- 1Department of Pathology, Shanghai Sixth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2The Key Laboratory of Experimental Teratology, Ministry of Education and Department of Pathology, School of Basic Medical Sciences, Shandong University, Jinan, Shandong, China
- 3Department of Environmental Health, Division of Health Risk Factors Monitoring and Control, Shanghai Municipal Center for Disease Control and Prevention, State Environmental Protection Key Laboratory of Environmental Health Impact Assessment of Emerging Contaminants, Shanghai, China
Background: Decabromodiphenyl ether (BDE209), an essential industrial flame retardant that is widely used, has recently been reported to be increasing in human serum. Due to the structural similarity between BDE209 and thyroid hormones, its toxic effects on the thyroid are of particular concern.
Methods: Original articles in the PubMed database were collected using the terms “BDE209”, “decabromodiphenyl ether”, “endocrine disrupting”, “thyroid”, “carcinogenesis”, “polybrominated diphenyl ethers”, “PBDEs,” and their synonyms from inception up to October of 2022.
Results: Of the 748 studies initially identified, 45 were selected, which emphasized the adverse effects of BDE209 on endocrine system. BDE209 may have a toxic effect not only on thyroid function but also on thyroid cancer tumorigenesis at multiple levels, such as by directly interfering with the TR, hypothalamic-pituitary-thyroid (HPT) axis, enzyme activity, and methylation. However, it is impossible to draw a definitive conclusion on the exact pathway of thyroid toxicity from BDE209.
Conclusions: Although the toxic effects of BDE209 on the thyroid have been well investigated, its tumorigenic effects remain unclear and further research is necessary.
1 Introduction
Decabromodiphenyl ether (BDE209) is a member of the polybrominated diphenyl ether (PBDE) family. As the most common brominated flame retardant (BFR), it is widely used in industrial and daily essentials such as electronic equipment, building materials, and textiles. The demand and application of PBDEs in China are extensive, and the production hubs are concentrated in Shandong and Jiangsu. The consumption amount of BDE209 is more than 75% of the total BFRs (1, 2) because it is inexpensive and convenient. However, due to its lack of binding effects from chemical bonds, BDE209 easily escapes from the matrix material into the environment (3). It is fat-soluble and is widely detected in the atmosphere, water resources, soil, animals, and human bodies (4, 5). BDE209 was included as a persistent organic pollutant (POP) under the Stockholm Convention in 2009. The Chinese Ministry of Environmental Protection and the National Health Commission of China launched the List of Priority Control Chemicals (first batch) in 2017, which included BDE209 as a priority control chemical. Nevertheless, due to its stability and permanence in the environment, the risks to human health still remain. It is reported that residents of the producing area have serum concentrations of BDE209 as high as 3100 ng/g lipid weights (lw) (4), which is associated with diseases such as thyroid dysfunction. Therefore, it is urgent to investigate the toxic effects and potential mechanisms of BDE209 on the thyroid gland.
2 Materials and methods
2.1 Search strategy
The present systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines for relevant studies published through October 2022 using the PubMed database (6). The search strategy was developed using the Medical Subject Headings (MeSH)-related terms/subheadings, using the terms “BDE209”, “decabromodiphenyl ether”, “endocrine disrupting”, “thyroid”, “carcinogenesis”, “polybrominated diphenyl ethers”, and “PBDEs”. The search strategy was modified for each database. Articles not written in English were excluded. Manual backward and forward citation searches were also conducted to ensure that no publications were missed.
2.2 Study selection
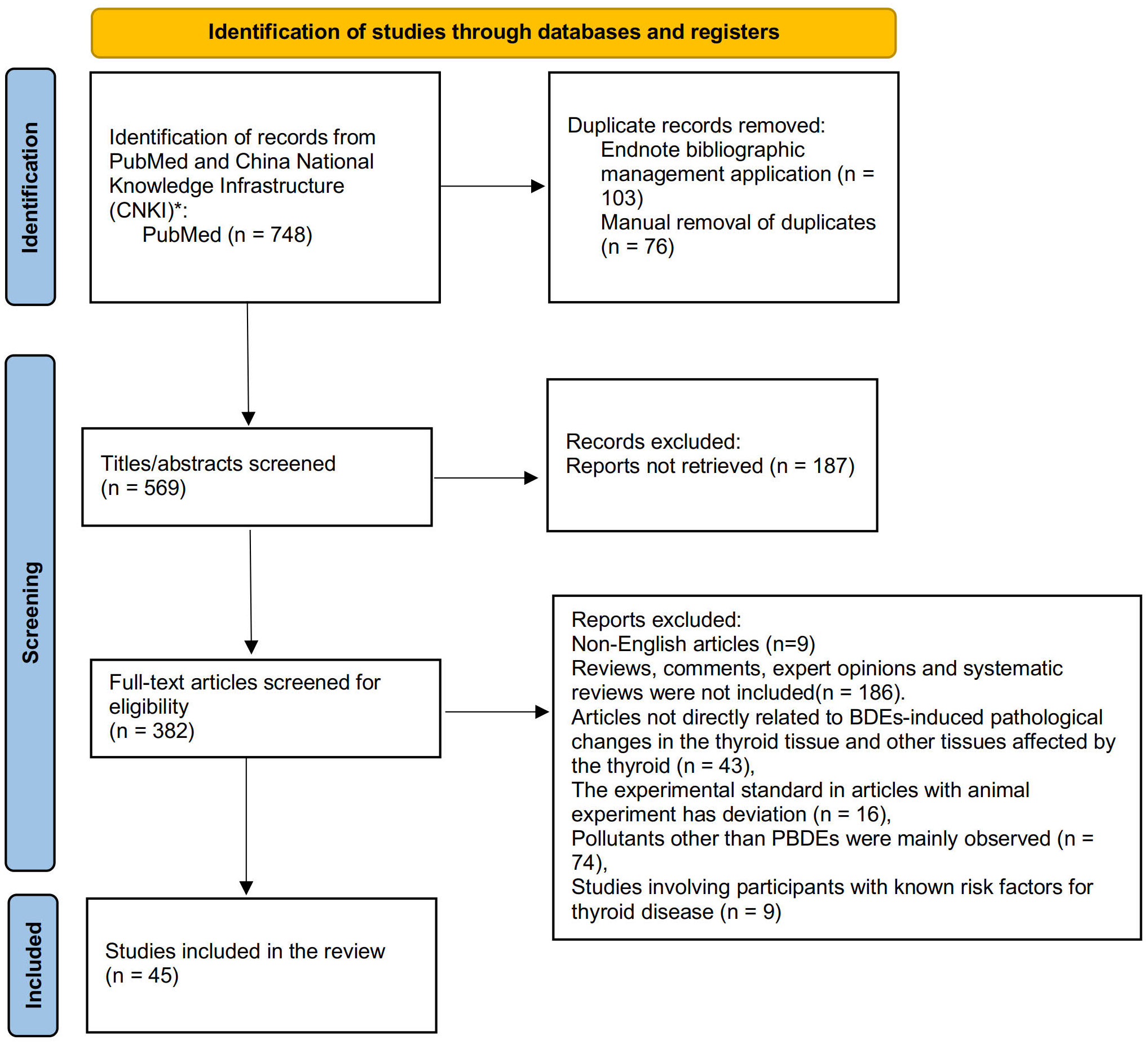
Figure 1 depicts the results of the PRISMA search strategy. After duplicate reports were removed, 382 articles were identified. The inclusion criteria for this systematic review are as follows:
1. Original articles describing the effect of BDE209 exposure on thyroid tissue and thyroid-affected tissues (e.g., normal thyroid tissue, thyroid tumor tissue, or blood).
