertil Steril (2018) 110(4):631. doi: 10.1016/j.fertnstert.2018.06.024
58. Wasielak-Politowska M, Kordowitzki P. Chromosome segregation in the oocyte: what goes wrong during aging. Int J Mol Sci (2022) 23(5):2880. doi: 10.3390/ijms23052880
59. Dai X, Gao T, Xia X, Cao F, Yu C, Li T, et al. Analysis of biochemical and clinical pregnancy loss between frozen-thawed embryo transfer of blastocysts and day 3 cleavage embryos in young women: a comprehensive comparison. Front Endocrinol (Lausanne (2021) 12:785658. doi: 10.3389/fendo.2021.785658
Keywords: artificial endometrial preparation, endometrial transformation time, frozen-thawed embryo transfer (FET), implantation rate, pregnancy outcome, window of implantation (WOI)
Citation: Liu L, Zhou H, Hu J, Sun X, Liu D and Huang G (2023) Association between duration of progesterone supplementation and clinical outcomes in artificial frozen-thawed embryo transfer cycles. Front. Endocrinol. 14:1193826. doi: 10.3389/fendo.2023.1193826
Received: 25 March 2023; Accepted: 22 June 2023;
Published: 27 July 2023.
Edited by:
Emre Pabuccu, Ufuk University, TürkiyeReviewed by:
Hongzhan Zhang, Shenzhen Zhongshan Urological Hospital, ChinaCopyright © 2023 Liu, Zhou, Hu, Sun, Liu and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling Liu, ZXllOTlAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.