- 1Department of Assisted Reproduction, Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2Department of Integrative Physiology and Biochemistry, University of Colorado at Boulder, Boulder, NV, United States
- 3Department of Obstetrics and Gynaecology, Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Background: ICSI (intracytoplasmic sperm injection) leads to a reduced male-to-female ratio at birth, whereas blastocyst transfer results in an increased male-to-female ratio. However, limited knowledge exists regarding the impact of these factors on the live birth rate for each gender. This study aimed to investigate the influence of patient characteristics and treatment parameters on the live birth rate for each gender, as well as the ultimate male-to-female ratio at birth in frozen-thawed embryo transfer (FET) cycles.
Method: This retrospective cohort study involved a total of 28,376 FET cycles and 9,217 subsequent deliveries, spanning from January 2003 to December 2015. The study consisted of two parts. First, logistic regression models were constructed to determine the factors influencing the male-to-female ratio among babies born after FET. Second, we aimed to investigate the mechanisms underlying this sex ratio imbalance by analyzing data from all transfer cycles. Generalized estimated equations were employed to assess the impact of risk factors on rates of male and female live births separately.
Results: ICSI resulted in a lower proportion of male offspring compared to in vitro fertilization (IVF) (50.1% vs. 53.7%, aOR: 0.87, 95% CI: 0.80-0.96). Conversely, blastocyst transfer yielded a higher proportion of male offspring than cleavage-stage embryo transfer (58.7% vs. 51.6%, aOR: 1.32, 95% CI: 1.17-1.48). Analysis of all cycles indicated that ICSI resulted in a reduced likelihood of male live birth in comparison to IVF (19.8% vs. 21.6%, aOR: 0.90, 95% CI: 0.83-0.97). However, the transfer of blastocysts rather than cleavage-stage embryos not only increased the chance of male live birth (26.9% vs. 20.2%, aOR: 1.70, 95% CI:1.56-1.85) but also facilitated female live birth (20.3% vs. 19.3%, aOR: 1.26, 95% CI: 1.15-1.39).
Conclusion: ICSI was associated with a reduction in the male-to-female sex ratio and a lower rate of male live births, while blastocyst transfer was associated with an increased male-to-female sex ratio at birth and a higher rate of male live births.
Introduction
Ever since the first birth accomplished through in vitro fertilization (IVF) in 1978 (1), the advent of IVF has brought approximately 10 million infants into the world (2). In recent years, IVF births have accounted for approximately 1% and 2% of all deliveries in China and in the United States, respectively (3, 4). Nevertheless, anxiety lingers in society regarding the potential repercussions of this artificial mode of conception, particularly with regard to its impact on the resulting offspring.
The general male-to-female ratio of all births (i.e., the proportion of male newborns) tends to hover at approximately 51.2% (male:female=105:100) (5); this ratio plays a critical role in facilitating social equilibrium and warding off undesirable socioeconomic consequences (6). The ratio itself, in turn, is dependent on a multitude of factors spanning the realms of biology (e.g., the age and body mass index (BMI) of the mother and father), the environment (e.g., exposure to pollutants and pesticides), society (e.g., gender selection and selective abortion), and economics (e.g., economic downturns and stressors) (7–17).
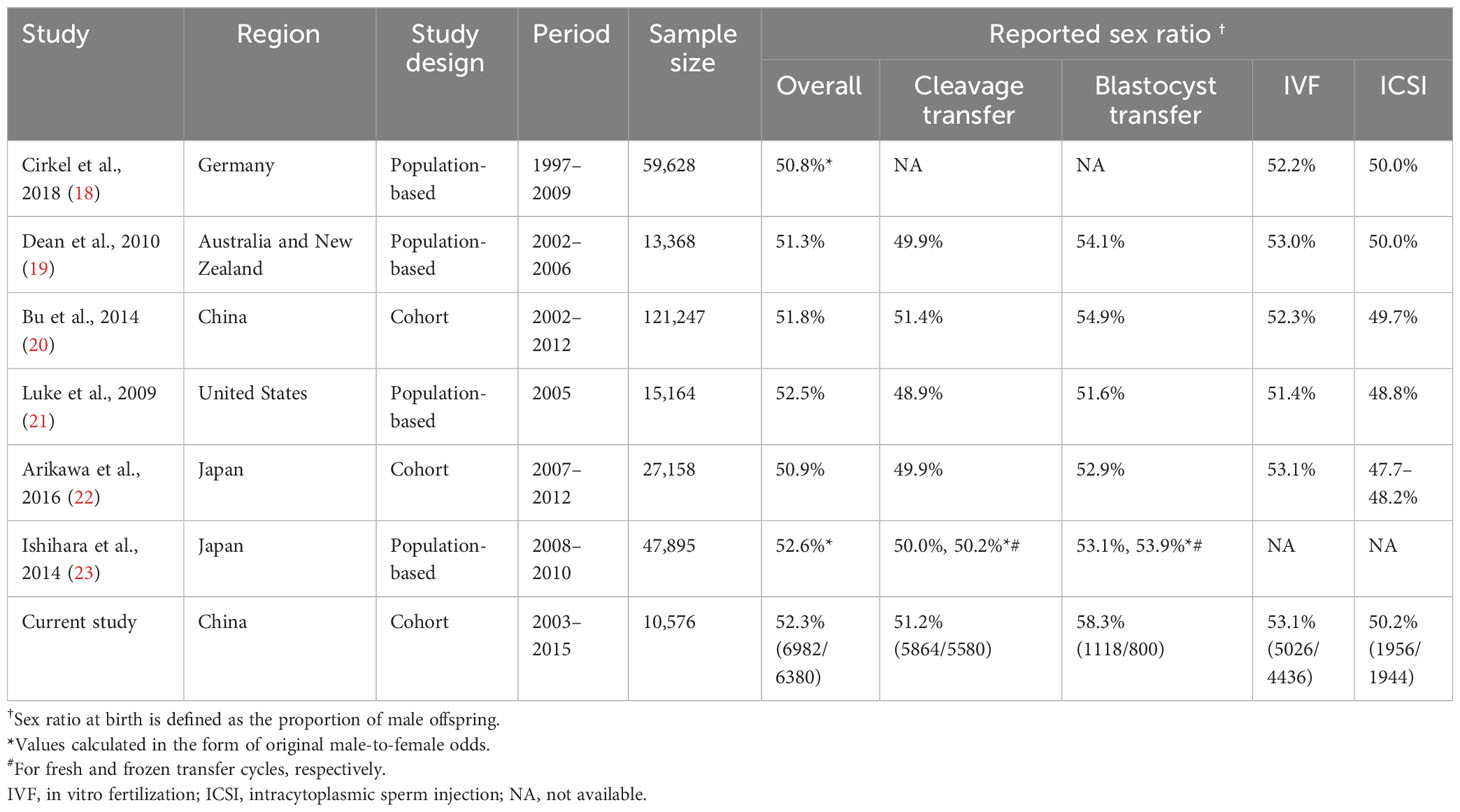
Additionally, the impact of procedures used in assisted reproduction technology (ART) on the male-to-female ratio cannot be disregarded. As presented in Table 1, studies have suggested that intracytoplasmic sperm injection (ICSI) may increase the proportion of female offspring by 2.2–5.4% compared to IVF (19–21, 24). In contrast, blastocyst transfer has been found to be associated with a sex-ratio imbalance, resulting in 2.7–3.8% more male offspring (19, 21, 25–27). Although these two outcomes have been extensively documented, there has been limited research investigating the relationships of various factors involved in ART (such as the underlying characteristics of infertile couples, reproductive history, and treatment interventions) with male-to-female ratio at birth. Furthermore, it remains unknown whether these factors have a gender-specific impact on the live birth rate.