2. Thyroid function tests and/or at least one imaging technique (thyroid ultrasound or radionuclide study), cytological confirmation (fine needle aspiration), or medical certificate issued by a hospital were used to diagnose the impact on thyroid function.
3. Original articles describing the evidence and possible mechanism of the association between PBDEs (especially BDE209) and thyroid disease across studies and summarizing them at different levels (thyroid endocrine disturbance, potential carcinogenicity).
Reviews, comments, expert opinions, and systematic reviews were not included. The titles and abstracts of the retained publications were independently screened by two authors (YW and XW) to determine whether they met the criteria for inclusion. Following that, the authors (ZL and SS) screened the full texts of the retained publications to assess their eligibility for inclusion.
3 Results
Through a rigorous search of the PubMed database, a total of 45 studies emphasizing the adverse effects of BDE209 on thyroid function were identified. These studies were divided into two broad categories based on their methodology: epidemiologic studies and studies on hypothesized mechanisms. Epidemiologic studies were summarized in Supplementary Table 1. Studies on hypothesized mechanisms were summarized in Supplementary Table 2.
4 Exposure and bioconcentration of BDE209 in the environment
PBDEs have the general chemical formula C12H (0–9)Br(10-1)O and include 209 congeners. BDE209 is the most commonly used PBDE and has the highest bromine content.
BDE209 is more likely to be particle-bound in the atmosphere and has higher lipid solubility on the ground (7). This has drawn increasing attention to its toxicity to human health. In addition to air, BDE209 can be dispersed in the natural environment through the movement of water currents, sediments, and soil (8). Because of its lipid solubility, BDE209 accumulates easily in organisms and produces biomagnification along the food chain. In nature, zebrafish larvae exposed to BDE209 accumulated 10 times more BDE209 than the control group (9). Terrestrial biota have higher levels of BDE209 than aquatic biota.
There has been research on the correlation between BDE209 exposure and human serum levels. The closer to the electronic recycling and BDE209 manufacturing sites, the more severe the contamination is. Serum concentrations of BDE209 in occupational workers from a PBDE manufacturing plant ranged from 67.4 to 109,000 ng/g lw, with a median of 3420 ng/g lw, contributing to 93.1% of the total PBDEs (10). Hu et al. found that BDE209 was the highest measured amount (accounting for 73.6% of the total PBDEs) when they evaluated the levels of PBDEs in the human serum of adult residents along the Yangtze River (11). Sabrina et al. found that the geometric mean plasma concentration of BDE209 was the highest of the PBDE congeners (18 ng/g lw, geometric standard deviation [GSD]: 2.8) among workers exposed to electronic waste recycling in Canada – 10 times higher than the control group (12). Detection rates for BDE209 were slightly higher in women (13).
In the general population, ingestion is the main route of exposure to BDE209. For the population with occupational exposure, the main modes are inhalation and skin exposure. For infants, these pathways also include placental and breast milk exposure. The exposure level, body burden, and toxic effect were higher in infants than in adults (14).
It is noteworthy that many studies have shown that BDE209 can affect thyroid hormone homeostasis in vivo (15). Thyroid hormone (TH) is the essential hormone for growth, development, and reproduction. It is more susceptible to being influenced by exogenous interfering substances when the thyroid system is still developing in the early stages of life (16). Debarshi et al. indicated that BDE209 could affect the reproductive potential of the testis, a THs-responsive organ, by altering THs status (17).
5 Thyroid endocrine disruption by BDE209
As an endocrine-disrupting chemical (EDC), BDE209 may affect thyroid function in several ways (18). As a congener of THs, the connection between BDE209 and thyroid metabolism and diseases has become a hot topic. BDE209 was the only component that had a positive correlation to T3 (ß-coefficient 0.079) and was the relatively largest contributor (66% in ΣPBDE) (15, 19).
In the research on occupational workers, urine and serum concentrations of BDE209 were positively correlated with total thyroxine (TT4, r = 0.270, p = 0.029) and total triiodothyronine (TT3, r = 0.232, p = 0.061). A 10-fold increase in serum BDE209 was associated with a 7.8% increase in TT4 and a 5.4% increase in TT3 (10). However, there were no significant associations between BDE209 in human hair and nail samples and THs or thyroid antibodies, which suggests that sample type and sample size may lead to statistical differences.
