- 1Department of Cardiology, The Second Affiliated Hospital of Nanchang University, Nanchang, Jiangxi, China
- 2Center for Prevention and Treatment of Cardiovascular Diseases, The Second Affiliated Hospital of Nanchang University, Nanchang, Jiangxi, China
- 3Jiangxi Provincial Cardiovascular Disease Clinical Medical Research Center, Nanchang, Jiangxi, China
- 4Jiangxi Sub-center of National Clinical Research Center for Cardiovascular Diseases, Nanchang, Jiangxi, China
Background: Remnant cholesterol (RC) and chronic kidney disease (CKD) have not been definitively linked in individuals with different characteristics. This study aims to investigate the relationship between serum RC level and CKD and examine possible effect modifiers in Chinese patients with hypertension.
Methods: Our study is based on the Chinese H-type Hypertension Project, which is an observational registry study conducted in real-world settings. The outcome was CKD, defined as an estimated glomerular filtration rate of less than 60 ml/min·1.73 m2. Multivariate logistic regression and smooth curve fitting were used to analyze the association between RC and CKD. Subgroup analyses were subsequently conducted to examine the effects of other variables.
Results: The mean age of the 13,024 patients with hypertension at baseline was 63.8 ± 9.4 years, and 46.8% were male. A conspicuous linear positive association was observed between RC level and CKD (per SD increment; odds ratio [OR], 1.15; 95% confidence interval [CI], 1.08–1.23). Compared with the lowest quartile group of RC, the risk of CKD was 53% higher (OR, 1.53; 95% CI, 1.26–1.86) in the highest quartile group. Furthermore, a stronger positive association between RC level and CKD was found among participants with a higher body mass index (BMI <24 vs. ≥24 kg/m2; P-interaction = 0.034) or current non-smokers (smoker vs. non-smoker; P-interaction = 0.024).
Conclusions: Among Chinese adults with hypertension, RC level was positively associated with CKD, particularly in those with a BMI of ≥24 kg/m2 and current non-smokers. These findings may help improve lipid management regimens in patients with hypertension.
1 Introduction
Chronic kidney disease (CKD) has high morbidity and mortality, particularly among people with diabetes and hypertension (1). By 2040, CKD is predicted to become the fifth leading cause of mortality worldwide (2). Common factors promoting the occurrence and development of CKD include diabetes, hypertension, dyslipidemia, and smoking (3). Lipid metabolism disorders are common in patients with CKD. Previous studies have shown various lipid concentrations and structural changes, including higher triglyceride (TG) levels, in patients with CKD (4, 5). Nevertheless, most of the existing research is focused on traditional lipid profiles.
Remnant cholesterol (RC) is a recently described lipid indicator. It is the cholesterol component of triglyceride-rich lipoproteins, consisting of cholesterol cargo of very low-density lipoprotein (VLDL) and medium-density lipoprotein (IDL) in the fasting state and chylomicron (CM) remnants in the non-fasting state (6). Previous research has shown that recurrent atherosclerotic cardiovascular disease (ASCVD) can occur even when the low-density lipoprotein cholesterol (LDL-C) concentration is reduced to an optimal level. This residual risk is believed to be caused by remnant cholesterol (RC) (7, 8). As a new cholesterol index, in the past ten years, RC has been confirmed by extensive research to be related to the initiation and progression of atherosclerosis and ASCVD (9–11).
Previous studies have demonstrated inconsistent results regarding the association between RC levels and CKD. Some studies have shown that elevated levels of RC or its components (comprising VLDL and IDL cholesterol) are associated with an increased risk of CKD, whereas others have not found this association. However, the hypertensive population is unique and is a high-risk subgroup for CKD. A discussion of the relationship between RC and CKD in patients with hypertension may provide a new direction for lipid management in populations with hypertension in the future. Therefore, the association between RC levels and CKD risk in a Chinese population with hypertension needs to be assessed.
2 Materials and methods
2.1 Study population and design
This cross-sectional study used observational data from the Chinese hypertension Registry. The study was conducted in July 2018 in Wuyuan, Jiangxi Province, China. A total of 14,268 participants aged ≥18 years were included after excluding those who were unable to provide informed consent or failed to complete follow-up for various reasons. Patients with hypertension are defined as those with systolic blood pressure (SBP) ≥140 mmHg and/or diastolic blood pressure (DBP) ≥90 mmHg with a previous diagnosis of hypertension or currently taking antihypertensive medication. This study was approved by the Ethics Committee of the Biomedical Institute of Anhui Medical University, and written informed consent was obtained from all study participants.
Among the 14,268 participants, we excluded those without hypertension (n = 34), those with missing data for total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), LDL-C (n = 7), use of lipid-lowering drugs (n = 506), and individuals with extreme values of RC (n = 697). Ultimately, 13,024 participants were included in the final analysis (Figure S1).
2.2 Data collection
We collected basic information on all the participants, including sex, age, height, weight, waist circumference (WC), drinking status, and smoking status. All blood samples were collected by professionals to examine fasting TC, TG, HDL-C, LDL-C, aspartate aminotransferase (AST), alanine transaminase (ALT), plasma homocysteine (Hcy), fasting blood glucose (FBG), and serum creatinine levels. All parameters were tested using a professional instrument (Beckman Coulter) at the Biaojia Biotechnology Laboratory, Shenzhen, China.