The objective of this study was to analyze the potentially differential impact of various risk factors on live birth rate for each gender in couples undergoing frozen-thawed embryo transfer (FET).
Methods
Ethical approval
This study was approved by the Institutional Review Board of Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine (SH9H-2021-T271-1).
Study design and population
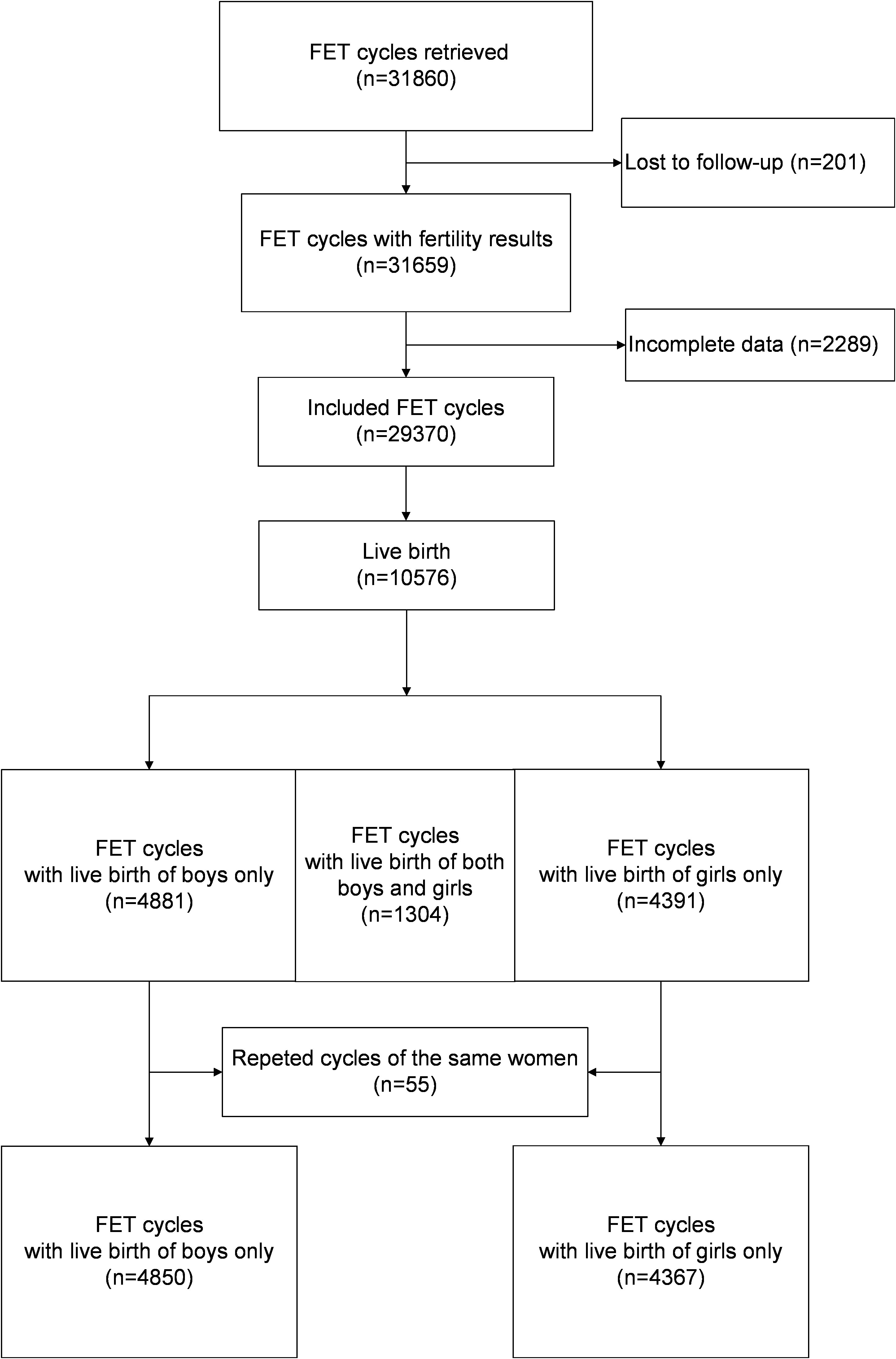
This retrospective cohort study was conducted at the Department of Assisted Reproduction of Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine. The study screened patients who underwent FET treatment between January 2003 and December 2015, ensuring that complete information was available. In the analyses of data on live births, patients who delivered twins of different genders and those with multiple deliveries during the study period were excluded.
Treatment
The IVF/ICSI procedures followed have been described in our previous publications (28). In brief, fertilization was performed either by IVF or by ICSI 4–6 hours after oocyte retrieval. On Day 3, embryos were evaluated according to the Cummins criteria (29). Embryos graded as I and II were cryopreserved on Day 3, while culture of embryos classified as III/IV was extended until Day 7 to enable the selection of morphologically good blastocysts using the Gardner and Schoolcraft grading system; blastocysts meeting the minimum requirement of 3CC were considered eligible for cryopreservation on Day 7 (29, 30).
Endometrial preparation and the FET procedure have previously been described in detail (28). In brief, endometrial preparation was conducted using modified natural cycles, mild stimulation cycles, or hormonal therapy treatments for patients with regular menstrual cycles, irregular menstrual cycles, or a history of thin endometrium, respectively. A progestin supplement was administered until 10 weeks of gestation after achievement of pregnancy.
Statistical analysis
Data are presented in the form of % (n/N). Between-groups differences were assessed using the Chi-square test or Fisher’s exact test, whichever was appropriate. Statistical significance was determined by a P-value <0.05, and odds ratio (ORs) and 95% confidence intervals (CIs) were calculated as indicators of statistically significant effects. Data analysis was conducted using the SPSS software package, version 23.0 (SPSS Inc., Chicago, USA).
