- Center for Reproductive Medicine, Guangdong Women and Children Hospital, Guangzhou, Guangdong, China
Objective: To investigate whether serum LH levels on hCG trigger day are associated with live birth rate (LBR) after fresh embryo transfer with GnRH antagonist regimen in different populations.
Methods: This study was a retrospective study. A total of 3059 fresh embryo transfers were divided into three populations: predicted normal ovarian responders (NOR) (n=2049), patients with PCOS (n=533), and predicted poor ovarian responders (POR) (n=477). Each population was stratified into three groups based on LH levels: < 25th percentile, 25–75th percentile, and > 75th percentile. The primary outcome of the study was LBR, and secondary outcomes included implantation, clinical pregnancy, and early pregnancy loss rates. Univariable and multivariable regression analyses were performed to adjust for potential confounders.
Results: In NOR, compared to the reference group (>75th percentile), LBR was significantly lower in the < 25th percentile group (adjusted OR=0.662; 95%CI, 0.508-0.863) and 25-75th percentile group (adjusted OR=0.791; 95%CI, 0.633-0.988). In PCOS patients, LBR decreased significantly in the < 25th percentile group (41.4%) compared to the 25-75th percentile group (53.7%) and > 75th percentile group (56.1%). In addition, the LBR was lower in the < 25th percentile group (33.6%) compared with the 25-75th percentile group (43.4%) and the>75th percentile group (42.0%) in POR, but this was not statistically significant.
Conclusions: High serum LH levels are associated with increased LBR after fresh embryo transfer in GnRH antagonist cycles, which may be attributable to higher implantation rate. LH may be a predictor of whether to schedule fresh embryo transfer in IVF cycles for better clinical outcomes.
Introduction
Over the past decade, gonadotropin-releasing hormone (GnRH) antagonist regimen has emerged as one of the leading controlled ovarian hyperstimulation (COH) regimens due to its comparable convenience, safety, and efficacy compared to GnRH agonist (1–3). The influence of GnRH antagonist on clinical outcomes of fresh embryo transfer has become a matter of debate (1, 3). Although some studies have not found significant difference in pregnancy outcomes between GnRH antagonists and agonists, others have reported that GnRH antagonists were associated with lower clinical pregnancy and live birth rates after fresh embryo transfer compared with GnRH agonists (4–8). A meta-analysis showed that in the general IVF population, when patients with polycystic ovary syndrome (PCOS) or poor responders were excluded, GnRH antagonists were associated with lower ongoing pregnancy rate compared to agonists (9).
Although the ideal number of oocytes and embryos can be obtained with COH, the implantation rate after fresh embryo transfer is still relatively low in GnRH antagonist cycles (1, 3). An important issue regarding the use of GnRH antagonist is the inability to predict factors that may affect pregnancy outcomes in fresh IVF cycles, which is critical in deciding whether fresh embryo transfer should be selected.
It has been demonstrated that LH not only plays an important role in follicle development, ovulation, and steroidogenesis, but also affects luteal function and endometrial development (10, 11). GnRH antagonist cause a rapid and profound inhibition of endogenous LH secretion, which occurs when follicle and endometrium development are most sensitive to LH activity (12–14). The function of LH in the ovary by binding to LH/choriogonadotropin receptor (LHCGR) is well known (15, 16). Previous studies have identified LHCGR expression in the uterus, suggesting that LH may affect endometrial receptivity and placentation (17, 18). However, the specific mechanism by which LH affects implantation process remains largely unknown.
The question of whether LH concentrations in GnRH antagonist cycles are associated with pregnancy outcomes after fresh embryo transfer remains relatively sparse and controversial. Previous studies have identified the possible effects of premature LH increase as a sign of premature luteinization, which may lead to reduced oocyte yield and poor embryo implantation as a result of elevated progesterone (19, 20). Nonetheless, LH levels during stimulation are not always associated with progesterone elevation, which may be related to inconsistent findings on whether it affects clinical outcomes (21). Some studies showed that LH levels did not affect clinical outcomes (22–24), and yet others found that low LH levels were associated with adverse pregnancy outcomes (25–28). These controversial results may be related to the heterogeneity of the populations enrolled in different studies, and various control methods for confounders among studies, which require further research to clarify.
The aim of this study was to investigate the association between LH levels on hCG trigger day and live birth rate (LBR), with a particular focus on different populations.
Materials and methods
Study design and participants
This study was a retrospective study performed at the Reproductive Medicine Center of Guangdong Women and Children’s Hospital. The Institutional Review Board of the hospital has approved the study protocol. This study included patients undergoing their first fresh embryo transfer using GnRH antagonist regimen between January 2014 and October 2020. The inclusion criteria were as follows: (i) maternal age ≤ 40 years; (ii) day-3 fresh embryo transfer; (iii) at least one embryo was available. The exclusion criteria were as follows: (i) uterine abnormalities and intrauterine adhesion; (ii) endometrium thickness on hCG trigger day < 7 mm; (iii) recurrent spontaneous abortion; (iv) hypothalamic or pituitary amenorrhea (29, 30); (v) core data missing. In this study, we conducted separate analyses of three populations: predicted normal ovarian responders (NOR), patients with PCOS and predicted poor ovarian responders (POR). PCOS was defined as patients who met two of the three criteria (oligo- and/or anovulation, hyperandrogenism, and PCOM) according to the revised Rotterdam Consensus (31). POR were considered: antral follicle count (AFC) < 5 or AMH < 1.1 ng/ml or previous adverse ovarian response. NOR were patients with normal ovarian reserve and regular menstrual cycle (9).
Ovarian stimulation
A flexible GnRH antagonist regimen was used for ovarian stimulation. In brief, recombinant follicle-stimulating hormone (Gonal-f; Merck Serono or Puregon; MSD, Organon) at a dose of 100 to 300 IU per day was administered on day 2 or 3 of the menstrual cycle. The doses were adjusted according to ovarian response assessed by ultrasound and measurement of serum hormone levels every 3 to 4 days. The GnRH antagonist (Ganirelix; MSD, Organon) was started when at least one follicle was ≥ 12 mm at a daily dose of 0.25 mg and continued until the day of hCG trigger. When at least three follicles measured 17 mm or at least two follicles reached 18 mm in diameter, hCG was administered at a dose of 6000 to 10000 IU to induce oocyte maturation. Oocyte retrieval was performed 35-36 hours later by transvaginal ultrasound-guided follicle aspiration, and oocytes were fertilized by either IVF or ICSI depending on sperm quality. Serum LH, estradiol (E2) and progesterone (P) levels were measured using an electrochemiluminescence immunoassay (Roche Diagnostics Inc., Germany) on the Roche Elecsys 2010 automated immunoassay analyser.
Embryo transfer and luteal phase support
On the third day after oocyte retrieval, a maximum of two embryos were routinely transferred. A good-quality embryo was defined as day 3 embryos with < 20% fragmentation, and regular-sized cells. Freeze-all strategy was performed in patients at high risk for ovarian hyperstimulation syndrome, abnormal endometrial morphology or thickness (e.g., endometrium thickness < 7 mm), and serum P levels ≥ 1.5 ng/ml during COH.
The luteal phase support was started one day after oocyte retrieval. Intramuscular progesterone (40 mg once daily) or a combination of vaginal progesterone sustained-release gel (Crinone 8%, 90mg once daily) and oral progesterone (Dydrogesterone, 10 mg twice daily) was administered until 10 weeks of gestation. The method of progesterone supplementation depends on patient preference, as there is no clear medical evidence that using one regimen is better than another (32–34).