Based on the previous description, the RC was calculated as RC = TC - (HDL-C) - (LDL-C). CKD was defined as an estimated glomerular filtration rate (eGFR) of less than 60 ml/min·1.73 m2. The eGFR was calculated according to the Chronic Kidney Disease Epidemiology Collaboration (12). Body mass index (BMI) was calculated by dividing the weight by the square of height.
2.3 Covariates
The selected covariates related to CKD included sex, age, WC, BMI, DBP, drinking status, smoking status, SBP, Hcy, TG, diabetes, coronary heart disease (CAD), stroke, and use of antihypertensive drugs.
2.4 Statistical analysis
For continuous variables, we used means with standard deviations (SDs) or median (interquartile range), and categorical variables included the characteristics of the study population (percentages). To demonstrate the relationship between RC levels and CKD more intuitively, we used a generalized additive model and smooth curve fitting (penalty-spline method). Multivariate logistic regression was used to evaluate the odds ratios (ORs) and 95% confidence intervals (CIs) for CKD in RC (as a continuous variable and quartiles). We established two models in which the fully adjusted model (Model II) was adjusted for sex, age, WC, BMI, drinking status, smoking status, SBP, DBP, diabetes, CAD, stroke, antihypertensive drugs, Hcy, and TG. Subgroup and interaction analyses were used to further explore the potential effect modifiers. A sensitivity analysis was performed to confirm the robustness of the relationship between RC levels and CKD. All statistical analyses were performed using the statistical package R (http://www.R-project.org) and EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA), and a two-tailed P <0.05 was considered statistically significant.
3 Results
3.1 Baseline characteristics of the study population
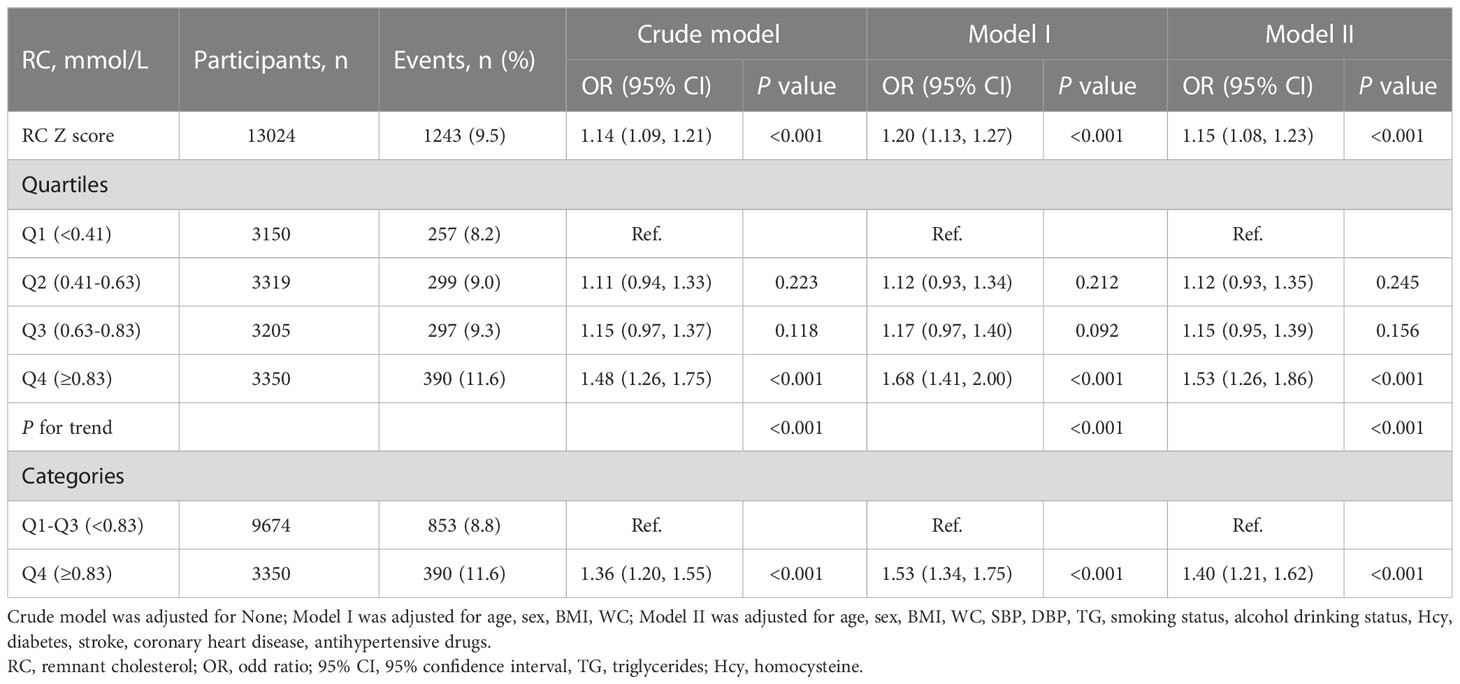
The baseline characteristics of the 13,024 participants are described in Table 1. Overall, their average age was 63.8 ± 9.4 years, and 6,099 participants were male, accounting for 46.8%. The baseline characteristics of the study participants were stratified according to RC quartiles. The RC ranges for the quartiles were ≤0.41 mmol/L, 0.41–0.63 mmol/L, 0.63–0.83 mmol/L, and ≥0.83 mmol/L. Compared with the lowest quartile of RC, the population in the highest quartile mostly comprised older female participants who did not smoke or drink at the time and were more likely to have a history of diabetes mellitus. The values of BMI, WC, TC, SBP, LDL-C, FBG, TG, and UA were also higher. HDL-C level and eGFR were lower, and a smaller proportion of people took antihypertensive drugs.