Factors analyzed in this study included maternal age (≤29, 30-34, 35-37, 38-40, 41-43, or ≥44), maternal BMI (<18.5, 18.5-24, or >24), partner age (≤29, 30-39, 40-49, or ≥50), duration of infertility (≤1, 2-4, 5-9, ≥10 years), previous miscarriages (0, 1, or ≥2), previous ectopic pregnancy (yes or no), tubal factor infertility (yes or no), PCOS (yes or no), endometriosis (yes or no), male factor infertility (yes or no), treatment year (2003-2009, 2010-2011, 2012-2013, or 2014-2015), fertilized method (IVF or ICSI), endometrial preparation for FET (natural cycle, hormone therapy treatment, or mild stimulation), endometrial thickness at transfer (≤8, 8-15, or ≥15 mm), number of embryos transferred (1, 2, or 3), and embryonic stage at transfer (cleavage or blastocyst).
First, we analyzed the male-to-female ratio among live-born babies. Univariate logistic regression was employed to examine the potential effects of various characteristics on the male-to-female ratio. Multivariate logistic regression analysis, using the conditional backward method, was conducted to identify variables with a significant influence on the male-to-female ratio among offspring, and to calculate adjusted odds ratios (aORs) and 95% confidence intervals (CIs). The significance of the models was assessed based on the −2 log likelihood, and their goodness of fit of models was evaluated using Nagelkerke’s R2.
Second, we investigated the impact of the aforementioned risk factors on live birth rate for each gender across all transfer cycles. Generalized estimated equation (GEE) models were conducted to address the issue of clustered data (multiple cycles for the same woman), and to calculate ORs and 95% CIs. Significant variables (defined as P < 0.2 in a Chi-square test or Fisher’s exact test) were included in multivariate models. GEE models were evaluated based on the quasi-likelihood under independence model criterion.
Finally, we investigated the relationships between each of the aforementioned characteristics and the gender of newborns in all cases of live births involving twins. A multivariate logistic regression model (using the simultaneous entry method) was constructed to calculate aORs and 95% CIs.
Results
Figure 1 illustrates the analysis of 29,370 cycles conducted between January 2003 and December 2015, with the aim of examining the relationships between various risk factors and the live birth rate for each gender. Among these cycles, a total of 10,576 cycles resulted in live births. After the exclusion of twin deliveries involving babies of different genders (n = 1,304) and repeated deliveries by the same women (n = 55), a total of 9,217 deliveries were included for assessment of the associations between the risk factors and the male-to-female ratio at birth; these consisted of 5,639 male babies and 5,047 female babies.
Association between risk factors and male-to-female ratio among all live-born babies
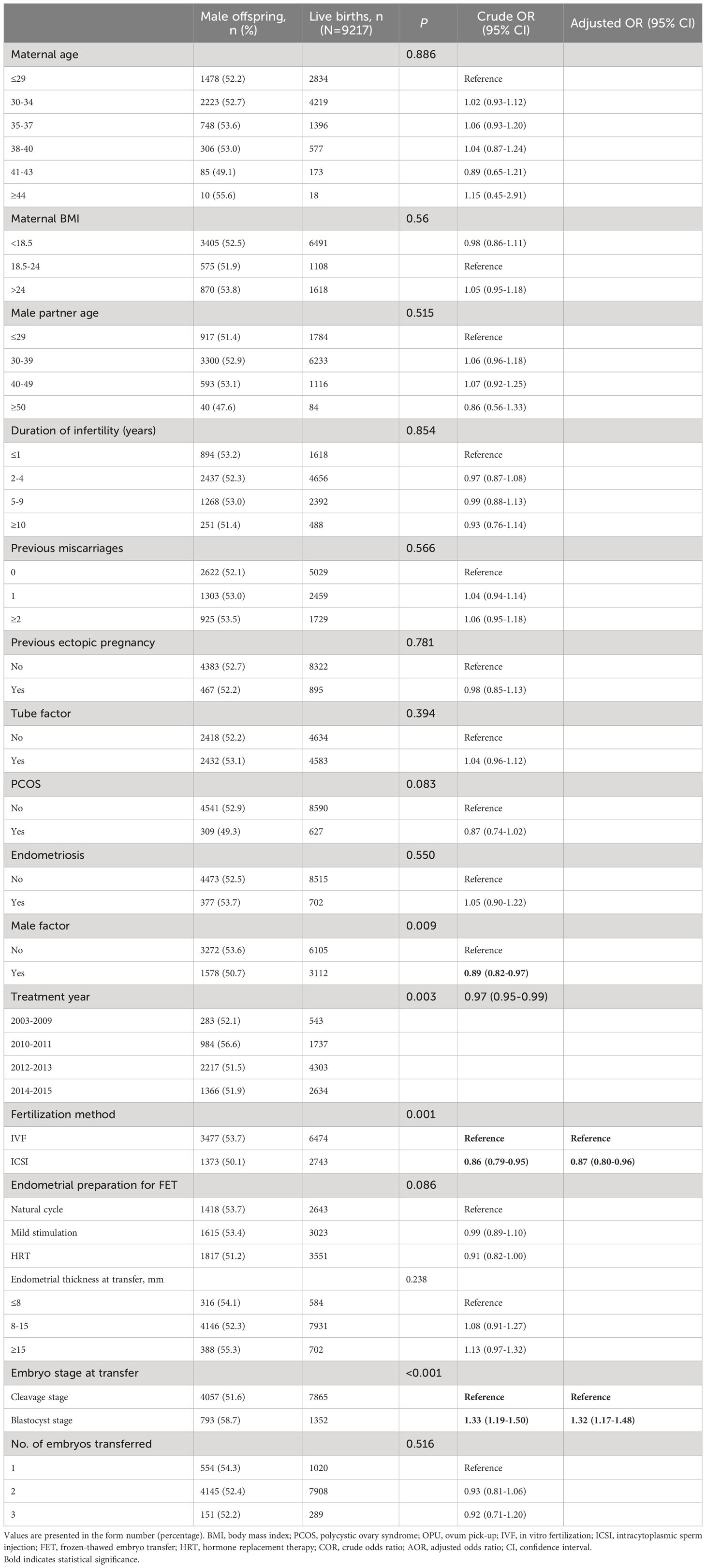
Table 2 presents the male-to-female ratio among all live-born babies when stratified based on patient and treatment characteristics. In comparison to IVF, ICSI resulted in a lower proportion of male offspring (50.1% vs. 53.7%, aOR: 0.87, 95% CI: 0.80–0.96). However, blastocyst transfer was associated with a higher likelihood of male offspring compared to cleavage embryo transfer (58.7% vs. 51.6%, aOR: 1.32, 95% CI: 1.17–1.48).
Association between risk factors and newborn gender among all transfer cycles
Table 3 presents a comprehensive overview of the male and female live birth rates based on patient and treatment characteristics. Table 4 shows the results of multivariate analysis. In the adjusted analysis, it was observed that women undergoing ICSI had a reduced likelihood of male live birth (aOR: 0.90, 95% CI: 0.83–0.97). The chances of both male and female live birth were increased when blastocysts were transferred rather than cleavage-stage embryos (male: aOR 1.70, 95% CI 1.56–1.85; female: aOR 1.26, 95% CI 1.15–1.39).