Outcome measures
The primary outcome of the study was LBR, which was defined as the delivery of a live infant after 24 gestational weeks. The secondary outcomes included implantation rate (number of intrauterine sacs divided by number of embryos transferred), clinical pregnancy (presence of at least one gestational sac in the uterine cavity at 5 weeks after embryo transfer), and early pregnancy loss (spontaneous loss of clinical pregnancy before 12 weeks of gestation). Cycle outcomes included oocyte yield (ratio of the number of oocytes retrieved to the number of follicles with an average diameter >10 mm on hCG trigger day), normal fertilization rate (ratio of the number of two pronuclear fertilized eggs to the number of oocytes for insemination), usable cleavage embryo rate (ratio of available embryos to cleavage embryos on day 3), and good-quality embryo rate (ratio of good-quality embryos to normally fertilized cleavage embryos on day 3).
Statistical analysis
All statistical analyses were performed using the statistical software package (SPSS, version 22.0). We used the Kolmogorov–Smirnov test to determine whether continuous variables were normally distributed. Continuous variables were presented as mean with standard deviation (mean ± SD) or median with interquartile range (median (Q1, Q3)), and categorical variables were described as number with percentage. The variables between live birth and non-live birth groups were compared using Student’s t-test or Mann-Whitney U test, and Chi-square or Fisher’s exact test, as appropriate. P-value < 0.05 was considered statistically significant.
We then performed separate analyses of three populations: NOR, patients with PCOS, and POR. Univariable and multivariable logistic regression analyses were used to identify potential confounding factors that may be independently associated with live birth for three populations. Confounding factors were assessed by univariable analysis and then added into multivariable regression model for adjustment. In multivariable models, variables with significance in the univariable analysis at P < 0.10 or more and variables that may potentially have an effect on live birth (e.g., body mass index (BMI)) were included. To assess the impact of LH level on the incidence of clinical outcomes, first, univariable and multivariable regression analyses were performed in three populations when the variable LH level on hCG trigger day was used as a continuous variable to adjust for confounders (Supplementary Tables 1, 3). Then, each population was stratified into three groups according to the interquartile range of LH levels on hCG trigger day: < 25th percentile group, 25–75th percentile group, and > 75th percentile group. Using the LH > 75th percentile group as the reference group, adjusted odds ratio (OR) and 95% confidence interval (CI) for LBR in other categories were calculated.
Results
Study population
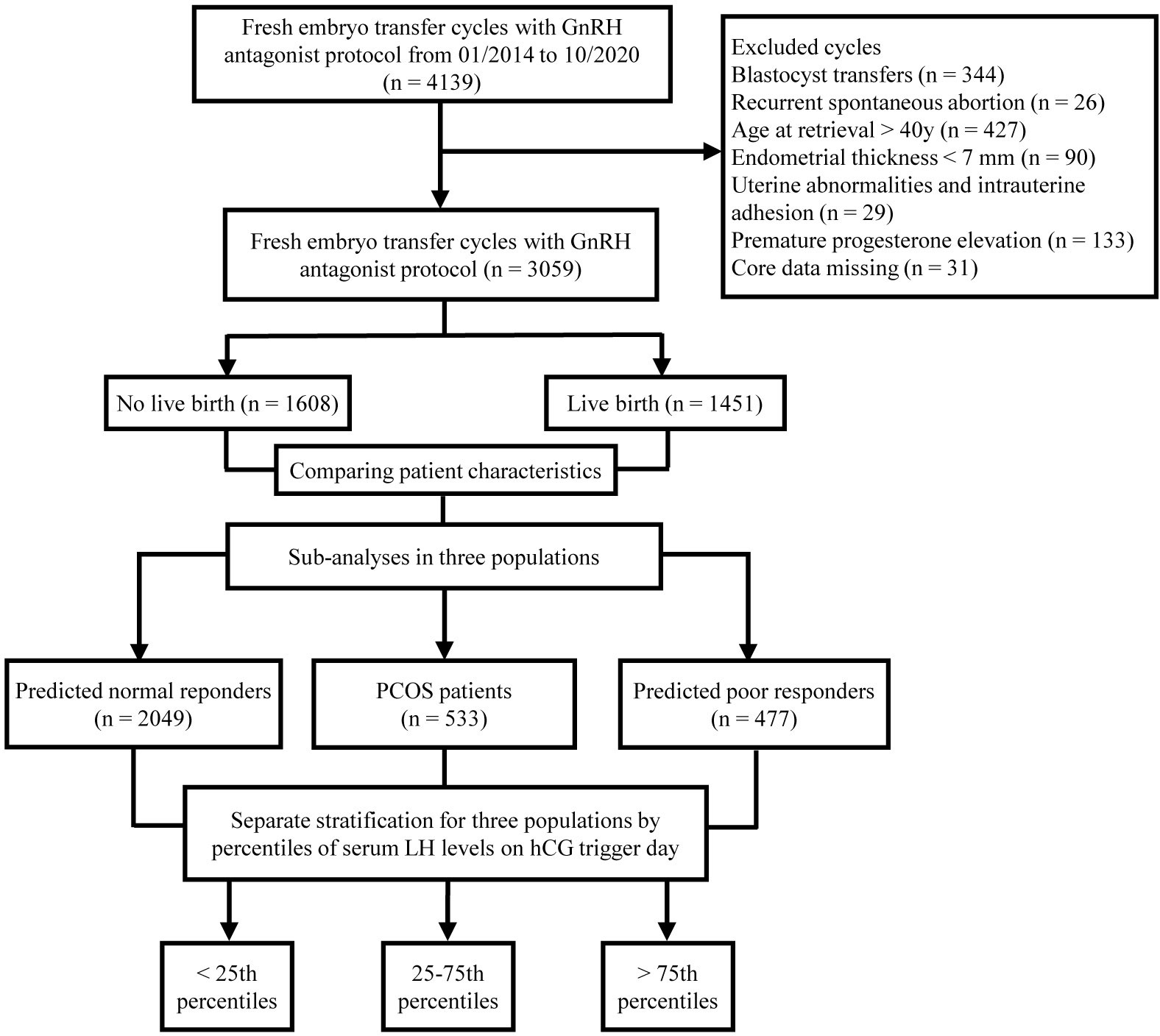
A total of 3059 patients who met the inclusion and exclusion criteria were included in this study. The flowchart of the study is shown in Figure 1. Among them, 1451 patients achieved live births, while 1608 patients did not achieve live births after fresh embryo transfer. Baseline characteristics were compared between patients who did and did not achieve live birth (Table 1). There were significant differences in terms of age, AFC, gonadotropin dose, days of stimulation, number of embryos transferred, rate of good-quality embryos transferred, and endometrial thickness on hCG trigger day between the two groups. Notably, serum LH levels on hCG trigger day were significantly higher in patients achieved live births than those of non-live births, while E2 and P levels did not show any significant differences (Table 1).