3.2 Association between RC and CKD
As shown in Table 2, multiple logistic regression models were constructed to assess the association between RC levels and CKD, and a positive association was observed. In a complete adjustment model, which adjusted for sex, age, WC, SBP, BMI, DBP, smoking status, drinking status; Hcy, TG, diabetes, stroke, CAD, and antihypertensive drugs, for each SD increment in RC, the risk of CKD increased by 15% (adjusted OR, 1.15; 95% CI: 1.08–1.23). Consistently, when serum RC level was assessed as quartiles, compared with the Q1, the adjusted ORs in the Q2, Q3, and Q4 were 1.12 (95% CI: 0.95–1.53), 1.15 (95% CI: 0.95–1.39), and 1.53 (95% CI: 1.26–1.86), respectively. Our research also found that compared with RC level <0.83mmol/L, RC level ≥0.83 mmol/L significantly increased the risk of CKD, (OR, 1.40; 95% CI: 1.21–1.62). In addition, we used a generalized additive model and smooth curve fitting (penalized spline method) to represent the association between RC levels and CKD. As shown in Figure 1, a linear relationship (P = 0.007) was observed between RC levels and CKD. We also analyzed the association between RC levels and eGFR. Moreover, the RC level was negatively associated with the eGFR. Further multiple linear regression analysis showed that for every SD increment in RC, eGFR decreased by 1.97 ml/min·1.73 m2 (95% CI: -2.29 to -1.65). Compared with the lowest quartile of RC, the eGFR of participants in the highest quartile decreased significantly (β = -6.35, 95% CI: -7.20 to -5.51) (Table S1).
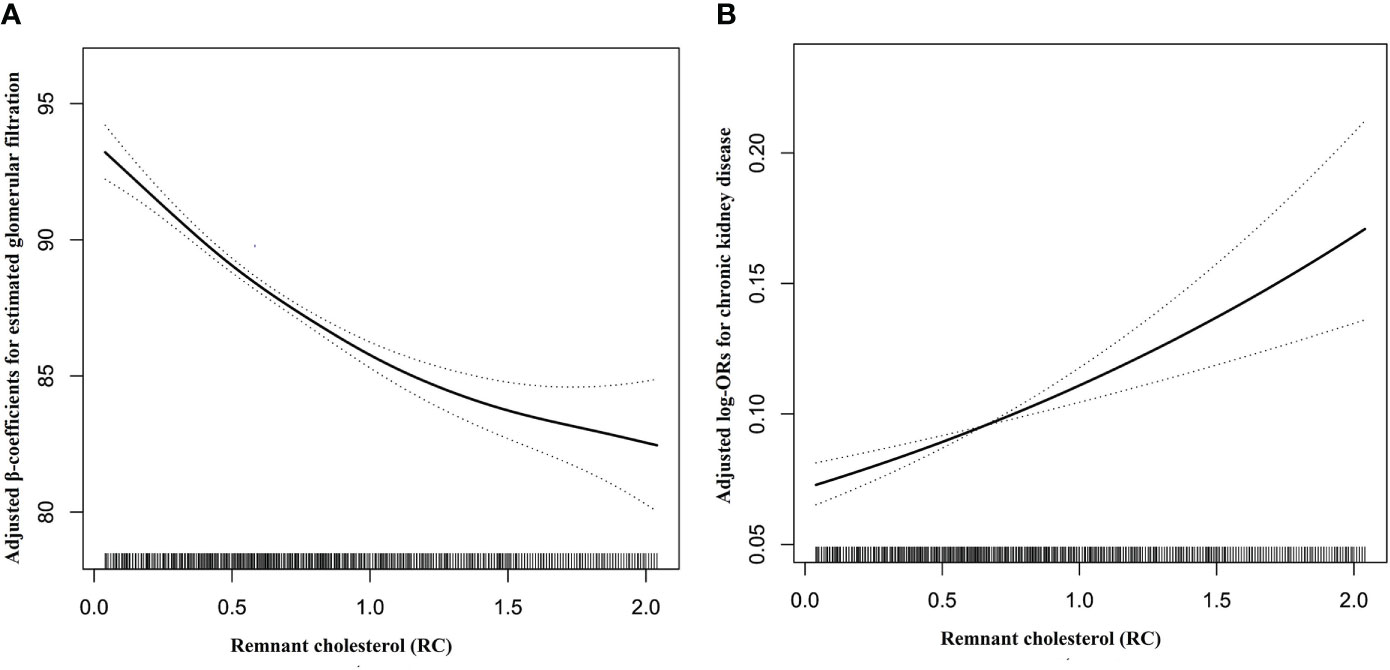
Figure 1 Dose-response relationships between RC and the risk of eGFR decline (A) and CKD (B). Adjustment factors included age, sex, BMI, WC, SBP, DBP, TG, smoking status, drinking status, Hcy, diabetes, stroke, coronary heart disease, and antihypertensive drugs at baseline.