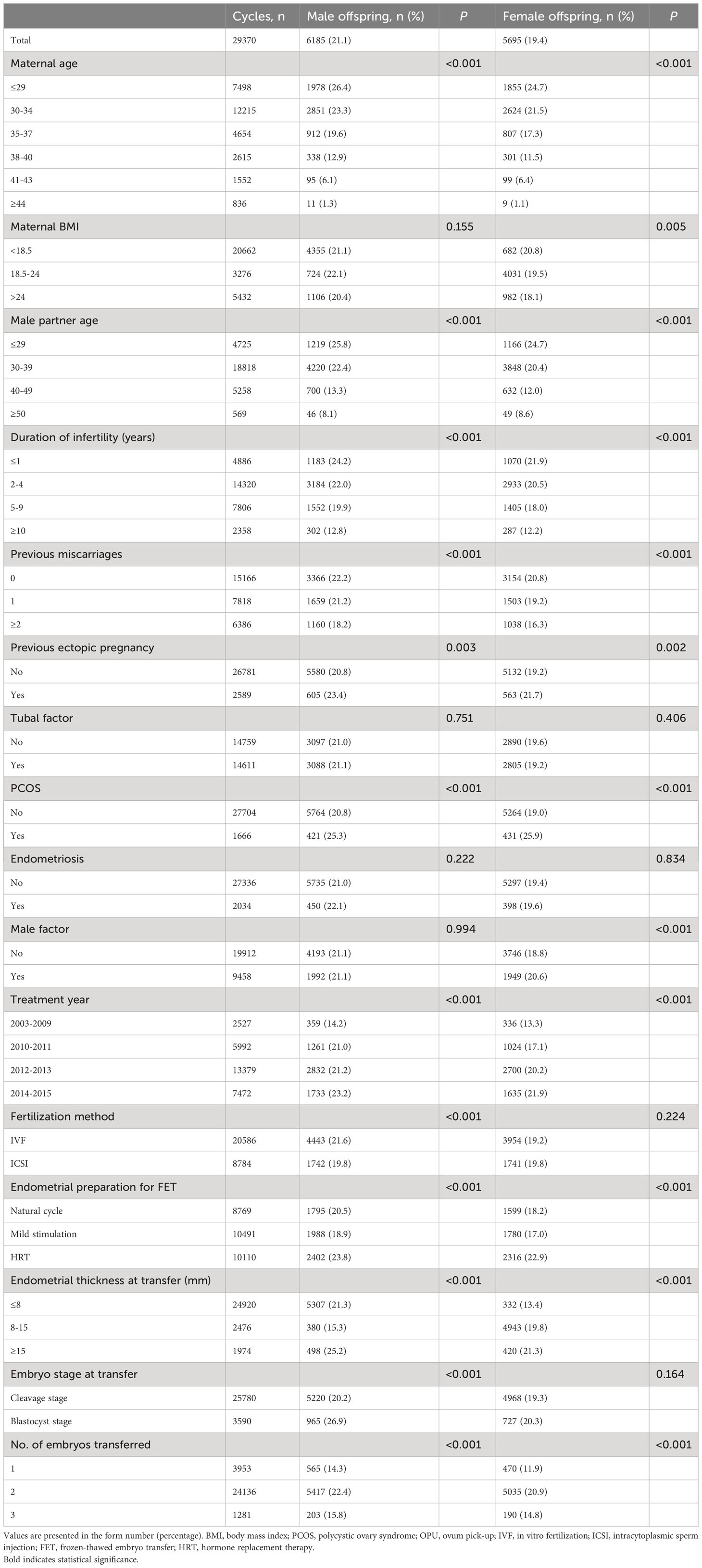
Table 3 Rates of births of male and female offspring by patient characteristics and treatment parameters for all transferred cycles.
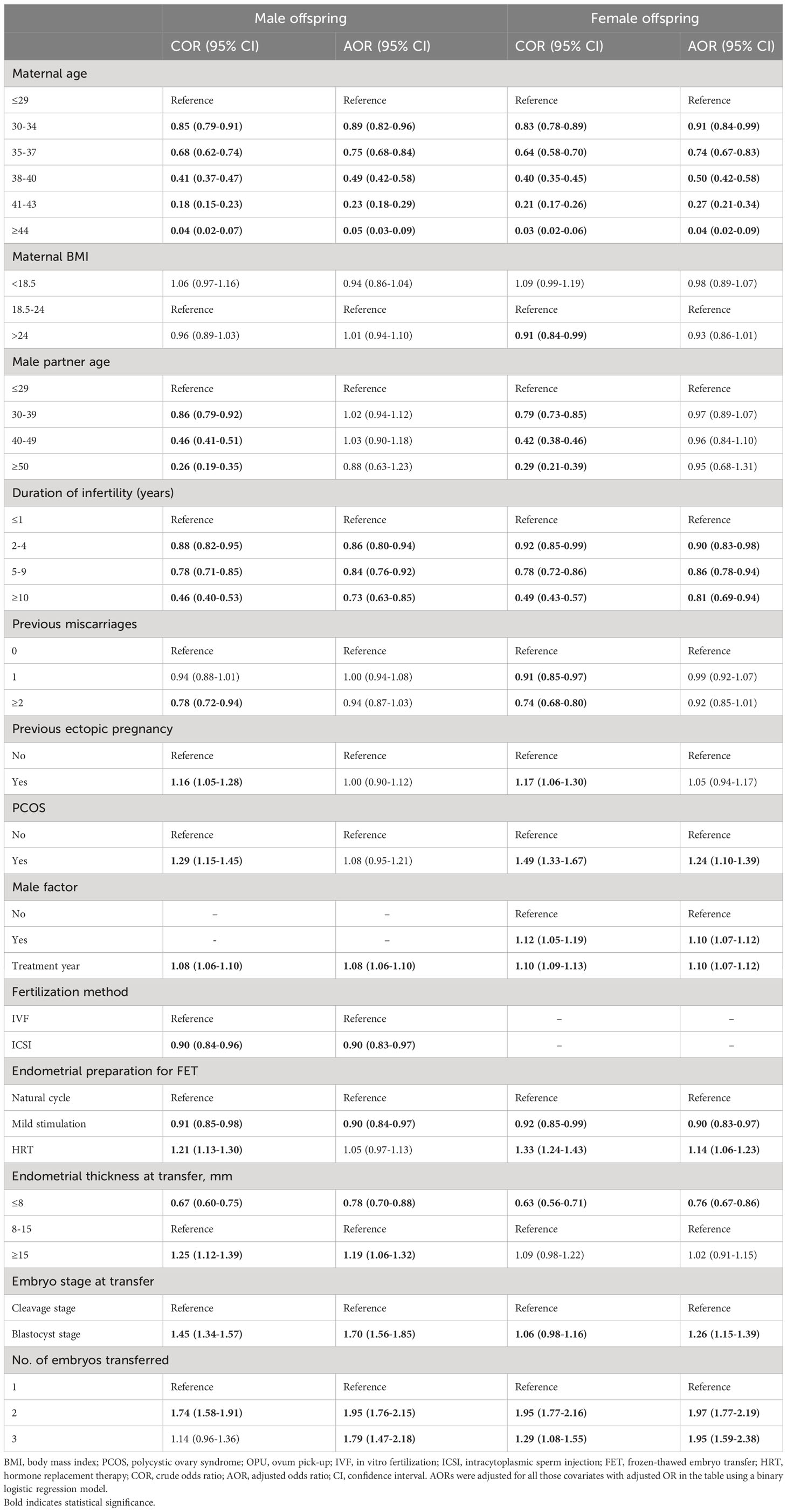
Table 4 ORs of live birth of male and female offspring according to patient characteristics and treatment parameters across all transferred cycles.