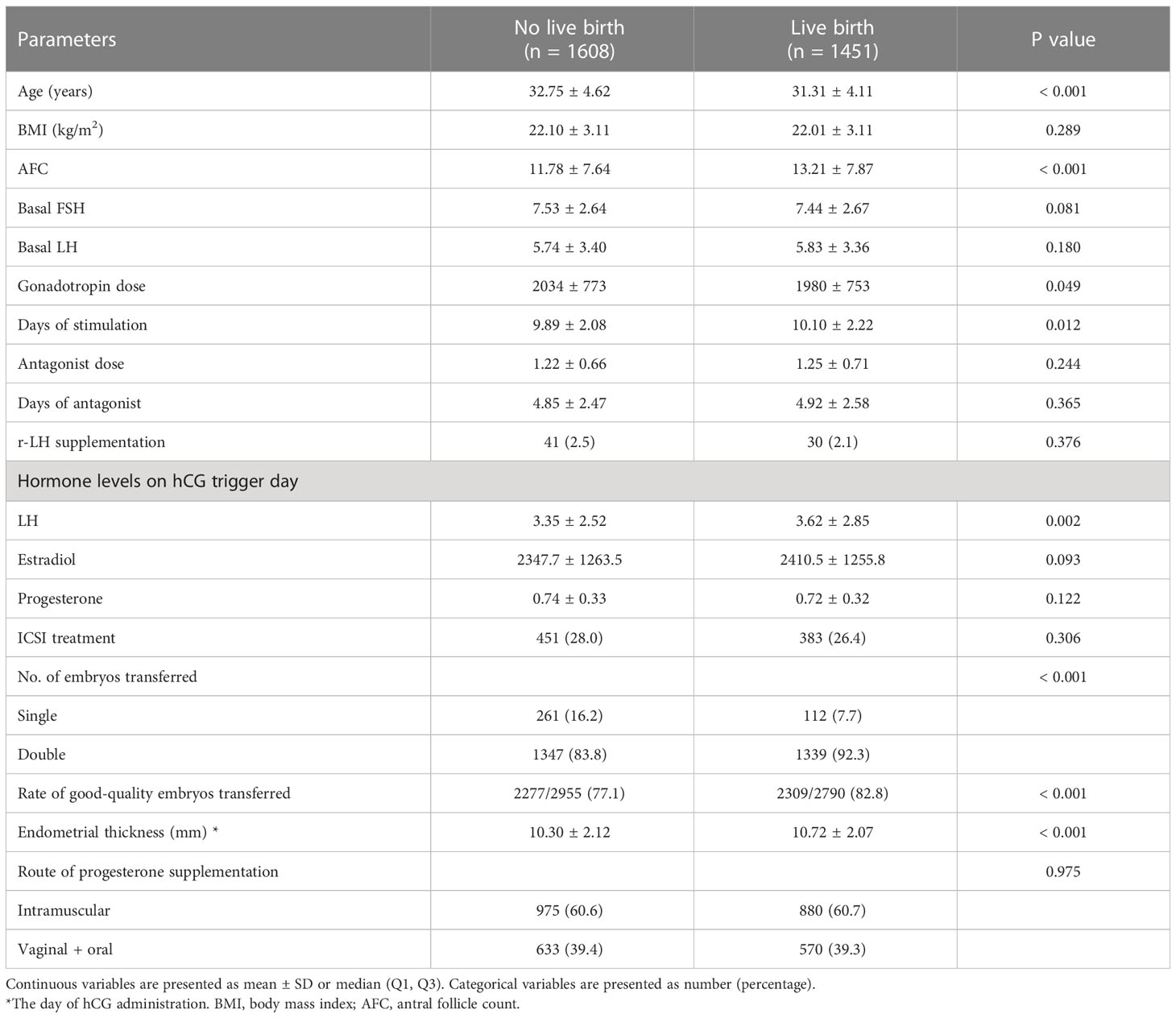
Table 1 A comparison of baseline characteristics according to whether patients achieved a live birth after fresh embryo transfer.
To investigate the relationship between LH levels and LBR in different populations, we divided patients into three categories: NOR (n=2049), those with PCOS (n=533) and POR (n=477). First, univariable and multivariable regression analyses were performed in three populations when the variable LH level on hCG trigger day was used as a continuous variable to adjust for confounders (Supplementary Tables 1, 3). Then, each population was stratified into three groups according to the interquartile range of LH levels on hCG trigger day. The interquartile range (25th to 75th) for LH in NOR was 1.62 to 3.86 mIU/ml; 2.25 to 5.68 mIU/ml in PCOS patients; and 2.14 to 4.72 mIU/ml in POR (Table 2).
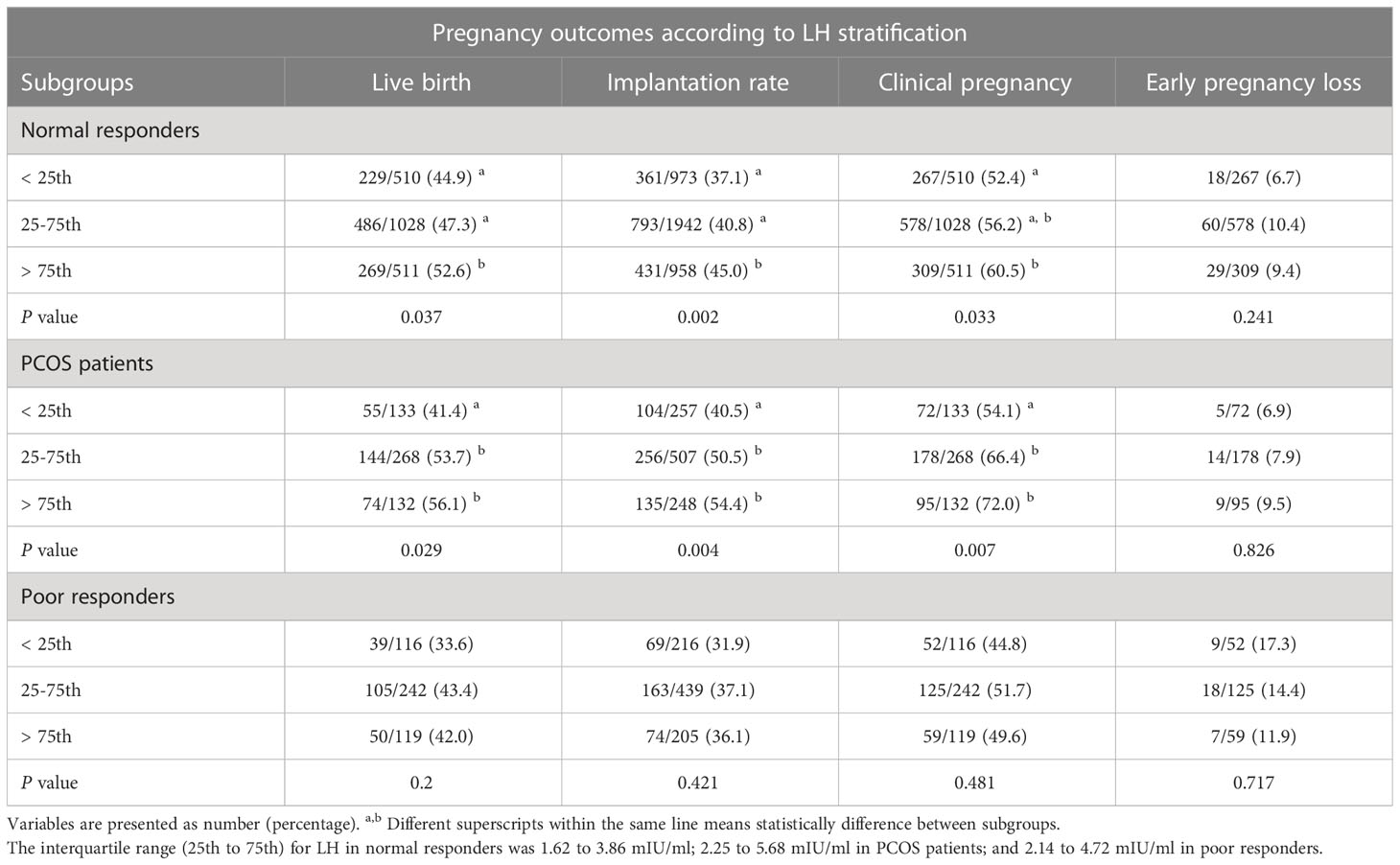
Table 2 Pregnancy outcomes after fresh embryo transfer according to LH stratification on hCG trigger day in different populations.