3.3 Subgroup analyses
To further explore the possible effect of modifiers on the relationship between RC levels and CKD, we performed a stratified analysis. In Figure 2, the association between RC level and increased CKD risk is significantly stronger in the subgroup of patients with BMI ≥24 kg/m2 than in those with BMI <24 kg/m2 and in non-smoking participants than in smoking patients (interaction P=0.034, 0.024 respectively). In participants with BMI ≥24 kg/m², for every unit increment in RC, the risk of CKD increased by 73% (OR, 1.73; 95% CI: 1.33–2.24; P = 0.034). Similarly, in current non-smokers, every additional unit of RC increased the risk of CKD by 60% (OR, 1.60; 95% CI: 1.32 1.95). However, sex, age, SBP, DBP, alcohol consumption status, diabetes, and antihypertensive drug use did not significantly modify the relationship between RC levels and CKD.
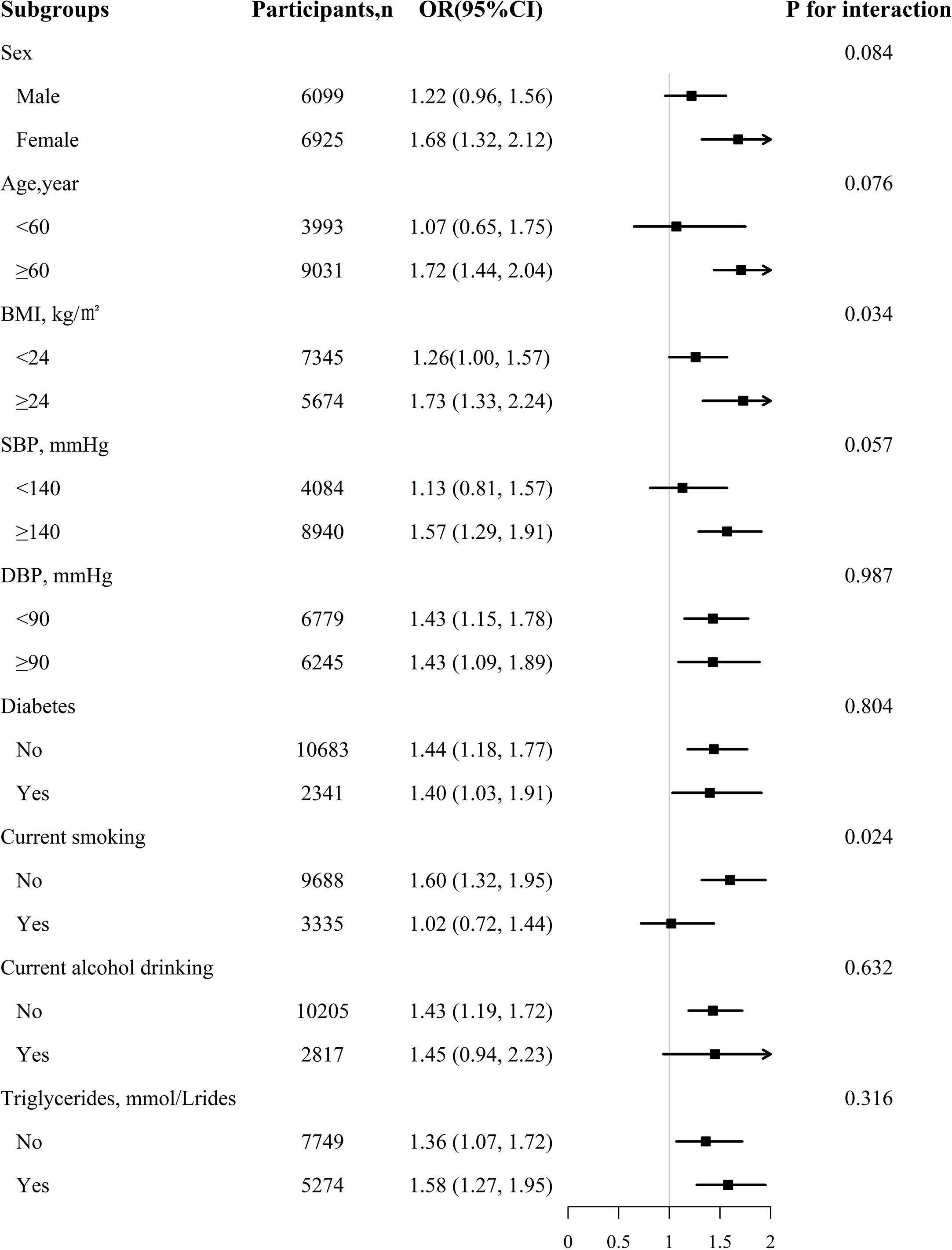
Figure 2 The association between RC and CKD in various subgroups. Each subgroup analysis adjusted, if not stratified, for age, sex, BMI, WC, SBP, DBP, TG, smoking status, drinking status, Hcy, diabetes, stroke, coronary heart disease, antihypertensive drugs at baseline.
3.4 Sensitivity analysis
Owing to the close association between the RC and TG levels, the concentration of RC increased with an increase in the TG level (9, 13). Therefore, we carried out the sensitivity analysis, which explored the relationship between RC levels and CKD in populations with hypertension and normal TG levels. In Table S2, for each SD increment in RC, the risk of CKD increases by 11% (OR, 1.11; 95% CI: 1.02–1.19). Compared with Q1, the adjusted ORs in Q2, Q3, and Q4 were 1.17 (95% CI: 0.92–1.49), 1.11 (95% CI: 0.87–1.41), and 1.42 (95% CI: 1.12–1.79), respectively. In the previous analysis, given that RC was calculated from TC, LDL-C, and HDL-C, no adjustments were made in the model, but their collinearity with RC was considered to be large. In order to verify whether the relationship between RC and CKD obtained in this study is independent of traditional lipid indexes, residual analysis was conducted (Table S3). The positive association between RC and CKD risk still exists after adjusting for TC residuals, LDL-C residuals, and HDL-C residuals. This shows that the relationship between RC and CKD is independent of traditional lipid indexes.