In the resident study, no significant associations between PBDEs and thyroid function were observed in 36 anglers in the state of New York. However, Bloom et al. indicated that PBDEs may be positively associated with FT4 when the sample size is increased by approximately nine times (20). In a cross-sectional study of 85 Alaskan natives, there were no significant associations between serum levels of BDE209 and either free or total T4. However, they were significantly associated with THs when BDE47, BDE153, and BDE209 were covariates in the same model (13).
The meta-analysis shows that the relationship between PBDE exposure and thyroid function follows an approximately u-shaped curve. The low level was negatively correlated with THs, while the high level is positively associated with THs (21). The reason for this difference may be related to the sample type, sample size, and different exposure times and doses of BDE209. Future studies need to expand their scope and study population for prospective longitudinal studies and in-depth analysis.
6 Endocrine disruption mechanisms of BDE209
6.1 Effects of BDE209 on the HPT Axis
BDE209 and its metabolites have chemical structures that are similar to those of T3 and T4, so they can bind with thyroid hormone receptors (TRs), thyroid binding globulin (TBG), transthyretin (TTR), and related enzymes. These interactions can have different effects on the synthesis and transport of THs, the accurate regulation of the hypothalamic-pituitary-thyroid (HPT) axis, and thyroid function. The HPT axis is a complicated negative feedback regulation system. Thyrotropin Releasing Hormone (TRH), which is produced by neuroendocrine cells in the hypothalamus, promotes the synthesis and release of thyroid-stimulating hormone (TSH) and THs (T4 and T3). Chevrier et al. found that there was a negative correlation between serum PBDEs and TSH during pregnancy, and the incidence of subclinical hyperthyroidism increased significantly, which may indirectly influence fetal growth (22).
6.2 Effects of BDE209 on thyroid function
Based on animal model studies, BDE209 produces direct and indirect toxic effects on the thyroid gland, and its endocrine-disrupting mechanism may be related to oxidative stress and enzyme activity (23). Higher doses of BDE209 may cause the reduction of thyroglobulin (TG) and thyroid peroxidase (TPO) by the atrophy of the rough endoplasmic reticulum or down-regulation of related genetic alterations (24). Further research showed that PBDEs increased thyroglobulin antibodies and TPO-Ab in the female population (r = 0.453, p = 0.045) (10, 25).
6.3 Effects of BDE209 on thyroid hormone conjugated enzyme
Although PBDEs can conjugate with enzymes involved in the synthesis and metabolism of THs, different congeners may have different substrate metabolic activities. The higher the brominated PBDEs, the stronger the binding affinities with TR and TTR (26). Therefore, BDE209 and its metabolites may disrupt TH homeostasis through strong competitive binding to thyroid hormone transporters (TBG and TTR) to increase fT4 and further reduce T4 half-life in vivo (27, 28). PBDEs can induce UDP-glucuronosyltransferases (UGTs) in the liver and eliminate thyroxine through the bile to cause THs dysfunction. Chen et al. showed that the decreased TT4 level and the increased TT3 level are accompanied by decreased UGT gene transcription after BDE209 exposure (29). Moreover, BDE209 can also conjugate with TR and competitively inhibit THs (30).
6.4 Effects of BDE209 on TH activation
Several animal studies show that BDE209 exposure can regulate the expression or activity of thyroid hormone deiodinases. BDE209 induced upregulation of deiodinase type I (Dio1) activity in human hepatocytes (31) and deiodinase type II (Dio2) activity in larvae (32). However, PBDEs down-regulated Dio2 activity in human glial cells (33). Long-term dietary exposure to BDE209 could cause significant reductions in T4 outer ring deiodination (T4-ORD) and inner ring deiodination (T4-IRD) (34). Qin et al. suggested that BDE209 reduces the expression of the deiodinase type III (Dio3) gene through induction of IG-DMR hypermethylation of the parent substance and inhibition of miR409-3p and miR668-3p expression. Reduced expression and insufficient activity of placental Dio3 lead to the direct exposure of fetal tissues to high levels of maternal active thyroid hormone, which endangers fetal brain development. This toxicity may persist into late fetal growth and early childhood.
BDE209 may enhance the absorption of heavy metals such as lead in earthworms and zebrafish, which would have a synergistic effect on the homeostasis of thyroid hormones. As a result, the comprehensive effect of BDE209 and heavy metals on the thyroid is more complicated and harmful (35–37).
6.5 Effects of BDE209 on thyroid inflammation
The research found that 31% of subjects who had been exposed to PBDEs had higher levels of thyroglobulin antibodies. Abnormal values in thyroglobulin antibodies could be seen in 80-90% of patients with chronic autoimmune thyroiditis and 50-60% of patients with Graves’ disease (25). Therefore, PBDE exposure may be a potential cause of susceptibility to autoimmune thyroid disease.
Different immunologic toxic effects of BDE209 were identified in 2021 (1): atrophy of immune organs (thymus and spleen); hepatomegaly accompanied by increased aspartate aminotransferase and oxidative stress; (2) changes in humoral (immunoglobulins) and cellular (lymphocyte proliferation and cytokine secretion) indices of immunity; (3) dose-dependent alterations in gene expression, leading to Th1/Th2 imbalance (38).
7 Potential carcinogenicity of BDE209
Many case-control studies have recently shown that BDE209 has potential carcinogenicity in biological matrices (39).
The National Toxicology Program (NTP) established an animal model to examine the toxicity of BDE209 in 1986. B6C3F1 mice (50 males and 50 females) were fed diets supplemented with different doses of BDE209 (25 and 50 g/kg) for 103 weeks. Compared to the control group, abnormal proliferation of thyroid follicular cells was found in the female and male groups, and the ratio of follicular thyroid adenomas increased (40). In 2011, Noyes et al. found that long-term dietary exposure to BDE209 caused cellular and structural atypia in thyroid follicles (16). Therefore, BDE209 may cause abnormal thyroid proliferation and thyroid follicular adenoma.