Predicted normal ovarian responders
In NOR, serum LH level on hCG trigger day as a continuous variable was positively associated with live birth in univariable analysis (OR=1.042; 95%CI, 1.002-1.083) (Supplementary Table 1). Pregnancy outcomes were then subdivided into three groups by LH stratification, as presented in Table 2. The LBR was significantly lower in the < 25th percentile (44.9%) and 25-75th percentile group (47.3%) than that of the>75th percentile group (52.6%). There was a significantly lower implantation rate for the < 25th percentile group (37.1%) and 25-75th percentile group (40.8%) compared with the>75th percentile group (45%). The clinical pregnancy rate was also found to be significantly lower in the < 25th percentile group than the>75th percentile group (52.4% vs. 60.5%). However, there was no significant difference in early pregnancy loss rate. Additionally, cycle outcomes including oocyte yield, normal fertilization rate, rate of usable cleavage embryos, and rate of good-quality embryos did not differ significantly between three groups (Supplementary Table 2).
After controlling for age, BMI, AFC, basal LH, gonadotropin dose, E2 and P levels on hCG trigger day, number of embryos transferred, and endometrial thickness on hCG trigger day, LH levels on hCG trigger day as a continuous variable were positively associated with live birth (OR=1.060; 95%CI, 1.016-1.105) (Table 3). Furthermore, when LH was stratified as a categorical variable, multivariable analysis revealed that LBR significantly decreased in the < 25th percentile group (adjusted OR=0.662; 95%CI, 0.508-0.863) and 25-75th percentile group (adjusted OR=0.791; 95%CI, 0.633-0.988), compared to the>75th percentile group (Figure 2). The other variables with a significant impact on live birth were age (adjusted OR=0.936; 95%CI, 0.914-0.958), number of embryos transferred (adjusted OR=2.897; 95%CI, 2.109-3.978) and endometrial thickness on hCG trigger day (adjusted OR=1.069; 95%CI, 1.023-1.116).
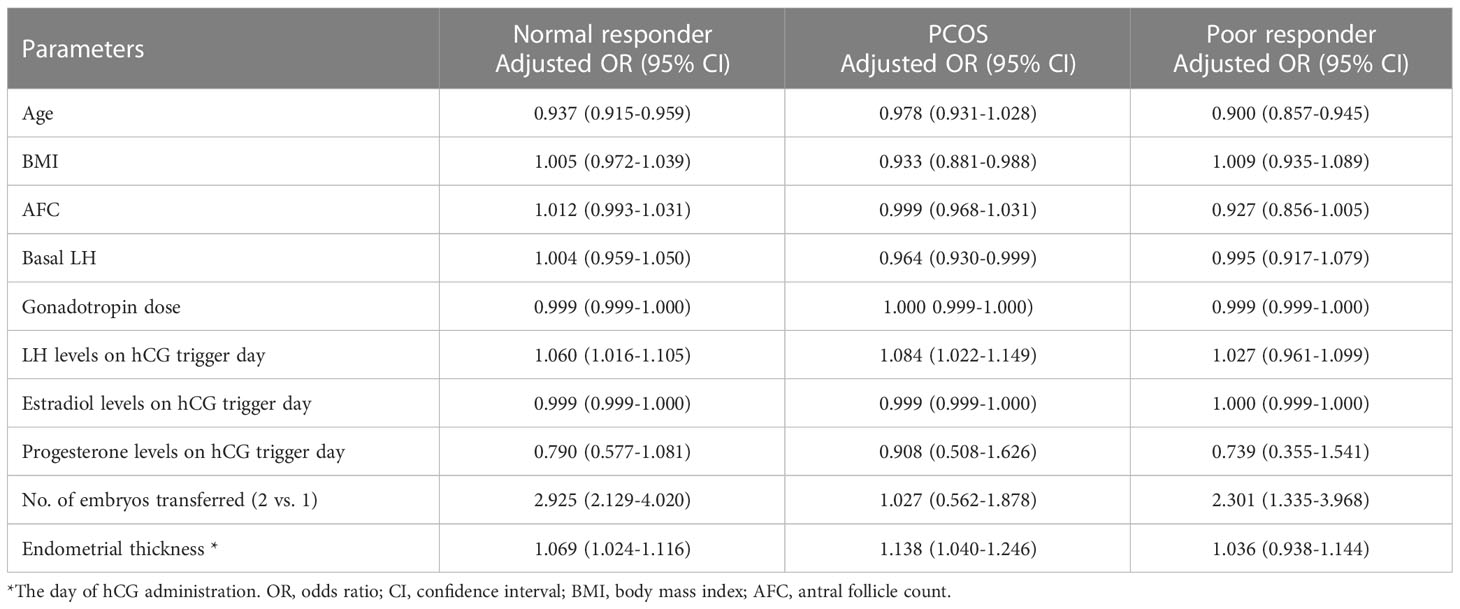
Table 3 Multivariable regression analysis for live birth after fresh embryo transfer for different populations.
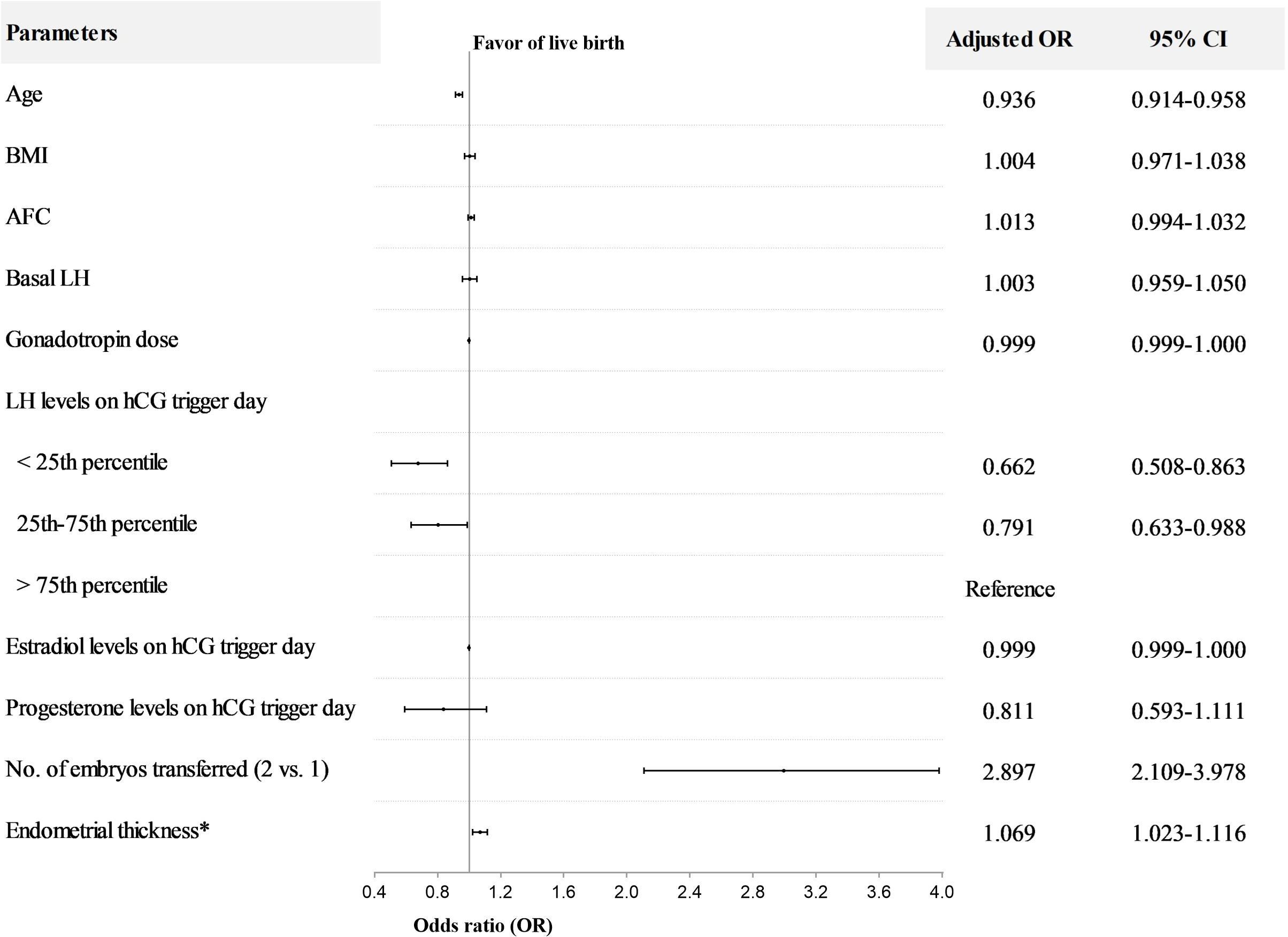
Figure 2 Multivariable regression analysis for live birth after fresh embryo transfer in normal responders. *The day of hCG administration. OR, odds ratio; CI, confidence interval; BMI, body mass index; AFC, antral follicle count. The interquartile range (25th to 75th) for LH was 1.62 to 3.86 mIU/ml in normal responders.