4 Discussion
In this study, we found that the RC level was positively associated with the risk of CKD in a Chinese population with hypertension. This positive association was only significant in patients with BMI ≥24 kg/m2 who did not smoke, not in those with BMI <24 kg/m2 who smoked. These findings suggest that BMI and smoking status are significant effect modifiers.
Previous studies have indicated that dyslipidemia is related to renal insufficiency (14, 15); however, most are limited to traditional lipid indices, such as TG and HDL-C (16, 17). Few studies have investigated the relationship between unconventional lipid profiles and kidney disease. Qi et al. (18) designed a prospective cohort study of 3,909 participants with normal eGFR and a baseline age of ≥40 years, and the results showed that elevated blood lipid levels during follow-up increased the risk of eGFR decline. Higher RC levels are associated with the early progression of renal injuries. A cross-sectional study conducted by Marcelino et al. (19) involving 395 non-diabetic individuals who did not receive statins showed that the RC level of patients with CKD increased and was positively associated with the CKD stage. A cross-sectional study of 146 participants with type 2 diabetes at different CKD stages showed that lower renal function was associated with increased concentrations of CM, VLDL, or both (20). Apolipoprotein (apo) B48 is a specific marker of CM. A study of 101 participants with diabetic nephropathy of different stages showed that plasma apob48 levels increased with the progression of diabetic nephropathy (21). In contrast, a prospective cohort study by Rahman et al. showed no independent association between VLDL cholesterol levels and kidney disease progression (22). Some of these results were consistent with our conclusions, whereas others were not. The reason for this may be the different selections of race, population, and sample sizes. Yan et al. (23) conducted a cross-sectional study of 7,356 participants aged ≥40 years in China showing that a higher RC level is an independent risk factor for CKD (OR: 1.344, 95% CI: 1.097–1.648). This result is mostly consistent with the results of our study. However, as a high-risk group for CKD, patients with hypertension need to be considered as a special population to analyze the relationship between RC levels and CKD. Previous studies have confirmed that RC is a powerful lipid component that promotes atherosclerosis, and RC can act on the arterial wall through oxidative stress and low-grade inflammation, resulting in endothelial dysfunction and atherosclerosis, which can lead to the development of ASCVD and CKD (6, 24, 25). Although previous studies have discussed the relationship between RC levels and CKD, no study has investigated this association in populations with hypertension.
As a risk factor for CKD, a high TG level is related to the occurrence and progression of CKD. Hypertriglyceridemia is the prevalent feature of lipid metabolism disorders in patients with CKD. Therefore, we investigated the relationship between RC levels and CKD under normal TG levels and concluded that even if the TG level was normal, an increase in RC level was independently and positively associated with the risk of CKD. In further subgroup analysis, the positive association was more significant for people with BMI ≥24 kg/m2 (Figure S2).
In addition, we found that BMI and smoking status significantly modified the relationship between RC levels and CKD in a Chinese population with hypertension. With the increase in RC levels, participants with BMI ≥24 kg/m2 who did not smoke had a greater risk of CKD. For overweight or obese populations, the possible mechanisms are as follows. First, obesity can cause abnormal lipid metabolism in the human body, including an increase in TG concentration (26). This will increase the RC level and, thus, the risk of CKD. Second, being overweight or obese is a risk factor for developing CKD (27). Its impacts on renal function may be associated with comorbidities, such as diabetes or hypertension. However, it also leads to inflammation, oxidative stress, activation of the renal angiotensin-aldosterone system, and insulin resistance through the production of hormones, such as adiponectin, leptin, and resistin, resulting in increased glomerular hypertension and permeability, and ultimately, CKD (28–30). In previous studies, the effect of smoking on eGFR was unclear. Some studies have suggested that smoking is negatively correlated with eGFR, whereas others have suggested that smoking could increase eGFR (31–33). In our study, CKD was defined as eGFR less than 60 ml/min·1.73 m2; therefore, current non-smokers had a higher risk of CKD because non-smokers had lower eGFR values than smokers. This may be because of the following mechanisms. First, previous literature reviews have shown that current smokers tend to weigh less than non-smokers. It is well known that the source of creatinine in serum is muscle cells; therefore, in earlier studies, it was also found that the serum creatinine content in smokers was low (34, 35). However, the lower serum creatinine level in smokers may not simply be explained by the fact that smokers have less body fat and muscle mass, as the lower serum creatinine level remains even after adjusting for BMI (36). Consequently, smokers may have increased creatinine excretion, thereby increasing the eGFR (37). Second, smoking causes recurrent transient decreases in renal plasma flow and eGFR. This small recurrent transient renal hypoperfusion may damage some glomeruli and, thus, lead to aging of the peribulbar blood vessels and glomeruli and result in residual glomerular compensatory hypertrophy and hyperfiltration, ultimately leading to an elevated eGFR (38, 39). However, this compensatory increase in eGFR is limited, and as smoking volume and duration increase, renal function will eventually be severely impaired. In addition, previous studies have confirmed that smoking can damage kidney function. Perhaps, when smoking seriously damages kidney function (40, 41), the damage caused by increased RC on kidney function is not obvious. However, no definitive conclusions have been drawn regarding the effect of smoking on eGFR. According to existing research results, we believe that smokers have an increased compensatory eGFR in the early stages and a downward trend in eGFR in the latter stages owing to the aggravated impairment of renal function. Further research is required to explore this relationship in detail.