In 2017, Hoffman et al. found that the concentration of BDE209 in indoor dust was significantly associated with the prevalence of papillary thyroid carcinoma (PTC). Participants with above-median BDE209 exposure had a 2.29-fold increased risk compared to those with low BDE209 exposure. High concentrations of BDE209 were significantly associated with PTC with BRAF wild-type (14.2 times, p<0.05) compared to the control group (41). Deziel et al. showed a negative correlation between BDE209 and the pathogenesis of thyroid cancer in 2018 (42). However, several recent studies offer a different opinion. In 2021, Zhang et al. found that BDE209 was positively correlated with the risk of thyroid cancer, and its co-exposure with heavy metals aggravated the risk of developing it (43).
The above studies suggested the potential toxicity and tumorigenic effects of BDE209 on the thyroid gland. The tumorigenic effects may be related to exposure levels, time, methods, and the types of experimental animals used.
8 Study of the carcinogenic mechanisms of BDE209
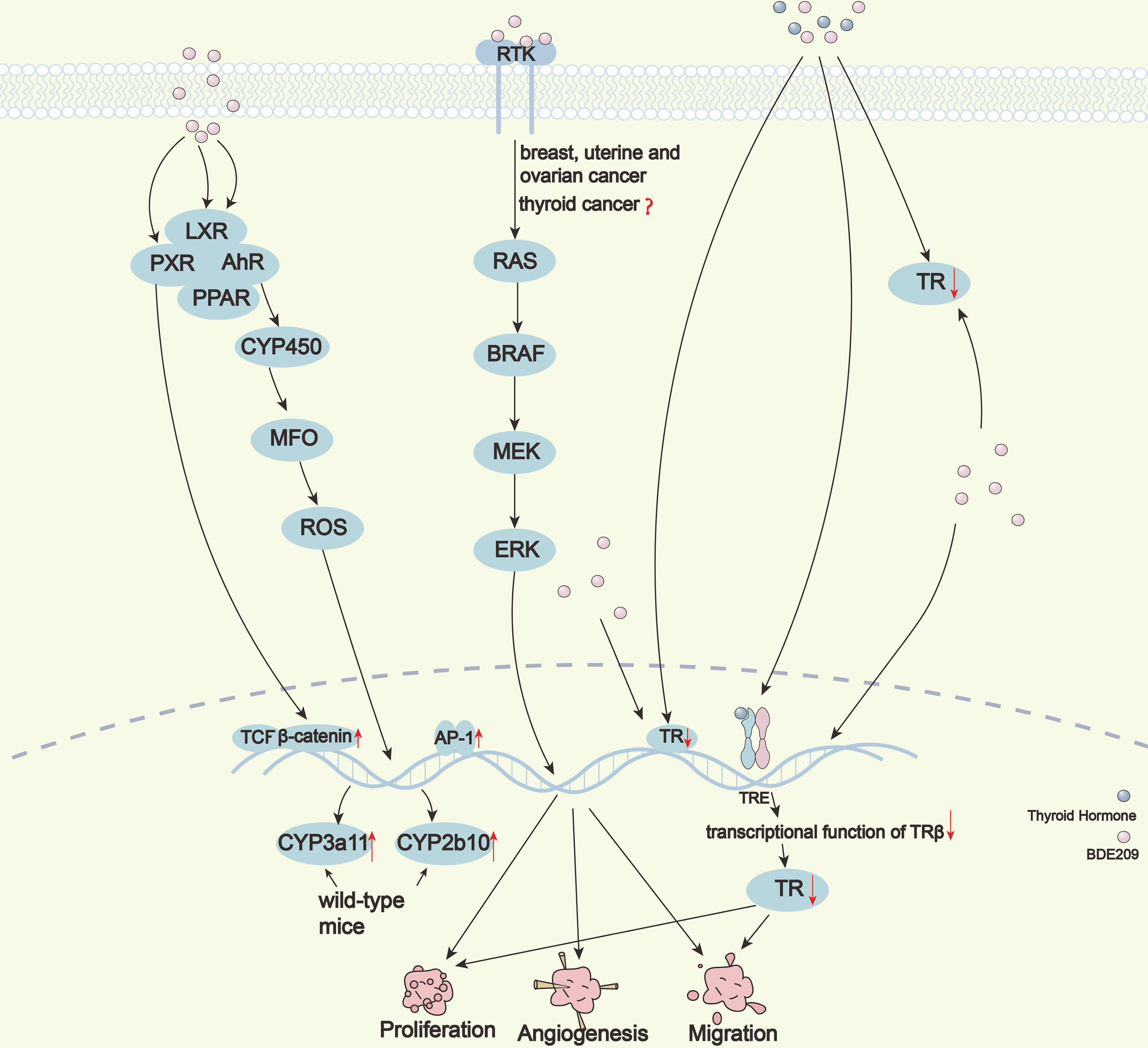
Although it has been widely reported that BDE209 induces thyroid cancer, studies on its potential carcinogenic mechanisms are relatively scarce. In a study using RNA-seq of thyroid tissue samples exposed to BDE209, 501 differentially expressed genes (DEGs) were screened out. After enrichment analysis on DEGs, it was found that BDE209 response-related genes can be significantly enriched in multiple KEGG and GO databases (44). The carcinogenic mechanism of BDE209 is multifaceted and relatively complex (Figure 2).
8.1 Interaction between BDE209 and transcription factors
Natàlia et al. predicted the proteins with which BDE209 may interact by computer target analysis. BDE209 could activate five types of key human transcription factors, such as the aryl hydrocarbon receptor (AhR), peroxisome proliferator response element (PPRE), TCF/ß-catenin, AP-1, and Oct-MLP (9). The low concentration of BDE209 can significantly activate AhR (45); however, different results have also been reported (46). BDE209 can activate the peroxisome proliferator-activated receptor (PPAR) pathway through indirect binding to lipid peroxidation-related receptors (47). High levels of BDE209 can activate TCF/ß-catenin. and AP-1 (46). BDE209 can affect the POU domain family of Oct-MLP, and these proteins are developmental regulatory factors of conservative evolution. These results can explain the carcinogenicity of BDE209 to a certain extent.