Women with PCOS
In PCOS patients, LH level on hCG trigger day as a continuous variable was positively associated with live birth in both univariable (OR=1.066; 95%CI, 1.011-1.124) and multivariable analyses (adjusted OR = 1.084; 95% CI, 1.022–1.149), with results similar to those of NOR (Supplementary Tables 1, 3). The PCOS patients were also stratified into three groups by LH stratification. The LBR in the < 25th percentile group (41.4%) was significantly lower than the 25-75th percentile group (53.7%) and >75th percentile group (56.1%). In addition, both implantation and clinical pregnancy rates decreased significantly in the < 25th percentile group compared to the 25-75th percentile group and >75th percentile group (Table 2). The incidence of early pregnancy loss did not differ among the three groups. In terms of cycle outcomes, the rate of good-quality embryos in the < 25th percentile group (76.4%) was significantly lower than the 25-75th percentile group (80.4%) and >75th percentile group (82.1%), while oocyte yield, normal fertilization rate, and rate of usable cleavage embryos did not show any difference between groups (Supplementary Table 2). After adjusting for confounders, the LBR decreased significantly in the < 25th percentile group compared with the>75th percentile group (adjusted OR=0.479; 95%CI, 0.277-0.828) (Figure 3). Moreover, for PCOS patients, BMI (adjusted OR=0.931; 95%CI, 0.878-0.987), basal LH (adjusted OR=0.962; 95%CI, 0.928-0.998), and endometrial thickness on hCG trigger day (adjusted OR=1.135; 95%CI, 1.036-1.244) were also independent predictors of live birth in multivariable model (Figure 3).
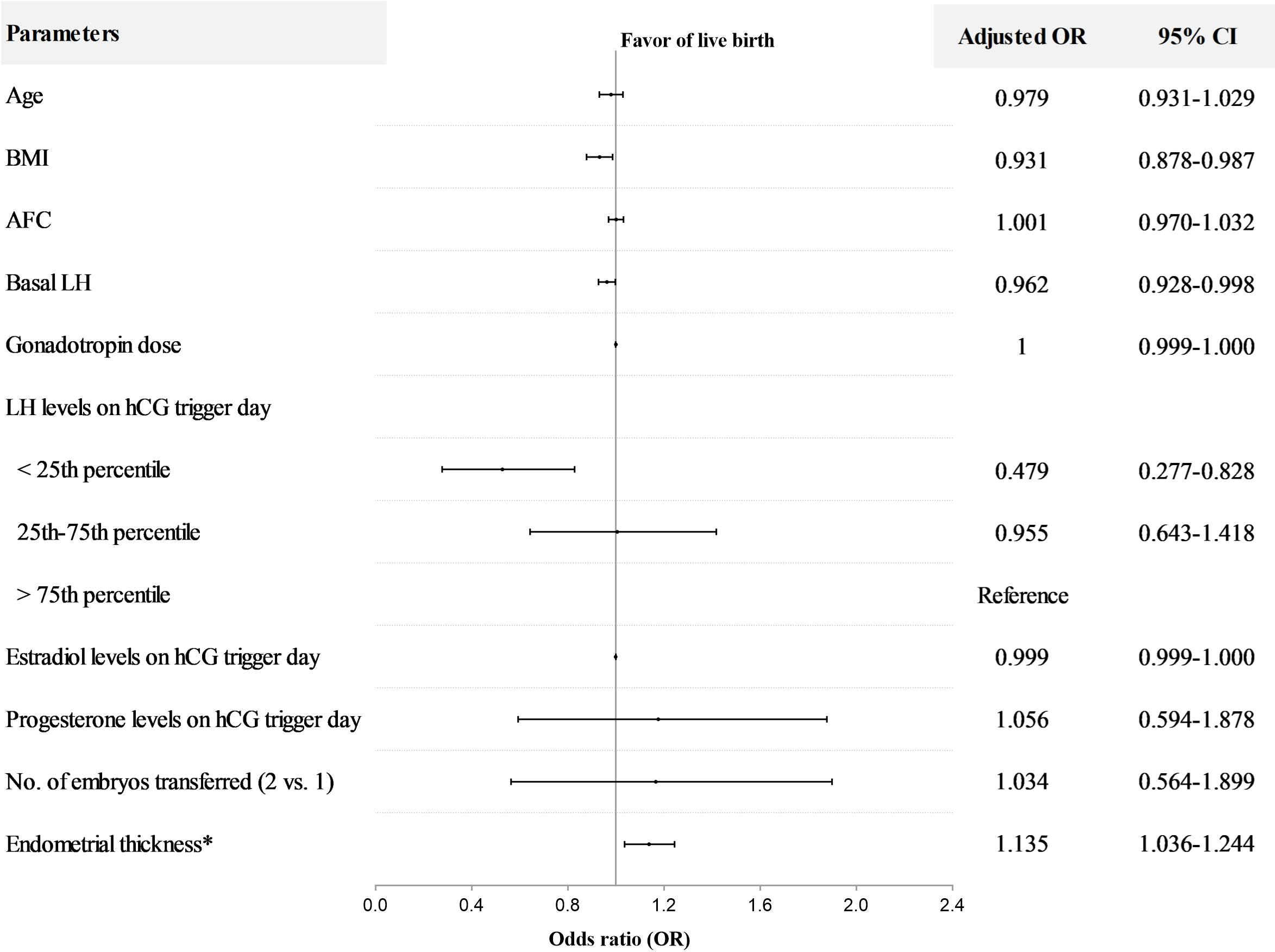
Figure 3 Multivariable regression analysis for live birth after fresh embryo transfer in PCOS patients. *The day of hCG administration. OR, odds ratio; CI, confidence interval; BMI, body mass index; AFC, antral follicle count. The interquartile range (25th to 75th) for LH was 2.25 to 5.68 mIU/ml in PCOS patients.
Predicted poor ovarian responders
In patients with POR, no evidence of a statistical association between LH levels as a continuous variable and live birth was found in both univariable (OR=1.008; 95%CI, 0.949-1.072) and multivariable analyses (adjusted OR=1.027; 95%CI, 0.961-1.099) (Supplementary Tables 1, 2). However, the LBR appeared to be lower in the < 25th percentile group (33.6%) compared with the 25-75th percentile group (43.4%) and the>75th percentile group (42.0%), but this was not statistically significant (Table 2). Cycle outcomes showed that oocyte yield decreased significantly in the < 25th percentile group (89.7%) compared with the 25-75th percentile group (93.4%) and>75th percentile group (92.8%). Nonetheless, other cycle outcomes did not present any significant difference among groups (Supplementary Table 2). In addition, controlling for major covariates, age was inversely associated with live birth (adjusted OR = 0.900; 95% CI, 0.857–0.945), whereas the number of embryos transferred was positively associated with live birth in POR (adjusted OR = 2.301; 95% CI, 1.335–3.968) (Table 3).