Association between risk factors and newborn gender among all live birth of twins
The results of the subgroup analysis are displayed in Table 5. When blastocyts were transferred, as opposed to cleavage-stage embryos, the likelihood of delivery of two male twins was significantly higher, whereas the likelihood of delivery of two female twins was noticeably lower (two male twins: aOR 1.78, 95% CI 1.42–2.24; two female twins: aOR 0.64, 95% CI 0.48–0.84).
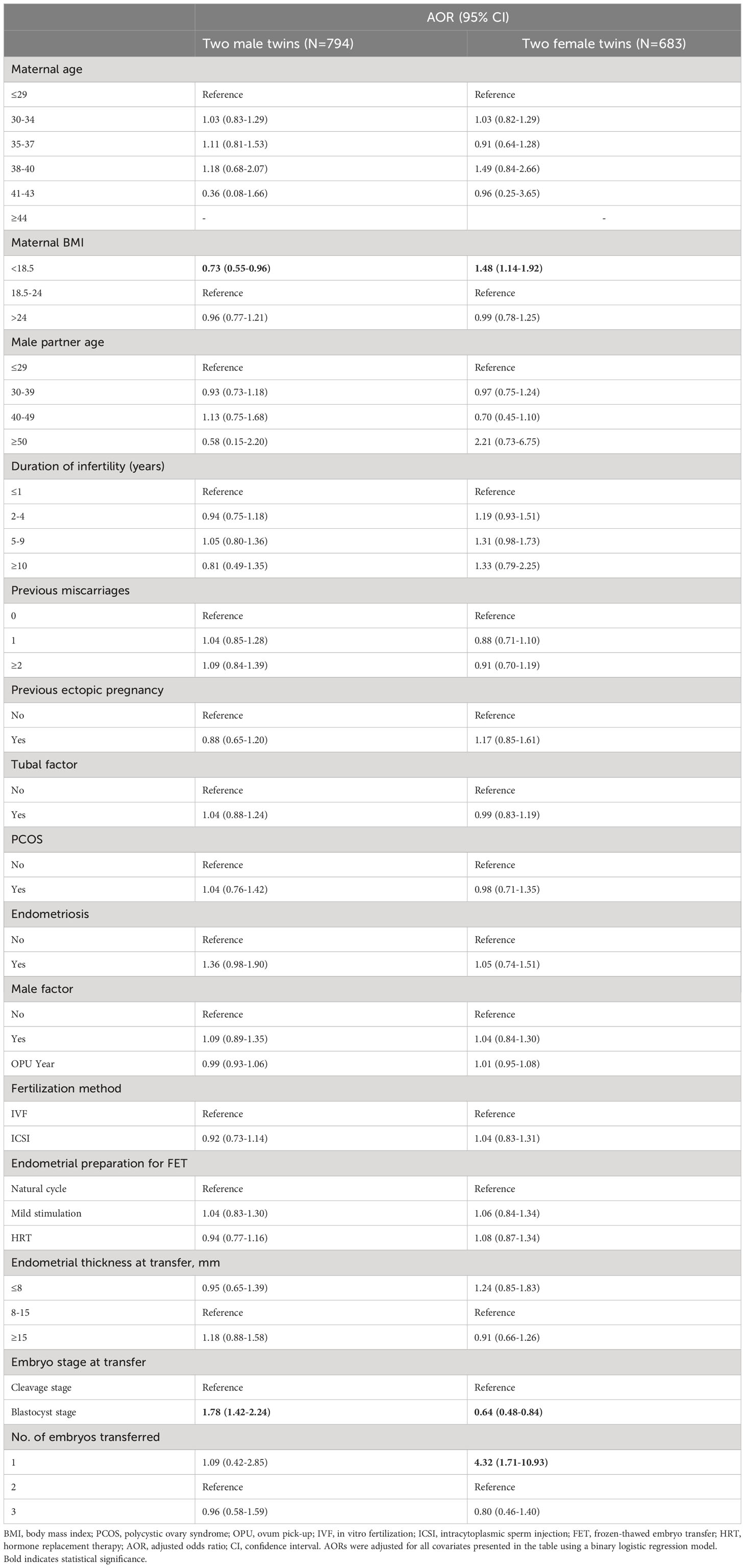
Table 5 AORs for live birth of two male twins and two female twins according to patient characteristics and treatment parameters.
Discussion
This retrospective cohort study not only confirmed previous findings regarding the association between blastocyst transfer and a skew towards male offspring, as well as the tendency for ICSI to result in fewer male offspring, but also expanded upon these findings by analyzing the rate of live births of each gender. The findings revealed that ICSI was linked to a lower rate of male live births, and that blastocyst transfer favored male live births over female live births.
Jacobsen et al. analyzed a population of over 800,000 babies born in Denmark between 1980 and 1993, establishing a natural reference point for the male-to-female ratio at birth of approximately 51.2% males (5). As summarized in Table 1, previous studies have reported male-to-female ratios following IVF ranging from 50.8% males to 52.6% males (18–23). In the present study, the overall male-to-female ratio was 52.3% males, surpassing the figures reported in previous studies. This discrepancy may be attributed to variations in the incidence of various risk factors. Our study involved a lower rate of use of ICSI (29.5%) compared to previous studies (44.8%–61.9%) (18, 19, 22), while a larger proportion of patients in our study underwent blastocyst transfer (14.5%) compared to a study conducted in 2014 (10.9%) (20).
The relationship between maternal age and the male-to-female ratio at birth remains controversial. Rueness et al. have reported a positive association between maternal age and male-to-female ratio at birth, attributing this association to an increased risk of miscarriage related to adverse events during pregnancy in female fetuses (7). Conversely, Matsuo et al. have reported that advanced maternal age is associated with a higher likelihood of female offspring (31). Beyond these two studies, most research has failed to establish a significant relationship between maternal age and the male-to-female ratio at birth (18, 21, 22, 32). In our study, we observed that advanced maternal age was associated with a decreased live birth rate for both genders, but did not influence the final male-to-female ratio.