As a lipid index, RC has been studied more in cardiovascular diseases; however, studies on the relationship between RC levels and CKD have not received much attention in the past, let alone in individuals with hypertension. The guideline of the European Atherosclerosis Association (42) defines a high RC level as a fasting RC level ≥0.8 mmol/L and/or postprandial RC level ≥0.9 mmol/L; however, our research found that RC level ≥0.83 mmol/L significantly increased the risk of CKD, (OR, 1.40; 95% CI: 1.21–1.62), which suggests that reducing RC levels below 0.8 can reduce the risk of cardiovascular disease and CKS in both the general population and the population with hypertension. In our future clinical studies, we aim to calculate the value of RC levels using a simple formula and evaluate whether further lipid-lowering treatments are required. Whether a further reduction in RC levels can reduce the incidence rate requires further prospective research.
5 Study strengths and limitations
To the best of our knowledge, this is the first study to investigate the association between RC levels and CKD in a Chinese population with hypertension. However, this study has several limitations. First, we could not establish a causal relationship between RC levels and CKD because of the cross-sectional nature of the study. Second, the study population included participants with hypertension from rural areas of southern China who were aged over 18 years. Therefore, the results of this study cannot be generalized to individuals of other ages, regions, or disease types.
6 Conclusion
In the present study, we observed an independent positive association between elevated RC levels and CKD risk in a Chinese population with hypertension. This relationship seems to be more significant in patients with BMI ≥24 kg/m2 and current non-smokers. RC ≥0.83 mmol/L seems to be a rough cut-off point, which can be used to suggest that patients with hypertension need further lipid-lowering treatment. However, further studies are required to verify this. Our results are significant for clinical lipid management in patients with hypertension.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the ethics committee of the Institute of biomedical research of Anhui Medical University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
TY, HB, and XC conceived and designed the study. TY, CD, and YX contributed to statistical analysis. TY drafted the manuscript. All authors contributed to the data collection and reviewed/edited the manuscript’s important intellectual content. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Cultivation of backup projects for National Science and Technology Awards (20223AEI91007), Jiangxi Science and Technology Innovation Base Plan - Jiangxi Clinical Medical Research Center (20223BCG74012), Science and Technology Innovation Base Construction Project (20221ZDG02010), Jiangxi Science and Technology Innovation Platform Project (20165BCD41005), Jiangxi Provincial Natural Science Foundation (20212ACB206019, 20224BAB206090), Key R&D Projects, Jiangxi (20203BBGL73173), Jiangxi Provincial Health Commission Science and Technology Project (202130440, 202210495, 202310528), Jiangxi Provincial Drug Administration Science and Technology Project (2022JS41), Fund project of the Second Affiliated Hospital of Nanchang University (2016YNQN12034, 2019YNLZ12010, 2021efyA01, 2021YNFY2024).
Acknowledgments
Thanks to all the investigators and subjects who participated in the China Hypertension Registry Study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1189574/full#supplementary-material
Supplementary Figure 1 | Flow chart of participants.
References
1. Kalantar-Zadeh K, Jafar TH, Nitsch D, Neuen BL, Perkovic V. Chronic kidney disease. Lancet (2021) 398(10302):786–802. doi: 10.1016/S0140-6736(21)00519-5
2. Foreman KJ, Marquez N, Dolgert A, Fukutaki K, Fullman N, McGaughey M, et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet (2018) 392(10159):2052–90. doi: 10.1016/S0140-6736(18)31694-5
3. Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. Lancet (2017) 389(10075):1238–52. doi: 10.1016/S0140-6736(16)32064-5
4. Bowe B, Xie Y, Xian H, Balasubramanian S, Al-Aly Z. Low levels of high-density lipoprotein cholesterol increase the risk of incident kidney disease and its progression. Kidney Int (2016) 89(4):886–96. doi: 10.1016/j.kint.2015.12.034
5. Harper CR, Jacobson TA. Managing dyslipidemia in chronic kidney disease. J Am Coll Cardiol (2008) 51(25):2375–84. doi: 10.1016/j.jacc.2008.03.025
6. Sandesara PB, Virani SS, Fazio S, Shapiro MD. The forgotten lipids: triglycerides, remnant cholesterol, and atherosclerotic cardiovascular disease risk. Endocr Rev (2019) 40(2):537–57. doi: 10.1210/er.2018-00184