Discussion
This study investigated the impact of serum LH levels on hCG trigger day on LBR after fresh embryo transfer in consideration of different populations for the first time. The absolute risk reduction of LBR was 7.7% in NOR and 14.7% in PCOS patients when comparing the low LH group with the high LH group. In POR, the low LH group appeared to have a lower LBR than the middle and high LH groups, but not significantly, which may be related to insufficient patients enrolled.
A few available studies have investigated the effect of LH levels on LBR after fresh embryo transfer with GnRH antagonist regimen. However, previous studies have yielded conflicting results. Contrary to our findings, Marviel et al. showed no significant difference in clinical outcomes between different LH levels on the day of hCG administration, divided into two groups based on an arbitrary threshold of 0.5 IU/L (n=270) (22). In their study, potential bias was reduced by only selecting relatively homogenous population without adjusting for confounders that could have had impact on clinical outcomes. Similarly, Griesinger et al. reported that LH concentrations on day 8 of stimulation were not associated with ongoing pregnancy rates (24). Their retrospective study included 1764 patients pooled from six clinical trials with different purposes, which resulted in a huge heterogenous population. Many confounders affecting pregnancy rate may not have been adequately accounted for, including endometrial thickness and the number of embryos transferred. The study by Luo et al. was consistent with our conclusion, and their results showed that low LH significantly reduced LBR after fresh embryo transfer (38.0% vs. 51.5%) (28). They included 1480 normogonadotropic women underwent COH, and arbitrarily divided patients into low and high LH groups with a cutoff of 4 IU/L, which may not be an appropriate threshold for stratification. In a retrospective study of 619 cycles, Chen et al. found that LH ≤ 0.8 mIU/ml during COH was associated with higher early pregnancy loss rate, but no significant difference in implantation and live birth rates (26). Most previous studies have failed to adequately consider different populations, and they used an arbitrary LH threshold for grouping. Some studies included small sample sizes or did not use LBR as the primary outcome, which may not be sufficient to draw valid conclusions.
The present study provides the largest (n=3059) and well-controlled analysis of the relationship between LH levels and LBR after fresh embryo transfer. The particular strength of this study is that it took into account patient types as comprehensively as possible, as well as the presence of various potential confounders. Great efforts were taken to minimize sources of bias, particularly through the use of univariable and multivariable regression models. Unlike previous studies, which either only included normogonadotropic women or did not differentiate between different populations, the present study aims to investigate the association between LH levels and LBR in three populations: NOR, patients with PCOS and POR. Confounding factors were also minimized by making each population more homogeneous. Most studies used only a single LH value to arbitrarily define low and high LH, which was insufficient to reflect the distribution of LH levels across the population, and statistical difference may be compromised when choosing inappropriate LH level for stratification. In our study, LH levels were used as both continuous and categorical variables to examine the effect of LH levels on clinical outcomes in three populations. We stratified different populations using the 25th and 75th percentiles of LH levels, respectively, and tried to find an appropriate LH cutoff for each population, which might be more applicable to clinical practice. Nowadays, clinicians are more familiar the “one size does not fit all” concept. The analyses of different populations provide new perspectives to objectively consider different treatments for personalized patient characteristics, thereby may contribute to better clinical outcomes.
The present study has certain limitations due to its retrospective design. The large sample size of this study may partially reduce selection and statistical bias, nevertheless, the numbers of PCOS and POR patients remained small, which may potentially affect statistical efficiency. In the present study, we did not find significant deleterious effect of LH levels on oocyte performance. However, we only included fresh transfer cycles to analyze oocyte performance and embryonic development, so the results should be interpreted with caution. When progesterone levels were ≥ 1.5 ng/ml during stimulation, we use freeze-all strategy to rule out adverse effect of premature luteinization on fresh IVF outcomes, based on previous studies (35–38). However, we could not exclude the possibility that there may still be an association between progesterone levels and LBR, as the cutoff value for progesterone levels to affect LBR has been inconsistent in previous studies. Thus, we included progesterone levels in the multivariable models. The role of adding recombinant LH (r-LH) remains controversial, despite numerous clinical trials on this issue (39–43). The r-LH supplementation in this study depended on the physician preference, and the number of cycles with r-LH supplementation (n=71) was insufficient to draw conclusion. Future studies are needed to further reveal whether r-LH supplementation has an impact on clinical outcomes, especially with different groupings of LH levels. Besides, LH levels can vary and fluctuate significantly, and measured levels in the same individual may be significantly different one hour later, which may affect the applicability of the results. In addition, the relatively young age of the patients included in this study may have limited its applicability.
It is of most clinical importance to identify patients who could obtain better pregnancy outcomes after fresh embryo transfer. It seems that high LH levels predict benefits in implantation and live birth rates, even when only one or two embryos are available for transfer, which can be explained in several ways. First, successful implantation requires synchronization of endometrial receptivity and embryo development (44, 45). Previous studies have shown that pregnancy outcomes in FET cycles were significantly higher than those in fresh IVF cycles using GnRH antagonists (28, 46). These results suggest that endometrial receptivity is impaired during COH, an effect thought to be mediated by the negative impact of non-physiological levels of hormones on embryo-endometrium asynchrony (47–49). One possible explanation for our findings is that low LH may lead to embryo-endometrial asynchrony and defective placentation by binding to LHCGRs, which are widely expressed in the female reproductive tract (17, 18). It is speculated that high LH level on hCG trigger day without concomitant elevated progesterone may be associated with good endometrial receptivity. Second, while this works for most patients, a small proportion of patients may exhibit unexpected responses and may require individual evaluation. Individual differences in LH levels may be partly related to LH genotype, which cannot be assessed in our daily clinical practice. Third, many studies have shown an association between high E2 and adverse pregnancy outcomes in IVF cycles (35–38, 50–52). Therefore, we also included E2 levels on hCG trigger day in our regression models, which was not a predictor of LBR. Finally, patients with low LH actually had a better prognosis than those with higher LH, with younger age, better ovarian reserve and a higher rate of good-quality embryos transferred (Supplementary Table 3), and cycle outcomes including those related to oocyte and embryo quality did not show very significant differences between different LH stratifications (Supplementary Table 2), suggesting that embryo quality may not be responsible for the difference in implantation rates due to LH levels. However, it is unclear if there is a relationship between LH levels on the trigger day and clinical outcomes of FET cycles, which needs further research to find out. In PCOS patients, previous studies have suggested that excessive basal LH may be detrimental to clinical outcomes (11), which was similar to our finding that high basal LH negatively affected LBR in the multivariable model. Notably, on the conversely, high LH on hCG trigger day had a positive effect on LBR in the model.