8.2 Inhibitory effect of BDE209 on thyroid hormone receptor
Thyroid hormone receptors (TRs) are ligand-dependent transcription factors that mediate the transcription of human growth- and development-related genes. PBDEs can bind to TRs due to the similarity of their chemical structures to THs, and the affinity increases significantly with increasing bromination. BDE209 has the highest degree of bromination and the strongest affinity with TRs among PBDEs (26, 48, 49). BDE209 may dissociate the DNA binding domain of TRs from thyroid hormone response elements (TRE) by competitively combining TRß with TH and then destroying TRß-mediated transcription (50). Recently, our research group demonstrated that BDE209 not only inhibits the transcriptional function of TRß but also directly blocks its gene expression, which then significantly promotes the proliferation of PTC cells both in vivo and in vitro (30). BDE209 may promote malignant transformation of thyroid follicular cells by inhibiting TRß at multiple levels, as supported by analysis of multiple public datasets (51).
8.3 BDE209-mediated thyroid injury through induction of oxidative stress
Reactive oxygen species (ROS) play an important role in cell damage and other aspects. When ROS in cells exceeds the capacity of antioxidant mechanisms, it is called oxidative stress. High levels of ROS may also activate signaling molecules as second messengers in intracellular signaling pathways that regulate cell proliferation and apoptosis (52). BDE47 has been shown to induce DNA damage via regulation of the ROS-induced oxidative stress response to mediate hepatocyte toxicity (53). Therefore, PBDEs could induce tissue damage and carcinogenicity through oxidative stress.
8.4 Upregulation of cytochrome genes induced by BDE209
Cytochrome is one of the important enzymes in mixed functional oxidase (MFO). It could form ROS complexes with molecular oxygen and oxidize into exogenous chemicals. In the cytochrome family, CYP2 and CYP3 are the main isoenzymes responsible for metabolizing drugs. Pacyniak et al. proved for the first time that BDE209 was an activator for exogenous nuclear receptors and that BDE209 could induce up-regulation of CYP3a11 and CYP2b10 mRNA levels by activating PXR in wild-type mice (54). Also, BDE209 can bind to AhR to regulate the function of downstream CYP450 (9). The abnormal expression of the CYP450 gene is associated with many types of tumors, including thyroid cancer. Furthermore, CYP450 can activate the oxidative metabolism of MFO to produce more ROS and oxidative stress, thereby increasing toxicity and becoming mutagenic or carcinogenic.
8.5 MAPK/PI3K pathways activated by BDE209
Thyroid cancer is usually driven by a few mutually exclusive somatic gene mutations or fusions involving the MAPK/PI3K pathways (55–58). Li et al. showed that BDE209 increases the incidence of breast, uterine, and ovarian cancers through the activation of the MAPK signaling pathway (59). Wang et al. found that BDE47 participated in regulating cell proliferation and was related to carcinogenesis in HepG2 cells through the DNA-PKcs/Akt pathway (60). However, it remains unknown whether BDE209 is involved in the pathogenesis of thyroid neoplasms through the MAPK/PI3K pathways.
9 Discussion
Because of its widespread use, common exposure, persistence, and bioaccumulation in the environment, the burden level of BDE209 in organisms is continuously increasing. Low-dose, long-term exposure to environmental BDE209 may pose an enormous threat to health. Moreover, the chronic toxic effect of BDE209 may be more severe in those subjects who are more sensitive to TH disruption, such as pregnant women and people with hyperthyroidism. Therefore, additional research is necessary to reveal its toxic dose and mechanisms of action.
Furthermore, as an endocrine pollutant, the relationship of BDE209 with thyroid diseases and thyroid cancer has been reported many times, but research is scarce on its carcinogenicity and its potential mechanisms in humans. Limited exposure studies of human environmental pollutants usually evaluate the effects of high-dose and short-term exposure, and it is difficult to obtain long-term, low-dose experimental exposure data.
BDE209 is easily metabolized into low-brominated diphenyl ethers and OH-PBDE, which could be affected by measurement error sources, including sample environmental contamination risk and analytical degradation risk. The features of environmental exposure in real life are the coexistence of multiple pollutants to produce synergistic or antagonistic toxicity, which causes much more obvious toxicological effects and more complicated mechanisms. THs are inversely associated with low- and high-brominated congeners, which may cause result errors. To provide a sufficient basis for the revision of the use standard for BDE209 products and the formulation of environmental pollution policy, further research is needed to standardize multiple risk factors, expand study sites, and study populations for in-depth analysis.