7. Mora S, Wenger NK, Demicco DA, Breazna A, Boekholdt SM, Arsenault BJ, et al. Determinants of residual risk in secondary prevention patients treated with high- versus low-dose statin therapy: the treating to new targets (TNT) study. Circulation (2012) 125(16):1979–87. doi: 10.1161/CIRCULATIONAHA.111.088591
8. Fruchart JC, Sacks FM, Hermans MP, Assmann G, Brown WV, Ceska R, et al. The residual risk reduction initiative: a call to action to reduce residual vascular risk in dyslipidaemic patient. Diabetes Vasc Dis Res (2008) 5(4):319–35. doi: 10.3132/dvdr.2008.046
9. Varbo A, Benn M, Tybjærg-Hansen A, Jørgensen AB, Frikke-Schmidt R, Nordestgaard BG, et al. Remnant cholesterol as a causal risk factor for ischemic heart disease. J Am Coll Cardiol (2013) 61(4):427–36. doi: 10.1016/j.jacc.2012.08.1026
10. Castañer O, Pintó X, Subirana I, Amor AJ, Ros E, Hernáez Á, et al. Remnant cholesterol, not LDL cholesterol, is associated with incident cardiovascular disease. J Am Coll Cardiol (2020) 76(23):2712–24. doi: 10.1016/j.jacc.2020.10.008
11. Burnett JR, Hooper AJ, Hegele RA. Remnant cholesterol and atherosclerotic cardiovascular disease risk. J Am Coll Cardiol (2020) 76(23):2736–9. doi: 10.1016/j.jacc.2020.10.029
12. Zhai Q, Dou J, Wen J, Wang M, Zuo Y, Su X, et al. Association between changes in lipid indexes and early progression of kidney dysfunction in participants with normal estimated glomerular filtration rate: a prospective cohort study. Endocrine (2022) 76(2):312–23. doi: 10.1007/s12020-022-03012-z
13. Bermudez-Lopez M, Forne C, Amigo N, Bozic M, Arroyo D, Bretones T, et al. An in-depth analysis shows a hidden atherogenic lipoprotein profile in non-diabetic chronic kidney disease patients. Expert Opin Ther Targets (2019) 23(7):619–30. doi: 10.1080/14728222.2019.1620206
14. Sonoda M, Shoji T, Kimoto E, Okute Y, Shima H, Naganuma T, et al. Kidney function, cholesterol absorption and remnant lipoprotein accumulation in patients with diabetes mellitus. J Atheroscl Thromb (2014) 21(4):346–54. doi: 10.5551/jat.20594
15. Hayashi T, Hirano T, Taira T, Tokuno A, Mori Y, Koba S, et al. Remarkable increase of apolipoprotein B48 level in diabetic patients with end-stage renal disease. Atherosclerosis (2008) 197(1):154–8. doi: 10.1016/j.atherosclerosis.2007.03.015
16. Rahman M, Yang W, Akkina S, Alper A, Anderson AH, Appel LJ, et al. Relation of serum lipids and lipoproteins with progression of CKD: the CRIC study. Clin J Am Soc Nephrol (2014) 9(7):1190–8. doi: 10.2215/CJN.09320913
17. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med (2009) 150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
18. Vaziri ND. Dyslipidemia of chronic renal failure: the nature, mechanisms, and potential consequences. Am J Physiology-Renal Physiol (2006) 290(2):F262–72. doi: 10.1152/ajprenal.00099.2005
19. Noels H, Lehrke M, Vanholder R, Jankowski J. Lipoproteins and fatty acids in chronic kidney disease: molecular and metabolic alterations. Nat Rev Nephrol (2021) 17(8):528–42. doi: 10.1038/s41581-021-00423-5
20. Kwan BCH, Kronenberg F, Beddhu S, Cheung AK. Lipoprotein metabolism and lipid management in chronic kidney disease. J Am Soc Nephrol (2007) 18(4):1246–61. doi: 10.1681/ASN.2006091006
21. Navaneethan SD, Schold JD, Arrigain S, Thomas G, Jolly SE, Poggio ED, et al. Serum triglycerides and risk for death in stage 3 and stage 4 chronic kidney disease. Nephrol Dialysis Transplant (2012) 27(8):3228–34. doi: 10.1093/ndt/gfs058
22. Zewinger S, Speer T, Kleber ME, Scharnagl H, Woitas R, Lepper PM, et al. HDL cholesterol is not associated with lower mortality in patients with kidney dysfunction. J Am Soc Nephrol (2014) 25(5):1073–82. doi: 10.1681/ASN.2013050482
23. Yan P, Xu Y, Miao Y, Bai X, Wu Y, Tang Q, et al. Association of remnant cholesterol with chronic kidney disease in middle-aged and elderly Chinese: a population-based study. Acta Diabetologica (2021) 58(12):1615–25. doi: 10.1007/s00592-021-01765-z
24. Varbo A, Benn M, Tybjærg-Hansen A, Nordestgaard BG. Elevated remnant cholesterol causes both low-grade inflammation and ischemic heart disease, whereas elevated low-density lipoprotein cholesterol causes ischemic heart disease without inflammation. Circulation (2013) 128(12):1298–309. doi: 10.1161/CIRCULATIONAHA.113.003008
25. Varbo A, Nordestgaard BG. Remnant cholesterol and triglyceride-rich lipoproteins in atherosclerosis progression and cardiovascular disease. Arteriosclerosis Thrombosis Vasc Biol (2016) 36(11):2133–5. doi: 10.1161/ATVBAHA.116.308305
26. Franssen R, Monajemi H, Stroes ES, Kastelein JJ. Obesity and dyslipidemia. Med Clinics North America (2011) 95(5):893–902. doi: 10.1016/j.mcna.2011.06.003