Our study identifies a possible mechanism underlying the alteration in sex ratio associated with ICSI (19–21, 24). Specifically, we found that ICSI was correlated with a decreased likelihood of male live births, and this may be attributable to selection preference in ICSI procedures. Unlike IVF, ICSI involves the artificial selection of spermatozoa, primarily based on their morphology and motility. A prospective randomized study has shown that intracytoplasmic morphologically selected sperm injection (IMSI), in which a high-magnification microscope is employed for sperm selection, results in a higher proportion of female embryos compared to standard ICSI (66.9% vs. 52.5%, respectively). Additionally, it was observed that morphologically normal spermatozoa were less likely to carry the Y chromosome (33). Consequently, Y-bearing spermatozoa might be less likely to be selected in the artificial selection process involved in ICSI, leading to a reduced chance of male live births. Furthermore, it is noteworthy that oocytes exhibit higher susceptibility to Y-bearing spermatozoa, which suggests that oocytes might have a greater tendency to be fertilized by Y-bearing spermatozoa in IVF compared to ICSI (34–36). However, it is important to note that the advantage of Y-bearing spermatozoa in IVF, in terms of fertilization chance, may be eliminated in ICSI procedures. Although ICSI is commonly recommended for patients with severe male factor infertility, such as severe oligoasthenoteratozoospermia (37), neither previous studies (21, 38) nor our current study have identified any association between male factor infertility and the male-to-female ratio at birth, suggesting that the ICSI procedure itself may act as an independent factor influencing the male-to-female ratio.
In line with previous studies (19, 21, 25–27), our findings also showed that blastocyst transfer was associated with a significantly higher male-to-female ratio at birth compared to cleavage embryo transfer. Remarkably, this association was also observed in the subgroup of all live births of twins. Furthermore, our study revealed a possible previously unrecognized mechanism underlying this association: a sex-related differential response to blastocyst culture in vitro. We found that blastocyst culture in vitro increased the chance of live birth by 70% for male embryos, whereas the increase for female embryos was only 26%. Two potential explanations for this effect can be considered.
First, it is possible that male embryos exhibit faster growth rates than female embryos, resulting in better morphological grades. Alfarawati et al. found that male embryos were 2.6 times more likely to develop into grade 5 or 6 blastocysts compared to female embryos. Additionally, they reported that among the slowest-growing embryos (grade ≤ 3), 60% (124 of 207) were female, while only 40% (83 of 207) were male (39). A study by Ray additionally showed that male embryos have more cells on Day 2 compared to female embryos (40). Similarly, Pergament et al. observed that the percentage of male embryos with four or more cells on Day 2 was six times higher than that of female embryos (41). Animal studies have indicated that male embryos tend to develop at a faster rate than female embryos, resulting in a higher proportion of good-quality male embryos on Day 3 (42–44). Dumoulin et al. counted blastocyst cell numbers and found that male blastocysts derived from ICSI had more cells than female blastocysts (106.00 ± 9.06 vs. 65.00 ± 9.17, P < 0.01) (45). One possible explanation for the delayed development observed in female embryos is their higher requirement for glucose during the pre-implantation stage compared to male embryos (46–48). Although several studies have shown that there is no sex imbalance among blastocysts (39, 49), it is important to consider the selection process of blastocysts for transfer, which was primarily based on morphological criteria, such as cell number and degree of tightness, as well as developmental stage according to the Gardner and Schoolcraft grade system (29). Hence, when embryos are assessed at roughly the same time point, male embryos (with their higher cell count) may tend to receive better grades, potentially resulting in an increased chance of selection of male embryos for transfer.
Second, there is a possibility that the in vitro environment may have an adverse effect in terms of X chromosome inactivation (XCI), which in turn may impair the development of female embryos. At the appropriate time, XCI is a crucial step in the normal development of female embryos (50). However, studies have suggested that an unphysiological environment might lead to precocious random XCI in human embryonic stem cells (51). In the context of bovine embryos, Oliveira et al. found that in vitro culture was associated with higher expression of XIST, a major controller of XCI, compared to in vivo conditions (52). Interference with the appropriate timing of XCI during in vitro culture could potentially disrupt the normal process of implantation and development, and even lead to early embryonic death.
To the best of our knowledge, this study is the first to explore the influence of risk factors on the live birth rate for each gender, providing new insights into the mechanisms underlying the skewed male-to-female ratio associated with IVF/ICSI and FET. Another key strength of this study lies in the comprehensive exploration of the association between the male-to-female ratio at birth and various factors involved in ART, including the clinical characteristics of infertile couples and treatment interventions, on which is there is little information in the existing literature. Additionally, the relatively large sample size of this study ensures more reliable modeling and reduces potential bias.
The major weakness of this study is its retrospective and non-randomized design, which introduces the possibility of unknown confounding factors. In addition, data on known confounders such as adverse environmental exposure and psychological conditions (14, 16, 17) were not available in our database. Another limitation is the absence of data on the gender of embryos with an outcome of embryonic death or miscarriage, which constrains further exploration of the underlying mechanisms contributing to the gender bias.
Conclusion
ICSI was found to be associated with a decreased male-to-female ratio and a lower rate of male live births in FET cycles, while blastocyst transfer was associated with an increased male-to-female ratio at birth and a higher likelihood of male live birth compared to female live birth.
Data availability statement
The data underlying this article cannot be shared publicly to protect the privacy of individuals included in the study.
Author contributions
TD, BL, and ZY conceived and designed this study. TD, JQ, SZ, JL, and DZ participated in data collection. TD, QX, JY, SZ, JL, and DZ analyzed and interpreted the data. QX, TD, XW, JL, and DZ participated in drafting the manuscript. All the authors participated in making revisions to the article, as well as reading and approving the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (grant numbers 82201912, 82271693 and 82071615) and by the Shanghai Sailing Program (21YF1423200).