Author contributions
Conceptualization, ZL, and SS; methodology, ZL; software, YW and XW; validation, ZL, and SS; formal analysis, ZL; investigation, SS; resources, XW; data curation, YW and XW; writing—original draft preparation, YW and XW; writing—review and editing, YW, XW, and ZL; visualization, ZL, and SS; supervision, ZL; project administration, ZL; funding acquisition, ZL. All authors read and approved the published version of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the National Natural Science Foundation of China (Grant no. 81972500), the Science and Technology Innovation Plan of Science and Technology Commission of Shanghai Municipality (STCSM) (Grant no. 20Z11900304), the Natural Science Fund of the Science and Technology Commission of Shanghai Municipality (STCSM) (Grant no. 23ZR1448200), and the Clinical Research Fund of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital (ZL 2020).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1183815/full#supplementary-material
References
1. Stubbings WA, Harrad S. Extent and mechanisms of brominated flame retardant emissions from waste soft furnishings and fabrics: a critical review. Environ Int (2014) 71:164–75. doi: 10.1016/j.envint.2014.06.007
2. Xiaowen J, Jue D, Xianchuan X, Yu C, Yu H, Long Q, et al. Pollution status and human exposure of decabromodiphenyl ether (Bde-209) in China. ACS omega (2017) 2(7):3333–48. doi: 10.1021/acsomega.7b00559
3. Solomon GM, Weiss PM. Chemical contaminants in breast milk: time trends and regional variability. Environ Health Perspect (2002) 110(6):A339–A47. doi: 10.1289/ehp.021100339
4. Songjie H, Mingyuan L, Jun J, Ying W, Yunjie B, Meng X, et al. Concentrations and trends of halogenated flame retardants in the pooled serum of residents of laizhou bay, China. Environ Toxicol Chem (2013) 32(6):1242–7. doi: 10.1002/etc.2172
5. Xinhui BI, Thomas GO, Jones KC. Exposure of electronics dismantling workers to polybrominated diphenyl Ethers,Polychlorinated biphenyls,and organochlorine pesticides in south China. Environ Sci Technol: ES&T (2007) 41(16):5647–53. doi: 10.1021/es070346a
6. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the prisma statement. PloS Med (2009) 6(7):336–41. doi: 10.1371/journal.pmed.1000097
7. Tittlemier SA, Halldorson T, Stern GA, Tomy GT. Vapor pressures, aqueous solubilities, and henry’s law constants of some brominated flame retardants. Environ Toxicol Chem (2002) 21(9):1804–10. doi: 10.1002/etc.5620210907
8. Van der Ven LTM, van de Kuil T, Leonards PEG, Slob W, Cantón RF, Germer S, et al. A 28-day oral dose toxicity study in wistar rats enhanced to detect endocrine effects of decabromodiphenyl ether (Decabde). Toxicol Lett (2008) 179(1):6–14. doi: 10.1016/j.toxlet.2008.03.003
9. Garcia-Reyero N, Escalon BL, Prats E, Stanley JK, Thienpont B, Melby NL, et al. Effects of bde-209 contaminated sediments on zebrafish development and potential implications to human health. Environ Int (2014) 63:216–23. doi: 10.1016/j.envint.2013.11.012
10. Chen T, Niu P, Kong F, Wang Y, Bai Y, Yu D, et al. Disruption of thyroid hormone levels by decabrominated diphenyl ethers (Bde-209) in occupational workers from a deca-bde manufacturing plant. Environ Int (2018) 120:505–15. doi: 10.1016/j.envint.2018.08.032
11. Ming-Jun H, Jin-Liang Z, Qian Z, Jia-Liu H, Wan-Jun Y, Zhen-Yu Z, et al. Thyroid hormones in relation to polybrominated diphenyl ether and metals exposure among rural adult residents along the Yangtze river, China. Int J Hygiene Environ Health (2021) 236. doi: 10.1016/j.ijheh.2021.113800
12. Gravel S, Lavoué J, Bakhiyi B, Lavoie J, Roberge B, Patry L, et al. Multi-exposures to suspected endocrine disruptors in electronic waste recycling workers: associations with thyroid and reproductive hormones. Int J Hygiene Environ Health (2020) 225(C). doi: 10.1016/j.ijheh.2019.113445
13. BS C, Pamela M, Samarys S-M, Vi W, Loren BC, vHF A, et al. Associations between serum polybrominated diphenyl ethers and thyroid hormones in a cross sectional study of a remote Alaska native population. Sci Rep (2018) 8(1). doi: 10.1038/s41598-018-20443-9
14. Ni K, Lu Y, Wang T, Kannan K, Gosens J, Xu L, et al. A review of human exposure to polybrominated diphenyl ethers (Pbdes) in China. Int J Hygiene Environ Health (2013) 216(6):607–23. doi: 10.1016/j.ijheh.2013.02.002
15. Guo L-C, Xiao J, Zhang Y, Yu S, Lin H, Su G, et al. Association between serum polybrominated diphenyl ethers, new flame retardants and thyroid hormone levels for school students near a petrochemical complex, south China. Chemosphere (2018) 202:476–82. doi: 10.1016/j.chemosphere.2018.03.120
16. Noyes PD, Hinton DE, Stapleton HM. Accumulation and debromination of decabromodiphenyl ether (Bde-209) in juvenile fathead minnows (Pimephales promelas) induces thyroid disruption and liver alterations. Toxicological sciences: an Off J Soc Toxicol (2011) 122(2):265–74. doi: 10.1093/toxsci/kfr105
17. Debarshi S, Kumar SS. Decabromodiphenyl ether (Bde-209) exposure to lactating mice perturbs steroidogenesis and spermatogenesis in adult Male offspring. Ecotoxicol Environ Saf (2021) 209. doi: 10.1016/j.ecoenv.2020.111783
18. Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, et al. Executive summary to edc-2: the endocrine society’s second scientific statement on endocrine-disrupting chemicals. Endocrine Rev (2015) 36(6):593–602. doi: 10.1210/er.2015-1093