27. de Vries APJ, Ruggenenti P, Ruan XZ, Praga M, Cruzado JM, Bajema IM, et al. Fatty kidney: emerging role of ectopic lipid in obesity-related renal disease. Lancet Diabetes Endocrinol (2014) 2(5):417–26. doi: 10.1016/S2213-8587(14)70065-8
28. Kovesdy CP, Furth SL, Zoccali C. Obesity and kidney disease: hidden consequences of the epidemic. Kidney Int (2017) 91(2):260–2. doi: 10.1016/j.kint.2016.10.019
29. Wahba IM, Mak RH. Obesity and obesity-initiated metabolic syndrome: mechanistic links to chronic kidney disease. Clin J Am Soc Nephrol (2007) 2(3):550–62. doi: 10.2215/CJN.04071206
30. Xu T, Sheng Z, Yao L. Obesity-related glomerulopathy: pathogenesis, pathologic, clinical characteristics and treatment. Front Med (2017) 11(3):340–8. doi: 10.1007/s11684-017-0570-3
31. Ishizaka N, Ishizaka Y, Toda E, Shimomura H, Koike K, Seki G, et al. Association between cigarette smoking and chronic kidney disease in Japanese men. Hypertens Res (2008) 31(3):485–92. doi: 10.1291/hypres.31.485
32. Yoon H, Park M, Yoon H, Son KY, Cho B, Kim S. The differential effect of cigarette smoking on glomerular filtration rate and proteinuria in an apparently healthy population. Hypertension Res (2009) 32(3):214. doi: 10.1038/hr.2008.37
33. Garcia-Esquinas E, Loeffler LF, Weaver VM, Fadrowski JJ, Navas-Acien A. Kidney function and tobacco smoke exposure in US adolescents. Pediatrics (2013) 131(5):e1415–23. doi: 10.1542/peds.2012-3201
34. Dales LG, Friedman GD, Siegelaub AB, Seltzer CC. Cigarette smoking and serum chemistry tests. J Chronic Dis (1974) 27(6):293–307. doi: 10.1016/0021-9681(74)90093-9
35. Savdie E, Grosslight GM, Adena MA. Relation of alcohol and cigarette consumption to blood pressure and serum creatinine levels. J Chronic Dis (1984) 37(8):617–23. doi: 10.1016/0021-9681(84)90111-5
36. Noborisaka Y, Ishizaki M, Nakata M, Yamada Y, Honda R, Yokoyama H, et al. Cigarette smoking, proteinuria, and renal function in middle-aged Japanese men from an occupational population. Environ Health Prev Med (2012) 17(2):147–56. doi: 10.1007/s12199-011-0234-x
37. Goetz FC, Jacobs DR Jr, Chavers B, Roel J, Yelle M, Sprafka JM, et al. Risk factors for kidney damage in the adult population of wadena, Minnesota. A prospective study Am J Epidemiol (1997) 145(2):91–102. doi: 10.1093/oxfordjournals.aje.a009091
38. Halimi JM, Giraudeau B, Vol S, Cacès E, Nivet H, Lebranchu Y, et al. Effects of current smoking and smoking discontinuation on renal function and proteinuria in the general population. Kidney Int (2000) 58(3):1285–92. doi: 10.1046/j.1523-1755.2000.00284.x
39. Remuzzi G. Cigarette smoking and renal function impairment. Am J Kidney Dis (1999) 33(4):807–10. doi: 10.1016/S0272-6386(99)70241-6
40. Pinto-Sietsma SJ, Mulder J, Janssen WM, Hillege HL, de Zeeuw D, de Jong PE, et al. Smoking is related to albuminuria and abnormal renal function in nondiabetic persons. Ann Intern Med (2000) 133(8):585–91. doi: 10.7326/0003-4819-133-8-200010170-00008
41. Fu YC, Xu ZL, Zhao MY, Xu K. The association between smoking and renal function in people over 20 years old. Front Med (Lausanne) (2022) 9:870278. doi: 10.3389/fmed.2022.870278
42. Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias: the task force for the management of dyslipidaemias of the European society of cardiology (ESC) and European atherosclerosis society (EAS) developed with the special contribution of the European association for cardiovascular prevention & rehabilitation (EACPR). Atherosclerosis (2016) 253:281–344. doi: 10.1016/j.atherosclerosis.2016.08.018
Keywords: remnant cholesterol, chronic kidney disease, Chinese hypertensive population, cross-sectional study, lipid metabolism
Citation: Yuan T, Ding C, Xie Y, Zhou X, Xie C, Wang T, Yu C, Zhou W, Zhu L, Bao H and Cheng X (2023) Association between remnant cholesterol and chronic kidney disease in Chinese hypertensive patients. Front. Endocrinol. 14:1189574. doi: 10.3389/fendo.2023.1189574
Received: 19 March 2023; Accepted: 02 June 2023;
Published: 21 June 2023.
Edited by:
Guiting Lin, University of California, San Francisco, United StatesReviewed by:
Yuxuan Song, Peking University People’s Hospital, ChinaQiangqiang He, Tsinghua University, China
Xiao-qiang Liu, Tianjin Medical University General Hospital, China
Hanping Shi, Capital Medical University, China
Copyright © 2023 Yuan, Ding, Xie, Zhou, Xie, Wang, Yu, Zhou, Zhu, Bao and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huihui Bao, aHVpaHVpX2Jhbzc3QDEyNi5jb20=; Xiaoshu Cheng, eGlhb3NodW1lbmZhbjEyNkAxNjMuY29t
 Ting Yuan
Ting Yuan Congcong Ding
Congcong Ding Yanyou Xie
Yanyou Xie Xinlei Zhou
Xinlei Zhou Chong Xie1,2,3
Chong Xie1,2,3 Tao Wang
Tao Wang Chao Yu
Chao Yu Wei Zhou
Wei Zhou Lingjuan Zhu
Lingjuan Zhu Huihui Bao
Huihui Bao Xiaoshu Cheng
Xiaoshu Cheng