Acknowledgments
The authors would like to express their sincerest gratitude to all the staff of the Department of Assisted Reproduction, Shanghai Ninth People’s Hospital, and all the patients who participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Steptoe PC, Edwards RG. Birth after the reimplantation of a human embryo. Lancet (1978) 2(8085):366. doi: 10.1016/s0140-6736(78)92957-4
2. Adamson GD, Dyer S, Zegers-Hochschild F, Chambers G, De Mouzon J, Ishihara O, et al. O-154 ICMART preliminary world report 2019. Hum Reprod (2023) 38 (Suppl 1):dead093.187. doi: 10.1093/humrep/dead093.187
3. De Geyter C, Wyns C, Calhaz-Jorge C, de Mouzon J, Ferraretti AP, Kupka M, et al. 20 years of the European IVF-monitoring Consortium registry: What have we learned? A comparison with registries from two other regions. Hum Reprod (2020) 35(12):2832–49. doi: 10.1093/humrep/deaa250
4. Yang X, Li Y, Li C, Zhang W. Current overview of pregnancy complications and live-birth outcome of assisted reproductive technology in mainland China. Fertil Steril (2014) 101(2):385–91. doi: 10.1016/j.fertnstert.2013.10.017
5. Jacobsen R, Møller H, Mouritsen A. Natural variation in the human sex ratio. Hum Reprod (1999) 14(12):3120–5. doi: 10.1093/humrep/14.12.3120
6. Chen R, Zhang L. Imbalance in china's sex ratio at birth: a review. J Econ Surv (2019) 33:1050–69. doi: 10.1111/joes.12309
7. Rueness J, Vatten L, Eskild A. The human sex ratio: Effects of maternal age. Hum Reprod (2012) 27(1):283–7. doi: 10.1093/humrep/der347
8. Ządzińska E, Rosset I, Mikulec A, Domański C, Pawłowski B. Impact of economic conditions on the secondary sex ratio in a post-communist economy. Homo (2011) 62(3):218–27. doi: 10.1016/j.jchb.2011.03.002
9. Zhu WX, Lu L, Hesketh T. China's excess males, sex selective abortion, and one child policy: Analysis of data from 2005 national intercensus survey. BMJ (2009) 338:b1211. doi: 10.1136/bmj.b1211
10. Macmahon B, Pugh TF. Sex ratio of white births in the United States during the Second World War. Am J Hum Genet (1954) 6(2):284–92.
11. Nicolich MJ, Huebner WW, Schnatter AR. Influence of parental and biological factors on the male birth fraction in the United States: An analysis of birth certificate data from 1964 through 1988. Fertil Steril (2000) 73(3):487–92. doi: 10.1016/s0015-0282(99)00576-2
12. Jacobsen R. Parental ages and the secondary sex ratio. Hum Reprod (2001) 16(10):2244. doi: 10.1093/humrep/16.10.2244
13. Fukuda M, Fukuda K, Shimizu T, Møller H. Decline in sex ratio at birth after Kobe earthquake. Hum Reprod (1998) 13(8):2321–2. doi: 10.1093/humrep/13.8.2321
14. Fukuda M, Fukuda K, Shimizu T, Andersen CY, Byskov AG. Parental periconceptional smoking and male: Female ratio of newborn infants. Lancet (2002) 359(9315):1407–8. doi: 10.1016/S0140-6736(02)08362-9
15. Cagnacci A, Renzi A, Arangino S, Alessandrini C, Volpe A. Influences of maternal weight on the secondary sex ratio of human offspring. Hum Reprod (2004) 19(2):442–4. doi: 10.1093/humrep/deh071
16. Catalano RA. Sex ratios in the two Germanies: a test of the economic stress hypothesis. Hum Reprod (2003) 18(9):1972–5. doi: 10.1093/humrep/deg370
17. Ryan JJ, Amirova Z, Carrier G. Sex ratios of children of Russian pesticide producers exposed to dioxin. Environ Health Perspect (2002) 110(11):A699–701. doi: 10.1289/ehp.021100699
18. Cirkel C, König IR, Schultze-Mosgau A, Beck E, Neumann K, Griesinger G. The use of intracytoplasmic sperm injection is associated with a shift in the secondary sex ratio. Reprod BioMed Online (2018) 37(6):703–8. doi: 10.1016/j.rbmo.2018.09.009
19. Dean JH, Chapman MG, Sullivan EA. The effect on human sex ratio at birth by assisted reproductive technology (ART) procedures–an assessment of babies born following single embryo transfers, Australia and New Zealand, 2002-2006. BJOG (2010) 117(13):1628–34. doi: 10.1111/j.1471-0528.2010.02731.x
20. Bu Z, Chen ZJ, Huang G, Zhang H, Wu Q, Ma Y, et al. Live birth sex ratio after in vitro fertilization and embryo transfer in China–an analysis of 121,247 babies from 18 centers. PLoS One (2014) 9(11):e113522. doi: 10.1371/journal.pone.0113522
21. Luke B, Brown MB, Grainger DA, Baker VL, Ginsburg E, Stern JE. Society for assisted reproductive technology writing group. The sex ratio of singleton offspring in assisted-conception pregnancies. Fertil Steril (2009) 92(5):1579–85. doi: 10.1016/j.fertnstert.2008.08.107
22. Arikawa M, Jwa SC, Kuwahara A, Irahara M, Saito H. Effect of semen quality on human sex ratio in in vitro fertilization and intracytoplasmic sperm injection: An analysis of 27,158 singleton infants born after fresh single-embryo transfer. Fertil Steril (2016) 105(4):897–904. doi: 10.1016/j.fertnstert.2015.12.009
23. Ishihara O, Araki R, Kuwahara A, Itakura A, Saito H, Adamson GD. Impact of frozen-thawed single-blastocyst transfer on maternal and neonatal outcome: An analysis of 277,042 single-embryo transfer cycles from 2008 to 2010 in Japan. Fertil Steril (2014) 101(1):128–33. doi: 10.1016/j.fertnstert.2013.09.025
24. Supramaniam PR, Mittal M, Ohuma EO, Lim LN, McVeigh E, Granne I, et al. Secondary sex ratio in assisted reproduction: An analysis of 1 376 454 treatment cycles performed in the UK. Hum Reprod Open (2019) 2019(4):hoz020. doi: 10.1093/hropen/hoz020
25. Hentemann MA, Briskemyr S, Bertheussen K. Blastocyst transfer and gender: IVF versus ICSI. J Assist Reprod Genet (2009) 26(8):433–6. doi: 10.1007/s10815-009-9337-3
26. Practice Committee of the American Society for Reproductive Medicine. Blastocyst production and transfer in clinical assisted reproduction. Fertil Steril (2004) 82(Suppl 1):S149–50. doi: 10.1016/j.fertnstert.2004.05.036
27. Chang HJ, Lee JR, Jee BC, Suh CS, Kim SH. Impact of blastocyst transfer on offspring sex ratio and the monozygotic twinning rate: A systematic review and meta-analysis. Fertil Steril (2009) 91(6):2381–90. doi: 10.1016/j.fertnstert.2008.03.066
28. Du T, Chen H, Fu R, Chen Q, Wang Y, Mol BW, et al. Comparison of ectopic pregnancy risk among transfers of embryos vitrified on day 3, day 5, and day 6. Fertil Steril (2017) 108(1):108–116.e1. doi: 10.1016/j.fertnstert.2017.05.027
29. Gardner DK, Schoolcraft WB. In vitro culture of human blastocyst. In: Jansen R, Mortimer D, editors. Towards reproductive certainty: infertility and genetics beyond 1999. Carnforth: Parthenon Press (1999). p. 378–88.