19. Huang F, Wen S, Li J, Zhong Y, Zhao Y, Wu Y. The human body burden of polybrominated diphenyl ethers and their relationships with thyroid hormones in the general population in northern China. Sci Total Environ (2014) 466:609–15. doi: 10.1016/j.scitotenv.2013.07.008
20. Bloom M, Spliethoff H, Vena J, Shaver S, Addink R, Eadon G. Environmental exposure to pbdes and thyroid function among new York anglers. Environ Toxicol Pharmacol (2007) 25(3):386–92. doi: 10.1016/j.etap.2007.12.004
21. Xuemin Z, Hailong W, Jing L, Zhongyan S, Weiping T, Xiaochun T. The correlation between polybrominated diphenyl ethers (Pbdes) and thyroid hormones in the general population: a meta-analysis. PloS One (2015) 10(5). doi: 10.1371/journal.pone.0126989
22. Jonathan C, HK G, Asa B, Myriam G, Andreas S, Brenda E. Polybrominated diphenyl ether (Pbde) flame retardants and thyroid hormone during pregnancy. Environ Health Perspect (2010) 118(10):1444–9. doi: 10.1289/ehp.1001905
23. Sarkar D, Joshi D, Singh SK. Maternal bde-209 exposure during lactation causes testicular and epididymal toxicity through increased oxidative stress in peripubertal mice offspring. Toxicol Lett (2019) 311:66–79. doi: 10.1016/j.toxlet.2019.04.028
24. Wang Y, Chen T, Sun Y, Zhao X, Zheng D, Jing L, et al. A comparison of the thyroid disruption induced by decabrominated diphenyl ethers (Bde-209) and decabromodiphenyl ethane (Dbdpe) in rats. Ecotoxicol Environ Saf (2019) 174:224–35. doi: 10.1016/j.ecoenv.2019.02.080
25. TM E, PV W, Pamela I, Lynda K, Robert C, Anderson HA. Hormone disruption by pbdes in adult Male sport fish consumers. Environ Health Perspect (2008) 116(12):1635–41. doi: 10.1289/ehp.11707
26. Ren X-M, Guo L-H, Gao Y, Zhang B-T, Wan B. Hydroxylated polybrominated diphenyl ethers exhibit different activities on thyroid hormone receptors depending on their degree of bromination. Toxicol Appl Pharmacol (2013) 268(3):256–63. doi: 10.1016/j.taap.2013.01.026
27. Meerts I, Zanden J, Luijks E, Leeuwen-Bol IV, Marsh G, Jakobsson E, et al. Potent competitive interactions of some brominated flame retardants and related compounds with human transthyretin in vitro. Oxford University Press (2000) 56(1):95–104. doi: 10.1093/toxsci/56.1.95
28. Hamers T, Kamstra JH, Sonneveld E, Murk AJ, Kester MH, Andersson PL, et al. In vitro profiling of the endocrine-disrupting potency of brominated flame retardants. Toxicological Sci (2006) 92:157–73. doi: 10.1093/toxsci/kfj187
29. Chen Q, Yu L, Yang L, Zhou B. Bioconcentration and metabolism of decabromodiphenyl ether (Bde-209) result in thyroid endocrine disruption in zebrafish larvae. Aquat Toxicol (2012) 110:141–8. doi: 10.1016/j.aquatox.2012.01.008
30. Xinpei W, Xiujie C, Qian Z, Feifei S, Ru Z, Tingting F, et al. Long-term exposure to decabromodiphenyl ether promotes the proliferation and tumourigenesis of papillary thyroid carcinoma by inhibiting trß. Cancers (2022) 14(11). doi: 10.3390/cancers14112772
31. SH M, KS M, Ruoting P J, Claudia G. Metabolism of polybrominated diphenyl ethers (Pbdes) by human hepatocytes in vitro. Environ Health Perspect (2009) 117(2):197–202. doi: 10.1289/ehp.11807
32. Wei L, Lifei Z, Jinmiao Z, Zijian W. Effects of decabromodiphenyl ether (Bde-209) on mrna transcription of thyroid hormone pathway and spermatogenesis associated genes in Chinese rare minnow (Gobiocypris rarus). Environ Toxicol (2014) 29(1):1–9. doi: 10.1002/tox.20767
33. Roberts SC, Bianco AC, Stapleton HM. Disruption of type 2 iodothyronine deiodinase activity in cultured human glial cells by polybrominated diphenyl ethers. Chem Res Toxicol (2015) 28(6):197–202. doi: 10.1021/acs.chemrestox.5b00072
34. Robinson DR, Kalyana-Sundaram S, Wu YM, Shankar S, Cao X, Ateeq B, et al. Functionally recurrent rearrangements of the mast kinase and notch gene families in breast cancer. Nat Med (2011) 17(12):1646–51. doi: 10.1038/nm.2580
35. Tseng L-H, Li M-H, Tsai S-S, Lee C-W, Pan M-H, Yao W-J, et al. Developmental exposure to decabromodiphenyl ether (Pbde 209): effects on thyroid hormone and hepatic enzyme activity in Male mouse offspring. Chemosphere (2008) 70(4):640–7. doi: 10.1016/j.chemosphere.2007.06.078
36. Zhang W, Liu K, Li J, Liang J, Lin K. Impacts of Bde209 addition on Pb uptake, subcellular partitioning and gene toxicity in earthworm (Eisenia fetida). J Hazardous Materials (2015) 300:737–44. doi: 10.1016/j.jhazmat.2015.08.014
37. Marijana C, Saša J, Vesna J, Sanja S, Slavica V, Ksenija D, et al. Combined effects of cadmium and decabrominated diphenyl ether on thyroid hormones in rats. Arhiv za higijenu rada i toksikologiju (2012) 63(3):255–62. doi: 10.2478/10004-1254-63-2012-2179
38. Taotao L, Baorui L, Zhehan Z, Weiwei F, Yao C, Yangyang D, et al. Short-term exposure of decabromodiphenyl ether in female adult Balb/C mice: immune toxicity and self-recovery. Toxicol Lett (2021) 342:26–37. doi: 10.1016/j.toxlet.2021.02.002
39. Briseis A-K, DC T, Mark P, Christopher K, Yawei Z, Andreas S, et al. Polybrominated diphenyl ethers and thyroid cancer risk in the prostate, colorectal, lung, and ovarian cancer screening trial cohort. Am J Epidemiol (2015) 181(11):883–8. doi: 10.1093/aje/kwu358
40. National Toxicology Program. Ntp toxicology and carcinogenesis studies of decabromodiphenyl oxide (Cas no. 1163-19-5) in F344/N rats and B6c3f1 mice (Feed studies). National Toxicology Program (1986) 309:1–242.