In conclusion, there is a positive association between serum LH levels on hCG trigger day and LBR after fresh embryo transfer in both NOR and PCOS patients. LH may be a predictor of whether to schedule fresh embryo transfer in IVF cycles for better clinical outcomes. Further studies are required to uncover the underlying mechanism to better understand the relationship between LH and LBR in GnRH antagonist cycles.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
RZ and FL conceived and designed the study. All the authors analyzed and interpreted the data. LH contributed to the data collection. RZ performed the statistical analysis and wrote the article. FL and XQZ revised the article. All the authors approved the final version of the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors thank all the staff of the Reproductive Medicine Center of Guangdong Women and Children’s Hospital for their cooperation and support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1191827/full#supplementary-material
References
1. Depalo R, Jayakrishan K, Garruti G, Totaro I, Panzarino M, Giorgino F, et al. GnRH agonist versus GnRH antagonist in in vitro fertilization and embryo transfer (IVF/ET). Reprod Biol Endocrinol (2012) 10:26. doi: 10.1186/1477-7827-10-26
2. Toftager M, Bogstad J, Løssl K, Prætorius L, Zedeler A, Bryndorf T, et al. Cumulative live birth rates after one ART cycle including all subsequent frozen-thaw cycles in 1050 women: secondary outcome of an RCT comparing GnRH-antagonist and GnRH-agonist protocols. Hum Reprod (2017) 32(3):556–67. doi: 10.1093/humrep/dew358
3. Al-Inany HG, Youssef MA, Ayeleke RO, Brown J, Lam WS, Broekmans FJ. Gonadotrophin-releasing hormone antagonists for assisted reproductive technology. Cochrane Database Syst Rev (2016) 4(4):Cd001750. doi: 10.1002/14651858.CD001750.pub4
4. Albano C, Felberbaum RE, Smitz J, Riethmüller-Winzen H, Engel J, Diedrich K, et al. Ovarian stimulation with HMG: results of a prospective randomized phase III European study comparing the luteinizing hormone-releasing hormone (LHRH)-antagonist cetrorelix and the LHRH-agonist buserelin. Eur Cetrorelix Study Group Hum Reprod (2000) 15(3):526–31. doi: 10.1093/humrep/15.3.526
5. Borm G, Mannaerts B. Treatment with the gonadotrophin-releasing hormone antagonist ganirelix in women undergoing ovarian stimulation with recombinant follicle stimulating hormone is effective, safe and convenient: results of a controlled, randomized, multicentre trial. Eur Orgalutran Study Group Hum Reprod (2000) 15(7):1490–8.
6. European and Middle East Orgalutran Study Group. Comparable clinical outcome using the GnRH antagonist ganirelix or a long protocol of the GnRH agonist triptorelin for the prevention of premature LH surges in women undergoing ovarian stimulation. Hum Reprod (2001) 16(4):644–51.
7. Fluker M, Grifo J, Leader A, Levy M, Meldrum D, Muasher SJ, et al. Efficacy and safety of ganirelix acetate versus leuprolide acetate in women undergoing controlled ovarian hyperstimulation. Fertil Steril (2001) 75(1):38–45. doi: 10.1016/S0015-0282(00)01638-1
8. Al-Inany H, Aboulghar M. GnRH antagonist in assisted reproduction: a cochrane review. Hum Reprod (2002) 17(4):874–85. doi: 10.1093/humrep/17.4.874
9. Lambalk CB, Banga FR, Huirne JA, Toftager M, Pinborg A, Homburg R, et al. GnRH antagonist versus long agonist protocols in IVF: a systematic review and meta-analysis accounting for patient type. Hum Reprod Update (2017) 23(5):560–79. doi: 10.1093/humupd/dmx017
10. Tavaniotou A, Albano C, Smitz J, Devroey P. Impact of ovarian stimulation on corpus luteum function and embryonic implantation. J Reprod Immunol (2002) 55(1-2):123–30. doi: 10.1016/S0165-0378(01)00134-6
11. Filicori M. The role of luteinizing hormone in folliculogenesis and ovulation induction. Fertil Steril (1999) 71(3):405–14. doi: 10.1016/S0015-0282(98)00482-8
12. Griesinger G, Diedrich K. Role of LH in ovarian stimulation: considerations. Reprod BioMed Online (2006) 12(4):404–6. doi: 10.1016/S1472-6483(10)61990-6
13. Huirne JA, van Loenen AC, Schats R, McDonnell J, Hompes PG, Schoemaker J, et al. Dose-finding study of daily GnRH antagonist for the prevention of premature LH surges in IVF/ICSI patients: optimal changes in LH and progesterone for clinical pregnancy. Hum Reprod (2005) 20(2):359–67. doi: 10.1093/humrep/deh601
14. Lyttle Schumacher BM, Mersereau JE, Steiner AZ. Cycle day, estrogen level, and lead follicle size: analysis of 27,790 in vitro fertilization cycles to determine optimal start criteria for gonadotropin-releasing hormone antagonist. Fertil Steril (2018) 109(4):633–7. doi: 10.1016/j.fertnstert.2017.12.021
15. Sacchi S, Sena P, Degli Esposti C, Lui J, La Marca A. Evidence for expression and functionality of FSH and LH/hCG receptors in human endometrium. J Assist Reprod Genet (2018) 35(9):1703–12. doi: 10.1007/s10815-018-1248-8
16. Lévy DP, Navarro JM, Schattman GL, Davis OK, Rosenwaks Z. The role of LH in ovarian stimulation: exogenous LH: let's design the future. Hum Reprod (2000) 15(11):2258–65. doi: 10.1093/humrep/15.11.2258
17. Rao CV. Multiple novel roles of luteinizing hormone. Fertil Steril (2001) 76(6):1097–100. doi: 10.1016/S0015-0282(01)02863-1
18. Gridelet V, Perrier d'Hauterive S, Polese B, Foidart JM, Nisolle M, Geenen V. Human chorionic gonadotrophin: new pleiotropic functions for an "Old" hormone during pregnancy. Front Immunol (2020) 11:343. doi: 10.3389/fimmu.2020.00343
19. Griesinger G, Dawson A, Schultze-Mosgau A, Finas D, Diedrich K, Felberbaum R. Assessment of luteinizing hormone level in the gonadotropin-releasing hormone antagonist protocol. Fertil Steril (2006) 85(3):791–3. doi: 10.1016/j.fertnstert.2005.08.048
20. Bosch E, Labarta E, Crespo J, Simón C, Remohí J, Jenkins J, et al. Circulating progesterone levels and ongoing pregnancy rates in controlled ovarian stimulation cycles for in vitro fertilization: analysis of over 4000 cycles. Hum Reprod (2010) 25(8):2092–100. doi: 10.1093/humrep/deq125
21. Bosch E, Valencia I, Escudero E, Crespo J, Simón C, Remohí J, et al. Premature luteinization during gonadotropin-releasing hormone antagonist cycles and its relationship with in vitro fertilization outcome. Fertil Steril (2003) 80(6):1444–9. doi: 10.1016/j.fertnstert.2003.07.002
22. Merviel P, Antoine JM, Mathieu E, Millot F, Mandelbaum J, Uzan S. Luteinizing hormone concentrations after gonadotropin-releasing hormone antagonist administration do not influence pregnancy rates in in vitro fertilization-embryo transfer. Fertil Steril (2004) 82(1):119–25. doi: 10.1016/j.fertnstert.2003.11.040
23. Doody K, Devroey P, Gordon K, Witjes H, Mannaerts B. LH concentrations do not correlate with pregnancy in rFSH/GnRH antagonist cycles. Reprod BioMed Online (2010) 20(4):565–7. doi: 10.1016/j.rbmo.2009.12.019
24. Griesinger G, Shapiro DB, Kolibianakis EM, Witjes H, Mannaerts BM. No association between endogenous LH and pregnancy in a GnRH antagonist protocol: part II, recombinant FSH. Reprod BioMed Online (2011) 23(4):457–65. doi: 10.1016/j.rbmo.2011.06.016
25. Esposito MA, Barnhart KT, Coutifaris C, Patrizio P. Role of periovulatory luteinizing hormone concentrations during assisted reproductive technology cycles stimulated exclusively with recombinant follicle-stimulating hormone. Fertil Steril (2001) 75(3):519–24. doi: 10.1016/S0015-0282(00)01745-3
26. Chen CD, Chiang YT, Yang PK, Chen MJ, Chang CH, Yang YS, et al. Frequency of low serum LH is associated with increased early pregnancy loss in IVF/ICSI cycles. Reprod BioMed Online (2016) 33(4):449–57. doi: 10.1016/j.rbmo.2016.07.001
27. Westergaard LG, Laursen SB, Andersen CY. Increased risk of early pregnancy loss by profound suppression of luteinizing hormone during ovarian stimulation in normogonadotrophic women undergoing assisted reproduction. Hum Reprod (2000) 15(5):1003–8. doi: 10.1093/humrep/15.5.1003
28. Luo Y, Liu S, Su H, Hua L, Ren H, Liu M, et al. Low serum LH levels during ovarian stimulation with GnRH antagonist protocol decrease the live birth rate after fresh embryo transfers but have no impact in freeze-all cycles. Front Endocrinol (Lausanne) (2021) 12:640047. doi: 10.3389/fendo.2021.640047
29. Gordon CM, Ackerman KE, Berga SL, Kaplan JR, Mastorakos G, Misra M, et al. Functional hypothalamic amenorrhea: an endocrine society clinical practice guideline. J Clin Endocrinol Metab (2017) 102(5):1413–39. doi: 10.1210/jc.2017-00131
30. Klein DA, Paradise SL, Reeder RM. Amenorrhea: a systematic approach to diagnosis and management. Am Fam Physician (2019) 100(1):39–48.
31. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril (2004) 81(1):19–25.
32. Abdelhakim AM, Abd-ElGawad M, Hussein RS, Abbas AM. Vaginal versus intramuscular progesterone for luteal phase support in assisted reproductive techniques: a systematic review and meta-analysis of randomized controlled trials. Gynecol Endocrinol (2020) 36(5):389–97. doi: 10.1080/09513590.2020.1727879
33. Jiang L, Luo ZY, Hao GM, Gao BL. Effects of intramuscular and vaginal progesterone supplementation on frozen-thawed embryo transfer. Sci Rep (2019) 9(1):15264. doi: 10.1038/s41598-019-51717-5
34. Penzias AS. Luteal phase support. Fertil Steril (2002) 77(2):318–23. doi: 10.1016/S0015-0282(01)02961-2
35. Elgindy EA. Progesterone level and progesterone/estradiol ratio on the day of hCG administration: detrimental cutoff levels and new treatment strategy. Fertil Steril (2011) 95(5):1639–44. doi: 10.1016/j.fertnstert.2010.12.065
36. Lee CI, Chen HH, Huang CC, Lin PY, Lee TH, Lee MS. Early progesterone change associated with pregnancy outcome after fresh embryo transfer in assisted reproduction technology cycles with progesterone level of >1.5 ng/ml on human chorionic gonadotropin trigger day. Front Endocrinol (Lausanne) (2020) 11:653. doi: 10.3389/fendo.2020.00653
37. Vuong LN, Pham TD, Dang VQ, Ho TM, Ho VNA, Norman RJ, et al. Live birth rates with a freeze-only strategy versus fresh embryo transfer: secondary analysis of a randomized clinical trial. Reprod BioMed Online (2019) 38(3):387–96. doi: 10.1016/j.rbmo.2018.12.012
38. De Cesare R, Morenghi E, Cirillo F, Ronchetti C, Canevisio V, Persico P, et al. The role of hCG triggering progesterone levels: a real-world retrospective cohort study of more than 8000 IVF/ICSI cycles. Front Endocrinol (Lausanne) (2020) 11:547684. doi: 10.3389/fendo.2020.547684
39. De Placido G, Alviggi C, Perino A, Strina I, Lisi F, Fasolino A, et al. Recombinant human LH supplementation versus recombinant human FSH (rFSH) step-up protocol during controlled ovarian stimulation in normogonadotrophic women with initial inadequate ovarian response to rFSH. a multicentre, prospective, randomized controlled trial. Hum Reprod (2005) 20(2):390–6.
40. Kolibianakis EM, Kalogeropoulou L, Griesinger G, Papanikolaou EG, Papadimas J, Bontis J, et al. Among patients treated with FSH and GnRH analogues for in vitro fertilization, is the addition of recombinant LH associated with the probability of live birth? a systematic review and meta-analysis. Hum Reprod Update (2007) 13(5):445–52. doi: 10.1093/humupd/dmm008
41. Hill MJ, Levens ED, Levy G, Ryan ME, Csokmay JM, DeCherney AH, et al. The use of recombinant luteinizing hormone in patients undergoing assisted reproductive techniques with advanced reproductive age: a systematic review and meta-analysis. Fertil Steril (2012) 97(5):1108–14.e1. doi: 10.1016/j.fertnstert.2012.01.130
42. Gizzo S, Andrisani A, Noventa M, Manfè S, Oliva A, Gangemi M, et al. Recombinant LH supplementation during IVF cycles with a GnRH-antagonist in estimated poor responders: a cross-matched pilot investigation of the optimal daily dose and timing. Mol Med Rep (2015) 12(3):4219–29. doi: 10.3892/mmr.2015.3904
43. Griesinger G, Shapiro DB. Luteinizing hormone add-back: is it needed in controlled ovarian stimulation, and if so, when? J Reprod Med (2011) 56(7-8):279–300.
44. Carson DD, Bagchi I, Dey SK, Enders AC, Fazleabas AT, Lessey BA, et al. Embryo implantation. Dev Biol (2000) 223(2):217–37. doi: 10.1006/dbio.2000.9767
45. Paria BC, Reese J, Das SK, Dey SK. Deciphering the cross-talk of implantation: advances and challenges. Science (2002) 296(5576):2185–8. doi: 10.1126/science.1071601
46. Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen-thawed embryo transfer in normal responders. Fertil Steril (2011) 96(2):344–8. doi: 10.1016/j.fertnstert.2011.05.050
47. Rackow BW, Kliman HJ, Taylor HS. GnRH antagonists may affect endometrial receptivity. Fertil Steril (2008) 89(5):1234–9. doi: 10.1016/j.fertnstert.2007.04.060
48. Li F, Zhang M, Zhang Y, Liu T, Qu X. GnRH analogues may increase endometrial Hoxa10 promoter methylation and affect endometrial receptivity. Mol Med Rep (2015) 11(1):509–14. doi: 10.3892/mmr.2014.2680
49. Gómez E, Ruíz-Alonso M, Miravet J, Simón C. Human endometrial transcriptomics: implications for embryonic implantation. Cold Spring Harb Perspect Med (2015) 5(7):a022996.
50. Wei D, Yu Y, Sun M, Shi Y, Sun Y, Deng X, et al. The effect of supraphysiological estradiol on pregnancy outcomes differs between women with PCOS and ovulatory women. J Clin Endocrinol Metab (2018) 103(7):2735–42. doi: 10.1210/jc.2018-00613
51. Forman R, Fries N, Testart J, Belaisch-Allart J, Hazout A, Frydman R. Evidence for an adverse effect of elevated serum estradiol concentrations on embryo implantation. Fertil Steril (1988) 49(1):118–22. doi: 10.1016/S0015-0282(16)59661-7
Keywords: GnRH antagonist, live birth rate, controlled ovarian stimulation (COS), ovarian response, luteinizing hormone (LH)
Citation: Zhou R, Dong M, Huang L, Zhu X, Wei J, Zhang Q, Liu D, Zhang X and Liu F (2023) Association between serum LH levels on hCG trigger day and live birth rate after fresh embryo transfer with GnRH antagonist regimen in different populations. Front. Endocrinol. 14:1191827. doi: 10.3389/fendo.2023.1191827
Received: 22 March 2023; Accepted: 06 June 2023;
Published: 05 July 2023.
Edited by:
Jan Tesarik, MARGen Clinic, SpainReviewed by:
Tom Kelsey, University of St Andrews, United KingdomJing Shu, Zhejiang Provincial People’s Hospital, China
Alexander Quaas, University Hospital of Basel, Switzerland
Maren Goeckenjan, Technical University Dresden, Germany
Copyright © 2023 Zhou, Dong, Huang, Zhu, Wei, Zhang, Liu, Zhang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fenghua Liu, bGl1c2hpbmUyMDA2QDE2My5jb20=; Xiqian Zhang, NjUxNTU3MDc1QHFxLmNvbQ==
 Ruiqiong Zhou
Ruiqiong Zhou Mei Dong
Mei Dong Li Huang
Li Huang Fenghua Liu
Fenghua Liu