30. Du T, Wang Y, Fan Y, Zhang S, Yan Z, Yu W, et al. Fertility and neonatal outcomes of embryos achieving blastulation on Day 7: Are they of clinical value? Hum Reprod (2018) 33(6):1038–51. doi: 10.1093/humrep/dey092
31. Matsuo K, Ushioda N, Udoff LC. Parental aging synergistically decreases offspring sex ratio. J Obstet Gynaecol Res (2009) 35(1):164–8. doi: 10.1111/j.1447-0756.2008.00836.x
32. Orzack SH, Stubblefield JW, Akmaev VR, Colls P, Munné S, Scholl T, et al. The human sex ratio from conception to birth. Proc Natl Acad Sci USA (2015) 112(16):E2102–11. doi: 10.1073/pnas.1416546112
33. Setti AS, Figueira RC, Braga DP, Iaconelli A Jr, Borges E Jr. Gender incidence of intracytoplasmic morphologically selected sperm injection-derived embryos: a prospective randomized study. Reprod BioMed Online (2012) 24(4):420–3. doi: 10.1016/j.rbmo.2012.01.007
34. Tarín JJ, García-Pérez MA, Hermenegildo C, Cano A. Changes in sex ratio from fertilization to birth in assisted-reproductive-treatment cycles. Reprod Biol Endocrinol (2014) 12:56. doi: 10.1186/1477-7827-12-56
35. Grant VJ, Chamley LW. Can mamMalian mothers influence the sex of their offspring peri-conceptually? Reproduction (2010) 140(3):425–33. doi: 10.1530/REP-10-0137
36. Ellis PJ, Yu Y, Zhang S. Transcriptional dynamics of the sex chromosomes and the search for offspring sex-specific antigens in sperm. Reproduction (2011) 142(5):609–19. doi: 10.1530/REP-11-0228
37. Esteves SC, Roque M, Bedoschi G, Haahr T, Humaidan P. Intracytoplasmic sperm injection for male infertility and consequences for offspring. Nat Rev Urol (2018) 15(9):535–62. doi: 10.1038/s41585-018-0051-8
38. Jacobsen R, Bostofte E, Skakkebaek NE, Hansen J, Moller H. Offspring sex ratio of subfertile men and men with abnormal sperm characteristics. Hum Reprod (2000) 15(11):2369–70. doi: 10.1093/humrep/15.11.2369
39. Alfarawati S, Fragouli E, Colls P, Stevens J, Gutiérrez-Mateo C, Schoolcraft WB, et al. The relationship between blastocyst morphology, chromosomal abnorMality, and embryo gender. Fertil Steril (2011) 95(2):520–4. doi: 10.1016/j.fertnstert.2010.04.003
40. Ray PF, Conaghan J, Winston RM, Handyside AH. Increased number of cells and metabolic activity in male human preimplantation embryos following in vitro fertilization. J Reprod Fertil (1995) 104(1):165–71. doi: 10.1530/jrf.0.1040165
41. Pergament E, Fiddler M, Cho N, Johnson D, Holmgren WJ. Sexual differentiation and preimplantation cell growth. Hum Reprod (1994) 9(9):1730–2. doi: 10.1093/oxfordjournals.humrep.a138783
42. Avery B, Madison V, Greve T. Sex and development in bovine in-vitro fertilized embryos. Theriogenology (1991) 35(5):953–63. doi: 10.1016/0093-691x(91)90306-x
43. Valdivia RP, Kunieda T, Azuma S, Toyoda Y. PCR sexing and developmental rate differences in preimplantation mouse embryos fertilized and cultured in vitro. Mol Reprod Dev (1993) 35(2):121–6. doi: 10.1002/mrd.1080350204
44. Boklage CE. The epigenetic environment: Secondary sex ratio depends on differential survival in embryogenesis. Hum Reprod (2005) 20(3):583–7. doi: 10.1093/humrep/deh662
45. Dumoulin JC, Derhaag JG, Bras M, Van Montfoort AP, Kester AD, Evers JL, et al. Growth rate of human preimplantation embryos is sex dependent after ICSI but not after IVF. Hum Reprod (2005) 20(2):484–91. doi: 10.1093/humrep/deh614
46. Garcia-Herreros M, Aparicio IM, Rath D, Fair T, Lonergan P. Differential glycolytic and glycogenogenic transduction pathways in male and female bovine embryos produced in vitro. Reprod Fertil Dev (2012) 24(2):344–52. doi: 10.1071/RD11080
47. Nasiri N, Karimian L, Hassani F, Gourabi H, Alipour H, Zolfaghari Z, et al. Total antioxidant capacity; A potential biomarker for non-invasive sex prediction in culture medium of preimplantation human embryos. Cell J (2019) 21(3):253–8. doi: 10.22074/cellj.2019.6115
48. Gardner DK, Wale PL, Collins R, Lane M. Glucose consumption of single post-compaction human embryos is predictive of embryo sex and live birth outcome. Hum Reprod (2011) 26(8):1981–6. doi: 10.1093/humrep/der143
49. Wang A, Kort J, Behr B, Westphal LM. Euploidy in relation to blastocyst sex and morphology. J Assist Reprod Genet (2018) 35(9):1565–72. doi: 10.1007/s10815-018-1262-x
50. Schulz EG, Heard E. Role and control of X chromosome dosage in mamMalian development. Curr Opin Genet Dev (2013) 23(2):109–15. doi: 10.1016/j.gde.2013.01.008
51. Lengner CJ, Gimelbrant AA, Erwin JA, Cheng AW, Guenther MG, Welstead GG, et al. Derivation of pre-X inactivation human embryonic stem cells under physiological oxygen concentrations. Cell (2010) 141(5):872–83. doi: 10.1016/j.cell.2010.04.010
Keywords: blastocyst transfer, FET, ICSI, IVF, male-to-female ratio
Citation: Du T, Xie Q, Ye J, Wang X, Qiu J, Yan Z, Zhang S, Zhao D, Lin J and Li B (2023) Factors affecting male-to-female ratio at birth in frozen-thawed embryo transfer cycles: a large retrospective cohort study. Front. Endocrinol. 14:1188433. doi: 10.3389/fendo.2023.1188433
Received: 17 March 2023; Accepted: 31 July 2023;
Published: 20 September 2023.
Edited by:
Gedis Grudzinskas, Self-employed, London, United KingdomReviewed by:
Angela Vidal, University of Lucerne, SwitzerlandMete Işıkoğlu, Future IVF Center, Türkiye
Copyright © 2023 Du, Xie, Ye, Wang, Qiu, Yan, Zhang, Zhao, Lin and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong Zhao, aGVuZHJ5ekBnbWFpbC5jb20=; Jiaying Lin, bGVtb25fMTExNEAxMjYuY29t; Bin Li, bGliaW5saWNjY0AxNjMuY29t
†These authors share first authorship
 Tong Du
Tong Du Qin Xie
Qin Xie Jing Ye
Jing Ye Xindi Wang2
Xindi Wang2 Zheng Yan
Zheng Yan Bin Li
Bin Li