41. Hoffman K, Lorenzo A, Butt CM, Hammel SC, Henderson BB, Roman SA, et al. Exposure to flame retardant chemicals and occurrence and severity of papillary thyroid cancer: a case-control study. Environ Int (2017) 107:235–42. doi: 10.1016/j.envint.2017.06.021
42. DN C, Javier A-G L, Huang H, Andreas S, Yawei Z. Exposure to polybrominated diphenyl ethers and a polybrominated biphenyl and risk of thyroid cancer in women: single and multi-pollutant approaches. Cancer epidemiol Biomarkers Prev (2019) 28(10):1755–64. doi: 10.1158/1055-9965.EPI-19-0526
43. Qian Z, Mingjun H, Huabing W, Qingshan N, Xuelei L, Jialiu H, et al. Plasma polybrominated diphenyl ethers, urinary heavy metals and the risk of thyroid cancer: a case-control study in China. Environ pollut (2021) 269. doi: 10.1016/j.envpol.2020.116162
44. Lin C, Junhua Y, Qinxiong R, Zehui L, Wei S, Shuhui G, et al. Toxic effects of decabromodiphenyl ether (Bde-209) on thyroid of broiler chicks by transcriptome profile analysis. Ecotoxicol Environ Saf (2021) 219. doi: 10.1016/j.ecoenv.2021.112305
45. Brown DJ, Ovarmeire IV, Goeyens L, Denison MS, De Vito MJ, Clark GC. Analysis of ah receptor pathway activation by brominated flame retardants. Chemosphere (2004) 55(11):1509–18. doi: 10.1016/j.chemosphere.2003.10.019
46. US EPA National Center for Environmental Assessment IO. Iris toxicological review of decabromodiphenyl ether. (2008).
47. Huang SC, Giordano G, Costa LG. Comparative cytotoxicity and intracellular accumulation of five polybrominated diphenyl ether congeners in mouse cerebellar granule neurons. Toxicological Sci (2010) 114(1):124–32. doi: 10.1093/toxsci/kfp296
48. McDonald TA. A perspective on the potential health risks of pbdes. Chemosphere (2002) 46(5):745–55. doi: 10.1016/S0045-6535(01)00239-9
49. Meerts IA, van Zanden JJ, Luijks EA, van Leeuwen-Bol I, Marsh G, Jakobsson E, et al. Potent competitive interactions of some brominated flame retardants and related compounds with human transthyretin in vitro. Toxicological Sci (2000) 56(1):95–104. doi: 10.1093/toxsci/56.1.95
50. Kingsley I, Toshiharu I, Junko K-K, Wataru M, Noriaki S, Noriyuki K. Disruption of thyroid hormone receptor-mediated transcription and thyroid hormone-induced purkinje cell dendrite arborization by polybrominated diphenyl ethers. Environ Health Perspect (2011) 119(2):168–75. doi: 10.1289/ehp.1002065
51. Yawei Z, GG L, Xuesong H, Cairong Z, KB A, Yong Z, et al. Do polybrominated diphenyl ethers (Pbdes) increase the risk of thyroid cancer? Biosci Hypotheses (2008) 1(4):195–9. doi: 10.1016/j.bihy.2008.06.003
52. Cheung EC, Vousden KH. The role of ros in tumour development and progression. Nat Rev Cancer (2022) 22(5):280–97. doi: 10.1038/s41568-021-00435-0
53. An J, Yin L, Shang Y, Zhong Y, Zhang X, Wu M, et al. The combined effects of Bde47 and bap on oxidatively generated DNA damage in L02 cells and the possible molecular mechanism. MutRes-Genetic Toxicol Environ Mutagenesis (2011) 721(2):192–8. doi: 10.1016/j.mrgentox.2011.02.002
54. Pacyniak EK, Cheng X, Cunningham ML, Crofton K, Klaassen CD, Guo GL. The flame retardants, polybrominated diphenyl ethers, are pregnane X receptor activators. Toxicological Sci (2007) 97(1):94–102. doi: 10.1093/toxsci/kfm025
55. Jakubíková I, Gabalec F, Beránek M, Žák P, Čáp J. The significance of Brafv600e mutation in thyroid cancer in terms of novel targeted therapies - overview of current knowledge and studies. Klinicka onkologie: casopis Ceske Slovenske onkologicke spolecnosti (2018) 31(5):339–44. doi: 10.14735/amko2018339
56. Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in thyroid cancer incidence and mortality in the united states, 1974-2013. JAMA (2017) 317(13):560–70. doi: 10.1001/jama.2017.2719
57. Xing M, Alzahrani AS, Carson KA, Viola D, Elisei R, Bendlova B, et al. Association between braf V600e mutation and mortality in patients with papillary thyroid cancer. JAMA (2013) 309(14):1493–501. doi: 10.1001/jama.2013.3190
58. Liu Z, Kakudo K, Bai Y, Li Y, Ozaki T, Miyauchi A, et al. Loss of cellular Polarity/Cohesiveness in the invasive front of papillary thyroid carcinoma, a novel predictor for lymph node metastasis; possible morphological indicator of epithelial mesenchymal transition. J Clin Pathol (2011) 64(4):325–9. doi: 10.1136/jcp.2010.083956
59. Zhi-Hua L, Xiao-Yan L, Na W, Jing-Si C, Yan-Hong C, Jin-Tao H, et al. Effects of decabrominated diphenyl ether (Pbde-209) in regulation of growth and apoptosis of breast, ovarian, and cervical cancer cells. Environ Health Perspect (2012) 120(4):541–6. doi: 10.1289/ehp.1104051
Keywords: BDE209, endocrine disrupting, carcinogenesis, thyroid hormone, environmental exposure
Citation: Wang Y, Wang X, Sui S and Liu Z (2023) Endocrine disrupting and carcinogenic effects of decabromodiphenyl ether. Front. Endocrinol. 14:1183815. doi: 10.3389/fendo.2023.1183815
Received: 10 March 2023; Accepted: 04 May 2023;
Published: 02 June 2023.
Edited by:
Luca De Toni, University of Padua, ItalyReviewed by:
Andrea Di Nisio, University of Padua, ItalyChiara Castellini, University of L’Aquila, Italy
Copyright © 2023 Wang, Wang, Sui and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiyan Liu, emhpeWFubGl1QHNoc211LmVkdS5jbg==
†These authors have contributed equally to this work
 Yi Wang1†
Yi Wang1† Xinpei Wang
Xinpei Wang Shaofeng Sui
Shaofeng Sui Zhiyan Liu
Zhiyan Liu